Abstract
In the long-term evolutionary process, species maintain a natural balance within certain limits through communication. As plants grow and function as producers, root enrichment fosters a dynamic rhizosphere microbiome, which serves not only as a disintegrator within the ecological niche but also as a medium for interaction between the host and the soil environment. The life cycle of fungi within the microbiome alternates between single-cell resting spores and multicellular trophic mycelia. This cycle not only establishes a stable rhizosphere environment but also plays a crucial role in regulating both intra- and interspecific information transmission, significantly impacting the environment and plant health. The rhizosphere microbiome, particularly the fungi it contains, can be harnessed to repair environmental damage and either promote the growth of the plant host or inhibit pathogens. However, the mechanisms underlying these actions remain inadequately understood, hindering the advancement of artificial regulation. Additionally, the variability of influencing factors, along with unstable genes and traits, poses challenges to industrial development. In conclusion, this paper focuses on the fungal components of the rhizosphere microbiome, introduces the mechanisms of communication and current applications, and further analyzes existing bottlenecks and potential solutions. The aim is to provide theoretical support for achieving green, sustainable agriculture through biological means.
1. Introduction
In natural ecosystems, species populations exhibit characteristic dynamics (e.g., regulated growth, niche differentiation) that arise from biotic interdependence (e.g., symbiosis, competition) as well as constraints imposed by abiotic factors (e.g., nutrient availability, temperature) and stochastic processes, collectively shaping balanced ecological networks. Microorganisms serve as primary producers and decomposers, playing a pivotal role in sustaining material circulation, energy flow, and ecosystem stability within natural systems. To secure competitive ecological niches, where microorganisms gain advantages in resource acquisition or functional expression, individual microorganisms engage in essential cellular activities and demonstrate acclimatory stress responses to cope with environmental challenges (e.g., nutrient scarcity, fluctuating pH, or oxidative stress). These stress-induced physiological adjustments often involve the secretion of diffusible signaling molecules (DSMs) or metabolites, which simultaneously mediate intraspecific coordination and act as cues to recruit compatible partners [1]. Soil, as a three-phase polydisperse medium (solid, liquid, gaseous), harbors extraordinarily diverse biological resources, including unique microbial communities and specialized invertebrates. While some taxa (e.g., certain protozoa) overlap with aquatic environments, soil’s distinct microhabitats (e.g., rhizospheres, soil pores) support high levels of taxonomic and functional uniqueness that differ from the free-living communities of air and bulk water [2]. The rhizosphere, which defined as the soil microdomain modified by plant root activities to possess distinct physical, chemical, and biological properties relative to bulk soil, is considered one of the most dynamic interfaces on Earth [3]. The microorganisms associated with the surrounding rhizosphere are collectively known as the “rhizosphere microbiome” and form a network of interactions with plant hosts and other soil-dwelling organisms across different trophic levels [4]. Analogous to the current hot point “gut microbiota”, the rhizosphere microbial community associated with the plant host constitutes an integrated system, which was discovered earlier and should receive more attention [5]. Through the plant’s structure, they facilitate the exchange of matter and energy, collectively encoding a greater number of genes than the host itself, a phenomenon termed the plant’s “second genome” [6].
The rhizosphere microbiome comprises a complex assemblage of plant roots, along with bacteria, fungi, archaea, algae, actinomycetes, protozoa, and viruses, exhibiting a total cell density ranging from 106 to 109 cells/cm2 [7]. Fungi constitute a significant portion of the rhizosphere microbial biomass and are instrumental in promoting plant growth and ameliorating soil quality due to their unique growth and metabolic patterns [8]. However, infections caused by toxigenic fungi in crops can lead to crop products contamination by mycotoxins. This contamination initiates during cultivation, whereby dormant spores attached to the surface or vegetative mycelia invade the plant. Upon detachment from the soil microenvironment, the ecosystem balance is disrupted, triggering the rapid activation of defensive mechanisms in toxigenic fungi, such as the upregulation of secondary metabolism, which results in the production of mycotoxins throughout the subsequent food chain, including collection, storage, transportation, and marketing. According to the Food and Agriculture Organization (FAO), annual mycotoxin contamination accounts for 25% of the global grain supply and has been reported in over 100 types of agricultural products, including food and feed, with oil crops being particularly susceptible [9]. Recently, the prevalence and distribution of mycotoxins have escalated due to the compounded effects of extreme climatic conditions and emerging pollutants [10].
In addition to fluctuations in temperature, humidity, pH, and other environmental factors that contribute to abnormal mycotoxin production and variability, disruption of the “soil–plant–fungus” equilibrium may also induce behavioral transformations in toxigenic fungi within the rhizosphere microbiome [3]. Communication signaling constitutes a critical facet of the adaptive resistance mechanisms employed by fungi. Furthermore, how does intraspecific communication, represented by quorum sensing, respond to influences from plant roots and other rhizosphere microorganisms? These emerging research directions remain active areas of investigation.
Additionally, the controlled regulation of microorganisms to safeguard host organisms, mitigate losses, promote growth, and enhance economic returns has emerged as a prominent focus in both agricultural and forestry sciences. Nature harbors immense biological resources, and the utilization of microbial agents and cell factories has shown remarkable potential. However, many of these effects remain inconsistent. The intricate biological interactions arising from ecological dynamics, along with incompletely elucidated internal mechanisms, constrain the advancement of rhizosphere microbiology research.
This review focuses on fungi within the rhizosphere microbiome, elaborating on the principles governing their assembly and establishment in response to dynamic host changes, as well as the current state of research in this field. It analyzes key regulatory aspects and proposes potential mechanisms that refine knowledge of microbial cell communication, findings intended to further propel the development of sustainable green agriculture.
2. Methodology
To ensure the review’s comprehensiveness, we conducted a systematic literature search across three core databases—Web of Science, Scopus, PubMed—with a timeframe focused on recent advances. We used tailored keyword combinations that integrated terms for the biological system (“rhizospheric fungi,” “rhizobiome”), target molecules (“diffusible signaling molecules,” “quorum sensing”), and ecological context (“plant-microbe interaction”), ensuring that we captured both foundational and cutting-edge research.
The study of rhizosphere interactions involves the intersection of a series of disciplines such as biochemistry, microbiology, bioinformatics, and genetics. Core methodological approaches include but are not limited to gas/liquid chromatography–mass spectrometry, biosensors, gene editing/sequencing, fluorescence tracking, molecular simulation, etc., in the research of rhizosphere interactions (see Table 1).

Table 1.
Advanced methods in research of rhizosphere interactions.
3. Assembly of Fungal Communities in the Rhizosphere Microbiome and Their Species Composition
3.1. Dynamic Building with Soil, Plant, and Fungi
The interrelationship among host roots, their associated microbiome, and the surrounding soil environment forms a cohesive system that engages in dynamic interactions to maintain ecological balance amid environmental fluctuations [11]. The biological activities and species characteristics within this system exhibit significant variability, reflecting the extensive influence of environmental factors. Furthermore, the adaptive feedback mechanisms of the rhizosphere microbiome in response to environmental changes help sustain the balance of this system [12,13]. This microbiome acts as a crucial regulator of material transformation during ecosystem evolution (see Figure 1).
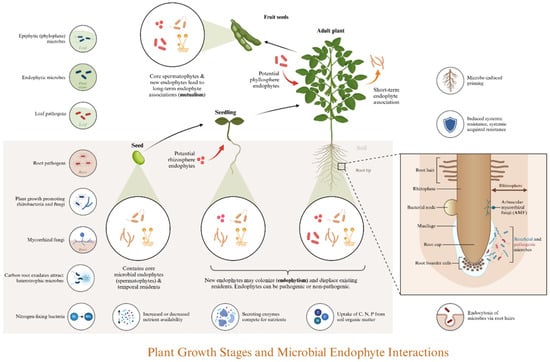
Figure 1.
Dynamic Changes in the Rhizosphere Microbiome Associated with Plant Hosts. As plants mature, microorganisms are progressively recruited to the rhizosphere in the soil, resulting in a balanced rhizosphere microbiome with varying compositions at different developmental stages. This microbiome includes neutral symbiotic, beneficial growth-promoting, harmful pathogenic bacteria and fungi, all of which directly and indirectly influence the growth of plant hosts.
The macro environment serves as a fundamental selective pressure for microorganisms, which in turn can influence soil properties [14]. Soil texture affects aeration, water retention, and nutrient content, thereby impacting the living environment. Additionally, soil composition influences microbial growth and metabolism, ultimately determining the assembly of the microbiome in a given environment [15]. Microorganisms can enhance soil aggregate structure by secreting extracellular polymers (EPS), which increase both stability and aeration [16]. Certain microbial strains contribute to soil nutrient cycling, including nitrogen fixation, organic matter decomposition, and the mineralization of organic carbon [17]. Furthermore, the metabolic activities of specific strains can alter soil pH, while some microorganisms possess the ability to degrade organic pollutants or immobilize heavy metals, thereby improving environmental quality [18].
Planting further enriches the diversity of microorganisms. Various host-related factors, including developmental stage and circadian rhythms, influence the structure of the root microbial community [19]. This structure is also partially determined by uncontrollable abiotic factors in the natural environment, which elucidates the challenges associated with culturing many environmental microorganisms, particularly endophytes [20]. Physically, the soil’s water release characteristics and aeration conditions in the rhizosphere are superior to those in non-rhizosphere areas due to the interpenetration of plant roots. Additionally, the diversity of root-associated microorganisms tends to decrease from the surrounding soil to the rhizosphere, root surface, and root interior, indicating a high degree of specialization [21]. Chemically, approximately 20% to 60% of photosynthates produced during plant growth are released into the rhizosphere as root exudates [22]. These exudates can be categorized into two primary categories: small molecular compounds (including amino acids, organic acids, sugars, alcohols, polyamines, fatty acids, purines, plant hormones, terpenes, flavonoids, benzoxazines, etc.) and macromolecular compounds (including polysaccharides and proteins). Root exudates serve multiple functions: (A) surfactants, such as lecithin, help create a hydrophobic environment; (B) organic matter, along with apoptotic roots and their exfoliations—including root hairs, epidermal cells, and root crowns—contributes to a nutrient-rich root environment, serving as vital sources of nutrients and energy; (C) small root exudates function as signaling molecules, with attractants and repellents facilitating the recruitment of rhizosphere microorganisms [23,24]. Particularly, quorum-sensing molecules (QSMs), including volatile compounds, regulate interactions among plants and microorganisms, as well as among microorganisms themselves [25].
The types and concentrations of root exudates vary across different root regions, resulting in distinct microbial niches on a spatial scale [26]. The absolute abundance of microorganisms is highest in the rhizosphere, followed by the root surface, within the roots, and lowest in the surrounding soil [27]. Additionally, characteristics such as motility, chemotaxis, and adhesion influence the chemotactic behavior of microorganisms as they assemble toward the root exudates of the plant host. This spatial heterogeneity allows rhizosphere microorganisms to exert varying effects on plants across various vertical and horizontal scales [28]. Based on absolute abundance to elucidate the assembly processes of the rhizosphere microbiome, Microorganisms are initially amplified in the rhizosphere soil and subsequently selected through interactions with the root, leading to the formation of a specific rhizosphere microbial community [29]. Furthermore, there are also “two-step selection” and “multi-step enrichment” models, which emphasize the gradual selection of microbial communities from the soil to the rhizosphere and ultimately into the roots [27,30]. Although the rhizosphere microbiome often fluctuates with changes in the soil environment, host genetics acts as a ‘selection filter’ that maintains consistent assembly of a core microbiome subset within a particular host genotype, regardless of soil conditions. Moreover, the rhizosphere microbiome undergoes a stabilization and host-specialization dynamic throughout plant growth: it shows high compositional variation in the early stages and becomes progressively less variable while more host-specific in the later stages [8].
