Candida albicans and non-albicans Isolates from Bloodstream Have Different Capacities to Induce Neutrophil Extracellular Traps
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
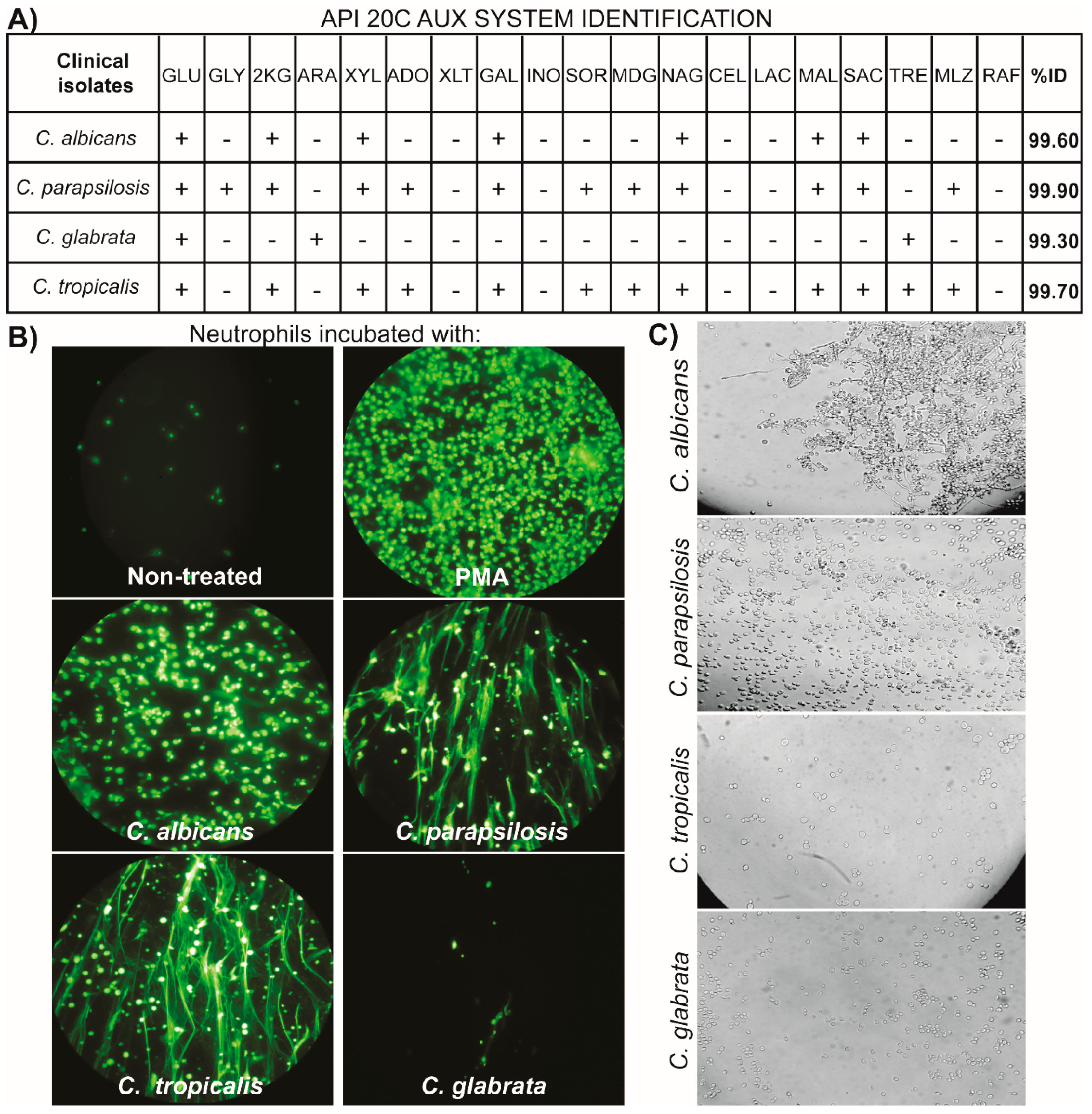
2.2. Candida spp. Strains
2.3. Candida spp. Growth Determination
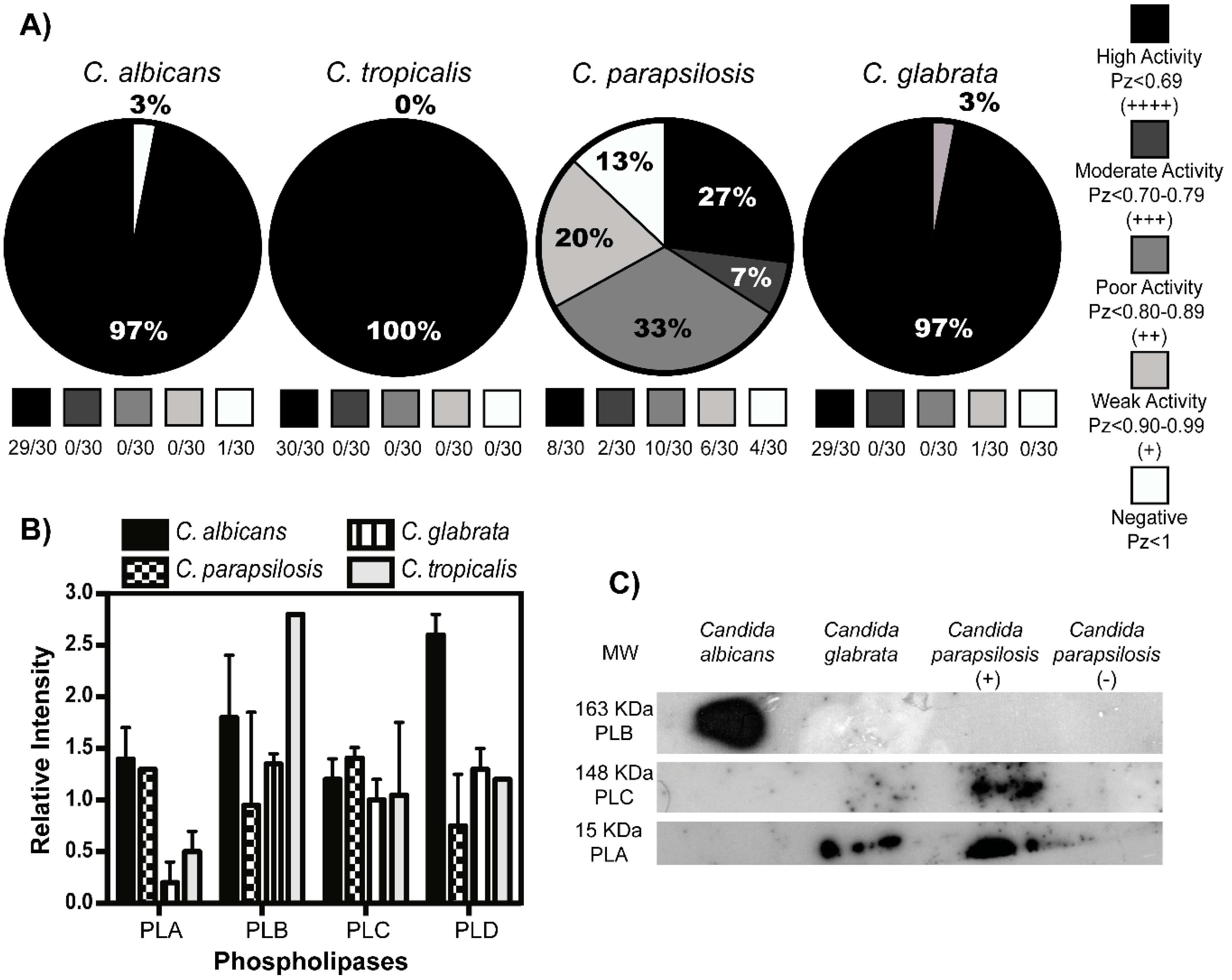
2.4. DNAse and Hemolysin Activity in Candida spp. Isolates
2.5. Production Kinetics and Determination of Activity of Fungal Phospholipases
2.6. Phospholipase Identification
2.7. Neutrophil Isolation
2.8. NETs Induction
2.9. NETs Quantification
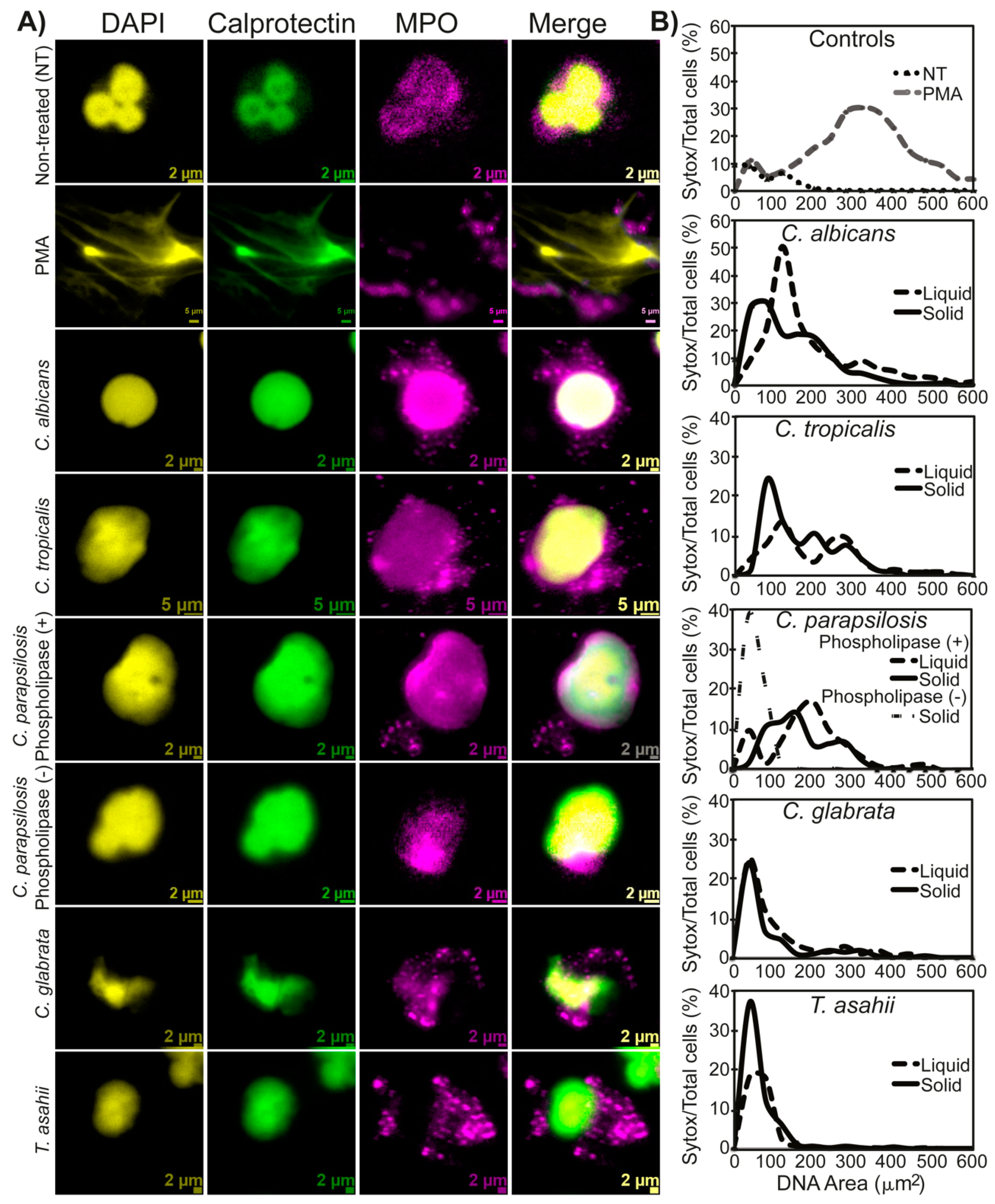
2.10. NETs Characterization
2.11. Statistical Analysis
3. Results
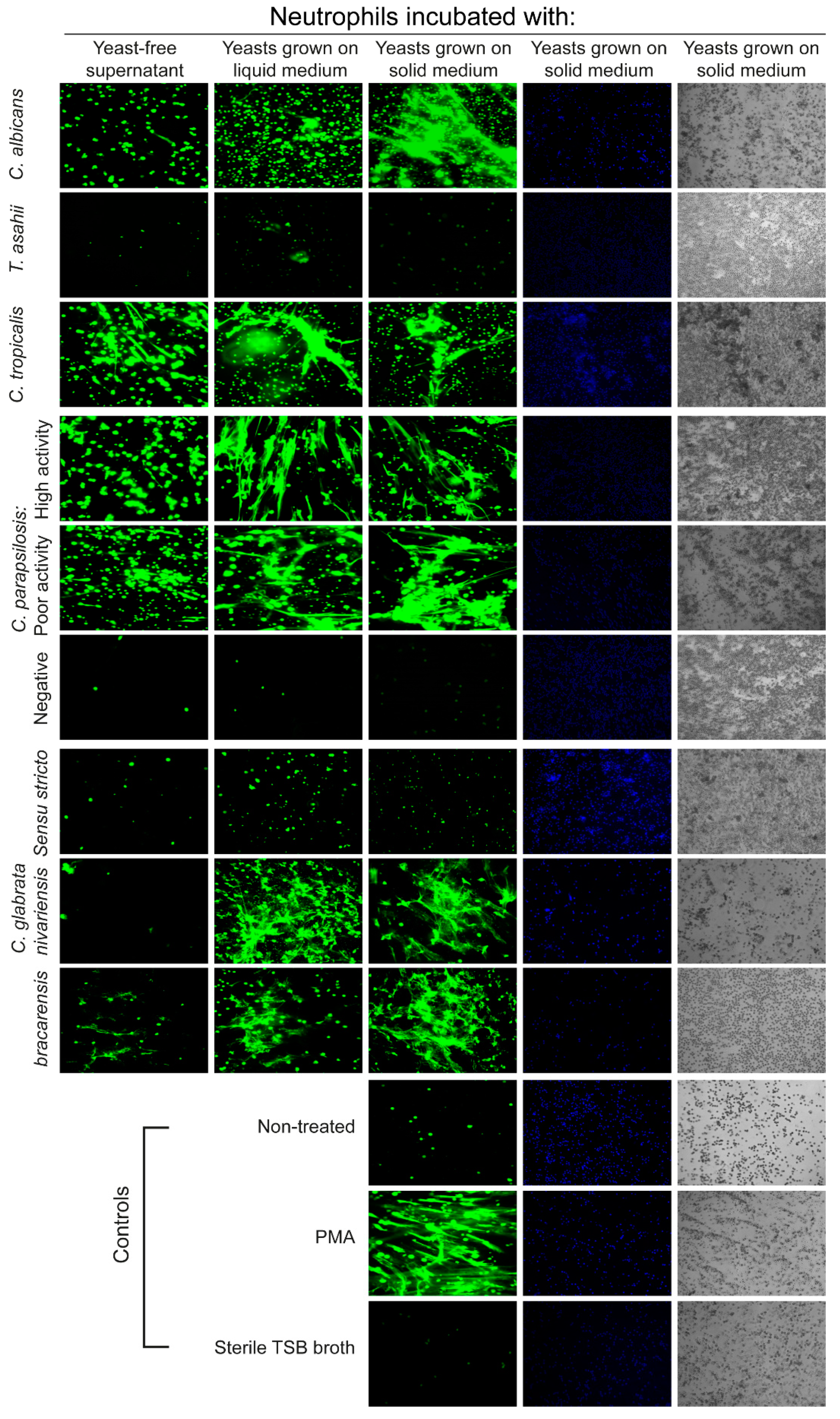
3.1. NETs Induction by Clinical Isolates of Candida
3.2. NETs Characterization
3.3. Fungal Phospholipases Production and NETs Induction
3.4. NETs Formation in Candida glabrata
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Ermert, D.; Zychlinsky, A.; Urban, C. Fungal and bacterial killing by neutrophils. Methods Mol. Biol. 2009, 470, 293–312. [Google Scholar] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Jenne, C.N.; Kubes, P. Nets tangle with hiv. Cell Host Microbe 2012, 12, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Bianchi, M.; Hakkim, A.; Brinkmann, V.; Siler, U.; Seger, R.A.; Zychlinsky, A.; Reichenbach, J. Restoration of net formation by gene therapy in cgd controls aspergillosis. Blood 2009, 114, 2619–2622. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against candida albicans. PLOS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Bachiega, T.F.; Dias-Melicio, L.A.; Fernandes, R.K.; de Almeida Balderramas, H.; Rodrigues, D.R.; Ximenes, V.F.; de Campos Soares, A.M. Participation of dectin-1 receptor on nets release against paracoccidioides brasiliensis: Role on extracellular killing. Immunobiology 2016, 221, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.S.; O’Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to candida albicans. J. Immunol. 