Abstract
Hydrogen is an efficient fuel which can be generated via water splitting, however hydrogen evolution occurs at high overpotential, and efficient hydrogen evolution catalysts are desired to replace state-of-the-art catalysts such as platinum. Here, we report an advanced electrocatalyst that has low overpotential, efficient charge transfers kinetics, low Tafel slope and durable. Carbon nanofibers (CNFs), obtained by carbonizing electrospun fibers, were decorated with MoS2 using a facile hydrothermal method. The imaging of catalyst reveals a flower like morphology that allows for exposure of edge sulfur sites to maximize the HER process. HER activity of MoS2 decorated over CNFs was compared with MoS2 without CNFs and with commercial MoS2. MoS2 grown over CNFs and MoS2-synthesized produced about 374 and 98 times higher current density at −0.30 V (vs. Reversible Hydrogen Electrode, RHE) compared with the MoS2-commercial sample, respectively. MoS2-commercial, MoS2-synthesized and MoS2 grown over CNFs showed a Tafel slope of 165, 79 and 60 mV/decade, capacitance of 0.99, 5.87 and 15.66 mF/cm2, and turnover frequency of 0.013, 0.025 and 0.54 s−1, respectively. The enhanced performance of MoS2-CNFs is due to large electroactive surface area, more exposure of edge sulfur to the electrolyte, and easy charge transfer from MoS2 to the electrode through conducting CNFs.
1. Introduction
Hydrogen, due to its high energy density of 141.86 MJ/kg and clean combustion, has a potential to be one of the most effective clean energy sources [1,2]. However, the majority of hydrogen is produced via steam reforming, which consumes fossil fuels and negates the goal of a cleaner and sustainable energy future. In this context, hydrogen production via electrochemical water splitting using a catalyst is attracting considerable attention due to its inherent higher efficiency and lower cost compared to steam reforming [3]. An ideal electro-catalyst for hydrogen evolution reaction (HER) should provide high current density at a lower overpotential [4]. The current state-of-the-art catalyst, platinum, is one of the most efficient electro-catalyst for HER, however, its high cost and limited availability has curtailed its use for hydrogen production. This prompted scientists to look for alternative cost-effective materials which have high stability and efficiency comparable to that of platinum [3,5,6]. Thus, the development of efficient electro-catalyst for HER using earth-abundant materials is crucial [5,7,8,9,10]. Several nanostructured materials have been synthesized and used for energy applications [11,12,13,14,15]. Segev-Bar and Haick have written a very informative review article on sensors for various applications using nanoparticles [16]. Earth-abundant NiO nanoparticles have been used for various energy related applications [17]. A highly active and stable carbon-shell-coated FeP nanoparticles as a non-Pt electrocatalyst for hydrogen production has been synthesized using iron oxide nanoparticles as a precursor [18]. Carbon-shell-coated FeP nanoparticles showed a low overpotential of 71 mV at 10 mA/cm2, which is comparable to that of a commercial Pt catalyst. Multicomponent nanoparticles such as Ni-Co-Se have been used for hydrogen evolution reaction [19]. The electrocatalytic properties of these nanoparticles were improved by growing them on reduced graphene oxide nanoflakes. Phuruangrat et al. have synthesized molybdenum trioxide (MoO3) nanowire having high aspect ratios (>200) and good crystallinity for hydrogen evolution reaction by decomposing (NH4)6Mo7O24·4H2O [20].
Other materials such as transition metal chalcogenides (TMCs), for example molybdenum disulfide (MoS2), are potential candidates for HER; however, it is still challenging to get catalytic performance close to platinum [21,22]. It is reported that the catalytic activities of MoS2 are mainly due to edge sulfur groups [23,24]. The lower catalytic properties of these materials are due to poor charge transport, low reactivity of active sites and less exposure of edge sulfur groups to the electrolyte [23,25,26]. Improving the charge transport properties and exposing large number of edge sulfur are the crucial steps to enhance the catalytic activities of MoS2 [5].
Several strategies such as exfoliation of TMC sheets, controlling the morphologies, and improving the charge transport by making composites and decorating them over conducting substrate/materials have been reported to improve the performance of TMCs towards HER [27,28,29,30,31,32]. Among various transition metal chalcogenides, MoS2, MoSe2, CoS2, CoSe2, FeS2, and NiS2 have shown high catalytic activities for HER applications [3,33,34,35,36,37]. Gopalakrishnan et al. have used liquid exfoliation method to synthesize nanostructured MoS2 quantum dots for hydrogen evolution from the water [27]. These nanostructured MoS2 quantum dots showed high electrocatalytic activity with low overpotential. They reported a current density of 120 mA/cm2 (at −0.4 V vs. SHE) with a Tafel slope of ~74 mV/decade. In another study, You et al. have studied the effect of morphology on the electrocatalytic performance of CoS [32]. CoS having various morphology such as hollow prisms, broken prisms and nanoparticles were synthesized using two-step microwave-assisted anion-exchange method and effect of morphology on HER activity was studied. Li et al. have improved the HER activity of MoS2 by decorating it over CNTs [31]. A Tafel slope of 40 and 60 mV/decade was reported for MoS2 and MoS2 decorated over CNT, respectively.
