Evolution of Potential Distribution Areas and Cultivation Zones of Morchella esculenta (L.) Pers. Under Climate Warming: Application of Ensemble Models and Production Dynamics Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Data Collection and Filtering
2.2. Selection and Processing of Environmental Variables
2.3. Ensemble Model Construction and Implementation
2.4. Spatial Pattern Changes in Suitable Distribution Areas
2.5. Multivariate Environmental Similarity Surfaces (MESS) and Divergence Analysis
2.6. Niche Differentiation Analysis
2.7. Centroid Migration Patterns
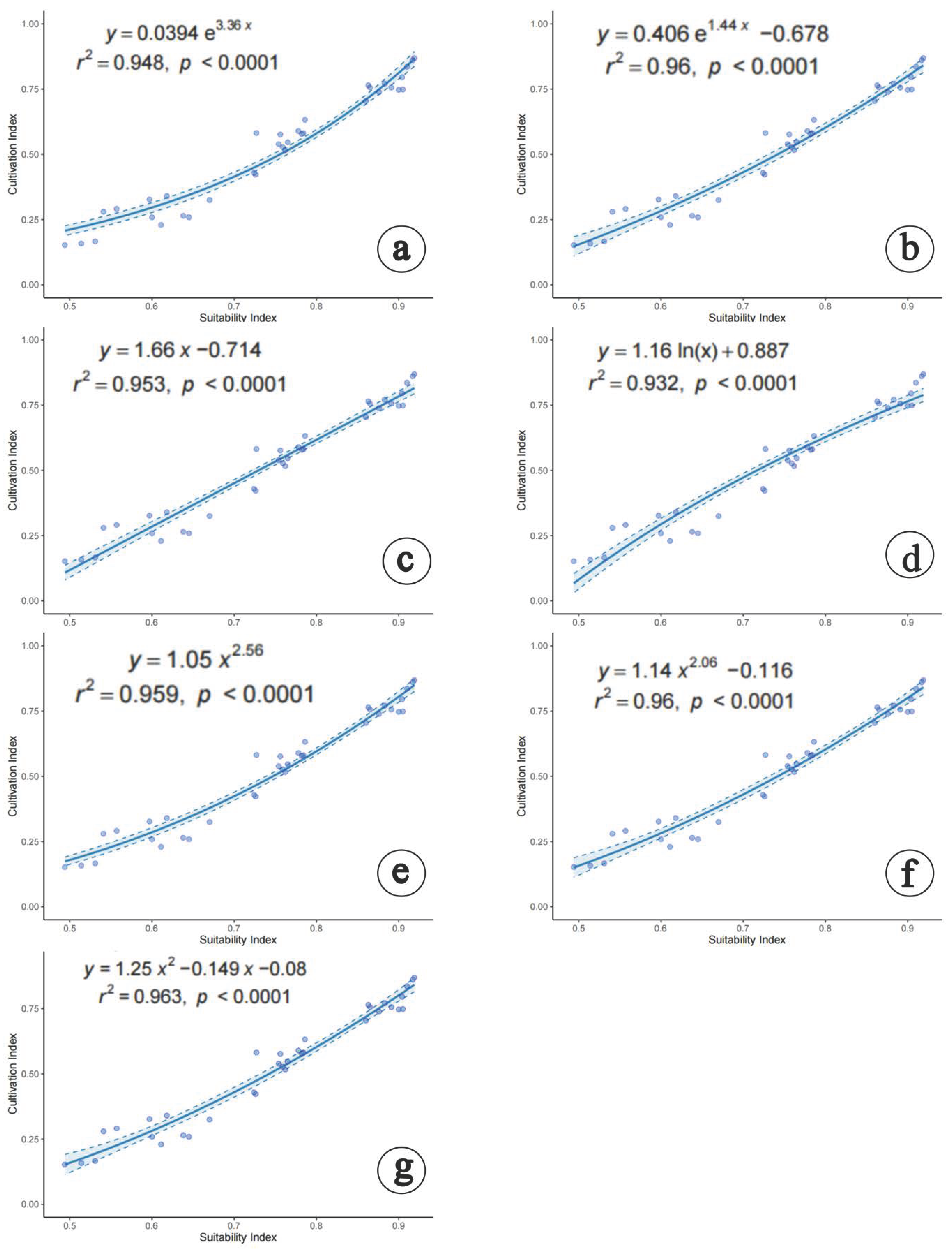
2.8. Modeling Cultivation Productivity–Environmental Suitability Relationships
3. Results
3.1. Multi-Model Prediction Outcomes and Ensemble Model Accuracy Validation
3.2. Environmental Factor Combinations Influencing M. esculenta Potential Distribution
3.3. Multivariate Environmental Similarity Surfaces (MESS) and Most Dissimilar Variables (MOD) Analysis
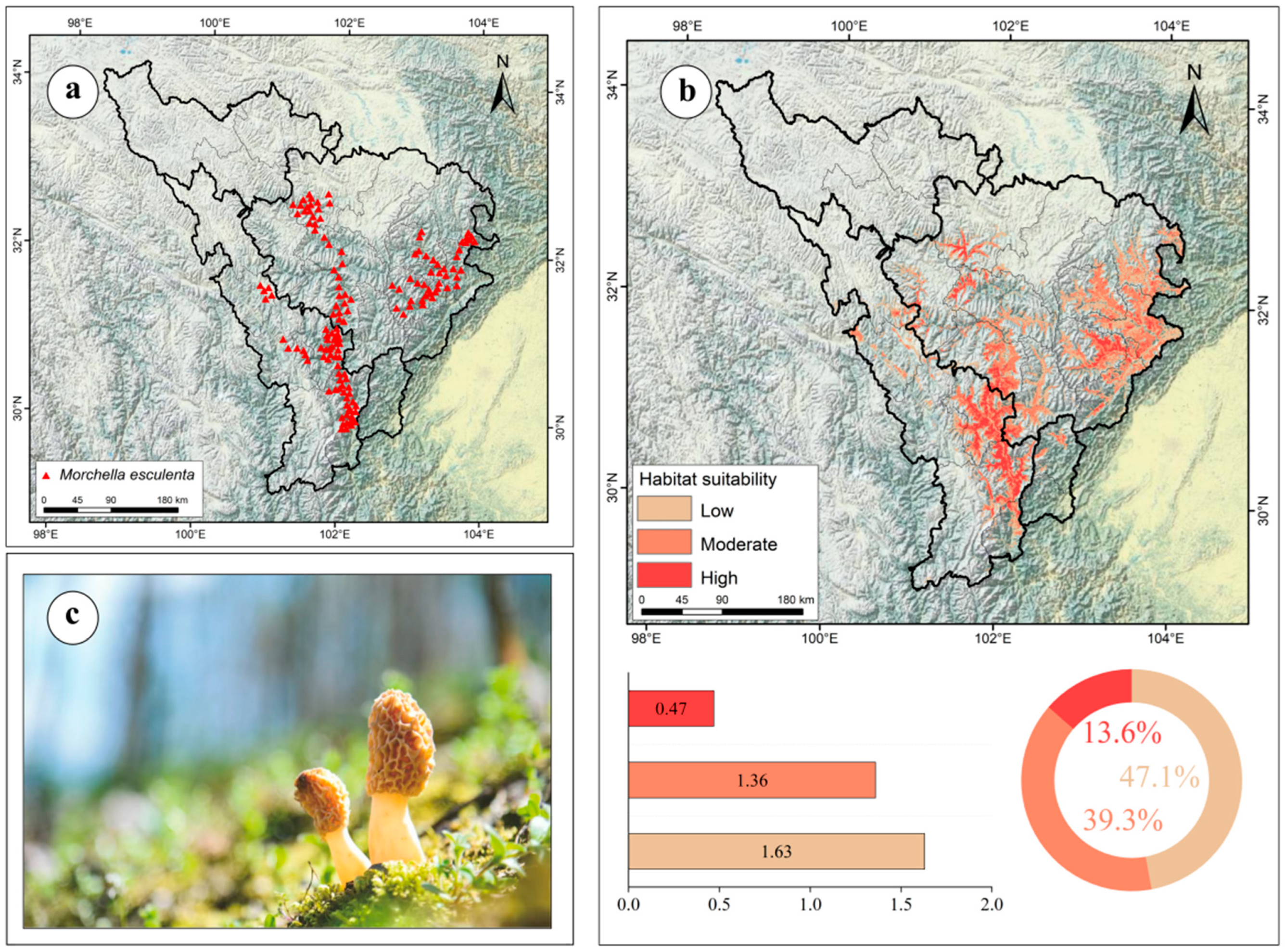
3.4. Current Potential Distribution of M. esculenta in the Upper Dadu–Min River Region
3.5. Future Potential Distribution of M. esculenta in the Upper Dadu–Min River Region
3.6. Future Potential Distribution Patterns of M. esculenta in the Upper Dadu–Min River Region
3.7. Analysis of Niche Shifts in M. esculenta Under Future Climates
3.8. Centroid Migration Trajectories Under Climate Change
3.9. Dynamics of Potential Cultivation Production Areas Across Periods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Donnell, K.; Rooney, A.P.; Mills, G.L.; Kuo, M.; Weber, N.S.; Rehner, S.A. Phylogeny and Historical Biogeography of True Morels (Morchella) Reveals an Early Cretaceous Origin and High Continental Endemism and Provincialism in the Holarctic. Fungal Genet. Biol. 2011, 48, 252–265. [Google Scholar] [CrossRef]
- Deng, G.; Li, J.; Liu, H.; Wang, Y. Volatile Compounds and Aroma Characteristics of Mushrooms: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 13175–13192. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Zhang, X. Cultivation, Nutritional Value, Bioactive Compounds of Morels, and Their Health Benefits: A Systematic Review. Front. Nutr. 2023, 10, 1159029. [Google Scholar] [CrossRef]
- Ullah, T.S.; Firdous, S.S.; Shaheen, H.; Manzoor, M.; Gillani, S.W.; Shier, W.T.; Ali, B.; Malik, T.; Ercisli, S.; Aljowaie, R.M.; et al. Metabolic and Proteomic Analysis of a Medicinal Morel (Morchella elata) from Western Himalayas, Kashmir. Metabolomics 2025, 21, 34. [Google Scholar] [CrossRef]
