Relationship Between Histomonas meleagridis Infection and Cecal Intestinal Microbiota of Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Parasite
2.4. Experimental Design
2.5. Sample Collection and Intestinal Lesion Score
2.6. Lesion Scoring Rules
2.7. DNA Extraction, Library Construction, and Sequencing
2.8. Bioinformatic Analysis
2.9. Statistical Analysis
3. Results
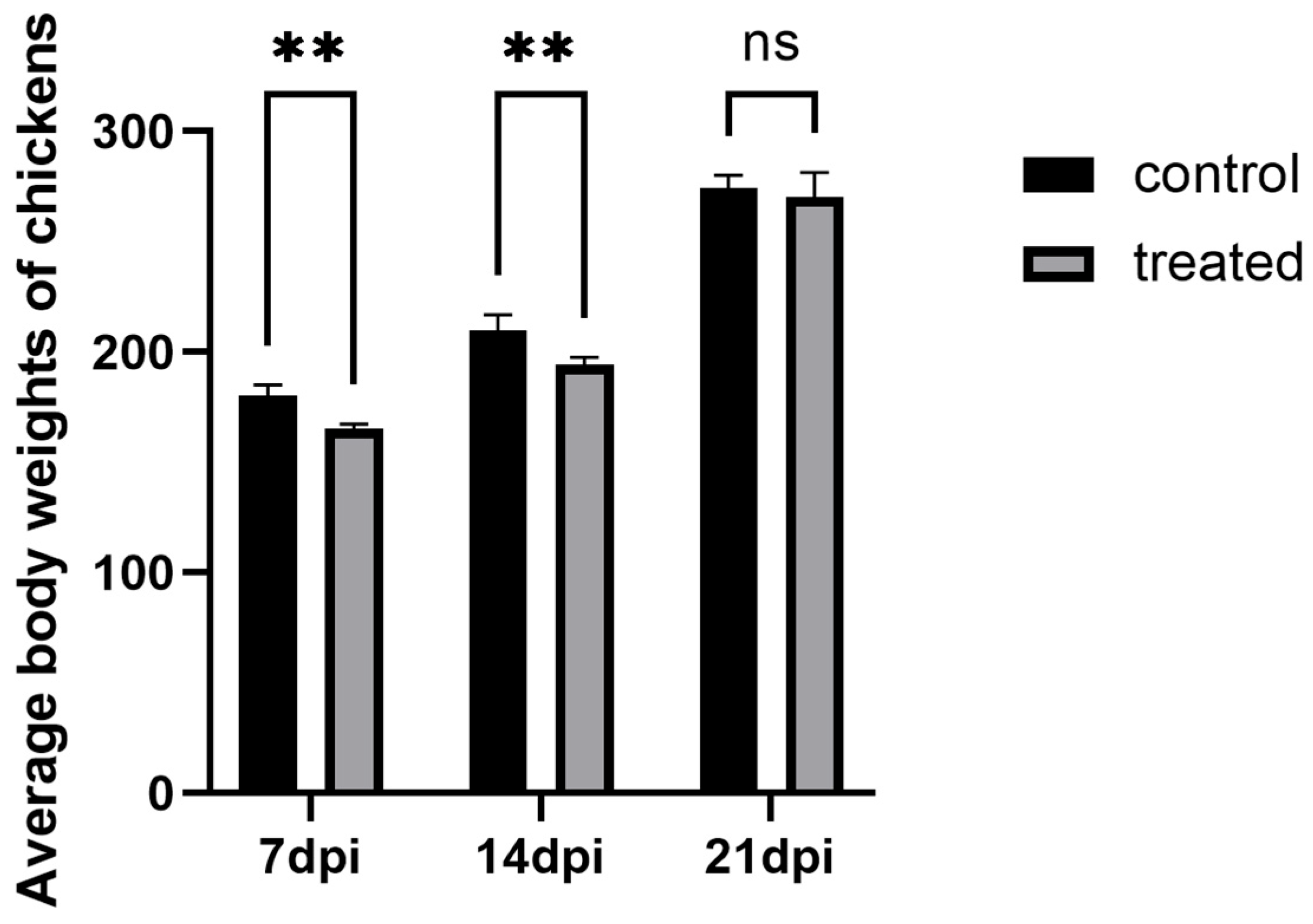
3.1. Growth Performance
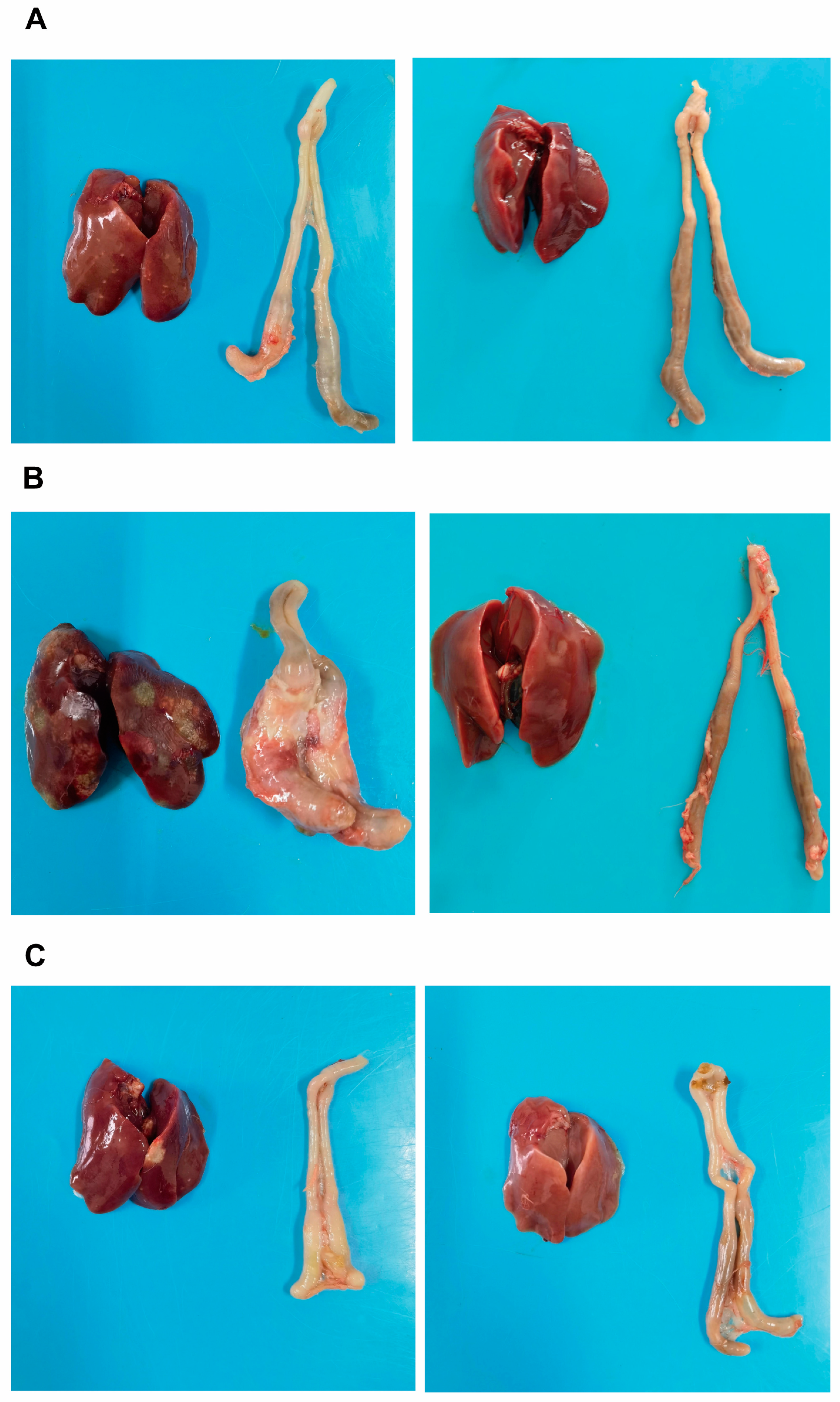
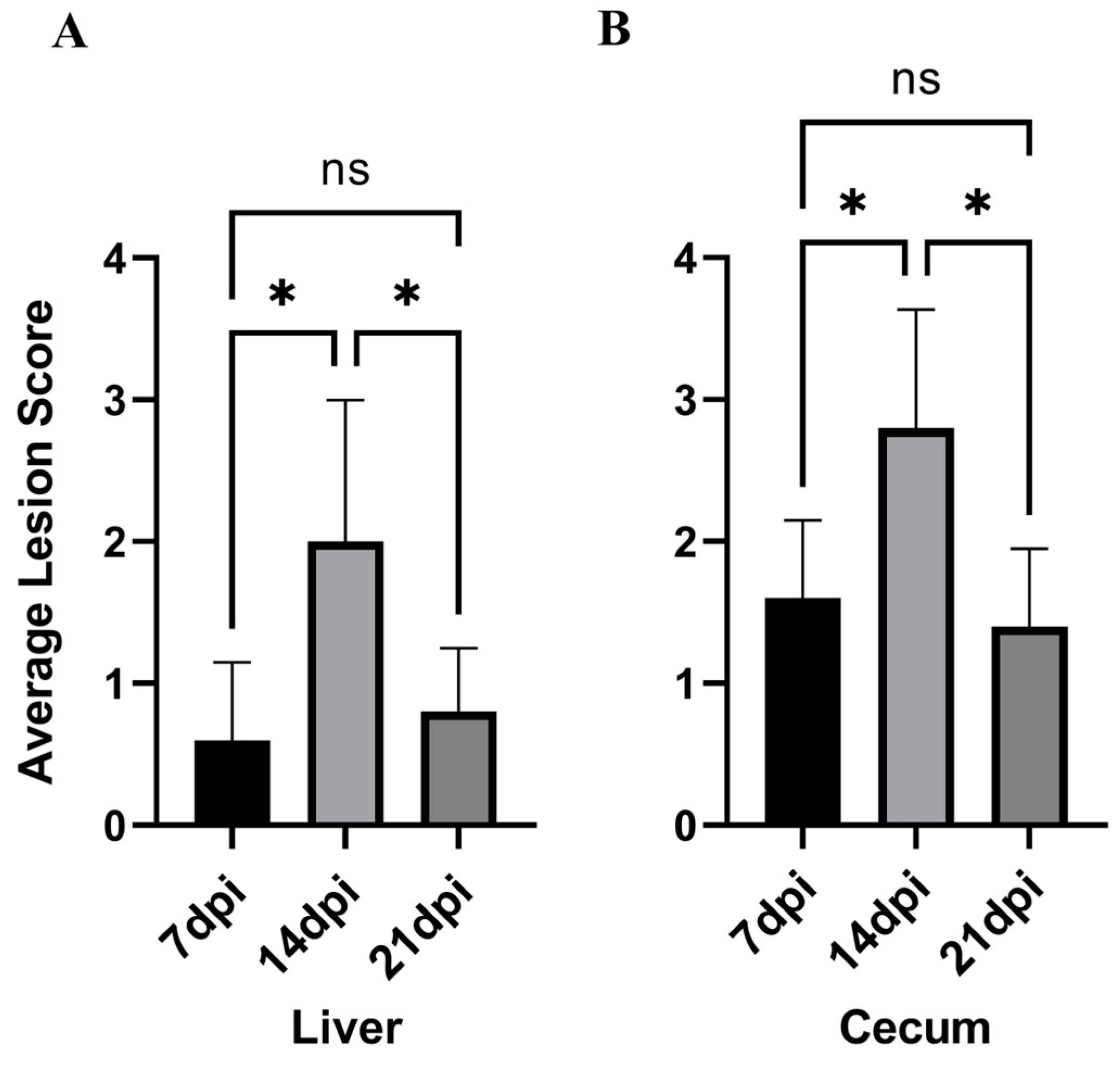
3.2. Intestinal Lesion and Intestinal Lesion Score
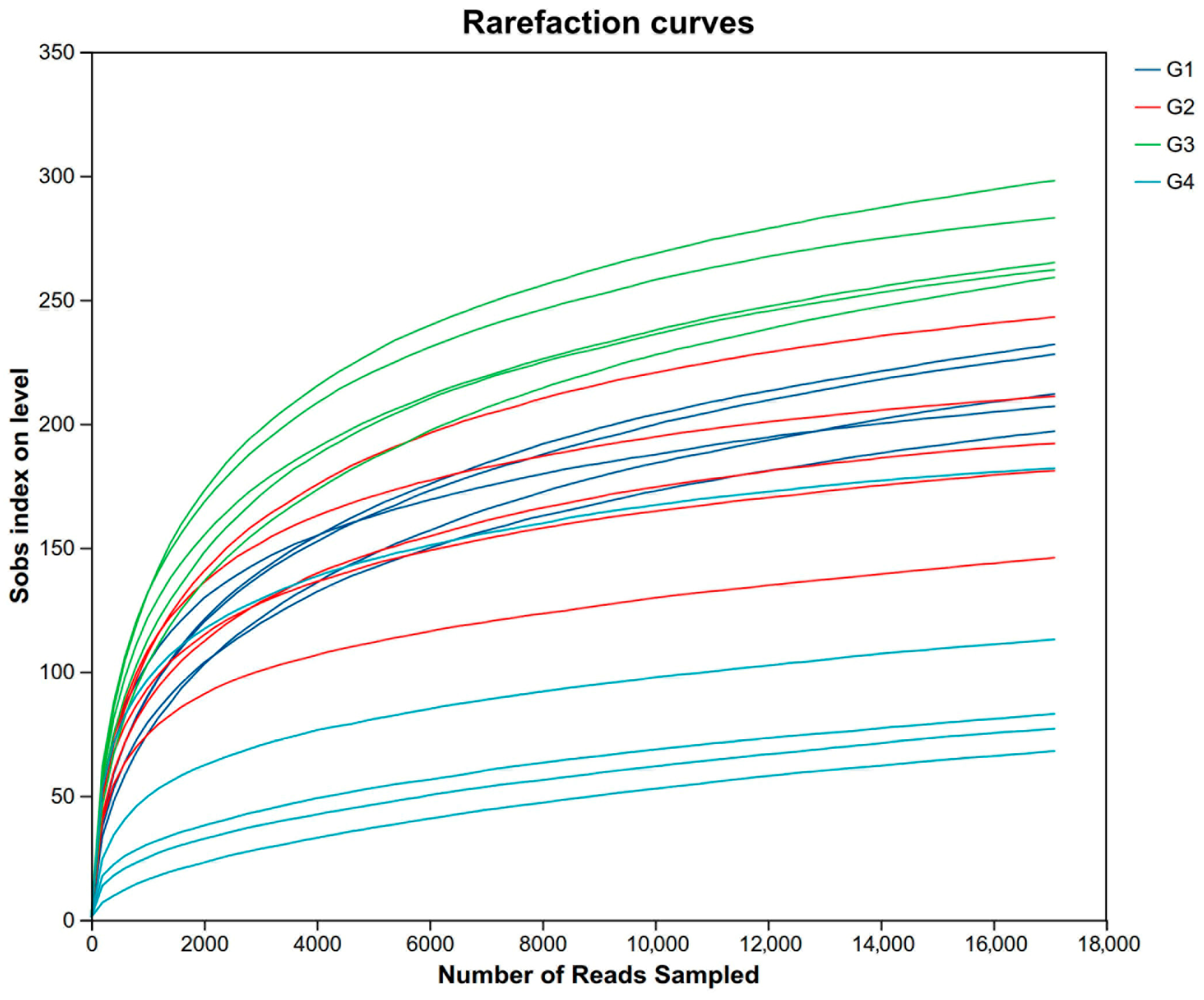
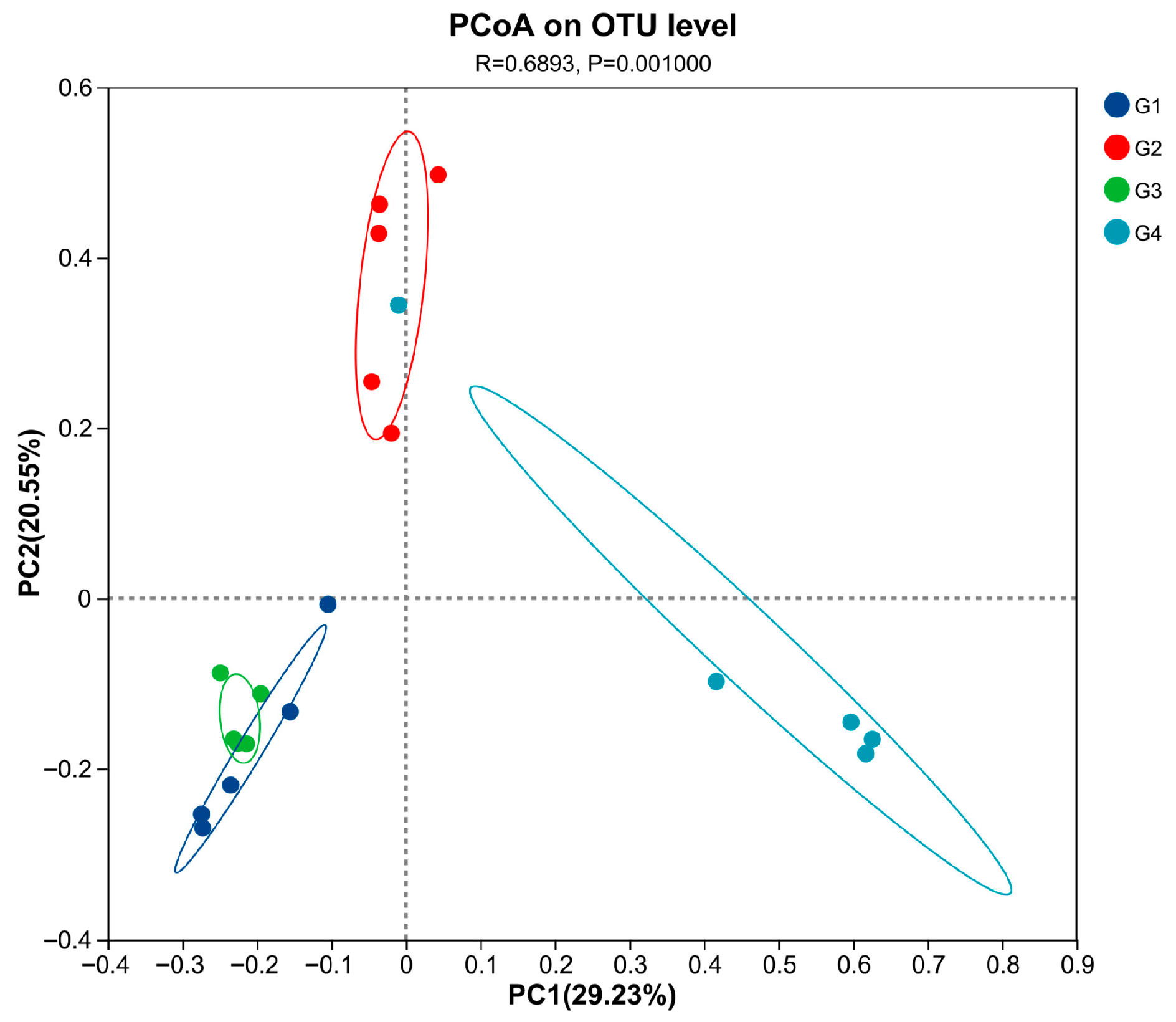