In this work, we report an improved performance of MoS2 by decorating it over carbon nanofibers (CNFs). Carbon nanofibers were prepared using electrospinning method followed by carbonization. MoS2 decorated over CNFs (MoS2-CNFs) was synthesized using a facile hydrothermal method. MoS2-CNFs offers several advantages: (1) high conductivity; (2) high electroactive surface area; (3) vertically grown over conducting CNFs exposing high density of edge sulfur; (4) high current density and low Tafel slope; (5) low charge transfer resistance; and (6) high turnover frequency, compared with commercial MoS2 as well as MoS2 synthesized using similar method. The detailed results are presented and discussed in the following sections.
2. Results and Discussion
The crystalline structure of MoS2-commercial, MoS2-synthesized and MoS2 decorated on CNFs were studied using X-ray diffraction (Figure 1a). The X-ray diffraction (XRD) patterns of MoS2-commercial well matches with diffraction patterns of hexagonal MoS2 phase with lattice parameters of a = 3.160 Å, b = 3.160 Å, and c = 12.295 Å, which are in good agreement with Joint Committee on Powder Diffraction Standards (JCPDS) file No. 00-006-0097. The (002) peak of MoS2-commercial sample was observed at 14.68°. However, for the MoS2-synthesized sample, the characteristic hexagonal basal plane peak associated with (002) plane was observed to shift to 9.58°, indicative of the increased d-spacing between these planes. A second order diffraction peak associated with (004) plane was observed around 18.3°. The MoS2 grown on CNFs shows similar patterns as the MoS2-synthesized with (002) and (004) peaks at 9.46° and 18.5°, respectively, demonstrating the reproducibility of the synthesis approach on different supports. The d spacing of all the samples was calculated using the Bragg’s law. A d spacing of 6.03, 9.23 and 9.35 Å was calculated for MoS2-commercial, MoS2-synthesized and MoS2 decorated on CNFs, respectively. Raman spectra of all the samples show similar nature, however, the intensity of the peaks was observed to be improved for synthesized MoS2 and MoS2 grown over CNFs (Figure 1b).
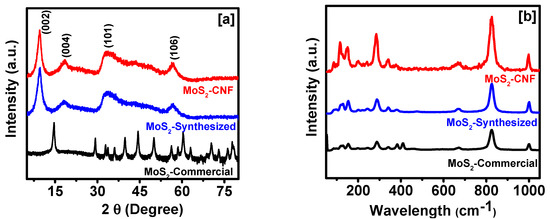
Figure 1.
(a) X-ray diffraction (XRD) patterns (log scale is for MoS2-commercial); and (b) Raman spectra of various MoS2 samples.
X-ray photoelectron spectroscopy was used to study the chemical nature of the prepared samples. The co-existence of 2H and 1T phase was confirmed by X-ray photoelectron spectroscopy (XPS) analysis. Figure 2 shows the XPS spectra of Mo 3d and S 2p regions of MoS2-commercial, MoS2-synthesized, and MoS2-CNFs. Two characteristics peaks around 229.4 and 232.5 eV were observed in the XPS spectrum of MoS2-commercial sample, indicating existence of the 2H phase of MoS2 [38]. However, the synthesized MoS2 and MoS2 grown over carbon nanofibers showed a shift of about 1 eV towards lower binding energy compared to that of MoS2-commercial sample, indicating presence of 1T phase in the synthesized MoS2 samples [38,39]. XPS spectra of S 2p region also showed the similar behavior, i.e., shift of the peaks of S 2p in MoS2-commercial sample towards lower binding energies for MoS2-synthesized samples, further confirming the existence of 1T phase in the synthesized samples [38]. Observation of new peak in the synthesized MoS2 samples indicated the presence of edge sulfur [40]. As evident from the XPS analysis, this facile synthesis results in a larger portion of the 1T phase of MoS2, without any additional exfoliation steps, which should be beneficial for its catalytic activities towards HER.
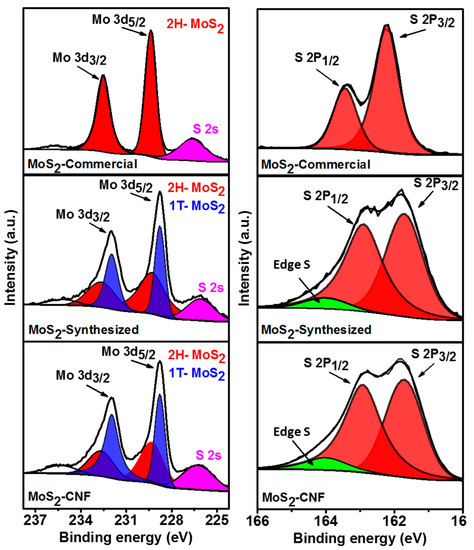
Figure 2.
