Abstract
Hypocrealean fungi are a well-documented group of fungi, with a wide range of ecological roles that include saprobic, parasitic, and endophytic forms, capable of thriving in diverse environments, both terrestrial and marine. Members in this group are abundant and widely distributed in marine environments around the world. However, the species diversity and distribution of this fungal group in Chinese seas is rarely reported. This study introduces five new species, namely Fusarium flavoides M.M. Wang & W. Li, Gliomastix fasciculata M.M. Wang & W. Li, Marquandomyces ulvae M.M. Wang & W. Li, Stephanonectria arenicola M.M. Wang & W. Li, and Verruciconidia oligospora M.M. Wang & W. Li, based on morphological characteristics and LSU-ITS-tef1-rpb2 phylogenetics. These new species were discovered from marine algae (Ulva spinulosa) and sediments (mud and sand). A detailed comparison of these new species and their close relatives is also presented.
1. Introduction
The order Hypocreales Lindau was established in 1897, and recently this fungal group is now considered to be one of the most substantial orders of the class Sordariomycetes [1], comprising approximately 320 genera in 15 families [2]. Historically, the classification of this order has undergone numerous revisions, with a modified characterization of the Hypocreales being proposed in 1970 [3]. Subsequent to this, a considerable number of species were assigned to this order, and an updated taxonomic system for this group of fungi was conducted. Morphologically, members of this order, also known as hypocrealean fungi, are frequently characterized by colorful sexual structures such as white, light orange, and black, and the typically asexual structures that were summarized as Acremonium-like, Cylindrocarpon-like, Fusarium-like, Paecilomyces-like, and so on [4,5,6,7].
Hypocrealean fungi exhibit a broad spectrum of host/habitat and ecological functions, encompassing saprophytes, parasites, and endophytes in soil and plants in terrestrial habitats [7,8]. Furthermore, these fungi have been identified in a wide range of marine environments around the world. For instance, studies using culturable methods have revealed that species in genera Acremonium Link and Fusarium Link have a worldwide distribution in sediments, corals, sponges, sea fans, and seaweeds [9,10,11,12,13]. However, the distribution of culturable hypocrealean fungi in Chinese seas has been sporadically reported, with the exception of Acremonium egyptiacum (J.F.H. Beyma) W. Gams, Emericellopsis maritima Beliakova, and Neocosmospora solani (Mart.) L. Lombard & Crous from marine carbs in Taiwan [14], Fusarium aseptatum Meng Li & L. Cai from mangrove sediment in Guangdong [15], and several species including A. egyptiacum and Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones & Samson from marine algae in Shandong [16]. Considering the high relative abundances of these fungi revealed by unculturable methods in the Yellow Sea of China [17] and their ecological roles (endophyte, parasite, pathogen, and saprotroph) identified by FungalTraits [18], we believe that the marine environments in China should be inhabited by diverse hypocrealean fungi that probably perform important ecological functions.
During the past decade, our group has conducted a long-term survey of fungal diversity in Chinese seas (e.g., [19,20,21,22]) and accumulated a number of hypocrealean strains from various marine samples such as algae, seawater, and sediment. The present study introduced five new species from marine algae and sediments in the intertidal zones, based on their morphology and culture characteristics. The taxonomic placements of these new species were confirmed by multi-locus phylogenetic analyses of LSU, ITS, tef1, and rpb2. Comparisons were made between the new species and their close relatives.
2. Materials and Methods
2.1. Sample Collection and Fungal Isolation
Algae samples were collected from intertidal zones in the Guangdong province (Shenzhen), while sediment samples (2–20 cm depth) were obtained from the Liaoning province (Dalian and Huludao) and the Shandong province (Qingdao, Weihai and Yantai). The collection of samples was conducted in accordance with the protocol described in Wang et al. [22]. The isolation of fungal strains from the algae and sediment samples was undertaken employing the protocol outlined by Wang et al. [22]. In summary, samples of algae and sediment were collected and stored in sterile bags in a 4 °C freezer and transferred to the laboratory promptly. The algae samples were then cut into 0.5 × (0.2–0.5) cm segments, surface-sterilised, and placed onto isolation media (tissue-isolation method). Segments were homogenised, diluted to a series of concentrations (10−1, 10−2, 10−3, and 10−4), and spread onto isolation media (dilution plate method). Sediment samples were directly dispersed onto isolated medium plates (direct isolation method), and suspended, cultivated by shaking, diluted (10−1, 10−2, 10−3, and 10−4), and plated onto isolation media (dilution plate method) [22]. Fifty types of media that are consistent with the formula of Wang et al. [22], including Martin medium (MM), 1/10 potato dextrose agar (1/10 PDA), 1/5 malt extract agar (1/5MEA), corn meal agar (CMA), and yeast extract peptone glucose agar (YPG), were used as isolation media in this study. Fungal isolates were picked up with a sterilised needle and transferred onto potato dextrose agar (PDA) plates when individual colonies were observed. All isolates examined in this study were deposited in Wei Li’s personal culture collection (WL). Type specimens of new species were deposited in the Fungarium of the Institute of Microbiology (HMAS; https://nmdc.cn/fungarium/, accessed on 15 January 2025), with the ex-type living cultures in the China General Microbiological Culture Collection Center (CGMCC; https://www.cgmcc.net/, accessed on 15 January 2025).
2.2. Morphological Observation
Considering that fungi often exhibit distinct colony morphological characteristics on different culture media, this study employed three different culture media to obtain a more comprehensive understanding of the cultural characteristics of the studied isolates. The isolates studied were incubated in the dark on PDA and oatmeal agar (OA) at 25 °C in the dark for seven days. Seven days later, culture characteristics including colony morphology, pigmentation, and odour were observed. Colours were assessed according to the colour charts of Kornerup and Wanscher [23]. Micromorphological characteristics were examined and photo-documented using water as a mounting medium under an Olympus BX53 microscope with differential interference contrast (DIC) optics [22]. For each species, 30 conidiophores, 30 conidiogenous cells, and 50 conidia were mounted and measured randomly, respectively [22].