2013, 190, 4136–4148. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.J.; Cabezas-Olcoz, J.; Kernien, J.F.; Wang, S.X.; Beebe, D.J.; Huttenlocher, A.; Ansari, H.; Nett, J.E. The extracellular matrix of candida albicans biofilms impairs formation of neutrophil extracellular traps. PLOS Pathog. 2016, 12, e1005884. [Google Scholar] [CrossRef] [PubMed]
- Zawrotniak, M.; Bochenska, O.; Karkowska-Kuleta, J.; Seweryn-Ozog, K.; Aoki, W.; Ueda, M.; Kozik, A.; Rapala-Kozik, M. Aspartic proteases and major cell wall components in candida albicans trigger the release of neutrophil extracellular traps. Front. Cell. Infect. Microbiol. 2017, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 2000, 13, 122–143. [Google Scholar] [CrossRef]
- Naglik, J.R.; Rodgers, C.A.; Shirlaw, P.J.; Dobbie, J.L.; Fernandes-Naglik, L.L.; Greenspan, D.; Agabian, N.; Challacombe, S.J. Differential expression of candida albicans secreted aspartyl proteinase and phospholipase b genes in humans correlates with active oral and vaginal infections. J. Infect. Dis. 2003, 188, 469–479. [Google Scholar] [CrossRef]
- Calderone, R.A.; Fonzi, W.A. Virulence factors of candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Banno, Y.; Yamada, T.; Nozawa, Y. Secreted phospholipases of the dimorphic fungus, candida albicans; separation of three enzymes and some biological properties. Sabouraudia 1985, 23, 47–54. [Google Scholar] [CrossRef]
- McLain, N.; Dolan, J.W. Phospholipase d activity is required for dimorphic transition in candida albicans. Microbiology 1997, 143, 3521–3526. [Google Scholar] [CrossRef]
