Abstract
Naturally colored fermented foods currently represent the trend toward a global demand for healthier products. This work produced naturally blue and green ice creams using C-phycocyanin (C-PC) and spirulina residual biomass (RB). The ice creams were assessed based on microbiological analysis, color stability over 6 months, antioxidant activity before and after in vitro digestion, and sensory evaluation. Considering the microorganisms that must be analyzed in accordance with Brazilian legislation, no growth was detected during the storage period. L*, a*, and b* were maintained according to the expected colors. The blue color was intoned over the shelf life (SC-PC *b −9.46 to −19.44 and MC-PC *b from −9.87 to −18.04). The antioxidant activity of the fermented ice creams SC-PC and SRB increased from 15.4 to 41.3 and from 15.3 to 38.0 µM TE/g, respectively, after bioaccessibility analysis. The C-PC ice cream’s appearance received the highest rating, with 70.26% of volunteers expressing a strong preference, highlighting its attractiveness. However, there were no significant differences compared to control samples in the global acceptance. The RB ice cream presented lower results for flavor but moderate acceptance. Thus, these fermented ice creams presented color stability over 6 months, and their antioxidant activity increased after in vitro digestion, highlighting their biological potential.
1. Introduction
Color plays a crucial role in the food experience, influencing sensory perception and consumer acceptance from the initial encounter with a food preparation or product [1,2,3,4,5]. It not only contributes to visual attractiveness but also sets expectations for the taste and quality of the food. Beyond aesthetics, color provides information about the content of the food, establishing an essential connection between color and flavor, a pivotal element in the gustatory experience [6,7,8,9,10,11]. However, a uniqueness arises when it comes to blue food coloring [2]. Natural blue is less common in nature than the prevalent green, yellow, and red, which have already been extensively commercialized by the industry. This makes natural blue food colorants an innovative prospect for the food industry, aiming to stand out in a highly competitive market [12].
The use of artificial colorants raises several concerns, especially regarding human health [13,14]. Studies have linked some synthetic colorants to allergic reactions and sensitivities, along with potential adverse effects such as carcinogenicity, hyperactivity, and hypersensitivity [15,16,17,18,19,20,21]. Faced with these concerns, consumer preference is growing for more natural and minimally processed foods [22,23]. In this context, the market is turning to sustainable alternatives, exploring coloring methods with natural ingredients such as plant extracts, fruits, and microalgae. This shift meets consumer expectations and reflects a broader transition towards sustainable and responsible food practices.
Specifically, blue colorants have prominence due to their scarcity in nature. Brilliant Blue FCF (E133) and Indigotine (E132) are commonly used in the food industry to impart blue tones to various products [24]. Although Brilliant Blue FCF has been the subject of discussions regarding potential associations with hyperactivity in children, both are considered safe in permitted amounts [25]. Transitioning to natural blue colorants can be a safe alternative for food products, especially considering the scarcity of this pigment in nature. This shift responds to consumer demands and contributes to a more sustainable approach in the food industry.
According to various authors and international regulatory entities, microalgae are touted as the food of the future due to their ability to provide antioxidant activity and nutritional boosts [26,27,28,29,30,31]. The naturally green biomass of spirulina, the commercial name of Limnospira (previously known as Arthrospira platensis), contains numerous bioactive compounds and natural pigments, including C-phycocyanin (C-PC), a phycobiliprotein with a fluorescent blue color [32]. Its health appeal is highlighted, with regular consumption linked to health benefits such as preventing non-transmissible chronic diseases such as obesity and even aiding cancer treatment [33,34,35]. After the extraction of C-PC, the residual biomass of spirulina (RB) remains nutritionally attractive for protein enrichment and, alternatively, as a natural green pigment for food coloring, avoiding disposal [36,37].
The most significant challenge when applying alternative ingredients to food products is related to consumer acceptance. To meet the demands of the new consumer profile worldwide, foods must not only taste good but also exhibit features such as being as similar and natural as possible, promoting health benefits, and being eco-friendly, among others [22,36]. The flavors formed during the fermentation process seems to be a way to improve microalgae acceptance as an ingredient [38].
One such product is ice cream, a delightful and highly consumed dessert that typically includes dairy ingredients such as milk, cream, and butter with optional additional substances such as caseinates, hydrolyzed milk proteins, and safe non-milk-derived ingredients. In Brazil, the Federal Legislation [39] defines ice creams as emulsions of fats and proteins or a mixture of water and sugar. It may be supplemented with other ice cream ingredients since they do not alter the product characterization. For instance, lactic acid bacteria (LAB) may naturally occur in dairy products or be added as a starter culture for creating fermented foods such as frozen yogurts and ice creams [40,41]. Several works have used spirulina and its constituents as ingredients in the literature [36,37,42,43,44]; on the market, we can already observe several products containing spirulina in their formulation, including desserts, bread, pasta, juices, and others.
However, biotechnological challenges for delivering probiotics through fermented ice cream encompass the cell stability and viability due to the freezing process and presence of different cell species [45]. Viability of Lactobacillus acidophilus was achieved over 90 days of storage when the fermentation process occurred before freezing the ice cream mixture. Once overcome, the enzyme complexes of LAB strains contribute to the release of various bioactive metabolites into the dairy matrix or the gut, thereby supporting the concept of the food–gut health axis. Fermented ice cream has been shown to combat the accumulation of pro-inflammatory IL-6 and IL-8, reduce ROS, and preserve Caco-2 integrity [46,47].
In this context, this work aimed to produce different formulations of naturally colored fermented ice cream using Lactobacillus acidophilus, Bifidobacterium, and Streptococcus thermophilus incorporated with C-PC (blue) or RB (green). Color stability over shelf life and antioxidant activity before and after bioaccessibility analysis were assessed. Sensory acceptance and the ice creams’ consumer profiles were examined.
2. Materials and Methods
2.1. Spirulina Biomass
Commercial dry organic spirulina biomass was kindly donated from Fazenda Tamanduá® (Santa Teresinha, Paraíba, Brazil, https://www.fazendatamandua.com.br/, accessed on 1 June 2024). The product is certified with the official Brazilian Organic Conformity Assessment and Biodynamic Federation Demeter International seals. The nutrition labeling composition per 100 g corresponds to 333.3 kcal, 20 g carbohydrates, 53.3 g protein, 6.6 g total fat, 2.6 g saturated fat, 6 g fiber, 200 mg calcium, 2 mg iron, and 1.26 g sodium. The biomass was stored in an ultra-freezer (−40 °C).
