Abstract
As a result of climate change, the phenology of grapes has been altered, mainly by increasing the sugar content and decreasing the acidity of ripe grapes. This shift, when the must is fermented, affects the quality of the wine. In this regard, the use of selected Saccharomyces and non-Saccharomyces yeasts to mitigate these undesirable effects in wine fermentations entails new strategies to improve their control and also to obtain wines better adapted to current consumer preferences. This work focuses on the use of a commercially available strain of Lachancea thermotolerans immobilized in biological support to form “microbial biocapsules”, comparing its effect with a free format and spontaneous fermentation on alcoholic fermentation and volatile compound composition. These biocapsules, consisting of yeast cells attached to fungal pellets, are being tested to improve wine sensory attributes and also to facilitate yeast inoculation in fermentative and clarification winemaking processes, as well as to reduce time and production costs. The composition of young wines obtained with L. thermotolerans, inoculated as free or biocapsule formats, were compared with those obtained by the traditional method of spontaneous fermentation using native yeast by quantifying 12 oenological variables and the contents in 12 major volatiles, 3 polyols, and 46 minor volatile compounds. The analytical data matrix underwent statistical analysis to compare and establish significant differences at p ≤ 0.05 level between the different wines obtained. Among the major volatiles and polyols, only ethyl acetate, 1,1-diethoxyethane, methanol, 2-methyl-1-butanol, acetoin, ethyl lactate, and glycerol showed significant differences in L. thermotolerans wines. Also, from the minor volatile metabolites, eight showed a significant dependence on the format used for L. thermotolerans, and the other nine volatiles were dependent on both yeast and inoculation format. Only 27 volatiles were selected as aroma-active compounds with odor activity values higher than 0.2 units. Statistical analysis showed a clear separation of the obtained wines into groups when subjected to Principal Component Analysis, and the fingerprinting of wines made with biocapsules shows intermediate values between the two remaining inoculation formats, particularly in the fruity/ripe fruit, green, and floral series. The organoleptic evaluation of wines results in significantly higher values in taste, overall quality, and total score for wines obtained with biocapsules.
1. Introduction
Over the last few years, new climatic conditions such as rising temperatures due to global climate change are causing severe alterations in the phenology of the wine produced in different wine-growing regions. These conditions influence the chemical and microbiological composition of the grapes, having an effect on the quality of the resulting wine [1]. This quality can be disrupted by changes in the sugar concentration and/or acidity of the wine, resulting in a loss of sensory quality. Therefore, many tools are being developed to prevent these modifications, in particular, the use or addition of selected yeast strains with specific metabolic traits. In the past, non-Saccharomyces yeasts were linked to unwanted aromas and flawed wines. However, it is now widely recognized that these yeasts play a role in producing important metabolites that positively influence the flavor and style of wines. These properties render them valuable biotechnological tools for tackling challenges derived from the chemical and microbiological composition of grape musts, particularly in warm grape-growing regions [2,3,4].
Lachancea thermotolerans is a non-Saccharomyces commonly used to manage the potential impacts of global warming. The main purpose of its use is to decrease the ethanol content and increase the acidity of wine. In contrast to S. cerevisiae, L. thermotolerans ferments sugars into both lactate and ethanol, redirecting carbon flux from ethanol towards lactate [5]. This leads to a significant increase in the production of lactic acid, a key attribute of the resulting wine [6]. Being a commonly used yeast, researchers are studying its various forms of inoculation, among which sequential inoculation has emerged as the most prominent [7]. However, the immobilization of this non-Saccharomyces yeast presents a less explored but highly potential alternative form of inoculation.
Compared to starters with suspended cells, yeast immobilization offers many opportunities for process development in the wine industry. Immobilization systems involve the attaching of yeast cells to physical supports that allow them to exchange metabolites with the medium while maintaining quality and safety standards [8]. These systems facilitate their removal from the medium and subsequent re-use, reduce the risk of microbial contamination, produce a higher density of yeast cells, which can increase metabolite production, and offer high resistance to inhibitory compounds. This streamlines processes and costs for the industry, facilitating continuous production and limiting separation techniques [9,10,11]. Different immobilization material supports have been used, such as organic or inorganic polymers, natural matrices, or membrane systems [9]. One of the most widely used has been calcium alginate supports. However, some studies have shown that they have some drawbacks to this carrier, such as the increase in calcium ions in the medium, which can produce insoluble salts of tartaric acid [12].
Another form of cell immobilization is the so-called “microbial biocapsules”. These are smooth, hollow, and elastic spheres composed of microbial cells attached to active or inactive spherical fungal hyphae (i.e., fungal pellet) of a generally recognized as safe (GRAS) filamentous fungus, which gives them high resistance and tolerance to fermentation conditions [13,14,15,16]. Puig-Pujol et al. (2013) [17] observed that sparkling wines, whose second fermentation was carried out using immobilized S. cerevisiae yeasts in biocapsules, were more sensorially appreciated than those produced with free cells. In addition, the biocapsules can be removed from the medium on demand, speeding up processes and reducing fermentation costs and time [18]. This also makes fermentation practices more sustainable and environmentally friendly, requiring fewer resources without compromising product quality [19].
This work aims to evaluate the effect of adding an inoculum of microbial biocapsules made of L. thermotolerans on the composition of wines obtained by fermentation of a must of the white grape Pedro Ximénez. To this end, the values of oenological variables and the contents in volatile metabolites of the wines are compared with those obtained when the fermentations of the same must are carried out with the addition of the same yeast in free format and also with wines obtained via spontaneous fermentation. This work contributes to the knowledge of the metabolism of bioimmobilized non-Saccharomyces yeasts.
2. Materials and Methods
2.1. Grape Must and Winemaking Conditions
White Pedro Ximénez grapes grown in the warm region of Montilla-Moriles (Córdoba, southern Spain) were harvested, crushed, and pressed in an industrial pneumatic press, obtaining a grape must with a density of 1088 g·L−1 (21.4 °Bx; 207.9 g·L−1 sugars) and 3.78 pH. This must was corrected by adding 1.5 g·L−1 of tartaric acid and 100 mg·L−1 of potassium meta-disulphite (K2S2O5) from Panreac-Applichem (Barcelona, Spain), bringing the pH to 3.41, and distributed into six 2 L Pyrex graduated cylinders, each filled with 1.75 L. The alcoholic fermentations were carried out at 20 °C for 14 days or until fermentation was complete (residual sugars < 4 g·L−1).