X-ray photoelectron spectroscopy (XPS) spectra of Mo 3d and S 2p for MoS2-Commercial, MoS2-Synthesized, and MoS2-CNFs.
The scanning electron microscopy images of MoS2 grown over CNFs at various magnifications show well-defined flower-like morphology (Figure 3a–c). The diameter of the flowers is in the range of 200–600 nm with well-organized nano-sheets. Inherently, MoS2 grows in sheets due to its intrinsic lamellar structure and these sheets aggregate to form flower-like structures composed of several vertically aligned nano-sheets. It is further seen that the MoS2 grows very uniformly over CNFs. A high magnification scanning electron microscopy (SEM) image confirms that the flower-like structure is made of many nano-sheet which is vertical on the surface of CNFs. Vertical growth of MoS2 is very beneficial for hydrogen evolution reaction as it is reported that the catalytic activity of MoS2 is due to edge sulfur [23]. Growing MoS2 vertically in nano-sheet form with direct contact with conducting CNFs and highly porous nature will provide high electro-catalytic activity, reduced resistance (facilitated electron transfer from MoS2 to CNFs) and minimal structural damage for a long-term run. Figure 3d shows the EDS spectrum of MoS2 grown over CNFs confirming the presence of carbon, molybdenum, and sulfur with molybdenum to sulfur ratio of approximately 1 to 2.
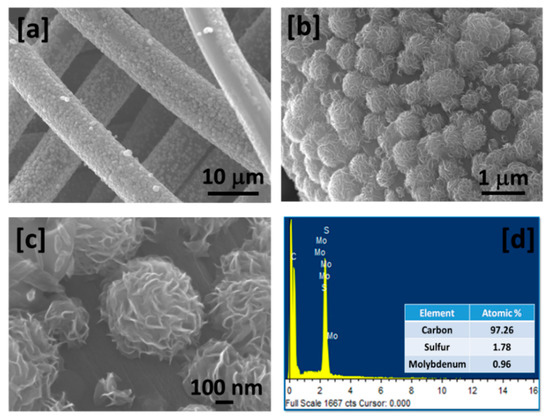
Figure 3.
(a–c) Scanning electron microscopy (SEM) images at various magnifications; and (d) Energy Dispersive x-ray Spectrum (EDS) of MoS2 grown over CNFs.
The electro-catalytic activity of the synthesized materials was performed by depositing the materials on glassy carbon electrode (details in experimental section). As a reference, we also carried out the measurements of commercial Pt/C (20% Pt) electrocatalyst. The IR corrected polarization curves of all the samples are shown in Figure 4a. As seen in Figure 4a, MoS2-commercial produces lower current densities (0.196 mA/cm2 at −0.3 V) compared to the MoS2-synthesized (19.1 mA/cm2 at −0.3 V) and MoS2 grown over CNFs (73.2 mA/cm2 at −0.3 V). The onset potential (defined at 1 mA/cm2) for MoS2-commercial, MoS2-synthesized, and MoS2 grown over CNFs was observed to be −435, −193 and −145 mV, respectively, vs. RHE. In addition, MoS2-synthesized and MoS2-CNFs offer an overpotential of 268 mV and 207 mV to generate a current density of 10 mA/cm2, respectively. The polarization study confirms that MoS2 grown over CNFs showed superior catalytic-activity for hydrogen evolution reaction compared to MoS2-commercial and MoS2-synthesized samples. MoS2 grown over CNFs and MoS2-synthesized produced about 374 and 98 times higher current density at −0.30 V (vs. RHE) compared with the MoS2-commercial sample, respectively. Youn et al. have reported an overpotential of 255 mV for MoS2 synthesized on carbon nanotube -graphene hybrid support at 10 mA/cm2 [41].
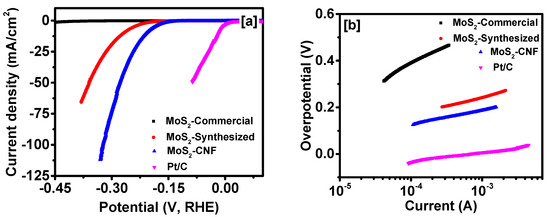
Figure 4.
(a) Polarization curves; and (b) Tafel slopes for various MoS2 samples.
The electrocatalytic activity was further analyzed using Tafel plots. Tafel plots of all the samples are given in Figure 4b. Commercial Pt/C electrocatalyst showed a Tafel slope of 39 mV/decade, whereas MoS2-commercial, MoS2-synthesized and MoS2 grown over CNFs showed a Tafel slope of 165, 79 and 60 mV/decade, respectively. The observed Tafel slope is among the best-reported slope for MoS2. For example, Tafel slopes of 87, 82 and 77 mV/decade were reported for defect-free MoS2 nanosheets, nanosized bulk MoS2 and MoS2 nanoparticles, respectively [42,43,44]. Lithium intercalated MoS2 showed a Tafel slope of 62 mV/decade [22]. Ren et al. have synthesized monolayer of MoS2 quantum dots and observed a Tafel slope of 59 mV/decade [45]. MoS2 grown on Toray paper, MWCNT, and CNT/graphene showed a Tafel slope of 120, 109 and 100 mV/decade, respectively [41,46,47]. Some important parameters for MoS2 based HER catalysts are given in Table S1 for comparison.