3.2. Species and Functions of Rhizosphere Fungi
Rhizosphere fungi encompass a phylogenetically and functionally diverse array of taxa, including symbionts that form mutualistic associations with plants, saprotrophs that drive organic matter cycling, regulators that modulate microbiome structure, and pathogens that occasionally disrupt plant health. These groups collectively shape rhizosphere function and plant fitness, with each contributing unique ecological roles [31]. Among the microbial inhabitants of the rhizosphere, fungi can establish a more reciprocal symbiotic relationship with higher plants compared to bacteria. They develop extensive rhizosphere mycelium through mycelial extension in the soil, which is influenced by mycelial secretions, occupying an irreplaceable ecological niche within the ecosystem [32]. The interactions between rhizosphere fungi and their plant hosts can be categorized into three types: neutral commensal, harmful pathogenic, and beneficial promoting relationships. The majority of rhizosphere fungi engage in neutral symbiosis with plants, demonstrating a relatively loose dependence while also being attracted to plant secretions. Although their influence may not be overtly positive or negative, under certain conditions, neutral fungi can exhibit either beneficial or detrimental effects on their host [33].
Beneficial rhizosphere fungi can influence their host through bidirectional regulatory mechanisms. Nutrient exchange and symbiotic formation are facilitated by signaling molecules secreted by plants, which stimulate fungal mycelia to accelerate growth and branching, thereby enabling them to approach and invade host roots [6]. This interaction expands the plant’s absorption area and enhances the utilization of nutrients and mineral elements. For example, arbuscular mycorrhizal fungi (AMF) are attracted by plant-secreted strigolactones, which prompt the fungi to bifurcate and form unique arbuscular structures that can supply over 70% of the total phosphorus acquired by the host plant [34]. Concurrently, fungi can dynamically regulate the balance of nutrient exchange through feedback regulatory pathways that prevent energy wastage and excessive reliance on the host. Furthermore, they are influenced by hormones and secondary metabolites [35]. AMF can secrete various hormones, such as abscisic acid [36], auxin [37], and vincristine, which promote root growth in plants and enhance their stress resistance. Additionally, fungi inhibit pathogens to protect their hosts. Rhizosphere fungi can cooperate with and promote the growth of beneficial microorganisms, such as azotobacter, phosphorolytic bacteria, and actinomycetes, thereby occupying a dominant ecological niche that mitigates pathogen colonization in the rhizosphere [38]. Moreover, their synergistic interactions regulate host root exudates to construct a defensive barrier that directly repels pathogen invasion. Plants inoculated with AMF can enhance resistance genes’ expression and elevate phenolic acids, flavonoids, enzymes and other anti-pathogen compounds, thereby alleviating pathogen infections [39]. Finally, specific metabolites produced by certain rhizosphere fungi can modify environmental conditions in the rhizosphere, such as pH and redox potential, which can inhibit pathogen survival.
The detrimental effects of fungi on their hosts encompass both direct disease and indirect inhibition; what is particularly concerning is that much of the damage occurs simultaneously. Mycelial infections of roots can cause physical damage and potentially lead to plant mortality, while also diminishing the host’s stress resistance through the production of toxic metabolites, thereby impeding normal growth and development. Common pathogenic and rot fungi include Fusarium, Alternaria, Verticillium, and Pythium, which can induce conditions such as root rot, wilt, and hollow disease (see Table 2). Although non-pathogenic fungi do not directly cause plant diseases, they can still be harmful by inhibiting beneficial rhizosphere microorganisms from competing with plants for nutrients, thereby indirectly reducing biomass. Additionally, these fungi may pose threats through mechanisms such as spore adhesion, rapid growth, and the metabolic accumulation of mycotoxins once they exit the soil environment.

Table 2.
Fungi associated with plant diseases in agricultural ecosystems and their ecological–agronomic contexts.
Recently, a novel classification system for the rhizosphere microbiome based on distinct assembly mechanisms be published, which delineates the microbiome into two dominant factors: environmental dominance, which is influenced by rhizosphere soil characteristics (approximately 96.5%), and plant genetic dominance, which is determined by the plant host genotype (less than 3.5%) [61]. This classification framework enhances our understanding of the assembly mechanisms of the rhizosphere microbiome and provides a new perspective for future research endeavors.
4. Inter- and Intra-Specific Communication of Rhizosphere Fungi
The rhizosphere microbiome represents a distinct microenvironment characterized by a diverse array of organisms and abiotic components within a specific spatiotemporal context. This microbiome constitutes a complex network of interrelated factors that sustaining a dynamic equilibrium and exhibit a certain degree of buffering capacity as a cohesive entity. Within the rhizosphere microbiome, various microbial species, along with fungi and plants, engage in interactions through direct contact facilitated by structures such as vesicles and mycelia, as well as through non-contact intercellular secretory systems that utilize diffusible molecules. Although multiple mechanisms underpin these interactions, our understanding of the precise processes involved remains insufficiently developed (see Figure 2).
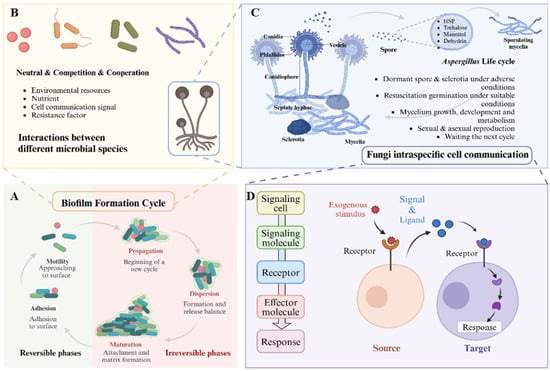
Figure 2.
Interspecific interactions and intraspecific communication mechanisms of fungi within the rhizosphere microbiome. (A) Biofilm formed on the root surface exemplifies the interaction among various microorganisms. (B) Within these biofilms, relationships among members can be categorized as neutral, competitive, or cooperative, influenced by factors such as environmental resources, nutrient availability, cellular communication signals, and resistance mechanisms. (C) For example, in fungal biofilms, particularly those involving Aspergillus, the life cycle of individual strains is profoundly affected by cellular communication. This process encompasses the activation of dormant spores under unfavorable conditions; when conditions improve, these spores commence germination, followed by hyphal growth, development, and metabolic activity, ultimately culminating in the next reproductive cycle through either sexual or asexual means. (D) Intraspecific cells respond to external stimuli through ligand-receptor interactions. Essential components of this process include the exogenous stimulus, the perception and secretion of signals by source cells, the reception of these signals by target cells, and the subsequent activation of intracellular pathways in response.
4.1. Hyphosphere
The hyphosphere is a specialized zone within the soil, characterized by the proliferation of fungal mycelia, which distinguishes it from typical soil environments. This zone closely overlaps with plant roots, forming the rhizosphere mycelium. During dynamic interactions with plants, specific microbial communities are recruited and ultimately develop into the rhizosphere microbiome. The mycelial network established by mycelia maintains the stability of this microenvironment and constitutes a diffusion network for signaling molecules, thereby expanding the sensing range of microbial interactions. This phenomenon is commonly referred to as the “fungal superhighway”, with typical filamentous fungi such as Aspergillus and Mucor play an important role [62,63].
In terms of resource allocation, the hyphosphere regulates the distribution of resources within the soil to mitigate competition among plants and maintain ecological balance. Organisms within the hyphosphere engage in cooperative and symbiotic relationships to compensate for the fungi’s limitations in utilizing organic nutrients, primarily by producing enzymes and facilitating the mineralization of organic matter [17]. Concurrently, the mycelium in the rhizosphere enhances the host plant’s nutrient absorption capabilities and facilitates the transfer of energy within the plant system [38]. Regarding interspecific communication, mycelium serves as a mediator for the transmission of information both within individual plants and among different species. The mycelial network in the soil can convey defense signals secreted by the host in response to pest attacks to neighboring plants, thereby assisting them in addressing potential threats [64]. For instance, the spores of non-spore-forming Streptomyces can activate the chemotactic mechanisms of soil bacteria such as Bacillus subtilis and Pseudomonas fluorescens [65], prompting their migration from the bulk soil to the nodule layer of plant roots [66]. This interaction fosters a more robust legume-rhizobia symbiosis, with Streptomyces playing a pivotal role in this ecological relationship.
4.2. Diffusible Signaling Molecule
Diffusible signaling molecules (DSMs) are synthesized within cells and subsequently secreted into the extracellular environment by both plant and microbial organisms. These molecules facilitate dynamic interactions in a non-contact manner, thereby expanding their influence [67]. A diverse array of signaling molecules exists, and even identical molecules can elicit markedly different responses, potentially attributable to variations in the signaling detection and response mechanisms across different species [68] (see Table 3).
The significance of plant-derived signaling molecules in mediating interactions between plants and rhizosphere fungi is noteworthy. Throughout their growth, plants generate a variety of molecules that orchestrate their physiological processes at various developmental stages [69]. For example, hormones such as indole-3-acetic acid (IAA), cytokinins (CTK), gibberellins (GA), ethylene (ET), and abscisic acid (ABA) play crucial roles in organ development [70]. Furthermore, in response to environmental stressors, plants emit volatile compounds such as nitric oxide (NO) that serve as signals to neighboring plants, thereby enhancing the collective responsiveness of plant populations [71]. Additionally, signaling molecules produced by plants not only function within the plant system but are also secreted into the rhizosphere to modulate the activity of microorganisms [72]. Conversely, microorganisms can synthesize and secrete analogs of plant signals that penetrate the host from the rhizosphere, thereby extending their influence to the aerial parts of the plant. For instance, the compound 3-oxo-C12-HSL, produced by Pseudomonas aeruginosa, can readily infiltrate plant tissues and impact their nutritional and defense mechanisms [73].
DSMs facilitate both intra- and interspecific communication among microbial populations. The rhizosphere is inhabited by over 30,000 microbial species, with cell densities ranging from 1010 to 1012 cells per gram of soil—approximately 1000 to 2000 times greater than those found in non-rhizosphere soils [27]. This elevated population density not only enhances the likelihood of cellular communication but also intensifies competition for survival resources among microorganisms. In response to the environmental pressures associated with high cell density, microorganisms employ quorum-sensing (QS) mechanisms to stabilize the spatial distribution of the rhizosphere microbiome [74]. Quorum-sensing molecules (QSMs) are synthesized intracellularly and subsequently secreted extracellularly, mediating adaptive behavioral changes in microorganisms [75]. While quorum sensing is prevalent in both bacterial and fungal species, the potential for QSMs to mediate cross-species regulation remains uncertain. Bacterial QSMs can be classified into N-acyl-homoserine lactones (AHLs) in Gram-negative bacteria, autoinducing peptides (AIPs) in Gram-positive bacteria, and autoinducer 2 (AI-2), which functions as a universal signaling molecule among both Gram-positive and Gram-negative bacteria [76]. In fungi, QSMs encompass alcohols, oxylipins, small peptides, aldehydes, and volatile organic compounds (VOCs) [75,77]. Beyond their role as communication molecules that can globally regulate transcriptional activity among microbial cells, QSMs can also exert direct influences on plant hosts, even in the absence of quorum-sensing mechanisms within the plant [64]. Once the concentration of QSMs within the rhizosphere microbiome surpasses a certain threshold, they can synchronize microbial community behavior and indirectly affect the host organism. At present, the types that can be used both as fungal QSMs and biocontrol products include peptides and aldehydes; however, more specific compounds have yet to be refined, indicating potential for further development.