3.3. Rarefaction Curve and the Change in the Alpha and Beta of Cecal Gut Microbiota
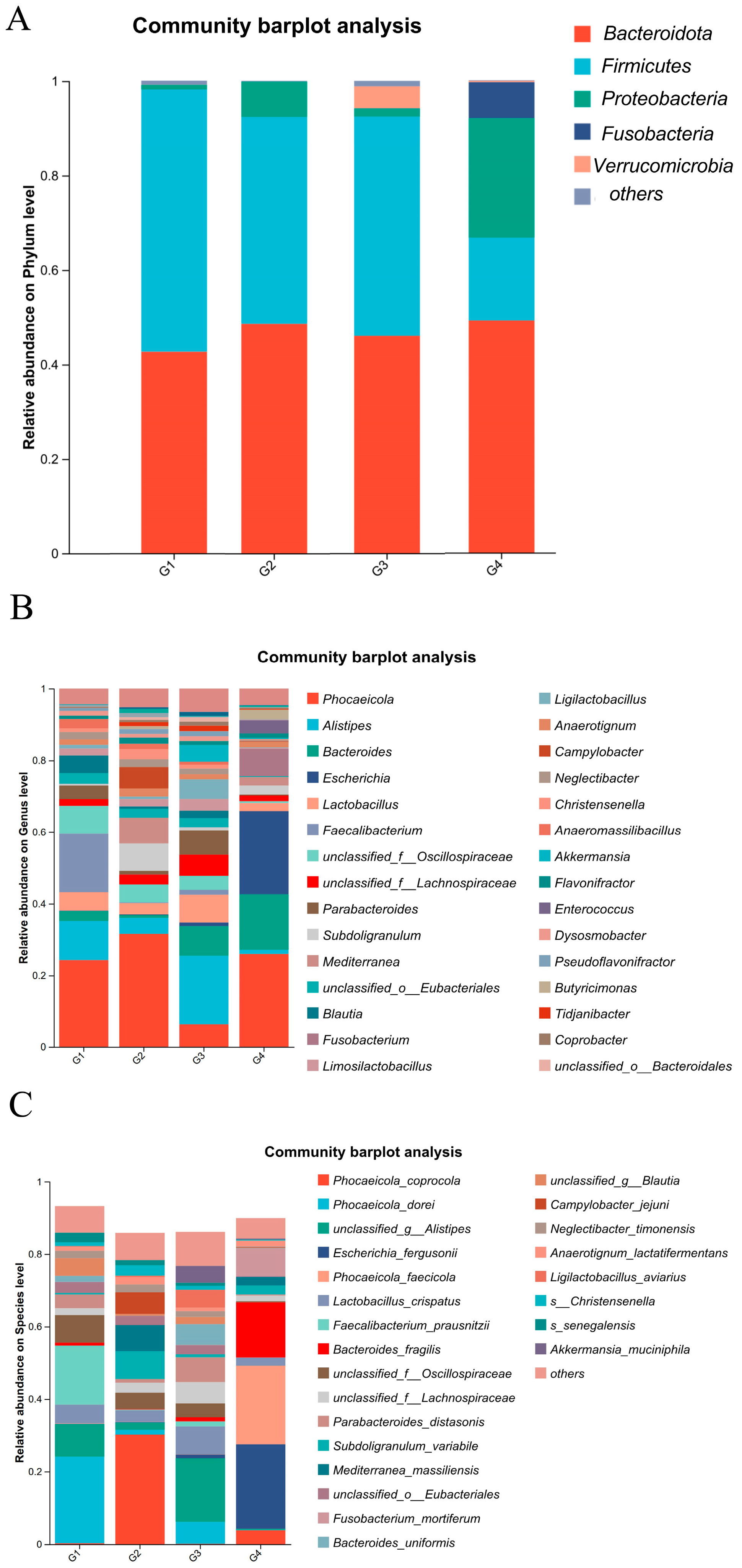
3.4. Analysis of the Composition and Differential Changes in Species Abundance of Cecal Microbiota in Chickens
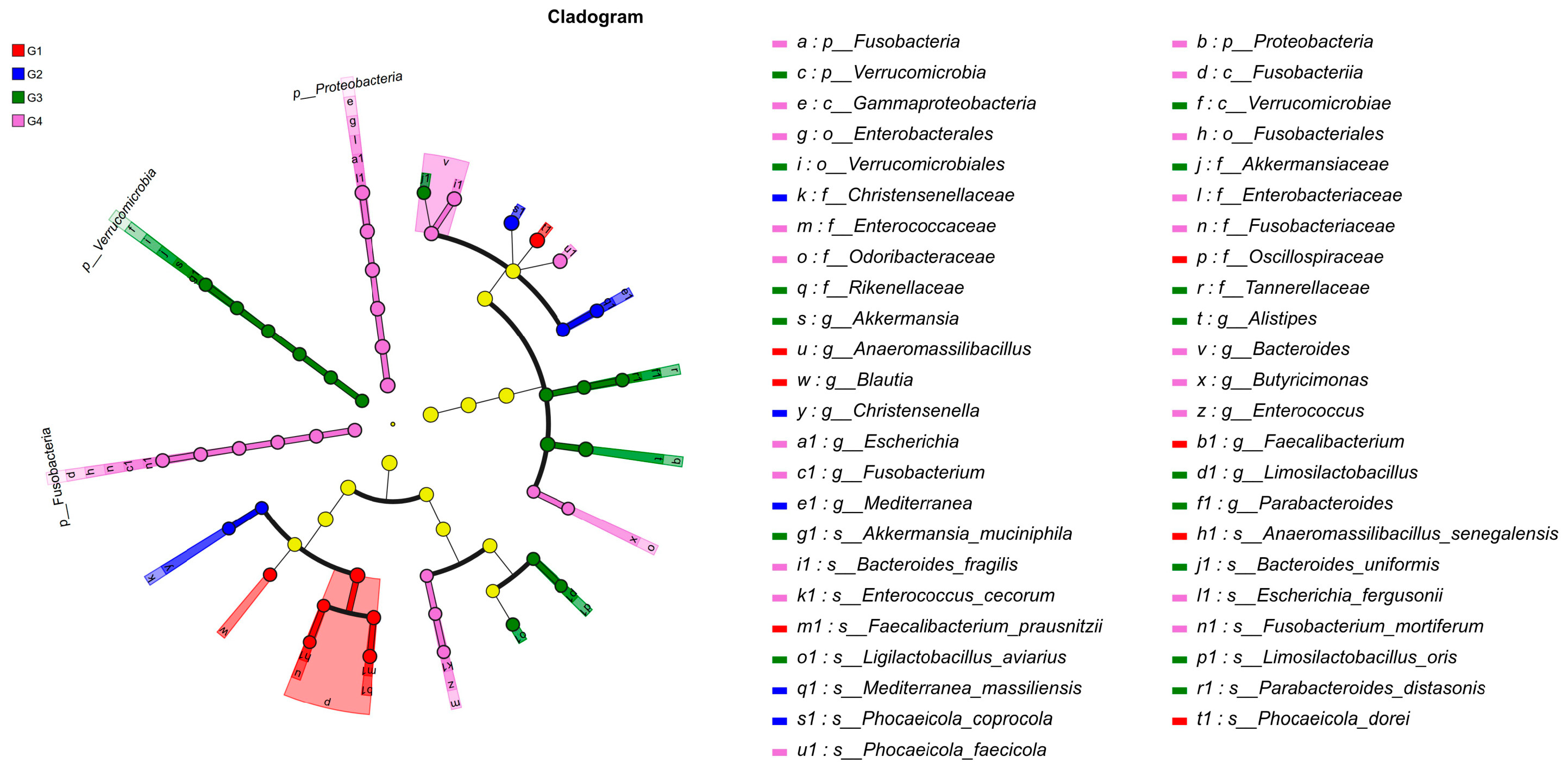
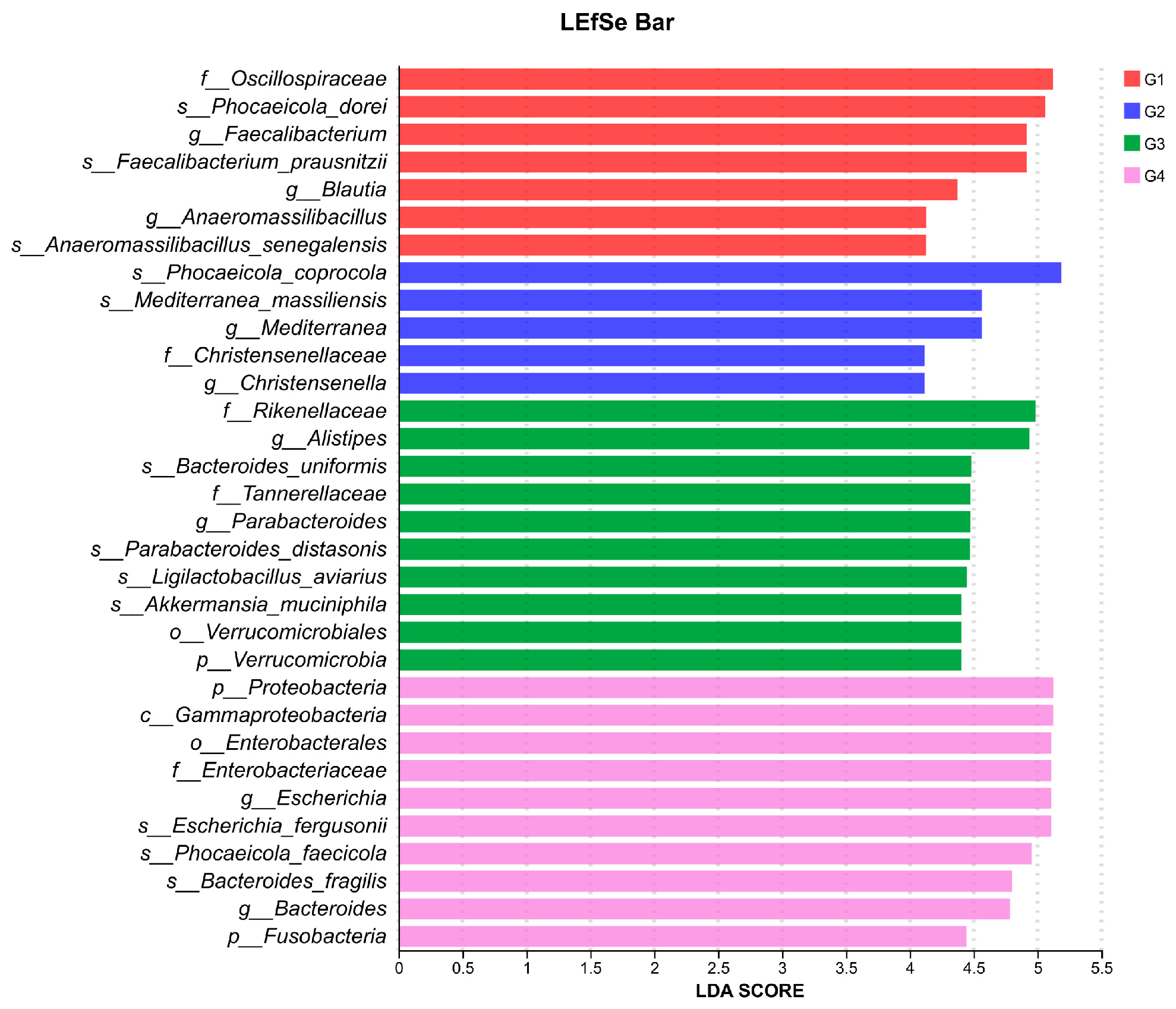
3.5. LEfSe-Based Discriminant Analysis of Multi-Level Species Differences in Cecal Microbiota Across Experimental Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beer, L.C.; Petrone-Garcia, V.M.; Graham, B.D.; Hargis, B.M.; Tellez-Isaias, G.; Vuong, C.N. Histomonosis in Poultry: A Comprehensive Review. Front. Vet. Sci. 2022, 9, 880738. [Google Scholar] [CrossRef]