2.2. Green Extraction and Purification of C-PC and Gettering of RB
Spirulina biomass was used as a source of C-PC, extracted according to Fratelli et al. [34] to standardize the particles. The spirulina biomass was milled and then filtered using a stainless steel test sieve (ASTM E11—140 MESH/Tyler 150), and only distilled water was used as a solvent. At the end of the extraction, the supernatant was separated from the rest of the solid material, designated in the present work as RB. Once the liquid fraction of the extract was obtained (C-PC), the samples were vacuum filtrated and kept in an ultra-freezer at −40 °C. The RB was frozen as well. Fractional precipitation with (NH4)2SO4 was performed [48] to obtain food-grade C-PC (purity > 0.7).
2.3. Analytical Methods
C-PC concentration (mg.mL−1) was calculated as described by Bennet and Bogorad [21] with the wavelength adapted by Moraes and Kalil [49] according to Equation (1), where the absorbance at 652 nm (A652) indicates the presence of allophycocyanin and the absorbance at 620 nm (A620) indicates the C-PC concentration [50]. The C-PC extract purity (EP) was calculated according to Equation (2) [24], where the absorbance at 280 nm (A280) indicates the protein concentration in the solution. The absorbances (A) were assessed using a UV–visible spectrophotometer (Varian, Cary-50®, Mulgrave, VIC, Australia).
C-PC = (A620 − 0.474 × A652)/5.34
EP = A620/A280
2.4. Formulation of the Fermented Ice Creams
2.4.1. Fermented Milk Base
The Laboratory of Food Bioactive Compounds (UNIFESP) research group fabricated the fermented milk base with varying sweeteners (Table S1). The fermented milk was prepared by combining pasteurized whole milk and a commercial dairy culture (containing Lactobacillus acidophilus, Bifidobacterium, and Streptococcus thermophilus), sucrose or maltodextrin, whole milk powder, and unflavored gelatin. The pasteurized whole milk was heated to 80 °C, and the dry ingredients were added gradually to guarantee the homogeneity of the mixture. The commercial dairy culture was dissolved when the mixture reached 40 °C. The mixture was transferred to properly packed containers and placed in a lab oven at 40 °C for four hours to obtain fermented milk [25].
2.4.2. Ice Cream Manufacture
According to previous studies, the formulated fermented milk was used as the base for developing the ice creams [42], and the Duas Rodas Industrial® recipe was adapted (Table S2). Sucrose or maltodextrin, whole milk powder, heavy cream, and a commercial stabilizer powder (Duas Rodas Industrial®, Super Liga Neutra®, Santa Catarina, RS, Brazil) were added to the fermented milk base and homogenized in a mixer. After 3 min, the mixture was frozen at −12 °C for 4 h. Then, an emulsifier (Duas Rodas Industrial®, Emustab®, Jaraguá do Sul, Santa Catarina, Brazil) and C-PC (0.25 mg.mL−1) were added in a new homogenization step for 5 min. The mixture was stored at −12 °C. The RB formulation underwent the same production stages but received RB instead of C-PC. The control formulations did not receive RB or C-PC but differed between maltodextrin (MC) and sucrose (SC). Besides the control ice creams (SC and MC), four formulations were prepared, including sucrose and C-PC (SC-PC), sucrose and residual biomass (SRB), maltodextrin and C-PC (MC-PC), and maltodextrin and residual biomass (MRB). Assays were carried out in triplicate, and samples of each ice cream were freeze-dried and stored to determine microbiological safety, antioxidant activity, and bioaccessibility.
2.5. Microbiological Quality
For the microbiological safety analysis (initial and final times), 1 g of each ice cream sample was evaluated after production (t = 0) and on the 182nd day (t = 182). The presence of coagulase-positive Staphylococcus aureus (48 h at 35 °C), Salmonella (18–24 h at 41.5 °C), and Enterobacteriaceae (24 h at 35 °C) as specified in Brazilian legislation by the National Health Surveillance Agency [51] was evaluated. Total coliforms at 45 °C and Escherichia coli (48 h at 35 °C) were also examined. Total aerobic mesophilic microorganisms (48 h at 32 °C) were counted using the rapid methodology of Petrifilm™ (3M) following the instructions of the manufacturer, a method validated by the Association of Analytical Communities [52]. All procedures were performed in triplicate, and the results are reported in CFU/g.
2.6. Color Stability of C-PC- and RB-Supplemented Ice Creams
The color stability of the products was monitored every seven days for 182 days using the L*, a*, and b* color system. The L*, a*, and b* values were determined for each formulation in triplicate at each point in time using a colorimeter (Minolta, model CM25D, Tokyo, Japan). The hue angle (h), which indicates the color angle (0°—red; 90°—yellow; 180°—green; 270°—blue; 360°—black), was calculated by Equation (3). L* indicates the brightness (0–100), a* indicates the amount of red (positive values) or green (negative values), and b* specifies the amount of yellow (positive values) or blue (negative values) in the samples. The color difference (ΔE, Equations (4)–(7)) was also calculated from the initial (t = 0) and final (t = 182) values of L*, a*, and b* of each formulation (SC, SC-PC, SRB, MC, MC-PC, and MRB).
when (−a*, +b*) or (−a*, −b*)
2.7. Antioxidant Activity, Total Phenolic Content, and Bioaccessibility of the Manufactured Samples
The freeze-dried samples were stirred with 20 mL methanol for 15 min to extract the antioxidant compounds from the matrices. Samples were filtered using filter paper, and the remaining solids were washed twice with an additional 20 mL methanol. The filtrate was concentrated using a rotary evaporator below 40 °C until a total volume of 15 to 25 mL was obtained [53,54]. The samples were filtered using 0.22 μm cellulose acetate membranes before the experiment. To determine the antioxidant activity against the peroxyl radical, the ORAC (oxygen radical absorption capacity) method was used according to Rodrigues et al. [55] with a spectrophotometer (Molecular Devices, Spectra Max®, San Jose, CA, USA). ABTS●+ analysis was also performed according to Re et al. [56]; the reaction’s absorbance was measured in a Cary-50® spectrophotometer (Santa Clara, CA, USA). Both antioxidant results are expressed as μmol.g of sample−1 of Trolox equivalents (TE).
The total phenolic compounds were determined by the Folin–Ciocalteu method, according to Singleton and Rossi’s procedure [57]. The results are expressed in gallic acid equivalents (GAE).100 g−1.
Aliquots of 2 g of freeze-dried samples were digested using the INFOGEST protocols [58,59]. Stock solutions of simulated salivary (SSF), gastric (SGF), and intestinal (SIF) fluids were prepared and diluted according to the INFOGEST protocol so that the recommended ionic composition of each digestive step was reached. The samples were kept at −40 °C until the analysis.
