Abstract
Aims: Sinus tachycardia after heart transplantation (HTX) due to cardiac graft denervation is associated with reduced post-transplant survival and requires adequate treatment. We analyzed the long-term effects of heart rate control with ivabradine or metoprolol succinate in HTX recipients. Methods: This observational retrospective single-center study analyzed the ten-year results of 110 patients receiving ivabradine (n = 54) or metoprolol succinate (n = 56) after HTX. Analysis included comparison of demographics, medications, heart rates, blood pressure values, echocardiographic features, cardiac catheterization data, cardiac biomarkers, and post-transplant survival including causes of death. Results: Both groups showed no significant differences concerning demographics or medications (except for ivabradine and metoprolol succinate). At 10-year follow-up, HTX recipients with ivabradine showed a significantly lower heart rate (72.7 ± 8.5 bpm) compared to baseline (88.8 ± 7.6 bpm; p < 0.001) and to metoprolol succinate (80.1 ± 8.1 bpm; p < 0.001), a significantly lower NT-proBNP level (588.4 ± 461.4 pg/mL) compared to baseline (3849.7 ± 1960.0 pg/mL; p < 0.001) and to metoprolol succinate (1229.0 ± 1098.6 pg/mL; p = 0.005), a significantly lower overall mortality (20.4% versus 46.4%; p = 0.004), and mortality due to graft failure (1.9% versus 21.4%; p = 0.001). Multivariate analysis showed a significantly decreased risk of death within 10 years after HTX in patients with post-transplant use of ivabradine (HR 0.374, CI 0.182–0.770; p = 0.008). Conclusions: In this single-center trial, patients with ivabradine revealed a significantly more pronounced heart rate reduction, a lower NT-proBNP level, and a superior 10-year survival after HTX.
1. Introduction
Elevated resting heart rates lead to increased myocardial oxygen demand, diastolic dysfunction and shortened diastolic filling resulting in decreased stroke volume and reduced myocardial perfusion [1,2,3,4,5,6,7]. Accordingly, increased morbidity and mortality have been linked to elevated resting heart rates in the general population as well as in patients with heart failure [8,9,10,11].
As a result of cardiac denervation after heart transplantation (HTX), the autonomous control of heart rate regulation by the vagus nerve is diminished, and HTX recipients usually suffer from inadequate sinus tachycardia [1,2,3,4,5,6,7]. Although a higher resting heart rate can help stabilize the patient in the early post-transplant stage by increasing cardiac output, in the long-term, higher resting heart rates in HTX recipients have been related to reduced post-transplant survival [6,7,12].
Given these circumstances, a specific and selective therapeutic agent with a minimum of possible side effects for the treatment of sinus tachycardia in HTX recipients is needed to achieve physiological resting heart rates [1,2,3,4,5,6,7]. Standard pharmacological management of tachycardia includes the use of beta blockers or non-dihydropyridine calcium channel blockers [1,2,3,4,5,6,7,13]. However, both drug classes are non-specific inhibitors of pacemaker activity and can cause side effects such as atrioventricular block, negative inotropy, hypotension, bronchospasm, depression, fatigue, and sexual dysfunction [1,2,3,4,5,6,7,14].
In contrast to beta blockers or calcium channel blockers, ivabradine is a specific and selective inhibitor of pacemaker activity [1,2,3,4,5,6,7,14,15,16,17,18]. Ivabradine inhibits the so-called funny current (If), also known as “pacemaker current”, generated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in pacemaker cells [1,2,3,4,5,6,7,14,15,16,17,18]. Although ivabradine is considered to exhibit an overall good safety profile, its heart rate-lowering properties can cause bradycardia and respective QT interval prolongation as the QT interval depends on heart rate [19,20,21,22]. Long-term effects of ivabradine on QT and QTc interval in HTX recipients are unknown, especially relating to the co-administration of potentially QT-prolonging drugs such as tacrolimus [23]. We therefore investigated the long-term effects (ten-year results) of heart rate control with ivabradine or metoprolol succinate in HTX recipients focusing on concomitant medications, heart rates, QT/QTc intervals, echocardiographic features, cardiac catheterization data, cardiac biomarkers, and survival after HTX.
2. Patients and Methods
2.1. Patients
We carried out this study in compliance with the ethical principles set forth in the Declaration of Helsinki. The institutional review board (IRB) of Heidelberg University, Heidelberg, Germany, issued ethics approval number: S-286/2015, Version 1.2, 28 July 2020. Written informed consent was obtained from patients for enrollment in the Heidelberg HTX Registry and for the clinical and scientific use of their data. The ethics approval does not require additional consent for this observational retrospective single-center as we used only routine clinical data [6,7,24,25,26].
We screened the medical data of all adult patients (≥18 years) for continuous post-transplant use of ivabradine or metoprolol succinate (in the following context referred to as metoprolol) who underwent HTX at Heidelberg Heart Center, Heidelberg, Germany, between 2006 and 2015. HTX recipients were excluded if they were only temporarily treated with ivabradine or metoprolol, received a combination of ivabradine and metoprolol, or were treated with additional antiarrhythmic drugs (amiodarone, digoxin/digitoxin, other beta blockers, or non-dihydropyridine calcium channel blockers) [6,7]. Patients who had undergone repeat HTX were also excluded [6,7].
2.2. Follow-Up
Follow-up of HTX recipients adhered to the routine clinical protocol of Heidelberg Heart Center [6,7,24,25,26]. As part of HTX surgery, patients routinely received a temporary pacemaker system consisting of an external pacing box and two epicardial pacing leads that were placed on the right atrium and ventricle during HTX surgery. The epicardial pacing leads remained in situ for about 10 days after HTX [26]. If clinically indicated, HTX recipients could be over-paced with this temporary pacemaker. As soon as HTX recipients were clinically stable, they were discharged and followed up at the HTX outpatient clinic. During the first visit at the HTX outpatient clinic, HTX recipients presented for baseline follow-up, which included routine assessment of resting heart rate and initialization of heart rate control with ivabradine or metoprolol succinate. HTX recipients were neither preselected nor randomized for treatment with ivabradine versus metoprolol to manage post-transplant heart rate. Individual physician practice and patient preference influenced the prescription of either drug, reflecting real-world data [6,7]. As the use of ivabradine in HTX recipients is still off-label, HTX recipients were informed beforehand about effects, adverse effects, contraindications, and the off-label use of ivabradine [6,7].
At baseline after HTX, the initial standard daily ivabradine dose was 10 mg (2 × 5.0 mg), and the initial standard daily metoprolol succinate dose was 100 mg (2 × 50 mg or 1 × 100 mg), which are both adequate starting doses. Slight variations in this scheme occurred due to clinical reasons (individual physician practice and/or patient preference). Adaption of the doses of ivabradine and metoprolol succinate were managed during follow-up visits, aiming for a durable resting heart rate ≤ 80 bpm. HTX recipients were seen monthly at the HTX outpatient clinic during the first six post-transplant months, then bimonthly until the end of the first year after HTX, and approximately three to four times per year thereafter. From five years after HTX onward, routine follow-up visits were reduced to once or twice annually (with additional visits as clinically needed) [6,7,24,25,26].
Standard follow-up assessments consisted of medical history, physical examination, systolic and diastolic blood pressure measurement, laboratory tests including immunosuppressive drug monitoring, resting 12-lead ECG, echocardiography, endomyocardial biopsy, annual chest X-ray, as well as annual 24 h Holter monitor [6,7,24,25,26].