To further understand the origin of superior performance in the electro-catalytic activities of MoS2-synthesized and MoS2 grown over CNFs, cyclic voltammogram (CV) were performed. All the CV measurements were performed in a potential range where no faradic processes were observed [48]. The CV was recorded for all the samples at various scan rates to determine the electrochemical double layer capacitance (Cdl) which is directly proportional to the electrochemical effective surface area of the samples [21]. Figure 5a shows the CV curves of MoS2-commercial, MoS2-synthesized, and MoS2 grown over CNFs at a scan rate of 50 mV/s. As seen in Figure 5a, the area under the CV curves is highest for MoS2-CNFs and lowest for MoS2-commercial samples, indicating improvement in the electrochemical effective surface area. The effect of scan rate on the CV curves of all the samples is shown in Figure S1. The CV curves were observed to be similar in shape even at higher scan rates indicating high electrochemical rate performance. Since electrochemical double layer capacitance is directly proportional to effective electrochemical surface area, Cdl was calculated by plotting difference in positive and negative current densities at 0.05 V (vs. Ag/AgCl) versus the scan rates (Figure 5b). The Cdl values of the samples were half of the slopes of Δj vs. scan rate plots [21]. The Cdl values of the MoS2-commercial, MoS2-synthesized, and MoS2 grown over CNFs were calculated to be 0.99, 5.87 and 15.66 mF/cm2, respectively. The effective electrochemical surface area of the MoS2-CNFs is ~16 times higher than that of MoS2-commercial and six times higher than that of MoS2-synthesized samples. The highest effective electrochemical surface area and vertical structure of MoS2 sheets over CNFs provide the best HER activity among the studied materials.
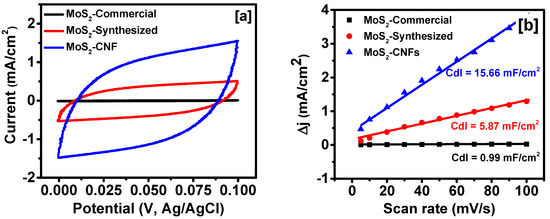
Figure 5.
(a) Cyclic voltammogram (CV) curves of all samples at 50 mV/s; and (b) scan rate dependence of the current densities of all the samples at 0.05 V vs. Ag/AgCl.
From the CV analysis, one can calculate turnover frequency (TOF) per site for these materials to estimate the intrinsic activity of the catalyst [49]. The TOF for MoS2-commercial, MoS2-synthesized and MoS2-CNFs at −200 mV was estimated to be 0.013, 0.025 and 0.54 s−1, respectively (Figure S2). The improved TOF for MoS2-CNFs could be due to better charge transfer from MoS2 to the electrode through conducting CNFs. Yan et al. have also observed an improvement in TOF by incorporating conducting CNTs in MoS2 [5]. The TOF varies from 0.9 to ~10−9 s−1 for platinum to transition metal catalysts, respectively [23].
In addition to the high effective electrochemical surface area and vertical structure of MoS2, the conductivity of the samples could play a major role in deciding the performance of these materials. Effective electron transfers and thus better electrical conductivity is required for improved HER performance. High electrical conductivity will facilitate electron transfer from MoS2 to the electrode. Electrochemical impedance spectroscopy was used to study this behavior. Nyquist plots of various MoS2 samples are shown in Figure 6a, with magnified Nyquist plots in low-frequency region. All the measured data were fitted using Randles circuit. The measured and simulated Nyquist plots for all the samples are shown in Figure S3 and the resulting fitting parameters are given in Table S2. The presence of semicircles in Nyquist plots indicates charge transfer process at the interface (electro-catalyst and electrolyte). Such charge transfer is associated with charge transfer resistance (Rct) and capacitance. In addition to Rct, such process also contains small series resistance (Rs) as shown in the inset of Figure 6a. Charge transfer resistance for MoS2-CNFs (19.6 Ω) was much lower than MoS2-synthesized (43.2 Ω) and MoS2-commercial (10.3 kΩ) samples. Lower charge transfer resistance would provide higher electro-catalytic activity due to reduced resistance at the interface. The capacitance estimated after fitting the data is proportional to the electroactive surface area of the catalyst [41]. The capacitance value for MoS2-commercial, MoS2-synthesized, and MoS2-CNFs was estimated to be 31.4, 806 and 5160 μF, respectively. Figure 6b shows the variation of impedance as a function of frequency for all the samples. As seen in Figure 6b, MoS2-CNF displayed the lowest impedance. The high electro-catalytic activity of MoS2-CNFs sample is due to lower charge transfer resistance and higher capacitance among other studied samples.

Figure 6.
