Abstract
Biorefineries (BRFs) that process the organic fraction of municipal solid waste and generate bioproducts and bioenergies have attracted attention because they can simultaneously address energy and environmental problems/needs. The objective of this article was to critically review the microbial production of hyaluronic acid (MPHA) and its production profile for its integration into a GBAER-type BRF (a type of BRF based on organic wastes) and to identify the environmental and economic sustainability aspects of the modified BRF that would confirm it as a sustainable option. It was found that the MPHA by selected strains of pathogenic Streptococci was moderate to high, although the trend to work with genetically transformed (GT) (innocuous) bacteria is gaining momentum. For instance, A GT strain of Corynebacterium glutamicum reached a maximum HA production of 71.4 g L−1. MPHA reports that use organic wastes as sources of carbon (C) and nitrogen (N) are scarce. When alternative sources of C and N were used simultaneously, HA production by S. zooepidemicus was lower than that with conventional sources. We identified several knowledge gaps that must be addressed regarding aspects of process scale-up, HA industrial production, economic feasibility and sustainability, and environmental sustainability of the MPHA.
Content
- Introduction
- Biorefineries that process organic wastes
- 2.1.
- General context
- 2.2.
- Biorefineries of GBAER type and the potential for integration of the microbial hyaluronic acid production
- Microbial production of hyaluronic acid
- 3.1.
- Hyaluronic acid and its significance/importance
- 3.2.
- Native hyaluronic acid-producing bacteria
- 3.3.
- Recombinant bacteria that produce hyaluronic acid
- 3.4.
- Microbial production of hyaluronic acid using organic waste
- Scale-up of hyaluronic acid production
- 4.1.
- Antecedents so far
- 4.2.
- Concluding remarks
- Economic aspects of hyaluronic acid production
- 5.1.
- Hyaluronic market and price
- 5.2.
- Cost and economic feasibility of microbial hyaluronic production of hyaluronic acid
- 5.3.
- Concluding remarks
- Environmental sustainability studies of HA production
- Conclusion and perspective
1. Introduction
At a global level, it is crucial to determine and implement the sustainability of processes and projects in three dimensions: environmental, economic, and social. Therefore, developing sustainable products and processes are practices currently relevant to companies, governments, and academia [1]. Gawel et al. [2] have reported that the bioeconomy is critical for sustainability. They define the bioeconomy as a circular economy based on biotechnology that is sustainable in producing, converting, and consuming resources and managing wastes.
Biorefineries (BRFs) are considered one of the main backbones of the bioeconomy since BRFs efficiently use resources, minimize waste, and generate a wide range of biobased products. In this way, BRFs can generate high-value-added products, commodities, and bioenergies such as biofuels and bioproducts that promote a greener and more circular economy [3]. For the above reasons, BRFs offer excellent advantages for developing modern societies. One of these advantages is obtaining renewable and sustainable energy to replace fossil fuel production from oil refining, a scarce natural resource [4]. The production and use of fossil-based energy are some of the most important causes that generate global warming and its consequences, such as climate change [5,6,7]. The search, development, and implementation of clean, non-polluting, renewable, and sustainable energy production technologies, such as BRFs, are issues of vital importance worldwide to carry out the necessary energy transition that allows the reduction or mitigation of greenhouse gas (GHG) emissions that cause global warming.
BRFs use different types of biomass as raw materials, such as solid organic waste, lignocellulosic biomass, and algae. Depending on their configurations, these BRFs can produce a range of biofuels, such as ethanol, biodiesel, methane, hydrogen, synthesis gas, electricity/heat, and bio-oil [8,9,10,11]; these biofuels generate lower GHG emissions than fossil fuels, which also offer critical environmental benefits. Palandri et al. [12] reported that ethanol and biodiesel emit up to 80% less CO2-equivalent (CO2e) emissions when produced with organic waste than gasoline or diesel. Busch et al. [13] showed that hydrogen production using waste biomass or municipal solid waste does not contribute to net CO2e emissions. In the case of biogas (methane), Burg et al. [14] found a reduction in emissions when biogas is used instead of gasoline and bituminous coal that reached 24% and 40%, respectively. According to their article, biogas had an emission factor of 56.4 t CO2 TJ−1, while gasoline and bituminous coal emissions were 73.8 t CO2 TJ−1 and 92.7 t CO2 TJ−1, respectively.
A second advantage of BRFs is that value-added products (VAPs) can also be obtained in addition to producing biofuels. VAPs have been an outstanding reason why BRF development has attracted attention in the last decade. VAPs may be co-products or byproducts of biofuel production processes or produced in specific stages of the BRF, depending on the configuration of the BRF and the raw material used. A wide range of VAPs has been reported, from food products [15] to chemicals and biomaterials with industrial applications [16,17,18]. However, several challenges should be overcome to achieve the sound operation of large-scale BRFs since they should be efficient, cost-effective, and sustainable. One of these challenges is the need for more standardization of the production of novel VAPs and efficient biofuel production processes.
Another challenge is identifying the environmental burdens of the production processes, the waste generated, and their respective management in the BRF to understand the ecological implications involved in the complete BRF system. Another related issue is the development of metrics that could quantify environmental sustainability and the other two types of sustainability (economic and social), thus allowing for a more objective comparison between BRFs or between a BRF and conventional technologies [19]. Also significant is the challenge of identifying and eventually developing the market for the commercialization of VAPs and their effect on the economic benefits (economic feasibility) of entire BRF systems. These aspects impact sustainability, and therefore, more scientific attention is required to determine the economic sustainability of BRFs.
The BRFs that process municipal solid waste (MSW) as a raw material constitute a configuration currently attracting great scientific attention [20,21,22,23]. This BRF configuration shows an additional advantage over other types of BRFs because, in addition to addressing the problems above, it also tackles the global problems of waste treatment and disposal, and the role of such BRFs in minimizing the negative impacts of conventional waste disposal. An annual generation of 2.01 billion tons of waste is estimated globally, composed primarily of food and pruning waste (44%), followed by dry recyclables such as paper and cardboard (17%), plastics (12%), glass (5%), and metal (4%), which together generate 1.6 billion of GHG emissions [24]. Moreover, the disposal of waste in the vast majority of low- and middle-income countries is carried out in open dumps and sanitary landfills, thus showing environmental problems due to GHG emissions, mainly methane (CH4), carbon dioxide (CO2), and nitrogen oxide (N2O), as well as adverse effects on human health and the environment, contaminating groundwater, surface water, soil, and air.
The Environmental Biotechnology and Renewable Energies Group (GBAER, for its acronym in Spanish) from CINVESTAV, IPN, Mexico, have developed a BRF that processes MSW where conventional recyclable materials such as aluminum, iron, and plastics are recovered, and the organic fraction of MSW (OFMSW) is separated and used as a feedstock for the BRF. This BRF consists of a network of physical, physicochemical, biological, and auxiliary processes that convert the OFMSW into bioenergies and bioproducts as final products [18,25].
This BRF configuration was coined “GBAER type” and has an “HMZS” arrangement, named in this way because of the production stages of the biofuels and products obtained: biohydrogen in stage (H), methane in stage (M), enzymes of industrial interest in stage (Z) and saccharified liquors in stage (S). According to the results reported by Escamilla-Alvarado et al. [26] and Sotelo-Navarro et al. [18], this configuration presents an attractive environmental sustainability, as determined by the life cycle assessment (LCA) technique, aligned to International Organization Standardization (ISO). The economic sustainability of the GBAER BRF is still to be determined because information on Mexican markets and the economic benefits is scarce, and it is a topic in which research and development still needs to be improved. However, positive economic results are expected to be obtained, as shown by BRFs that process food waste and produce biodiesel and biogas in countries such as the USA and India [27]. Ladakis et al. [21], found that BRFs that process MSW from Greek regions and generate VAPs such as lipids, proteins, and succinic acid showed positive economic results. Jacob-Lopes et al. [28] conducted a market review for several VAPs (for instance, bioactive compounds used as food additives) produced by macroalgae-based BRFs. They reported that these products had a consolidated market share in the USA, Japan, and Brazil, and presented positive economic benefits.
The GBAER proposes that the BRF with the HMZS configuration integrates hyaluronic acid (HA) microbial production using the saccharified liquors produced in the S stage, thus obtaining a high VAP such as HA. It is known that HA is a product with a well-defined and dynamic market, and according to global market forecasts, its economic value, which is already very high, will increase in the future in the short and long term [29]. Therefore, including HA production in the GBAER-type BRF could consolidate the economic feasibility and provide economic sustainability to the BRF. Ucm et al. [30] have also generally considered the idea of HA production in BRFs, proposing HA production by microbial conversion using Streptomyces as an extension of lignocellulosic BRFs. This precedent indicates the current interest in developing high VAPs with an identified and consolidated market to achieve the sustainability of BRFs.
HA has different biomedical, cosmetic, food, health, and medical applications [31,32,33]. It is naturally produced by microorganisms such as Streptococcus [33,34]. Other microbes do not produce HA naturally and can be converted to HA-producers through genetic transformation (GT). Moreover, some reports of high HA production using this approach exist [35,36,37]. The HA-producing microorganisms fundamentally require a source of carbon (C) and a source of nitrogen (N). In most cases published in the open literature, these sources are based on glucose and a yeast extract (see Section 3 below). The investigation of the microbial production of HA (MPHA) to improve the HA production yield has increased scientific interest [38,39,40,41]. However, other important topics that deserve scientific attention need to be addressed, such as using organic waste as a source of C and N in HA production and the economic aspects and environmental impacts of the microbial HA production process.
The objective of this study was to review the microbial production of HA critically, report on the use of organic waste as a substrate, identify/define the stages of microbial production, describe the types of microorganisms that produce HA and their HA production profiles, and discuss the integration of the production of HA in a GBAER-type BRF. The content of this article encompasses six topics. Section 2 addresses BRFs that process organic waste, presenting the biofuels and VAPs that these BRFs produce and emphasizing the GBAER-type BRF. Section 3 addresses MPHA, processes, and production profiles. Section 4 reviews the scaling of the MPHA process at an industrial level. Section 5 deals with the economic aspect of MPHA, continuing in Section 6 with a review of its environmental sustainability. Finally, the conclusions and perspectives are presented.
2. Biorefineries That Process Organic Waste
This section is devoted to the definition of BRFs that use organic waste as feedstock, short descriptions of the main BRF variants, the discussion of the main and auxiliary principles that should ideally rule BRF design, as well as the development of a BRF by our group. The motive of this section is that the review intends, in the end, to foster/consider the integration of the MPHA into organic waste-based BRFs.
2.1. General Context
The development of BRFs has emerged as a sustainable alternative to oil refineries [42,43] that, due to (i) the scarcity of oil resources and (ii) GHG emissions from the burning of fossil fuels, have arisen strong environmental, social, and political concerns. On the one hand, these concerns have encouraged the search for energy alternatives to reduce the dependence on oil and, on the other hand, the development of technological and political options to reduce GHG emissions and mitigate climate change.
Using biomass as a renewable resource (preferably organic waste) through its transformation into a BRF system is a strategy that is of great interest today and continues to be developed. In a BRF, bioenergy, biofuels, chemicals, and biomaterials are produced by integrating several technologies and processes [10,44]. Food and fiber are sometimes obtained, depending on the raw material and process combination within the BRF [8,45]. The different types of biomass and processes used in BRFs include lignocellulosic biomass and organic waste [9,11].
The BRFs that process lignocellulosic biomass are based on several agricultural, forestry, and urban pruning wastes. Through biochemical conversion methods, such as anaerobic digestion and fermentation, as well as thermochemical conversion methods, such as combustion, pyrolysis, gasification, and liquefaction, the production of biofuels such as bioethanol, biobutanol, biogas, bio-oil, and biohydrogen has been reported. Likewise, the production of biomaterials such as nanocellulose, bioplastics, biochemicals (benzene, toluene, and xylene), phenols, and xylitol, in addition to functional materials such as fibers, hydrogels, and vinylin have been reported, and all of these have different industrial applications [9,16,17,46].
BRFs that process algal biomass produce lipids, proteins, carbohydrates, and pigments, depending on the species of micro or macroalgae and the conversion processes used. The production of biofuels such as bio-oil and syngas has been reported when selected streams are subjected to pyrolysis and hydrothermal liquefaction. Biodiesel can be obtained if lipids are transesterified; bioalcohols and biogas can be obtained if biomass is subjected to fermentation and anaerobic (methanogenic) digestion, respectively [47,48]. Narayanan et al. [15] reported that the biomass of both microalgae and macroalgae also presents a significant potential for biohydrogen production based on dark fermentation.
Among the high VAPs that can be obtained from the algal BRF configuration, there are products for human food and animal feed, such as omega-3 and carotenoids, and non-fuel products, such as proteins, pigments (astaxanthin, lutein, echinenone, chlorophyll, and fucoxanthin), carbohydrates and algal extracts used in cosmetics, olefins, and biochar, as well as secondary metabolites (polyphenols, pigments, tocopherols, phytosterols, vitamins, and terpenoids [15,48,49]). Several of these products are value-added compounds [50,51]. Some algal biomass BRF systems show high production costs, high energy consumption, and still relatively low productivity of algal biomass and biomolecules, which make the commercialization of this type of BRF product not viable [15].