Table 3.
Diffusible Signaling Molecules (DSMs) Produced by Rhizospheric Fungi and Their Communication Roles.
Table 3.
Diffusible Signaling Molecules (DSMs) Produced by Rhizospheric Fungi and Their Communication Roles.
| Fungi | Produced Molecules | Communication Type (Intraspecific/Interspecific/Interkingdom) | Function | References |
|---|---|---|---|---|
| Candida spp. | Farnesol Tyrosol γ-butyrolactone Phenylethanol, 3,4-Dihydroxyphenyl ethanol | Intraspecific | Regulate morphological transitions (yeast-to-hyphae); control biofilm maturation and quorum sensing; coordinate virulence gene expression. | [78,79] |
| Aspergillus spp. | Gliotoxin Pyomelanin 1-Octen-3-olCyclopiazonic acid | Intraspecific & Interspecific (bacteria/fungi) | Mediate competition in microbial communities via antimicrobial activity; regulate fungal sporulation. | [80,81] |
| Trichoderma spp. | 6-Pentyl-α-pyrone (6-PAP) Trichovirin Volatile terpenes Trichodermin Chitinase-inducing factors | Interspecific & Interkingdom (Plant) | Inhibit phytopathogens (e.g., Fusarium oxysporum); induce plant systemic resistance via jasmonic acid signaling. | [82] |
| Penicillium spp. | Penicillic acid Sesquiterpenoids Roquefortine C PR-toxin Citrinin | Intraspecific & Interspecific | Regulate fungal growth; modulate plant defense responses in fruits (e.g., apples). | [83] |
| Rhizopus spp. | Putrescine Cadaverine Spermidine Ethylene precursor (1-aminocyclopropane -1- carboxylic acid) | Intraspecific & Interkingdom (Plant) | Promote fungal spore germination; enhance plant root elongation and nutrient uptake. | [84] |
| Arbuscular Mycorrhizal Fungi (AMF) e.g., Glomus spp. | Strigolactone analogs Flavonoid-inducing factors Mycorrhizal lipochitooligosaccharides (Myc-LCOs) Strigolactone mimics | Interkingdom (Plant–Fungus) | Trigger fungal spore germination and hyphal branching, stimulate plant root colonization and phosphate transport; facilitating the establishment of mycorrhizal symbiotic relationships. | [85] |
| Saccharomyces cerevisiae | 2-Phenylethanol Maltol Isoamyl alcohol 2-Methylpropanol | Intraspecific | Regulates yeast flocculation and fermentation processes; participate in signal transmission for nutrient exchange during symbiosis with plants; inhibit bacterial biofilm formation (e.g., E. coli). | [86,87] |
| Fusarium spp. | Fusaric acid Fumonisins Beauvericin Zearalenone | Interspecific & Interkingdom (Plant) | Suppress plant immune responses; modulate plant hormone signaling (auxin pathways); facilitate fungal invasion of cereal crops (e.g., wheat). | [88] |
| Metarhizium spp. | Volatile organic compounds (e.g., 1-octen-3-ol, 3-Octanone) Destruxins | Interkingdom (plants/insects) | Stimulate plant growth via auxin-like activity; attract insect hosts for fungal pathogenesis. | [89] |
| Piriformospora indica | Indole-3-acetic acid (IAA) Gibberellin A3 Sphingolipids | Interkingdom (plants) | Promote plant growth through hormone signaling; enhance stress tolerance. | [90] |
4.3. Biofilm
Biofilm formation is a significant phenomenon associated with quorum sensing. As the density of microorganisms in a specific area increases, the concentration of secreted quorum-sensing molecules reaches a threshold that triggers the aggregation of free microorganisms into a multicellular biofilm that adheres to a host surface [91]. It is estimated that between 40% and 80% of microorganisms on Earth exist in biofilm form, which enhances communication and cooperation among these organisms, thereby enabling them to exploit ecological niches more effectively [92].
Alpkvist has proposed a three-dimensional model of biofilm architecture, which consists of an inner structure comprising the basal layer, conditional layer, and connection layer, arranged from the innermost to the outermost layer, with the outermost part enveloped by the biofilm layer [93]. Extracellular polymeric substances (EPS) secreted by microorganisms, including polysaccharides, proteins, nucleic acids, and lipids, account for over 90% of the biofilm’s composition [94]. Various protein components, particularly matrix proteins, play a crucial role in promoting the formation, development, and maturation of biofilms. Additionally, extracellular DNA (eDNA) and lipids present on the biofilm surface can interact with positively charged elements within the matrix, thereby contributing to the stability of the biofilm structure [95].
Biofilms that are firmly attached to plant roots create a relatively independent and enclosed environment, which enhances the colonization potential of internal microorganisms and strengthens the interactions among microbes, plants, and soil [96]. Additionally, the interior of the biofilm encapsulates and stabilizes a greater quantity of nutrients, thereby improving resource utilization by microorganisms and enhancing their resilience to various environmental stresses, including fluctuations in temperature, osmotic pressure, and pH levels. Biofilms also serve as significant mechanisms for virulence and resistance [97]. During biofilm formation, microorganisms can initiate a series of defensive responses, including the synthesis of antibiotics, degrading enzymes, and other antibacterial substances, as well as inducing resistance to antimicrobials and evading the host immune system. This enhanced capability also facilitates more stable horizontal gene transfer through the exchange of extracellular genetic material within the biofilm [98].
5. Application and Development
The rhizosphere microbiome is a crucial component and medium for plant interactions with soil and the atmosphere. It is characterized by its dynamic nature and its ability to sustain the stability of both the plant host and the soil environment. Notably, this multifunctional assemblage comprises microorganisms that exhibit strong self-healing properties and considerable potential for artificial manipulation. Consequently, rhizosphere microorganisms are expected to play an increasingly important role in agricultural production, environmental protection, biotechnology, and various other fields. However, the lack of clarity regarding the regulatory mechanisms governing individual microorganisms, as well as their adaptive strategies in response to environmental uncertainties, presents challenges to their development. Therefore, research and applications concerning rhizosphere microorganisms are focused on advancing more precise and efficient methodologies.
5.1. Ecological Restoration
Characteristic species or communities within the rhizosphere microbiome have been employed as indicators of overall soil health. This approach facilitates multifaceted monitoring of various soil types, including sandy soils, those with acidic or alkaline properties, and soils differing in metal content and organic pollutants [99]. Rhizosphere microorganisms are essential for the healthy growth of their host plants, as they possess sensitive defense mechanisms against environmental stressors [100]. For example, they produce EPS with diverse types and structures that help retain water, enhance the absorption of metal ions and organic matter, and play a significant role in the remediation of contaminated soil and water (see Table 4).
Exopolysaccharides secreted by rhizosphere microorganisms constitute over 90% of biofilm composition, which includes polysaccharides, proteins, nucleic acids, and lipids. These exopolysaccharides exhibit significant biosorption and flocculation properties, including scleroglucan, beta-glucan, split-fold polysaccharide, pullulan, and galactan, among others [101]. Investigations have identified genes associated with polysaccharide synthesis in biofilms, ranging from 12 to 17 kilobases in length, which primarily participate in the processes of monosaccharide addition, acylation of monosaccharides, monosaccharide polymerization, and polysaccharide secretion [102]. The anionic groups of polysaccharides, such as hydroxyl, carboxyl, amino, and sulfate, are rich in coordination atoms like oxygen and nitrogen, which can form complexes with ions. Additionally, these groups can interact with metal ions and organic matter, encapsulating them through alterations in membrane structure, thereby facilitating the degradation and metabolism of pollutants through the synergistic actions of biofilm constituents [103].

Table 4.
Fungi for ecological restoration.
Table 4.
Fungi for ecological restoration.
| Type | Contaminant | Application | Repair | Species | References |
|---|---|---|---|---|---|
| Cellulose decomposing fungi | micropollutants | Forest and grassland | Secreting cellulase and lignin enzyme to degrade large plants and promote ecological cycle | Phanaerochaete chrysosporium Pleurotus ostreatus | [104] |
| arbuscular mycorrhizal fungi | Barren land | Sandy land | Form symbiotic relationship with plant roots to enhance plant resistance to abiotic stresses, thus improving soil structure | Rhizophagus Diversispora epigaea Gigaspora rosea | [105] |
| Halophilic fungi | Rigid, deteriorating soil | Mining areas Saline-alkali land | Adapt and improve soil structure and fertility through metabolism to promote vegetation recovery | Aspergillus glaucus | [106] |
| Plastic degrading fungi | Plastic polymer | litter-piled field | Biological reactions occur with plastics through the enzymes produced, breaking chemical bonds between plastic molecules or polymers |
Alternaria alternate
Aspergillus tubingensis Penicillium oxalicum Pestalotiopsis microspora | [107] |
| Pesticide-tolerant fungi | Organochlorine, phosphorus and other pesticides | Overused farmland | Secretory enzymes form a degradation system, decomposes pesticide molecules into inorganic compounds through co-metabolism and mineralization |
Phanerochaete
Acremonium sp. Rhodococcus sp. Paecilomyces lilacinus | [108,109] |
| basidiomycetes | polycyclic aromatic hydrocarbons, polychlorinated biphenyls | Soil Water | intracellular segregation, organic acid precipitation and metal binding proteins | Aspergillus niger Podospora anserina | [110] |
| Mushroom | Heavy metal, Dyes, etc. | Wastewater and field | Mycelium cell wall has ion adsorption capacity | Trachyderma lucidum Phoenix mushroom | [111,112] |
| Algae | water contamination | Water | Photosynthesis absorbs carbon dioxide and nutrients such as nitrogen, phosphorus and ammonia in wastewater. Both adsorption and desorption mechanisms are more effective in living cells than in dead cells | Phycoremediation | [113] |
Biofilms have specialized pollutant degradation functions, enabling the release and diffusion of functional genes (as single/double-stranded DNA or circular plasmids) [114]. These genetic materials enter new hosts through gene recombination and conjugation, facilitating horizontal gene transfer across same or different microbial species, effectively enhance the biofilm’s ability to remediate contaminated soil. Consequently, some studies aim to improve microorganisms’ pollutant degrading metabolic capacity through gene editing, aiming to apply these engineered organisms in agricultural settings [115].
However, engineered bacteria and fungi have limited natural survival, and the repair cycle remains lengthy. Efforts to modify their competitive dynamics with native microorganisms via antisense RNA and suicide genes have had minimal impact. Moreover, uncontrollable environmental factors, as well as pollutants (which may have synergistic effects and concealment), further complicate bioremediation. Beyond conventional inorganic heavy metals and microplastics, new persistent organic pollutants (e.g., endocrine disruptors, antibiotics) have emerged. Both single-species and colony biofilms are primarily effective in remediating similar pollutants in contaminated soils; however, research on the remediation of composite pollutants in diverse ecosystems remains limited [116].
