Importance of Imaging Assessment Criteria in Predicting the Need for Post-Dilatation in Transcatheter Aortic Valve Implantation with a Self-Expanding Bioprosthesis
Abstract
1. Introduction
- It aims to assess the incidence of PD in patients undergoing transfemoral TAVI with a self-expandable system and to identify imaging criteria derived from echocardiography and computed tomography that may causally contribute to, and therefore predict, the need for balloon PD;
- We evaluate the clinical impact of PD by analyzing procedural outcomes and long-term all-cause mortality.
2. Materials and Methods
2.1. Study Cohort
2.2. Ethics Declaration
2.3. Data Collection
2.4. Transthoracic Echocardiography (TTE)
2.5. Computed Tomography Angiography and Calcium Scoring
2.6. TAVI Procedure and PD
- Structural assessment of the valve prosthesis using rotational fluoroscopy: insufficient spontaneous expansion affecting the entire circumference (“underexpansion”) and/or localized infolding;
- Functional assessment using angiography and instantaneous transvalvular pressure measurement: unacceptable paravalvular leakage and/or residual stenosis.
- Valve dislocation/embolization: implantation height;
- Annulus rupture: eccentric sub-/supra-/valvular calcifications;
- Hemodynamic intolerance of rapid ventricular pacing: left ventricular dysfunction, arrhythmia;
- Lack of benefit from PD: concomitant (coronary, valvular) heart disease or extracardiac disease, individual life expectancy, age.
2.7. Clinical Outcomes
2.8. Statistical Analysis
3. Results
4. Discussion
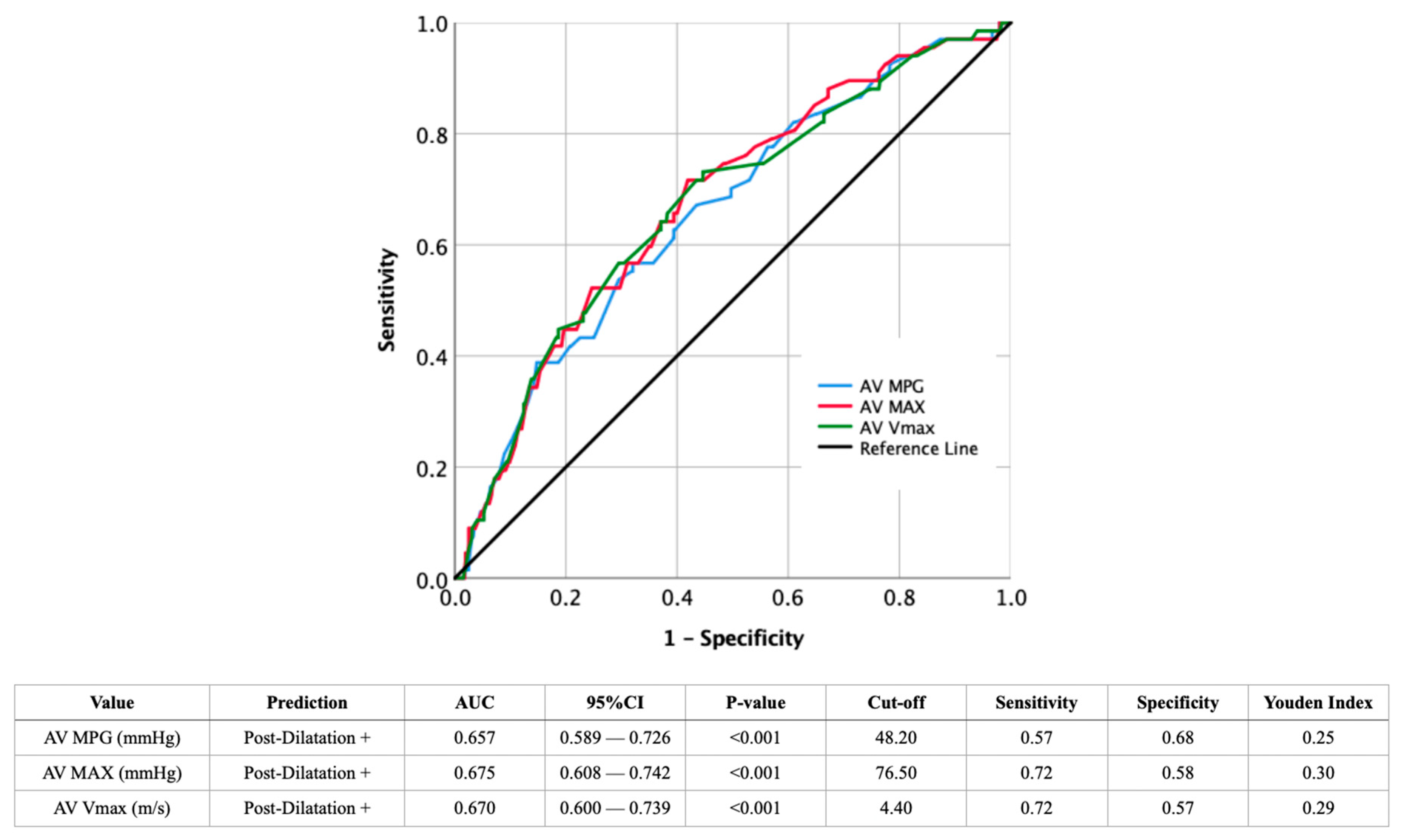
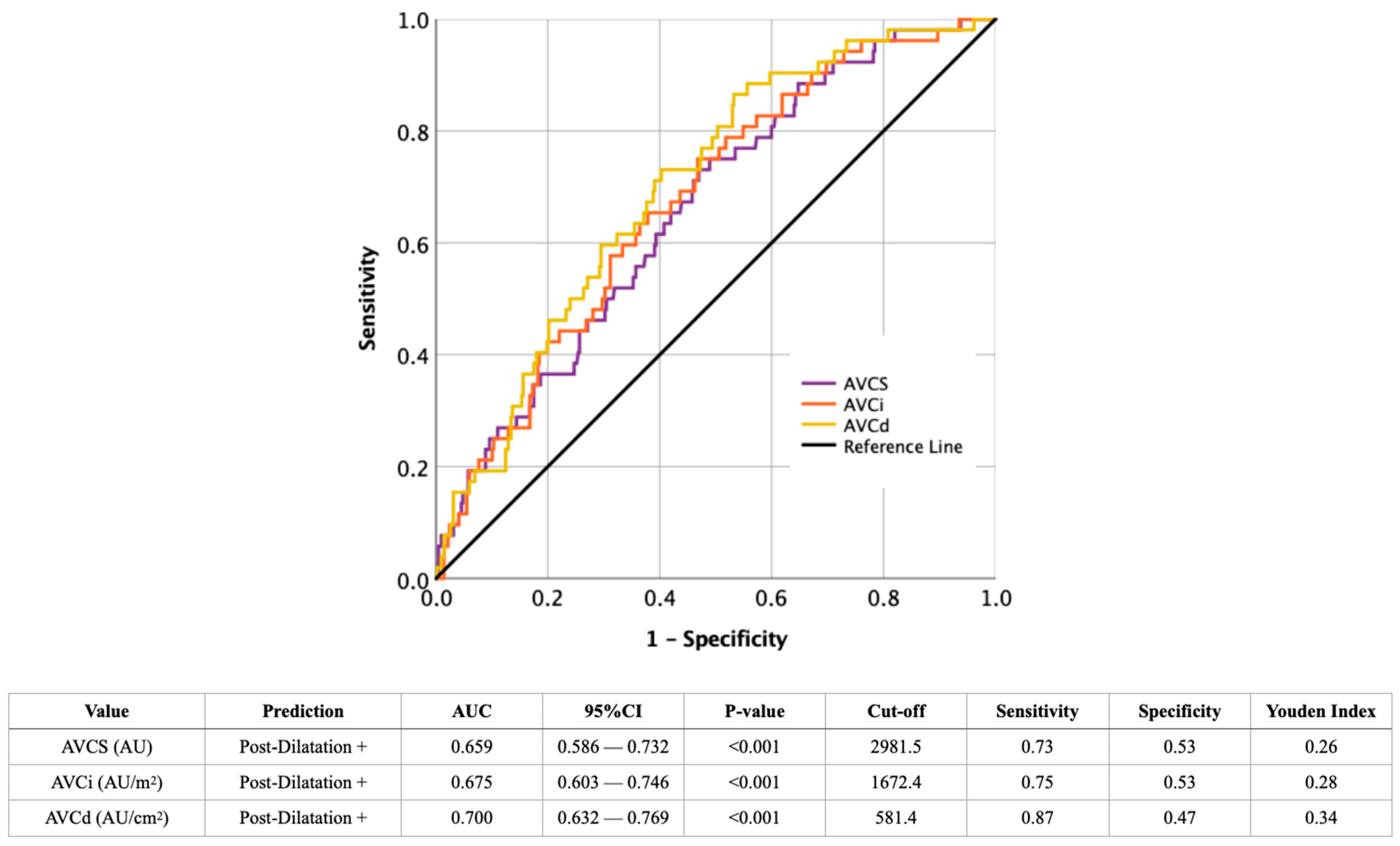
4.1. Predicting the Probability of Post-Dilatation from Imaging Findings
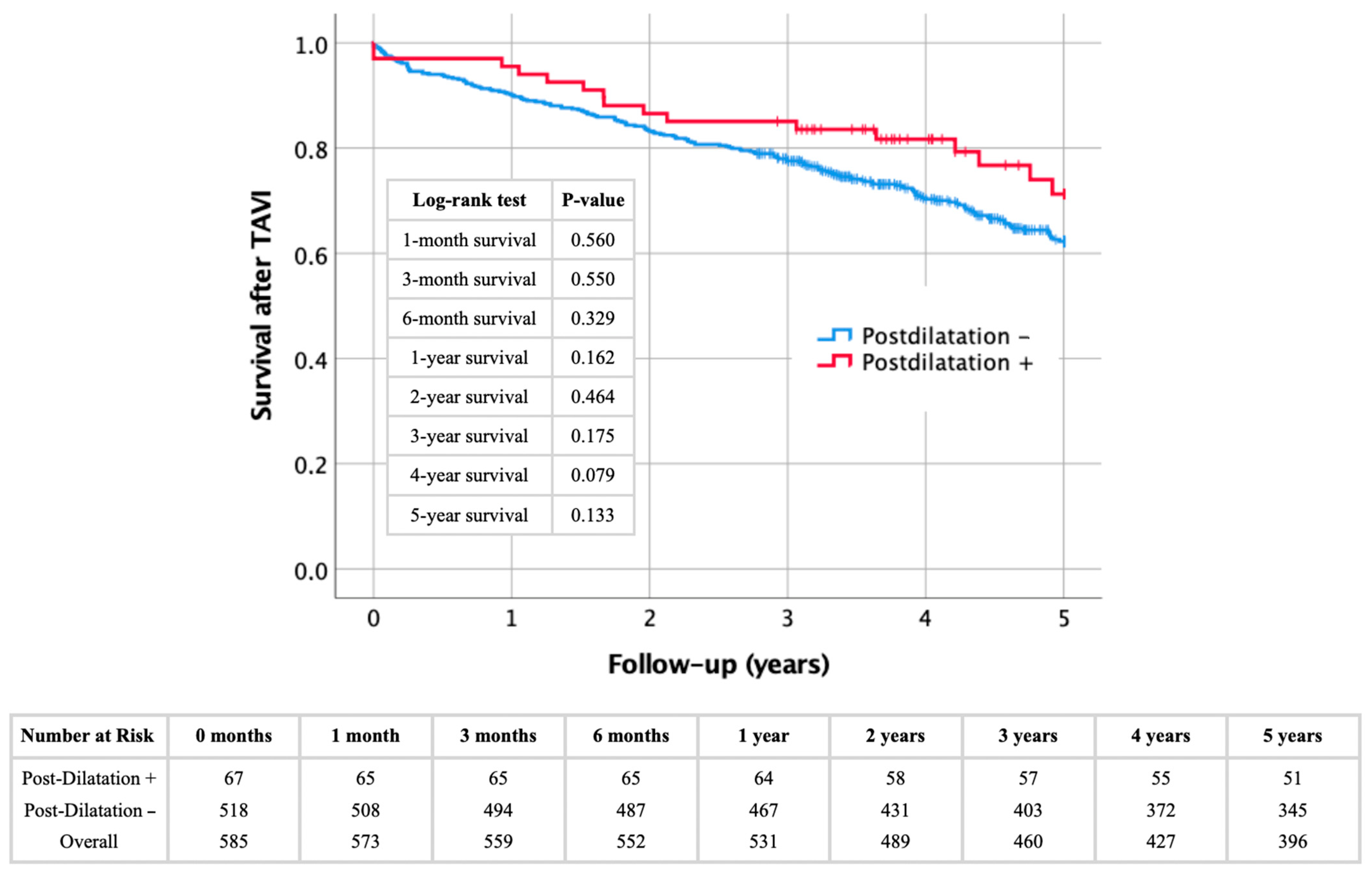
4.2. Impact of Post-Dilatation on Procedural and Longterm Outcomes
4.3. Clinical Relevance
4.4. Limitations