2.2. Microorganisms and Inoculation Conditions
Three fermentation strategies were tested, each in duplicate. The first was carried out with wild (or native) yeasts from the grapes (hereafter referred to as WY), using the traditional method of spontaneous fermentation as control. The other two strategies were conducted with L. thermotolerans, a non-Saccharomyces active dry yeast (ADY) available as Laktia™ (Lallemand®, Barcelona, Spain ) inoculated on the must in free or immobilized format. Free format is a conventional method (referred to as LT in this work), while the second is an immobilization of this yeast in microbial biocapsules (hereafter named BC).
2.2.1. Free-Format Procedure
L. thermotolerans ADY were rehydrated following the manufacturer’s instructions and precultured in a synthetic medium with 50 g·L−1 of glucose, 2.8 g·L−1 tartaric acid, 2.4 g·L−1 potassium bitartrate, and 200 mg·L−1 diammonium hydrogen phosphate (DAP). An aliquot of this preculture was added to the respective grape must to obtain a yeast population of 2 × 106 cells mL−1.
2.2.2. Active Dry Yeast Immobilization Procedure
ADY of L. thermotolerans were rehydrated and pre-cultured in the same way as for the free format described above. The rehydrated yeasts were pelleted via centrifugation (7000 rpm, 15 min; Beckman Coulter J2-HS Centrifuge, ø 30 cm), and a portion was used for immobilization in the form of microbial biocapsules. Parallelly, spores of filamentous fungus (ff) Aspergillus oryzae UCD 76-2 (FST 76-2) from Phaff Culture Collection at the University of California, Davis (USA), previously grown for 7 days at 28 °C on sporulation agar medium consisting of (g·L−1) corn meal agar, 17; yeast extract, 1; glucose, 2; and agar, 20, were harvested and added to a vessel with sterile distilled water. These spores were vortexed and sonicated for 5 min to obtain a homogeneous suspension. A controlled spore population was inoculated to reach a final population of 1 × 106 spores/mL into a fungal pellet culture medium (FPM) consisting of (g·L−1) glucose, 60; yeast extract, 3; NaNO3, 3; K2HPO4, 1; MgSO4, 0.5; KCl, 0.5; and FeSO4, 0.01, and adjusted to pH 5.5 with HCl and cultured for 3 days at 175 rpm, 30 °C, to form the fungal pellets. Fungal pellets were inactivated by autoclave (1 atm overpressure, 20 min, 121 °C). ADY and inactive ff pellets were immersed in a 50 mL Falcon tube with sterile distilled water in a ratio of 1:1 wet weight ADY:ff pellet. This suspension was subjected to vacuum infusion (<0.3 atm pressure) for 1 min using a Bonsenkitchen system (Oakwood, GA, USA), which forces the yeast cell suspension into the tight hyphae matrix of the ff pellets. To ensure infusion, the absorbance at 580 nm was measured in the cell suspension before and after the use of vacuum; a 20% reduction in OD580 was obtained. Ff pellets with encapsulated yeast cells were submerged into a flask with YPD solution and cultivated overnight at 175 rpm, 28 °C, to increase the cell population from inside the pellets. Finally, the obtained biocapsules were rinsed with sterile DI water to remove the surface cells, as loose cells could lead to leakage during the subsequent fermentation. The immobilized yeast cell population per gram of biocapsule was calculated. For this purpose, the biocapsules were subjected to a cell-carrier detachment step before cell counting and inoculation. This involved immersing five randomly weighted biocapsules per culture flask in a 0.1 M NaCl solution, disrupting them with a tissue grinder (Kisker Biotech, Steinfurt, Germany), and sonicating them for 20 min until a homogeneous suspension was obtained. This technique enabled the mixing of yeast cells and hyphal segments of A. oryzae, with the yeast cells and hyphal debris being easily differentiated under a 40x objective microscope. A specific weight of microbial biocapsules was finally inoculated to the grape must to reach a yeast population of 2 × 106 cells mL−1. The immobilization methodology is subjected to a provisional patent (application number: 63/411,843).
2.3. Chemical Analysis
The oenological variables ethanol, titratable acidity, volatile acidity, pH, and reducing sugars were quantified following the protocols established by OIV, (2021) [20]. Lactic and malic acids were measured using reflectometry with Reflectoquant™ (Merck®, Darmstadt, Germany). Absorbances at 280, 420, 520, and 620 nm were measured using an Agilent Cary 60 UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Glutathione levels were determined via Ultra Performance Liquid Chromatography coupled with tandem Mass Spectrometry (LC-MS/MS) employing an Acquity H-Class UPLC (Waters, Mildford, MA, USA) and QTRAP 5500 Mass Detector (Sciex, Concord, ON, Canada).
Major volatile compounds and polyols were analyzed by gas chromatography in the Agilent 6890 GC (Agilent technologies, Santa Clara, CA, USA) equipped with a Flame Ionization Detector (FID) and a capillary column CP-WAX 57 CB (60 m; 0.25 mm; 0.4 µm film thickness) by direct injection of the wine. Briefly, 10 mL of wine sample was added with 1 mL of a 1.018 gL−1 of 4-methyl-2-pentanol (CAS 108-11-2) solution as internal standard and 0.2 g of solid calcium trioxide carbonate (CaCO3). The mixture was then stirred for 30 s in an ultrasonic bath and centrifuged at 5000 rpm for 10 min (2 °C). Lastly, 0.7 µL of the supernatant was injected into the GC inlet. Absolute quantification of methanol, higher alcohols (1-propanol, isobutanol, 2-methyl and 3-methyl-1-butanol, and 2-phenylethanol), acetaldehyde, acetoin, ethyl acetate, and the polyols glycerol and 2,3-butanediol (both levo and meso forms) was carried out using a calibration table built with standard solutions. These solutions contained a known concentration of compounds and underwent the same treatment as the samples. The identification and absolute quantification of minor volatile compounds was performed using the SBSE-TD-GC-MS (Stir Bar Sorptive Extraction–Thermal Desorption–Gas Chromatography–Mass Spectrometry) analytical platform. This platform consists of an Agilent-7890A GC coupled to an MSD 5975C (Wilmington, DE, USA) and a Gerstel Multi-Purpose Sampler (MPS) (GmbH & Co. KG—Mülheim an der Rhur, Germany). The software Chemstation v. 02. 02. 1431 (Agilent) and Maestro v. 1. 0. (Gerstel) were used for the platform control and chromatographic data processing. The minor volatile compounds were extracted by SBSE technique, using a Twister (10 mm long, 0.5 mm-thick film) coated with polydimethylsiloxane (PDMS), which preferentially adsorbs low polar and apolar compounds. Briefly, the procedure was as follows: 1 mL of wine sample, 0.1 mL of internal standard solution (0.4116 gL−1 hexyl butyrate in absolute ethanol), and 8.9 mL of buffered solution 12% (v/v) ethanol containing 2.6 gL−1 tartaric acid and 2.2 gL−1 potassium bitartrate (pH 3.5) were added to a 10 mL vial. Then, the twister was placed in the vial and stirred at 1200 rpm and 20 °C for 120 min to favor the adsorption of compounds in a Variomag Multipoint 15 magnetic stirrer (Thermo Fisher Scientific, Waltham, MA, USA). The twister was removed, water rinsed, and dried after this time and placed in a desorption tube to be transferred by MPS to the Thermal Desorption Unit (TDU), where the volatiles were desorbed and transferred to the GC system. An HP-5MS-fused silica capillary column (60 m × 0.25 mm i.d., 0.25 μm film) from Agilent Technologies was used and an initial oven temperature of 50 °C (2 min) was then increased from 4 °C/min to 190 °C during 10 min. The MSD operated at 70 eV in the electron impact mode (EI), with a mass range of 35–550 Da at a temperature of 150 °C. All samples were analyzed in triplicate. Quantification of minor volatile compounds was performed using a calibration table built with standard solutions, containing a known concentration of each compound.