Compared to animal and plant polysaccharides, microbial exopolysaccharides have drawbacks (instability, vulnerability to crop loss, soil pollution, climate change) but also advantages (low toxicity, biodegradability, sustainable use potential). Functional biofilms developed through Biofilm Integrated Nanofiber Display (BIND) technology have enhanced bioactive. Nguyen et al. engineered Escherichia coli biofilms to express amyloid CsgA fusion protein within the extracellular matrix, facilitating self-assembly into an extracellular amyloid nanofiber network [117]. This innovation improves biofilm stability and substrate adhesion, effectively transforming it into tailorable biological repair material. Subsequently, it is essential to clarify EPS functional groups’ characteristics and classification, and explore strategies (genetic manipulation, synthetic biology, etc.) to boost EPS yield in modified strains, such as A. niger has been widely used as cell factory for polysaccharide production [118]. It is important to examine the functions and synthesis mechanisms of multifunctional biofilms, as well as the combined repair capabilities of multiple strains.
Furthermore, integrating multi-omics approaches, such as metagenomics, with systems biology methodologies is essential for achieving a comprehensive understanding of the commonalities across various terrestrial and aquatic ecosystems. This integration will facilitate the development of programmable biofilm materials or artificially optimized biofilm communities that exhibit a broad degradation spectrum, high repair efficiency, and robust repair capabilities tailored to specific requirements. Ultimately, these advancements aim to enhance the restorative capacity of biofilms within ecological environments.
5.2. Host Growth Promotion
In agricultural and horticultural systems, rhizosphere microorganisms are widely deployed as biostimulants to foster crop growth and boost yields. Hormone analogs synthesized by these microorganisms mimic endogenous plant hormones, influencing not only on local plant tissues but also indirectly modulating the aboveground biomass and overall performance of host plants [119,120]. This regulatory impact is mediated by altering traits such as root system architecture and mineral nutrient acquisition, thereby achieving long-term impact across various spatial scales. For instance, research conducted by Shen Qirong’s team demonstrated that volatile organic compounds (VOCs) produced by beneficial rhizosphere bacteria can enhance the productivity of individual plant communities by stimulating phenotypic variation, resulting in a pronounced overproduction effect [121].
Plants and microorganisms have coevolved to establish highly mutualistic symbiotic relationship. Rhizosphere microorganisms serve as indicators of plant health, as their community composition and metabolite profiles accurately reflect the physiological status of host plants, which can be utilized to develop targeted plant health management strategies. Monitoring rhizosphere microbial dynamics enables the early detection of plant diseases [122,123]. Conversely, the application of beneficial microbial activation signals can indirectly fortify crop defense mechanisms and reduce reliance on chemical pesticides. In transgenic crop research, efforts are focused not only on modifying genes that directly influence crop growth indices but also on introducing genes that mediate the assembly of beneficial rhizosphere microbial communities, thereby optimizing the microenvironment to indirectly promote crop growth [124]. Bacteria are particularly amenable to such biotechnological development due to their unicellular nature. For instance, bioselenium nanoparticles (SeNPs) synthesized by selenium bacteria can induce chemotaxis and biofilm formation in plant growth-promoting rhizobacteria (PGPRs) in a dose-dependent manner. This approach reveals a novel method for recruiting beneficial soil microorganisms and proposes innovative strategies for accurately regulating plant-associated microbial communities [125].
Additionally, fungi such as Trichoderma spp. are common host plant growth promoters [126]. As one of the three major microbial resources globally, due to survival advantages, strong adsorption cell structure, and ability to secrete extracellular enzymes (cellulase, chitinase, protease, phytase, etc.) and functional factors, it has been developed into pesticides, fertilizers, growth promoters, and soil remediation agents. Across Trichoderma species and strains, growth-promoting characteristics converge on synthesizing regulators/metabolites, modulating nutrient availability, regulating physiological processes, and enhancing stress resistance (see Table 5).

Table 5.
Trichoderma used to promote plant growth.
Regulatory effects of including modulating soil microbial diversity/enzyme activity, optimizing seedling biomass allocation, promoting tillering/earing, facilitating signal perception/transmission, inducing chlorophyll/soluble protein and adjusting K/Na ratio (T. longibrachiatum HL167 [141]); activating hormone signaling for defense (T. longibrachiatum H9 [142]); enhancing nutrient absorption with heat resistance (T. longibrachiatum MD30); producing growth-promoting volatile organics (T. koningi T-51 [140]); and regulating HCN while stimulating ammonia (T. yunnanense TM10 [143]). Some (T. harzianum T22 [131]) also accelerate germination, boost seedling vitality, and induce oxidative damage protection.
5.3. Enhanced Host Resistance
Throughout long-term evolution, plants have developed a variety of defense mechanisms that are activated in response to environmental stressors. These mechanisms include enhanced physical barriers, such as the cuticle structure, as well as the secretion of fungicides and other bioactive compounds, collectively referred to as systemic acquired resistance (SAR) [144]. This adaptive response enhances the plant’s defense against pathogens, nematodes, and parasitic plants, enabling localized disease control during the initial stages of infection. Upon subsequent infection by the same pathogen, the plant’s SAR can facilitate a more rapid and effective resistance response. Microorganisms can bolster plant stress resistance, particularly through the colonization of the rhizosphere by specific beneficial microbial species, which can trigger induced systemic resistance (ISR) in plants [144]. This ISR enhances the resistance of neighboring plants without directly activating their own defense responses, which is influenced by competition among microorganisms [145].
Exploitation competition occurs when microorganisms consume limited resources and rapidly occupy spatial niches, thereby forming a physical barrier that effectively excludes pathogens. Rhizosphere microorganisms can chelate insoluble iron oxides or iron hydroxides by secreting ferriferous carriers, which allows them to quickly utilize iron chelates through specific extracellular receptors, thereby limiting the availability of essential nutrients to competing microorganisms and indirectly diminishing their survival capabilities [146]. Conversely, interference competition involves antagonistic interactions among microorganisms, wherein they secrete effectors to inhibit the growth of competitors. Antagonistic substances that can directly suppress pathogen growth include bacteriocins, antibiotics, lysozymes, and proteases [147]. Additionally, interference signals can indirectly inhibit other microorganisms by disrupting cell communication through mechanisms such as quorum quenching (QQ) [148]. This disruption impedes quorum sensing, preventing pathogens from accurately perceiving population density and hindering their ability to form biofilms, thereby weakening their pathogenicity. These interference signals are known as quorum-sensing inhibitors (QSIs), which include VOCs such as ethylene, jasmonic acid (JA), and salicylic acid (SA), as well as fatty acids (FAs) and their derivatives [149].
The application of rhizosphere microbial-induced systemic resistance offers opportunities for the development of safer and more environmentally sustainable biological strategies aimed at enhancing plant quality and combating plant diseases. Research has demonstrated that the EPS produced by Bacillus thuringiensis can limit the spread of Sclerotinia sclerotiorum in rapeseed through ISR [150]. Qian et al. revealed that rhizosphere bacteria inhibit the quorum-sensing system by blocking the synthesis of the quorum-sensing molecule AHL in pathogenic bacteria [151]. This finding represents the first documented instance of QQ that does not involve the degradation of QSMs but rather interferes with their synthesis. Directly applying artificially optimized microbial strains to crop cultivation soil or utilizing fermentation products in the plant rhizosphere can help reduce soil biological barriers and improve crop yields. Research has shown that the application of the proteolytic Bacillus OSUB18 as a root infusion can activate ISR in Arabidopsis, enhancing the plant’s resistance to Pseudomonas syringae and Botrytis cinerea [152]. Currently, a summary of commonly utilized biocontrol microorganisms is presented in Table 6, which highlights the five most prevalent microbial pesticide varieties: Bacillus thuringiensis, Bacillus subtilis, Heliothis armigera nucleopolyhedrovirus (HaNPV), Metarhizium anisopliae CQMa421, and Bacillus polymyxa KN-03.
Furthermore, research has demonstrated that the combination of multiple bacterial or fungal strains often yields more significant effects than the application of a single strain, including unexpected outcomes that cannot be achieved by any individual strain. For instance, the ARC bactericide developed by Li’s research team consists of four distinct bacteria: Bacillus amyloliquefaciens, Brevibacillus laterosporus, Bacillus mucilaginosus, and Enterobacter ludwigii. Remarkably, it shows substantial benefits in improving quality, fixing nitrogen, and inhibiting A. flavus only when all four strains coexist. This phenomenon is believed to be linked to specific signaling molecules generated through their interactions; however, the precise mechanisms underlying this effect remain to be elucidated [153]. Currently, ARC is widely used in peanut and soybean cultivation across various provinces in China.

Table 6.
Microbial agent products for pathogen control.
Table 6.
Microbial agent products for pathogen control.
| Genus | Species | Applied | Control | Product (Country) | References |
|---|---|---|---|---|---|
| Bacillus | B. amyloliquefaciens B. cepacia B. cereus B. laterosporus B. lichenifomis B. subtilis B. thuringiensis B. velezensis | Various grain Cotton Vegetable Fruit Arabidopsis | Sheath blight Blight Chlorotic wilt Black rot, Anthrax, Rice blast Lepidoptera, Root-knot nematode | ARVATICO (China) Double nickel (Canada) XenTari WG (Canada) Companion (USA) Provilar (USA) | [154,155] |
| Pseudomonas | P. fluorescens P. putida P. brassicacaerum P. oryzihabitans P. marginali | Tomato, potato, cucumber, rape, strawberry, Cotton, Various grain, Tobacco | Bacterial wilt, Plague, Gray mold, Root rot, Black shank | GaoTian (China) | [156,157,158] |
| Agrobacterium | A. amazonense A. radiobacter A. rhizoides A. larrymoorei | Fruit tree, rice | Root cancerosis | K599 (China) | [159] |
| Collimonas | C. fungivorans | Tomato, cabbage, watermelon, jujube | Scab Black rot | Complex biological agent (China) | [160] |
| Comamonas | C. acidovorans C. aquatica | Rice Kiwi | Sheath blight | Ca30 (China) | [161] |
| Trichoderma | T. harzianum T. longibrachiatum T. viride T. asperellum T. atroviride | Various grain, vegetable, fruit. Agricultural and forestry fields | Dry rot, Wilt, Verticillium | Trianum (Canada) TrichoPlus (USA) RootShield Plus (USA) Trichodex®, Trianum® (Holland) | [162,163] |
| Streptomyces | S. jingyangensis S. bungoensis S. fimbriatus S.lydicus S.griseoviridis | Vegetable, Pear | Anthrax, Gray mold Black blot | TJ561 (China) Actinovate®, Mycostop® | [164] |
| Aspergillus | A. oryzae A. niger A. awamori A. glaucus A. terreus | Rice Maize Peanut | Blast | CGMCCNo.7127 (China) | [165] |
| Penicillum | P. frequentans P. baileii P. citri P.P.P. | Cucumber Apple Maize Wheat Cotton Sesame Tobacco | Wilt Verticillium Anthracnose Root rot botrytis | MoonBiotech (China) | [166] |
| Paecilomyces | P. lilacinus | Various fruits and vegetables, tobacco, rice, oil crops | Plant pathogenic nematode | JBC033 (China) | [167] |
| Beauveria | B. bassiana | Forest, nurserie, lawn, field | Grub, ostrinia nubilalis Dendrolimus Empoasca vitis | Tackler (India) Tezpetix Beauve (USA) ARBIOGY (China) | [168,169] |
| Metarhizium | M. anisopliae M. ablum M. cylindrosporum M. acridum M. lepidiotae M. globosum M.robertsii M.majus M.rileyi | Agriculture and forestry, fruit trees, fields | Scarabean beetle Weevil Wireworm Lepidoptera Hemiptera | JULIXIN (China) | [170,171,172] |
6. Discussion
The interactions between plant and microbial communities are more intricate than mere pairwise relationships, which constitute a dynamic, multi-layered network woven from metabolic crosstalk, signal transduction, and co-evolutionary feedback loops [63]. These interactions operate across spatial scales, from the microenvironments of root hairs to entire agroecosystems. Such complexity renders obsolete the reductionist approaches of the past, which isolated individual microorganisms or plant species for study. It is now imperative to adopt a systems ecology perspective, one that integrates metagenomic data, metabolomic profiling, and environmental monitoring to capture the full breadth of interactions. This shift in framework is not merely a methodological choice but a scientific necessity, yet it presents profound challenges, from the computational hurdles of modeling 5000+ interacting species in a single soil sample to the technical limitations of tracking transient quorum-sensing molecules like acyl-homoserine lactones in real time [173].