Complete 10-year follow-up data after HTX were available for all 110 HTX recipients; no HTX recipient was lost to follow-up. As part of post-transplant care, patients were regularly assessed for medication use, tolerability, and potential complications to support adherence [6,7,24,25,26].
2.3. Post-Transplant Medications
Post-transplant pharmacotherapy, including immunosuppressive regimens, followed the center’s standard [6,7,24,25,26]. Perioperatively, HTX recipients received anti-thymocyte globulin-based immunosuppression induction therapy. Most HTX recipients in this study received tacrolimus and mycophenolic acid as immunosuppressive drug regime, given the fact that cyclosporine A was subsequently replaced by tacrolimus as the primary immunosuppressive drug from 2006 onward. Patients also received steroids (prednisolone), which were gradually tapered and, when clinically feasible, discontinued six months after HTX [6,7,24,25,26].
2.4. Statistical Analysis
Data analysis was performed with MedCalc (Version 23.2.1, MedCalc Software Ltd., Ostend, Belgium) and presented as mean ± standard deviation (SD) or as count (n) with percentage (%). Mean difference (MD) with 95% confidence interval (CI) was used for measures of association. Depending on data type and study question, statistical testing employed—as appropriate in each case—Student’s t-test, Mann–Whitney U-test, analysis of variance (ANOVA), Kruskal–Wallis test, chi-squared test, or Fisher’s exact test. Heart rates [measured by beats per minute (bpm)] between HTX recipients with ivabradine or metoprolol were annually assessed by resting 12-lead ECG over a period of ten years after HTX and graphically displayed by a box-plot diagram. Post-transplant survival between groups was graphically compared with Kaplan–Meier curves and the log-rank test. Figures were created with CorelDRAW Graphics Suite 2025 (Version 26.0.0.101; Corel Corporation, Ottawa, ON, Canada). A p-value of <0.050 denoted statistical significance [6,7,24,25,26].
We performed large-scale univariate analyses to search for intergroup differences between HTX recipients with ivabradine or metoprolol. Analyzed variables included recipient data, recipient previous open-heart surgery, recipient principal diagnosis for HTX, donor data, transplant sex mismatch, perioperative data, immunosuppressive drug therapy, post-transplant concomitant medications, resting ECGs, 24 h-Holter monitors, QT/QTc intervals, blood pressure values, echocardiographic features, cardiac catheterization data, and cardiac biomarkers after HTX [6,7,24,25,26,27].
Causes of death within ten years after HTX were categorized into the following groups: graft failure, acute rejection, infection/sepsis, malignancy, and thromboembolic event/bleeding. Analysis of 10-year post-transplant mortality between HTX recipients with ivabradine or metoprolol further included a multivariate analysis (Cox regression model) with the following eight clinically relevant parameters: recipient age, recipient body mass index (BMI), recipient estimated glomerular filtration rate (eGFR), donor age, donor BMI, transplant sex mismatch, total ischemic time, and administration of ivabradine after HTX. We did not include additional variables in this multivariate analysis for 10-year mortality after HTX to avoid biased regression coefficients and to ensure a stable number of events (deceased patients) per analyzed variable [6,7,24,25,26].
3. Results
3.1. Demographics and Post-Transplant Medications
After applying the exclusion criteria, a total of 110 HTX recipients were selected, including 54 patients with ivabradine after HTX (54 of 110 [49.1%]) and 56 patients with metoprolol after HTX (56 of 110 [50.9%]).
In terms of demographics, we found no statistically significant differences between both groups regarding recipient data, recipient previous open-heart surgery, recipient principal diagnosis for HTX, donor data, transplant sex mismatch, or perioperative data (all p ≥ 0.050). Demographic and clinical characteristics at baseline are presented in Table 1.

Table 1.
Demographic and clinical characteristics at baseline.
Analysis of post-transplant medications including the immunosuppressive drug regimen revealed no statistically significant differences between both groups (all p ≥ 0.05) except for the administration of ivabradine or metoprolol. Post-transplant medications at baseline, including the immunosuppressive drug regimen, are given in Table 2.

Table 2.
Post-transplant medications at baseline.
3.2. Drug Dosage and Side Effects
At baseline after HTX, HTX recipients with ivabradine were administered a mean daily dose of 9.8 mg ± 2.9 mg ranging from 5.0 mg to 15.0 mg, and HTX recipients with metoprolol were administered a mean daily dose of 98.0 mg ± 44.4 mg ranging from 47.5 mg to 190.0 mg. At 5-year follow-up after HTX, the mean daily ivabradine dose was 10.7 mg ± 3.6 mg ranging from 5.0 mg to 15.0 mg, and the mean daily metoprolol dose was 112.7 mg ± 51.0 mg ranging from 47.5 mg to 190.0 mg. At 10-year follow-up after HTX, the mean daily ivabradine dose was 10.8 mg ± 3.4 mg ranging from 5.0 mg to 15.0 mg, and the mean daily metoprolol dose was 111.6 mg ± 51.1 mg ranging from 47.5 mg to 190.0 mg.
Patients generally tolerated ivabradine well. Mild and temporary side effects were infrequent, with phosphenes reported transiently in three individuals (5.6%) during the initial treatment period. No HTX recipient with ivabradine reported symptomatic bradycardia (0.0%), while six HTX recipients with metoprolol reported symptomatic bradycardia with heart rates < 60 bpm (10.7%; p = 0.013). Only one HTX recipient with ivabradine reported intermittent dizziness (1.9%), whereas eight HTX recipients with metoprolol reported intermittent dizziness (14.3%; p = 0.017). No HTX recipient with ivabradine reported fatigue (0.0%), while five HTX recipients with metoprolol reported fatigue (8.9%; p = 0.025).
3.3. Post-Transplant Heart Rates
At baseline after HTX, there was no statistically significant difference in average heart rates (resting ECG) between HTX recipients starting treatment with ivabradine or metoprolol (ivabradine at baseline: 88.8 ± 7.6 bpm versus metoprolol at baseline: 86.9 ± 9.5 bpm, p = 0.257). At 5-year follow-up after HTX, patients with ivabradine had a statistically significant lower average heart rate (resting ECG) in comparison to baseline after HTX (ivabradine at 5-year follow-up: 73.4 ± 9.0 bpm versus ivabradine at baseline: 88.8 ± 7.6 bpm, p < 0.001) and to HTX recipients with metoprolol (ivabradine at 5-year follow-up: 73.4 ± 9.0 bpm versus metoprolol at 5-year follow-up: 80.7 ± 10.3 bpm, p < 0.001). At 10-year follow-up after HTX, patients with ivabradine continued to have a statistically significant lower average heart rate (resting ECG) in comparison to baseline after HTX (ivabradine at 10-year follow-up: 72.7 ± 8.5 bpm versus ivabradine at baseline: 88.8 ± 7.6 bpm, p < 0.001) and to HTX recipients with metoprolol (ivabradine at 10-year follow-up: 72.7 ± 8.5 bpm versus metoprolol at 10-year follow-up: 80.1 ± 8.1 bpm, p < 0.001). Figure 1 illustrates the 10-year trajectory of average heart rates (resting ECG) in HTX recipients treated with ivabradine versus metoprolol.