- Hess, M.; Liebhart, D.; Bilic, I.; Ganas, P. Histomonas meleagridis—New Insights into an Old Pathogen. Vet. Parasitol. 2015, 208, 67–76. [Google Scholar] [CrossRef]
- Liu, D.; Kong, L.; Tao, J.; Xu, J. An Outbreak of Histomoniasis in Backyard Sanhuang Chickens. Korean J. Parasitol. 2018, 56, 597–602. [Google Scholar] [CrossRef]
- Liebhart, D.; Sulejmanovic, T.; Grafl, B.; Tichy, A.; Hess, M. Vaccination against Histomonosis Prevents a Drop in Egg Production in Layers Following Challenge. Avian Pathol. 2013, 42, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Liebhart, D.; Hess, M. Spotlight on Histomonosis (Blackhead Disease): A Re-Emerging Disease in Turkeys and Chickens. Avian Pathol. 2020, 49, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.G.; Wang, S.; Rong, J.; Chen, C.; Hou, Z.F.; Liu, D.D.; Tao, J.P.; Xu, J.J. Inhibitory Effects of Different Plant Extracts on Histomonas meleagridis In Vitro and In Vivo in Chickens. Vet. Parasitol. 2025, 337, 110487. [Google Scholar] [CrossRef]
- Dubey, J.P.; Paker, C.; Graham, D.; Hargis, B.M.; Jenkins, M.C. Histomonas meleagridis Infections in Turkeys in the USA: A Century of Progress, Resurgence, and Tribute to Its Early Investigators, Theobald Smith, Ernst Tyzzer, and Everett Lund. J. Parasitol. 