Biorefineries that processes organic waste (non-edible and biogenic waste) include the OFMSW, paper, and food waste [11]. The treatment of food waste and OFMSW in BRFs has generated scientific interest because, in addition to obtaining a renewable energy source, it exhibits excellent environmental and economic benefits by developing a waste management system that valorizes waste and avoids dumping or its disposal in landfills [52,53,54]. The bioprocesses in BRFs that process food waste are anaerobic digestion, hydrolysis, microbial fermentation, and hydrothermal carbonization; from these processes, butanol, biogas, methane, hydrogen, biomethane (series production of H2 and CH4), ethanol, electricity, heat, organic acids, biopolymers, proteins, enzymes, and biodegradable plastics are obtained [52,55]. In the conversion of the OFMSW, bioconversion technologies use the metabolic activities of living organisms through fermentation, digestion, and solubilization processes to produce biofuels such as methane, bioethanol, biohydrogen, and bioelectricity, and VAPs such as organic acids (oxalic, lactic, citric, gluconic, succinic, and fumaric acids), bioplastics, and enzymes (proteases, amylases, and lipases) [53,56]. The BRFs that process the OFMSW benefit human health and environmental quality and play an important role in the circular economy. BRFs are generally considered sustainable because they generate minimal waste and thus reduce the pressure on the environment and ecosystem services [23,57,58].
However, although BRF processes are sustainable concepts and practices, there are still needs to (i) develop general sustainability metrics and (ii) establish efficient and cost-effective methods to convert biomass into biofuels and value-added products [17,19,54]. In this way, although developing high-value co-products can have significant commercial potential [48], there are still great challenges for these byproducts to become marketable.
2.2. Biorefineries of the GBAER Type and the Potential for the Integration of Microbial Hyaluronic Acid Production
This subsection focuses on developing a Mexican model of BRFs and the possible integration of the microbial production of hyaluronic acid (MPHA) as a significant stage of VAP generation. For the last 12 years, the Environmental Biotechnology and Renewable Energies Group (GBAER) has been engaged in the conceptual design and lab- and bench-scale development of organic waste-based BRFs using the sustainability approach as a central principle. These BRFs rely on the OFMSW. Four auxiliary principles have been applied to help achieve BRF sustainability [25]. The auxiliary principles are the following: (i) principle of cascading, (ii) principle of non-conflict food bioenergy, (iii) principle of a neutral carbon footprint, and (iv) principle of the production of value-added compounds [25].
The cascading principle seeks to maximize the use of the organic waste fed to the BRF by subjecting it to a network of series–parallel processes that allow a variety of bioenergies and VAPs to be obtained. Thus, the organic waste processed in the first stage yields one or more products, and the exit waste streams enter another stage where, in turn, bioenergy or additional products are obtained. In the GBAER, the reverse cascade approach was chosen; the first stage of the BRF is the generation of bioenergies, followed by a second subset of stages devoted to biomaterial production and ending with a third subset of stages that produce bioenergies from secondary waste streams of the BRF. This arrangement (i) ensures the early obtainment of energy for the operation of the BRF, thus minimizing the use of fossil energy, and (ii) it can be advantageous for countries and regions that are fossil fuel importers. In contrast, in some developed countries, the direct cascade is applied, where the first stage of the BRF is dedicated to obtaining bioproducts. At the same time, some bioenergy is generated in the downstream stages. This arrangement may mean that the BRF might only sometimes be self-sufficient from an energy point of view.
Our GBAER BRF complies with the second auxiliary principle. Using OFMSW as a raw material in the BRF, there is no conflict between food production vis-á-vis energy production. This exciting feature can lead to positive social impacts; that is, it contributes to social sustainability. Furthermore, the energy obtained in the BRF is renewable, has a carbon footprint close to neutral, and generates adequate management and handling of waste. This, in turn, avoids the discharge of the OFMSW to the environment or burying it in landfills, as is the practice in Mexico [59,60,61,62], thereby obtaining positive environmental impacts. Finally, VAPs with industrial applications and market value with potential economic benefits can be obtained (enzymes, bionanoparticles, and succinic acid [18,25]). The fourth auxiliary principle can contribute to the economic feasibility and sustainability of the GBAER BRF.
The core GBAER BRF that processes the OFMSW produces biohydrogen by dark fermentation and produces methane by methanogenesis from the fermented organic waste for hydrogen production [63,64]; these stages, biohydrogen and methane production, were assigned the names H and M, respectively. After stage H, the production stage of value-added hydrolytic enzymes was also integrated (Z stage). Trichoderma reesei and extracted fermented solids are used as substrates. The solids spent from the Z stage were further subjected to enzymatic hydrolysis to obtain saccharified liquors; the latter are rich in glucose and xylose. This stage was called S (Figure 1) [65]. Due to the production stages that make up the core GBAER BRF, the name BRF-HMZS was coined. This BRF performed well as an alternative technology for the disposal of MSW concerning sanitary landfills in Mexico, avoiding emissions of 128 kg CO2e (ton MSW)−1 and reducing eutrophication impacts by up to 98%, ozone layer depletion impacts by 42%, and photochemical oxidation [26].
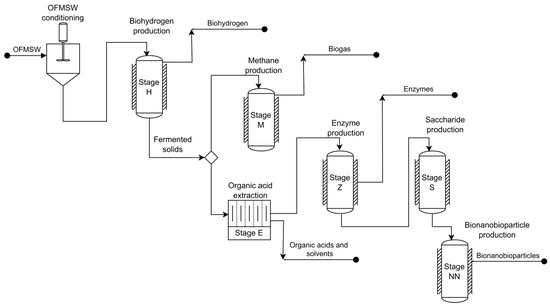
Figure 1.
Configuration of the HMEZS biorefinery developed by the GBAER. The bioenergies obtained were hydrogen and methane in this BRF developed by the GBAER. At the same time, the products that provide added value were organic acids and solvents, a cellulolytic and xylanolytic enzyme concentrate, and bionanobioparticles.
This core BRF was expanded with a solvent extraction of organic acids and low-molecular-weight solvents (Stage E) from a fraction of the fermented solid stream generated in the H stage and with a final stage of bionanobioparticle (BNBP) production (stage NN) for the environmental industry. Bionanobioparticles (BNBPs) are nanodecorated bioparticles (BPs) with iron-material nanoparticles (magnetite, mixed oxides, etc.) [66]. The bioparticles, in turn, are biological catalysts sampled from anaerobic fluidized bed reactors [67,68] treating saccharified liquors (the latter are produced in the BRF [69]). The BPs consist of granular activated carbon (mesh 30) colonized by an anaerobic methanogenic consortium [68]. This consortium is immobilized on the carrier and typically forms a biofilm, thus leading to BPs. With adequate acclimation, BPs can degrade chlorinated organic compounds and surfactants [60,61,62,63,64,65,66,67,68,69,70,71,72]. The nano decoration is carried out using the biological reduction power (bioreduction) of the biomass immobilized in the BPs with a mixture of a FeCl3 solution and a degradable source of carbon (such as saccharified liquors from the BRF) in an ancillary methanogenic bioreactor [66,69]. In this way, iron-material nanoparticles are formed on the surface of the bioparticles, leading to BNBPs. The latter typically show enhanced degradation of organo-chlorinated compounds in polluted waters [66].
The NN stage used the saccharified liquors from the S stage. The expanded BRF was coined HMEZS-NN [18,69]. Its general scheme is shown in Figure 1. The GBAER BRF were self-sufficient in electric energy (with a significant amount of electrical energy for export) but deficient in thermal energy for heating several processes. The environmental sustainability assessment of the BRF HMEZS-NN, which was evaluated by the environmental LCA methodology, presented a sound environmental balance with no significant impacts on the global warming category. However, the Z and NN stages, that is, the production of enzymes and bionanobioparticles, accounted for 90% of the effects on 6 of the 18 environmental impacts evaluated, which were freshwater eutrophication, freshwater and marine ecotoxicities, non-carcinogenic toxicity to humans, and scarcity of fossil resources [18]. In this BRF-HMEZS-NN configuration, economic sustainability remains to be evaluated, which is decisive to knowing whether the byproducts that have added value compensate for their cost of production and repair the negative environmental impacts that are generated. Although economic sustainability is the desired criterion, economic sustainability generally differs from economic feasibility. So far, there has yet to be a consensus and straightforward method to determine economic sustainability. In contrast, the economic feasibility determination is well-known in textbooks of chemical and process engineering [73].
The GBAER proposed the BRF-HMEZS-HA configuration, in which the S stage saccharified liquors are used to produce HA (HA stage) of microbial origin. The latter constitutes an additional or alternative VAP that could diversify the BRF’s product menu. Our hypothesis revolves around using saccharified liquors from the S stage (exhibiting high glucose and xylose concentrations) as carbon sources in the MPHA. As we will cover below, one of the main features of MPHA is the need for carbon sources, regardless of the type of microbial strain. On the other hand, HA has a very high added value [31,74] and could increase the economic sustainability of the BRF.
3. Microbial Production of Hyaluronic Acid
This section reviews the main subject of MPHA. It consists of four subsections, namely, (i) the hyaluronic acid nature and its importance; (ii) the MPHA by bacteria that naturally produce HA; (iii) the MPHA by recombinant microbes, and (iv) the use of organic wastes as sources of C and N. The latter subsection is a key subject for the possible integration of MPHA into the BRF, and so far, there is no systematic information.
3.1. Hyaluronic Acid and Its Significance/Importance
HA is a linear polysaccharide constituted of 2000 to 25,000 units (disaccharides) of N-acetylglucosamine and glucuronic acid joined alternately by β-1-3 and β-1-4 glycosidic bonds, as shown in Figure 2. HA is negatively charged, highly hydrophilic, and forms viscous and dense network at high molecular weight [75,76].
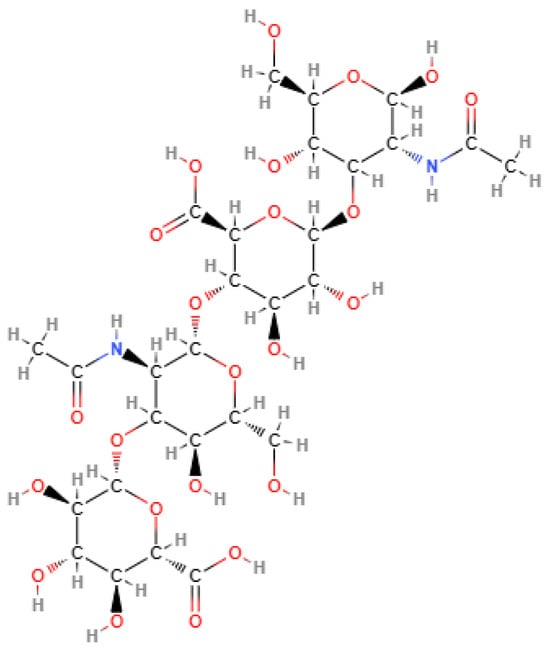
Figure 2.
Chemical structure of hyaluronic acid. Source, NCBI [77].
Hyaluronic acid is a very attractive product for a great variety of medical, biomedical, pharmaceutical, chemical, and food applications due to its characteristics of high hygroscopicity, viscoelasticity, biocompatibility, and antigenicity. This is coupled with the fact that it does not generate toxic products when degraded [31,34,75,78]. Hyaluronic acid has been used as an adjunct in drugs, as a means of drug absorption and administration, as a scaffold in tissue engineering, and for intra-articular administration, among some of its applications. In ophthalmology, HA is used as an adjunct in artificial tears, eye drops, and intravitreal injections, as an in situ gel and nanoparticle coating for drug absorption and delivery, and as a hydrogel in corneal tissue engineering [69]. In rheumatology, intra-articular administration of HA improves negative symptoms and decreases the use of non-steroidal anti-inflammatory drugs in patients with osteoarthritis [79]. HA is also a scaffold material in bone regeneration. Zhai et al. [80] reported that coverage of dental implants with HA improves their osseointegration and acts as part of a complement to periodontitis. Covering the surgical area (interior and exterior) with HA improves and accelerates the tissue healing process and the remodeling of the dental bone, enamel, dental pulp, root canal, temporomandibular joint, mucosa, and salivary glands.
Currently, HA has a potential focus in cancer treatment as a drug carrier and in the design of nanoparticles or liposomes encapsulated with or conjugated to therapeutic agents [75,81]. It is also used to detect CD44 hyaladherins (CD44, cluster of differentiation 44), which are used in the diagnosis of specific tumors [82]. Other uses are as a specific magnetic resonance imaging (MRI) contrast agent for cancer diagnosis and treatment [75,82]. Hyaluronic acid has also been tested as a carbon source for forming fluorescent C dots to spot small metal ions such as Fe3+ in water. Since an increase or deficiency of iron can induce severe physiological damage and biological obstacles such as kidney failure, heart disease, liver injuries, and cancer, it is essential to monitor and determine Fe3+ levels in biological, medical, and environmental samples [83]. Regarding food applications, the use of HA as an edible film or coating that improves food products’ quality and shelf life [84,85]. However, it is most frequently used in dermatology as a dermal filler to treat wrinkles and for facial rejuvenation [86,87,88].
The physical, physical–chemical, and biological functions of HA are generally related to the molecular weight (MW). This and HA’s purity indicate its quality [32,89]. HA can be classified into three categories: (i) high MW, (ii) low MW, and (iii) oligosaccharides [32,75]. The MW of the first type of HA is greater than 1 MDa; it exhibits anti-inflammatory activity and is used for the recovery and repair of joint tissue and cartilage degeneration, respectively, and in plastic and cosmetic surgeries [32,90]. The MW of the second type of HA is between 0.01 and 1 MDa; the human body better absorbs this type, and it has pro-inflammatory activity and promotes tissue remodeling in wound healing [75,91]. The MW of the third type of HA is less than 0.01 MDa, and it has several food, health, and medical applications [32].