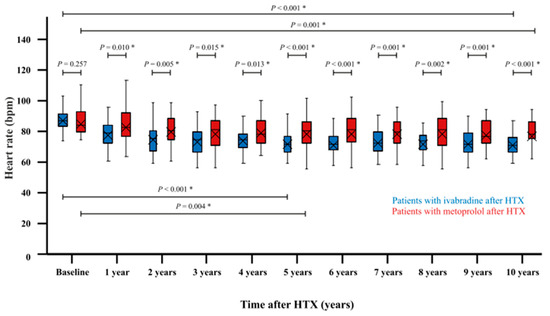
Figure 1.
The 10-year course of average heart rates (resting ECG) in patients with ivabradine or metoprolol after HTX. There is no statistically significant difference in average heart rates (resting ECG) between HTX recipients starting treatment with ivabradine or metoprolol (p = 0.257). At 5-year follow-up after HTX, patients with ivabradine show a statistically significant lower average heart rate (resting ECG) in comparison to baseline after HTX (p < 0.001) and to patients with metoprolol at 5-year follow-up (p < 0.001). At 10-year follow-up after HTX, patients with ivabradine continue to have a statistically significant lower average heart rate (resting ECG) in comparison to baseline after HTX (p < 0.001) and to patients with metoprolol at 10-year follow-up (p < 0.001). BPM = beats per minute; HTX = heart transplantation; and * = statistically significant (p < 0.050).
Analysis of average heart rates (24 h-Holter monitor) at baseline showed no statistically significant difference between HTX recipients starting treatment with ivabradine or metoprolol (ivabradine at baseline: 86.2 ± 9.8 bpm versus metoprolol at baseline: 85.6 ± 9.0 bpm, p = 0.756). At 5-year follow-up after HTX, patients with ivabradine had a statistically significant lower average heart rate (24 h-Holter monitor) in comparison to baseline after HTX (ivabradine at 5-year follow-up: 72.5 ± 7.1 bpm versus ivabradine at baseline: 86.2 ± 9.8 bpm, p < 0.001) and to patients with metoprolol (ivabradine at 5-year follow-up: 72.5 ± 7.1 bpm versus metoprolol at 5-year follow-up: 79.9 ± 8.1 bpm, p < 0.001). At 10-year follow-up after HTX, patients with ivabradine continued to have a statistically significant lower average heart rate (24 h-Holter monitor) in comparison to baseline after HTX (ivabradine at 10-year follow-up: 70.9 ± 7.0 bpm versus ivabradine at baseline: 86.2 ± 9.8 bpm, p < 0.001) and to patients with metoprolol (ivabradine at 10-year follow-up: 70.9 ± 7.0 bpm versus metoprolol at 10-year follow-up: 79.1 ± 8.4 bpm, p < 0.001). Average heart rates (24 h-Holter monitor) of patients with ivabradine or metoprolol after HTX are given in Table 3.

Table 3.
Resting ECG, 24 h-Holter monitor, QT/QTc intervals, and blood pressure values.
3.4. QT/QTc Intervals
No statistically significant differences in baseline QT/QTc intervals were observed between the two groups (QT: p = 0.337, respectively, QTc: p = 0.672). At 5-year follow-up after HTX, both groups showed a statistically significant longer QT interval compared to baseline (ivabradine at 5-year follow-up: 391.9 ± 23.4 ms versus ivabradine at baseline: 365.5 ± 21.1 ms, p < 0.001, respectively, metoprolol at 5-year follow-up: 383.5 ± 23.8 ms versus metoprolol at baseline: 369.4 ± 21.2 ms, p = 0.004). In contrast, there was no statistically significant change concerning QTc interval at 5-year follow-up after HTX compared to baseline (ivabradine: p = 0.560, respectively, metoprolol p = 0.165). QTc intervals at 5-year follow-up after HTX did not differ significantly between patients treated with ivabradine and those receiving metoprolol (p = 0.199). At 10-year follow-up after HTX, both groups continued to show a statistically significant longer QT interval compared to baseline (ivabradine at 10-year follow-up: 392.8 ± 26.4 ms versus ivabradine at baseline: 365.5 ± 21.1 ms, p < 0.001, respectively, metoprolol at 10-year follow-up: 389.9 ± 21.3 ms versus metoprolol at baseline: 369.4 ± 21.2 ms, p < 0.001). There was still no statistically significant change concerning QTc interval at 10-year follow-up after HTX compared to baseline (ivabradine: p = 0.341, respectively, metoprolol p = 0.148). Likewise, there was no statistically significant difference between patients with ivabradine or metoprolol concerning QTc interval at 10-year follow-up after HTX (p = 0.423). QT/QTc intervals of patients with ivabradine or metoprolol after HTX are presented in Table 3.
3.5. Blood Pressure Values
Assessment of blood pressure values showed no statistically significant differences between HTX recipients with ivabradine or metoprolol concerning systolic blood pressure or diastolic blood pressure at baseline (p = 0.821, respectively, p = 0.469), at 5-year follow-up after HTX (p = 0.782, respectively, p = 0.790), and at 10-year follow-up after HTX (p = 0.963, respectively, p = 0.932). Blood pressure values are shown in Table 3.
3.6. Post-Transplant Mortality and Causes of Death
The Kaplan–Meier estimator showed a significantly better 5-year post-transplant survival (p = 0.022) and 10-year post-transplant survival (p = 0.003) in HTX recipients with ivabradine in comparison to HTX recipients with metoprolol. Kaplan–Meier estimators are displayed in Figure 2 and Figure 3.
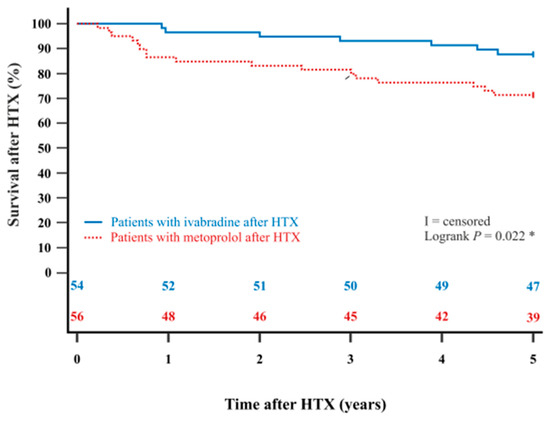
Figure 2.
The 5-year survival after HTX between patients with ivabradine or metoprolol after HTX (Kaplan–Meier estimator). Patients with ivabradine after HTX have a significantly higher 5-year post-transplant survival than patients with metoprolol after HTX (p = 0.022). HTX = heart transplantation; * = statistically significant (p < 0.050).
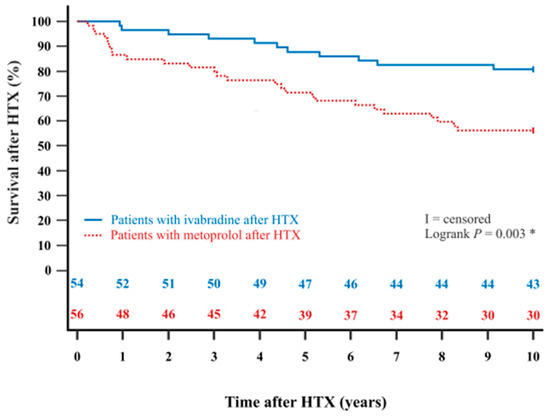
Figure 3.
The 10-year survival after HTX between patients with ivabradine or metoprolol after HTX (Kaplan–Meier estimator). Patients with ivabradine after HTX have a significantly higher 10-year post-transplant survival than patients with metoprolol after HTX (p = 0.003). HTX = heart transplantation; * = statistically significant (p < 0.050).