- Chen, X.; Wu, L.; Lei, Y.; Tang, H.; Yan, Z.; Zhu, S.; Wen, T.; Zhu, Z. A Polysaccharide from Morchella esculenta Mycelia: Structural Characterization and Protective Effect on Antioxidant Stress on PC12 Cells against H2O2-Induced Oxidative Damage. Int. J. Biol. Macromol. 2025, 298, 139886. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Jin, X.; Liu, B.; Zhang, C.; Narbad, A.; Zhao, J.; Chen, W.; Tian, F.; Yu, L.; Zhai, Q. Morchella esculenta Polysaccharides Ameliorate Obesity by Enriching Bifidobacterium and Bacteroides. Food Biosci. 2025, 68, 106552. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, X.; Cai, M.; Gu, H.; E, H.; Li, X.; Zhang, Y.; Lu, H.; Zhou, C. Metabolomics Analysis of Morchella sp. from Different Geographical Origins of China Using UPLC-Q-TOF-MS. Front. Nutr. 2022, 9, 865531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Han, L.; Wang, M.; Tang, M.; Chen, H.; Ran, W.; Li, Z. Characteristics and risk assessment of chilling injury on Morchella esculenta in Western Sichuan Plateau, China. J. Appl. Ecol. (Ying Yong Sheng Tai Xue Bao) 2023, 34, 1541–1546. [Google Scholar] [CrossRef]
- Li, P.; Dai, X.; Chen, X.; Shao, Q. Recent Advances in the Bioactive Polysaccharides and Other Key Components from Morchella spp. and Biological Activities: A Review. Carbohydr. Res. 2025, 554, 109515. [Google Scholar] [CrossRef]
- Nisa, S.U.; O’Donnell, K.; Badshah, H.; Shah, S.A.; Mumtaz, A.S. Genetic Diversity of True Morels (Morchella) in Pakistan Inferred from Multilocus DNA Sequence Data Augmented by a Retrospective Analysis of Pakistani and Indian Morel Sequences in GenBank. Mycologia 2025, 117, 418–434. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Y.; Kang, Y.; Yan, T.; Yue, H. The Growth–Climate Relationships of Three Dominant Subalpine Conifers on the Baima Snow Mountain in the Southeastern Tibetan Plateau. Plants 2024, 13, 1645. [Google Scholar] [CrossRef] [PubMed]
- Ai, N. On the geography and environment of the Tibetan-Yi Corridor. J. Southwest Minzu Univ. (Humanit. Soc. Sci.) 2007, 2, 15–16. [Google Scholar]
- Deng, X.; Sun, Q. Prediction of Climate Change Impacts on the Distribution of an Umbrella Species in Western Sichuan Province, China: Insights from the MaxEnt Model and Circuit Theory. Diversity 2025, 17, 67. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Cong, N.; Yu, X.; Lin, Y.; Liu, T.; Qi, G.; Ren, P. Modeling Ecological Resilience of Alpine Forest under Climate Change in Western Sichuan. Forests 2023, 14, 1769. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Fonseca, G.A.B.; da Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global Biodiversity Conservation Priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef]