2.8. Sensory Analysis
For the sensory analysis, a new batch was prepared, and considering the color results, the analysis was conducted after 21 days of ice cream color maturation. The sensory evaluation was conducted with 74 consumers, aged 16 to 74, in a mixed group of males and females. The volunteers answered a questionnaire and rated the appearance, aroma, flavor, and overall acceptance of the products using a 9-point hedonic scale composed of the following options: (1) dislike extremely, (2) dislike much, (3) dislike moderately, (4) dislike slightly, (5) neither dislike nor like, (6) like slightly, (7) like moderately, (8) like much, (9) like extremely. Additionally, general questions regarding consumption habits, frequency of consumption, and ice cream determinant features to purchase the product were also applied during the tests. The ice cream portions (25 g) were separately offered in a random sequence in paper cups coded with 3-digit numbers and maintained in ice until the tasting tests. The evaluation was conducted in a climate-controlled (20–25 °C) sensory evaluation laboratory with separate booths. The panelists rinsed their mouths with water between samples to minimize residual effects.
2.9. Statistical Analysis
The assays were carried out independently in triplicate and compared by applying an analysis of variance (ANOVA), Tukey’s post hoc test, and a t-test to compare the means, using a degree of significance of 95% (p < 0.05). For the sensory analysis, the individuals were treated as random effects, and the Q-Q plots and residual histograms indicate a good fit for normality. The data were subjected to both descriptive and quantitative methods of analysis, and the formulations’ effects on the attributes were evaluated using average and standard error.
3. Results
3.1. Microbiological Quality and Shelf Life
Microbiological analysis confirmed the products’ safety for consumption over a 6-month evaluated shelf life (Table S3), according to the current Brazilian legislation [44]. No Salmonella or coagulase-positive Staphylococcus were detected, and counts of coliforms at 45 °C, E. coli, and Enterobacteriaceae were all less than 10 CFU/g. A balance between physical properties, sensory acceptability, and microbial safety is always desired but not always achieved; fortunately, the results obtained in the present work have accomplished this equilibrium.
3.2. Color Stability of C-PC- and RB-Supplemented Ice Creams
The color variation of the formulated ice creams over 182 days is presented in Figure 1. All the formulations had statistical differences between t = 0 and t = 182 days for L*, a*, b*, and hue angle (p < 0.05), with the exception of the a* and hue angle parameters in the SRB formulation, pointing out that the green color of the SRB formulation (Figure 1C) was the most stable in the presence of sucrose over the evaluated time. The other formulations changed more drastically considering the luminosity parameter, until 21 days for SC, MC (Figure 1A,B), SC-PC, and MC-PC (Figure 1C,D) and until 49 days for SRB and MRB (Figure 1E,F). Qualitatively, after this initial time, the color was stable. The final values of L*, a*, and b* aligned visually with the expected colors (yellowish for controls, greenish for RB addition, and blueish for C-PC-supplemented ice creams), albeit with statistical differences over the evaluated six months of shelf life.
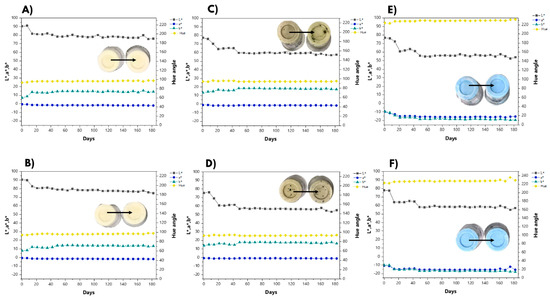
Figure 1.
Color stability of different ice cream formulations: (A) sucrose control (SC); (B) maltodextrin control (MC); (C) sucrose with residual biomass (SRB); (D) maltodextrin with residual biomass (MRB); (E) sucrose with C-PC (SC-PC); (F) maltodextrin with C-PC (MC-PC).
In the control formulations (Figure 1A,B), the b* values were positive. The hue angles were 93.64° ± 0.52 (SC) and 93.98° ± 0.39 (MC) at the initial time point, indicating slightly yellowish coloration, as expected due to the ingredients used to develop the ice cream, especially the heavy cream and the powdered whole milk. At 182 days, the hue angles were 97.90° ± 0.73 (SC) and 97.61° ± 1.68 (MC), significantly changing over the evaluation time period. For both samples, L* decreased and b* increased (p < 0.05) from the initial time point to the 182nd day, changing more markedly around the 21st day (Figure 1A,B), which resulted in a decrease in brightness and an increase in yellow color. SRB and MRB showed negative a* values and positive b* values, indicating the green color in these samples, as expected, due to the green color of spirulina RB.
The hue angles on days 0 and 182 were 94.48° ± 2.44 and 95.22° ± 2.43 (SRB) and 92.93° ± 0.16 and 93.93° ± 0.43 (MRB), respectively. These values, combined with positive b* values, indicated a stable color closer to yellow than green; in other words, the most detected color was that of the base ingredients, even though the visually achieved color was green.
3.3. Antioxidant Activity, Total Phenolic Content, and Bioaccessibility of the Manufactured Samples
The ORAC and ABTS methods determined the antioxidant activity to provide a broader evaluation of the antioxidant capacity of the biomass, C-PC, and RB from spirulina separately and in ice creams before and after bioaccessibility analysis (Table 1).

Table 1.
ABTS and ORAC methods determined the antioxidant activity of the formulated ice creams (SC, SC-PC, SRB, MC, MC-PC, and MRB) before and after in vitro digestion, as well as the total phenolic content of the formulated ice creams without digestion.
3.4. Sensory Analysis
For the sensory evaluation, 74 volunteers evaluated three formulations (SC, SC-P, and SRB); the average age was 28 years (Figure 2).
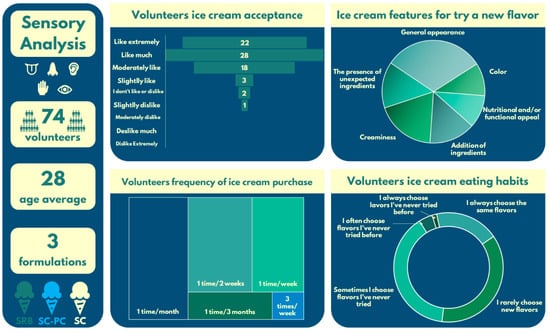
Figure 2.
Panoramic scheme to understand the volunteers’ attitudes towards the ice creams.
Overall, 67.56% of them answered that they like very much/extremely like ice cream, followed by 24.32% who moderately like ice creams. Furthermore, 29.72% of the panelists used to purchase the product at least once a month, as we ideally expect of people who participate in this sort of study. Interestingly, the highest rating was given to the appearance of the C-PC ice cream (Figure 3), with 70.26% of volunteers expressing “like much” and “like extremely”, drawing notable attention to the blue ice cream’s attractiveness.