When examining causes of death, significantly fewer patients in the ivabradine group died from graft failure within five years after HTX (0.0% versus 16.0%, MD: 16.0%, CI: 6.4–25.6%, p = 0.002), and within ten years after HTX (1.9% versus 21.4%, MD: 19.5%, CI: 8.2–30.8%, p = 0.001) in comparison to the metoprolol group. No significant differences were observed between the two groups regarding acute rejection, infection/sepsis, malignancy, or thromboembolic events/bleeding at 5- or 10-year follow-up after HTX (all p ≥ 0.050). Table 4 presents the causes of death within five and ten years after HTX.

Table 4.
Causes of death after HTX.
Multivariate analysis for 5-year mortality after HTX showed that patients in the ivabradine group had a significantly reduced risk of death within five years after HTX (HR: 0.388, 95% CI: 0.159–0.949, p = 0.038) and within ten years after HTX (HR: 0.374, 95% CI: 0.182–0.770, p = 0.008), whereas the other seven included variables (recipient age, recipient body mass index, recipient estimated glomerular filtration rate, donor age, donor body mass index, transplant sex mismatch, and total ischemic time) showed no statistically significant effect on 5-year or 10-year post-transplant mortality. The multivariate analysis for 5-year and 10-year mortality after HTX can be found in Table 5.

Table 5.
Multivariate analysis for mortality after HTX.
3.7. Post-Transplant Echocardiographic Features
Analysis of echocardiographic features at baseline showed no statistically significant differences between patients with ivabradine or metoprolol regarding left ventricular (LV) mass, LV mass index, left ventricular ejection fraction (LVEF), mitral annular plane systolic excursion (MAPSE), early diastolic mitral inflow peak velocity (E) to late diastolic mitral inflow peak velocity (A) ratio (E/A), E to early diastolic mitral annular velocity (e′) ratio (E/e′), deceleration time (DT) of E (DT-E), left atrial (LA) diameter, or systolic pulmonary artery pressure (PAP) (all p ≥ 0.050).
At 10-year follow-up after HTX, the ivabradine group showed a statistically significant reduction in LV mass (p = 0.004) and LV mass index (p = 0.001) towards normal values, whereas no such effect over time was observed in the metoprolol group in terms of LV mass (p = 0.874) or the LV mass index (p = 0.916). Furthermore, patients in the metoprolol group had a slight but significant decrease in LVEF (p < 0.001) and MAPSE (p < 0.001) at 10-year follow-up after HTX, while patients in the ivabradine group had no statistically significant change in LVEF (p = 0.314) or MAPSE (p = 0.515).
Assessment of diastolic parameters showed that patients in the metoprolol group experienced a significant decrease in E/A ratio (p = 0.005), an increase in E/e′ ratio (p = 0.002), and a stable DT-E (p = 0.117) at 10-year follow-up after HTX. In contrast, patients in the ivabradine group over time showed a stable E/A ratio (p = 0.169), a stable E/e′ ratio (p = 0.987), and a decrease in DT-E (p < 0.001). Additionally, patients with ivabradine showed an unaltered LA diameter (p = 0.076) and a lower systolic PAP (p = 0.003) at 10-year follow-up after HTX, whereas patients with metoprolol had an enlarged LA diameter (p < 0.001) and no decrease in systolic PAP (p = 0.915). Echocardiographic features after HTX are presented in Table 6.

Table 6.
Echocardiographic features after HTX.
3.8. Post-Transplant Cardiac Catheterization Data and Cardiac Biomarkers
Cardiac catheterization data showed no statistically significant differences between HTX recipients with ivabradine or metoprolol in coronary artery disease, coronary stenting, or high-sensitivity cardiac troponin T at baseline, at 5-year follow-up after HTX, or at 10-year follow-up after HTX (all p ≥ 0.050).
Patients in the ivabradine group showed a statistically significant lower left ventricular end-diastolic pressure (LVEDP) at 5-year follow-up after HTX (ivabradine group: 12.0 ± 3.7 mmHg versus metoprolol group: 17.1 ± 2.6 mmHg; p < 0.001) and at 10-year follow-up after HTX (ivabradine group: 10.4 ± 3.4 mmHg versus metoprolol group: 16.7 ± 2.5 mmHg; p < 0.001) as well as a statistically significant lower N-terminal prohormone of the brain natriuretic peptide (NT-proBNP) at 5-year follow-up after HTX (ivabradine group: 555.4 ± 541.9 pg/mL versus metoprolol group: 1021.0 ± 862.8 pg/mL; p = 0.004) and at 10-year follow-up after HTX (ivabradine group: 588.4 ± 461.4 pg/mL versus metoprolol group: 1229.0 ± 1098.6 pg/mL; p = 0.005). Cardiac catheterization data and cardiac biomarkers after HTX are presented in Table 7.

Table 7.
Cardiac catheterization data and cardiac biomarkers after HTX.
4. Discussion
4.1. Long-Term Management of Resting Heart Rates After Heart Transplantation
This study is the first to present 10-year data comparing heart rate control with ivabradine versus metoprolol in HTX recipients. We found that ivabradine was associated with a significantly better long-term heart rate reduction, which correlated with a lower NT-proBNP level, and an improved 10-year survival after HTX. Long-term management of resting heart rates after HTX is of high clinical relevance for HTX recipients as the current guidelines do not specifically address this issue and transplant cardiologists need to rely on the limited available studies for guidance [1,2,3,4,5,6,7]. Accordingly, center-specific practices have emerged over time but are not available to the public for review. The purpose of this study was therefore to provide insights into this complex topic.
In terms of resting heart rates, HTX recipients with ivabradine or metoprolol showed comparable resting heart rates at baseline after HTX. At 5-year and 10-year follow-up after HTX, the ivabradine group had significantly lower resting heart rates compared to both their baseline and the metoprolol group. The ivabradine group sustained a significantly lower resting heart rate than the metoprolol group over the entire 10-year period, indicating superior heart rate control with ivabradine. Comparable findings on the effectiveness of ivabradine in reducing heart rate among HTX recipients over shorter observation periods have been reported by Boeken and colleagues [28] as well as by Dos Santos and colleagues [29].
Variations in resting heart rate outcomes between HTX recipients with ivabradine or metoprolol could partially stem from differences in daily drug dosages. At baseline after HTX, the mean daily ivabradine dose was about 10 mg (2 × 5.0 mg), and the mean daily metoprolol dose was about 100 mg (2 × 50 mg or 1 × 100 mg), which are both adequate starting doses. Although both groups were titrated upwards over time, neither group reached the maximum doses (2 × 7.5 mg for ivabradine; 2 × 100 mg or 1 × 200 mg for metoprolol) due to reports of temporary asymptomatic bradycardia (heart rate < 60 bpm) during patient self-monitoring [4,5,6,7]. Therefore, while greater heart rate reductions may have been possible with higher doses in both groups, the similarity in dose titration suggests that dosage differences had minimal impact on overall heart rate outcomes.
Additionally, analysis of 24 h-Holter monitors at 5-year and 10-year follow-up after HTX showed significantly lower average heart rates in the ivabradine group compared to both their baseline and the metoprolol group, while there was no significant difference in average heart rates between both groups at baseline. These findings underscore the superior efficacy of ivabradine in reducing heart rate in denervated cardiac grafts following HTX, where autonomic control is lost. Ivabradine exerts its effect by directly inhibiting sinoatrial node activity, thereby lowering heart rate independently of autonomic input [14,15,16,17,18]. In contrast, metoprolol, a beta-adrenergic blocker, reduces heart rate indirectly by attenuating sympathetic stimulation via beta-receptor inhibition [30]. Consequently, in the context of post-transplant autonomic denervation, the effectiveness of beta-blockers is inherently limited [2,6,7].