- Takahashi, M.; Banno, Y.; Nozawa, Y. Secreted candida albicans phospholipases: Purification and characterization of two forms of lysophospholipase-transacylase. J. Med. Vet. Mycol. 1991, 29, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Rhee, S.G.; Billah, M.M.; Hannun, Y.A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991, 5, 2068–2077. [Google Scholar] [CrossRef]
- Lennartz, M.R. Phospholipases and phagocytosis: The role of phospholipid-derived second messengers in phagocytosis. Int. J. Biochem. Cell Biol. 1999, 31, 415–430. [Google Scholar] [CrossRef]
- Gray, R.D.; Lucas, C.D.; MacKellar, A.; Li, F.; Hiersemenzel, K.; Haslett, C.; Davidson, D.J.; Rossi, A.G. Activation of conventional protein kinase c (pkc) is critical in the generation of human neutrophil extracellular traps. J. Inflamm. 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for nadph oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012, 92, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Radic, M. Opposition between pkc isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front. Immunol. 2013, 4, 38. [Google Scholar] [CrossRef]
- Beheshti, F.; Smith, A.G.; Krause, G.W. Germ tube and chlamydospore formation by candida albicans on a new medium. J. Clin. Microbiol. 1975, 2, 345–348. [Google Scholar]
- Anil, S.; Samaranayake, L.P. Brief exposure to antimycotics reduces the extracellular phospholipase activity of candida albicans and candida tropicalis. Chemotherapy 2003, 49, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Kadir, T.; Gumru, B.; Uygun-Can, B. Phospholipase activity of candida albicans isolates from patients with denture stomatitis: The influence of chlorhexidine gluconate on phospholipase production. Arch. Oral Biol. 2007, 52, 691–696. [Google Scholar] [CrossRef]