2.3. DNA Extraction, PCR Amplification and Sequencing
Genomic DNA was extracted from fungal mycelia grown on PDA after 7 days, using a modified CTAB protocol as described in Guo et al. [24]. Briefly, the process of fungal DNA extraction involved the grinding of fresh mycelia with CTAB buffer [24] and quartz sand, followed by incubation at 60 °C for 30 min. Subsequently, 500 μL of a solution of phenol–chloroform (1:1) was added, mixed thoroughly, and subjected to centrifugation (11,900× g, 15 min). The aqueous phase was transferred, mixed with chloroform–isoamyl alcohol (24:1) and centrifuged again. The DNA was precipitated using 50 µL of 5M KOAc and 400 µL of isopropanol, mixed gently, centrifuged (9200× g, 2 min), washed twice with 70% ethanol, air-dried, and finally resuspended in 100 µL of TE buffer (10 mM Tris-HCl, 1 mM EDTA).
Four loci, including partial large subunit ribosomal RNA gene (LSU), 5.8S nuclear ribosomal RNA gene with the two flanking internal transcribed spacer (ITS) regions, partial translation elongation factor gene (tef1) and partial DNA-directed RNA polymerase II second largest subunit gene (rpb2), were amplified with the primer pairs ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′)/ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [25], LR0R (5′-ACCCGCTGAACTTAAGC-3′)/LR5 (5′-TCCTGAGGGAAACTTCG-3′) [26,27], EF-983F (5′-GCYCCYGGHCAYCGTGAYTTYAT-3′)/EF2218R (5′-ATGACACCRACRGCRACRGTYTG-3′) [28] and RPB2-5F2 (5′-GGGGWGAYCAGAAGAAGGC-3′)/RPB2-7cR (5′-CCCATRGCTTGYTTRCCCAT-3′) [29,30], respectively. The PCR amplifications were performed in a total volume of 30 μL containing 15 μL of 2 × Rapid Taq Plus Master Mix (Dye Plus) (Vazyme, Nanjing, China), 1 μL of each primer (0.1 μM), 1 μL of genomic DNA (1–10 ng), and 12 μL of ddH2O. The PCR amplifications of LSU, ITS, and tef1 were set as follows: an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 48 °C (LSU), 52 °C (ITS), and 56 °C (tef1), respectively, for 1 min, and extension at 72 °C for 1 min, and a final extension step at 72 °C for 10 min. The PCR amplification for rpb2 was set as described by Wang et al. [31]. PCR products for four loci were purified and sequenced in both directions using an ABI DNA analyzer by SinoGenoMax company (Beijing, China). Consensus sequences were obtained using SeqMan of the Lasergene software package v. 14.1 (DNAstar, Madison, WI, USA).
2.4. Evolutionary Lineage Modeling
The sequences of the hypocrealean strains examined in this study and the reference strains are listed in Table 1. For each locus, sequences were aligned using ClustalX 2.1 [32], and the alignments were manually adjusted where necessary. The best-fitting nucleotide-substitution modes according to the Akaike Information Criterion (AIC) were selected using mrmodeltest2 [33]. Alignments derived from this study were deposited in TreeBASE (submission ID 31992), and taxonomic novelties were deposited in Fungal Names (https://nmdc.cn/fungalnames/, accessed on 15 January 2025).

Table 1.
Strains examined in this study, with information on source, origin and GenBank accessions of the sequences.
Phylogenetic analyses of the combined dataset were performed using Bayesian inference (BI) and maximum-likelihood (ML) methods. The BI analyses were conducted using MrBayes v. 3.2.1 following the protocol of Wang et al. [31], with optimization of each locus treated as a partition in combined analyses, based on the Markov Chain Monte Carlo (MCMC) approach [34]. All characters were equally weighted, and gaps were treated as missing data. The stationarity of the analyses was determined by examining the standard deviation of split frequencies (<0.01) and –ln likelihood plots in AWTY [35]. The ML analyses were conducted using PhyML v. 3.0 [36], with 1000 bootstrap replicates. The general time-reversible model was applied with an invariable gamma-distribution rate variation (GTR+I+G).
3. Results
3.1. Phylogenetic Analyses
Analyses of the hypocrealean fungal phylogeny were conducted by using a combined LSU (875 bp), ITS (634 bp), tef1 (849 bp) and rpb2 (908 bp) dataset. For the BI analysis, the GTR+I+G model was selected for the LSU, ITS and rpb2 loci, while the GTR+G model was used for the tef1 locus. The phylogeny showed that our five new species were clustered into five genera in three families (Bionectriaceae Samuels & Rossman, Clavicipitaceae (Lindau) Earle, and Nectriaceae Tul. & C. Tul.), namely Fusarium flavoides, Gliomastix fasciculata, Marquandomyces ulvae, Stephanonectria arenicola, and Verruciconidia oligospora (Figure 1). In order to provide a more accurate illustration of the genetic relationships between the five new species and other species within the same genus, phylogenetic analyses were conducted for each genus based on multi-locus datasets (Supplementary Figure S1).
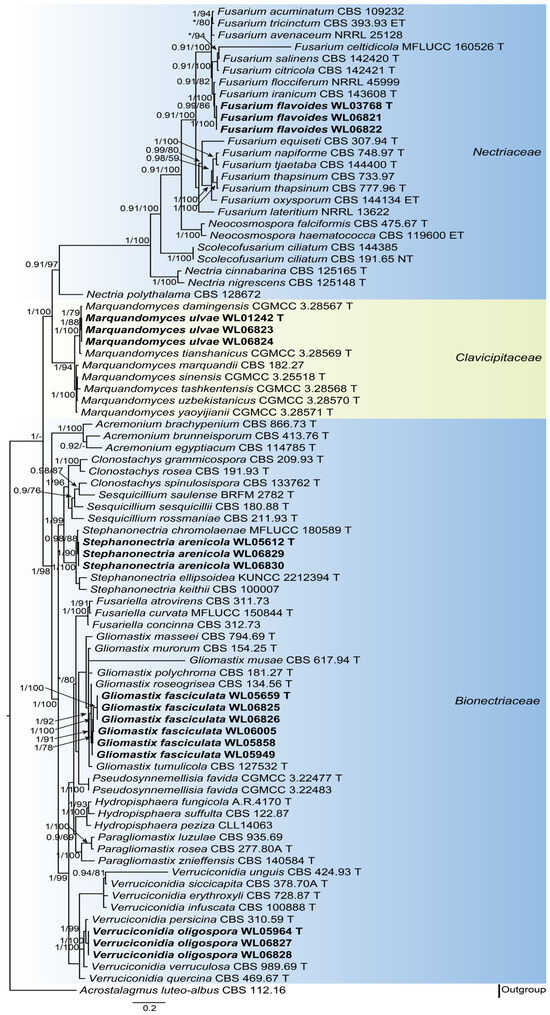
Figure 1.