4.2. Long-Term Results of Side Effects, Blood Pressure, and QT/QTc Interval
Since an elevated resting heart rate after HTX has been associated with reduced post-transplant survival [6,7,12], effective management of sinus tachycardia is essential. Ivabradine is a highly specific and selective inhibitor of the If current, also referred to as the pacemaker current, which is mediated by HCN channels in pacemaker cells of the sinoatrial node (SAN), the heart’s primary pacemaker [1,2,3,4,5,6,7,14,15,16,17,18]. Through this mechanism, ivabradine effectively reduces the resting heart rate without exerting significant effects on other parameters of cardiac function. Despite evidence supporting the safety and efficacy of ivabradine in achieving heart rate reduction in patients after HTX, its application in this patient population remains off-label and necessitates vigilant clinical monitoring [1,2,3,4,5,6,7]. Moreover, its use may be associated with extracardiac adverse effects, such as luminous visual disturbances (phosphenes), which are attributed to the inhibition of similar channels in the retina. These visual phenomena typically emerge approximately 40 days after initiation of therapy, are transient in nature, and generally do not interfere with daily activities [1,2,3,4,5,6,7,14]. In contrast, metoprolol is a non-selective inhibitor of pacemaker activity, which can lead to a range of cardiac and systemic side effects, including decreased contractility, atrioventricular block, low blood pressure, bronchospasm, fatigue, depression, and sexual dysfunction [1,2,3,4,5,6,7,14].
In our study, only three HTX recipients with ivabradine (5.6%) reported transient experiences of phosphenes, while patients with ivabradine had a significantly lower percentage of symptomatic bradycardia with heart rates < 60 bpm (p = 0.013), intermittent dizziness (p = 0.017), and fatigue (p = 0.025) in comparison to patients with metoprolol after HTX. Our findings are consistent with those of Boeken and colleagues [28] as well as with those of Lage-Gallé and colleagues [31] who both reported no substantial adverse effects associated with ivabradine use in HTX recipients [28,31].
With respect to blood pressure, HTX recipients with ivabradine or metoprolol exhibited comparable systolic and diastolic values at baseline after HTX. In the ivabradine group, systolic and diastolic blood pressure remained stable over time, with no significant differences observed between the two groups at both the 5-year and 10-year follow-up after HTX. Furthermore, we observed no significant difference between groups regarding the use of antihypertensive agents. These results align with previous studies reporting no significant impact of ivabradine on systolic or diastolic blood pressure [1,2,3,4,5,6,7,14].
Ivabradine‘s heart rate-lowering properties can lead to bradycardia and QT interval prolongation as the QT interval is inherently rate-dependent [19,20,21,22]. However, from a clinical point of view, the QTc interval is more relevant for assessing the risk of cardiac arrhythmias. In our study, no significant differences were observed between the ivabradine and metoprolol groups in either QT or QTc intervals at baseline after HTX. At 5-year and 10-year follow-up after HTX, patients with ivabradine as well as with metoprolol exhibited a statistically significant prolongation of the QT interval compared to baseline, which corresponds with the observed reduction in heart rate. However, in both groups, QTc intervals remained stable over time, with no significant changes detected at 5-year and 10-year follow-up after HTX compared to baseline. Moreover, there were no statistically or clinically meaningful differences in QT or QTc intervals between the two groups throughout the study period. Additionally, there was no significant difference between both groups regarding the use of tacrolimus (p = 0.771), a known QT-prolonging immunosuppressant [23,32,33].
4.3. Long-Term Survival After Heart Transplantation
Regulation of resting heart rate is of critical importance in HTX recipients as elevated heart rates have been associated with increased post-transplant mortality [6,7,12]. Ivabradine has demonstrated both safety and efficacy in managing elevated heart rates in this patient population [1,2,3,4,5,6,7]. However, existing evidence is largely confined to short- and medium-term outcomes, with long-term data currently lacking [1,2,3,4,5,6,7,28,29,31]. In the present study, we observed a significantly improved 10-year post-transplant survival in HTX recipients treated with ivabradine (p = 0.003), along with a markedly lower percentage of graft failure-related mortality (1.9% vs. 21.4%, p = 0.001). Comprehensive analyses of demographics and post-transplant medications, including immunosuppressive drug therapy, revealed no statistically significant differences between groups that could account for the observed survival benefit. Furthermore, multivariate analysis confirmed a significantly reduced risk of mortality within ten years after HTX for patients receiving ivabradine (HR: 0.374, 95% CI: 0.182–0.770, p = 0.008), suggesting a favorable impact on long-term survival, extending previous observations of improved short- and mid-term outcomes in HTX recipients [6,7].
In contrast, Dos Santos and colleagues [29] reported no statistically significant reduction in all-cause mortality at 3-year follow-up after HTX in patients treated with ivabradine (p = 0.13). However, the authors acknowledged that their study was not powered to detect differences in mortality, noting that a substantially larger sample size with extended follow-up would be needed [29].
The underlying mechanisms contributing to this improved survival in HTX recipients with ivabradine may extend beyond heart rate control. Ivabradine has been associated with enhanced endothelial function, increased sarcoplasmic reticulum calcium uptake, reduced expression of pro-inflammatory cytokines (including tumor necrosis factor alpha), decreased oxidative stress, normalization of mRNA expression across multiple signaling pathways, and reductions in cardiomyocyte apoptosis and hypertrophy [34,35,36]. In addition to these pleiotropic effects, ivabradine-induced heart rate reduction may also contribute to improved long-term post-transplant survival through favorable alterations in echocardiographic parameters, such as LV mass and diastolic function [6,7].
4.4. Long-Term Effects of Heart Rate Control After Heart Transplantation
Several mechanisms are likely involved in the cardioprotective effects of ivabradine following HTX [1,2,3,4,5,6,7,37,38,39]. Its primary action—heart rate reduction—prolongs diastolic time, thereby enhancing coronary perfusion, ventricular filling, and diastolic function [34,35,36,37,38,39]. Improved diastolic performance may, in part, be mediated by increased sarcoplasmic reticulum calcium uptake and enhanced SERCA (sarcoplasmic/endoplasmic reticulum calcium ATPase) activity [36]. In the present study, ivabradine-treated HTX recipients exhibited significantly higher E/A ratios and lower E/e′ ratios, consistent with prior reports [36,37].
Beyond its effects on diastolic function, ivabradine has been shown to influence systolic function and myocardial structure through modulation of cardiomyocytes and the extracellular matrix [38,39]. In our cohort, HTX recipients receiving ivabradine had a significantly higher LVEF and a marked reduction in LV mass and the LV mass index toward normative values. These findings suggest that ivabradine may attenuate adverse cardiac remodeling and cardiomyocyte hypertrophy, thereby improving LV function through reduced hypoxia and myocardial oxygen demand [36].
Each heartbeat imposes energetic stress on cardiac metabolism and mechanical stress on the endothelium [35,40]. Elevated heart rates therefore amplify pulsatile wall stress, potentially leading to endothelial dysfunction, inflammation, structural degradation, and microvascular coronary disease [35,36,37,38]. Notably, ivabradine has been reported to stimulate angiogenesis and enhance microvascular perfusion with long-term use [34,35,36,37,38,39]. Although our study found no significant differences between HTX recipients with ivabradine or metoprolol in coronary artery disease, coronary stenting, or high-sensitivity cardiac troponin T, patients treated with ivabradine had a significantly lower LVEDP and NT-proBNP level—findings that may reflect improved microvascular integrity and changes in cardiac metabolism.