- The retrospective design limits the ability to establish causal relationships between variables and the need for PD. Although significant associations were identified, prospective studies are required to confirm these findings and assess their real-world applicability. We focused on key echocardiographic and radiological factors, but other potential predictors, such as specific anatomical features or calcium distribution, were not analyzed;
- The study was conducted at a single center with a relatively homogeneous patient population and small sample size, limiting the statistical power of our analyses and the generalizability to more diverse groups. Patient selection, procedural expertise, and device-specific factors might have influenced the outcomes. Larger, multicenter studies are needed to validate the findings and to establish whether they are transferable to other clinical and procedural settings;
- The use of second- and third-generation self-expanding TAVI devices may not fully reflect the performance of newer systems, such as the Evolut Fx, currently in use; the findings are not generalizable to other self-expanding platforms with different designs and radial strengths such as the Acurate or Navitor systems. Of course, our results cannot be applied to balloon-expanded TAVI systems, which generally have significant lower post-dilatation rates compared with self-expanding systems due to their different deployment technology;
- As a systematic limitation, it should be pointed out that the multitude of anatomical, morphological, functional and clinical criteria that contributed to the operators’ decisions whether or not to post-dilate—which were inherently made intraprocedurally and under limited time resources—could not be assessed in this retrospective analysis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic Stenosis |
| AV | Aortic Valve |
| AV MAX | Aortic Valve Maximum Pressure Gradient |
| AV MPG | Aortic Valve Mean Systolic Pressure Gradient |
| AV Vmax | Aortic Valve Maximal Systolic Transvalvular Flow Velocity |
| AVCS | Aortic Valve Calcium Score |
| AVCi | Aortic Valve Calcium Index |
| AVCd | Aortic Valve Calcium Density |
| AUC | Area Under the Curve |
