Distribution of Airborne Fungi in Vehicles and Its Association with Usage Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Area of Study
2.2. Survey Design
2.3. Sampling Method
2.4. Fungal Quantitation and Identification
2.5. Statistical Analysis
2.5.1. Data Preprocessing
2.5.2. Univariate Analysis
2.5.3. Correlation Analysis
2.5.4. Multivariate Modeling (GLM)
2.5.5. Redundancy Analysis (RDA)
2.5.6. Relative Abundance Analysis
3. Results
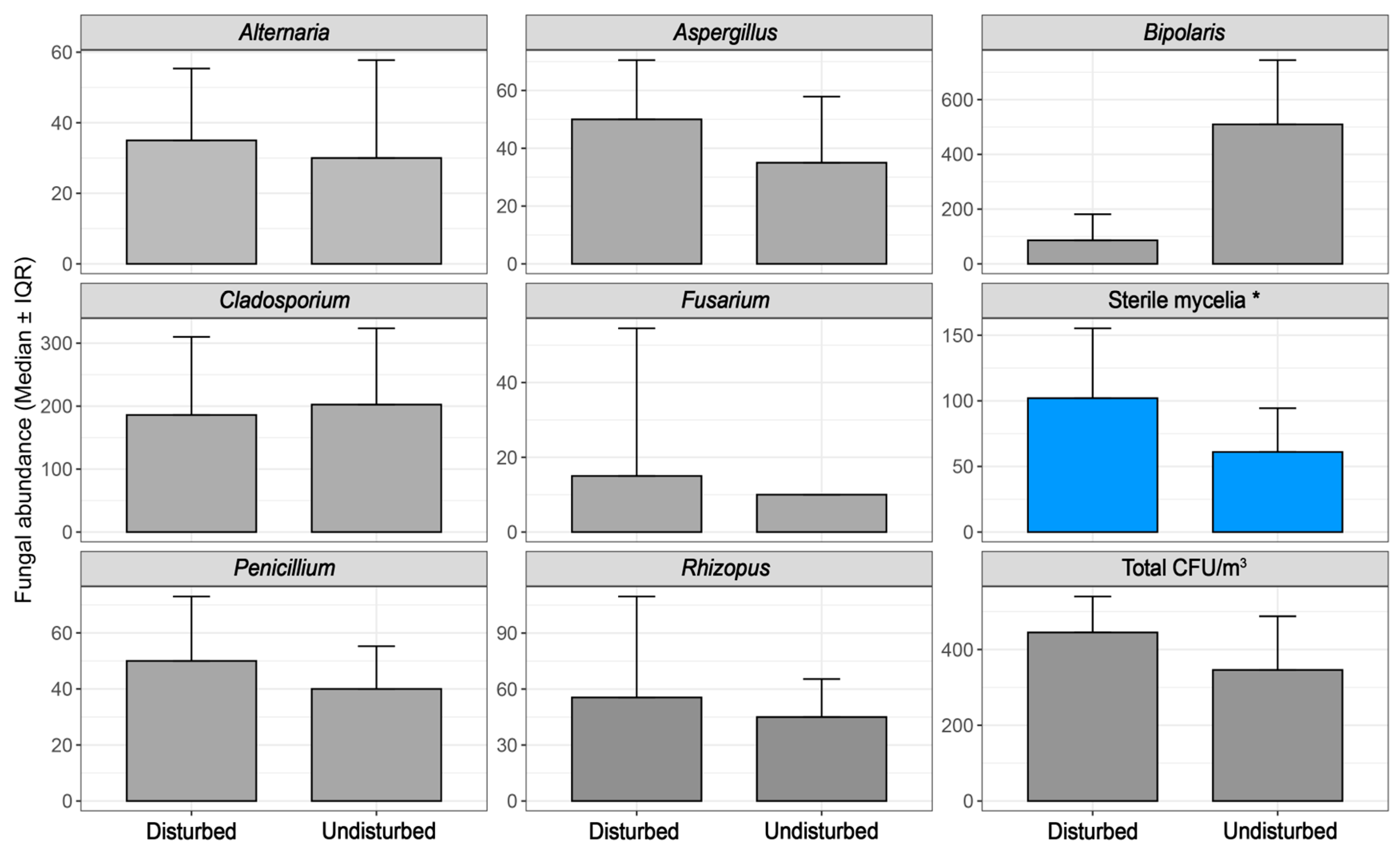
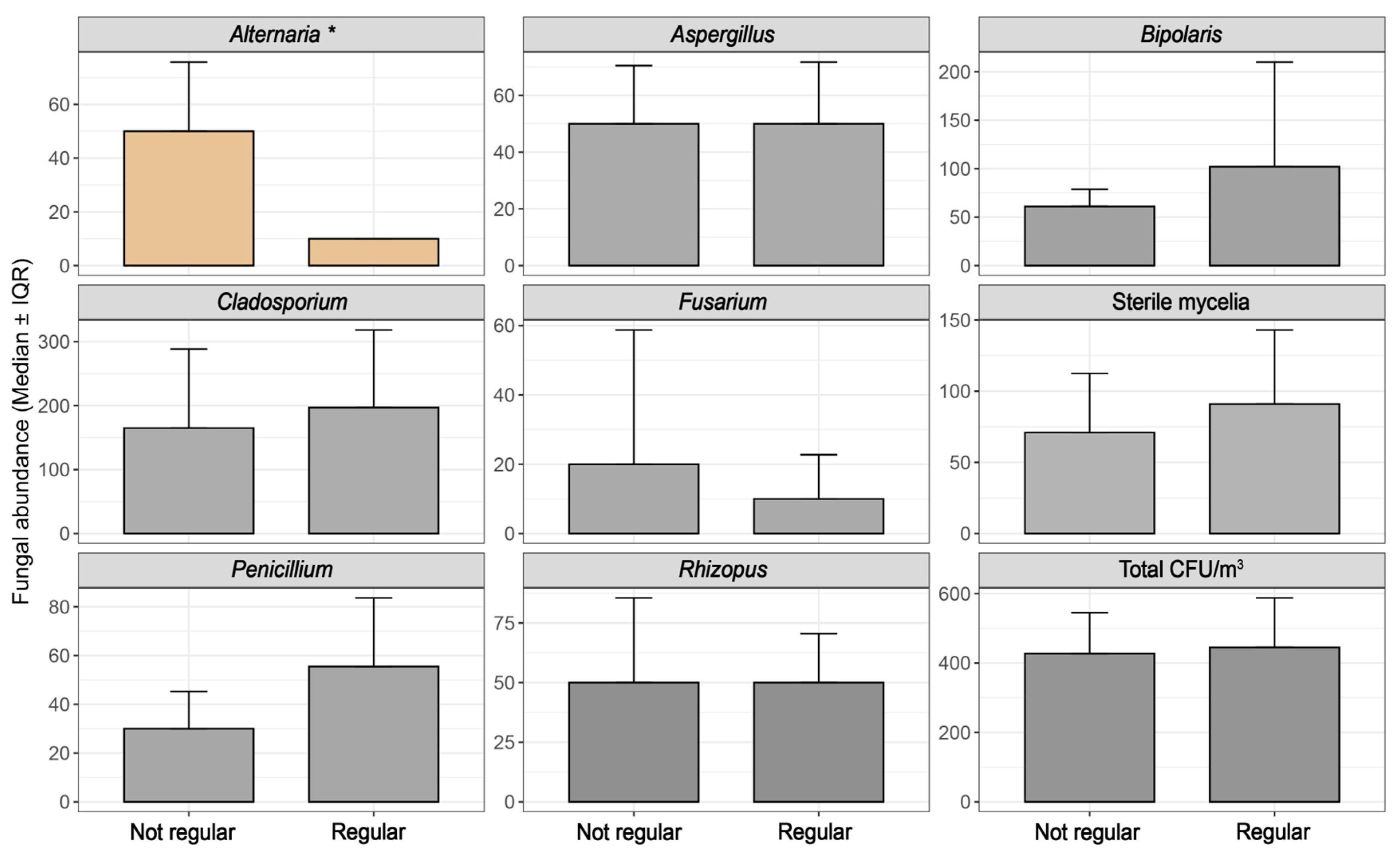
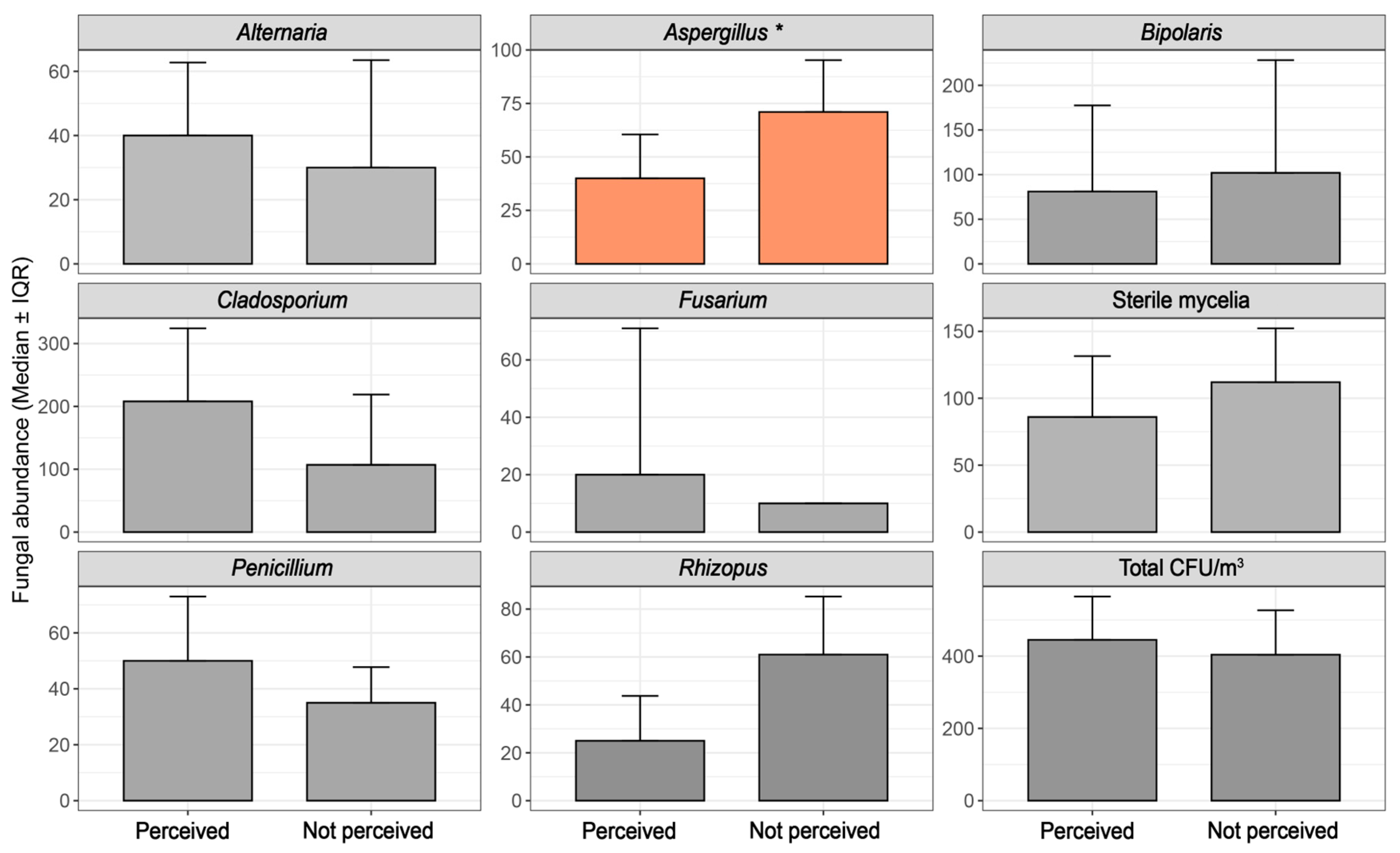
3.1. Univariate Analysis
3.2. Redundancy Analysis (RDA)
3.3. Relative Abundance Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INRIX 2024 Global Traffic Scorecard. Available online: https://inrix.com/scorecard-city/?city=Monterrey&index=62#form-download-the-full-report (accessed on 10 August 2025).