Improvement in microvascular perfusion, reduction in myocardial oxygen demand and oxidative stress, as well as vascular protective effects are all clinically relevant, given the fact that approximately one out of three HTX recipients develops cardiac allograft vasculopathy within five years after HTX due to coronary inflammation, endothelial dysfunction, and fibroproliferative remodeling [41].
In summary, our findings suggest that ivabradine not only provides effective heart rate control after HTX but also exerts beneficial effects at multiple levels—enhancing systolic and diastolic function, mitigating hypertrophic remodeling, and supporting coronary microvascular health through angiogenic and anti-oxidative mechanisms [34,35,36,37,38,39,40,41]. Importantly, HTX recipients with ivabradine rapidly achieved a durable resting heart rate ≤ 80 bpm and sustained a resting heart rate ≤ 80 bpm over the entire 10-year study period. This is of great clinical importance as non-transplant participants with a resting heart rate > 80 bpm had a greater risk for both cardiovascular disease and all-cause mortality in a large epidemiological study [42]. Based upon our findings, resting heart rates > 80 bpm in HTX recipients should be addressed and treated with ivabradine, if possible, to achieve an adequate long-term heart rate control after HTX with a resting heart rate ≤ 80 bpm. However, further investigation—ideally through large, multicenter randomized controlled trials—is warranted to explore these findings in greater detail and to validate the long-term benefits of ivabradine in the post-transplant setting.
4.5. Study Limitations
Our results were based on an observational retrospective single-center study with 110 adult patients receiving HTX at Heidelberg Heart Center between 2006 and 2015. Hereof, 54 HTX recipients had ivabradine, and 56 HTX recipients had metoprolol. Though this participant number seems rather small at first glance, to our knowledge, this is the largest study analyzing long-term effects (10-year results) of heart rate control with ivabradine or metoprolol in HTX recipients. It therefore provides important and clinically needed data to the limited available literature in this field [1,2,3,4,5,6,7].
Given the known limitations of the study design used, our findings should be interpreted with caution as the non-randomized study design may be subject to selection bias and unmeasured confounders. Nevertheless, we were able to use highly detailed data of 110 HTX recipients, as our patients received standardized treatment and follow-up, reducing the likelihood of selection bias and potential confounders [6,7,24,25,26].
The Devereux formula was used to calculate LV mass, which carries the limitation of a two-dimensional assessment. Assessment of echocardiographic features is generally subject to the examiner‘s experience. Therefore, echocardiographic video files were independently assessed by two cardiologists [6,7].
We did not perform a randomization of HTX recipients regarding the use of ivabradine or metoprolol as individual physician practice and patient preference influenced the prescription reflecting real-world data. However, we could not detect significant differences between groups in terms of demographics or concurrent drugs reducing the likelihood of selection bias [6,7,24,25,26].
We also did not perform routine exercise-based testing such as exercise ECG, treadmill exercise ECG, or a 6 min walk test in HTX recipients at Heidelberg Heart Center but rather performed stress cardiac MRI or cardiac catheterization with biopsy in cases of clinical deterioration, changes in LVEF, or suspicion of relevant myocardial ischemia. We therefore had to rely on a resting 12-lead ECG and 24 h Holter monitor data, which were routinely performed [6,7,24,25,26].
Regarding the use of ivabradine or metoprolol over a period of ten years, temporary changes in medications cannot be ruled out completely, as routine follow-up visits at Heidelberg Heart Center were reduced to once or twice annually, five years after HTX. However, HTX recipients, in general, have a very high rate of medication adherence as this is crucial for their survival. In addition, patients were routinely asked about their medication adherence at each follow-up and change in medications was standardly performed only after consultation [6,7,24,25,26].
Finally, our results should be considered hypothesis-generating, especially in terms of post-transplant survival, as multiple factors may affect post-transplant survival. It is further unknown whether our findings are attributed to differences between ivabradine and metoprolol or ivabradine and beta blockers in general. Therefore, to confirm our findings, further research is needed, ideally through large, prospective randomized controlled multicenter trials to investigate the long-term effects of heart rate control with ivabradine or metoprolol in patients after HTX [6,7,24,25,26].
5. Conclusions
As sinus tachycardia after HTX due to cardiac graft denervation is associated with reduced post-transplant survival, we performed an observational retrospective single-center study analyzing the ten-year post-transplant results of 54 HTX recipients with ivabradine and 56 HTX recipients with metoprolol. Noteworthy, HTX recipients were neither preselected nor randomized for treatment with ivabradine versus metoprolol to manage post-transplant heart rate. Individual physician practice and patient preference influenced the prescription of either drug, reflecting real-world data [6,7]. At 10-year follow-up, HTX recipients with ivabradine showed a significantly lower average heart rate compared to baseline (p < 0.001) and to metoprolol (p < 0.001) as well as a significantly lower NT-proBNP level compared to baseline (p < 0.001) and to metoprolol succinate (p = 0.005). Moreover, HTX recipients with ivabradine had a significantly lower overall mortality (20.4% versus 46.4%, p = 0.004) and mortality due to graft failure (1.9% versus 21.4%, p = 0.001) in comparison to HTX recipients with metoprolol at 10-year follow-up. In addition, multivariate analysis showed a significantly decreased risk of death within ten years after HTX in patients with post-transplant use of ivabradine (HR 0.374, CI 0.182–0.770; p = 0.008). In summary, based upon our findings, ivabradine appears to be a safe, specific, and selective long-term treatment of sinus tachycardia in HTX recipients, which was associated with a significantly better heart rate reduction, a lower NT-proBNP level, and a superior 10-year survival after HTX.
Author Contributions
Conceptualization, F.F.D., A.C.A., R.R., and A.-K.R.; methodology, F.F.D., A.C.A., R.R., and A.-K.R.; validation, F.F.D., A.C.A., R.R., M.H., and A.-K.R.; formal analysis, F.F.D., A.C.A., R.R., and A.-K.R.; investigation, F.F.D., A.C.A., R.R., M.H., and A.-K.R.; resources, F.F.D., A.C.A., R.R., M.H., P.E., N.F., and A.-K.R.; data curation, F.F.D., A.C.A., R.R., and A.-K.R.; writing—original draft preparation, F.F.D., A.C.A., R.R., and A.-K.R.; writing—review and editing, F.F.D., A.C.A., R.R., and A.-K.R.; visualization, F.F.D., A.C.A., R.R., and A.-K.R.; supervision, F.F.D., A.C.A., R.R., M.H., P.E., N.F., and A.-K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fondation Coeur—Daniel Wagner, Fondation de Luxembourg (F.F.D. and R.R.). For the publication fee we acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding program ‘Open Access Publikationskosten’ as well as by Heidelberg University.
Institutional Review Board Statement
This study was performed in accordance with the ethical standards of the Declaration of Helsinki. Approval was granted by the institutional review board (IRB) of Heidelberg University (ethics approval number: S-286/2015, Version 1.2, 28 July 2020).
Informed Consent Statement
We obtained written informed consent from patients for their inclusion in the Heidelberg HTX Registry and the clinical and scientific use of their data. The ethics approval does not require additional consent for this observational study as only routine clinical data were used.