HA was initially obtained from tissues such as rooster comb, shark skin, and bovine eyeballs, among other types of tissues. Since this form is found in a complex linked to other biopolymers, complex procedures are required to obtain the pure compound and prevent contamination by toxins. These procedures include digestion with proteases, precipitation of HA ion pairs, membrane ultrafiltration, solvent-free precipitation and lyophilization [92]. After extraction, the HA-containing solution is treated to eliminate the bacteria and proteins attached to the HA framework. Bactericide substances and proteolytic enzymes are used at this stage [34]. The HA extraction and purification procedures are expensive and can cause cross infections [93].
Additionally, the extraction of HA from animal tissues presents other disadvantages, such as the limited availability of animal tissues and the low rate of HA extraction [34]. Another major problem is that HA from animal tissue may be bound to undesirable contaminating cellular proteins, such as hyaluronidase. The latter is a HA-specific binding protein because there is the possibility that these proteins provoke an immune response in addition to the potential risk of contamination with nucleic acid prions (in the case of bovine tissues) and viruses (in the case of avian tissues) that could cause the transmission of infectious diseases [94]. MPHA has been developed in recent decades to avoid the risk of viral infection across species. It exhibits lower production costs, easier and more efficient purification processes, and higher HA production yields than animal tissue extraction [34].
The MPHA process consists of five main steps, as shown in Figure 3. These steps range from propagating the HA-producing bacteria to HA quantification and characterization methods.

Figure 3.
Main phases of the hyaluronic acid production process.
The process begins with the preculture of the selected microorganism to generate the seed culture that will later be inoculated in phase II. During phase I, different nutritional media are used that provide sources of C, N, vitamins, and minerals to allow abundant growth of microorganisms [39,95]. In phase II, the MPHA is carried out under physical–biochemical conditions of temperature, pH, stirring speed, and aeration rate. At this stage, the culture medium consists of sources of C, N, vitamins, and minerals that can be optimized to increase HA production. The C sources can be glucose, fructose, and maltose [38,96,97,98]; the N sources are typically ammonium sulfate and yeast extract [36,95]. Thiamine, riboflavin, and pyridoxine can be added as vitamins [99,100], and sodium, potassium, and calcium can be added as minerals [38,101,102]. Other growth factors can be peptone and tryptone [103,104]. The cultivation methods in the MPHA that have been reported include three types: (i) batch, where a closed container is loaded and operated for a specific period, afterwards, the culture is harvested and HA is extracted; (ii) fed-batch, where a reactor or container is loaded with a microbial inoculum and intermittently or continuously fed with the substrate, but no exit streams exist; and (iii) continuous, in this configuration, the microorganisms grow in a closed container and are constantly fed the substrate, whereas spent broth is constantly wasted out of the bioreactor.
In phase III, collection and purification are carried out for subsequent characterization. The MPHA occurs intracellularly, and most of the HA is eliminated from the cells as capsules, and so three steps are distinguished: (i) release of the capsular material, (ii) precipitation of the supernatant, and (iii) centrifugation [105,106]. The release of HA from the capsular material involves detergent and cold ethanol extraction. The release of HA favored by surfactant addition and the decrease of the dielectric constant of the HA-containing aqueous system by solvent addition lead to better intramolecular interactions of the polymer. This, in turn, increases the molecular weight of the HA, followed by its precipitation. Afterward, the enlarged polymer is separated by centrifugation [107]. The compound cetyl-pyridinium chloride is commonly added to react with the centrifuged pellet of HA, forming a complex. Then, it is dissolved in ethanol at 5 °C. A second method is the release of the HA capsule with SDS, precipitation with ethanol, and centrifugation.
In phase IV, HA is quantified using two main methods: the carbazole and turbidimetric techniques [35,36]. Finally, phase V determines the MW, and the quality parameters determine the rheological properties and responses to physiological effects that define HA applications [40,103].
3.2. Native Hyaluronic Acid-Producing Bacteria
Two types of bacteria produce HA naturally, namely, Streptococcus groups A and C and Pasteurella multocida [33,34]. Streptococci are firmicute bacteria of the class bacilli and order Lactobacillus [108] (p. 2110). They belong to the family Streptococcaceae and are Gram-positive bacteria (BG+), non-sporulating, non-motile, and typically pathogenic to animals. Their shape is spherical or ovoid. Cells appear arranged in chains or forming couples, surrounded by an extensive extracellular capsular material where HA is synthesized as part of their self-defense against invading organisms [76].
The Streptococcus strains that produce HA are S. zooepidemicus, S. pyrogenes, S. equisimiles, and S. thermophilus. Streptococcus equi subsp. equi and predominantly S. equi subsp. zooepidemicus are commonly used in commercial HA production [31,109,110]. HA is synthesized by HA synthase (HAS). In S. equi subsp zooepidemicus, HA is synthesized by the has operon composed of 5 genes: (i) hasA, which encodes hyaluronate; (ii) hasB encoding UDP-glucose dehydrogenase; (iii) hasC encoding UDP-glucose pyrophosphorylase; (iv) hasD encoding N-acetyl-glucosamine-1-phosphate uridyltransferase (glmU); and (v) hasE encoding glucosa-6-phosphate isomerase (pgi) [111,112,113,114]. Table 1 presents the HA production profiles of the Streptococcus strains under natural production conditions. Five types of different strains are reported, with Streptococcus zooepidemicus strains ATCC 39920 and 35246 being the most used.

Table 1.
Native hyaluronic acid-producing strains: culture conditions, hyaluronic acid production, and molecular weight.
The most used type of fermentation in HA production is batch type, and little has been reported on continuous or fed-batch processes. To our knowledge, the largest reported bioreactor capacity for Streptococcus zooepidemicus strains is 10 L. The temperature and pH conditions were standardized at 37 °C and pH 7, but stirring and aeration speed parameters varied from 180 to 600 rpm and 0.5 to 2 vvm, respectively. In the culture media, the primary source of C used was glucose, although it can also be sucrose or maltose. On the other hand, the N source was yeast extract in all cases, although some authors, such as Huang et al., Rangaswamy & Jain, and Yuan et al. [38,95,97], added tryptone or casein enzyme hydrolysate to increase the N source. The culture medium supplements used in all cases were sulfates and/or phosphates, such as magnesium sulphate (MgSO4), dipotassium phosphate (K2HPO4), monopotassium phosphate (KH2PO4), and potassium sulphate (K2SO4). Jagannath & Ramachandran [99] have added trace metals such as calcium chloride (CaCl2), iron(II) sulfate (FeSO4), ammonium sulphate ((NH4)2SO4), manganese(II) chloride (MnCl2), zinc sulfate (ZnSO4), and copper(II) sulfate (CuSO4); amino acids such as alanine, glycine, leucine, isoleucine, valine, methionine, serine, threonine, proline, asparagine, arginine, lysine, ornithine, glutamate, aspartate, phenylalanine, tryptophan, histidine, cysteine, adenine, guanine, and uracil; and vitamins like calcium pantothenate, pridoxamine, rivoflavin, thiamine, pyridoxamine, cyanocobalmin, folic acid, and biotin; as well as glumanite and lipoic acid [100], ascorbic acid [115,116] and beta-glycerophosphate [96].
Table 1 shows that the highest HA production reported with Streptococcus zooepidemicus was obtained using the WSH-24 strain, which exhibited a production of 6.6 g L−1 in a stage culture strategy. The latter consisted of a fed-batch culture in the first stage, where cell growth was achieved, whereas HA was synthesized in a second batch culture [117]. However, the polymer MW was not reported.
In the case of the Streptococcus equi strain, the fermentation conditions of temperature and agitation, 33 °C and 187 rpm, respectively, were lower than those reported for Streptococcus zooepidemicus, and the pH was slightly higher (7.8). The sources of C, N, and supplements did not vary from Streptococcus zooepidemicus. Streptococcus equi produced the highest HA content among bacteria that naturally produce HA, with a value of 12.01 g L−1 and a low MW of 0.079 MDa in a batch process [104]. This production is two times higher than the highest production reported with Streptococcus zooepidemicus in fed-batch culture [117], although this value in Streptococcus equi was achieved in low-capacity bioreactors.
Genetic transformation techniques have been used on Streptococcus strains to increase HA production (Table 2). The mutagenesis technique has been employed with Streptococcus zooepidemicus [89,119,120]. In some cases, the elimination or amplification of hyaluronidases [39,121], as well as the overexpression of synthases [122,123], have been implemented. Like the production of HA using Streptococcus zooepidemicus without GT, most fermentations were batch type. They used low-capacity bioreactors (0.2 to 5 L), except in one study [108], which was the only one to report the production of HA on a pilot scale (70 L). The operation conditions were established at 37 °C, pH 7, and agitation of 150–600 rpm. When aeration was applied, the values were in the range of 0.5 to 2 vvm.

Table 2.
Native hyaluronic acid-producing strains with genetic transformations, culture conditions, production, and molecular weight.
The sources of C and N in the culture media were generally glucose and yeast extract, respectively. However, peptone and casein hydrolysate were used as a supplementary sources of N. Mineral supplements included MgSO4 and K2HPO4 and trace metals such as CaCl2, FeSO4, MnSO4, ZnCl2, and CuSO4, as is the case in the study repoted by Lu et al. [119]. Among vitamins and other additives, glutamine, glutamate, and oxalic acid addition have been reported [120], as well as pyruvate, N-acetyl glucosamine, and phosphatidylcholine [39].
With these GT strains, HA production has been slightly improved in most cases compared to Streptococcus zooepidemicus without GT. HA concentrations up to 6.7 to 8.5 g L−1 were achieved [119,120,122] and generally with a high MW greater than 1 MDa (Table 2). A recent study by Zhang et al. [39] implemented an experimental strategy combining Streptococcus zooepidemicus 39,920 mutagenesis with the construction and amplification of a recombinant plasmid associated with a two-stage semi-continuous fermentation process. With this approach, HA production reached 29.38 g L−1, which is so far the highest HA production in Streptococcus. However, its MW still needs to be reported. The simple expression of hasA, hasB, and glmu has been performed in Streptococcus thermophilus [123]. The production of HA was carried out in a batch process in a low-capacity bioreactor. The temperature, pH, and agitation conditions for Streptococcus thermophilus varied from those used to cultivate Streptococcus zooepidemicus. For instance, higher temperature conditions (40 °C) and lower pH and agitation (6.8 and 100 rpm, respectively) were required with Streptococcus thermophilus. However, this strategy presented a low HA productivity of 1.2 g L−1 with a low MW.
3.3. Recombinant Bacteria That Produce Hyaluronic Acid
The polysaccharide cell capsule of Streptococcus is a virulence factor because it is the place where several endotoxins produced by the microbe are found, along with HA, which is a natural component of the host organism [33,110]. Due to safety concerns about the pathogenicity of the genus Streptococci, other Gram-positive and Gram-negative microorganisms classified as GRAS (generally recognized as safe) have been studied and transformed. Although these GRAS microorganisms do not produce HA naturally, it has been possible for the bacteria to produce that compound through GT. This result is very convenient since the GRAS microbes do not produce endotoxins [94]. The GT microorganisms can be grouped as Gram-positive bacteria (BG+), Streptococcus, Gram-negative bacteria (BG−), and fungi [33].
The use of recombinant microorganisms to produce HA has consisted of introducing and expressing one or more genes responsible for producing the acid in the bacterium that naturally produces HA into an innocuous bacterium. The first one is generally a strain of Streptococcus; the second one can belong to a comprehensive series of genera. Genetic transformation can also be accompanied by strategies that block or attenuate metabolic pathways that divert HA production to other compounds, such as lactate–acetate or pentose phosphate. Among the recipient innocuous bacteria that naturally do not produce HA, we highlight several BG+, such as Lactobacillus acidophilus, Corynebacterium glutamicum, Streptomyces albulus, Synechococcus, Bacillus subtilis, and Lactococcus lactis. Table 3 compiles information on innocuous microbes and their performed GT, their culture conditions, and the corresponding HA production after the intervention.

Table 3.
Recombinant bacteria for the production of hyaluronic acid, culture conditions, hyaluronic acid production, and molecular weight.
HA production was mainly carried out in batch bioreactors in these recombinant bacteria. However, to a lesser extent, fed-batch type bioreactors have also been reported. Bioreactor capacities in these trials were typically low (at lab scale), with a maximum of 5 L. The culture conditions of temperature, pH, agitation, and aeration varied among the different bacteria. The highest temperatures of 38 °C and 37 °C were set for Lactobacillus acidophilus and Bacillus subtilis cultures, while the lowest temperature was used in the production with Streptomyces albulus and Lactococcus lactis (30 °C). Cultures were set at pH 7, except for Streptomyces albulus, whose experiments were performed at pH = 4.2. Agitation varied between 200 and 800 rpm, and aeration, when used, was 2 to 5 vvm.
The sources of C and N and the supplements were similar to those used with Streptococcus strains (glucose or sucrose and yeast extract, respectively). The most common supplements were MgSO4, K2HPO4, and KH2PO4. For Corynebacterium glutamicum and Bacillus subtilis, trace elements such as cations in the salts CaCl2, FeSO4, MnSO4, ZnCl2, and CuSO4 were reported. Other additives, such as pyruvate, Isopropyl-β-D-thiogalactoside, N-morpholino propane sulfonic acid, and citric acid, and ascorbic acid, were added in all cultures.
Genetically transformed bacteria generally produced higher amounts of the polymer than natural HA producers (except for Lactobacillus acidophilus). These GT bacteria can reach productions greater than 6.2 g L−1 (up to 74 g L−1) and produce the three HA types: high MW, low MW, and oligosaccharides. C. glutamicum strains showed the highest HA production. The authors of [36] reported a strain in fed-batch culture that produced 28.7 g L−1 of HA with a MW of 0.21 MDa, while Wang et al. [101] achieved the highest HA production observed in fed-batch culture with a value of 74.1 g L−1 of 0.053 MDa HA. It is recognized that the production of HA by other GT bacteria has yet to be explored. Among these are Escherichia coli and Agrobacterium sp., although these have shown very low HA production levels. The types of strains and production of these BG- are gathered in Table 4.