| AUROC | Area Under the Receiver Operating Characteristic Curve |
| BSA | Body Surface Area |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CT | Computed Tomography |
| CTA | Computed Tomography Angiography |
| ECG | Electrocardiogram |
| ESC | European Society of Cardiology |
| IQR | Interquartile Range |
| IVSD | Interventricular Septal Thickness |
| LVEF | Left Ventricular Ejection Fraction |
| LVEDD | Left Ventricular End-Diastolic Diameter |
| MDCT | Multi-Detector Computed Tomography |
| OR | Odds Ratio |
| PD | Post-Dilatation |
| PVR | Paravalvular Regurgitation |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| TEE | Transesophageal Echocardiography |
| TTE | Transthoracic Echocardiography |
| TAVI | Transcatheter Aortic Valve Implantation |
| VARC-3 | Valve Academic Research Consortium-3 |
| YI | Youden Index |
References
- Tamburino, C.; Valvo, R.; Crioscione, E.; Reddavid, C.; Picci, A.; Costa, G.; Barbanti, M. The path of transcatheter aortic valve implantation: From compassionate to low-risk cases. Eur. Heart J. Suppl. 2020, 22 (Suppl. L), L140–L145. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.S.; Redwood, S.R.; Allen, C.J.; Hurrell, H.; Chehab, O.; Rajani, R.; Prendergast, B.; Patterson, T. A 20-year journey in transcatheter aortic valve implantation: Evolution to current eminence. Front. Cardiovasc. Med. 2022, 9, 971762. [Google Scholar] [CrossRef] [PubMed]
- Nombela-Franco, L.; Rodés-Cabau, J.; DeLarochellière, R.; Larose, E.; Doyle, D.; Villeneuve, J.; Bergeron, S.; Bernier, M.; Amat-Santos, I.J.; Mok, M.; et al. Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc. Interv. 2012, 5, 499–512. [Google Scholar] [CrossRef] [PubMed]
- McInerney, A.; Vera-Urquiza, R.; Tirado-Conte, G.; Marroquin, L.; Jimenez-Quevedo, P.; Nuñez-Gil, I.; Pozo, E.; Gonzalo, N.; de Agustín, J.A.; Escaned, J.; et al. Pre-dilation and post-dilation in transcatheter aortic valve replacement: Indications, benefits and risks. Interv. Cardiol. 2021, 16, e28. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lal, S. Post-dilation in transcatheter aortic valve replacement: A systematic review and meta-analysis. J. Interv. Cardiol. 2017, 30, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Sengoku, K.; Ichibori, Y.; Mizote, I.; Maeda, K.; Kuratani, T.; Sawa, Y.; Sakata, Y. The role of echocardiography in transcatheter aortic valve implantation. Cardiovasc. Diagn. Ther. 2018, 8, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Gurvitch, R.; LaBounty, T.M.; Min, J.K.; Wood, D.; Johnson, M.; Ajlan, A.M.; Wijesinghe, N.; Webb, J.G. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc. Imaging 2011, 4, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, W.; Zhang, T.; Han, C.; Wang, Z.; Pei, X.; Li, X.; Zhao, Z.; Wang, P.; Han, J.; et al. Quantity and location of aortic valve calcification predicts paravalvular leakage after transcatheter aortic valve replacement: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1170979. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; A Leipsic, J.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, L.F.; Soliman, O.I.; van Gils, L.; Vletter, W.B.; Van Mieghem, N.M.; Ren, B.; Galema, T.W.; Schultz, C.; de Jaegere, P.P.; Di Biase, M.; et al. Relation between calcium burden, echo-cardiographic stent frame eccentricity and paravalvular leakage after CoreValve transcatheter aortic valve implantation. J. Cardiovasc. Imaging 2017, 18, 648–653. [Google Scholar] [CrossRef]
- Hagar, A.; Li, Y.; Wei, X.; Peng, Y.; Xu, Y.; Ou, Y.; Wang, Z.; Wang, X.; Shah, J.-P.; Sihag, V.; et al. Incidence, predictors, and outcome of paravalvular leak after transcatheter aortic valve implantation. J. Interv. Cardiol. 2020, 2020, 8249497. [Google Scholar] [CrossRef] [PubMed]
- Tzamtzis, S.; Viquerat, J.; Yap, J.; Mullen, M.; Burriesci, G. Numerical analysis of the radial force produced by the Medtronic-CoreValve and Edwards-SAPIEN after transcatheter aortic valve implantation. Med. Eng. Phys. 2013, 35, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ring, L.; Shah, B.N.; Bhattacharyya, S.; Harkness, A.; Belham, M.; Oxborough, D.; Pearce, K.; Rana, B.S.; Augustine, D.X.; Robinson, S.; et al. Echocardiographic assessment of aortic stenosis: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2021, 8, G19–G59. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, K.E.; Gough, A.; Segurado, R.; Barry, M.; Sugrue, D.; Hurley, J. Is valve choice a significant determinant of paravalvular leak post-transcatheter aortic valve implantation? A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2014, 45, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Pibarot, P.; Webb, J.; Rodes-Cabau, J.; Herrmann, H.C.; Williams, M.; Makkar, R.; Szeto, W.Y.; Main, M.L.; Thourani, V.H.; et al. Outcomes with post-dilation following transcatheter aortic valve replacement: The PARTNER I trial. JACC Cardiovasc. Interv. 2014, 7, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Kleczyński, P.; Dziewierz, A.; Daniec, M.; Bagieński, M.; Rzeszutko, Ł.; Sorysz, D.; Trębacz, J.; Sobczyński, R.; Tomala, M.; Dudek, D. Impact of post-dilatation on the reduction of paravalvular leak and mortality after transcatheter aortic valve implantation. Kardiol. Pol. 2017, 75, 742–748. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daneault, B.; Koss, E.; Hahn, R.T.; Kodali, S.; Williams, M.R.; Généreux, P.; Paradis, J.-M.; George, I.; Reiss, G.R.; Moses, J.W.; et al. Efficacy and safety of postdilatation to reduce paraval-vular regurgitation during balloon-expandable transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 2013, 6, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.S.; Regazzoli, D.; Barbanti, M.; Fiorina, C.; Adamo, M.; Angelillis, M.; De Carlo, M.; Bellini, B.; Montorfano, M.; Mangieri, A.; et al. Impact of balloon post-dilation on valve durability and long-term clinical outcomes after self-expanding transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2024, 103, 209–218. [Google Scholar] [CrossRef] [PubMed]
| Post-Dilatation+ | Post-Dilatation− | p | |
|---|---|---|---|
| n/% | 67/11.5 | 518/88.5 | - |
| Sex (male)—n/% | 27/40.3 | 262/50.6 | 0.113 |