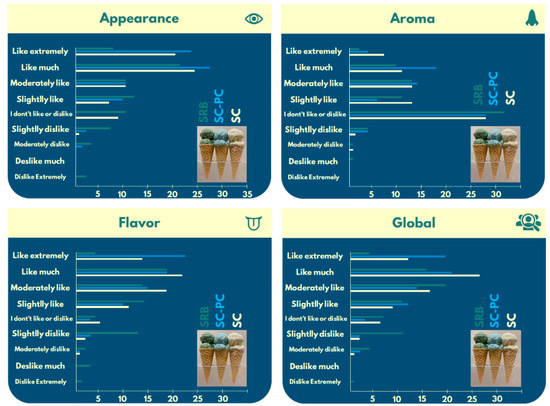
Figure 3.
Acceptability scores for the appearance, aroma, flavor, and global aspects of the ice cream formulations (control, C-PC, and RB).
4. Discussion
In a previous study, frozen yogurt fermented with L. bulgaricus and S. thermophilus maintained microbial stability over 60 days of storage [40]. In this study, the microbiological analysis of the fermented samples showed stability over 6 months, with no harmful bacterial growth detected.
The optical properties of food, including its color and overall appearance, play a key role in consumer acceptance and affect its sensory acceptability, which relates to how the food appeals to the human senses, such as flavor, aroma, and appearance. Additionally, microbial safety is paramount to prevent foodborne illnesses and ensure consumer well-being. This balance has been successfully achieved in the present work. This signifies that the authors have created a food product that meets the desired physical properties and sensory attributes and ensures microbial safety. Such an accomplishment is fundamental to ensure the natural pigment potential in the food development process. The microbiological safety was also evaluated by Misturini Rodrigues [60], who produced frozen yogurt, and the product offered to the sensorial panelists was also safe.
Considering the results from the color analysis, in each ice cream containing C-PC (Figure 1E,F), the measured b* parameter was negative. The hue angles at the initial time point for SC-PC and MC-PC were 224.23° ± 0.53 and 223.15° ± 0.16, respectively. Combined with the b* values, the results indicated the blue color in both formulations but were less pronounced than those reported by Amarante et al. [42]. They observed hue values close to 261.90° in the initial analysis of milk-based ice creams with C-PC added, most likely due to the more yellowish color of the fermented milk base made with the addition of whole milk powder, to the detriment of the milk base used by the authors. Amazingly, the b* values reported here increased more than twofold (p < 0.05), reinforcing the blue color gain. This increase occurred around the 42nd day (Figure 1E,F), demonstrating the desired intonation of the color after this storage time. By the 182nd day, the hue angles were 231.80° ± 1.16 and 229.40° ± 1.62 for SC-PC and MC-PC, respectively, showing a significant increase (p < 0.05) in the blue color over the 6-month shelf life.
According to Figure 1, the most affected parameter was L*, which clearly indicates a darkening in the colors [61]. This is a desirable result since the loss of luminosity implies stronger colors, which occurred around the 42nd day (Figure 1E,F). Therefore, the challenge of preserving the color stability of C-PC was addressed, likely due to the low storage temperatures and the absence of high temperatures in the production process. These results align with those obtained by Mohammadi-Gouraji et al. [62], where the maintenance of C-PC color was observed in another dairy derivative, natural yogurt.
Table 1 confirms that even the control samples of our ice cream presented antioxidant activity and phenolic compounds. The values increased after we added the natural pigments from spirulina. This was expected and desired, since one of the reasons to use yogurt as a base for our ice creams was to incorporate the precious features acquired with the fermentation process. Many studies have found that fermentation positively affects the antioxidant activity of manufactured milk-based products [63,64]. Peptides and free amino acids released during milk fermentation increase the antioxidant capacity of products and inhibit lipid peroxidation [65]. Considering the results, there is probably a synergistic effect regarding these fermentation-derived molecules and the natural pigments added, leading to a positive increment in the antioxidant activity of the ice creams.
The ice cream formulations presented antioxidant activities against ABTS●+ and peroxyl radicals before and after in vitro digestion (Table 1). Before the digestion, the antioxidant activities of MRB and MC-PC measured by ORAC showed no statistical difference. Still, MRB was significantly statistically superior (p < 0.05) compared to the other formulations, and for the ABTS method, MRB and MC-PC presented statistical differences among the formulations. However, after the digestion, both ORAC and ABTS methods revealed no significant difference (p < 0.05) between the antioxidant activities of MC-PC, MRB, SC-PC, and SRB, but differences were significant when compared to controls, evidencing that neither maltodextrin nor sucrose changed the positive biological effects of RB and C-PC, which implicates sucrose as a better option to produce the ice creams considering the cost.
Comparing before and after digestion, it was concluded that the digestion process led to a significant increase (p < 0.05) in the antioxidant activity of the SC-PC and SRB formulations in the ORAC method due to the release of the target biomolecules from the soluble fibers, proteins, and carbohydrates they were linked to in the ice cream complex matrix. In this regard, Lamothe et al. [66] demonstrated that milk and polyphenol-rich beverages act synergically to reduce polyunsaturated fatty acid oxidation during gastrointestinal digestion due to the interaction between proteins from milk and polyphenols, which protects the biomolecules and consequently improves the antioxidant activity. This result is further supported by the significant decrease (p < 0.05) in the SC formulation in the ABTS assay, as it was a blank sample with no bioactive compounds from spirulina to be released. Furthermore, Amarante et al. [42] formulated an ice cream containing C-PC and observed an increase of 2 to 13 times in antioxidant activity in digested ice creams compared to the control ice cream (without adding C-PC) in the same conditions, indicating that the C-PC and its biological effects remained active and increased in the final product. Even with the previous literature review, we have yet to find another study that has considered antioxidant activity after digestion. These results are congruent with the 2.7-fold and 2.5-fold increments in the antioxidant activity of the SC-PC and SRB ice creams after the bioaccessibility test (41.3 ± 2.6 and 38.0 ± 4.3), respectively, with the ORAC method. The greater results achieved by Amarante et al. [42] (ORAC: 134.63 ± 15.68; ABTS: 1425.19 ± 54.93 μmol TE.g−1) are probably due to the 1.1 purity of C-PC. This work agrees with Amarante et al. [42], who observed increased health benefits of ice creams after digestion. Additionally, both studies reached color stability, corroborating their reproducibility and possibility of scaling up in the industry. The results show the importance of the great potential of C-PC as a natural pigment as well as an innovative ingredient to be used in the food industry and, to the best of our knowledge, gives the first perspective on using RB with these technology functions as well.
One significant distinction of the fermentation process lies in its ability to alter food’s overall flavor profile. What was once solely a method for food preservation has now evolved into a matter of preference rather than necessity. Consumers of ice cream, particularly in recent years, have exhibited increased expectations not only for cleanliness but also for more robust and distinctive flavor profiles [46]. This shift is evident in the multitude of frozen yogurt brands that have thrived in this highly competitive market [67]. From a scientific perspective, this change in consumer preference presents us with the challenge of integrating fermented products to create novel flavor profiles. These processes’ advantages include enhancing the microbiota and fostering beneficial biological effects through fermentation [46,47]. In the present work, the antioxidant activity was the biological effect studied.