2024, 110, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Liebhart, D.; Ganas, P.; Sulejmanovic, T.; Hess, M. Histomonosis in Poultry: Previous and Current Strategies for Prevention and Therapy. Avian Pathol. 2017, 46, 1–18. [Google Scholar] [CrossRef]
- McDougald, L.R. Blackhead Disease (Histomoniasis) in Poultry: A Critical Review. Avian Dis. 2005, 49, 462–476. [Google Scholar] [CrossRef]
- Regmi, P.R.; Shaw, A.L.; Hungerford, L.L.; Messenheimer, J.R.; Zhou, T.; Pillai, P.; Omer, A.; Gilbert, J.M. Regulatory Considerations for the Approval of Drugs Against Histomoniasis (Blackhead Disease) in Turkeys, Chickens, and Game Birds in the United States. Avian Dis. 2016, 60, 725–730. [Google Scholar] [CrossRef]
- Beer, L.C.; Graham, B.D.M.; Barros, T.L.; Latorre, J.D.; Tellez-Isaias, G.; Fuller, A.L.; Hargis, B.M.; Vuong, C.N. Evaluation of Live-Attenuated Histomonas meleagridis Isolates as Vaccine Candidates against Wild-Type Challenge. Poult. Sci. 2022, 101, 101656. [Google Scholar] [CrossRef]
- Chen, Q.G.; Kong, L.M.; Rong, J.; Chen, C.; Wang, S.; Hou, Z.-F.; Liu, D.-D.; Tao, J.-P.; Xu, J.-J. Evaluation of an Attenuated Chicken-Origin Histomonas meleagridis Vaccine for the Prevention of Histomonosis in Chickens. Front. Vet. Sci. 2024, 11, 1491148. [Google Scholar] [CrossRef]
- Hess, M.; Liebhart, D.; Grabensteiner, E.; Singh, A. Cloned Histomonas meleagridis Passaged in Vitro Resulted in Reduced Pathogenicity and Is Capable of Protecting Turkeys from Histomonosis. Vaccine 2008, 26, 4187–4193. [Google Scholar] [CrossRef]