Nevertheless, the rhizosphere microbiome has demonstrated a remarkable resilient and effective tool in agricultural practice, even amid incomplete mechanistic understanding. The urgency to unlock this potential has intensified in recent years, driven by two global imperatives: the “double reduction” policy targeting pesticide and fertilizer usage (a response to widespread soil degradation, such as cadmium contamination in 16% of Chinese farmland [174], and eutrophication of 50% of U.S. lakes [175]) and the race to achieve carbon neutrality (which demands agricultural systems that sequester 0.5–1.0 Pg of carbon annually). Together, these goals have transformed the development of innovative biological pesticides and biofertilizers from a niche pursuit into a strategic priority. However, the industrialization of these microbial products faces a labyrinth of technical barriers including the short shelf life of microbial agents, inconsistent efficacy, poor adaptability, and slow action, which stymied market penetration.
To overcome these challenges, researchers are turning to biomimicry that is adhering to the principles of natural systems to design more robust, cost-efficient solutions. Advances in genetic engineering technology are taking this further, shifting focus to synthetic microbial communities (SMCs) [176], as well as products that exhibit long-term stability and extended shelf life [177]. Beyond product design, the future of microbial agriculture lies in holistic integration into crop management, for example, rather than one-time inputs, “full-cycle application” is gaining traction [178,179,180].
This evolution reflects more than technological progress; it represents a fundamental shift in humanity’s relationship with nature. For decades, agriculture sought to dominate ecosystems—now, we collaborate with them. The rhizosphere microbiome once hidden has become a cornerstone of this approach. As we deepen understanding and refine engineering, we move closer to a future where agriculture is not just productive, but regenerative: feeding 9.7 billion people by 2050 while healing degraded soils and mitigating climate change.
Author Contributions
Conceptualization, J.G.; validation, A.D., J.L. and J.X.; writing—original draft preparation, J.G.; writing—review and editing, J.G.; supervision, Z.L. and A.F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Pioneer” and ”Leading Goose” R&D Program of Zhejiang (Grant No. 2024SSYS0103).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fuhrman, J.A. Microbial community structure and its functional implications. Nature 2009, 459, 193–199. [Google Scholar] [CrossRef]
- Kayiranga, A.; Isabwe, A.; Yao, H.; Shangguan, H.; Coulibaly, J.L.K.; Breed, M.; Sun, X. Distribution patterns of soil bacteria, fungi, and protists emerge from distinct assembly processes across subcommunities. Ecol. Evol. 2024, 14, e11672. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y.H. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar] [CrossRef] [PubMed]
- Pantigoso, H.A.; Newberger, D.; Vivanco, J.M. The rhizosphere microbiome: Plant-microbial interactions for resource acquisition. J. Appl. Microbiol. 2022, 133, 2864–2876. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Feng, G.; Limpens, E.; Bonfante, P.; Xie, X.; Zhang, L. Cross-kingdom nutrient exchange in the plant–arbuscular mycorrhizal fungus–bacterium continuum. Nat. Rev. Microbiol. 2024, 22, 773–790. [Google Scholar] [CrossRef]
- Khade, O.; Sruthi, K. The rhizosphere microbiome: A key modulator of plant health and their role in secondary metabolites production. In Biotechnology of Emerging Microbes; Sarma, H., Joshi, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 327–349. [Google Scholar]
- Luo, C.; He, Y.; Chen, Y. Rhizosphere microbiome regulation: Unlocking the potential for plant growth. Curr. Res. Microb. Sci. 2025, 8, 100322. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- O’Brien, A.M.; Harrison, T.L. Host match improves root microbiome growth. Nat. Microbiol. 2021, 6, 1103–1104. [Google Scholar] [CrossRef]
- Lü, H.; Tang, G.-X.; Huang, Y.-H.; Mo, C.-H.; Zhao, H.-M.; Xiang, L.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Li, Q.-X. Response and adaptation of rhizosphere microbiome to organic pollutants with enriching pollutant-degraders and genes for bioremediation: A critical review. Sci. Total Environ. 2024, 912, 169425. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Whalen, E.D.; Grandy, A.S.; Geyer, K.M.; Morrison, E.W.; Frey, S.D. Microbial trait multifunctionality drives soil organic matter formation potential. Nat. Commun. 2024, 15, 10209. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.S.; Du, D.L. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Nizamani, M.M.; Hughes, A.C.; Qureshi, S.; Zhang, Q.; Tarafder, E.; Das, D.; Acharya, K.; Wang, Y.; Zhang, Z.-G. Microbial biodiversity and plant functional trait interactions in multifunctional ecosystems. Appl. Soil Ecol. 2024, 201, 105515. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Védère, C.; Lebrun, M.; Honvault, N.; Aubertin, M.-L.; Girardin, C.; Garnier, P.; Dignac, M.-F.; Houben, D.; Rumpel, C. How does soil water status influence the fate of soil organic matter? A review of processes across scales. Earth-Sci. Rev. 2022, 234, 104214. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Photosynthesis. In Plant Physiological Ecology; Lambers, H., Chapin, F.S., Pons, T.L., Eds.; Springer: New York, NY, USA, 2008; pp. 11–99. [Google Scholar]
- Afzal, M.R.; Naz, M.; Yu, Y.; Yan, L.; Wang, P.; Mohotti, J.; Hao, G.F.; Zhou, J.-J.; Chen, Z.; Zhang, L.; et al. Root exudates: The rhizospheric frontier for advancing sustainable plant protection. Resour. Environ. Sustain. 2025, 21, 100249. [Google Scholar] [CrossRef]
- Fan, X.Y.; Ge, A.-H.; Qi, S.S.; Guan, Y.; Wang, R.; Yu, N.; Wang, E. Root exudates and microbial metabolites: Signals and nutrients in plant-microbe interactions. Sci. China Life Sci. 2025, 68, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zou, Y.; Zheng, J.; Qiu, S.; Liu, L.; Wei, C. Quorum sensing-mediated microbial interactions: Mechanisms, applications, challenges and perspectives. Microbiol. Res. 2023, 273, 127414. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.; Zhalnina, K.; Kosina, S.; Northen, T.R.; Sasse, J. The core metabolome and root exudation dynamics of three phylogenetically distinct plant species. Nat. Commun. 2023, 14, 1649. [Google Scholar] [CrossRef]
- Poria, V.; Singh, S.; Nain, L.; Singh, B.; Saini, J.K. Rhizospheric Microbial Communities: Occurrence, Distribution, and Functions. In Microbial Metatranscriptomics Belowground; Nath, M., Bhatt, D., Bhargava, P., Choudhary, D.K., Eds.; Springer: Singapore, 2021; pp. 239–271. [Google Scholar]
- Wang, Y.; Ebrahimi, A.; Chen, G.; Zhang, Z.; Zhu, K.; Franklin, S.; Jin, Y.; Liu, Y.; Wang, G. Active motility and chemotactic movement regulate the microbial early-colonization and biodiversity. Geoderma 2025, 460, 117419. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, Y.; Chang, H.; Wang, C.; Yang, J.; Shi, J.; Gao, J.; Yang, W.; Lan, L.; Wang, Y.; et al. An SHR–SCR module specifies legume cortical cell fate to enable nodulation. Nature 2021, 589, 586–590. [Google Scholar] [CrossRef]
- Wu, S.-H.; Luo, M.-X.; Chang, J.-T.; Chen, Y.; Liao, P.-C. Unravelling the dynamics of soil microbial communities under the environmental selection and range shift process in afforestation ecosystems. Sci. Total Environ. 2023, 898, 165476. [Google Scholar] [CrossRef]
- Pattnaik, S.S.; Busi, S. Rhizospheric fungi: Diversity and potential biotechnological applications. In Recent Advancement in White Biotechnology Through Fungi; Yadav, A.N., Mishra, S., Singh, S., Gupta, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–84. [Google Scholar]
- Wang, Z.; Li, T.; Wen, X.; Liu, Y.; Han, J.; Liao, Y.; DeBruyn, J.M. Fungal communities in rhizosphere soil under conservation tillage shift in response to plant growth. Front. Microbiol. 2017, 8, 1301. [Google Scholar] [CrossRef]
- Suyal, D.C.; Prasad, P.; Sahu, B.; Soni, R.; Goel, R. Rhizosphere fungi and their plant association: Current and future prospects. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Sharma, V.K., Shah, M.P., Parmar, S., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 339–356. [Google Scholar]
- Tang, B.; Man, J.; Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal fungi benefit plants in response to major global change factors. Ecol. Lett. 2023, 26, 2087–2097. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.-C.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Vanneste, S.; Pei, Y.; Friml, J. Mechanisms of auxin action in plant growth and development. Nat. Rev. Mol. Cell Biol. 2025, 26, 648–666. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, A.A.; Babalola, O.O. Fungi that promote plant growth in the rhizosphere boost crop growth. J. Fungi 2023, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Ghorui, M.; Chowdhury, S.; Balu, P.; Das, K.; Sunar, K. Boosting plant immunity: The functional role and mechanism of arbuscular mycorrhizal fungi in resistance. In Plant Microbiome and Biological Control: Emerging Trends and Applications; Mathur, P., Roy, S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 195–219. [Google Scholar]