(a) Nyquist plot; inset figures show the zoomed Nyquist plot near origin (left) and equivalent circuit (right), and (b) variation of Z vs. frequency for various MoS2 samples at 0.45 V vs. Ag/AgCl.
In addition to high HER catalytic activities, durable performance of a catalyst is required for practical applications. The long-term stability of the MoS2-CNFs was studied using chronoamperometry and cyclic voltammetry measurements. Figure 7 shows a variation of current density as a function of time. As seen in Figure 7, the performance of MoS2-CNFs is very stable up to about 18 hours of measurement. In fact, a small improvement in current density was observed during this study. The inset of Figure 7 clearly shows the evolution of hydrogen during measurement. Figure 8a shows the polarization curves of MoS2-CNFs before and after 9000 cycles. As seen, the current density of the catalyst improved after the cyclic performance. For example, at an overpotential of 200 mV, the current density increased from 7.9 to 14.7 mA/cm2, although there was no significant change in the Tafel slope (Figure 8b), suggesting that kinetics is almost same before and after the long-term testing. The stability performance of the MoS2-CNFs was further investigated using electrochemical impedance spectroscopy (EIS). The Nyquist plots and variation of impedance as a function of frequency before and after stability test is shown are Figure 8c,d. The charge transfer resistance and impedance of the MoS2-CNFs decreased significantly. Our study indicates that MoS2 grown over conducting substrate could be a potential candidate for electro-catalyst.

Figure 7.
Time-dependent current density curve for MoS2-CNFs (inset shows the evolution of hydrogen during chronoamperometric measurements).
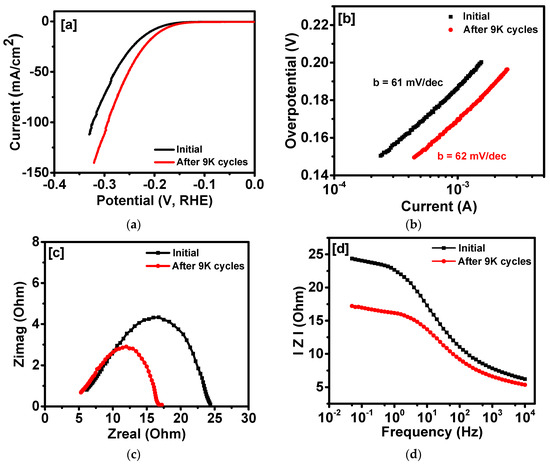
Figure 8.
(a) Polarization curves; (b) corresponding Tafel slopes; (c) Nyquist plots; and (d) variation of impedance vs. frequency for a MoS2-CNFs sample before and after stability test (Electrochemical impedance spectroscopy (EIS) data are measured at −0.45 V vs. Ag/AgCl).
3. Experimental Details
3.1. Synthesis
Carbon nanofibers were prepared using a simple electrospinning method followed by a heat treatment process. Briefly, a solution for electrospinning was prepared by dissolving 0.5 g of polyacrylonitrile (PAN) in 5 mL of N,N-dimethylformamide (DMF). The solution was transferred to 5 mL syringe with a stainless-steel needle (21 gauge). A high-voltage power supply (Gamma High Voltage Research, Product HV power Supply) was used as a high voltage source. The positive terminal of the source was connected to the stainless needle and negative terminal to collector plate made of aluminum foil. A voltage of 15 kV, volume feed rate of 0.6 mL/h and needle tip-to-aluminum foils distance of 12 cm was maintained during the electrospun process. The collected fibers were stabilized at 400 °C for 2 h (5 °C/min) and carbonized at 800 °C for 1 h (5 °C/min) under N2 atmosphere.
MoS2 decorated CNFs were prepared using a facile hydrothermal method. In a typical synthesis, 20 mg of CNFs were dispersed in 15 mL DMF using bath sonication for 1 h. In this, 75 mg of ammonium tetrathiomolybdate was added and dissolved using bath sonication for another 30 min. Then, the mixture was transferred into an autoclave and maintained at 210 °C for 18 h. The reactor was cooled down to room temperature and the black precipitate was filtered. The precipitate was washed subsequently with DI-water and anhydrous ethanol and finally dried at 60 °C for 24 h. MoS2 without CNFs was synthesized using the above method except having CNFs in the solution.
3.2. Characterization
MoS2-commercial, MoS2-synthesized, and MoS2 decorated on CNFs were structurally and electrochemically characterized. X-ray diffraction (XRD), Raman spectroscopy and scanning electron microscopy (SEM) were used for structural characterization. The XRD spectra of all the samples were taken using Shimadzu X-ray diffractometer. CuKα1 was used as a source to produce X-ray. Raman measurements were performed using an Ar+ laser at a wavelength of 514.5 nm as the excitation source (Model Innova 70, Coherent). X-ray photoelectron spectroscopy (XPS) was used to detect the nitrogen and oxygen doping and chemical bonding in carbon nanofibers. A Thermo Scientific Kα XPS system was used to record XPS spectra. The X-ray power of 75 W at 12 kV was used for the experiment with a spot size of 400 mm2. The XPS data acquisition was performed using the “Advantage v5.932” software provided with the instrument. Scanning electron microscope imaging was carried out using a JEOL 7000 FE SEM equipped with electron backscatter diffraction (EBSD), secondary electron (SE), backscattered electron (BE) and transmission electron (TE) detectors.