Fifty percent majority rule consensus tree from a Bayesian analysis based on a four-locus combined dataset (LSU-ITS-tef1-rpb2) showing the phylogenetic relationships of these hypocrealean fungi. The Bayesian posterior probabilities (PP > 0.9) and PhyML bootstrap support values (BS > 50%) are displayed at the nodes (PP/BS). Nodes with PP value below 0.9 are displayed as “*”. The tree was rooted to Acrostalagmus luteo-albus CBS 112.16. Ex-type cultures are indicated with “T”, epi-type with “ET” and neo-type with “NT”. New species introduced in this paper are marked in bold.
3.2. Taxonomy of the Novel Species
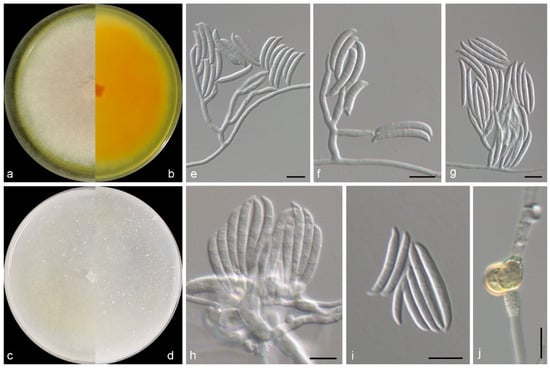
Figure 2.
Morphological characters of Fusarium flavoides (from ex-type WL03768). (a–d) Colonies on PDA and OA after 7 d; (e–h) conidiophores and conidiogenous cells; (i) conidia; (j) chlamydospores. Bars: (e–j) = 10 μm.
Classification: Ascomycota, Sordariomycetes, Hypocreales, Nectriaceae, Fusarium
FungalNames: FN 572360
Etymology: Epithet refers to the cultural characteristics that yellow pigmentation produced on PDA, “flav-” (yellow) + “-oides” (similar to) = flavoides.
Typus: China, Shandong Province, Yantai city (120.70° E, 37.81° N), from intertidal sediment of a sand beach, June 2014, X.M. Bian (HMAS 353470, holotype designated here, dried culture on PDA; culture ex-type CGMCC 3.28711 = WL03768).
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 2–3 μm wide. Conidiophores mostly aggregated, erect, straight, arising directly from aerial or substratal mycelium, branched, 30–60 μm long, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides solitary, lateral, terminal, subcylindrical, hyaline, thick-, smooth-walled, 7.0–20.5 × 2.5–4.5 μm, commonly with conspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Macroconidia 2–3-septate, falciform, with a pointed to blunt apical cell and a poorly developed to well-developed foot-shaped basal cell, curved, hyaline, thin-, smooth-walled, eguttulate, 20.0–36.5 × 2.5–5.7 μm; microconidia not observed. Chlamydospores 0–1-septate, subglobose, hyaline and smooth in young and becoming golden and verrucous with maturity, 8.3–9.7 × 6.2–9 μm. Sexual morph not observed.
Culture characteristics—Colonies on OA at 25 °C after 7 days reaching 40–45 diam, flat, villose, sparse, colony margin erose, surface white in the centre, yellowish white (2A2) at the margin; reverse white in the centre, yellowish white (2A2) at the margin. Colonies on PDA at 25 °C after 7 days reaching 60–65 mm diam, flat, felty, colony margin filamentous, surface reddish white (9A2) in the centre, white at the margin; reverse light orange (5A5) in the centre, white at the margin, with light yellow (3A4) pigmentation.
Other examined isolates: China, Shandong Province, Yantai city (120.70° E, 37.81° N), from intertidal sediment of a sand beach, June 2014, X.M. Bian (WL06821); ibid., WL06822.
Notes: In this study, a new Fusarium species, namely F. flavoides from intertidal sediment, was introduced. Phylogenetically, this species was closely related to F. flocciferum Corda and F. iranicum Torbati, Arzanlou & Sand.-Den., but differs by 71 bp and 81 bp in the LSU-ITS-tef1-rpb2 dataset, respectively (Figure 1). Morphologically, the above species could be distinguished in the septation, shape and size of macroconidia (2–3-septate, falciform, with a pointed to blunt apical cell and a foot-shaped basal cell, 20–36.5 × 2.5–5.7 μm in F. flavoides vs. 3–5-septate, falcate, with tapering apical cells and distinctly pedicellate basal cells, 16–60 × 3–4 μm in F. flocciferum, and 0–5-septate, falcate, 15.5−20 × 3–4.5 μm in F. iranicum) [37,38,39,40]. These species differ in locations and hosts/habitats: F. flavoides was discovered from intertidal sediment in China, while F. flocciferum and F. iranicum was mostly from soil, plants, and other fungi in Germany, Iran, Netherlands, and other countries [8,41,42]. The new species is similar to F. thapsinum Klittich, J.F. Leslie, P.E. Nelson & Marasas in the production of yellowish pigment, but differs in the absence of microconidia (present in F. thapsinum) and septation and size of macroconidia (3–8-septate, 24–64 × 3–4 μm in F. thapsinum) [43]. Moreover, F. flavoides is far from F. thapsinum in the LSU-ITS-tef1-rpb2 phylogeny (Figure 1).
Gliomastix fasciculata M.M. Wang & W. Li sp. nov., Figure 3.
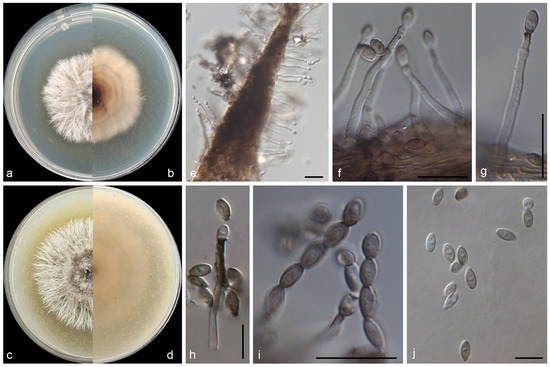
Figure 3.
Morphological characters of Gliomastix fasciculata (from ex-type WL05659). (a–d) Colonies on PDA and OA after 7 d; (e–h) conidiophores and conidiogenous cells; (i) conidia in chains; (j) conidia. Bars: (e–j) = 10 μm.
Classification: Ascomycota, Sordariomycetes, Hypocreales, Bionectriaceae, Gliomastix
FungalNames: FN 572361
Etymology: Epithet refers to the fascicular conidiophores of this species, “fasciculata” in Latin means “fascicular”.