Table 4.
Strains of recombinant Gram-negative bacteria.
In batch fermentation, the HA production studies with GT-BG- have been carried out in very low-capacity bioreactors (0.25 L and 0.5 L). The temperature in experiments with E. coli has been set at 37 °C, while in Agrobacterium sp., the temperature was 30 °C. The pH was 7 in the E. coli cultures, but in Agrobacterium sp., the corresponding value was not reported. Agitation was similar in both cultures, with values between 200 and 250 rpm. Incidentally, in neither case was the application of aeration recorded.
Regarding C and N sources, in the same way as the BG+ GT case (Table 3), glucose and yeast extract have been used, respectively. Similarly to what was commented above, MgSO4, K2HPO4, and KH2PO4 supplements were used, although in BG- (Table 4), the use of antibiotics was greater. In the case of E. coli, the use of isopropyl β-D-1-thiogalactopyranoside, chloramphenicol, ampicillin, and kanamycin was observed, while casamino acids and sodium citrate were used as additives for Agrobacterium sp. However, HA production with BG- GT has been deficient so far (<1 g L−1 HA). E. coli can produce low and high MW HA, whereas, in Agrobacterium sp., the production of high MW HA with a value of 1.56 MDa was observed.
The utilization of fungi for HA production is still scarce. Among these, we found Kluyveromyces lactis, Pichia pastoris, and Ogataea polymorpha, whose GT consisted of the introduction of HA-synthesizing genes from naturally producing bacteria, generally Pasteurella multocida. Table 5 shows the types of fungi, cultivation conditions, and HA production achieved. The application of batch bioreactors was predominant. A capacity up to 2.5 L was observed. The operation temperature depended on the fungal strain, and 26 °C, 30 °C, and 37 °C were set for Pichia pastoris, Kluyveromyces lactis, and Ogataea polymorpha, respectively.

Table 5.
Types of fungi, culture conditions, production, and molecular weight of the hyaluronic acid produced by recombinant strains.
The pH and stirring speed were lower for K. lactis (pH = 6, 200 rpm), while for P. pastoris, the pH was 7. For P. pastoris and Ogataea polymorpha, the stirring rate reached 500 rpm. With all the strains, the primary sources of C and N were glucose and yeast extract supplemented with peptone. The leading mineral supplements were MgSO4 and K2HPO4. The use of vitamins and other additives, such as glutamine, glutamic acid, and oxalic acid, was also important.
Fungi produced very little HA; the highest yield reached was 1.89 g L−1 in K. lactis [132], but the MWs were high: 2.09 MDa in K. lactis [132] and 1.2–2.5 MDa in P. pastoris [133].
3.4. Microbial Production of Hyaluronic Acid Using Organic Waste
In MPHA, the two vital conditions for the growth of bacteria or fungi are the sources of C and N. In all the cases reviewed, the sources were typically glucose or sucrose as a C source and yeast extract as an N source. Nowadays, few studies have explored the use of alternative organic substrates, and even fewer have explored the use of organic waste as alternative sources of C and N. Table 6 shows the different studies that have evaluated the use of particular organic waste as a source of C for HA production, the types of microbial strains, and their HA production levels. The microorganism used in these works was Streptococcus zooepidemicus, which naturally produces HA. No BG+ GT or BG− GT has been tested with residual carbon sources. Generally, batch bioreactors were used, and the highest capacity was 5 L. The culture conditions were similar to those reported in Table 1: 37 °C, pH between 7 and 8, a low agitation rate between 100 and 300 rpm, and aeration ranging from 0.5 vvm to 1 vvm. The organic materials used as alternative sources of C that have been reported so far are starch, cashew apple juice, palm sap, and cane molasses. In several of these cases, industrial-grade glucose was added. For the N source, no waste source was observed; yeast extract continued to be used, along with the addition of MgSO4 and KH2PO4 as supplements. Under these conditions, low HA production levels were observed, and the HA had a low MW. Only the experiments where starch was used as the C source and peptone was used as the N source yielded HA levels similar (6.7 g L−1) to those reached by Streptococcus strains with conventional C and N sources.

Table 6.
Microbial strains and organic waste as carbon sources used in hyaluronic acid production.
Studies that have explored the use of organic waste as a source of N are also very scarce and always use Streptococcus zooepidemicus (Table 7). No reports for HA production with residual N sources using BG+ GT or BG− GT exist.

Table 7.
Microbial strains and organic waste as nitrogen sources used in hyaluronic acid production.
Finally, Table 8 shows the studies that have simultaneously evaluated the use of alternative C and N sources. In all cases where alternative sources of C and N were evaluated, natural HA-producing strains of Streptococcus zooepidemicus were used under similar culture conditions.

Table 8.
Microbial strains and organic waste sources of carbon and nitrogen used to produce hyaluronic acid.
Batch bioreactors with an up to 5 L capacity have been used. The temperature conditions were maintained at 37 °C. However, the pH was slightly lower (6.7), whereas the agitation speed was higher (500 rpm) compared to studies where only an alternative C source was used (Table 6). The alternative sources of nitrogen studied were peptone obtained from fish viscera, cheese whey, and fermented corn liquor, to which small amounts of conventional N source (yeast extract) were also added. In addition, the conventional source of C (glucose or sucrose) and the supplements of MgSO4, KH2PO4, and K2HPO4 were maintained. In these cases, high MW HA production reached 4 g L−1.
As seen in Table 8, these cases when alternative sources of C and N were simultaneously used exhibited low-to-moderate HA production, between 33% and 50% of the maximum observed for Streptococcus using conventional C and N sources.
Arslan and Aydogan [144] reported the highest production of this type of HA, reaching 3.54 g L−1 HA (Table 8), and Vazquez et al. [143] observed that the HA was a high MW. The HA production shown by any of the three ways of using organic waste as sources of C and N is generally similar, except when starch with glucose is used. Differences were found in the HA’s MW. In studies where only an alternative source of C was used, HA had a low MW, typically 0.001 to 0.98 MDa [136,137,138], while it had a high MW (1.8 to 3.8 MDa) when using only an alternative source of N or alternative sources of both C and N [140,141,143].
To our knowledge, there are no reports in the open literature on the use of organic waste from MSW or the OFMSW for the microbial production of HA. Knowledge about this aspect is vital to consider the integration of microbial HA production in GBAER-type biorefineries where a good amount of saccharified liquors from the hydrolysis of several internal solid streams of the BRF would be available.
4. Scale-Up of Hyaluronic Acid Production
The efficiency/feasibility of MPHA at an industrial/commercial level on a full scale is critical to fostering its continued application and the practical success of obtaining this VAP. This section focuses on this important topic and is subdivided into (i) the revision of reported experiences and (ii) concluding remarks.
4.1. Antecedents So Far
According to Chong et al. [76], around the 1980s, commercial microbial production of HA began using strains from group C streptococci, such as S. equi subsp. zooepidemicus and Streptococcus equi subsp. equi, and batch processes. Other bioreactor configurations were also reported, such as fed-batch or continuous fermentation. However, the instability of the HA-producing strains of Streptococcus was evident, and until 2005, these configurations had yet to be applied at an industrial level. Until recently, open literature reports that address the microbial production of HA in bioreactors with a capacity greater than 10 L have been very scarce. There is an urgent need to produce more publications on the experience of scaling the production process. In this field, the experiences reported are mainly at the bench scale.
The studies shown in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8 indicate that HA production is carried out in bioreactor sizes of a maximum capacity of 10 L, except the study reported by Im et al. [120], which is the only one that analyzes the experimental production of HA on a pilot scale in a 75 L capacity bioreactor. In general, Streptococcus zooepidemicus strains are used in these studies (see Table 1 and Table 2). In contrast, the experiments for BG+ GT (that show the highest production of HA) have been performed in bioreactors of up to 5 L (see Table 3). On the other hand, studies of organic wastes as sources of C and/or N using Streptococcus zooepidemicus strains (see Table 6, Table 7 and Table 8) and those on HA production using BG- GT have been carried out at a lab scale.
The study reported by Im et al. [120] for HA production on a pilot scale using a 75 L fermenter containing 45 L of working volume achieved a product concentration of 6.94 g L−1 with a high MW (5.9 MDa). The strain used was Streptococcus zooepidemicus that was genetically transformed by mutagenesis using a culture medium enriched with glutamine, glutamate, and oxalic acid. The operating conditions were 36 °C, 0.5 vvm, and 400 rpm, and a pH of 7 for 24 h. Two six-bladed disc turbine impellers provided agitation, with impeller and vessel diameters of 120 mm and 350 mm, respectively. In addition to maintaining high HA production yields in process scaling, another essential aspect is guaranteeing a high HA purity. Marketing of HA requires high levels of purity and thorough removal of endogenous toxins from Streptococci strains [30,109]. Ferreira et al. and Ucm et al. [30,74] recognize that the approach for MPHA must consist of three main stages: the upstream section, primary HA collection, and purification. Figure 4 shows the general scheme of the large-scale microbial HA production process.
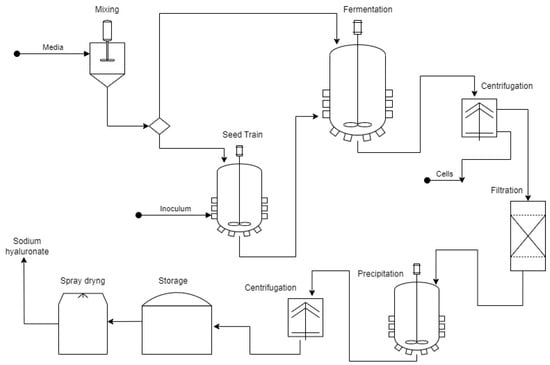
Figure 4.
Microbial production process for hyaluronic acid. Adapted from [30,74].
4.2. Concluding Remarks
The information available so far on the scale-up of HA production points out the need to promote studies and experiments on pilot and commercial scales so that the industrial MPHA will have solid design and operation bases. In addition, the drivers of findings and developments obtained at laboratory scale to practical production levels can be accelerated with multiple benefits. This is a vital area of the HA R & D frontier that can easily last a decade.
Tackling this will boost the MPHA and have significant positive consequences on HA’s availability, quality, and price in the national and global markets. It will also expand the number of beneficiaries from the virtuous use of the HA produced, especially in medical and human health applications. On the other hand, the possible and, in principle, attractive integration of MPHA into BRFs (such as GBAER-type BRFs) depends to a large extent on HA production becoming a mature industrial option with reliable and optimized performance.
It is necessary to focus efforts on the evaluation of MPHA on an industrial scale to verify that cultivation conditions and source rates of N and C, as well as supplements, match the productions reported on a small scale. According to Ucm et al. [28], these actions are vital to accelerating technological improvements to obtain higher HA yields and understanding their industrial applications.
5. Economic Aspects of Hyaluronic Acid Production
The economic aspects of any production system are crucial to determining whether a project will be successful. This section addresses the economic issues of MPHA. The section is divided in (i) a short description of the hyaluronic acid market and price; (ii) results and a discussion of available information on the costs and economic feasibility of microbial production of hyaluronic acid; and (iii) concluding remarks.
5.1. Hyaluronic Acid Market and Price
The economic aspects of any production system are crucial to determining whether a project will be successful. Although it is known that the global HA market is highly profitable, an economic analysis of HA production processes has yet to be reported. Grand View Research [29] reported that in 2022, the market value was valued at USD 9.4 billion and showed an upward trend, maintaining an average annual growth rate of 7.58% (and the forecast for 2030 predicts that its value will reach USD 16.8 billion). It has been reported that the price of HA depends on the application and purity required [31]. De Oliveira et al. [31] indicated that the sale price varied between USD 1000 and USD 5000 per kg, whereas Ferreira et al. [74] stated that it varies between EUR 1500 and EUR 4000 per kg. Both estimates represent attractive sales prices; however, knowing HA’s production cost is essential to determine this bioproduct’s added value.
5.2. Costs and Economic Feasibility of Microbial Production of Hyaluronic Acid
A few studies reported the costs of microbial HA production. Among them, the reports by Amado et al. [141,142] suggest that cheese whey or fermented corn liquor is a lower-cost alternative source of N than commercial peptones such as tryptone. In this way, organic waste is a low-cost alternative for HA production and can also be a strategy to revalue that waste. In the study by Amado et al. [141], the production cost of HA was estimated, considering the commercial costs of peptones and sugars. They found that the cost of HA production was 72% lower when using cheese whey than commercial peptones. The HA produced with cheese whey as an N source was EUR 0.27 g−1, while the HA produced with commercial peptones had a cost of EUR 0.91 g−1.
Rohit et al. [138] evaluated the technical–economic viability of MPHA and estimated its economic performance. They found that the use of palmyra palm sugar performed better than the use of glucose. The results also showed that the combination of peptone soy and palmyra palm sugar performed the best, obtaining in the sale of HA a profit of USD 3.02 for each USD nutrient invested in the production of HA. Similar results were observed when using organic waste as substrates for HA production in the study by Vazquez et al. [143], who compared the cost of HA production using marine wastes as sources of C and N with the corresponding cost using glucose and yeast extract. Indeed, for the second case, the cost was EUR 2.4 per g of HA, while in the first case (use of wastewater from mussel processing as a C source and tuna peptones as an N source), the cost was only EUR 1.4 per g of HA.