- Montoya, A.M.; Sanchez Gonzalez, A.; Palma-Nicolas, J.P.; Gomez-Trevino, A.; Gonzalez, J.G.; Gonzalez, G.M. Genotyping, extracellular compounds, and antifungal susceptibility testing of trichosporon asahii isolated from mexican patients. Med. Mycol. 2015, 53, 505–511. [Google Scholar] [CrossRef]
- Gonzalez, A.S.; Bardoel, B.W.; Harbort, C.J.; Zychlinsky, A. Induction and quantification of neutrophil extracellular traps. Methods Mol. Biol. 2014, 1124, 307–318. [Google Scholar]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill candida albicans yeast and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.J.; Kernien, J.F.; Hoyer, A.R.; Nett, J.E. Mechanisms involved in the triggering of neutrophil extracellular traps (nets) by candida glabrata during planktonic and biofilm growth. Sci. Rep. 2017, 7, 13065. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Kruger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [PubMed]
- Pniewska, E.; Sokolowska, M.; Kuprys-Lipinska, I.; Przybek, M.; Kuna, P.; Pawliczak, R. The step further to understand the role of cytosolic phospholipase a2 alpha and group x secretory phospholipase a2 in allergic inflammation: Pilot study. BioMed Res. Int. 2014, 2014, 670814. [Google Scholar] [CrossRef]
- Stone, R.M.; Weber, B.L.; Spriggs, D.R.; Kufe, D.W. Phospholipase c activates protein kinase c and induces monocytic differentiation of hl-60 cells. Blood 1988, 72, 739–744. [Google Scholar]
- Charpentier, G.; Behue, N.; Fournier, F. Phospholipase c activates protein kinase c during induction of slow na current in xenopus oocytes. Pflugers Arch. 1995, 429, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the raf-mek-erk pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Sardi Jde, C.; Pitangui