- Mi, C.; Ma, L.; Yang, M.; Li, X.; Meiri, S.; Roll, U.; Oskyrko, O.; Pincheira-Donoso, D.; Harvey, L.P.; Jablonski, D.; et al. Global Protected Areas as Refuges for Amphibians and Reptiles under Climate Change. Nat. Commun. 2023, 14, 1389. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lenoir, J.; Chen, I.C. Limited Evidence for Range Shift–Driven Extinction in Mountain Biota. Science 2025, 388, 741–747. [Google Scholar] [CrossRef]
- Grant, L.; Vanderkelen, I.; Gudmundsson, L.; Fischer, E.; Seneviratne, S.I.; Thiery, W. Global Emergence of Unprecedented Lifetime Exposure to Climate Extremes. Nature 2025, 641, 374–379. [Google Scholar] [CrossRef]
- Wan, J.N.; Wang, S.W.; Leitch, A.R.; Leitch, I.J.; Jian, J.-B.; Wu, Z.Y.; Xin, H.-P.; Rakotoarinivo, M.; Onjalalaina, G.E.; Gituru, R.W.; et al. The Rise of Baobab Trees in Madagascar. Nature 2024, 629, 1091–1099. [Google Scholar] [CrossRef]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Wen, X.; Fang, G.; Chai, S.; He, C.; Sun, S.; Zhao, G.; Lin, X. Can Ecological Niche Models Be Used to Accurately Predict the Distribution of Invasive Insects? A Case Study of Hyphantria cunea in China. Ecol. Evol. 2024, 14, e11159. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.; Yang, L.; Chen, K.; Shama, Z.; Jiang, X.; Yang, J.; Zhao, G.; Huang, Y. Prediction of the Potential Distribution and Conservation Strategies of the Endangered Plant Tapiscia sinensis. Forests 2024, 15, 1677. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Jiang, X.; Chen, H.; Liu, M.; Wang, R. Potential Geographical Distribution of the Edangred Plant Isoetes under Human Activities Using MaxEnt and GARP. Glob. Ecol. Conserv. 2022, 38, e02186. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, X.; Sun, J.; Li, T.; Wang, Q.; Ye, X.; Fan, B. Analysis of the Distribution Pattern of Chinese Ziziphus jujuba under Climate Change Based on Optimized Biomod2 and MaxEnt Models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Zhou, X.; Zhang, X.; Zhao, G.; Zhang, F. Prediction of Potential Distribution Areas and Priority Protected Areas of Agastache Rugosa Based on Maxent Model and Marxan Model. Front. Plant Sci. 2023, 14, 1200796. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A Review of Evidence about Use and Performance of Species Distribution Modelling Ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Wen, X.; Zhao, G.; Cheng, X.; Chang, G.; Dong, X.; Lin, X. Prediction of the Potential Distribution Pattern of the Great Gerbil (Rhombomys opimus) under Climate Change Based on Ensemble Modelling. Pest Manag. Sci. 2022, 78, 3128–3134. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Sun, C.; Zuo, J.; Shi, X.; Liu, X.; Liu, H. Diverse Inter-Annual Variations of Winter Siberian High and Link with Eurasian Snow in Observation and BCC-CSM2-MR Coupled Model Simulation. Front. Earth Sci. 2021, 9, 761311. [Google Scholar] [CrossRef]