- Darsaraei, H.; Afshan, N.U.S.; Afzal, S.; Zafar, I.; Anwar, A.; Goetz, M.; Khodaparast, S.A.; Braun, U.; Khodaparast, G.; Braun, S. A re-assessment of Erysiphe polygoni: Phylogenetic analyses suggest an undescribed species. Sydowia 2023, 75, 233–242. [Google Scholar]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, population biology and management of rhizoctonia seedling disease of soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Li, Y.; Yang, N.; Mu, T.; Wu, X.; Zhao, C. Diversity of mycoviruses present in strains of binucleate rhizoctonia and multinucleate rhizoctonia, causal agents for potato stem canker or black scurf. J. Fungi 2023, 9, 214. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, S.; Li, F.; Zhang, S.; Zhang, H.; Sun, R.; Li, G. Research Overview and Prospects of the Downy Mildew in Cruciferous Vegetables. Acta Hortic. Sinica 2025, 52, 1233–1250. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Y.; Hao, Y.; Lu, J. Genetic diversity and population structure of Plasmopara viticola in China. Eur. J. Plant Pathol. 2014, 140, 365–376. [Google Scholar] [CrossRef]
- Zeng, X.-Y. A checklist for identifying Meliolales species. Mycosphere 2017, 8, 218–359. [Google Scholar] [CrossRef]
- Murria, S.; Kaur, N.; Arora, N.; Mahal, A.K. Field reaction and metabolic alterations in grape (Vitis vinifera L.) varieties infested with anthracnose. Sci. Hortic. 2018, 235, 286–293. [Google Scholar] [CrossRef]
- Ma, J.; Xiao, X.; Wang, X.; Guo, M. Colletotrichum spaethianum Causing Anthracnose on Polygonatum cyrtonema in Anhui Province, China. Plant Dis. 2021, 105, 509. [Google Scholar] [CrossRef]
- Mohammadi, A.; Bahramikia, S. Molecular identification and genetic variation of Alternaria species isolated from tomatoes using ITS1 sequencing and inter simple sequence repeat methods. Curr. Med. Mycol. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, R.; Cardoza, R.E.; Rubio, M.B.; Gutiérrez, S.; Monte, E. Chapter 10 - Secondary Metabolism and Antimicrobial Metabolites of Trichoderma. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 125–137. [Google Scholar]
- Dong, S.; Zhou, S. Potato late blight caused by Phytophthora infestans: From molecular interactions to integrated management strategies. J. Integr. Agric. 2021, 21, 3456–3466. [Google Scholar] [CrossRef]
- Van Tran, Q.; Ha, C.V.; Vvedensky, V.V.; Han, V.-C. Current status and characterization of Phytophthora species associated with gummosis of citrus in Northern Vietnam. J. Phytopathol. 2023, 171, 478–488. [Google Scholar] [CrossRef]
- Chandrasekaran, U.M.; Perumal, N.; Balakrishnan, A.; Ravikumar, P. Biocontrol potential of Acremonium sclerotigenum (Moreau & R. Moreau ex Valenta) W. Gams Against Wheat Oat Rust Fungi (Puccinia Species). Indian Phytopathol. 2024, 77, 739–746. [Google Scholar] [CrossRef]
- Castillejo, M.-Á.; Fondevilla-Aparicio, S.; Fuentes-Almagro, C.; Rubiales, D. Quantitative analysis of target peptides related to resistance against Ascochyta blight (Peyronellaea pinodes) in pea. J. Proteome Res. 2020, 19, 1000–1012. [Google Scholar] [CrossRef]
- Cai, J.; Ma, D.Y.; Yu, F.; Qiang, S. Expression analysis of chickpea defense-related genes induced by Ascochyta rabiei. J. Plant Prot. 2019, 46, 1121–1131. [Google Scholar]
- Liu, X.; Li, B.; Cai, J.; Zheng, X.; Feng, Y.; Huang, G. Colletotrichum species causing anthracnose of Rubber trees in China. Sci. Rep. 2018, 8, 10435. [Google Scholar] [CrossRef]
- Qu, Z.; Ren, X.; Du, Z.; Hou, J.; Li, Y.; Yao, Y.; An, Y. Fusarium mycotoxins: The major food contaminants. mLife 2024, 3, 176–206. [Google Scholar] [CrossRef]
- Šliková, S.; Gregová, E.; Pastirčák, M. Toxin accumulation in avena species after different spray inoculation by Fusarium graminearum and F. culmorum. Cereal Res. Commun. 2019, 47, 656–668. [Google Scholar] [CrossRef]
- Riedling, O.L.; David, K.T.; Rokas, A. Global patterns of species diversity and distribution in the biomedically and biotechnologically important fungal genus Aspergillus. bioRxiv. 2024. [Google Scholar]
- He, Z.; Abeywickrama, P.D.; Wu, L.; Zhou, Y.; Zhang, W.; Yan, J.; Shang, Q.; Zhou, Y.; Li, S. Diversity of Cytospora species associated with trunk diseases of Prunus persica (Peach) in northern China. J. Fungi 2024, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dun, C.; Tang, T.; Duan, Y.; Guo, X.; You, J. Boeremia exigua causes leaf spot of walnut trees (Juglans regia) in China. Plant Dis. 2022, 106, 1993. [Google Scholar] [CrossRef]
- Xun, W.; Liu, Y.; Ma, A.; Yan, H.; Miao, Y.; Shao, J.; Zhang, N.; Xu, Z.; Shen, Q.; Zhang, R. Dissection of rhizosphere microbiome and exploiting strategies for sustainable agriculture. New Phytol. 2024, 242, 2401–2410. [Google Scholar] [CrossRef]
- Barto, E.K.; Weidenhamer, J.D.; Cipollini, D.; Rillig, M.C. Fungal superhighways: Do common mycorrhizal networks enhance below ground communication? Trends Plant Sci. 2012, 17, 633–637. [Google Scholar] [CrossRef]
- Rodríguez, L.A.Y.; Gutiérrez, P.E.Á.; Gunde-Cimerman, N.; Jiménez, J.C.C.; Gutiérrez-Cepeda, A.; Ocaña, A.M.F.; Batista-García, R.A. Exploring extremophilic fungi in soil mycobiome for sustainable agriculture amid global change. Nat. Commun. 2024, 15, 6951. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, R.; Song, Y.; Lu, J.; Zhou, B.; Song, F.; Zhang, L.; Huang, Q.; Gong, J.; Lei, J.; et al. Pyoluteorin-deficient Pseudomonas protegens improves cooperation with Bacillus velezensis, biofilm formation, co-colonizing, and reshapes rhizosphere microbiome. NPJ Biofilms Microbiomes 2024, 10, 145. [Google Scholar] [CrossRef]
- Song, H.; Li, N.; Zhang, R.; Hu, X.; Zhang, W.; Zhang, B.; Li, X.; Wang, Z.; Xin, J. Study on signal transmission mechanism of arbuscular mycorrhizal hyphal network against root rot of Salvia miltiorrhiza. Scientific. Reports. 2023, 13, 16936. [Google Scholar]
- He, Y.-W.; Deng, Y.; Miao, Y.; Chatterjee, S.; Tran, T.M.; Tian, J.; Lindow, S. DSF-family quorum sensing signal-mediated intraspecies, interspecies, and inter-kingdom communication. Trends Microbiol. 2023, 31, 36–50. [Google Scholar] [CrossRef]
- Maitra, P.; Hrynkiewicz, K.; Szuba, A.; Jagodziński, A.M.; Al-Rashid, J.; Mandal, D.; Mucha, J. Metabolic niches in the rhizosphere microbiome: Dependence on soil horizons, root traits and climate variables in forest ecosystems. Front. Plant Sci. 2024, 15, 1344205. [Google Scholar] [CrossRef]
- Gasperini, D.; Howe, G.A. Phytohormones in a universe of regulatory metabolites: Lessons from jasmonate. Plant Physiol. 2024, 195, 135–154. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gaurav, A.K.; Singh, S.; Yadav, S.; Bhowmick, S.; Abeysinghe, S.; Verma, J.P. The bioactive potential of phytohormones: A review. Biotechnol. Rep. 2022, 35, e00748. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sharma, P.; Singh, P.; Kumar, V.; Yadav, P.; Sharma, A. Nitric oxide: An emerging warrior of plant physiology under abiotic stress. Nitric Oxide 2023, 140–141, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Yun, B.-W. Nitric oxide acts as a key signaling molecule in plant development under stressful conditions. Int. J. Mol. Sci. 2023, 24, 4782. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Yadav, V.K.; Kalia, M.; Dohare, S.; Sharma, D.; Agarwal, V. Pseudomonas aeruginosa auto inducer3-oxo-C12-HSL exerts bacteriostatic effect and inhibits Staphylococcus epidermidis biofilm. Microb. Pathog. 2017, 110, 612–619. [Google Scholar] [CrossRef]
- Moreno-Gámez, S.; Hochberg, M.E.; van Doorn, G.S. Quorum sensing as a mechanism to harness the wisdom of the crowds. Nat. Commun. 2023, 14, 3415. [Google Scholar] [CrossRef]
- Padder, S.A.; Prasad, R.; Shah, A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018, 210, 51–58. [Google Scholar] [CrossRef]
- Wu, L.; Luo, Y. Bacterial quorum sensing systems and their role in intestinal bacteria-host crosstalk. Front. Microbiol. 2021, 12, 611413. [Google Scholar] [CrossRef]
- Kim, S.-R.; Yeon, K.-M. Quorum sensing as language of chemical signals. Fundamentals of Quorum Sensing, Analytical Methods and Applications in Membrane Bioreactors. Compr. Anal. Chem. 2018, 81, 57–94. [Google Scholar]
- Shirtliff, M.E.; Krom, B.P.; Meijering, R.A.; Peters, B.M.; Zhu, J.; Scheper, M.A.; Harris, M.L.; Jabra-Rizk, M.A. Farnesol-induced apoptosis in Candida albicans. Antimicrob. Agents Chemother. 2009, 53, 2392–2401. [Google Scholar] [CrossRef]
- Dižová, S.; Bujdáková, H. Properties and role of the quorum sensing molecule farnesol in relation to the yeast Candida albicans. Pharmazie 2017, 72, 307–312. [Google Scholar] [PubMed]
- Soltani, J. Secondary metabolite diversity of the genus Aspergillus: Recent advances. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 275–292. [Google Scholar]
- Vadlapudi, V.; Borah, N.; Yellusani, K.R.; Gade, S.; Reddy, P.; Rajamanikyam, M.; Vempati, L.; Gubbala, S.; Chopra, P.; Upadhyayula, S.; et al. Aspergillus secondary metabolite database, a resource to understand the secondary metabolome of Aspergillus genus. Sci. Rep. 2017, 7, 7325. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Metin, B. Penicillium roqueforti secondary metabolites: Biosynthetic pathways, gene clusters, and bioactivities. Fermentation 2023, 9, 836. [Google Scholar] [CrossRef]
- Mou, W.; Kao, Y.T.; Michard, E.; Simon, A.A.; Li, D.; Wudick, M.M.; Lizzio, M.A.; Feijó, J.A.; Chang, C. Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat. Commun. 2020, 11, 4082. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, J.; Lian, X.; Wei, B.; Ma, X.; Wu, P. Effect of Glomus intraradices on root morphology, biomass production and phosphorous use efficiency of Chinese fir seedlings under low phosphorus stress. Front. Plant Sci. 2023, 13, 1095772. [Google Scholar] [CrossRef]
- Gao, P.; Yang, S.; Jiang, Q.; Yu, P.; Yang, F.; Zhang, X.; Zhang, Z.; Liu, S.; Xia, W. Effect of quorum-sensing molecule 2-phenylethanol on fermentation performance of Saccharomyces cerevisiae 31 in simulated sour fish. Food Microbiol. 2025, 132, 104822. [Google Scholar] [CrossRef]
- Maillet, F.; Poinsot, V.; André, O.; Puech-Pagès, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A.; et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef]