Nde, S.; Voltan, A.R.; Braz, J.D.; Machado, M.P.; Fusco Almeida, A.M.; Mendes Giannini, M.J. In vitro paracoccidioides brasiliensis biofilm and gene expression of adhesins and hydrolytic enzymes. Virulence 2015, 6, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Coco, B.; Sherry, L.; Bagg, J.; Lappin, D.F. In vitro candida albicans biofilm induced proteinase activity and sap8 expression correlates with in vivo denture stomatitis severity. Mycopathologia 2012, 174, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Nailis, H.; Kucharikova, S.; Ricicova, M.; van Dijck, P.; Deforce, D.; Nelis, H.; Coenye, T. Real-time pcr expression profiling of genes encoding potential virulence factors in candida albicans biofilms: Identification of model-dependent and -independent gene expression. BMC Microbiol. 2010, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Kernien, J.F.; Johnson, C.J.; Nett, J.E. Conserved inhibition of neutrophil extracellular trap release by clinical candida albicans biofilms. J. Fungi 2017, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, S.D.; Kook, M.; Lee, H.Y.; Ghim, J.; Choi, Y.; Zabel, B.A.; Ryu, S.H.; Bae, Y.S. Phospholipase d2 drives mortality in sepsis by inhibiting neutrophil extracellular trap formation and down-regulating cxcr2. J. Exp. Med. 2015, 212, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Curcic, S.; Holzer, M.; Frei, R.; Pasterk, L.; Schicho, R.; Heinemann, A.; Marsche, G. Neutrophil effector responses are suppressed by secretory phospholipase a2 modified hdl. Biochim. Biophys. Acta 2015, 1851, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.T. Role of phospholipases in fungal fitness, pathogenicity, and drug development-lessons from cryptococcus neoformans. Front. Microbiol. 