- Milovac, J.; Ingwersen, J.; Warrach-Sagi, K. Global Top Soil Texture Data Compatible with the WRF Model Based on the Harmonized World Soil Database (HWSD) at 30 Arc-Second Horizontal Resolution Version 1.21; World Data Center for Climate: Hamburg, Germany, 2018. [Google Scholar]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Global Terrestrial Human Footprint Maps for 1993 and 2009. Sci. Data 2016, 3, 160067. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.; Xiang, W.; Chen, L.; Ouyang, S. Predicting Potential Suitable Habitats of Chinese Fir under Current and Future Climatic Scenarios Based on Maxent Model. Ecol. Inform. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Xu, C. Study on Taiwania cryptomerioides under Climate Change: MaxEnt Modeling for Predicting the Potential Geographical Distribution. Glob. Ecol. Conserv. 2020, 24, e01313. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The Art of Modelling Range-Shifting Species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Geange, S.W.; Pledger, S.; Burns, K.C.; Shima, J.S. A Unified Analysis of Niche Overlap Incorporating Data of Different Types. Methods Ecol. Evol. 2011, 2, 175–184. [Google Scholar] [CrossRef]
- Sun, J.; Jiao, W.; Wang, Q.; Wang, T.; Yang, H.; Jin, J.; Feng, H.; Guo, J.; Feng, L.; Xu, X.; et al. Potential Habitat and Productivity Loss of Populus deltoides Industrial Forest Plantations Due to Global Warming. For. Ecol. Manag. 2021, 496, 119474. [Google Scholar] [CrossRef]
- Chen, F.H.; Dong, G.H.; Zhang, D.J.; Liu, X.Y.; Jia, X.; An, C.B.; Ma, M.M.; Xie, Y.W.; Barton, L.; Ren, X.Y.; et al. Agriculture Facilitated Permanent Human Occupation of the Tibetan Plateau after 3600 B. P. Science 2015, 347, 248–250. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A Significant Upward Shift in Plant Species Optimum Elevation During the 20th Century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Zhu, K.; Woodall, C.W.; Clark, J.S. Failure to Migrate: Lack of Tree Range Expansion in Response to Climate Change. Glob. Change Biol. 2012, 18, 1042–1052. [Google Scholar] [CrossRef]
- Alfonso-Corrado, C.; Naranjo-Luna, F.; Clark-Tapia, R.; Campos, J.E.; Rojas-Soto, O.R.; Luna-Krauletz, M.D.; Bodenhorn, B.; Gorgonio-Ramírez, M.; Pacheco-Cruz, N. Effects of Environmental Changes on the Occurrence of Oreomunnea mexicana (Juglandaceae) in a Biodiversity Hotspot Cloud Forest. Forests 2017, 8, 261. [Google Scholar] [CrossRef]
- Jiménez-García, D.; Peterson, A.T. Climate Change Impact on Endangered Cloud Forest Tree Species in Mexico. Rev. Mex. Biodivers. 2019, 90, 1–14. [Google Scholar] [CrossRef]