Only some studies also address the economic analysis as a project on an industrial scale. Cerminati et al. and Ferreira et al. [74,145] reported evaluations of microbial HA production processes based on simulation and published information at the lab scale. For instance, Ferreira et al. [74] simulated the HA production process using Streptococcus zooepidemicus strains to carry out the economic evaluation of the process considering an HA production of 5 g L−1 and an annual production of 20,000 t in 35 m3 fed-batch bioreactors. In this evaluation, investment and operation costs were considered, including the installation cost, the cost of raw materials such as glucose and yeast extract, the cost of materials and equipment, and the cost of operators’ salaries. In this way, a unit production cost was estimated between USD 1691 and 1449 per kg of HA, an internal rate of return of 42.5% to 53.1% and an investment recovery period of 2.35 to 1.88 years.
Cerminati et al. [145] simulated the microbial production of HA with two types of bacteria: Streptococcus equi ATCC6580 and two recombinant strains of Bacillus subtilis, A164Δ5 and 3NA. The simulation considered a bioreactor volume of 400 m3 and an HA sales cost of USD 200 kg−1 (value taken from the literature). The results showed that the highest HA production per year can be achieved using the Bacillus subtilis 3NA strain with production of 1,426,255 kg per year, followed by the Streptococcus equi strain with production of 1,222,257 kg per year. The results of the abovementioned studies are very encouraging and confirm the economic viability of HA production. According to Ferreira et al. [74], the highest production costs of HA using Streptococcus at an industrial level are in plant maintenance and depreciation, followed by the costs of workers’ salaries, the cost of the required consumables for HA collection and purification processes (such as microfiltration and ultrafiltration membrane), and the cost of glucose and yeast extract. In this way, the use of organic waste as an alternative source of C and N could minimize the cost of conventional C and N sources. Additionally, according to Cerminati et al. [145], recombinant strains, such as B. subtilis, can increase the production of HA compared with that of the Streptococcus strain. Therefore, using both strategies (using organic waste as an alternative source of C and N, and the development of recombinant strains) could minimize the unit cost of production, obtaining more significant economic benefits from the HA production.
5.3. Concluding Remarks
In general, the information available on the economic issues of the MPHA is scarce and not systematic. On the one hand, there is evidence that the global HA market is highly profitable. However, the economic analysis of the HA production processes has yet to be systematically reported. Only a couple of articles have addressed the economic analysis as a project on an industrial scale, and one is a simulation study. The results of these papers indicate the economic feasibility of MPHA and suggest that use of waste sources of C and N can decrease production costs, and MPHA by GT microbes can be superior to that of native HA-producing bacteria. Thus, these results indicate areas of further research, although this recommendation should be considered cautiously because it is based on only two references.
6. Environmental Sustainability Studies of Hyaluronic Acid Production
Evidence was sought on environmental sustainability studies for microbial production of HA carried out with systems analysis tools, such as LCA (LCA of HA production and environmental impacts of HA production). To the best our knowledge, no references were published in the 2005–2024 period. The previous discussion strongly suggests a priority research area for 2024 onwards because every technology must have a sustainability assessment. According to the consensus of broad communities of specialists, the sustainability of technological development is a requirement that, if it is not fulfilled or improved, the technology will eventually be abandoned as unviable.
The LCA is one of the internationally accepted tools for evaluating environmental sustainability. It is a methodological framework that estimates and analyzes environmental impacts attributable to the life cycle of a product associated with the manufacture and use of technologies, products, or services during their life cycle stages [146,147,148]. The LCA was standardized under ISO standards since 2006, and this methodology has been established in the ISO 14040 series, which provides a rigorous technical framework [149,150,151].
The term “life cycle” refers to the main activities involved in a product’s useful life, a process, or a service, from its manufacture, use, and maintenance to its final disposal, including acquiring the raw materials required to manufacture the product. When evaluating all life stages of a product in the LCA, these stages are considered interdependent, which means that one operation gives way to the next. In this way, the cumulative environmental effects of all stages of a product’s life cycle can be estimated. A life cycle assessment can be used as a comparison method (i.e., the enhancements of one process or product can be compared to the background status).
In a LCA, strategies are developed that are essential to making the production process more efficient, which, in turn, will significantly reduce the levels of environmental pollution by considering better use of the resources and the recycling of materials involved. The LCA is a systematic approach and is carried out in four phases established by the ISO 14040:2006 and 14044:2006 [152,153]. These phases are (1) the definition of the objective and scope; (2) inventory analysis; (3) impact evaluation; and (4) interpretation.
In stage 1 of defining the objective and scope, technical information is detailed, such as the functional unit, the system’s limits, the study’s assumptions and limits, the categories of environmental impacts, the methods used, and the location of environmental loads. In stage 2, all emissions released into the environment and resources extracted from the environment are detailed throughout the product’s life cycle; the use of energy, water, and materials is identified and quantified, as are emissions to the environment (atmospheric emissions, solid waste disposal, and wastewater discharge). In stage 3, life cycle impacts and possible environmental and human effects due to using and consuming energy, water, and materials are determined. The results obtained, called indicators of environmental interventions, are translated into environmental impacts using an impact analysis method.
Finally, in the interpretation phase, the information from the life cycle inventory results is identified, quantified, reviewed, and evaluated, and conclusions and recommendations are generated.
An evaluation of the environmental impacts generated by HA production is one aspect that has yet to be addressed in the literature and is a critical key to knowing the sustainability of the production system, confirm the product or the technology, and pursue its applications and design without using the precautionary principle. Environmental concerns have been reported in MPHA due to the use of complex media [145]. According to Cerminati et al. [145], these media are prone to contamination because waste with high organic loads is generated, multiple operations are required to isolate the product at high quality, and they may contain impurities not acceptable by regulatory agencies.
For this reason, it is also essential to know the waste generated from the HA production process. Table 9 shows a few studies that report the residues of MPHA. The residues resulting from the production of HA reported in all cases are biomass and lactic acid; in some cases, acetic acid, formic acid, and succinic acid are also reported. Except for the studies reported by Cheng et al. and Hmar et al. [36,154,155], the production of HA was performed by strains of Streptococcus zooepidemicus, in which residues represented around 90% to 94% of the total products obtained. Biomass production is between 1 and 2.5 times more than HA production, and lactic acid is between 7 and 18 times more. In cases where acetic acid production is reported, it is between 1 and 2 times higher than the production of HA.

Table 9.
Products obtained in the microbial production of hyaluronic acid.
In the study reported by Hmar et al. [155], a GT Lactococcus lactis strain was used for HA production. In general, residues were similar to those observed for Streptococcus zooepidemicus, which represented 94% of the total products obtained. With this Lactococcus lactis strain, a high biomass production was observed, and acetic acid showed a similar accumulation ratio to biomass production, which was seven times higher than HA. Lactic acid was produced in a lower quantity, reaching 2.6 times the production of HA for strains of Streptococcus zooepidemicus. With this strain, the production of formic acid was also reported and reached seven times the production of HA, as well as the production of biomass and acetic acid. In the studies reported by Cheng et al. [36,154], a GT strain of Corynebacterium glutamicum was the microbe used for HA production. They found that the production of concomitant waste was reduced, representing only between 10% and 40% of the total products obtained. All the waste products were nearly half the produced HA. Interestingly, the amount of biomass obtained was half of the HA produced.
In the case of the possible integration of MPHA into BRF plants, the way to manage or use the concomitant wastes in the system is decisive, as well as the way to find/develop technologies with lower cost and lower environmental impacts for the treatment of said waste and thus achieve a positive environmental balance. According to Cerminati et al. [145], in the simulation of industrial MPHA, using Streptococcus included costs for waste treatment. In contrast, in the case of using B. subtilis, these costs were not considered. The reason for not considering these costs was because the biomass was directed to further production of biogas or animal feed since the culture medium is based on inorganic salts that avoid effluents with high loads of organic matter. In this way, developing the HA production process with organic waste as alternative sources of C and N and recombinant bacteria can be part of a strategy to develop biorefinery systems that process the OFMSW and produce environmentally and economically sustainable VAPs.
7. Conclusions and Perspective
Developing BRFs that process OFMSW from which bioenergies and value-added bioproducts are obtained is a promising option to simultaneously solve energy and environmental problems in modern societies. An issue that is very important to promote is the obtaining of high VAPs with proven high prices and consolidated markets, such as HA, so that, in this way, the BRF become both environmentally and economically sustainable.
Our critical review of MPHA identified key HA-producing strains, their culture conditions, and their HA production profiles. Two types of natural HA-producing pathogenic bacteria from Streptococcus have shown the highest HA production: S. equi. ssp. equi and S. zooepidemicus. The first exhibited a production of 12.0 g L−1, while the second showed a production of 6.6 g L−1 and reached a production of 29.4 g L−1 when subjected to GT. Microorganisms that are not natural producers of HA, either Gram-positive or Gram-negative bacteria or fungi, are typically innocuous. However, when subjected to GT through the introduction or expression of the HA-synthesizing genes of naturally producing bacteria, they manage to produce HA. An example is GT Corynebacterium glutamicum. It is the bacteria that produced the highest HA yield, reaching 74.1 g L−1.
We found that little has been explored in the use of organic wastes as sources of C and N; these studies have only been carried out with non-recombinant Streptococcus zooepidemicus strains. The highest HA production observed was 6.7 g L−1 when starch was used as an alternative source of C and yeast extract as a conventional source of N. When an alternative source of N (cheese whey or fermented corn liquor) was used, or when both alternative sources of C and N were supplemented simultaneously, the HA productions only reached 4 g L−1 and 3.54 g L−1, respectively.
Before considering integrating HA’s production process into the BRF system, knowledge gaps about three aspects of HA production need to be addressed. The first issue concerns the need for more open information on scaling up the HA production process and industrial production. So far, all the research reported was performed at a laboratory scale, and only one case study was carried out at a pilot scale. The new information will foster knowledge for the sound design and operation of production processes.
The second aspect is economic analyses of HA production; scientific and technical results on this issue are very scarce. Fortunately, the first reports are encouraging and support the economic viability of HA production. In a couple of cases, the economy of MPHA has been evaluated at an industrial level by simulation and exhibited an internal rate of return of ca. 53% and an investment recovery period of 1.9 years. Additionally, it was found that the MPAH with Streptococcus zooepidemicus, which uses organic wastes as sources of C and N, showed reductions of up to 72% in the production cost compared to the MPHA with conventional sources of C and N.
Finally, the third issue is the need for more information on the environmental sustainability of MPHA. No scientific research has been reported, and little has been described on waste generation and discharges. However, several organic product concomitants with high organic strength have been identified in half a dozen cases. The residues for MPHA that have been reported are (biomass) mainly organic acids, such as formic, acetic, succinic, and lactic acids, which are generated in much more significant quantities than the amount of produced HA. Typically, those compounds can represent up to 94% of the total products from the HA production process. Therefore, this is another area that needs more R&D, i.e., to verify the potential for improving environmental sustainability and identify possible virtuous fates for generated wastes instead of their mere discharge into the environment.
No studies were found on using biorefinery saccharified liquors (from the S stage) for MPHA. However, given their sugar content, it seems promising to use them as a C source for HA production. Research on this subject is a must if we want to integrate the MPHA into an expanded and sustainable GBAER-type BRF.
Author Contributions
Conceptualization, H.M.P.-V. and G.P.-M.; methodology, H.M.P.-V. and G.P.-M.; software, G.P.-M.; validation, H.M.P.-V., G.P.-M., T.P.-N., A.P.-V., E.C.-Q., N.R.-O., J.G.-M. and P.X.S.-N.; formal analysis, G.P.-M. and H.M.P.-V.; investigation H.M.P.-V., G.P.-M., E.C.-Q., T.P.-N., A.P.-V., P.X.S.-N., N.R.-O. and J.G.-M.; resources, H.M.P.-V. and G.P.-M.; data curation, H.M.P.-V. and G.P.-M.; writing—original draft preparation, G.P.-M. and H.M.P.-V.; writing—review and editing, H.M.P.-V., G.P.-M., T.P.-N., E.C.-Q., P.X.S.-N., A.P.-V., N.R.-O. and J.G.-M.; visualization, G.P.-M.; supervision, H.M.P.-V. and G.P.-M.; project administration, H.M.P.-V. and G.P.-M.; funding acquisition, H.M.P.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
In principle, there are none of our own experimental data involved. However, the pool of references that were collected and reviewed for this work is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Notation
The following abbreviations are used in this manuscript:
| BG+ | Gram-positive bacteria |
| BG− | Gram-negative bacteria |
| BNBPs | Bionanobioparticles |
| BPs | Bioparticles |
| BRFs | Biorefineries |
| CO2e | Carbon dioxide-equivalent |
| E | Extraction stage in a biorefinery (producing a concentrate of low-molecular-weight organic acids and solvents) |
| GBAER | Environmental Biotechnology and Renewable Energies Group |
| GRAS | Generally recognized as safe |
| GT | Genetic transformation |
| GHG | Greenhouse gas |
| H | Biohydrogen-producing stage in a biorefinery |
| HA | Hyaluronic acid |
| M | Methane-producing stage in a biorefinery |
| MRI | Magnetic resonance imaging |
| LCA | Life cycle assessment |
| MW | Molecular weight |
| MSW | Municipal solid waste |
| MPHA | Microbial production of hyaluronic acid |
| NN | Bionanobioparticle-producing stage in a biorefinery |
| OFMSW | Organic fraction of municipal solid waste |
| S | Saccharified liquor-producing stage in a biorefinery |
| VAPs | Value-added products |
| Z | Enzyme-producing stage in a biorefinery |
References
- Vilochani, S.; McAloone, T.C.; Pigosso, D.C.A. Consolidation of Management Practices for Sustainable Product Development: A Systematic Literature Review. Sustain. Prod. Consum. 2024, 45, 115–125. [Google Scholar] [CrossRef]
- Gawel, E.; Pannicke, N.; Hagemann, N. A Path Transition towards a Bioeconomy-The Crucial Role of Sustainability. Sustainability 2019, 11, 3005. [Google Scholar] [CrossRef]
- Wagh, M.S.; Sowjanya, S.; Chandra, P.; Chakraborty, A.; Amrit, R.; Mishra, B.; Kumar, A.; Kishore, Y. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process–A Sustainable Symphony. Process Saf. Environ. Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Energy Institute. Oil Reserves, 1980 to 2020. Available online: https://ourworldindata.org/grapher/oil-proved-reserves?tab=chart (accessed on 8 January 2024).