All major and minor volatiles described in this work were identified and confirmed by GC-MS using the same Agilent 7890-MSD 5975C (Agilent technologies, Santa Clara, CA, USA, and Wilmington, DE, USA) described before and the same capillary column and settings for temperature and carrier helium gas programs used for their analysis. Compound identification was performed by comparing the peak data of compounds with mass spectra libraries NIST08 and Wiley7 and consulting the NIST data base from the Web of Chemistry. A second identification of the compounds analyzed was performed by subjecting a mixture of commercially available pure compounds to the same analytical conditions as the samples. Reagents and pure chemical compounds for identification and quantification were provided by Sigma-Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany). The importance of volatile aroma compounds in the organoleptic properties of foods is related to their content and odor perception threshold (OPT). The odor activity value (OAV) is a widely used measure to evaluate the contribution of a specific compound to the overall aroma and it is calculated by dividing its concentration by its OPT.
2.4. Sensorial Analysis
Blind sensory analyses were carried out by a tasting panel, consisting of eight experienced judges from the Department of Agricultural Chemistry, Edaphology, and Microbiology at the University of Córdoba, Spain. They evaluated the three wines using the OIV (2021) [20] official tasting sheet. The panel comprised 5 males and 3 females, who assessed the wines based on attributes for sight, smell, and taste. These attributes included limpidity, visual aspects other than limpidity, genuineness, positive intensity, harmonious persistence, and quality. The judges were also tasked with scoring samples for overall quality on a scale of 0 to 100 points, taking into account the previously scored attributes. Prior to the analysis, all samples were stored for 24 h at 4 °C. The wine samples (30 mL) were presented to the tasters at room temperature (20 °C) in standardized wine glasses (NF V09-110 AFNOR, 1995), in accordance with the requirements of ISO 3591 norms [20]. The wines were poured in random order, labeled with four-digit codes, and with a 1 min break between samples.
2.5. Statistical Analysis
All numerical data were pre-treated with two normalization filters (mean centering and autoscaling). The analytical data matrix was subjected to statistical analysis using the Statgraphics statistical software package (Centurion XVI v. 16. 1. 11). Two-way analysis of variance (ANOVA) and least significant test (LSD Fisher’s test), were performed to establish significant differences between the three wines. Differences at p ≤ 0.05 were considered to be statistically significant. The data matrix of the concentrations of major volatile compounds and polyols and those of minor volatile compounds was statistically autoscaled and then subjected to a Principal Component Analysis (PCA) by using the PLS_toolbox v. 8. 5. 2 of Matlab R2016a v. 9. 0. 0. 341360 (Natick, MA, USA).
3. Results and Discussion
3.1. Oenological Variables
The fermentation process was monitored by measuring the density. All the fermentations started with a density of 1088 g·L−1 and were considered finished on the 14th day of fermentation when their values decreased close to 990 g·L−1 (Supplementary Material Figure S1). The musts fermented in the traditional way (WY) showed the lowest fermentation rate during the first four days, compared to those inoculated with L. thermotolerans in free or biocapsule format. The highest fermentation rate of free LT yeast was similar to that reported by Lúquez-Caravaca et al. 2023 [15] and Puig-Pujol et al. 2013 [17], where fermentation kinetics with free cells were faster than those with immobilized cells. As noted in these studies, this could be due to the slight stress on the immobilized cells and/or the effect of the support matrix, which causes nutrient diffusion limitations.
Significant differences at p ≤ 0.05 level were observed in several general variables of the obtained wines (Table 1). The pH values showed two homogeneous groups (HGs) without significant differences between free and immobilized L. thermotolerans yeasts, both increasing about 0.2 units compared to the control wines. On the other hand, the volatile and titratable acidity data (expressed as g of acetic acid/L and g of tartaric acid/L, respectively) had three HGs, and the acidities showed lower values in the control wines. According to Moreno-García et al. (2018) [9], immobilized yeast cells are subjected to different conditions than free ones, causing potential changes in their metabolism, for instance, an increase in wine volatile acidity.

Table 1.
Oenological variables of wines. Mean values and standard deviations. Different letters in the same row indicate statistical differences in the normalized and scaled data at the 0.05 level according to Fisher’s least significant difference test, denoted in the table as homogeneous groups (HGs). WY: obtained by spontaneous fermentation; LT: wine using Lachancea thermotolerans yeast; BC: wine using Lachancea thermotolerans yeast immobilized in microbial biocapsules.
The ethanol content ranged between 12.3 ± 0.3 and 13.95 ± 0.05% (v/v) for the assays with LT and WY, respectively. It is well-known that L. thermotolerans has a lower ethanol production than S. cerevisiae during alcoholic fermentation, which predominates in the later stages of fermentation. As Wang et al. (2023) [4] noted, while S. cerevisiae requires complex genetic engineering to diminish their ethanol production, L. thermotolerans is more readily available and efficient for this purpose. The sugar content is reduced below 5 g·L−1, the level which is considered as dry wines, although wines from immobilized LT have higher values (2.4 g·L−1) followed by LT in free format (0.59 g·L−1) and wild yeast (0.17 g·L−1). These results show the greater efficiency of the traditional method of carrying out the alcoholic fermentation, using wild yeasts, compared to fermentations with non-Saccharomyces. Further, an important and significant difference in glutathione levels was observed between wines from wild yeast and those from L. thermotolerans, which was about 6 mg·L−1 higher in LT in free format and 2.6 mg·L−1 in immobilized format. The glutathione contents in grapes depend on several factors, including grape variety, vintage, climate, geographic location, ripeness, and viticultural practices. This content will remain in the must, albeit at low concentrations of around a few milligrams per liter [21].