3.3. Electrochemical Measurements
All electrochemical measurements were performed using a VersaSTAT 4-500 electrochemical workstation (Princeton Applied Research, Oak Ridge, TN, USA). Three-electrode measurement method was used for the electrochemical testing. A graphite rod as a counter electrode, Ag/AgCl as a reference electrode and samples on glassy carbon electrode as a working electrode was used. Ten milligrams of sample was dispersed using bath sonicator in 1 mL water–ethanol (1:1 v/v) solution containing 25 μL Nafion solution (5 wt %). Ten microliters of the solution was drop cast over glassy carbon electrode (diameter 5 mm) and dried before electrochemical testing. HER performance of commercial platinum/carbon (20% Pt over graphitic carbon, from Sigma, St. Louis, MO, USA) was also performed under a similar condition for comparison. All the electrochemical measurements were conducted in an N2 saturated 0.5 M H2SO4 electrolyte at room temperature. Electrochemical impedance spectroscopy (EIS) studies were performed in a frequency range of 0.05 Hz to 10 kHz with an applied 10 mV of AC amplitude for all measurements. The electrochemical surface area was determined by cyclic voltammetry at different scan rates in the non-faradic region.
4. Conclusions
MoS2 and MoS2 decorated carbon nanofibers were synthesized using a facile method. The structural characterizations confirm the formation of MoS2 with flower-like structure, and the vertical attachment of MoS2 sheets on CNFs. The electrochemical characterizations revealed the excellent electroactivity of MoS2-CNFs, which is due to its high conductivity, high electroactive surface area, and the vertical structure of MoS2 exposing high density of edge sulfur to the electrolyte. They provided a high current density, low Tafel slope, low charge transfer resistance and high turnover frequency compared with commercial MoS2 as well as MoS2 synthesized using a similar method. MoS2-commercial, MoS2-synthesized, and MoS2 grown over CNFs showed a Tafel slope of 165, 79 and 60 mV per decade, respectively. MoS2-CNFs showed outstanding stability for the long run. Thus, MoS2-CNFs could be a promising cheap electrocatalyst for hydrogen evolution reaction due to its high electroactivity and durability.
Supplementary Materials
The following are available online at http://www.mdpi.com/2311-5629/3/4/33/s1.
Acknowledgments
Ram K. Gupta expresses his sincere acknowledgment to the Polymer Chemistry Program, Pittsburg State University and KINBRE (Grant # P20GM103418) for providing financial and research support.
Author Contributions
Ram K. Gupta conceived the project and designed the experiments. Ram K. Gupta also performed synthesis, some structural and electrochemical characterizations, interpreted and analyzed the data, and prepared the manuscript. C. Zhang, Z. Wang, S. Bhoyate, T. Morey and Brooks L. Neria performed some electrochemical characterizations. Venkata Vasiraju performed Raman characterizations. Soubantika Palchoudhury recorded the S.E.M. images. Felio Perez performed XPS measurements. All the authors reviewed and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, M.-R.; Liang, J.-X.; Zheng, Y.-R.; Xu, Y.-F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.-H. An Efficient Molybdenum Disulfide/cobalt Diselenide Hybrid Catalyst for Electrochemical Hydrogen Generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6673. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lei, Z.; Wu, P. Facile Preparation of 3D MoS2/MoSe2 Nanosheet–graphene Networks as Efficient Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2015, 3, 16337–16347. [Google Scholar] [CrossRef]
- Zhou, W.; Hou, D.; Sang, Y.; Yao, S.; Zhou, J.; Li, G.; Li, L.; Liu, H.; Chen, S. MoO2 Nanobelts@nitrogen Self-Doped MoS2 Nanosheets as Effective Electrocatalysts for Hydrogen Evolution Reaction. J. Mater. Chem. A 2014, 2, 11358–11364. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, X.; Liu, Z.; Wang, J.-Y.; Lee, J.-M.; Wang, X. Facile Synthesis of Low Crystalline MoS2 Nanosheet-Coated CNTs for Enhanced Hydrogen Evolution Reaction. Nanoscale 2013, 5, 7768–7771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Tang, C.; Liu, D.; Wang, J.; Asiri, A.M.; Sun, X. A Self-Standing Nanoporous MoP2 Nanosheet Array: An Advanced pH-Universal Catalytic Electrode for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2016, 4, 7169–7173. [Google Scholar] [CrossRef]