- IPCC. Summary for Policymakers: Climate Change 2022–Impacts, Adaptation and Vulnerability_Working Group II Contribution to the Sixth Assessment Report of the Intergovernamental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, X.; Tan, Z.; Liu, C.; Hu, H.; Yuan, H.; Peng, S.; Cai, X. Assessment of the Global Energy Transition: Based on Trade Embodied Energy Analysis. Energy 2023, 273, 127274. [Google Scholar] [CrossRef]
- Grande-Acosta, G.; Islas-Samperio, J. Towards a Low-Carbon Electric Power System in Mexico. Energy Sustain. Dev. 2017, 37, 99–109. [Google Scholar] [CrossRef]
- Pérez, G.; Islas-Samperio, J.M. Sustainability Evaluation of Non-Toxic Jatropha Curcas in Rural Marginal Soil for Obtaining Biodiesel Using Life-Cycle Assessment. Energies 2021, 14, 2746. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Khoo, K.S.; Gupta, V.K.; Sharma, M.; Show, P.L.; Yap, P.S. Exploitation of Lignocellulosic-Based Biomass Biorefinery: A Critical Review of Renewable Bioresource, Sustainability and Economic Views. Biotechnol. Adv. 2023, 69, 108265. [Google Scholar] [CrossRef]
- Culaba, A.B.; Mayol, A.P.; San Juan, J.L.G.; Ubando, A.T.; Bandala, A.A.; Concepcion, R.S.; Alipio, M.; Chen, W.H.; Show, P.L.; Chang, J.S. Design of Biorefineries towards Carbon Neutrality: A Critical Review. Bioresour. Technol. 2023, 369, 128256. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Palandri, C.; Giner, C.; Debnath, D. Biofuels’ Contribution to Date to Greenhouse Gas Emission Savings. In Biofuels, Bioenergy and Food Security; Academic Press: New York, NY, USA, 2019; pp. 145–162. [Google Scholar] [CrossRef]
- Busch, P.; Kendall, A.; Lipman, T. A Systematic Review of Life Cycle Greenhouse Gas Intensity Values for Hydrogen Production Pathways. Renew. Sustain. Energy Rev. 2023, 184, 113588. [Google Scholar] [CrossRef]
- Burg, V.; Bowman, G.; Haubensak, M.; Baier, U.; Thees, O. Valorization of an Untapped Resource: Energy and Greenhouse Gas Emissions Benefits of Converting Manure to Biogas through Anaerobic Digestion. Resour. Conserv. Recycl. 2018, 136, 53–62. [Google Scholar] [CrossRef]
- Narayanan, M. Promising Biorefinery Products from Marine Macro and Microalgal Biomass: A Review. Renew. Sustain. Energy Rev. 2024, 190, 114081. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Francis, R.R.; Jeyakumar, R.B.; Saratale, R.G.; Ashokkumar, V.; Bhatia, S.K.; Kumar, V.; Kumar, G. Lignocellulosic Biomass Conversion via Greener Pretreatment Methods towards Biorefinery Applications. Bioresour. Technol. 2023, 369, 128328. [Google Scholar] [CrossRef]
- Zhao, Y.; Shakeel, U.; Saif, M.; Rehman, U.; Li, H.; Xu, X.; Xu, J. Lignin-Carbohydrate Complexes (LCCs ) and Its Role in Biore Fi Nery. J. Clean. Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]
- Sotelo-Navarro, P.X.; Poggi-Varaldo, H.M.; Chargoy-Amador, J.P.; Sojo-Benitez, A.; Pérez-Angón, M.A.; Sánchez-Pérez, R. Impactos Ambientales de Una Biorrefinería Tipo HMEZS-NN. Rev. Int. Contam. Ambient. 2022, 38, 48–57. [Google Scholar]
- Sierra-Gachuz, H.; Sotelo-Navarro, P.X.; Poggi-Varaldo, H.M.; Escamilla-Alvarado, C.; Sojo-Benitez, A.; Barrera-Cortés, J.; Gonzáles-Cardoso, G. Capítulo 1.1. Evaluación de La Sostenibilidad Ambiental de Las Biorrefinerías de La Familia H-M-Z-S. In Ambiente y Bioenergía. Perspectivas y Avances de La Sostenibilidad; Sotelo-Navarro, P.X., Tecorralco-Bobadilla, A.L., Escamilla-Alvarado, C., Hernández-Flores, G., Nava-Bravo, I., López-Díaz, J.A., Poggi-Varaldo, H.M., Eds.; ABIAER: Ciudad de México, Mexico, 2022. [Google Scholar]
- Duan, Y.; Tarafdar, A.; Kumar, V.; Ganeshan, P.; Rajendran, K.; Shekhar Giri, B.; Gómez-García, R.; Li, H.; Zhang, Z.; Sindhu, R.; et al. Sustainable Biorefinery Approaches towards Circular Economy for Conversion of Biowaste to Value Added Materials and Future Perspectives. Fuel 2022, 325, 124846. [Google Scholar] [CrossRef]
- Ladakis, D.; Stylianou, E.; Ioannidou, S.-M.; Koutinas, A.; Pateraki, C. Biorefinery Development, Techno-Economic Evaluation and Environmental Impact Analysis for the Conversion of the Organic Fraction of Municipal Solid Waste into Succinic Acid and Value-Added Fractions. Bioresour. Technol. 2022, 354, 127172. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Tabatabaei, M.; Tsapekos, P.; Rafiee, S.; Aghbashlo, M.; Lindeneg, S.; Angelidaki, I. Environmental Life Cycle Assessment of Different Biorefinery Platforms Valorizing Municipal Solid Waste to Bioenergy, Microbial Protein, Lactic and Succinic Acid. Renew. Sustain. Energy Rev. 2020, 117, 109493. [Google Scholar] [CrossRef]
- Shah, A.V.; Singh, A.; Sabyasachi Mohanty, S.; Kumar Srivastava, V.; Varjani, S. Organic Solid Waste: Biorefinery Approach as a Sustainable Strategy in Circular Bioeconomy. Bioresour. Technol. 2022, 349, 126835. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Munoz-Paez, K.M.; Escamilla-Alvarado, C.; Robledo-Narváez, P.N.; Ponce-Noyola, M.T.; Calva-Calva, G.; Ríos-Leal, E.; Galíndez-Mayer, J.; Estrada-Vázquez, C.; Ortega-Clemente, A.; et al. Biohydrogen, Biomethane and Bioelectricity as Crucial Components of Biorefinery of Organic Wastes: A Review. Waste Manag. Res. 2014, 32, 353–365. [Google Scholar] [CrossRef]
- Escamilla-Alvarado, C.; Poggi-Varaldo, H.M.; Ponce-Noyola, M.T. Bioenergy and Bioproducts from Municipal Organic Waste as Alternative to Landfilling: A Comparative Life Cycle Assessment with Prospective Application to Mexico. Environ. Sci. Pollut. Res. 2017, 24, 25602–25617. [Google Scholar] [CrossRef]
- Li, R. Techno-Economic and Environmental Characterization of Municipal Food Waste-to-Energy Biorefineries: Integrating Pathway with Compositional Dynamics. Renew. Energy 2024, 223, 120038. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive Food Compounds from Microalgae: An Innovative Framework on Industrial Biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Grand View Research. Hyaluronic Acid Market Size, Share & Trends Analysis Report By Application (Dermal Fillers, Osteoarthritis, Ophthalmic, Vesicoureteral Reflux), By Region (MEA, EU, North America, APAC), And Segment Forecasts, 2023–2030 Fuente. Available online: https://www.grandviewresearch.com/industry-analysis/hyaluronic-acid-market%0A (accessed on 25 July 2023).
- Ucm, R.; Aem, M.; Lhb, Z.; Kumar, V.; Taherzadeh, M.J.; Garlapati, V.K.; Chandel, A.K. Comprehensive Review on Biotechnological Production of Hyaluronic Acid: Status, Innovation, Market and Applications. Bioengineered 2022, 13, 9645–9661. [Google Scholar] [CrossRef]
- de Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.V.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic Basis for Hyper Production of Hyaluronic Acid in Natural and Engineered Microorganisms. Microb. Cell Fact. 2016, 15, 119. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, Y.; Huang, Y.; Li, S.; Xu, H.; Su, E. Current Advances in the Biosynthesis of Hyaluronic Acid with Variable Molecular Weights. Carbohydr. Polym. 2021, 269, 118320. [Google Scholar] [CrossRef]
- Shikina, E.V.; Kovalevsky, R.A.; Shirkovskaya, A.I.; Toukach, P.V. Prospective Bacterial and Fungal Sources of Hyaluronic Acid: A Review. Comput. Struct. Biotechnol. J. 2022, 20, 6214–6236. [Google Scholar] [CrossRef]
- Rigo, D.; da Silva, L.M.; Fischer, B.; Colet, R.; Dallago, R.M.; Zeni, J. Hyaluronic Acid–from Production to Application: A Review. Biointerface Res. Appl. Chem. 2023, 13. [Google Scholar] [CrossRef]
- Chahuki, F.F.; Aminzadeh, S.; Jafarian, V.; Tabandeh, F.; Khodabandeh, M. Hyaluronic Acid Production Enhancement via Genetically Modification and Culture Medium Optimization in Lactobacillus Acidophilus. Int. J. Biol. Macromol. 2019, 121, 870–881. [Google Scholar] [CrossRef]
- Cheng, F.; Yu, H.; Stephanopoulos, G. Engineering Corynebacterium Glutamicum for High-Titer Biosynthesis of Hyaluronic Acid. Metab. Eng. 2019, 55, 276–289. [Google Scholar] [CrossRef]
- Jin, P.; Kang, Z.; Yuan, P.; Du, G.; Chen, J. Production of Specific-Molecular-Weight Hyaluronan by Metabolically Engineered Bacillus Subtilis 168. Metab. Eng. 2016, 35, 21–30. [Google Scholar] [CrossRef]
- Huang, W.C.; Chen, S.J.; Chen, T.L. The Role of Dissolved Oxygen and Function of Agitation in Hyaluronic Acid Fermentation. Biochem. Eng. J. 2006, 32, 239–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; Xu, G.; Han, R.; Zhou, J.; Ni, Y. Efficient Production of Hyaluronic Acid by Streptococcus Zooepidemicus Using Two-Stage Semi-Continuous Fermentation. Bioresour. Technol. 2023, 377, 128896. [Google Scholar] [CrossRef]
- Yoshimura, T.; Shibata, N.; Hamano, Y.; Yamanaka, K. Heterologous Production of Hyaluronic Acid in an ε-Poly-L-Lysine Producer, Streptomyces Albulus. Appl. Environ. Microbiol. 2015, 81, 3631–3640. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, F.; Zheng, B.; Yu, H. Enhancing Single-Cell Hyaluronic Acid Biosynthesis by Microbial Morphology Engineering. Synth. Syst. Biotechnol. 2020, 5, 316–323. [Google Scholar] [CrossRef]
- Aristizábal-Marulanda, V.; Cardona Alzate, C.A. Methods for Designing and Assessing Biorefineries: Review. Biofuels, Bioprod. Biorefining 2019, 13, 789–808. [Google Scholar] [CrossRef]
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Ferreira, B.S. Biowaste Biorefinery in Europe: Opportunities and Research & Development Needs. N. Biotechnol. 2015, 32, 100–108. [Google Scholar] [CrossRef]
- Sills, D.L.; Van Doren, L.G.; Beal, C.; Raynor, E. The Effect of Functional Unit and Co-Product Handling Methods on Life Cycle Assessment of an Algal Biorefinery. Algal Res. 2020, 46, 101770. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Kishore Pant, K.; Aminabhavi, T.M. Integrated Biorefinery Processes for Conversion of Lignocellulosic Biomass to Value Added Materials: Paving a Path towards Circular Economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef]
- Wu, W.; Chang, J.S. Integrated Algal Biorefineries from Process Systems Engineering Aspects: A Review. Bioresour. Technol. 2019, 291, 121939. [Google Scholar] [CrossRef]
- Javed, M.U.; Mukhtar, H.; Hayat, M.T.; Rashid, U.; Mumtaz, M.W.; Ngamcharussrivichai, C. Sustainable Processing of Algal Biomass for a Comprehensive Biorefinery. J. Biotechnol. 2022, 352, 47–58. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Hemalatha, M.; Chakraborty, D.; Chatterjee, S.; Ranadheer, P.; Kona, R. Algal Biorefinery Models with Self-Sustainable Closed Loop Approach: Trends and Prospective for Blue-Bioeconomy. Bioresour. Technol. 2020, 295, 122128. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed Biorefinery Concept for Sustainable Use of Marine Resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Llano, T.; Arce, C.; Gallart, L.E.; Perales, A.; Coz, A. Techno-Economic Analysis of Macroalgae Biorefineries: A Comparison between Ethanol and Butanol Facilities. Fermentation 2023, 9, 340. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kabir, M.; Mehjabin, A.; Oishi, F.T.Z.; Ahmed, S.; Mannan, S.; Mofijur, M.; Almomani, F.; Badruddin, I.A.; Kamangar, S. Waste Biorefinery to Produce Renewable Energy: Bioconversion Process and Circular Bioeconomy. Energy Rep. 2023, 10, 3073–3091. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. From Garbage to Treasure: A Review on Biorefinery of Organic Solid Wastes into Valuable Biobased Products. Bioresour. Technol. Rep. 2023, 24, 101610. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, Y.; Tian, J.; Zhao, J.; Ye, N.; Zhang, Y.; Chen, L. Review of Waste Biorefinery Development towards a Circular Economy: From the Perspective of a Life Cycle Assessment. Renew. Sustain. Energy Rev. 2021, 139, 110716. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Singh, A.K.; Sonkar, S.; Shadangi, K.P.; Srivastava, R.K.; Gupta, V.K.; Parikh, J.; Sahoo, U.K.; Govarthanan, M. Biorefinery Solutions for Food Processing Wastes: A Sustainable Bioeconomic Perspective. Ind. Crops Prod. 2023, 205, 117488. [Google Scholar] [CrossRef]