- Frąk, M.; Gładyszewska-Fiedoruk, K.; Łuniewski, M.; Teleszewski, T. Microbiological tests of air quality in car cabins—Preliminary tests. J. Ecol. Eng. 2024, 25, 323–331. [Google Scholar] [CrossRef]
- Yamamoto, N.; Shendell, D.G.; Winer, A.M.; Zhang, J. Residential air exchange rates in three major US metropolitan areas: Results from the Relationship Among Indoor, Outdoor, and Personal Air study 1999–2001. Indoor Air 2010, 20, 85–90. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Fernández-Gracia, M.D.; Elizondo-Zertuche, M.; Orué, N.; Treviño-Rangel, R.d.J.; Rodríguez-Sánchez, I.P.; Adame-Rodríguez, J.M.; Zapata-Morín, P.A.; Robledo-Leal, E. Culturable Airborne Fungi in Downtown Monterrey (Mexico) and Their Correlation with Air Pollution over a 12-Month Period. Atmosphere 2023, 14, 983. [Google Scholar] [CrossRef]
- Gołofit-Szymczak, M.; Stobnicka-Kupiec, A.; Górny, R.L. Impact of air-conditioning system disinfection on microbial contamination of passenger cars. Air Qual. Atmos. Health 2019, 12, 1127–1135. [Google Scholar] [CrossRef]
- Farian, E.; Wójcik-Fatla, A. Mycological contamination of cabin filters as a potential source of air pollution inside passenger vehicles. Air Qual. Atmos. Health 2025, 18, 111–125. [Google Scholar] [CrossRef]
- Aquino, S.; de Lima, J.E.A.; Nascimento, A.P.B.D.; Reis, F.C. Analysis of fungal contamination in vehicle air filters and their impact as a bioaccumulator on indoor air quality. Air Qual. Atmos. Health 2018, 11, 1143–1153. [Google Scholar] [CrossRef]
- Elizondo-Zertuche, M.; Martínez-Carranza, K.; Orue, N.; de Jesús Treviño-Rangel, R.; Robledo-Leal, E. Managing raw materials of vegetable origin increases fungal indoor concentration in food companies. J. Food Sci. Technol. 2020, 57, 794–798. [Google Scholar] [CrossRef]
- Verhoeff, A.P.; van Wijnen, J.H.; Boleij, J.S.M.; Brunekreef, B.; van Reenen-Hoekstra, E.S.; Samson, R.A. Enumeration and identification of airborne viable mould propagules in houses. A field comparison of selected techniques. Allergy 1990, 45, 275–284. [Google Scholar] [CrossRef]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2023. Available online: https://www.R-project.org/ (accessed on 10 August 2025).
- Shelton, B.G.; Kirkland, K.H.; Flanders, W.D.; Morris, G.K. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 2002, 68, 1743–1753. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Zapata-Morín, P.; Reyna-Martinez, R.; Orue, N.; Treviño-Rangel, R.d.J.; Elizondo-Zertuche, M.; Adame-Rodríguez, J.; Becerra-Siller, Y.; Sánchez-Ovalle, V.; Robledo-Leal, E. The Influence of Carpeting, Human Activity and Number of Beds on Airborne Fungi Concentration in Hotel Bedrooms. Appl. Sci. 2021, 11, 6773. [Google Scholar] [CrossRef]
- Górny, R.L.; Reponen, T.; Willeke, K.; Schmechel, D.; Robine, E.; Boissier, M.; Grinshpun, S.A. Fungal fragments as indoor air biocontaminants. Appl. Environ. Microbiol. 2002, 68, 3522–3531. [Google Scholar] [CrossRef]
- Viegas, C.; Moreira, R.; Faria, T.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Aspergillus prevalence in air conditioning filters from vehicles: Taxis for patient transportation, forklifts, and personal vehicles. Arch. Environ. Occup. Health 2019, 74, 341–349. [Google Scholar] [CrossRef]
- Grydaki, N.; Colbeck, I.; Whitby, C. Characterization of bioaerosols associated with commuter transport micro-environments using high throughput sequencing. Sci. Total Environ. 2024, 957, 177539. [Google Scholar] [CrossRef] [PubMed]
- Aime, C. Fungal Biodiversity, 2nd ed.; Crous, P.W., Verkley, G.J.M., Groenewald, J.Z., Houbraken, J., Eds.; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019. [Google Scholar]
- Sharpe, T.; McGill, G.; Dancer, S.J.; King, M.F.; Fletcher, L.; Noakes, C.J. Influence of ventilation use and occupant behaviour on surface microorganisms in contemporary social housing. Sci. Rep. 2020, 10, 11841. [Google Scholar] [CrossRef] [PubMed]
- Kalwasińska, A.; Burkowska, A.; Wilk, I. Microbial air contamination in indoor environment of a university library. Ann. Agric. Environ. Med. 2012, 19, 25–29. [Google Scholar] [PubMed]