In the present work, we assumed that the use of a starter culture including Lactobacillus acidophilus, Bifidobacterium, and Streptococcus thermophiles was a positive strategy, since it is known that the fermentation of milk-based products using these microorganisms increases antioxidant activity [68,69,70]. Furthermore, adding bioactive compounds after the fermentation process improves the quality of the compounds offered in ready-to-eat products, mainly because their bioaccessibility and bioavailability depend on the individual microbiota of each consumer [53,71]. Therefore, even consumers with the most compromised microbiota can take advantage of the positive biological effects when consuming these products regularly, combating non-chronic, non-communicable diseases such as diabetes and obesity [72].
Besides the ORAC and ABTS methods, a total phenolics assay was conducted (Table 1) since they are also molecules with antioxidant properties. Phenolic compounds from spirulina identified by gas chromatography–mass spectrophotometry (GC-MS) were benzophenone chloroacetyl hydrazine, propane diamine 2-butyl, dihydro-methyl-phenyl acridine, isoquinoline, piperidin-1-dihydrodibenzo, carbonic acid, methyl-2-chloroethyl ester, pyrrolidine, oxazolidine, and dinitrobenzene [73]. According to the total phenolic content study, all the samples presented phenolic compounds for the following reasons: (i) even after C-PC extraction, other biomolecules such as phenolics are retained, as was observed by Niccolai et al. [74] who found an improvement in phenolic and C-PC contents in crostini at 2, 6, and 10 g/100g spirulina concentrations; (ii) the phenolics are provided by the ingredients contained in the preparations. As already reported in the literature, the natural colorants and cow milk used in the ice cream formulations naturally contain phenolic compounds [75]. MC-PC showed the highest content of phenolic compounds (p < 0.05) among the samples, probably due to the migration of the phenolic compounds during extraction [73] being more pronounced in those samples with C-PC extracted from the spirulina matrix. However, the ice creams with C-PC added (SC-PC and MC-PC) did not present a significant difference between them, indicating that the carbohydrate (maltodextrin or sucrose) did not affect the phenolic content, considering the conditions and concentrations evaluated in the present work. This result associated with the increment in the bioactivity of the SC-PC and SRB samples after in vitro digestion led to performance of a sensory analysis of only sucrose-sweetened formulations.
The microbiological security and shelf life of the developed products showed great stability considering both parameters after 182 days of storage. These results aligned with the color stability, confirming the ice creams’ industrial feasibility and corroborating the importance of academic studies for manufacturing innovative products, especially considering that ice creams are very appealing for young people. Therefore, offering a quality product is necessary to ensure the healthy consumption of colored foodstuff.
From the consumer’s preference results, the appearance, aroma, flavor, and global aspects were evaluated. Although the preliminary data acquired confirmed that most of them rarely choose new flavors and sometimes choose flavors that they have never tried before, a positive response with the acceptance of the formulated ice creams was achieved more expressively for the naturally colored blue ice cream.
In terms of the data acquired to understand how the volunteers behave when choosing an ice cream flavor, most of them stated they rarely choose new flavors/and sometimes choose flavors that they have never tried before, showing a particularly conservative behavior regarding their ice cream purchase habits. Appearance was declared the most important attribute by 62.16% of volunteers when trying new ice cream flavors. Creaminess and the addition of an unexpected ingredient were also pointed out by them to catch their attention to try ice creams.
Visual perception, including appearance and color, plays a significant role in how food and its taste are perceived by consumers [76]. The highest rating was given to the appearance of C-PC ice cream (Figure 3), with 70.26% of volunteers expressing “like much” and “like extremely”, highlighting the blue ice cream’s attractiveness, but no significant differences were detected compared to SC ice creams in the global parameter. In this sense, the appearance ratio and color stability of C-PC over the six-month period demonstrate the potential of this natural colorant to replace the commonly used Brilliant Blue synthetic dye (E133) [77] in ice cream formulations. Thus, new fermented products loaded with bioactive compounds could be launched, with no differences in the flavor perception compared to the conventional ice cream yogurt-like flavor despite the blue color, but with significant differences in antioxidant activity after in vitro digestion.
Another point to consider is the consumer type evaluating the developed product. Some authors have studied consumer behavior, separating tasters into categories to assess willingness to purchase a food product related to previous knowledge. The work developed by Lucas and Brunner [78], which evaluated attitudes and perceptions concerning microalgae uptake among Swiss citizens, showed that consumers included in the category ‘microalgae supporters and health eaters’ showed the highest scores for all the microalgae-based scales proposed by the study. This group profile is less neophobic and composed of 70% female and 63% non-omnivore people, suggesting the target market for algae-based products, such as the ice creams developed in this work. Furthermore, they are more food-involved and interested in environmental protection; have shown (p < 0.001) more oriented behavior towards price, food quality, and alternative protein consumption; and had the highest interest in food healthiness compared to the conservative group [78].
In contrast, consumers unfamiliar with microalgae-based food tend to find fishy, earthy, or muddy odors and off-flavors unpleasant, leading to restricted acceptance of these products [79,80]. Volatile organic compounds from spirulina were characterized by Prasetiyo et al. [81] using GC-MS. Some of the identified compounds were floral, balsamic, fish-like, powdery, rancid, alkali, and woody, among others. Remarkably, the aroma of the SRB ice cream was indicated by 44.59% of the volunteers as “I don’t like or dislike”, compared to 39.18% in the control samples, suggesting that the unpleasant volatile organic compounds were not perceived, probably due to the biomass’ broken cell walls and their evaporation during the RB processing. The aroma indifference, well-perceived appearance, and stable color throughout the shelf life are pivotal points for using RB in fermented ice creams.
5. Conclusions
The present study successfully addressed the challenge of maintaining the activity and stability of C-PC (C-phycocyanin) in a complex matrix for six months. The findings indicated that adding C-PC extract and RB from spirulina contributed significantly to the high scavenging capacities observed in the ice creams. Notably, the antioxidant activity of SC-PC and SRB was significantly enhanced after the digestion process, pointing out the biological potential of the ice creams when bioactive compounds are released from the digested matrix. This research highlights the use of RB as a reliable food coloring agent for ice creams and C-PC’s ability to maintain its blue color over 6 months. Considering their respective advantages, the RB with sucrose formulation appears more suitable for scaling up production due to its cost-effectiveness since it is a residue from spirulina biomass after C-PC extraction. Both the blue C-PC and green RB were stable and hold great potential for further exploration due to their health benefits, and C-PC offers innovative color properties. Finally, these results are valuable starting points for developing new products incorporating natural pigments with antioxidant properties, particularly considering the sensory analysis. It is worth noting that the appearance of the C-PC ice creams scored even higher than that of the control ice cream, but no significative differences were found in the overall acceptance, indicating their potential to enhance consumer experience and appeal when consuming fermented ice creams with improved biological potential that are naturally colored and microalgae-based.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation10060304/s1, Table S1. Fermented milk formulation containing sucrose or maltodextrin. Table S2. Formulation of ice creams using fermented milk as a basis containing sucrose or maltodextrin: controls; C-PC or RB added. Table S3. Microbiological quality of the formulated ice creams (initial and final times).