- Escamilla-Alvarado, C.; Pérez-Pimienta, J.A.; Ponce-Noyola, T.; Poggi-Varaldo, H.M. An Overview of the Enzyme Potential in Bioenergy-Producing Biorefineries. J. Chem. Technol. Biotechnol. 2017, 92, 906–924. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chatterjee, S.; Althuri, A.; Palani, S.G.; Venkata Mohan, S. Sustainable Enzymatic Treatment of Organic Waste in a Framework of Circular Economy. Bioresour. Technol. 2023, 370, 128487. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente y Recursos Naturales. Diagnóstico Básico Para la Gestión Integral de Los Residuos; Secretaría de Medio Ambiente y Recursos Naturales: Ciudad de México, Mexico, 2020. [Google Scholar]
- Olay-Romero, E.; Turcott-Cervantes, D.E.; Hernández-Berriel, M.d.C.; Lobo-García de Cortázar, A.; Cuartas-Hernández, M.; de la Rosa-Gómez, I. Technical Indicators to Improve Municipal Solid Waste Management in Developing Countries: A Case in Mexico. Waste Manag. 2020, 107, 201–210. [Google Scholar] [CrossRef]
- Reyna-Bensusan, N.; Wilson, D.C.; Smith, S.R. Uncontrolled Burning of Solid Waste by Households in Mexico Is a Significant Contributor to Climate Change in the Country. Environ. Res. 2018, 163, 280–288. [Google Scholar] [CrossRef]
- Sánchez-Arias, M.; Riojas-Rodríguez, H.; Catalán-Vázquez, M.; Terrazas-Meraz, M.A.; Rosas, I.; Espinosa-García, A.C.; Santos-Luna, R.; Siebe, C. Socio-Environmental Assessment of a Landfill Using a Mixed Study Design: A Case Study from México. Waste Manag. 2019, 85, 42–59. [Google Scholar] [CrossRef]
- Escamilla-Alvarado, C.; Ponce-Noyola, M.T.; Poggi-Varaldo, H.M.; Ríos-Leal, E.; García-Mena, J.; Rinderknecht-Seijas, N. Energy Analysis of In-Series Biohydrogen and Methane Production from Organic Wastes. Int. J. Hydrogen Energy 2014, 39, 16587–16594. [Google Scholar] [CrossRef]
- Escamilla-Alvarado, C.; Ponce-Noyola, T.; Ríos-Leal, E.; Poggi-Varaldo, H.M. A Multivariable Evaluation of Biohydrogen Production by Solid Substrate Fermentation of Organic Municipal Wastes in Semi-Continuous and Batch Operation. Int. J. Hydrog. Energy 2013, 38, 12527–12538. [Google Scholar] [CrossRef]
- Escamilla-Alvarado, C.; Poggi-Varaldo, H.M.; Ponce-Noyola, T.; Ríos-Leal, E.; Robles-Gonzalez, I.; Rinderknecht-Seijas, N. Saccharification of Fermented Residues as Integral Part in a Conceptual Hydrogen-Producing Biorefinery. Int. J. Hydrogen Energy 2015, 40, 17200–17211. [Google Scholar] [CrossRef]
- Romero-Cedillo, L.; Poggi-Varaldo, H.M.; Santoyo-Salazar, J.; Escamilla-Alvarado, C.; Matsumoto-Kuwabara, Y.; Ponce-Noyola, M.T.; Bretón-Deval, L.; García-Rocha, M. Biological Synthesis of Iron Nanoparticles Using Hydrolysates from a Waste-Based Biorefinery. Environ. Sci. Pollut. Res. 2020, 27, 28649–28669. [Google Scholar] [CrossRef]
- Garibay-Orijel, C.; Hoyo-Vadillo, C.; Ponce-Noyola, T.; García-Mena, J.; Poggi-Varaldo, H.M. Impact of Long-Term Partial Aeration on the Removal of 2,4,6-Trichlorophenol in an Initially Methanogenic Fluidized Bed Bioreactor. Biotechnol. Bioeng. 2006, 94, 949–960. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Bárcenas-Torres, J.D.; Moreno-Medina, C.U.; García-Mena, J.; Garibay-Orijel, C.; Ríos-Leal, E.; Rinderknecht-Seijas, N. Influence of Discontinuing Feeding Degradable Cosubstrate on the Performance of a Fluidized Bed Bioreactor Treating a Mixture of Trichlorophenol and Phenol. J. Environ. Manag. 2012, 113, 527–537. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M. Análisis de Ciclo de Vida de Una Biorrefinería Que Procesa La Fracción Orgánica de Residuos Urbanos y Produce Bioenergías, Ácidos Orgánicos, Enzimas, y Nanobiopartículas, Limited ed. Final Report, Diploma, Available upon request; CADISAC: Ciudad de México, México, 2019. [Google Scholar]
- Bretón-Deval, L.; Rios-Leal, E.; Poggi-Varaldo, H.M.; Ponce-Noyola, T. Biodegradability of Nonionic Surfactant Used in the Remediation of Groundwaters Polluted with PCE. Water Environ. Res. 2016, 88, 2159–2168. [Google Scholar] [CrossRef]
- Breton-Deval, L.; Rossetti, S.; Ríos-Leal, E.; Matturro, B.; Poggi-Varaldo, H.M. Effect of Coupling Zero-Valent Iron Side Filters on the Performance of Bioreactors Fed with a High Concentration of Perchloroethylene. J. Environ. Eng. 2016, 142, 986–994. [Google Scholar] [CrossRef]
- Moreno-Medina, C.U.; Poggi-Varaldo, H.M.; Breton-Deval, L.; Rinderknecht-Seijas, N. Effect of Sudden Addition of PCE and Bioreactor Coupling to ZVI Filters on Performance of Fluidized Bed Bioreactors Operated in Simultaneous Electron Acceptor Modes. Environ. Sci. Pollut. Res. 2017, 24, 25534–25549. [Google Scholar] [CrossRef]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Desing and Economics for Chemical Engineers; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation. Processes 2021, 9, 241. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial Hyaluronic Acid Production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 24759. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/24759 (accessed on 4 June 2024).
- Chang, W.H.; Liu, P.Y.; Lin, M.H.; Lu, C.J.; Chou, H.Y.; Nian, C.Y.; Jiang, Y.T.; Hsu, Y.H.H. Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef]
- Greenberg, D.D.; Stoker, A.; Kane, S.; Cockrell, M.; Cook, J.L. Biochemical Effects of Two Different Hyaluronic Acid Products in a Co-Culture Model of Osteoarthritis. Osteoarthr. Cartil. 2006, 14, 814–822. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The Application of Hyaluronic Acid in Bone Regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent Advantage of Hyaluronic Acid for Anti-Cancer Application: A Review of “3S” Transition Approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef]
- Kim, S.; Moon, M.J.; Poilil Surendran, S.; Jeong, Y.Y. Biomedical Applications of Hyaluronic Acid-Based Nanomaterials in Hyperthermic Cancer Therapy. Pharmaceutics 2019, 11, 306. [Google Scholar] [CrossRef]
- Wen, Q.L.; Pu, Z.F.; Yang, Y.J.; Wang, J.; Wu, B.C.; Hu, Y.L.; Liu, P.; Ling, J.; Cao, Q. Hyaluronic Acid as a Material for the Synthesis of Fluorescent Carbon Dots and Its Application for Selective Detection of Fe3+ Ion and Folic Acid. Microchem. J. 2020, 159, 105364. [Google Scholar] [CrossRef]
- Martin, A.A.; Sassaki, G.L.; Sierakowski, M.R. Effect of Adding Galactomannans on Some Physical and Chemical Properties of Hyaluronic Acid. Int. J. Biol. Macromol. 2020, 144, 527–535. [Google Scholar] [CrossRef]
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current Applications of Exopolysaccharides from Lactic Acid Bacteria in the Development of Food Active Edible Packaging. Curr. Opin. Food Sci. 2021, 40, 33–39. [Google Scholar] [CrossRef]
- Billon, R.; Hersant, B.; Meningaud, J.P. Rhéologie Des Acides Hyaluroniques: Principes Fondamentaux et Applications Cliniques En Rajeunissement Facial. Ann. Chir. Plast. Esthétique 2017, 62, 261–267. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and Delivery Mechanisms of Hyaluronic Acid Used for Topical/Transdermal Delivery–A Review. Int. J. Pharm. 2020, 578, 119127. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic Acid, a Promising Skin Rejuvenating Biomedicine: A Review of Recent Updates and Pre-Clinical and Clinical Investigations on Cosmetic and Nutricosmetic Effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Duan, X.J.; Yang, L.; Zhang, X.; Tan, W.S. Effect of Oxygen and Shear Stress on Molecular Weight of Hyaluronic Acid Produced by Streptococcus zooepidemicus. J. Microbiol. Biotechnol. 2008, 18, 718–724. [Google Scholar]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan Fragments: An Information-Rich System. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Ke, C.; Wang, D.; Sun, Y.; Qiao, D.; Ye, H.; Zeng, X. Immunostimulatory and Antiangiogenic Activities of Low Molecular Weight Hyaluronic Acid. Food Chem. Toxicol. 2013, 58, 401–407. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic Acid: A Natural Biopolymer with a Broad Range of Biomedical and Industrial Applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological Production of Hyaluronic Acid: A Mini Review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef]
- Rangaswamy, V.; Jain, D. An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol. Lett. 2008, 30, 493–496. [Google Scholar] [CrossRef]
- Blank, L.M.; McLaughlin, R.L.; Nielsen, L.K. Stable Production of Hyaluronic Acid in Streptococcus Zooepidemicus Chemostats Operated at High Dilution Rate. Biotechnol. Bioeng. 2005, 90, 685–693. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, Z.; Wang, B.; Wu, L.; Zhang, L. Process Control and Optimization of Hyaluronic Acid Production by Streptococcus zooepidemicus. Agric. Biotechnol. 2015, 56–60. [Google Scholar]
- Liu, L.; Wang, M.; Du, G.; Chen, J. Enhanced Hyaluronic Acid Production of Streptococcus Zooepidemicus by an Intermittent Alkaline-Stress Strategy. Lett. Appl. Microbiol. 2008, 46, 383–388. [Google Scholar] [CrossRef]
- Jagannath, S.; Ramachandran, K.B. Influence of Competing Metabolic Processes on the Molecular Weight of Hyaluronic Acid Synthesized by Streptococcus zooepidemicus. Biochem. Eng. J. 2010, 48, 148–158. [Google Scholar] [CrossRef]
- Chong, B.F.; Nielsen, L.K. Aerobic Cultivation of Streptococcus Zooepidemicus and the Role of NADH Oxidase. Biochem. Eng. J. 2003, 16, 153–162. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L.; Huang, H.; Wang, H.; Zhang, T.; Chen, J.; Du, G.; Kang, Z. Eliminating the Capsule-like Layer to Promote Glucose Uptake for Hyaluronan Production by Engineered Corynebacterium Glutamicum. Nat. Commun. 2020, 11, 3120. [Google Scholar] [CrossRef]
- Woo, J.E.; Seong, H.J.; Lee, S.Y.; Jang, Y.S. Metabolic Engineering of Escherichia Coli for the Production of Hyaluronic Acid From Glucose and Galactose. Front. Bioeng. Biotechnol. 2019, 7, 351. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, Z.; Ren, Y.; Zhao, Y.; Zhao, G. Efficient Production of High-Molecular-Weight Hyaluronic Acid with a Two-Stage Fermentation. RSC Adv. 2018, 8, 36167–36171. [Google Scholar] [CrossRef]
- Güngör, G.; Gedikli, S.; Toptaş, Y.; Akgün, D.E.; Demirbilek, M.; Yazıhan, N.; Aytar Çelik, P.; Denkbaş, E.B.; Çabuk, A. Bacterial Hyaluronic Acid Production through an Alternative Extraction Method and Its Characterization. J. Chem. Technol. Biotechnol. 2019, 94, 1843–1852. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, J.L.; Huang, W.C.; Chen, H.L. Fermentation Process Development for Hyaluronic Acid Production by Streptococcus Zooepidemicus ATCC 39920. Korean J. Chem. Eng. 2009, 26, 428–432. [Google Scholar] [CrossRef]
- Schulte, S.; Doss, S.S.; Jeeva, P.; Ananth, M.; Blank, L.M.; Jayaraman, G. Exploiting the Diversity of Streptococcal Hyaluronan Synthases for the Production of Molecular Weight–Tailored Hyaluronan. Appl. Microbiol. Biotechnol. 2019, 103, 7567–7581. [Google Scholar] [CrossRef]