Typus: China, Shandong Province, Weihai city (122.18° E, 37.50° N), from intertidal sediment of a sand beach, November 2020, M.M. Wang and Y. Zheng (HMAS 353471, holotype designated here, dried culture on PDA; culture ex-type CGMCC 3.28712 = WL05659).
Mycelium consisting of septate, hyaline, smooth-, thin-walled hyphae, up to 2.5 μm wide when young, becoming thick-walled, up to 3.5 μm wide in old cultures. Conidiophores solitary or aggregated, erect, straight or slightly flexuose at base, arising directly from superficial hyphae, unbranched, 1-septate at base, hyaline, becoming dark brown at the top with age, slightly rough-walled. Phialides lateral, terminal, subulate, 20.0–31.0 × 1.5–3.5 μm wide at base, hyaline at first, dark brown in old cultures, thick-, slightly rough-walled, with conspicuous periclinal thickening and dark brown flared collarettes; polyphialides not observed. Conidia aseptate, ovoid to ellipsoid with truncate, hyaline, thin-walled at beginning, brown to olivaceous brown with age, thick-, smooth-walled in old cultures, 2.8–4.7 × 2.0–3.1 μm, guttulate, arranged in long dry chains. Chlamydospores and sexual morph not observed.
Culture characteristics—Colonies on OA at 25 °C after 7 days reaching 40–45 diam, filamentous, crateriform, dusty, colony margin filamentous, surface olive grey (3D1) in the centre, white at the margin; reverse brownish orange (6C4) in the centre, white at the margin, with greyish brown (6D3) pigmentation. Colonies on PDA at 25 °C after 7 days reaching 40–45 mm diam, filamentous, crateriform, dusty, colony margin filamentous, surface olive grey (3D1) in the centre, white at the margin; reverse cognac (6E7) in the centre, white at the margin.
Other examined isolates: China, Shandong Province, Weihai city (122.18° E, 37.50° N), from intertidal sediment of a sand beach, November 2020, M.M. Wang and Y. Zheng (WL06825); ibid., WL06826; ibid., Qingdao city (120.47° E, 36.09° N), from intertidal sediment of a sand beach, March 2021, Y. Zheng, Z.H. Pan and Y.R. Ma (WL05858); Liaoning Province, Dalian city, from intertidal sediment of sand beach, May 2021, Y. Zheng and Z.H. Pan (WL05949); ibid., Huludao city (120.80° E, 40.49° N), from intertidal sediment of a sand beach, Y. Zheng and Z.H. Pan (WL06005).
Notes: Phylogenetically, the newly introduced species was closely related to G. polychroma (J.F.H. Beyma) Matsush., G. roseogrisea (S.B. Saksena) Summerb., and G. tumulicola (Kiyuna, An, Kigawa & Sugiy.) Summerb. (Figure 1), but differs by 176 bp, 92 bp and 148 bp in the LSU-ITS-tef1-rpb2 dataset, respectively. Morphologically, the four species distinct in the shape, size and pigmentation of conidia (ovoid to ellipsoid, 2.8–4.7 × 2–3.1 μm, brown to olivaceous brown with age in G. fasciculata, vs. ellipsoid, oviform, 3–7.5 × 2–4 μm in G. polychroma, mostly tear-shaped, 4.9–6.5 × 2.6–4.1 μm, greyish-black in G. roseogrisea, 4–5 × 2–3 μm, blackish brown in G. tumulicola) [44,45,46]. The above species differ in the hosts/habitats and geographic distribution in that G. fasciculata was retrieved from intertidal sediments in China, while G. polychroma, G. roseogrisea, and G. tumulicola from Hevea brasiliensis, painting, and soil in India, Indonesia, and Japan [44,45,46].
Marquandomyces ulvae M.M. Wang & W. Li sp. nov., Figure 4.
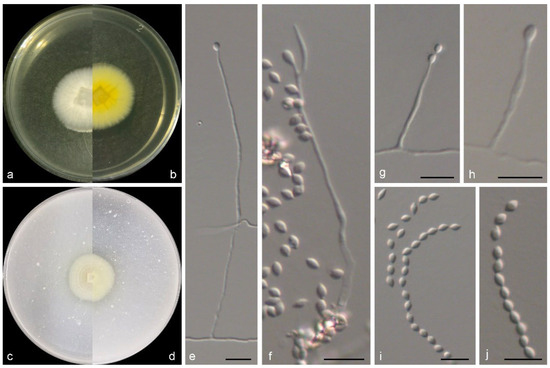
Figure 4.
Morphological characters of Marquandomyces ulvae (from ex-type WL01242). (a–d) Colonies on PDA and OA after 7 d; (e–h) conidiophores and conidiogenous cells; (i,j) conidia in chains. Bars: (e–j) = 10 μm.
Classification: Ascomycota, Sordariomycetes, Hypocreales, Clavicipitaceae, Marquandomyces
FungalNames: FN 572362
Etymology: Epithet refers to the host genus of the type specimen, Ulva.
Typus: China, Guangdong Province, Shenzhen city, from marine algae Ulva spinulosa, May 2014, M.M. Wang (HMAS 353472, holotype designated here, dried culture on PDA; culture ex-type CGMCC 3.28716 = WL01242).
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 3 μm wide. Conidiophores solitary, hyaline, erect, arising from superficial hyphae, unbranched or poorly branched, 15–100 μm long, 2–2.5 μm wide at base. Phialides terminal or lateral, cylindrical or subulate, hyaline, thin-, smooth-walled, 15.0–35.0 × 2.0–2.5 μm wide at base; polyphialides not observed. Conidia aseptate, oval with sharp ends, hyaline, thin-, smooth-walled, 2.5–5 × 2–3.3 μm, arranged in dry, long chains. Chlamydospores and sexual morph not observed.
Culture characteristics—Colonies on OA at 25 °C after 7 days reaching 10–12 diam, circular, flat, colony margin erose, surface white; reverse white. Colonies on PDA at 25 °C after 7 days reaching 10–15 mm diam, circular, flat, colony margin erose, surface white; reverse light yellow (2A5) in the centre, white at the margin, with pale yellow (2A3) pigmentation.
Other examined isolates: China, Guangdong Province, Shenzhen city, from marine algae Ulva spinulosa, May 2014, M.M. Wang (WL06823); ibid., WL06824.