Throughout alcoholic fermentation, the initial content of reduced glutathione (GSH) decreases initially, only to rise again due to cell lysis and ex-novo synthesis by yeasts. The yeast can take up GSH from the extracellular medium through the Opt1p/Hgt1p transporter and secrete it via the GSH/proton antiporter Gex1p [22]. This allows for GSH assimilation from the must and secretion into the extracellular medium, depending on various factors. In the case of BC, the exchange of GSH between the yeast and the extracellular medium could be disrupted probably due to a limitation on cell mass transfer, resulting in a lower concentration of GSH in the wine. L. thermotolerans has been reported as a significant producer of glutathione against S. cerevisiae [23]. This finding is of interest to the wine industry as it can help avoid detrimental changes in color and oxidative aromas, thus preserving sensory characteristics and stability. Additionally, it can promote a healthier product by reducing SO2 addition requirements [24,25]. Lactic acid concentration tends to increase when using LT. This yeast strain is known for its high lactic acid production, as confirmed by multiple recent studies [7,16,23]. Malic acid levels are higher in wines made with wild yeast than those of LT and BC. WY shows increased absorbance at 420, 520, and IPT. According to Vaquero et al. (2022) [26] in fermentations containing more lactic acid, the absorbance values are lower. Vicente et al. (2023) [1] reported some differences in color intensity related to anthocyanin changes in the coloration of red wines due to the changes in pH. Upon analyzing the various parameters, it is observed that the most significant differences are obtained between the wines from WY and LT inoculated in free format.
3.2. Major Volatile Compounds and Polyols
Upon analysis of the major volatile compounds, significant differences were observed for all compounds except for 3-methyl-1-butanol and 2,3-butanediol (meso) (Table 2). The main differences were found between wild yeast and those made with L. thermotolerans in both free and immobilized formats: acetaldehyde, 1-propanol, isobutanol, 2,3-butanediol (levo), diethyl succinate and 2-phenylethanol; exhibited 2 HG. The concentrations varied among the spontaneous and the inoculated fermentations, but there were no significant differences between free and immobilized L. thermotolerans fermentations as reported by Peinado et al. (2005) [13] for most of these compounds in S. cerevisiae. With the exception of 2,3-butanediol (levo) and diethyl succinate, L. thermotolerans fermentations produced larger quantities of these compounds. Non-Saccharomyces yeasts generally exhibit stronger abilities to produce 2-phenyletanol than S. cerevisiae. This is attributed to their enzyme and metabolite production, which are associated with several genes that regulate the synthesis of higher aromatic alcohols [4]. Additionally, acetaldehyde was found in larger concentrations in L. thermotolerans in this study. This compound is a by-product of fermentative glycolysis and is mostly formed during the active growth phase of yeast. It serves as a precursor metabolite for the synthesis of acetate, acetoin, and ethanol [27]. Ethyl acetate, 2-methyl-1-butanol, acetoin, and ethyl lactate showed three significant differences between WY and LT in both free and immobilized forms. LT also produces high amounts of ethyl lactate due to the formation of high levels of lactic acid [28]. Methanol was the only compound that was comparable in WY and free LT, and its concentration increased in the BC. In summary, only the contents of ethyl acetate, 1,1-diethoxyethane, methanol, 2-methyl-1-butanol, acetoin, ethyl lactate, and glycerol in wines show significant differences with respect to the inoculation format of LT used for fermentation. According to Lúquez-Caravaca et al. (2023) [15], most of the quantified parameters did not show significant differences when comparing the BC with free yeast.

Table 2.
Mean and deviation values of the major volatile compounds and polyols (mg·L−1) quantified in wines. Different letters in the same row indicate statistical differences in the normalized and scaled data at the 0.05 level according to Fisher’s least significant difference test, denoted in the table as homogeneous groups (HGs). WY: obtained by spontaneous fermentation; LT: Lachancea thermotolerans yeast in free format; BC: Lachancea thermotolerans yeast in immobilized format. CAS: identification number assigned by the Chemical Abstracts Service. OPT: odor perception threshold (mg·L−1). OS: odorant series.
A principal components analysis (PCA) was conducted on the data matrix, resulting in a biplot that explains 79.47% of the overall variance in the first two components (Figure 1). The samples were categorized separately. Spontaneous fermentation was observed towards the left-hand side of the graph, exhibiting negative values in Component 1 (PC1), while both free and immobilized L. thermotolerans appeared to shift towards positive values. It is important to note that PC1 is more significantly affected by compounds such as 2,3-butanediol and diethyl succinate. The two inoculation forms of L. thermotolerans are differentiated in Component 2 (PC2). The free form is displaced upwards on the graph with positive values, while the biocapsules are displaced towards negative values. This result is consistent with that found by García-Martínez et al. 2012 [14] for fermentations carried out with S. cerevisiae, where major volatile compounds could differentiate the produced wines with free and immobilized cells in biocapsules.
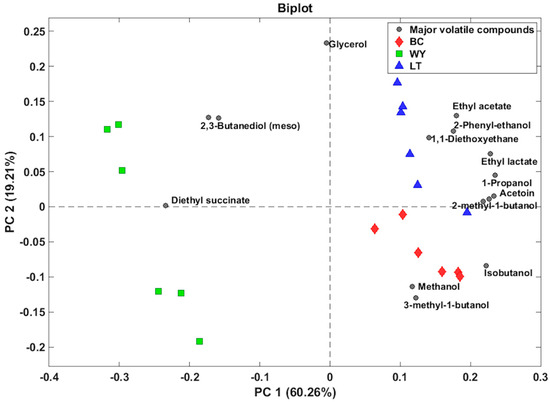
Figure 1.
Principal component analysis biplot of the 15 quantified volatile compounds by GC-FID in the wines. Circles represent the variables and their coordinates of the load to each component. Other geometric figures represent the scores of the wines in the two components. WY: obtained by spontaneous fermentation; LT: wine using Lachancea thermotolerans yeast; BC: wine using Lachancea thermotolerans yeast immobilized.