- Darino, M.; Jaiswal, N.; Darma, R.; Kroll, E.; Urban, M.; Xiang, Y.; Srivastava, M.; Kim, H.; Myers, A.; Scofield, S.; et al. The Fusarium graminearum Effector Protease FgTPP1 Suppresses Immune Responses and Facilitates Fusarium Head Blight Disease. Mol. Plant-Microbe Interact. 2025, 38, 297–314. [Google Scholar]
- Wood, M.J.; Kortsinoglou, A.M.; Khoja, S.; Kouvelis, V.N.; Myrta, A.; Midthassel, A.; Butt, T.M. Metarhizium brunneum (Hypocreales: Clavicipitaceae) and Its Derived Volatile Organic Compounds as Biostimulants of Commercially Valuable Angiosperms and Gymnosperms. J. Fungi 2022, 8, 1052. [Google Scholar]
- Hussien, R.H.M.; Ghareeb, S.; Kalmosh, F.S. Metarhizium species and their volatile organic compounds: Promising alternatives for the two-spotted spider mite, Tetranychus urticae management. BioControl 2025, 70, 345–355. [Google Scholar] [CrossRef]
- Cheng, C.; Li, D.; Wang, B.; Liao, B.; Qu, P.; Liu, W.; Zhang, Y.; Lu, P. Piriformospora indica colonization promotes the root growth of Dimocarpus longan seedlings. Sci. Hortic. 2022, 301, 111137. [Google Scholar] [CrossRef]
- Saxena, P.; Joshi, Y.; Rawat, K.; Bisht, R. Biofilms: Architecture, Resistance, Quorum Sensing and Control Mechanisms. Indian J. Microbiol. 2019, 59, 3–12. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Alpkvist, E.; Picioreanu, C.; Loosdrecht, M.C.M.; Heyden, A. Three-dimensional biofilm model with individual cells and continuum EPS matrix. Biotechnol. Bioeng. 2006, 94, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; van Hullebusch, E.D.; Little, B.J.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. Microbial extracellular polymeric substances in the environment, technology and medicine. Nat. Rev. Microbiol. 2025, 23, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.; Kumawat, R.; Prasanna, R.; Jothieswari, D.; Debnath, R.; Ikbal, A.M.A.; Palit, P.; Rawat, R.; Gopikrishna, K.; Tiwari, O. Microbial exopolysaccharides: Classification, biosynthetic pathway, industrial extraction and commercial production to unveil its bioprospection: A comprehensive review. Int. J. Biol. Macromol. 2025, 297, 139917. [Google Scholar] [CrossRef]
- Li, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Biofilms formation in plant growth-promoting bacteria for alleviating agro-environmental stress. Sci. Total Environ. 2024, 907, 167774. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Philipp, L.-A.; Bühler, K.; Ulber, R.; Gescher, J. Beneficial applications of biofilms. Nat. Rev. Microbiol. 2024, 22, 276–290. [Google Scholar] [CrossRef]
- Wang, H.R.; Du, X.R.; Zhang, Z.Y.; Feng, F.J.; Zhang, J.M. Rhizosphere interface microbiome reassembly by arbuscular mycorrhizal fungi weakens cadmium migration dynamics. iMeta 2023, 2, e133. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Nomura, K.; Wang, X.; Sohrabi, R.; Xu, J.; Yao, L.; Paasch, B.C.; Ma, L.; Kremer, J.; Cheng, Y.; et al. A plant genetic network for preventing dysbiosis in the phyllosphere. Nature 2020, 580, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-T.; Nguyen, T.-D.; Nguyen, T.-T.; Pham, M.-N.; Nguyen, P.-T.; Nguyen, T.-U.T.; Huynh, T.-T.; Nguyen, H.-T. Rhizosphere bacterial exopolysaccharides: Composition, biosynthesis, and their potential applications. Arch. Microbiol. 2024, 206, 388. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Kallman, J.; Liu, X.; Meng, M.; Lin, J. Polysaccharide biosynthetic pathway profiling and putative gene mining of Dendrobium moniliforme using RNA-Seq in different tissues. BMC Plant Biol. 2019, 19, 521. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; He, Y.; Ji, R. Biodegradation of phenolic pollutants and bioaugmentation strategies: A review of current knowledge and future perspectives. J. Hazard. Mater. 2024, 469, 133906. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Śliżewska, W.; Struszczyk-Świta, K.; Marchut-Mikołajczyk, O. Metabolic potential of halophilic filamentous fungi—Current perspective. Int. J. Mol. Sci. 2022, 23, 4189. [Google Scholar] [CrossRef]
- Okal, E.J.; Heng, G.; Magige, E.A.; Khan, S.; Wu, S.; Ge, Z.; Zhang, T.; Mortimer, P.; Xu, J. Insights into the mechanisms involved in the fungal degradation of plastics. Ecotoxicol. Environ. Saf. 2023, 262, 115202. [Google Scholar] [CrossRef]
- Serdar, O.; Yıldırım, N.; Onder Erguven, G.; Ketenalp, Z.; Cikcikoglu Yildirim, N.; Durmus, B. The Bioremediation of Industrial Effluents by Phanerochaete Chrysosporium: The COD, TOC Removal Efficiency and Biochemical Assessment for Dreissena Polymorpha. ChemistrySelect 2024, 9, e202304258. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Y.; Yang, M.; Zhang, J.; Lin, D. Earthworms enhance the bioremediation of tris (2-butoxyethyl) phosphate-contaminated soil by releasing degrading microbes. J. Hazard. Mater. 2023, 452, 131303. [Google Scholar] [CrossRef] [PubMed]
- Treu, R.; Falandysz, J.; Health, P.B. Mycoremediation of hydrocarbons with basidiomycetes—A review. J. Environ. Sci. Health 2017, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Corral-Bobadilla, M.; González-Marcos, A.; Vergara-González, E.P.; Alba-Elías, F. Bioremediation of waste water to remove heavy metals using the spent mushroom substrate of Agaricus bisporus. Water 2019, 11, 454. [Google Scholar] [CrossRef]
- Sahithya, K.; Mouli, T.; Biswas, A. Remediation potential of mushrooms and their spent substrate against environmental contaminants: An overview. Biocatal. Agric. Biotechnol. 2022, 42, 102323. [Google Scholar] [CrossRef]
- Kandasamy, S.; Narayanan, M.; He, Z.; Liu, G.; Ramakrishnan, M.; Thangavel, P.; Pugazhendhi, A.; Raja, R.; Carvalho, I.S. Current strategies and prospects in algae for remediation and biofuels: An overview. Biocatal. Agric. Biotechnol. 2021, 35, 102045. [Google Scholar] [CrossRef]
- Mishra, S.; Huang, Y.; Li, J.; Wu, X.; Zhou, Z.; Lei, Q.; Bhatt, P.; Chen, S. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 2022, 294, 133609. [Google Scholar] [CrossRef]
- Imam, A.; Kumar Suman, S.; Kanaujia, P.K.; Ray, A. Biological machinery for polycyclic aromatic hydrocarbons degradation: A review. Bioresour. Technol. 2022, 343, 126121. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, X.; Xiong, J.; Yan, B.; Gao, R.; Liu, S.; Zong, M.; Ge, J.; Lou, W. Hydrolase mimic via second coordination sphere engineering in metal-organic frameworks for environmental remediation. Nat. Commun. 2023, 14, 5974. [Google Scholar] [CrossRef]
- Nguyen, P.Q.; Botyanszki, Z.; Tay, P.K.R.; Joshi, N.S. Programmable biofilm-based materials from engineered curli nanofibres. Nat. Commun. 2014, 5, 4945. [Google Scholar] [CrossRef]
- Meyer, V.; Wu, B.; Ram, A.F. Aspergillus as a multi-purpose cell factory: Current status and perspectives. Biotechnol. Lett. 2011, 33, 469–476. [Google Scholar] [CrossRef]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Mechanisms and Impact of Rhizosphere Microbial Metabolites on Crop Health, Traits, Functional Components: A Comprehensive Review. Molecules 2024, 29, 5922. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, O.S.; Mazumder, M.; Kini, S.; Hill, E.D.; Aow, J.S.B.; Phua, S.M.L.; Elejalde, U.; Kjelleberg, S.; Swarup, S. Volatile methyl jasmonate from roots triggers host-beneficial soil microbiome biofilms. Nat. Chem. Biol. 2024, 20, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Raza, W.; Jiang, G.; Yi, Z.; Fields, B.; Greenrod, S.; Friman, V.; Jousset, A.; Shen, Q.; Wei, Z. Bacterial volatile organic compounds attenuate pathogen virulence via evolutionary trade-offs. ISME J. 2023, 17, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Solomon, W.; Janda, T.; Molnár, Z. Unveiling the significance of rhizosphere: Implications for plant growth, stress response, and sustainable agriculture. Plant Physiol. Biochem. 2024, 206, 108290. [Google Scholar] [CrossRef]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.-M.; Zhou, X.; Zhang, A.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Wang, Y.; Wang, M.; Xie, X.; Chang, H.; Wang, L.; Qu, J.; Sun, K.; He, W.; et al. Mycorrhizal symbiosis modulates the rhizosphere microbiota to promote rhizobia–legume symbiosis. Mol. Plant 2021, 14, 503–516. [Google Scholar] [CrossRef]
- Alvi, G.B.; Iqbal, M.S.; Ghaith, M.M.S.; Haseeb, A.; Ahmed, B.; Qadir, M.I. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have anti-bacterial activities. Sci. Rep. 2021, 11, 4811. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases—A review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Sehim, A.E.; Hewedy, O.A.; Altammar, K.A.; Alhumaidi, M.S.; Abd Elghaffar, R.Y. Trichoderma asperellum empowers tomato plants and suppresses Fusarium oxysporum through priming responses. Front. Microbiol. 2023, 14, 1140378. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Manfra, M.; De Nisco, M.; Tenore, G.; Troisi, J.; Di Fiori, R.; Novellino, E. Trichoderma harzianum strain T-22 induces changes in phytohormone levels in cherry rootstocks (Prunus cerasus × P. canescens). Plant Growth Regul. 2011, 65, 421–425. [Google Scholar] [CrossRef]
- Contina, J.B.; Dandurand, L.M.; Knudsen, G.R. Use of GFP-tagged Trichoderma harzianum as a tool to study the biological control of the potato cyst nematode Globodera pallida. Appl. Soil Ecol. 2017, 115, 31–37. [Google Scholar] [CrossRef]
- Borges, J.; Muniz, P.; Peixoto, G.; Oliveira, T.; Elizabeth, D.; Rodrigues, F.; Costa Carvalho, D.D. Promotion of Seedling Growth and Production of Wheat by Using Trichoderma spp. J. Agric. Sci. 2018, 10, 267. [Google Scholar] [CrossRef]
- Shakeri, J.; Foster, H.A. Proteolytic activity and antibiotic production by Trichoderma harzianum in relation to pathogenicity to insects. Enzym. Microb. Technol. 2007, 40, 961–968. [Google Scholar] [CrossRef]
- Chowdappa, P.; Kumar, S.M.; Lakshmi, M.J.; Upreti, K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- Jena, B.; Senapati, A.; Priyadarshinee, L.; Sen, A.; Sciences, P. Efficacy of T harzianum isolates in plant growth promotion and BLB management in rice. GSC Biol. Pharm. Sci. 2022, 20, 221–226. [Google Scholar] [CrossRef]