Notes: The genus Marquandomyces Samson, Houbraken & Luangsa-ard was established in 2020, with M. marquandii (Massee) Samson, Houbraken & Luangsa-ard as the type species [47]. In this study, a new species of this genus was introduced, namely M. ulvae. Phylogenetically, this species was closed related to M. damingensis X.C. Wang, L.Y. Peng & W.Y. Zhuang and M. tianshanicus X.C. Wang, L.Y. Peng, Gafforov & W.Y. Zhuang (Figure 1), but differs in 57 bp and 63 bp in the LSU-ITS-tef1 dataset, respectively. Morphologically, M. ulvae was distinguished from the latter two species in the conidiogenous structures, and shape and size of conidia (conidiophores often reduced to phialides, conidia oval, 2.5–5 × 2–3.3 μm in M. ulvae, vs. conidiophores terverticillate or biverticillate, conidia ellipsoidal to fusiform, 3.0–4.0 × 2.5–3.0 µm in M. damingensis, and conidiophores terverticillate, conidia ellipsoidal to fusiform, 3.0–4.0 × 2.0–3.0 µm in M. tianshanicus) [48]. Otherwise, M. ulvae discovers from marine algae in China, differs from M. damingensis and M. tianshanicus from soil in China and Uzbekistan, respectively [48].
Stephanonectria arenicola M.M. Wang & W. Li sp. nov., Figure 5.
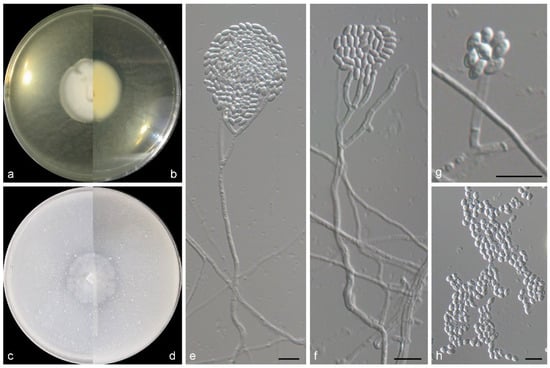
Figure 5.
Morphological characters of Stephanonectria arenicola (from ex-type WL05612). (a–d) Colonies on PDA and OA after 7 d; (e–g) conidiophores and conidiogenous cells; (h) conidia in chains. Bars: (e–h) = 10 μm.
Classification: Ascomycota, Sordariomycetes, Hypocreales, Bionectriaceae, Stephanonectria
FungalNames: FN 572363
Etymology: Epithet refers to the habitat of the type specimen, sand.
Typus: China, Shandong Province, Weihai city (122.19° E, 37.50° N), from intertidal sediment of a sand beach, November 2020, M.M. Wang and Y. Zheng (HMAS 353473, holotype designated here, dried culture on PDA; culture ex-type CGMCC 3.28726 = WL05612).
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 3 μm wide. Conidiophores solitary or aggregate, (sub-)erect, arising from submerged and superficial hyphae, unbranched or branched, bearing 1–3 phialides per node, up to ca. 50 μm long, 2–3.5 μm wide at base, with 1–3 septa, hyaline, rough-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, hyaline, thick-, smooth or slightly rough-walled, 9.5–30.5 × 2.3–3.5 μm; polyphialides not observed. Conidia aseptate, broad ellipsoid, occasionally with a slightly apiculate bases and rounded apices, hyaline, thin-, slightly rough-walled, 4–6.5 × 2.8–4.3 μm, forming conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics—Colonies on OA at 25 °C after 7 days reaching 10–12 diam, irregular, flat, colony margin undulate, surface white; reverse white. Colonies on PDA at 25 °C after 7 days reaching 10–15 mm diam, circular, raised, colony margin entire, surface white; reverse pale yellow (3A1) in the centre, white at the margin.
Other examined isolates: China, Shandong Province, Weihai city (122.19° E, 37.50° N), from intertidal sediment of a sand beach, November 2020, M.M. Wang and Y. Zheng (WL06829); ibid., WL06830.
Notes: The new species Stephanonectria arenicola is the fourth species of the genus Stephanonectria Schroers & Samuels after S. chromolaenae R.H. Perera & K.D. Hyde, S. ellipsoidea S.C. He, D.P. Wei & Jayaward., and S. keithii (Berk. & Broome) Schroers & Samuels. Morphologically, S. arenicola could be distinguished in the conidiogenous structures, and shape and size of conidia (conidiophores solitary or aggregate, unbranched or branched, bearing 1–3 phialides per node, conidia aseptate, broad ellipsoid, 4–6.5 × 2.8–4.3 μm in S. arenicola, vs. conidiophores sporodochial, densely arranged, irregularly branched, conidia ellipsoidal, 4.5–5.6 × 2–2.5 μm in S. chromolaenae, conidiophores sporodochial, irregularly branched, conidia oblong with obtuse ends, 6.2–6.8 × 3.3–3.7 μm in S. ellipsoidea, and conidiophores sporodochial, branched, condia ellipsoidal, 2.9–7.6 × 1.6–5.2 μm in S. keithii) [49,50,51]. Furthermore, the newly identified species exhibit distinct differences from the three other known species in habitats/hosts. Stephanonectria arenicola is found in intertidal sediment, while S. chromolaenae has been observed in the dead stem of Chromolaena odorata, S. ellipsoidea in dried fruit of a woody plant and S. keithii in the stalks of Brassica [49,50,51].
Verruciconidia oligospora M.M. Wang & W. Li sp. nov., Figure 6.
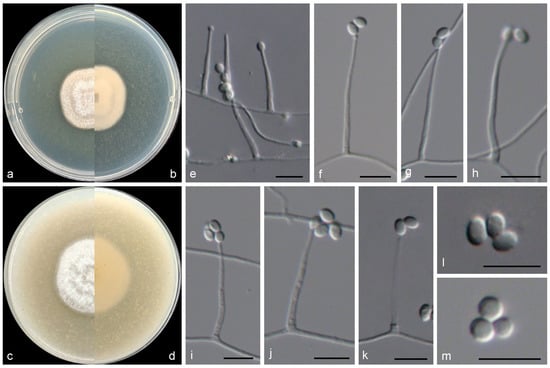
Figure 6.
Morphological characters of Verruciconidia oligospora (from ex-type WL05964). (a–d) Colonies on PDA and OA after 7 d; (e–k) conidiophores and conidiogenous cells; (l,m) conidia. Bars: (e–m) = 10 μm.
Classification: Ascomycota, Sordariomycetes, Hypocreales, Bionectriaceae, Verruciconidia
FungalNames: FN 572364
Etymology: Epithet refers to the less sporulation than other known species in the genus Verruciconidia.