| Age (years)—mean ± SD | 80.4 ± 5.4 | 82.3 ± 5.0 | 0.004 |
| Height (cm)—mean ± SD | 166.4 ± 8.2 | 168.1 ± 9.8 | 0.172 |
| Weight (kg)—mean ± SD | 71.8 ± 14.2 | 73.1 ± 14.5 | 0.497 |
| BMI (kg/m2)—mean ± SD | 25.9 ± 4.5 | 25.8 ± 4.4 | 0.825 |
| BSA (m2)—mean ± SD | 1.8 ± 0.2 | 1.8 ± 0.2 | 0.463 |
| LVEF (%)—median ± IQR | 55.0 ± 15.0 | 55.0 ± 10.0 | 0.417 |
| IVSD (mm)—median ± IQR | 14.0 ± 3.3 | 13.0 ± 2.4 | 0.265 |
| LVEDD (mm)—median ± IQR | 4.6 ± 1.0 | 4.6 ± 0.9 | 0.660 |
| AV MPG (mmHg)—median ± IQR | 47.0 ± 17.3 | 44.0 ± 11.8 | <0.001 |
| AV MAX (mmHg)—median ± IQR | 82.0 ± 20.5 | 74.0 ± 19.0 | <0.001 |
| AV Vmax (m/s)—median ± IQR | 4.6 ± 0.6 | 4.3 ± 0.5 | <0.001 |
| Annulus Diameter (mm)—median ± IQR | 25.0 ± 2.3 | 25.0 ± 3.0 | 0.850 |
| Annulus Area (cm2)—median ± IQR | 467.5 ± 87.0 | 472.0 ± 124.3 | 0.797 |
| AVCS (AU)—median ± IQR | 4123.5 ± 2264.3 | 2813.5 ± 2060.0 | <0.001 |
| AVCi (AU/m2)—median ± IQR | 2406.0 ± 1060.6 | 1562.7 ± 1114.4 | <0.001 |
| AVCd (AU/cm2)—median ± IQR | 865.7 ± 299.3 | 598.2 ± 376.4 | <0.001 |
| Pre-dilatation performed—n/% | 1/1.5 | 11/2.1 | 0.732 |
| Post-Dilatation Binary Logistic Regression | Univariate | Multivariable | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| LVEF | 0.789 (0.419–1.487) | 0.464 | ||
| IVSD | 1.036 (0.824–1.304) | 0.762 | ||
| LVEDD | 0.613 (0.147–2.561) | 0.502 | ||
| AV MPG | 1.607 (1.261–2.049) | <0.001 | 0.788 (0.470–1.321) | 0.366 |
| AV MAX | 1.679 (1.314–2.145) | <0.001 | 1.187 (0.455–3.100) | 0.726 |
| AV Vmax | 1.780 (1.361–2.328) | <0.001 | 1.424 (1.039–1.950) | 0.028 |
| Annulus Diameter | 0.961 (0.725–1.274) | 0.783 | ||
| Annulus Area | 0.984 (0.743–1.304) | 0.911 | ||
| AVCS | 1.636 (1.269–2.111) | <0.001 | 0.953 (0.531–1.710) | 0.872 |
| AVCi | 1.680 (1.293–2.184) | <0.001 | 1.248 (0.370–4.215) | 0.721 |
| AVCd | 1.794 (1.387–2.321) | <0.001 | 1.618 (1.227–2.132) | 0.001 |
| Post-Dilatation+ | Post-Dilatation− | p | |
|---|---|---|---|
| Major Vascular Complications after TAVI | 0/0.0 | 18/3.5 | 0.121 |