- Téllez-Valdés, O.; D¡ Vila-Aranda, P. Protected Areas and Climate Change: A Case Study of the Cacti in the Tehuacán-Cuicatlán Biosphere Reserve, México. Conserv. Biol. 2003, 17, 846–853. [Google Scholar] [CrossRef]
- Guo, B.; Zang, W.; Yang, F.; Han, B.; Chen, S.; Liu, Y.; Yang, X.; He, T.; Chen, X.; Liu, C.; et al. Spatial and Temporal Change Patterns of Net Primary Productivity and Its Response to Climate Change in the Qinghai-Tibet Plateau of China from 2000 to 2015. J. Arid Land 2020, 12, 1–17. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Pilz, D.; McLain, R.; Alexander, S.; Villarreal-Ruiz, L.; Berch, S.; Wurtz, T.L.; Parks, C.G.; McFarlane, E.; Baker, B.; Molina, R.; et al. Ecology and Management of Morels Harvested from the Forests of Western North America; General Technical Report PNW-GTR-710; U.S. Department of Agriculture: Washington, DC, USA; Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2007; Volume 710, p. 161. [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent Plant Diversity Changes on Europe’s Mountain Summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef]
- Wurtz, T.L.; Wiita, A.L.; Weber, N.S.; Pilz, D. Harvesting Morels after Wildfire in Alaska; Research Note PNW-RN-546; U.S. Department of Agriculture: Washington, DC, USA; Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2005; Volume 546, p. 31. [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhao, G.; Yang, J.; Yang, L.; Yang, Y.; Jiaba, W.; Shama, Z.; Yang, J. Evolution of Potential Distribution Areas and Cultivation Zones of Morchella esculenta (L.) Pers. Under Climate Warming: Application of Ensemble Models and Production Dynamics Models. J. Fungi 2025, 11, 475. https://doi.org/10.3390/jof11070475
Huang Y, Zhao G, Yang J, Yang L, Yang Y, Jiaba W, Shama Z, Yang J. Evolution of Potential Distribution Areas and Cultivation Zones of Morchella esculenta (L.) Pers. Under Climate Warming: Application of Ensemble Models and Production Dynamics Models. Journal of Fungi. 2025; 11(7):475. https://doi.org/10.3390/jof11070475
Chicago/Turabian StyleHuang, Yi, Guanghua Zhao, Jingtian Yang, Liyong Yang, Yang Yang, Wuzhi Jiaba, Zixi Shama, and Jian Yang. 2025. "Evolution of Potential Distribution Areas and Cultivation Zones of Morchella esculenta (L.) Pers. Under Climate Warming: Application of Ensemble Models and Production Dynamics Models" Journal of Fungi 11, no. 7: 475. https://doi.org/10.3390/jof11070475
APA StyleHuang, Y., Zhao, G., Yang, J., Yang, L., Yang, Y., Jiaba, W., Shama, Z., & Yang, J. (2025). Evolution of Potential Distribution Areas and Cultivation Zones of Morchella esculenta (L.) Pers. Under Climate Warming: Application of Ensemble Models and Production Dynamics Models. Journal of Fungi, 11(7), 475. https://doi.org/10.3390/jof11070475