2010, 1, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, S.; Sun, L.; Li, W.; Wei, Q.; Ashman, R.B.; Hu, Y. Different virulence of candida albicans is attributed to the ability of escape from neutrophil extracellular traps by secretion of dnase. Am. J. Transl. Res. 2017, 9, 50–62. [Google Scholar]
- Gupta, A.; Gupta, A.; Varma, A. Candida glabrata candidemia: An emerging threat in critically ill patients. Indian J. Crit. Care Med. 2015, 19, 151–154. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Garcia, L.; Jimenez-Valdes, R.J.; Hernandez-Bello, R.; Palma-Nicolas, J.; Gonzalez, G.M.; Sanchez-Gonzalez, A. Candida albicans and non-albicans Isolates from Bloodstream Have Different Capacities to Induce Neutrophil Extracellular Traps. J. Fungi 2019, 5, 28. https://doi.org/10.3390/jof5020028
Campos-Garcia L, Jimenez-Valdes RJ, Hernandez-Bello R, Palma-Nicolas J, Gonzalez GM, Sanchez-Gonzalez A. Candida albicans and non-albicans Isolates from Bloodstream Have Different Capacities to Induce Neutrophil Extracellular Traps. Journal of Fungi. 2019; 5(2):28. https://doi.org/10.3390/jof5020028
Chicago/Turabian StyleCampos-Garcia, Lizbeth, Rocio Jimena Jimenez-Valdes, Romel Hernandez-Bello, Jose Palma-Nicolas, Gloria Maria Gonzalez, and Alejandro Sanchez-Gonzalez. 2019. "Candida albicans and non-albicans Isolates from Bloodstream Have Different Capacities to Induce Neutrophil Extracellular Traps" Journal of Fungi 5, no. 2: 28. https://doi.org/10.3390/jof5020028
APA StyleCampos-Garcia, L., Jimenez-Valdes, R. J., Hernandez-Bello, R., Palma-Nicolas, J., Gonzalez, G. M., & Sanchez-Gonzalez, A. (2019). Candida albicans and non-albicans Isolates from Bloodstream Have Different Capacities to Induce Neutrophil Extracellular Traps. Journal of Fungi, 5(2), 28. https://doi.org/10.3390/jof5020028