- Jiang, N.; Bogoev, L.; Popova, M.; Gul, S.; Yano, J.; Sun, Y. Electrodeposited Nickel-Sulfide Films as Competent Hydrogen Evolution Catalysts in Neutral Water. J. Mater. Chem. A 2014, 2, 19407–19414. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W. (David). Porous Molybdenum Carbide Nano-Octahedrons Synthesized via Confined Carburization in Metal-Organic Frameworks for Efficient Hydrogen Production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-F.; Wang, C.-H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly Active and Durable Nanostructured Molybdenum Carbide Electrocatalysts for Hydrogen Production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Pumera, M.; Sofer, Z.; Ambrosi, A. Layered Transition Metal Dichalcogenides for Electrochemical Energy Generation and Storage. J. Mater. Chem. A 2014, 2, 8981–8987. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Zhang, C.; Bhoyate, S.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Perez, F.; Gupta, B.K.; Gupta, R.K. Flower-Shaped Cobalt Oxide Nano-Structures as an Efficient, Flexible and Stable Electrocatalyst for the Oxygen Evolution Reaction. Mater. Chem. Front. 2017, 1, 1580–1584. [Google Scholar] [CrossRef]
- Adhikari, H.; Neupane, D.; Ranaweera, C.K.; Candler, J.; Gupta, R.K.; Sapkota, S.; Shen, X.; Mishra, S.R. Template-Free Synthesis of Hierarchical Mixed-Metal Cobaltites: Electrocapacitive and Theoretical Study. Electrochim. Acta 2017, 225, 514–524. [Google Scholar] [CrossRef]
- Bhoyate, S.; Mensah-Darkwa, K.; Kahol, P.K.; Gupta, R.K. Recent Development on Nanocomposites of Graphene for Supercapacitor Applications. Curr. Graphene Sci. 2017, 1, 26–43. [Google Scholar] [CrossRef]
- Aloqayli, S.; Ranaweera, C.K; Wang, Z.; Siam, K.; Kahol, P.K.; Tripathi, P.; Srivastava, O.N.; Gupta, B.K.; Mishra, S.R.; Perez, P.; et al. Nanostructured Cobalt Oxide and Cobalt Sulfide for Flexible, High Performance and Durable Supercapacitors. Energy Storage Mater. 2017, 8, 68–76. [Google Scholar] [CrossRef]
- Alkhalaf, S.; Ranaweera, C.K.; Kahol, P.K.; Siam, K.; Adhikari, H.; Mishra, S.R.; Perez, F.; Gupta, B.K.; Ramasamy, K.; Gupta, R.K. Electrochemical Energy Storage Performance of Electrospun CoMn2O4 Nanofibers. J. Alloy. Compd. 2017, 692, 59–66. [Google Scholar] [CrossRef]
- Segev-Bar, M.; Haick, H. Flexible Sensors Based on Nanoparticles. ACS Nano 2013, 7, 8366–8378. [Google Scholar] [CrossRef] [PubMed]
- Naponiello, G.; Venditti, I.; Zardetto, V.; Saccone, D.; Di Carlo, A.; Fratoddi, I.; Barolo, C.; Dini, D. Photoelectrochemical Characterization of Squaraine-Sensitized Nickel Oxide Cathodes Deposited via Screen-Printing for p-type Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2015, 356, 911–920. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kim, H.; Yoo, J.M.; Lee, K.S.; Kim, T.; Shin, H.; Sinha, A.K.; Kwon, S.G.; et al. Large-Scale Synthesis of Carbon-Shell-Coated FeP Nanoparticles for Robust Hydrogen Evolution Reaction Electrocatalyst. J. Am. Chem. Soc. 2017, 139, 6669–6674. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Jahromi, A.R.T; Ensafi, A.A. Ni-Co-Se Nanoparticles Modified Reduced Graphene Oxide Nanoflakes, An Advance Electrocatalyst for Highly Efficient Hydrogen Evolution Reaction. Electrochim. Acta 2016, 213, 423–431. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Hamb, D.J.; Thongtem, S.; Lee, J.S. Electrochemical Hydrogen Evolution Over MoO3 Nanowires Produced by Microwave-Assisted Hydrothermal Reaction. Electrochem. Commun. 2009, 11, 1740–1743. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Huang, Y.; Sun, J.; Zhu, Z.; Nielsen, R.J.; He, R.; Bao, J.; Goddard, W.A., III; Chen, S.; et al. Efficient Hydrogen Evolution by Ternary Molybdenum Sulfoselenide Particles on Self-Standing Porous Nickel Diselenide Foam. Nat. Commun. 2016, 7, 12765. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Kong, D.; Sun, J.; Hymel, T.M.; Cui, Y. Electrochemical Tuning of MoS2 Nanoparticles on Three-Dimensional Substrate for Efficient Hydrogen Evolution. ACS Nano 2014, 8, 4940–4947. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the Surface Structure of MoS2 to Preferentially Expose Active Edge Sites for Electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Fullon, R.; Yang, J.; de Carvalho Castro e Silva, C.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G.; et al. The Role of Electronic Coupling between Substrate and 2D MoS2 Nanosheets in Electrocatalytic Production of Hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, H.; Dong, S.; Liu, Y.; Tai Nai, C.; Suk Shin, H.; Young Jeong, H.; Liu, B.; Ping Loh, K. High Yield Exfoliation of Two-Dimensional Chalcogenides Using Sodium Naphthalenide. Nat. Commun. 