Data Availability Statement
The original contributions presented in this study are included in this article; further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all the supporters of the HTX Program at Heidelberg Heart Center.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, R.; Haverich, A.; Strüber, M.; Simon, A.; Pichlmaier, M.; Bara, C. Effects of Ivabradine on Allograft Function and Exercise Performance in Heart Transplant Recipients with Permanent Sinus Tachycardia. Clin. Res. Cardiol. 2008, 97, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bobylev, D.; Stiefel, P.; Haverich, A.; Bara, C. Lasting Reduction of Heart Transplant Tachycardia with Ivabradine is Effective and Well Tolerated: Results of 48-Month Study. Clin. Res. Cardiol. 2012, 101, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Doesch, A.O.; Celik, S.; Ehlermann, P.; Frankenstein, L.; Zehelein, J.; Koch, A.; Katus, H.A.; Dengler, T.J. Heart Rate Reduction after Heart Transplantation with Beta-Blocker Versus the Selective If Channel Antagonist Ivabradine. Transplantation 2007, 84, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Doesch, A.O.; Ammon, K.; Konstandin, M.; Celik, S.; Kristen, A.; Frankenstein, L.; Buss, S.; Hardt, S.; Sack, F.U.; Katus, H.A.; et al. Heart Rate Reduction for 12 Months with Ivabradine Reduces Left Ventricular Mass in Cardiac Allograft Recipients. Transplantation 2009, 88, 835–841. [Google Scholar] [CrossRef]
- Doesch, A.O.; Mueller, S.; Erbel, C.; Gleissner, C.A.; Frankenstein, L.; Hardt, S.; Ruhparwar, A.; Ehlermann, P.; Dengler, T.; Katus, H.A. Heart Rate Reduction for 36 Months with Ivabradine Reduces Left Ventricular Mass in Cardiac Allograft Recipients: A Long-Term Follow-Up Study. Drug Des. Devel. Ther. 2013, 7, 1323–1328. [Google Scholar] [CrossRef]
- Rivinius, R.; Helmschrott, M.; Ruhparwar, A.; Rahm, A.K.; Darche, F.F.; Thomas, D.; Bruckner, T.; Ehlermann, P.; Katus, H.A.; Doesch, A.O. Control of Cardiac Chronotropic Function in Patients after Heart Transplantation: Effects of Ivabradine and Metoprolol Succinate on Resting Heart Rate in the Denervated Heart. Clin. Res. Cardiol. 2018, 107, 138–147. [Google Scholar] [CrossRef]
- Rivinius, R.; Helmschrott, M.; Rahm, A.K.; Darche, F.F.; Thomas, D.; Bruckner, T.; Doesch, A.O.; Katus, H.A.; Ehlermann, P. Five-Year Results of Heart Rate Control with Ivabradine or Metoprolol Succinate in Patients after Heart Transplantation. Clin. Res. Cardiol. 2022, 111, 141–153. [Google Scholar] [CrossRef]
- Custodis, F.; Roggenbuck, U.; Lehmann, N.; Moebus, S.; Laufs, U.; Mahabadi, A.A.; Heusch, G.; Mann, K.; Jöckel, K.H.; Erbel, R.; et al. Resting Heart Rate is an Independent Predictor of All-Cause Mortality in the Middle Aged General Population. Clin. Res. Cardiol. 2016, 105, 601–612. [Google Scholar] [CrossRef]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; SHIFT Investigators. Ivabradine and Outcomes in Chronic Heart Failure (SHIFT): A Randomised Placebo-Controlled Study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef]
- Böhm, M.; Swedberg, K.; Komajda, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; SHIFT Investigators. Heart Rate as a Risk Factor in Chronic Heart Failure (SHIFT): The Association Between Heart Rate and Outcomes in a Randomised Placebo-Controlled Trial. Lancet 2010, 376, 886–894. [Google Scholar] [CrossRef]
- Münzel, T.; Hahad, O.; Gori, T.; Hollmann, S.; Arnold, N.; Prochaska, J.H.; Schulz, A.; Beutel, M.; Pfeiffer, N.; Schmidtmann, I.; et al. Heart Rate, Mortality, and the Relation with Clinical and Subclinical Cardiovascular Diseases: Results from the Gutenberg Health Study. Clin. Res. Cardiol. 2019, 108, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.D.; McComb, J.M.; Dark, J.H. Heart Rate and Late Mortality in Cardiac Transplant Recipients. Eur. Heart J. 1993, 14, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Dobre, D.; Borer, J.S.; Fox, K.; Swedberg, K.; Adams, K.F.; Cleland, J.G.; Cohen-Solal, A.; Gheorghiade, M.; Gueyffier, F.; O’Connor, C.M.; et al. Heart Rate: A Prognostic Factor and Therapeutic Target in Chronic Heart Failure. The Distinct Roles of Drugs with Heart Rate-Lowering Properties. Eur. J. Heart Fail. 2014, 16, 76–85. [Google Scholar] [CrossRef]
- Tardif, J.C.; Ford, I.; Tendera, M.; Bourassa, M.G.; Fox, K.; INITIATIVE Investigators. Efficacy of Ivabradine, a New Selective I(f) Inhibitor, Compared with Atenolol in Patients with Chronic Stable Angina. Eur. Heart J. 2005, 26, 2529–2536. [Google Scholar] [CrossRef]
- DiFrancesco, D. Characterization of Single Pacemaker Channels in Cardiac Sino-Atrial Node Cells. Nature 1986, 324, 470–473. [Google Scholar] [CrossRef]
- DiFrancesco, D. The Contribution of the ‘Pacemaker’ Current (if) to Generation of Spontaneous Activity in Rabbit Sino-Atrial Node Myocytes. J. Physiol. 1991, 434, 23–40. [Google Scholar] [CrossRef]
- DiFrancesco, D.; Camm, J.A. Heart Rate Lowering by Specific and Selective I(f) Current Inhibition with Ivabradine: A New Therapeutic Perspective in Cardiovascular Disease. Drugs 2004, 64, 1757–1765. [Google Scholar] [CrossRef]
- Thollon, C.; Bedut, S.; Villeneuve, N.; Cogé, F.; Piffard, L.; Guillaumin, J.P.; Brunel-Jacquemin, C.; Chomarat, P.; Boutin, J.A.; Peglion, J.L.; et al. Use-Dependent Inhibition of hHCN4 by Ivabradine and Relationship with Reduction in Pacemaker Activity. Br. J. Pharmacol. 2007, 150, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Haechl, N.; Ebner, J.; Hilber, K.; Todt, H.; Koenig, X. Pharmacological Profile of the Bradycardic Agent Ivabradine on Human Cardiac Ion Channels. Cell. Physiol. Biochem. 2019, 53, 36–48. [Google Scholar] [CrossRef]
- Hancox, J.C.; Melgari, D.; Dempsey, C.E.; Brack, K.E.; Mitcheson, J.; Ng, G.A. hERG Potassium Channel Inhibition by Ivabradine May Contribute to QT Prolongation and Risk of Torsades De Pointes. Ther. Adv. Drug Saf. 2015, 6, 177–179. [Google Scholar] [CrossRef]