3.3. Minor Volatile Compounds
Table 3 shows the concentrations of minor volatile compounds expressed in micrograms per litter and the homogeneous groups obtained at a significance level of p ≤ 0.05 for each compound quantified. Forty-six compounds were quantified, including 8 acetates, 11 ethyl esters, 4 other esters, 4 alcohols, 4 lactones, 8 carbonyl compounds, 5 terpenes and derivatives, and 2 miscellaneous compounds. Many of the flavor compounds in wine are by-products of the alcoholic fermentation process, which is largely influenced by the type of yeast used [30]. During fermentation, a significant amount of acetates with fruity and floral properties are produced, including 2-phenylethyl acetate. This compound enhances the wine’s fruity aromas and adds complexity to its overall aroma [4]. However, according to Nedović et al. (2015) [27], some compounds, such as ethyl dodecanoate, are more commonly attributed to immobilized cells than to free cell systems. Additionally, studies by Wang et al. (2023) [4] have shown that mixed fermentation of Saccharomyces with non-Saccharomyces can enhance the production of these compounds, adding complexity to the wine aroma. During fermentation, yeasts produce ethanol and glycerol through their own metabolism. The production of other higher alcohols plays an important role in the taste of wine. The right amount of these alcohols can make a wine fuller and rounder, thereby improving its taste. However, if the alcohol content is too high, it can produce a peculiar off-flavor odor that decreases the quality of the wine [4,27]. It is well established that non-Saccharomyces yeasts compete with Saccharomyces yeasts for must sugars in the early stages of fermentation and that they have different capacities to produce volatile compounds, with Metschnikowia pulcherrima and L. thermotolerans standing out for their production of higher alcohols [4]. However, according to Vicente et al. (2021) [25], the contents of certain alcohols may be decreased due to the effect of L. thermotolerans.

Table 3.
Mean and deviation contents of minor volatile compounds (μg·L−1) in wines. Different letters in the same row indicate statistical differences in the normalized and scaled data at the 0.05 level according to Fisher’s least significant difference test, denoted in the table as homogeneous groups (HGs). WY: obtained by spontaneous fermentation; LT: using Lachancea thermotolerans yeast in free format; BC: with Lachancea thermotolerans immobilized. CAS: identification number assigned by the Chemical Abstracts Service. OPT: odor perception threshold (μg·L−1). OS: odorant series. * Indicates odor-active compounds and bold numbers indicate a dependence only on the format used to inoculate LT.
Ester and terpene families, commonly found in wines, are highly volatile metabolites and have the strongest odorants. Esters are produced during alcoholic fermentation, largely by the enzymatic pathway of yeasts. Qualitatively, they are the most abundant family of volatile metabolites and generally contribute to their fruity nuances. This study shows the content of 22 esters, i.e., half of the volatile metabolites quantified. On the other hand, terpenes are metabolites that are especially abundant in aromatic varieties such as Muscat. They can be found in free and volatile form or in a non-volatile form in combination with mono- or disaccharides, forming the so-called “hidden grape aroma”. These combinations can be hydrolyzed by some yeasts with beta-glucosidase activities. In general, the family of monoterpenes shows floral aromas, and their increase in contents leads to an increase in the aromatic complexity of wines [4].
Although the most significant differences in the composition of minor volatiles in the three wines are due to the yeast used as inoculum, some compounds show statistically significant differences according to the inoculation format used for L. thermotolerans. Thus, in the acetates group, isoamyl acetate and ethyl phenylacetate showed 3 HG, which means a different concentration in each obtained wine, while only octyl acetate has no differences among them. For the 11 ethyl esters quantified, only ethyl isobutyrate and ethyl hexadecanoate have three HGs. Among the four compounds grouped as other esters, only 2-phenylethyl butanoate has a significant dependence on the inoculation format, showing a higher concentration in BC wines. Also, 1-hexanol is the only higher alcohol whose content is dependent on the inoculum format with higher concentrations in wines from BC. In the lactone group, only β-damascenone showed three HGs, and together with γ-nonalactone, it also showed higher concentrations in BC. Benzaldehyde, octanal, nonanal, and 2-phenylacetaldehyde among the eight carbonyl compounds quantified showed three HGs; benzaldehyde, 2-phenylacetaldehyde, and furfural had higher contents in BC wines and the lowest content in octanal and nonanal. Compounds grouped as terpenes and derivatives showed no significant differences between their contents in LT and BC wines, except for nerolidol with higher content in LT. Limonene and geranyl acetone had the highest contents in WY wines. Finally, 2-pentylfuran also exhibited higher concentrations in these wines.
Figure 2 illustrates the two principal components that account for 66.33% of the total variance provided by the data matrix built with all the minority compounds quantified. The first principal component (PC1) accounts for 47.22% of the variability, while the second component (PC2) accounts for 19.11% of the variance. In Figure 2a, minor volatile compounds can clearly differentiate the three elaborated fermentations, separating each of them into distinct groups. Wines fermented with L. thermotolerans yeast were positioned on the left-hand side of the graph, with negative values on the PC1 axis, while the WY strain moved toward the right-hand side of the graph with positive values. Figure 2b illustrates that WY, located to the right, is primarily influenced by compounds such as hexyl acetate, 2-phenylethyl acetate, ethyl 3-methylbutyrate, ethyl heptanoate, 2-methoxy-4-ninylphenol, limonene, and 2-pentylfuran, as indicated by the loading values of >0.20. The weight of each load is presented in Table S1 of the Supplementary Material. Wines made with L. thermotolerans, whether in free format or in biocapsules, are primarily influenced by carbonyl compounds. The most significant contributors to this aspect are ethyl isobutyrate, octanal, 2-phenylacetaldehyde, and decanal. Although the differences between the types of yeast used for winemaking are clearly shown, the inoculation format of L. thermotolerans can also be observed to separate into two groups, as shown in Figure 2a, mainly in PC2. In the graph, BC is shifted upwards. This vector dimension is mainly defined by the influence of ethyl esters, such as ethyl 2-methylbutyrate, ethyl decanoate, ethyl dodecanoate, and ethyl tetradecanoate, and by 2-phenylethyl butanoate, hexanol, γ-nonalactone, furfural, and benzaldehyde. Previous research with immobilized S. cerevisiae has shown positive effects on the fruity character of final products due to an improved ratio of esters to alcohols [27]. The above results are consistent with this research. The free format moves towards negative values, with nerolidol having the greatest influence. This is attributed to the expression of genes that code key enzymes involved in ester formation, such as alcohol acetyltransferases encoded by the genes ATF1 and ATF2 [27].
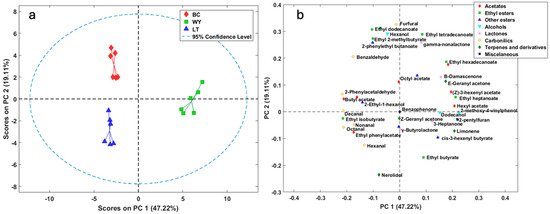
Figure 2.