Typus: China, Liaoning Province, Dalian city (122.99° E, 39.50° N), from intertidal sediment of a mud beach, May 2021, Y. Zheng and Z.H. Pan (HMAS 353474, holotype designated here, dried culture on PDA; culture ex-type CGMCC 3.28727 = WL05964).
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, up to 3 μm wide. Conidiophores solitary, (sub-)erect, arising from submerged and superficial hyphae, unbranched, hyaline, slightly rough-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, hyaline, thick, slightly rough-walled, 20.0–35.0 × 2.0–3.0 μm wide at base; polyphialides not observed. Conidia aseptate, ellipsoid to globose, hyaline, thin-, smooth-walled, 3.3–5.7 × 2.5–4.5 μm. Chlamydospores and sexual morph not observed.
Culture characteristics—Colonies on OA at 25 °C after 7 days reaching 15–20 diam, circular, crateriform, filamentous, colony margin entire, surface white; reverse white. Colonies on PDA at 25 °C after 7 days reaching 15–20 mm diam, circular, raised, filamentous, colony margin erose, surface white; reverse white.
Other examined isolates: China, Liaoning Province, Dalian city (122.99° E, 39.50° N), from intertidal sediment of a mud beach, May 2021, Y. Zheng and Z.H. Pan (WL06827); ibid., WL06828.
Notes: Verruciconidia L.W. Hou, L. Cai & Crous is a newly genus that established in 2023, with seven species including its type species V. verruculosa (W. Gams & Veenb.-Rijks) L.W. Hou, L. Cai & Crous [7]. In this study, the eighth species was introduced, namely V. oligospora. Phylogenetically, V. oligospora showed close relationship with V. persicina (Nicot) L.W. Hou, L. Cai & Crous and V. verruculosa (Figure 1), but differed by 61 bp and 99 bp in the LSU-ITS-tef1-rpb2 dataset, respectively. Morphologically, V. oligospora could be recognized in the shape and size of conidia (ellipsoid to globose, 3.3–5.7 × 2.5–4.5 μm in V. oligospora, vs. ellipsoid, ovoid, 4.2–6.2 × 2.4–3 μm in V. persicina, and ellipsoid, 4.5–6.2 × 2.5–3.5 μm in V. verruculosa) [7]. Additionally, the aforementioned species exhibit variations in their localities and hosts/habitats. For instance, V. oligospora from intertidal sediment in China, while V. persicina was discovered from coastal sand under Ammophila arenaria in France and V. verruculosa from agricultural soil in the Netherlands [7].
4. Discussion
The five new species described in this study were found to be phylogenetically well-located in five genera in three families in the order Hypocreales, with high supported values (BI = 0.97–1 and BS = 100; Figure 1). These include Gliomastix Guég., Stephanonectria and Verruciconidia in Bionectriaceae, Marquandomyces in Clavicipitaceae, and Fusarium Link in Nectriaceae, as determined by the LSU-ITS-tef1-rpb2 dataset (Figure 1). Morphologically, these species exhibited typical characteristics of Acremonium Link, yet differed from their closely related species in terms of conidiophores and shape and size of conidia, respectively. For example, all the known Stephanonectria species form sporodochial conidiophores [49,50,51] in contrast to the newly introduced species S. arenicola forms aerial conidiophores. Verruciconidia oligospora sp. nov. differs from its sister species in the size of conidia (3.3–5.7 × 2.5–4.5 μm in V. oligospora, vs. 4.2–6.2 × 2.4–3 μm in V. persicina and 4.5–6.2 × 2.5–3.5 μm in V. verruculosa) [7].
The hypocrealean fungi perform a variety of pivotal ecological functions within their respective environments, a fact that is exemplified by the five genera mentioned in this study. Members of the Fusarium (approximately 400 species) [52] are renowned pathogens of a wide range of plants and animals, and are frequently isolated from terrestrial and aquatic environments as documented in the FungalTraits database [18]. Species of Gliomastix (approximately 20 species) [52] are frequently documented as saprotrophic fungi [18] and are commonly found in soil, plants or air [7]. The genus Marquandomyces includes seven known species that have been extensively reported to play multiple crucial functions in agriculture and environmental remediation, for instance, as effective biocontrol agent against nematodes, notably reducing root galling in tomato and increasing the heat weights of lettuce [48]. The genus Stephanonectria was formerly considered to be saprotrophic fungi associated with litter, plants and soil [18]. The genus Verruciconidia has been found to be associated with air, plants and soil on a global scale [7]. The preponderance of evidence indicated that this group of fungi exhibits a wide geographical distribution and a robust capacity for environmental adaptation.
The broad ecological adaptability of hypocrealean fungi is attributable to their metabolic versatility, which concomitantly positions them as pivotal candidates for biotechnological innovation. The cross-sectoral utility of these fungi is evident in their capacity to fulfil specialised enzymatic and biochemical functions. A case in point is the mycoprotein synthesised by F. venenatum Nirenberg in the context of food manufacturing [53]. This mycoprotein is characterised by its high protein and cellulose content, and it has been commercialized as a sustainable meat alternative under the brand name Quorn [53]. In the field of chemical engineering, cutinase, a fungal enzyme produced by F. oxysporum Schltdl., has been shown to possess the capacity for polyethylene terephthalate modification [54]. Similarly, laccase-like enzymes derived from G. murorum (Corda) S. Hughes have demonstrated the ability to oxidise a wide range of organic compounds [55]. In the field of agriculture, Beauveria bassiana (Bals.-Criv.) Vuill. and Lecanicillium lecanii (Zimm.) Zare & W. Gams are effective entomopathogens that are capable of significantly reducing the survival and reproduction of insect pests like Aphis gossypii Glover through the mechanisms of direct infection and the production of toxic metabolites [56]. In the field of biomedicine, chlorinated orsellinic aldehydes derived from A. sclerotigenum (Moreau & R. Moreau ex Valenta) W. Gams have been shown to exhibit a high level of antifungal activity [57].