Author Contributions
Conceptualization, A.R.C.B.; methodology, M.B., C.F., M.A. and A.R.C.B.; investigation, A.R.C.B.; data curation, M.B. and C.F.; writing—original draft preparation, M.B., C.F. and M.A.; writing—review and editing, M.B., C.F., M.A. and A.R.C.B.; visualization, A.R.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP” through the grants n° 2020/06732-7, 2022/06293-9, and 2023/00857-0. The authors also acknowledge CAPES for financial support. M.A. acknowledges the “Juan de la Cierva” fellowship with code JDC2022-049934-I.
Institutional Review Board Statement
Ethical approval for the involvement of human subjects in this study was granted by the Universidade Federal de São Paulo Research Ethics Committee, reference number CAAE: 28670120.0.0000.5505, approved in June 2020.
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank Fazenda Tamanduá® for donating the organic spirulina used in the present work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Q.J.; Mielby, L.A.; Junge, J.Y.; Bertelsen, A.S.; Kidmose, U.; Spence, C.; Byrne, D.V. The Role of Intrinsic and Extrinsic Sensory Factors in Sweetness Perception of Food and Beverages: A Review. Foods 2019, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Landim Neves, M.I.; Silva, E.K.; Meireles, M.A.A. Natural Blue Food Colorants: Consumer Acceptance, Current Alternatives, Trends, Challenges, and Future Strategies. Trends Food Sci. Technol. 2021, 112, 163–173. [Google Scholar] [CrossRef]
- Echegaray, N.; Guzel, N.; Kumar, M.; Guzel, M.; Hassoun, A.; Lorenzo, J.M. Recent Advancements in Natural Colorants and Their Application as Coloring in Food and in Intelligent Food Packaging. Food Chem. 2023, 404, 134453. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, B.; Sigurdson, G.T.; Lao, F.; González-Miret, M.L.; Heredia, F.J.; Giusti, M.M. Assessment of the Color Modulation and Stability of Naturally Copigmented Anthocyanin-Grape Colorants with Different Levels of Purification. Food Res. Int. 2018, 106, 791–799. [Google Scholar] [CrossRef]
- Buchweitz, M. Natural Solutions for Blue Colors in Food. In Handbook on Natural Pigments in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2016; pp. 355–384. [Google Scholar]
- Spence, C. Background Colour & Its Impact on Food Perception & Behaviour. Food Qual. Prefer. 2018, 68, 156–166. [Google Scholar] [CrossRef]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for Snack Enrichment: Nutritional, Physical and Sensory Evaluations. LWT Food Sci. Technol. 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Özbal, B.; Çelekli, A.; Gün, D.; Bozkurt, H. Effect of Arthrospira Platensis Incorporation on Nutritional and Sensory Attributes of White Chocolate. Int. J. Gastron. Food Sci. 2022, 28, 100544. [Google Scholar] [CrossRef]
- Üstün-Aytekin, Ö.; Çoban, I.; Aktaş, B. Nutritional Value, Sensory Properties, and Antioxidant Activity of a Traditional Kefir Produced with Arthrospira platensis. J. Food Process Preserv. 2022, 46, e16380. [Google Scholar] [CrossRef]
- Gremski, L.A.; Coelho, A.L.K.; Santos, J.S.; Daguer, H.; Molognoni, L.; do Prado-Silva, L.; Sant’Ana, A.S.; Rocha, R.d.S.; da Silva, M.C.; Cruz, A.G.; et al. Antioxidants-Rich Ice Cream Containing Herbal Extracts and Fructooligossaccharides: Manufacture, Functional and Sensory Properties. Food Chem. 2019, 298, 125098. [Google Scholar] [CrossRef]
- Monteiro, M.J.P.; Costa, A.I.A.; Fliedel, G.; Cissé, M.; Bechoff, A.; Pallet, D.; Tomlins, K.; Pintado, M.M.E. Chemical-Sensory Properties and Consumer Preference of Hibiscus Beverages Produced by Improved Industrial Processes. Food Chem. 2017, 225, 202–212. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food Colorants: Challenges, Opportunities and Current Desires of Agro-Industries to Ensure Consumer Expectations and Regulatory Practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Tokuda, H.; Sridhar, R.; Balasubramanian, V.; Takayasu, J.; Bu, P.; Enjo, F.; Takasaki, M.; Konoshima, T.; Nishino, H. Cancer Chemopreventive Activity of Synthetic Colorants Used in Foods, Pharmaceuticals and Cosmetic Preparations. Cancer Lett. 1998, 129, 87–95. [Google Scholar] [CrossRef] [PubMed]
- El-Wahab, H.M.F.A.; Moram, G.S.E.-D. Toxic Effects of Some Synthetic Food Colorants and/or Flavor Additives on Male Rats. Toxicol. Ind. Health 2013, 29, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A.; Nurudeen, J.; Orovwigho, U.; Ahiabuike, J. Assessment of the Health Implications of Synthetic and Natural Food Colourants—A Critical Review. Pharm. Biosci. J. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food Colors: Existing and Emerging Food Safety Concerns. Crit. Rev. Food. Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Nigg, J.T.; Lewis, K.; Edinger, T.; Falk, M. Meta-Analysis of Attention-Deficit/Hyperactivity Disorder or Attention-Deficit/Hyperactivity Disorder Symptoms, Restriction Diet, and Synthetic Food Color Additives. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 86–97.e8. [Google Scholar] [CrossRef] [PubMed]
- Harley, J.P.; Matthews, C.G.; Eichman, P. Synthetic Food Colors and Hyperactivity in Children: A Double-Blind Challenge Experiment. Pediatrics 1978, 62, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Feketea, G.; Tsabouri, S. Common Food Colorants and Allergic Reactions in Children: Myth or Reality? Food Chem. 2017, 230, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Hannuksela, M.; Haahtela, T. Hypersensitivity Reactions to Food Additives. Allergy 1987, 42, 561–575. [Google Scholar] [CrossRef]
- Olas, B.; Białecki, J.; Urbańska, K.; Bryś, M. The Effects of Natural and Synthetic Blue Dyes on Human Health: A Review of Current Knowledge and Therapeutic Perspectives. Adv. Nutr. 2021, 12, 2301–2311. [Google Scholar] [CrossRef]
- Nakamoto, M.M.; Assis, M.; de Oliveira Filho, J.G.; Braga, A.R.C. Spirulina Application in Food Packaging: Gaps of Knowledge and Future Trends. Trends Food Sci. Technol. 2023, 133, 138–147. [Google Scholar] [CrossRef]