- Cavalcanti, A.D.D.; Melo, B.A.G.; Oliveira, R.C.; Santana, M.H.A. Recovery and Purity of High Molar Mass Bio-Hyaluronic Acid Via Precipitation Strategies Modulated by PH and Sodium Chloride. Appl. Biochem. Biotechnol. 2019, 188, 527–539. [Google Scholar] [CrossRef]
- Morris, C.G. Academic Press Dictionary of Science and Technology; Gulf Professional Publishing: Houston, TX, USA, 1992. [Google Scholar]
- Cavalcanti, A.D.D.; de Melo, B.A.G.; Ferreira, B.A.M.; Santana, M.H.A. Performance of the Main Downstream Operations on Hyaluronic Acid Purification. Process Biochem. 2020, 99, 160–170. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial Production of Hyaluronic Acid: Current State, Challenges, and Perspectives. Microb. Cell Fact. 2011, 10, 99. [Google Scholar] [CrossRef]
- Blank, L.M.; Hugenholtz, P.; Nielsen, L.K. Evolution of the Hyaluronic Acid Synthesis (Has) Operon in Streptococcus Zooepidemicus and Other Pathogenic Streptococci. J. Mol. Evol. 2008, 67, 13–22. [Google Scholar] [CrossRef]
- Crater, D.L.; Dougherty, B.A.; Van de Rijn, I. Molecular Characterization of HasC from an Operon Required for Hyaluronic Acid Synthesis in Group A Streptococci. Demonstration of UDP-Glucose Pyrophosphorylase Activity. J. Biol. Chem. 1995, 270, 28676–28680. [Google Scholar] [CrossRef]
- Crater, D.L.; Van de Rijn, I. Hyaluronic Acid Synthesis Operon (Has) Expression in Group A Streptococci. J. Biol. Chem. 1995, 270, 18452–18458. [Google Scholar] [CrossRef]
- Dougherty, B.A.; van de Rijn, I. Molecular Characterization of HasB from an Operon Required for Hyaluronic Acid Synthesis in Group A Streptococci. Demonstration of UDP-Glucose Dehydrogenase Activity. J. Biol. Chem. 1993, 268, 7118–7124. [Google Scholar] [CrossRef]
- Huang, W.C.; Chen, S.J.; Chen, T.L. Production of Hyaluronic Acid by Repeated Batch Fermentation. Biochem. Eng. J. 2008, 40, 460–464. [Google Scholar] [CrossRef]
- Badle, S.S.; Jayaraman, G.; Ramachandran, K.B. Ratio of Intracellular Precursors Concentration and Their Flux Influences Hyaluronic Acid Molecular Weight in Streptococcus Zooepidemicus and Recombinant Lactococcus Lactis. Bioresour. Technol. 2014, 163, 222–227. [Google Scholar] [CrossRef]
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Enhanced Hyaluronic Acid Production by a Two-Stage Culture Strategy Based on the Modeling of Batch and Fed-Batch Cultivation of Streptococcus zooepidemicus. Bioresour. Technol. 2008, 99, 8532–8536. [Google Scholar] [CrossRef]
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Comparative Study on the Influence of Dissolved Oxygen Control Approaches on the Microbial Hyaluronic Acid Production of Streptococcus zooepidemicus. Bioprocess Biosyst. Eng. 2009, 32, 755–763. [Google Scholar] [CrossRef]
- Lu, J.F.; Zhu, Y.; Sun, H.L.; Liang, S.; Leng, F.F.; Li, H.Y. Highly Efficient Production of Hyaluronic Acid by Streptococcus Zooepidemicus R42 Derived from Heterologous Expression of Bacterial Haemoglobin and Mutant Selection. Lett. Appl. Microbiol. 2016, 62, 316–322. [Google Scholar] [CrossRef]
- Im, J.H.; Song, J.M.; Kang, J.H.; Kang, D.J. Optimization of Medium Components for High-Molecular-Weight Hyaluronic Acid Production by Streptococcus Sp. ID9102 via a Statistical Approach. J. Ind. Microbiol. Biotechnol. 2009, 36, 1337–1344. [Google Scholar] [CrossRef]
- Pourzardosht, N.; Rasaee, M.J. Improved Yield of High Molecular Weight Hyaluronic Acid Production in a Stable Strain of Streptococcus Zooepidemicus via the Elimination of the Hyaluronidase-Encoding Gene. Mol. Biotechnol. 2017, 59, 192–199. [Google Scholar] [CrossRef]
- Zakeri, A.; Rasaee, M.J.; Pourzardosht, N. Enhanced Hyluronic Acid Production in Streptococcus Zooepidemicus by over Expressing HasA and Molecular Weight Control with Niscin and Glucose. Biotechnol. Rep. 2017, 16, 65–70. [Google Scholar] [CrossRef]
- Izawa, N.; Serata, M.; Sone, T.; Omasa, T.; Ohtake, H. Hyaluronic Acid Production by Recombinant Streptococcus Thermophilus. J. Biosci. Bioeng. 2011, 111, 665–670. [Google Scholar] [CrossRef]
- Cheng, F.; Gong, Q.; Yu, H.; Stephanopoulos, G. High-Titer Biosynthesis of Hyaluronic Acid by Recombinant Corynebacterium Glutamicum. Biotechnol. J. 2016, 11, 574–584. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, J.; Chen, X.; Tang, D.; Su, D.; Yao, W.; Gao, X. Metabolic Engineering of Bacillus Subtilis for the Efficient Biosynthesis of Uniform Hyaluronic Acid with Controlled Molecular Weights. Bioresour. Technol. 2013, 132, 427–431. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Zhao, X.; Shao, Y.; Wu, M.; Ma, T. Regulation of Hyaluronic Acid Molecular Weight and Titer by Temperature in Engineered Bacillus Subtilis. 3 Biotech. 2019, 9, 225. [Google Scholar] [CrossRef]
- Jeeva, P.; Shanmuga Doss, S.; Sundaram, V.; Jayaraman, G. Production of Controlled Molecular Weight Hyaluronic Acid by Glucostat Strategy Using Recombinant Lactococcus Lactis Cultures. Appl. Microbiol. Biotechnol. 2019, 103, 4363–4375. [Google Scholar] [CrossRef]
- Sunguroğlu, C.; Sezgin, D.E.; Aytar Çelik, P.; Çabuk, A. Higher Titer Hyaluronic Acid Production in Recombinant Lactococcus Lactis. Prep. Biochem. Biotechnol. 2018, 48, 734–742. [Google Scholar] [CrossRef]
- Lai, Z.W.; Teo, C.H. Cloning and Expression of Hyaluronan Synthase (HasA) in Recombinant Escherichia Coli BL21 and Its Hyaluronic Acid Production in Shake Flask Culture. Malays. J. Microbiol. 2019, 15, 575–582. [Google Scholar] [CrossRef]
- Yu, H.; Stephanopoulos, G. Metabolic Engineering of Escherichia Coli for Biosynthesis of Hyaluronic Acid. Metab. Eng. 2008, 10, 24–32. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, R.R. Recombinant Synthesis of Hyaluronan by Agrobacterium Sp. Biotechnol. Prog. 2007, 23, 1038–1042. [Google Scholar] [CrossRef]
- Gomes, A.M.V.; Netto, J.H.C.M.; Carvalho, L.S.; Parachin, N.S. Heterologous Hyaluronic Acid Production in Kluyveromyces Lactis. Microorganisms 2019, 7, 294. [Google Scholar] [CrossRef]
- Jeong, E.; Shim, W.Y.; Kim, J.H. Metabolic Engineering of Pichia Pastoris for Production of Hyaluronic Acid with High Molecular Weight. J. Biotechnol. 2014, 185, 28–36. [Google Scholar] [CrossRef]
- Manfrão-Netto, J.H.C.; Queiroz, E.B.; Rodrigues, K.A.; Coelho, C.M.; Paes, H.C.; Rech, E.L.; Parachin, N.S. Evaluation of Ogataea (Hansenula) Polymorpha for Hyaluronic Acid Production. Microorganisms 2021, 9, 312. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, X.; Yang, L.; Kong, Z. A Serum-Free Medium for Colony Growth and Hyaluronic Acid Production by Streptococcus Zooepidemicus NJUST01. Appl. Microbiol. Biotechnol. 2006, 72, 168–172. [Google Scholar] [CrossRef]
- Pires, A.M.B.; Macedo, A.C.; Eguchi, S.Y.; Santana, M.H.A. Microbial Production of Hyaluronic Acid from Agricultural Resource Derivatives. Bioresour. Technol. 2010, 101, 6506–6509. [Google Scholar] [CrossRef]
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.L.C.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus Zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293. [Google Scholar] [CrossRef]
- Rohit, S.G.; Jyoti, P.K.; Subbi, R.R.T.; Naresh, M.; Senthilkumar, S. Kinetic Modeling of Hyaluronic Acid Production in Palmyra Palm (Borassus Flabellifer) Based Medium by Streptococcus Zooepidemicus MTCC 3523. Biochem. Eng. J. 2018, 137, 284–293. [Google Scholar] [CrossRef]
- Duffeck, H.C.B.P.; Pan, N.C.; Saikawa, G.I.A.; da Rocha, S.P.D.; Baldo, C.; Celligoi, M.A.P.C. Biomedical Potential of Hyaluronic Acid from Streptococcus Zooepidemicus Produced in Sugarcane Molasses. Brazilian J. Dev. 2020, 6, 49963–49980. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Pastrana, L.; Piñeiro, C.; Teixeira, J.A.; Pérez-Martín, R.I.; Amado, I.R. Production of Hyaluronic Acid by Streptococcus Zooepidemicus on Protein Substrates Obtained from Scyliorhinus Canicula Discards. Mar. Drugs 2015, 13, 6537–6549. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Cheese Whey: A Cost-Effective Alternative for Hyaluronic Acid Production by Streptococcus zooepidemicus. Food Chem. 2016, 198, 54–61. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Microbial Production of Hyaluronic Acid from Agro-Industrial by-Products: Molasses and Corn Steep Liquor. Biochem. Eng. J. 2017, 268, 181–187. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Montemayor, M.I.; Fraguas, J.; Murado, M.A. Hyaluronic Acid Production by Streptococcus Zooepidemicus in Marine By-Products Media from Mussel Processing Wastewaters and Tuna Peptone Viscera. Microb. Cell Fact. 2010, 9, 46. [Google Scholar] [CrossRef]
- Arslan, N.P.; Aydogan, M.N. Evaluation of Sheep Wool Protein Hydrolysate and Molasses as Low-Cost Fermentation Substrates for Hyaluronic Acid Production by Streptococcus Zooepidemicus ATCC 35246. Waste Biomass Valorization 2021, 12, 925–935. [Google Scholar] [CrossRef]
- Cerminati, S.; Leroux, M.; Anselmi, P.; Peirú, S.; Alonso, J.C.; Priem, B.; Menzella, H.G. Correction to: Low Cost and Sustainable Hyaluronic Acid Production in a Manufacturing Platform Based on Bacillus Subtilis 3NA Strain (Applied Microbiology and Biotechnology, (2021), 105, 8, (3075-3086), 10.1007/S00253-021-11246-6). Appl. Microbiol. Biotechnol. 2021, 105, 6529. [Google Scholar] [CrossRef]
- Kloepffer, W. Life Cycle Sustainability Assessment of Products. Int. J. LCA 2008, 13, 89–95. [Google Scholar] [CrossRef]
- Guinée, J.B.; Heijungs, R.; Huppes, G.; Zamagni, A.; Masoni, P.; Buonamici, R.; Ekvall, T.; Rydberg, T. Life Cycle Assessment: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 90–96. [Google Scholar] [CrossRef]
- Lago-Olveira, S.; Arias, A.; Rebolledo-Leiva, R.; Feijoo, G.; González-García, S.; Moreira, M.T. Monitoring the Bioeconomy: Value Chains under the Framework of Life Cycle Assessment Indicators. Clean. Circ. Bioeconomy 2024, 7, 100072. [Google Scholar] [CrossRef]
- Valdivia, S.; Ugaya, C.M.L.; Hildenbrand, J.; Traverso, M.; Mazijn, B.; Sonnemann, G. A UNEP/SETAC Approach towards a Life Cycle Sustainability Assessment–Our Contribution to Rio+20. Int. J. Life Cycle Assess 2013, 18, 1673–1685. [Google Scholar] [CrossRef]
- Costa, D.; Quinteiro, P.; Dias, A.C. A Systematic Review of Life Cycle Sustainability Assessment: Current State, Methodological Challenges, and Implementation Issues. Sci. Total Environ. 2019, 686, 774–787. [Google Scholar] [CrossRef]
- Sinkko, T.; Sanyé-Mengual, E.; Corrado, S.; Giuntoli, J.; Sala, S. The EU Bioeconomy Footprint: Using Life Cycle Assessment to Monitor Environmental Impacts of the EU Bioeconomy. Sustain. Prod. Consum. 2023, 37, 169–179. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management–Life Cycle Assessment—Principles and Framework. International Standard Organization: Geneva, Switzerland, 2006.
- ISO 14044:2006 + A1:2018; Environmental Management–Life Cycle Assessment–Requirements and Guidelines. International Standard Organization: Geneva, Switzerland, 2018.
- Cheng, F.; Luozhong, S.; Guo, Z.; Yu, H.; Stephanopoulos, G. Enhanced Biosynthesis of Hyaluronic Acid Using Engineered Corynebacterium Glutamicum Via Metabolic Pathway Regulation. Biotechnol. J. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Hmar, R.V.; Prasad, S.B.; Jayaraman, G.; Ramachandran, K.B. Chromosomal Integration of Hyaluronic Acid Synthesis (Has) Genes Enhances the Molecular Weight of Hyaluronan Produced in Lactococcus Lactis. Biotechnol. J. 2014, 9, 1554–1564. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).