Principal component analysis of the 46 quantified compounds by SBSE-GC-MSD in the wines. (a) The scores of the first two components for the three wine types obtained. (b) The loadings of the 46 analyzed variables corresponding to the quantified minor compounds. WY: obtained by spontaneous fermentation; LT: wine using L. thermotolerans yeast; BC: wine using L. thermotolerans yeast immobilized.
3.4. Odor Activity Value
The OAV is a useful indicator of the contribution of a volatile compound to the overall aroma of wines and other food products. According to Niu et al. (2020) [39] and Guclu et al. (2016) [40], a volatile compound with an OAV higher than 0.2 is considered to have a significant influence on the aroma of foods and beverages. In this work, a total of 27 compounds among the major and minor volatiles quantified in WY were selected as aroma-active compounds with OAV ≥ 0.2, and 17 showed an OAV ≥ 1. Also, 26 and 27 volatile compounds for LT and BC, respectively, showed OAV ≥ 0.2, and 18 in both conditions have OAV ≥ 1 (Table 2 and Table 3).
The most potent odorants identified among the major volatiles were acetaldehyde, ethyl acetate, 3-methyl-1-butanol, and 2-phenylethanol, and the odorants among the minor volatiles were isoamyl acetate, 2-phenylethyl acetate, cis-3-hexenyl butyrate, β-damascenone, nonanal, 2-phenylacetaldehyde, and limonene. All these compounds have five or more OAVs. Some compounds, such as ethyl-3-methylbutyrate and 2-pentylfuran, are identified as odor-active only in WY, whereas 2-phenylacetaldehyde and 1,1-diethoxyethane are odor-active only in LT and BC.
Each volatile compound was grouped into one or more odorant series (OS) according to its aroma descriptor. In this way, 11 OS were selected, and the OAV for each of them was calculated as the addition of the OAVs for the minor and major volatiles integrated into it. The data matrix of these data was subjected to a multiple variable analysis (MVA) in order to obtain a footprint or sunray plot for each condition studied (Figure 3). These plots allow us to visualize in an objective, simple, and useful way the differences between the wines obtained by the three inoculation formats carried out. All the wines showed different footprints; in summary, the wine obtained with the same yeast but in a different format (LT and BC) showed similar footprints compared to the control wines (WY). In LT and BC wines, the OS associated with creamy (6) and waxy (11) aroma descriptors have the highest values. and in contrast, WY showed a higher OAV for the chemical (1), ripe (2) and green fruit (3), floral (5), citrus (7), herbaceous (8), toasted (9), and honey (10) OS. LT had the smallest shape footprint, while BC was intermediate between WY and LT. This effect is clearly observed in the fruity/ripe fruit, green, and floral series, where the values for BC are intermediate between WY and LT.
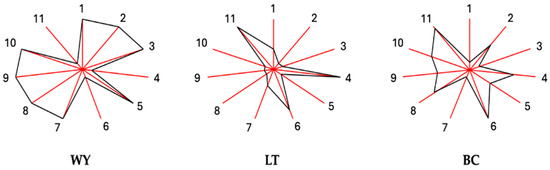
Figure 3.
Footprints obtained via multivariate data analysis of the odor activity values (OAV) contributing to the odorant series for each of the tests. Each ray of the polygon and its number corresponds to an odorant series. 1: chemical; 2: fruity/ripe fruit; 3: green fruit; 4: green; 5: floral; 6: creamy; 7: citrus; 8: herbaceous; 9: toasty/smoky; 10: honey; 11: waxy. The end of the ray is the mean value plus three standard deviations, and the origin is the mean minus three standard deviations. WY: wine obtained via spontaneous fermentation; LT: wine from free L. thermotolerans yeast; BC: wine from immobilized. L. thermotolerans yeast.
3.5. Organoleptic Evaluation
Of the attributes evaluated (Table 4), there were no significant differences in smell between the different wines. While the scores for sight were different between wild yeast and L. thermotolerans, it was the taste and overall quality that differentiated the yeast in biocapsules format from the free form and wild yeast. This difference resulted in a higher total score for the microbial biocapsules (BC), obtaining 78.64 points. Previous studies comparing L. thermotolerans and Saccharomyces yeasts have also shown higher scores for the sight attribute, which may be related to their lower absorption values and higher glutathione content, which produces less golden hues [23]. In studies comparing wines made with free and immobilized yeasts, similar sensory profiles were obtained [17,41]. No significant differences in several of the analyzed volatile compounds were found between wines made with free and microbial biocapsules. However, the use of microbial biocapsules to ferment with L. thermotolerans resulted in an improvement in organoleptic properties. This study, in agreement with Lúquez-Caravaca et al. (2023) [15], shows that the perceived taste quality and overall quality can be improved by using microbial biocapsules while maintaining the bioactivity of immobilized cells.

Table 4.
Mean scores, standard deviations, and homogeneous group values for the organoleptic attributes tested. Different letters in the same row indicate statistical differences in the normalized and scaled data at the 0.05 level according to Fisher’s least significant difference test, denoted in the table as homogeneous groups (HGs). WY: obtained by spontaneous fermentation; LT: wine using Lachancea thermotolerans yeast; BC: wine using Lachancea thermotolerans yeast immobilized.
4. Conclusions
The use of Lachancea thermotolerans, inoculated in free or immobilized format, on musts with a high content of fermentable sugars and low acidity, allows obtaining wines with lower ethanol content and higher acidity. This yeast strain increases the lactic acid and glutathione levels and decreases the absorbance values at 420, 520, and 280 nm, all of which have a positive effect on the visual appearance of the wine obtained.
Among the 58 volatile metabolites and 3 polyols quantified in this study, only 27 were identified as aroma-active compounds with odor activity values greater than 0.2. Ethyl acetate, 1,1-diethoxyethane, methanol, 2-methyl-1-butanol, acetoin, ethyl lactate, and glycerol showed significant differences between the free and immobilized formats used to inoculate L. thermotolerans. Also, eight minor volatiles showed a significant dependence on the inoculation format and nine were dependent on both the yeast and inoculation formats tested. The Principal Component Analysis performed on the data matrix of the major and minor volatiles also allowed us to classify the three wines by their scores in two components, which explains 79.47% and 66.33%, respectively, of the total variance.
The fingerprinting obtained by the multiple variable analysis applied to the odor activity values of volatiles, grouped in 11 odorant series, exhibit different profiles for the wines obtained, inoculating the must with L. thermotolerans in free or immobilized format and the wild yeasts in the fruity/ripe fruit, green and floral series.