- Cabrita, M.; Simões, S.; Álvarez-Castillo, E.; Castelo-Branco, D.; Tasso, A.; Figueira, D.; Guerrero, A.; Raymundo, A. Development of Innovative Clean Label Emulsions Stabilized by Vegetable Proteins. Int. J. Food Sci. Technol. 2023, 58, 406–422. [Google Scholar] [CrossRef]
- Arnold, L.E.; Lofthouse, N.; Hurt, E. Artificial Food Colors and Attention-Deficit/Hyperactivity Symptoms: Conclusions to Dye For. Neurotherapeutics 2012, 9, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Rambler, R.M.; Rinehart, E.; Boehmler, W.; Gait, P.; Moore, J.; Schlenker, M.; Kashyap, R. A Review of the Association of Blue Food Coloring with Attention Deficit Hyperactivity Disorder Symptoms in Children. Cureus 2022, 14, e29241. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chew, K.W.; Chang, C.K.; Munawaroh, H.S.H.; Kumar, P.S.; Huy, N.D.; Show, P.L. Perspective of Spirulina Culture with Wastewater into a Sustainable Circular Bioeconomy. Environ. Pollut. 2021, 284, 117492. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Cysewski, G.R. Potential Health Benefits of Spirulina MicroalgaePotential health benefits of Spirulina microalgae* A review of the existing literature. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Gurreri, L.; Calanni Rindina, M.; Luciano, A.; Lima, S.; Scargiali, F.; Fino, D.; Mancini, G. Environmental Sustainability of Microalgae-Based Production Systems: Roadmap and Challenges towards the Industrial Implementation. Sustain. Chem. Pharm. 2023, 35, 101191. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A Novel Ingredient in Nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banecjee, U.C. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Fratelli, C.; Burck, M.; Amarante, M.C.A.; Braga, A.R.C. Antioxidant Potential of Nature’s “Something Blue”: Something New in the Marriage of Biological Activity and Extraction Methods Applied to C-Phycocyanin. Trends Food Sci. Technol. 2021, 107, 309–323. [Google Scholar] [CrossRef]
- Silva-Neto, A.F.; Fratelli, C.; Pucci, V.G.; Boldarine, V.T.; Ferreira, Y.A.M.; Telles, M.M.; Braga, A.R.C.; Oyama, L.M. C-Phycocyanin Extracted from Spirulina Using a Green Solvent Approach Presents an Anti-Obesity Characteristic in Mice Fed a Hyperlipidic Diet. J. Funct. Foods 2023, 108, 105747. [Google Scholar] [CrossRef]
- Fratelli, C.; Bürck, M.; Silva-Neto, A.F.; Oyama, L.M.; De Rosso, V.V.; Braga, A.R.C. Green Extraction Process of Food Grade C-Phycocyanin: Biological Effects and Metabolic Study in Mice. Processes 2022, 10, 1793. [Google Scholar] [CrossRef]
- Salgado, M.T.S.F.; Fernandes e Silva, E.; Matsumoto, A.M.; Mattozo, F.H.; de Amarante, M.C.A.; Kalil, S.J.; de Souza Votto, A.P. C-Phycocyanin Decreases Proliferation and Migration of Melanoma Cells: In Silico and in Vitro Evidences. Bioorg. Chem. 2022, 122, 105757. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Nunes, M.C.; Raymundo, A. The Experimental Development of Emulsions Enriched and Stabilized by Recovering Matter from Spirulina Biomass: Valorization of Residue into a Sustainable Protein Source. Molecules 2023, 28, 6179. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, C.; Nunes, M.C.; De Rosso, V.V.; Raymundo, A.; Braga, A.R.C. Spirulina and Its Residual Biomass as Alternative Sustainable Ingredients: Impact on the Rheological and Nutritional Features of Wheat Bread Manufacture. Front. Food Sci. Technol. 2023, 3, 1258219. [Google Scholar] [CrossRef]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira Platensis as Natural Fermentation Booster for Milk and Soy Fermented Beverages. Foods 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. RDC N° 266; ANVISA: Brasilia, Brazil, 2005. [Google Scholar]
- Cedillos, R.; Aleman, R.S.; Page, R.; Olson, D.W.; Boeneke, C.; Prinyawiwatkul, W.; Aryana, K. Influence of Hesperidin on the Physico-Chemical, Microbiological and Sensory Characteristics of Frozen Yogurt. Foods 2024, 13, 808. [Google Scholar] [CrossRef]
- Akarca, G.; Kilinç, M.; Denizkara, A.J. Quality Specification of Ice Creams Produced with Different Homofermentative Lactic Acid Bacteria. Food Sci. Nutr. 2024, 12, 192–203. [Google Scholar] [CrossRef]
- de Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Kalil, S.J. Colour Stability and Antioxidant Activity of C-Phycocyanin-Added Ice Creams after in Vitro Digestion. Food Res. Int. 2020, 137, 109602. [Google Scholar] [CrossRef]
- El Baky, H.H.A.; El Baroty, G.S.; Ibrahem, E.A. Functional Characters Evaluation of Biscuits Sublimated with Pure Phycocyanin Isolated from Spirulina and Spirulina Biomass. Nutr. Hosp. 2015, 32, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Sözeri Atik, D.; Gürbüz, B.; Bölük, E.; Palabıyık, İ. Development of Vegan Kefir Fortified with Spirulina Platensis. Food Biosci. 2021, 42, 101050. [Google Scholar] [CrossRef]
- Arslan, A.A.; Gocer, E.M.C.; Demir, M.; Atamer, Z.; Hinrichs, J.; Kücükcetin, A. Viability of Lactobacillus Acidophilus ATCC 4356 Incorporated into Ice Cream Using Three Different Methods. Dairy Sci. Technol. 2016, 96, 477–487. [Google Scholar] [CrossRef]
- Polo, A.; Tlais, A.Z.A.; Filannino, P.; Da Ros, A.; Arora, K.; Cantatore, V.; Vincentini, O.; Nicolodi, A.; Nicolodi, R.; Gobbetti, M.; et al. Novel Fermented Ice Cream Formulations with Improved Antiradical and Anti-Inflammatory Features. Fermentation 2023, 9, 117. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Yılmaz, B.; Şahin, T.Ö.; Güneşliol, B.E.; Ayten, Ş.; Russo, P.; Spano, G.; Rocha, J.M.; Bartkiene, E.; Özogul, F. Dairy Lactic Acid Bacteria and Their Potential Function in Dietetics: The Food–Gut-Health Axis. Foods 2021, 10, 3099. [Google Scholar] [CrossRef]
- de Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Moraes, C.C.; Kalil, S.J. Design Strategies for C-Phycocyanin Purification: Process Influence on Purity Grade. Sep. Purif. Technol. 2020, 252, 117453. [Google Scholar] [CrossRef]
- Moraes, C.C.; Kalil, S.J. Strategy for a Protein Purification Design Using C-Phycocyanin Extract. Bioresour. Technol. 2009, 100, 5312–5317. [Google Scholar] [CrossRef]