- Arif, S.; Munis, M.F.H.; Liaquat, F.; Gulzar, S.; Haroon, U.; Zhao, L.; Zhang, Y. Trichoderma viride establishes biodefense against clubroot (Plasmodiophora brassicae) and fosters plant growth via colonizing root hairs in pak choi (Brassica campestris spp. chinesnsis). Biol. Control 2023, 183, 105265. [Google Scholar] [CrossRef]
- Nykiel-Szymańska, J.; Bernat, P.; Słaba, M. Biotransformation and detoxification of chloroacetanilide herbicides by Trichoderma spp. with plant growth-promoting activities. Pestic. Biochem. Physiol. 2020, 163, 216–226. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Valencia-Cantero, E.; Santoyo, G. Plant growth-promoting bacteria potentiate antifungal and plant-beneficial responses of Trichoderma atroviride by upregulating its effector functions. PLoS ONE 2024, 19, e0301139. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, N.; Pang, Q.; Khan, R.A.A.; Xu, Q.; Wu, C.; Liu, T. A salt-tolerant strain of Trichoderma longibrachiatum HL167 is effective in alleviating salt stress, promoting plant growth, and managing fusarium wilt disease in cowpeas. J. Fungi 2023, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Ayyandurai, M.; Akila, R.; Manonmani, K.; Theradimani, M.; Vellaikumar, S. Phytostimulation and growth promotion activities of Trichoderma spp. on groundnut (Arachis hypogaea L.) crop. J. Appl. Nat. Sci. 2021, 13, 1172–1179. [Google Scholar] [CrossRef]
- Prismantoro, D.; Jefferson, T.A.; Akbari, S.I.; Miranti, M.; Mispan, M.S.; Doni, F. In vitro inhibition mechanisms of Trichoderma yunnanense TM10 against Pyricularia oryzae and Rhizoctonia solani causing blast and sheath blight diseases in rice (Oryza sativa L.). All Life 2024, 17, 2422845. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Z.; Hao, B.; Diao, F.; Zhang, J.; Bao, Z.; Guo, W. Arbuscular mycorrhizal fungi alter microbiome structure of rhizosphere soil to enhance maize tolerance to La. Ecotoxicol. Environ. Saf. 2021, 212, 111996. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Liu, J.; Li, X.; Wang, X.; Dai, C.; Zhang, T.; Carrión, V.; Wei, Z.; Cao, F.; et al. Crop rotation and native microbiome inoculation restore soil capacity to suppress a root disease. Nat. Commun. 2023, 14, 8126. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis—Model and research methods. Soil Biol. Biochem. 2011, 43, 883–894. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Chormey, D.S.; Bakirdere, S.; Turan, N.B.; Engin, G.O. Fundamentals of Quorum Sensing. In Analytical Methods and Applications in Membrane Bioreactors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 117–149. [Google Scholar]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Wang, M.; Geng, L.; Jiao, S.; Wang, K.; Xu, W.; Shu, C.; Zhang, J. Bacillus thuringiensis exopolysaccharides induced systemic resistance against Sclerotinia sclerotiorum in Brassica campestris L. Biol. Control 2023, 183, 105267. [Google Scholar]
- Vadakkan, K. Quorum sensing in rhizosphere microbiome: Minding some serious business. In Rhizosphere Engineering; Dubey, R.C., Kumar, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 383–394. [Google Scholar]
- Yang, P.; Zhao, Z.; Fan, J.; Liang, Y.; Bernier, M.C.; Gao, Y.; Zhao, L.; Opiyo, S.; Xia, Y. Bacillus proteolyticus OSUB18 triggers induced systemic resistance against bacterial and fungal pathogens in Arabidopsis. Front. Plant Sci. 2023, 14, 1078100. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ma, F.; Li, P.-W.; Zhang, W.; Ding, X.-X.; Zhang, Q.; Li, M.; Wang, Y.; Xu, B. Effect of ozone on aflatoxins detoxification and nutritional quality of peanuts. Food Chem. 2014, 146, 284–288. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Nascimento, I.; Rodrigues, A.A.C.; Andrade, K.S.P.; Cunha, W.L.; Moraes, F.H.R.; de Sousa, F.A. Microbiolização de sementes de arroz com Bacillus spp. na redução de patógenos. Res. Soc. Dev. 2020, 9, e189108138. [Google Scholar] [CrossRef]
- Kumar, P.; Pandhi, S.; Mahato, D.K.; Kamle, M.; Mishra, A. Bacillus-based nano-bioformulations for phytopathogens and insect–pest management. Egypt. J. Biol. Pest Control 2021, 31, 128. [Google Scholar] [CrossRef]
- Boro, M.; Sannyasi, S.; Chettri, D.; Verma, A.K. Microorganisms in biological control strategies to manage microbial plant pathogens: A review. Arch. Microbiol. 2022, 204, 666. [Google Scholar] [CrossRef]
- Patel, J.S.; Kharwar, R.N.; Singh, H.B.; Upadhyay, R.S.; Sarma, B.K. Trichoderma asperellum (T42) and Pseudomonas fluorescens (OKC)-enhances resistance of pea against Erysiphe pisi through enhanced ROS generation and lignifications. Front. Microbiol. 2017, 8, 306. [Google Scholar] [CrossRef]
- Rahanandeh, H.; Safari Motlagh, M.R.; Hamzeh, A. Protease enzymes from Pseudomonas fluorescens and Pseudomonas aeruginosa as potential bioagents against Pratylenchus loosi. Can. J. Plant Pathol. 2024, 46, 465–477. [Google Scholar] [CrossRef]
- Song, G.Q.; Prieto, H.; Orbovic, V. Agrobacterium-mediated transformation of tree fruit crops: Methods, progress, and challenges. Front. Plant Sci. 2019, 10, 226. [Google Scholar] [CrossRef]
- Doan, H.K.; Maharaj, N.N.; Kelly, K.N.; Miyao, E.M.; Davis, R.M.; Leveau, J.H.J. Antimycotal activity of Collimonas isolates and synergy-based biological control of Fusarium wilt of tomato. Phytobiomes J. 2020, 4, 64–74. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, B.; Yan, Y.; Cheng, Z.; Li, Z.; Han, L.; Sun, Y.; Zheng, Y.; Xia, Y. Comamonas-dominant microbial community in carbon poor aquitard sediments revealed by metagenomic-based growth rate investigation. Sci. Total Environ. 2024, 912, 169203. [Google Scholar] [CrossRef]
- Nguvo, K.J.; Gao, X. Weapons hidden underneath: Bio-control agents and their potentials to activate plant induced systemic resistance in controlling crop Fusarium diseases. J. Plant Dis. Prot. 2019, 126, 177–190. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- LeBlanc, N. Bacteria in the genus Streptomyces are effective biological control agents for management of fungal plant pathogens: A meta-analysis. BioControl 2022, 67, 111–121. [Google Scholar] [CrossRef]
- Benoit-Gelber, I.; Gruntjes, T.; Vinck, A.; van Veluw, J.G.; Wösten, H.A.B.; Boeren, S.; Vervoort, J.J.M.; de Vries, R.P. Mixed colonies of Aspergillus niger and Aspergillus oryzae cooperatively degrading wheat bran. Fungal Genet. Biol. 2017, 102, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Vibha. Evaluation of fungal and bacterial bioagents against Rhizoctonia solani on Solanum melongena (L.). Arch. Phytopathol. Plant Prot. 2011, 44, 743–750. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, J.; Li, M.; Fang, F.; Hu, J.; Sun, Z.; Zhang, A.; Gao, X.; Li, J. Synergistic effect of Bacillus subtilis and Paecilomyces lilacinus in alleviating soil degradation and improving watermelon yield. Front. Microbiol. 2023, 13, 1101975. [Google Scholar] [CrossRef]
- Geremew, D.; Shiberu, T.; Leta, A. Isolation, morphological characterization, and screening a virulence of Beauveria bassiana and Metarhizium robertsii as bioagents. F1000Research 2023, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.; Tariq, M.; Mukhtar, T.; Gulzar, A. On the virulence of the entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae (Ascomycota: Hypocreales), against the diamondback moth, Plutella xylostella (L.)(Lepidoptera: Plutellidae). Egypt. J. Biol. Pest Control 2021, 31, 86. [Google Scholar] [CrossRef]
- de Almeida Moura, I.; Pereira, I.D.S.; Pires, R.A.; Ribeiro, O.L.; de Oliveira Monteiro, C.M.; de Souza Rocha, L.; de Souza Perinotto, W.M. First report of Metarhizium anisopliae sl action on gastrointestinal ruminant nematodes in the free-living stage and its persistence in soil. Biocontrol Sci. Technol. 2023, 33, 539–554. [Google Scholar] [CrossRef]
- Manjunatha, C.; Velavan, V.; Rangeshwaran, R.; Mohan, M.; Kandan, A.; Sivakumar, G.; Shylesha, A.N.; Kumar, M.; Pramesh, D.; Sujithra, M.; et al. Assessment of bio-formulations of indigenous strains of Bacillus thuringiensis, Metarhizium robertsii and Metarhizium majus for management of the rhinoceros beetle, Oryctes rhinoceros L., in field. Egypt. J. Biol. Pest Control 2023, 33, 70. [Google Scholar] [CrossRef]
- Xue, R.; Du, G.; Chen, C.; Chen, B.; Peng, Y. Oleic acid improves the conidial production and quality of Metarhizium rileyi as a biocontrol agent. Biocontrol Sci. Technol. 2023, 33, 758–771. [Google Scholar] [CrossRef]
- Synowiec, C.; Fletcher, E.; Heinkel, L.; Salisbury, T. Getting rigor right: A framework for methodological choice in adaptive monitoring and evaluation. Glob. Health Sci. Pract. 2023, 11, e2200243. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bai, Z.X.; Bo, W.H.; Lin, J.; Yang, J.J.; Chen, T. Analysis and evaluation of heavy metal pollution in farmland soil in China: A Meta-analysis. Huan Jing Ke Xue 2024, 45, 2913–2925. [Google Scholar]
- Scholz, M.J.; Obenour, D.R.; Morrison, E.S.; Elser, J.J. A critical eutrophication–climate change link. Nat. Sustain. 2025, 8, 222–223. [Google Scholar] [CrossRef]
- Yan, Q.; Ming, Y.; Liu, J.; Yin, H.; He, Q.; Li, J.; Huang, M.; He, Z. Microbial biotechnology: From synthetic biology to synthetic ecology. Adv. Biotechnol. 2025, 3, 1. [Google Scholar] [CrossRef]
- Gadar, K.; McCarthy, R.R. Using next generation antimicrobials to target the mechanisms of infection. npj Antimicrob. Resist. 2023, 1, 11. [Google Scholar] [CrossRef]
- Pearson, A.J.; Mukherjee, K.; Fattori, V.; Lipp, M. Opportunities and challenges for global food safety in advancing circular policies and practices in agrifood systems. npj Sci. Food 2024, 8, 60. [Google Scholar] [CrossRef]
- Jansson, J.K.; McClure, R.; Egbert, R.G. Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 2023, 41, 1716–1728. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).