This study has expanded the corpus of knowledge concerning the ecological adaptation of the hypocrealean fungi. While seven Fusarium species have previously been reported from diverse host/habitat (mangrove algae, plants, sponges and sediments; etc.) in the marine environment with a wide geographic distribution and dominant presence [15,58,59] and G. murorum has been isolated from sediment of marine environments [60,61], members of the other three genera Marquandomyces, Stephanonectria and Verruciconidia were newly documented in marine environments. The present study lends further credence to the idea that hypocrealean fungi may be largely represented in marine environments. In the future, the study of these fungi will be pursued, including their species diversity, geographical distribution, host/substrate correlation and possible application value.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11070476/s1, Figure S1: Fifty percent majority rule consensus tree from a Bayesian analysis based on three-locus combined datasets (ITS-tef1-rpb2) showing the phylogenetic relationships of Fusarium (a), Gliomastix (b), Marquandomyces (c), Stephanonectria (d) and Verruciconidia (e). The Bayesian posterior probabilities (PP > 0.9) and PhyML bootstrap support values (BS > 50%) are displayed at the nodes (PP/BS). Ex-type cultures are indicated with “T”, epi-type with “ET” and neo-type with “NT”. New species introduced in this paper are marked in bold.
Author Contributions
Conceptualization, M.-M.W. and W.L.; methodology, M.-M.W., W.-Y.M. and Y.-H.T.; software, M.-M.W.; investigation, M.-M.W.; resources, M.-M.W.; data curation, M.-M.W., W.-Y.M. and M.-Y.S.; writing, M.-M.W. and W.L.; visualization, M.-M.W.; supervision, W.L.; project administration, M.-M.W.; funding acquisition, M.-M.W. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 32470017, 32370012, 32070008), the STU Scientific Research Initiation Grant (NTF23018T, NTF23002), and the Science and Technology Fundamental Resources Investigation Program (2019FY100700).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequences generated in this study (PV020684-PV020719, PV023180-PV023190, and PV050414-PV050430) were submitted to GenBank (https://www.ncbi.nlm.nih.gov, accessed on 15 January 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Wijayawardene, N.; Hyde, K.; Dai, D.; Sánchez-García, M.; Goto, B.; Saxena, R.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Rogerson, C.T. The Hypocrealean Fungi (Ascomycetes, Hypocreales). Mycologia 1970, 62, 865–910. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Houbraken, J.; Varga, J.; Frisvad, J.C. Polyphasic taxonomy of the heat resistant ascomycete genus Byssochlamys and its Paecilomyces anamorphs. Persoonia 2009, 22, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Salgado, C.; Hirooka, Y.; Rossman, A.Y.; Samules, G.J. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Stud. Mycol. 2011, 68, 57–78. [Google Scholar] [CrossRef]
- Lombard, L.; van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef]
- Hou, L.W.; Giraldo, A.; Groenewald, J.Z.; Rämä, T.; Summerbell, R.C.; Huang, G.Z.; Cai, L.; Crous, P.W. Redisposition of acremonium-like fungi in Hypocreales. Stud. Mycol. 2023, 105, 23–203. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- Jones, E.B.G. Fifty years of marine mycology. Fungal Divers. 2011, 50, 73–112. [Google Scholar] [CrossRef]
- Morrison-Gardiner, S. Dominant fungi from Australian coral reefs. Fungal Divers. 2002, 9, 105–121. [Google Scholar]
- Zalar, P.; de Hoog, G.; Schroers, H.-J.; Crous, P.; Groenewald, J.; Gunde-Cimerman, N. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaeros-permum, with descriptions of seven new species from hypersaline environments. Stud. Mycol. 2007, 58, 157–183. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G. Diversity of fungal isolates from three Hawaiian marine sponges. Microb. Res. 2007, 164, 233–241. [Google Scholar] [CrossRef]
- Hong, J.-H.; Jang, S.; Heo, Y.M.; Min, M.; Lee, H.; Lee, Y.M.; Lee, H.; Kim, J.-J. Investigation of marine-derived fungal diversity and their exploitable biological activities. Mar. Drugs 2015, 13, 4137–4155. [Google Scholar] [CrossRef]
- Shaumi, A.; Cheng, U.-C.; Guo, S.Y.; Jones, E.B.G.; Chan, T.-Y.; Pang, K.-L. Diversity of fungi isolated from carapace and gut of the marine crab Portunus sanguinolentus in northern waters of Taiwan. Bot. Mar. 2023, 66, 301–307. [Google Scholar] [CrossRef]
- Li, M.; Raza, M.; Song, S.; Hou, L.; Zhang, Z.-F.; Gao, M.; Huang, J.-E.; Liu, F.; Cai, L. Application of culturomics in fungal isolation from mangrove sediments. Microbiome 2023, 11, 272. [Google Scholar] [CrossRef]
- Wang, X.; Ji, G.; Cun, J.; Xu, P.; Wang, X.; Ren, G.; Li, W. Screening of insecticidal and antifungal activities of the culturable fungi isolated from the intertidal zones of Qingdao, China. J. Fungi 2022, 8, 1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Ma, Y.Y.; Cai, L.; Tedersoo, L.; Bahram, M.; Burgaud, G.; Long, X.D.; Zhang, S.M.; Li, W. Seasonal dynamics of mycoplankton in the Yellow Sea reflect the combined effect of riverine inputs and hydrographic conditions. Mol. Ecol. 2021, 30, 3624–3637. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Nilsson, R.H.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Cheng, X.L.; Li, W.; Zhang, T.Y. A new species of Phaeoisaria from intertidal marine sediment collected in Weihai, China. Mycotaxon 2014, 127, 17–24. [Google Scholar] [CrossRef]
- Cheng, X.L.; Li, W.; Cai, L. Molecular phylogeny of Ascotricha, including two new marine algaeassociated species. Mycologia 2015, 107, 490–504. [Google Scholar] [CrossRef]
- Wang, M.-M.; Shenoy, B.D.; Li, W.; Cai, L. Molecular phylogeny of Neodevriesia, with two new species and several new combinations. Mycologia 2019, 109, 965–974. [Google Scholar] [CrossRef]