- Patil, G.; Chethana, S.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Fractionation and purification of the phycobiliproteins from Spirulina platensis. Bioresour. Technol. 2008, 99, 7393–7396. [Google Scholar] [CrossRef]
- ANVISA. RDC N° 331; ANVISA: Brasilia, Brazil, 2019. [Google Scholar]
- AOAC. Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Braga, A.R.C.; de Souza Mesquita, L.M.; Martins, P.L.G.; Habu, S.; de Rosso, V.V.; Mesquita, L.M.d.S.; Martins, P.L.G.; Habu, S.; de Rosso, V.V. Lactobacillus Fermentation of Jussara Pulp Leads to the Enzymatic Conversion of Anthocyanins Increasing Antioxidant Activity. J. Food Compos. Anal. 2018, 69, 162–170. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Mercadante, A.Z. HPLC–PDA–MS/MS of Anthocyanins and Carotenoids from Dovyalis and Tamarillo Fruits. J. Agric. Food Chem. 2007, 55, 9135–9141. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.B.; Faria, A.F.; Mercadante, A.Z. Microcapsules Containing Antioxidant Molecules as Scavengers of Reactive Oxygen and Nitrogen Species. Food Chem. 2012, 134, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1997, 28, 49–55. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Misturini Rodrigues, L.; Gonzales Domiciano, M.; Araujo de Almeida, E.; Sereia, M.J.; Peron, A.P.; da Silva, R. Production of Bioactive and Functional Frozen Yogurt through Easy-to-Make Microspheres Incorporation. J. Food Sci. Technol. 2024, 61, 192–200. [Google Scholar] [CrossRef]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-Enriched Yogurt and Its Antibacterial and Physicochemical Properties during 21 Days of Storage. LWT 2019, 102, 230–236. [Google Scholar] [CrossRef]
- Şanlıdere Aloğlu, H.; Öner, Z. Determination of Antioxidant Activity of Bioactive Peptide Fractions Obtained from Yogurt. J. Dairy Sci. 2011, 94, 5305–5314. [Google Scholar] [CrossRef]
- Sabokbar, N.; Khodaiyan, F.; Moosavi-Nasab, M. Optimization of Processing Conditions to Improve Antioxidant Activities of Apple Juice and Whey Based Novel Beverage Fermented by Kefir Grains. J. Food Sci. Technol. 2014, 52, 3422–3432. [Google Scholar] [CrossRef]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Potential of Yogurts Produced from Milk of Cows Fed Fodder Supplemented with Herbal Mixture with Regard to Refrigerated Storage. Appl. Sci. 2023, 13, 10469. [Google Scholar] [CrossRef]
- Lamothe, S.; Guérette, C.; Dion, F.; Sabik, H.; Britten, M. Antioxidant Activity of Milk and Polyphenol-Rich Beverages during Simulated Gastrointestinal Digestion of Linseed Oil Emulsions. Food Res. Int. 2019, 122, 149–156. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, E.; Preci, D.; Zeni, J.; Steffens, C.; Steffens, J. Desenvolvimento de Frozen Yogurt de Iogurte Em Pó de Leite de Ovelha. Rev. Ceres 2018, 65, 7–15. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative Activity of Probiotics. Arch. Med. Sci. 2021, 17, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of Blueberry and Blackberry Juices Using Lactobacillus Plantarum, Streptococcus Thermophilus and Bifidobacterium Bifidum: Growth of Probiotics, Metabolism of Phenolics, Antioxidant Capacity in Vitro and Sensory Evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Amaretti, A.; di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant Properties of Potentially Probiotic Bacteria: In Vitro and in Vivo Activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Giaconia, M.A.; Ramos, S.d.P.; Fratelli, C.; Assis, M.; Mazzo, T.M.; Longo, E.; de Rosso, V.V.; Braga, A.R.C.; Ramos, P.; Fratelli, C.; et al. Fermented Jussara: Evaluation of Nanostructure Formation, Bioaccessibility, and Antioxidant Activity. Front. Bioeng. Biotechnol. 2022, 10, 814466. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of Anthocyanins: Gaps in Knowledge, Challenges and Future Research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Alshuniaber, M.A.; Krishnamoorthy, R.; AlQhtani, W.H. Antimicrobial Activity of Polyphenolic Compounds from Spirulina against Food-Borne Bacterial Pathogens. Saudi J. Biol. Sci. 2021, 28, 459. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; Granchi, L.; Tredici, M.R. Vegetable Oils Protect Phycocyanin from Thermal Degradation during Cooking of Spirulina-Based “Crostini”. LWT 2021, 138, 110776. [Google Scholar] [CrossRef]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total Phenolic Compounds in Milk from Different Species. Design of an Extraction Technique for Quantification Using the Folin–Ciocalteu Method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.T.; Ou, L.C.; Luo, M.R.; Hutchings, J.B. Optimisation of Food Expectations Using Product Colour and Appearance. Food Qual. Prefer. 2012, 23, 49–62. [Google Scholar] [CrossRef]
- Ferreira, L.G.B.; Faria, R.X.; Ferreira, N.C.D.S.; Soares-Bezerra, R.J. Brilliant Blue Dyes in Daily Food: How Could Purinergic System Be Affected? Int. J. Food Sci. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.F.; Brunner, T.A. Attitudes and Perceptions towards Microalgae as an Alternative Food: A Consumer Segmentation in Switzerland. Algal. Res. 2024, 103386. [Google Scholar] [CrossRef]
- Colonia, B.S.O.; de Melo Pereira, G.V.; de Carvalho, J.C.; Karp, S.G.; Rodrigues, C.; Soccol, V.T.; Fanka, L.S.; Soccol, C.R. Deodorization of Algae Biomass to Overcome Off-Flavors and Odor Issues for Developing New Food Products: Innovations, Trends, and Applications. Food Chem. Adv. 2023, 2, 100270. [Google Scholar] [CrossRef]
- Marques, C.; Lise, C.C.; Bonadimann, F.S.; Mitterer-Daltoé, M.L. Flash Profile as an Effective Method for Assessment of Odor Profile in Three Different Fishes. J. Food Sci. Technol. 2019, 56, 4036. [Google Scholar] [CrossRef]
- Prasetiyo, H.; Purwaningsih, S.; Setyaningsih, I.; Nurilmala, M.; Uju, U.; Tarman, K. Off-Odour Identification from Volatile Organic Compounds (VOCs) of Spirulina. BIO Web. Conf. 2024, 92, 02006. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).