2014, 5, 2995. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 Quantum Dot-Interspersed Exfoliated MoS2 Nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gao, Q.; Li, Z. Effects of Additives on Morphology and Hydrogen Evolution Activities of Nickel Films Prepared by Electrodepositing. Int. J. Electrochem. Sci. 2015, 10, 8823–8833. [Google Scholar]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Wu, J.; Tao, L.; Shen, A.; Huo, J.; Wang, S. Carbon-Coated MoS2 Nanosheets as Highly Efficient Electrocatalysts for the Hydrogen Evolution Reaction. Nanotechnology 2016, 27, 045402. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; Kim, S.O. Molybdenum sulfide/N-Doped CNT Forest Hybrid Catalysts for High-Performance Hydrogen Evolution Reaction. Nano Lett. 2014, 14, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Jiang, N.; Sun, Y. Morphology–activity Correlation in Hydrogen Evolution Catalyzed by Cobalt Sulfides. Inorg. Chem. Front. 2016, 3, 279–285. [Google Scholar] [CrossRef]
- Cummins, D.R.; Martinez, U.; Sherehiy, A.; Kappera, R.; Martinez-Garcia, A.; Schulze, R.K.; Jasinski, J.; Zhang, J.; Gupta, R.K.; Lou, J.; et al. Efficient Hydrogen Evolution in Transition Metal Dichalcogenides via a Simple One-Step Hydrazine Reaction. Nat. Commun. 2016, 7, 11857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Hu, W.H.; Li, X.; Dong, B.; Shang, X.; Han, G.Q.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. Facile One-Pot Synthesis of CoS2-MoS2/CNTs as Efficient Electrocatalyst for Hydrogen Evolution Reaction. Appl. Surf. Sci. 2016, 384, 51–57. [Google Scholar] [CrossRef]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro- and Nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-Abundant Metal Pyrites (FeS2, CoS2, NiS2, and Their Alloys) for Highly Efficient Hydrogen Evolution and Polysulfide Reduction Electrocatalysis. J. Phys. Chem. C 2014, 118, 21347–21356. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 Nanoparticles Grown on Carbon Fiber Paper: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T Phase MoS2 Nanosheets as Supercapacitor Electrode Materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Smith, R.J.; McEvoy, N.; Berner, N.C.; McCloskey, D.; Nerl, H.C.; O’Neill, A.; King, P.J.; Higgins, T.; Hanlon, D.; et al. Edge and Confinement Effects Allow in Situ Measurement of Size and Thickness of Liquid-Exfoliated Nanosheets. Nat. Commun. 2014, 5, 4576. [Google Scholar] [CrossRef] [PubMed]
- Youn, D.H.; Han, S.; Kim, J.Y.; Kim, J.Y.; Park, H.; Choi, S.H.; Lee, J.S. Highly Active and Stable Hydrogen Evolution Electrocatalysts Based on Molybdenum Compounds on Carbon Nanotube-Graphene Hybrid Support. ACS Nano 2014, 8, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gao, D.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Li, Y.; Li, M. Size-Dependent Enhancement of Electrocatalytic Oxygen-Reduction and Hydrogen-Evolution Performance of MoS2 Particles. Chem. A Eur. J. 2013, 19, 11939–11948. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, G.; Ding, F.; Li, X.; Zhen, S.; Xue, Y.; Yan, Y.; Liu, T.; Sun, K. A Bulky and Flexible Electrocatalyst for Efficient Hydrogen Evolution Based on the Growth of MoS2 Nanoparticles on Carbon Nanofiber Foam. J. Mater. Chem. A 2015, 3, 5041–5046. [Google Scholar] [CrossRef]
- Ren, X.; Pang, L.; Zhang, Y.; Ren, X.; Fan, H.; Liu, S. (Frank). One-Step Hydrothermal Synthesis of Monolayer MoS2 Quantum Dots for Highly Efficient Electrocatalytic Hydrogen Evolution. J. Mater. Chem. A 2015, 3, 10693–10697. [Google Scholar] [CrossRef]
- Bonde, J.; Moses, P.G.; Jaramillo, T.F.; Nørskov, J.K.; Chorkendorff, I. Hydrogen Evolution on Nano-Particulate Transition Metal Sulfides. Faraday Discuss. 2008, 140, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum Sulfides—efficient and Viable Materials for Electro—And Photoelectrocatalytic Hydrogen Evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. Component-Controllable WS2(1−x)Se2x Nanotubes for Efficient Hydrogen Evolution Reaction. ACS Nano 2014, 8, 8468–8476. [Google Scholar] [CrossRef] [PubMed]
- Benck, J.D.; Chen, Z.; Kuritzky, L.Y.; Forman, A.J.; Jaramillo, T.F. Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production: Insights into the Origin of Their Catalytic Activity. ACS Catal. 2012, 2, 1916–1923. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).