- Wang, M.-M.; Yang, S.-Y.; Li, Q.; Zheng, Y.; Ma, H.-H.; Tu, Y.-H.; Li, W.; Cai, L. Microascaceae from the marine environment, with descriptions of six new species. J. Fungi 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1978. [Google Scholar]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000, 147, 617–630. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to the Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Sun, B.L. Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 4599–4603. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Sung, G.-H.; Hywel-Jones, N.L.; Sung, J.-M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.; Wilgenbusch, J.C.; Warren, D.L.; Swofford, D.L. AWTY (are we there yet?): A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 2008, 24, 581–583. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate Maximum-Likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, Surrey, 1971. [Google Scholar]
- Gerlach, W.; Nirenberg, H. The genus Fusarium—A pictorial atlas. In Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem; Kommissionsverlag P. Parey: Berlin, Germany, 1982; Volume 209, pp. 1–406. [Google Scholar]
- Sandoval-Denis, M.; Guarnaccia, V.; Polizzi, G.; Crous, P. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia 2018, 40, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Torbati, M.; Arzanlou, M.; Sandoval-Denis, M.; Crous, P.W. Multigene phylogeny reveals new fungicolous species in the Fusarium tricinctum species complex and novel hosts in the genus Fusarium from Iran. Mycol. Prog. 2019, 18, 119–133. [Google Scholar] [CrossRef]
- Corda, A.C.J. Deutschlands Flora, Abt. III. Die Pilze Dtschl. 1828, 2, 1–35. [Google Scholar]
- Torbati, M.; Arzanlou, M.; da Silva Santos, A.C. Fungicolous Fusarium Species: Ecology, Diversity, Isolation, and Identification. Curr. Microbiol. 2021, 78, 2850–2859. [Google Scholar] [CrossRef]
- Klittich, C.J.R.; Leslie, J.F.; Nelson, P.E.; Marasas, W.F.O. Fusarium thapsinum (Gibberella thapsina): A new species in section Liseola from sorghum. Mycologia 1997, 89, 643–652. [Google Scholar] [CrossRef]
- Matsushima, T. Icones Microfungorum a Matsushima Lectorum; The Nippon Printing & Publishing Co., Ltd.: Tokyo, Japan, 1975; pp. 1–209. [Google Scholar]
- de Hoog, G.S. Atlas of Clinical Fungi; Centraalbureau voor Schimmelcultures (CBS): Utrecht, The Netherlands, 2000; pp. 1–1126. [Google Scholar]
- Summerbell, R.C.; Gueidan, C.; Schroers, H.-J.; de Hoog, G.S.; Starink, M.; Arocha Rosete, Y.; Guarro, J.; Scott, J.A. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Stud. Mycol. 2011, 68, 139–162. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Khonsanit, A.; Thanakitpipattana, D.; Tasanathai, K.; Noisripoom, W.; Lamlertthon, S.; Himaman, W.; Houbraken, J.; Samson, R.A.; Luangsa-ard, J. Revisiting Metarhizium and the description of new species from Thailand. Stud. Mycol. 2020, 95, 171–251. [Google Scholar] [CrossRef]
- Peng, L.-Y.; Wang, Y.-F.; Song, H.; Urinboev, I.; Zhuang, W.-Y.; Gafforov, Y.; Wang, X.-C. Five new species of Marquandomyces (Clavicipitaceae, Ascomycota) from Asia. J. Fungi 2025, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Schroers, H.-J.; Samuels, G.J.; Gams, W. Stephanonectria, a new genus of the Hypocreales (Bionectriaceae), and its sporodochial anamorph. Sydowia 1999, 51, 114–126. [Google Scholar]
- He, S.C.; Wei, D.P.; Bhunjun, C.S.; Zhao, Q.; Jayawardena, R.S. A new species of Stephanonectria (Bionectriaceae) from southwestern China. Asian J. Mycol. 2023, 6, 98–106. [Google Scholar] [CrossRef]
- Perera, R.H.; Hyde, K.D.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Bundhun, D.; Camporesi, E.; Akulov, A.; Liu, J.K.; Liu, Z.Y. Profile of Bionectriaceae, Calcarisporiaceae, Hypocreaceae, Nectriaceae, Tilachlidiaceae, Ijuhyaceae fam. nov., Stromatonectriaceae fam. nov. and Xanthonectriaceae fam. nov. Fungal Divers. 2023, 118, 95–271. [Google Scholar] [CrossRef]
- Hyde, K.D.; Noorabadi, M.T.; Thiyagaraja, V.; He, M.Q.; Johnston, P.R.; Wijesinghe, S.N.; Armand, A.; Biketova, A.Y.; Chethana, K.W.T.; Erdoğdu, M.; et al. The 2024 Outline of Fungi and fungus-like taxa. Mycosphere 2024, 15, 5146–6239. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.; Casper, H.H. Molecular, phylogenetic morphological and mycotoxin data support reidentification of the Quorn mycoprotein fungus as Fusarium venenatum. Fungal Genet. Biol. 1998, 23, 57–67. [Google Scholar] [CrossRef]
- Dimarogona, M.; Nikolaivits, E.; Kanelli, M.; Christakopoulos, P.; Sandgren, M.; Topakas, E. Structural and functional studies of a Fusarium oxysporum cutinase with polyethylene terephthalate modification potential. Biochim. Biophys. Acta-Gen. Subj. 2015, 11, 2308–2317. [Google Scholar] [CrossRef]
- Fernández-Remacha, D.; González-Riancho, C.; Osua, M.L.; Arce, A.G.; Montánchez, I.; García-Lobo, J.M.; Estrada-Tejedor, R.; Kaberdin, V.R. Analysis of laccase-like enzymes secreted by fungi isolated from a cave in northern Spain. MicrobiologyOpen 2022, 11, e1279. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Keyhani, N.O.; Xia, Y.X.; Xie, J.Q. The potential and limitations of entomopathogenic fungi as biocontrol agents for insect pest management. Entomol. Gen. 2024, 44, 797–811. [Google Scholar] [CrossRef]
- Huo, R.; Tu, Y.; Liu, C.; Zi, G.; Shi, Y.; Ren, J.; Cai, L.; Liu, L. New antifungal chlorinated orsellinic aldehydes from the deep-sea-derived fungus Acremonium sclerotigenum LW14. Mycology 2025, 1–10. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Pang, K.-L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J.; et al. An online resource for marine fungi. Fungal Divers. 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Devadatha, B.; Abdel-Wahab, M.A.; Dayarathne, M.C.; Zhang, S.-N.; Hyde, K.D.; Liu, J.-K.; Bahkali, A.H.; Sarma, V.V.; Tibell, S.; et al. Phylogeny of new marine Dothideomycetes and Sordariomycetes from mangroves and deep-sea sediments. Bot. Mar. 2020, 63, 155–181. [Google Scholar] [CrossRef]
- Cecchi, G.; Cutroneo, L.; Piazza, S.D.; Capello, M.; Zotti, M. Culturable fungi from dredged and marine sediments from six ports studied in the framework of the SEDITERRA Project. J. Soils Sediments 2021, 21, 1563–1573. [Google Scholar] [CrossRef]
- Georgieva, M.L.; Bilanenko, E.N.; Georgiev, A.A.; Bubnova, E.N. Filamentous fungi in the sediments of the East Siberian and Laptev Seas. Microbiology 2024, 93, 364–368. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).