- Meklin, T.; Husman, T.; Vepsäläinen, A.; Vahteristo, M.; Koivisto, J.; Halla-Aho, J.; Hyvärinen, A.; Moschandreas, D.; Nevalainen, A. Indoor air microbes and respiratory symptoms of children in moisture damaged and reference schools. Indoor Air 2002, 12, 175–183. [Google Scholar] [CrossRef]
- Knox, R.B.; Suphioglu, C.; Taylor, P.; Desai, R.; Watson, H.C.; Peng, J.L.; Bursill, L.A. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: Implications for asthma and air pollution. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1997, 27, 246–251. [Google Scholar] [CrossRef]
- Gołofit-Szymczak, M.; Wójcik-Fatla, A.; Stobnicka-Kupiec, A.; Górny, R.L. Filters of automobile air conditioning systems as in-car source of exposure to infections and toxic moulds. Environ. Sci. Pollut. Res. 2023, 30, 108188–108200. [Google Scholar] [CrossRef]
- Bush, R.K.; Prochnau, J.J. Alternaria-induced asthma. J. Allergy Clin. Immunol. 2004, 113, 227–234. [Google Scholar] [CrossRef]
- Vasconcelos, K.R.; Ferri, A.S.; da Silva, A.I.; Leite Júnior, D.P. Characterization and epidemiological relevance of bioaerosol in air-conditioning filters in light and heavy vehicles: An environmental and public health issue. Glob. J. Res. Anal. 2023, 12, 1–13. [Google Scholar] [CrossRef]
- Rautiala, S.; Reponen, T.; Hyvärinen, A.; Nevalainen, A.; Husman, T.; Vehviläinen, A.; Kalliokoski, P. Exposure to airborne microbes during the repair of moldy buildings. Am. Ind. Hygiene Assoc. J. 1996, 57, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: A review of the epidemiologic evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.A.; Rosenbaum, P.F.; Anagnost, S.E.; Hunt, A.; Abraham, J.L. Indicators of airborne fungal concentrations in urban homes: Understanding the conditions that affect indoor fungal exposures. Sci. Total Environ. 2015, 517, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Magyar, U.; Husain, S. Invasive Aspergillosis in the Current Era. Infect. Dis. Clin. N. Am. 2025, 39, e33–e60. [Google Scholar] [CrossRef]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef]
- Latgé, J.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, 10–1128. [Google Scholar] [CrossRef]
- Li, D.; Amburgey-Crovetti, K.; Applebach, E.; Steen, T.Y.; Calderone, R. The Dual Pathogen Fusarium: Diseases, Incidence, Azole Resistance, and Biofilms. J. Fungi 2025, 11, 294. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Denk, U.; Pöll, V.; Rid, R.; Breitenbach, M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008, 145, 58–86. [Google Scholar] [CrossRef]



| Species | Variable | p-Value |
|---|---|---|
| Penicillium | Parking environment | 0.00617 |
| Cladosporium | Musty/moldy odor | 0.00076 |
| Rhizopus | Musty/moldy odor | 0.00034 |
| Alternaria | Air filter maintenance | 0.00747 |
| Alternaria | Parking environment | 0.02616 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Villarreal, R.A.; Elizondo-Zertuche, M.; Orué-Arreola, N.; Adame-Rodríguez, J.; Gordillo-Mata, L.E.; González-Enríquez, M.; Ortega-Castillo, B.; Zapata-Morín, P.A.; Robledo-Leal, E. Distribution of Airborne Fungi in Vehicles and Its Association with Usage Patterns. J. Fungi 2025, 11, 725. https://doi.org/10.3390/jof11100725
Rodríguez-Villarreal RA, Elizondo-Zertuche M, Orué-Arreola N, Adame-Rodríguez J, Gordillo-Mata LE, González-Enríquez M, Ortega-Castillo B, Zapata-Morín PA, Robledo-Leal E. Distribution of Airborne Fungi in Vehicles and Its Association with Usage Patterns. Journal of Fungi. 2025; 11(10):725. https://doi.org/10.3390/jof11100725
Chicago/Turabian StyleRodríguez-Villarreal, Raúl Asael, Mariana Elizondo-Zertuche, Nydia Orué-Arreola, Juan Adame-Rodríguez, Larissa E. Gordillo-Mata, Miguel González-Enríquez, Brandon Ortega-Castillo, Patricio Adrián Zapata-Morín, and Efrén Robledo-Leal. 2025. "Distribution of Airborne Fungi in Vehicles and Its Association with Usage Patterns" Journal of Fungi 11, no. 10: 725. https://doi.org/10.3390/jof11100725
APA StyleRodríguez-Villarreal, R. A., Elizondo-Zertuche, M., Orué-Arreola, N., Adame-Rodríguez, J., Gordillo-Mata, L. E., González-Enríquez, M., Ortega-Castillo, B., Zapata-Morín, P. A., & Robledo-Leal, E. (2025). Distribution of Airborne Fungi in Vehicles and Its Association with Usage Patterns. Journal of Fungi, 11(10), 725. https://doi.org/10.3390/jof11100725