- Melgari, D.; Brack, K.E.; Zhang, C.; Zhang, Y.; El Harchi, A.; Mitcheson, J.S.; Dempsey, C.E.; Ng, G.A.; Hancox, J.C. hERG Potassium Channel Blockade by the HCN Channel Inhibitor Bradycardic Agent Ivabradine. J. Am. Heart Assoc. 2015, 4, e001813. [Google Scholar] [CrossRef]
- Savelieva, I.; Camm, A.J. Novel If Current Inhibitor Ivabradine: Safety Considerations. Adv. Cardiol. 2006, 43, 79–96. [Google Scholar]
- Ozkanlar, Y.; Nishijima, Y.; da Cunha, D.; Hamlin, R.L. Acute Effects of Tacrolimus (FK506) on Left Ventricular Mechanics. Pharmacol. Res. 2005, 52, 307–312. [Google Scholar]
- Darche, F.F.; Helmschrott, M.; Rahm, A.K.; Thomas, D.; Schweizer, P.A.; Bruckner, T.; Ehlermann, P.; Kreusser, M.M.; Warnecke, G.; Frey, N.; et al. Atrial Fibrillation before Heart Transplantation is a Risk Factor for Post-Transplant Atrial Fibrillation and Mortality. ESC Heart Fail. 2021, 8, 4265–4277. [Google Scholar] [CrossRef] [PubMed]
- Darche, F.F.; Fabricius, L.C.; Helmschrott, M.; Rahm, A.K.; Ehlermann, P.; Bruckner, T.; Sommer, W.; Warnecke, G.; Frey, N.; Rivinius, R. Oral Anticoagulants after Heart Transplantation-Comparison between Vitamin K Antagonists and Direct Oral Anticoagulants. J. Clin. Med. 2023, 12, 4334. [Google Scholar] [CrossRef] [PubMed]
- Darche, F.F.; Heil, K.M.; Rivinius, R.; Helmschrott, M.; Ehlermann, P.; Frey, N.; Rahm, A.K. Early Pacemaker Dependency after Heart Transplantation is Associated with Permanent Pacemaker Implantation, Graft Failure and Mortality. J. Cardiovasc. Dev. Dis. 2024, 11, 394. [Google Scholar] [CrossRef]
- Vandenberk, B.; Vandael, E.; Robyns, T.; Vandenberghe, J.; Garweg, C.; Foulon, V.; Ector, J.; Willems, R. Which QT Correction Formulae to Use for QT Monitoring? J. Am. Heart Assoc. 2016, 5, e003264. [Google Scholar] [CrossRef] [PubMed]
- Boeken, U.; Albert, A.; Mehdiani, A.; Sowinski, B.; Westenfeld, R.; Aubin, H.; Saeed, D.; Akhyari, P.; Lichtenberg, A. Efficacy and Safety of Ivabradine Application in the Early Period after Heart Transplantation. J. Heart Lung Transplant. 2019, 38, S292. [Google Scholar] [CrossRef]
- Dos Santos, C.C.; Rossi Neto, J.M.; Finger, M.A.; Timerman, A.; Contreras, C.; Chaccur, P. Ivabradine plus Conventional Treatment vs Conventional Treatment Alone in Reducing the Mean Heart Rate in Heart Transplant Recipients: A Randomized Clinical Trial. Clin. Transplant. 2021, 35, e14227. [Google Scholar] [CrossRef]
- Halpert, I.; Goldberg, A.D.; Levine, A.B.; Levine, T.B.; Kornberg, R.; Kelly, C.; Lesch, M. Reinnervation of the Transplanted Human Heart as Evidenced from Heart Rate Variability Studies. Am. J. Cardiol. 1996, 77, 180–183. [Google Scholar] [CrossRef]
- Lage-Gallé, E.; Romero-Rodríguez, N.; Nevado-Portero, J.; Guisado-Rasco, A.; Sobrino-Márquez, M.; Machuca, M.G.; Fernández-Quero, M.; Campos-Pareja, A.; Ballesteros-Pradas, S.; Martínez-Martínez, A. Safety and Effectiveness of Ivabradine after Cardiac Transplantation. Transplant. Proc. 2010, 42, 3191–3192. [Google Scholar] [CrossRef]
- Minematsu, T.; Ohtani, H.; Sato, H.; Iga, T. Sustained QT Prolongation Induced by Tacrolimus in Guinea Pigs. Life Sci. 1999, 65, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.C.; So, S.; Marsh, J.W.; Murphy, A.M. QT Prolongation and Torsades de Pointes after Administration of FK506. Transplantation 1992, 53, 929–930. [Google Scholar] [CrossRef]
- Kröller-Schön, S.; Schulz, E.; Wenzel, P.; Kleschyov, A.L.; Hortmann, M.; Torzewski, M.; Oelze, M.; Renné, T.; Daiber, A.; Münzel, T. Differential Effects of Heart Rate Reduction with Ivabradine in Two Models of Endothelial Dysfunction and Oxidative Stress. Basic Res. Cardiol. 2011, 106, 1147–1158. [Google Scholar] [CrossRef]
- Mangiacapra, F.; Colaiori, I.; Ricottini, E.; Balducci, F.; Creta, A.; Demartini, C.; Minotti, G.; Di Sciascio, G. Heart Rate Reduction by IVabradine for Improvement of ENDothELial Function in Patients with Coronary Artery Disease: The RIVENDEL study. Clin. Res. Cardiol. 2017, 106, 69–75. [Google Scholar] [CrossRef]
- Sabbah, H.N.; Gupta, R.C.; Kohli, S.; Wang, M.; Zhang, K.; Rastogi, S. Heart Rate Reduction with Ivabradine Improves Left Ventricular Function and Reverses Multiple Pathological Maladaptations in Dogs with Chronic Heart Failure. ESC Heart Fail. 2014, 1, 94–102. [Google Scholar] [CrossRef]
- Fischer-Rasokat, U.; Honold, J.; Lochmann, D.; Wolter, S.; Liebetrau, C.; Fichtlscherer, S.; Möllmann, H.; Spyridopoulos, I.; Hamm, C.W. β-Blockers and Ivabradine Differentially Affect Cardiopulmonary Function and Left Ventricular Filling Index. Clin. Res. Cardiol. 2016, 105, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.; Barbier, S.; Chagraoui, A.; Richard, V.; Henry, J.P.; Lallemand, F.; Renet, S.; Lerebours, G.; Mahlberg-Gaudin, F.; Thuillez, C. Long-term Heart Rate Reduction Induced by the Selective I(f) Current Inhibitor Ivabradine Improves Left Ventricular Function and Intrinsic Myocardial Structure in Congestive Heart Failure. Circulation 2004, 109, 1674–1679. [Google Scholar] [CrossRef]
- Monnet, X.; Colin, P.; Ghaleh, B.; Hittinger, L.; Giudicelli, J.F.; Berdeaux, A. Heart Rate Reduction During Exercise-Induced Myocardial Ischaemia and Stunning. Eur. Heart J. 2004, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yang, M.; Wang, H.; Shen, C.; Wu, M.; Xu, H.; Wu, Y.; Li, Y.; Li, X.; Huang, T.; et al. Moderate Heart Rate Reduction Promotes Cardiac Regeneration through Stimulation of the Metabolic Pattern Switch. Cell Rep. 2022, 38, 110468. [Google Scholar] [CrossRef]
- Chih, S.; Chong, A.Y.; Mielniczuk, L.M.; Bhatt, D.L.; Beanlands, R.S. Allograft Vasculopathy: The Achilles’ Heel of Heart Transplantation. J. Am. Coll. Cardiol. 2016, 68, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Minton, D.; Lee, D.C.; Sui, X.; Fayad, R.; Lavie, C.J.; Blair, S.N. Protective Role of Resting Heart Rate on All-Cause and Cardiovascular Disease Mortality. Mayo Clin. Proc. 2013, 88, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).