The organoleptic evaluation showed significant differences between wines inoculated with L. thermotolerans in free format or as microbial biocapsules and those fermented with wild yeasts in terms of sight, taste, overall quality, and total score. The wines from biocapsules had the best scores in taste, overall quality, and total score. As a consequence, the application of the biocapsule immobilization system to non-Saccharomyces yeasts is revealed as a promising winemaking technique, whose use for sequential fermentations opens new possibilities to minimize the impact of climate change in wineries and to produce new types of wines according to the new tendencies of young consumers. However, to fully understand the potential of this technique, further research is needed, particularly on the bio-immobilization of other non-Saccharomyces yeasts and their impact on the production of metabolites that affect both the analytical and sensory quality of the resulting wines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation10060303/s1, Table S1. Loadings obtained from the data matrix of minor volatile compounds quantified by SBSE-GC-MSD and used as chemical variables to build the principal component analysis of wines. The variables with the highest loadings in each principal component are shown in bold.
Author Contributions
Conceptualization, J.M. and J.M.-G.; methodology, J.M.; software, R.M.-C.; formal analysis, R.M.-C.; investigation, J.M., J.M.-G. and R.M.-C.; resources, J.M., T.G.-M. and J.C.M.; writing—original draft preparation, J.M. and R.M.-C.; writing—review and editing, J.M.-G., T.G.-M. and J.C.M.; visualization, R.M.-C. and J.M.; supervision, J.M. and J.M.-G.; funding acquisition, J.M., T.G.-M. and J.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Junta Andalucía (Spain): Consejería de Economia, Conocimiento, Empresas y Universidad. Call PAIDI 2020: Projects of collaborative interest in the field of Innovation Ecosystems of the International Centers of Excellence (ceiA3). Grant number PYC20 RE 068 UCO. “Relationship of grape quality, its yeast microbiota and wine quality with the viticultural terroir”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are thankful to the staff at the Central Service for Research Support (SCAI) of the University of Córdoba for their assistance in UHPLC-MS-MS measurements.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Vicente, J.; Navascués, E.; Benito, S.; Marquina, D.; Santos, A. Microsatellite Typing of Lachancea thermotolerans for Wine Fermentation Monitoring. Int. J. Food Microbiol. 2023, 394, 110186. [Google Scholar] [CrossRef] [PubMed]
- Moreno García, J. Proteomic and Metabolomic Study of Wine Yeasts in Free and Immobilized Formats, Subjected to Different Stress Conditions; UCOPress: Cordoba, Spain, 2017; p. 181. [Google Scholar]
- Nisiotou, A.; Mallouchos, A.; Tassou, C.; Banilas, G. Indigenous Yeast Interactions in Dual-Starter Fermentations May Improve the Varietal Expression of Moschofilero Wine. Front. Microbiol. 2019, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, G.; Peng, Y.; Xu, N.; Xie, Y.; Zhou, H.; Liang, H.; Zhan, J.; Huang, W.; You, Y. Mechanisms and Effects of Non-Saccharomyces Yeast Fermentation on the Aromatic Profile of Wine. J. Food Compos. Anal. 2023, 124, 105660. [Google Scholar] [CrossRef]
- Battjes, J.; Melkonian, C.; Mendoza, S.N.; Haver, A.; Al-Nakeeb, K.; Koza, A.; Schrubbers, L.; Wagner, M.; Zeidan, A.A.; Molenaar, D.; et al. Ethanol-Lactate Transition of Lachancea thermotolerans Is Linked to Nitrogen Metabolism. Food Microbiol. 2023, 110, 104167. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Sanarica, L.; Zara, I.; Pisarra, C.; Gambacorta, G.; Natrella, G.; Cardinale, M. Cultivar-Dependent Effects of Non-Saccharomyces Yeast Starter on the Oenological Properties of Wines Produced from Two Autochthonous Grape Cultivars in Southern Italy. Foods 2022, 11, 3373. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airén Wines Fermented by Sequential Inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; García-Martínez, T. Using an Electronic Nose and Volatilome Analysis to Differentiate Sparkling Wines Obtained under Different Conditions of Temperature, Ageing Time and Yeast Formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, J.; García-Martinez, T.; Moreno, J.; Mauricio, J.C.; Ogawa, M.; Luong, P.; Bisson, L.F. Impact of Yeast Flocculation and Biofilm Formation on Yeast-Fungus Coadhesion in a Novel Immobilization System. Am. J. Enol. Vitic. 2018, 69, 278–288. [Google Scholar] [CrossRef]
- López-Menchero, J.R.; Ogawa, M.; Mauricio, J.C.; Moreno, J.; Moreno-García, J. Effect of Calcium Alginate Coating on the Cell Retention and Fermentation of a Fungus-Yeast Immobilization System. LWT 2021, 144, 111250. [Google Scholar] [CrossRef]
- Gosset, M.; Roques, C.; Taillandier, P. Microbial Biofilms in Oenology. OENO One 2022, 56, 167–184. [Google Scholar] [CrossRef]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of Two Yeast Strains in Free, Bioimmobilized or Immobilized with Alginate Forms on the Aromatic Profile of Long Aged Sparkling Wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Mauricio, J.C. Use of a Novel Immobilization Yeast System for Winemaking. Biotechnol. Lett. 2005, 27, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, T.; Puig-Pujol, A.; Peinado, R.A.; Moreno, J.; Mauricio, J.C. Potential Use of Wine Yeasts Immobilized on Penicillium chrysogenum for Ethanol Production. J. Chem. Technol. Biotechnol. 2012, 87, 351–359. [Google Scholar] [CrossRef]
- Lúquez-Caravaca, L.; Ogawa, M.; Rai, R.; Nitin, N.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Jiménez-Uceda, J.C.; Moreno-García, J. Yeast Cell Vacuum Infusion into Fungal Pellets as a Novel Cell Encapsulation Methodology. Appl. Microbiol. Biotechnol. 2023, 107, 5715–5726. [Google Scholar] [CrossRef]
- Pastor-Vega, N.; Carbonero-Pacheco, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T.; Nitin, N.; Ogawa, M.; Rai, R.; Moreno-García, J. Flor yeast immobilization in microbial biocapsules for Sherry wine production: Microvinification approach. World J. Microbiol. Biotechnol. 2023, 39, 271. [Google Scholar] [CrossRef] [PubMed]
- Puig-Pujol, A.; Bertran, E.; García-Martínez, T.; Capdevila, F.; Mínguez, S.; Mauricio, J.C. Application of a New Organic Yeast Immobilization Method for Sparkling Wine Production. Am. J. Enol. Vitic. 2013, 64, 386–394. [Google Scholar] [CrossRef]
- García-Martínez, T.; Moreno, J.; Mauricio, J.C.; Peinado, R. Natural Sweet Wine Production by Repeated Use of Yeast Cells Immobilized on Penicillium chrysogenum. LWT 2015, 61, 503–509. [Google Scholar] [CrossRef]
- Ogawa, M.; Bisson, L.F.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. New Insights on Yeast and Filamentous Fungus Adhesion in a Natural Co-Immobilization System: Proposed Advances and Applications in Wine Industry. Appl. Microbiol. Biotechnol. 2019, 103, 4723–4731. [Google Scholar] [CrossRef] [PubMed]
- OIV. Available online: https://www.oiv.int/en (accessed on 7 February 2024).