| Pacemaker after TAVI—n/% | 5/7.5 | 68/13.5 | 0.187 |
| Stroke after TAVI—n/% | 1/1.5 | 14/2.7 | 0.554 |
| Post-Dilatation Binary Logistic Regression | Univariate | |
|---|---|---|
| Odds Ratio (95% CI) | p | |
| Major Vascular Complications after TAVI | 0.000 (0.000–.) | 0.998 |
| Pacemaker after TAVI | 0.534 (0.207–1.375) | 0.193 |
| Stroke after TAVI | 0.544 (0.070–4.207) | 0.560 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammerer, M.; Hasenbichler, P.; Schörghofer, N.; Knapitsch, C.; Clodi, N.; Hoppe, U.C.; Hergan, K.; Boxhammer, E.; Scharinger, B. Importance of Imaging Assessment Criteria in Predicting the Need for Post-Dilatation in Transcatheter Aortic Valve Implantation with a Self-Expanding Bioprosthesis. J. Cardiovasc. Dev. Dis. 2025, 12, 296. https://doi.org/10.3390/jcdd12080296
Hammerer M, Hasenbichler P, Schörghofer N, Knapitsch C, Clodi N, Hoppe UC, Hergan K, Boxhammer E, Scharinger B. Importance of Imaging Assessment Criteria in Predicting the Need for Post-Dilatation in Transcatheter Aortic Valve Implantation with a Self-Expanding Bioprosthesis. Journal of Cardiovascular Development and Disease. 2025; 12(8):296. https://doi.org/10.3390/jcdd12080296
Chicago/Turabian StyleHammerer, Matthias, Philipp Hasenbichler, Nikolaos Schörghofer, Christoph Knapitsch, Nikolaus Clodi, Uta C. Hoppe, Klaus Hergan, Elke Boxhammer, and Bernhard Scharinger. 2025. "Importance of Imaging Assessment Criteria in Predicting the Need for Post-Dilatation in Transcatheter Aortic Valve Implantation with a Self-Expanding Bioprosthesis" Journal of Cardiovascular Development and Disease 12, no. 8: 296. https://doi.org/10.3390/jcdd12080296
APA StyleHammerer, M., Hasenbichler, P., Schörghofer, N., Knapitsch, C., Clodi, N., Hoppe, U. C., Hergan, K., Boxhammer, E., & Scharinger, B. (2025). Importance of Imaging Assessment Criteria in Predicting the Need for Post-Dilatation in Transcatheter Aortic Valve Implantation with a Self-Expanding Bioprosthesis. Journal of Cardiovascular Development and Disease, 12(8), 296. https://doi.org/10.3390/jcdd12080296