- Kritzinger, E.C.; Bauer, F.F.; Du Toit, W.J. Role of Glutathione in Winemaking: A Review. J. Agric. Food Chem. 2013, 61, 269–277. [Google Scholar] [CrossRef] [PubMed]
- De Vero, L.; Bonciani, T.; Verspohl, A.; Mezzetti, F.; Giudici, P. High-Glutathione Producing Yeasts Obtained by Genetic Improvement Strategies: A Focus on Adaptive Evolution Approaches for Novel Wine Strains. AIMS Microbiol. 2017, 3, 155–170. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. Chemometric Differentiation of White Wines from a Low-Aromatic Grape Obtained by Spontaneous Fermentation, Enriched with Non-Saccharomyces, or with a High-Glutathione-Producing Saccharomyces Yeast. Fermentation 2023, 9, 1023. [Google Scholar] [CrossRef]
- Binati, R.L.; Larini, I.; Salvetti, E.; Torriani, S. Glutathione Production by Non-Saccharomyces Yeasts and Its Impact on Winemaking: A Review. Food Res. Int. 2022, 156, 111333. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. An Integrative View of the Role of Lachancea Thermotolerans in Wine Technology. Foods 2021, 10, 2878. [Google Scholar] [CrossRef]
- Vaquero, C.; Escott, C.; Heras, J.M.; Carrau, F.; Morata, A. Co-Inoculations of Lachancea thermotolerans with Different Hanseniaspora Spp.: Acidification, Aroma, Biocompatibility, and Effects of Nutrients in Wine. Food Res. Int. 2022, 161, 111891. [Google Scholar] [CrossRef]
- Nedović, V.; Gibson, B.; Mantzouridou, T.F.; Bugarski, B.; Djordjević, V.; Kalušević, A.; Paraskevopoulou, A.; Sandell, M.; Šmogrovičová, D.; Yilmaztekin, M. Aroma Formation by Immobilized Yeast Cells in Fermentation Processes. Yeast 2015, 32, 173–216. [Google Scholar] [CrossRef]
- Comuzzo, P.; del Fresno, J.M.; Voce, S.; Loira, I.; Morata, A. Emerging Biotechnologies and Non-Thermal Technologies for Winemaking in a Context of Global Warming. Front. Microbiol. 2023, 14, 1273940. [Google Scholar] [CrossRef]
- Ogawa, M.; Vararu, F.; Moreno-Garcia, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Analyzing the Minor Volatilome of Torulaspora delbrueckii in an Alcoholic Fermentation. Eur. Food Res. Technol. 2022, 248, 613–624. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Du, G.; Gao, Y.T.; Wang, L.W.; Meng, D.; Li, B.J.; Brennan, C.; Wang, M.Y.; Zhao, H.; Wang, S.Y.; et al. The Effect of Carbonic Maceration during Winemaking on the Color, Aroma and Sensory Properties of ‘Muscat Hamburg’ Wine. Molecules 2019, 24, 3120. [Google Scholar] [CrossRef] [PubMed]
- Cometto-Muñiz, J.E.; Cain, W.S.; Abraham, M.H.; Gil-Lostes, J. Concentration-Detection Functions for the Odor of Homologous n-Acetate Esters. Physiol. Behav. 2008, 95, 658–667. [Google Scholar] [CrossRef]
- Zhu, L.X.; Zhang, M.M.; Shi, Y.; Duan, C.Q. Evolution of the Aromatic Profile of Traditional Msalais Wine during Industrial Production. Int. J. Food Prop. 2019, 22, 911–924. [Google Scholar] [CrossRef]
- Pardo, E.; Rico, J.; Gil, J.V.; Orejas, M. De Novo Production of Six Key Grape Aroma Monoterpenes by a Geraniol Synthase-Engineered S. cerevisiae Wine Strain. Microb. Cell Fact. 2015, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Petersen, M.A.; Liu, J.; Toldam-Andersen, T.B.; Ebeler, S.E.; Hopfer, H. Influence of Pre-Fermentation Treatments on Wine Volatile and Sensory Profile of the New Disease Tolerant Cultivar Solaris. Molecules 2015, 20, 21609–21625. [Google Scholar] [CrossRef]
- Song, X.; Dai, F.; Yao, J.; Li, Z.; Huang, Z.; Liu, H.; Zhu, Z. Characterization of the Volatile Profile of Feijoa (Acca sellowiana) Fruit at Different Ripening Stages by HS-SPME-GC/MS. LWT 2023, 184, 115011. [Google Scholar] [CrossRef]
- Martín-García, F.J.; Palacios-Fernández, S.; López de Lerma, N.; García-Martínez, T.; Mauricio, J.C.; Peinado, R.A. The Effect of Yeast, Sugar and Sulfur Dioxide on the Volatile Compounds in Wine. Fermentation 2023, 9, 541. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative Analysis of Headspace Volatile Compounds Using Comprehensive Two-Dimensional Gas Chromatography and Their Contribution to the Aroma of Chardonnay Wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Q.; Xiao, Z.; Mao, H.; Zhang, J. Characterization of Odor-Active Volatiles and Odor Contribution Based on Binary Interaction Effects in Mango and Vodka Cocktail. Molecules 2020, 25, 1083. [Google Scholar] [CrossRef]
- Guclu, G.; Sevindik, O.; Kelebek, H.; Selli, S. Determination of Volatiles by Odor Activity Value and Phenolics of cv. Ayvalik Early-Harvest Olive Oil. Foods 2016, 5, 46. [Google Scholar] [CrossRef]
- Fernández-Fernández, E.; Rodríguez-Nogales, J.M.; Vila-Crespo, J.; Falqué-López, E. Application of Immobilized Yeasts for Improved Production of Sparkling Wines. Fermentation 2022, 8, 559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).