- Bilic, I.; Hess, M. Interplay between Histomonas meleagridis and Bacteria: Mutualistic or Predator–Prey? Trends Parasitol. 2020, 36, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.K.; Quijada, N.M.; Dzieciol, M.; Hatfaludi, T.; Bilic, I.; Selberherr, E.; Liebhart, D.; Hess, C.; Hess, M.; Paudel, S. Co-Infection of Chicken Layers with Histomonas meleagridis and Avian Pathogenic Escherichia Coli Is Associated with Dysbiosis, Cecal Colonization and Translocation of the Bacteria from the Gut Lumen. Front. Microbiol. 2020, 11, 586437. [Google Scholar] [CrossRef]
- Ganas, P.; Liebhart, D.; Glösmann, M.; Hess, C.; Hess, M. Escherichia Coli Strongly Supports the Growth of Histomonas meleagridis, in a Monoxenic Culture, without Influence on Its Pathogenicity. Int. J. Parasitol. 2012, 42, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Tellez-Isaias, G.; Eisenreich, W. (Eds.) Alternatives to Antibiotics Against Pathogens in Poultry; Springer Nature: Cham, Switzerland, 2024; ISBN 978-3-031-70479-6. [Google Scholar]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the Chicken Gastrointestinal Tract: Influence on Health, Productivity and Disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Qamar, A.; Waheed, J.; Hamza, A.; Mohyuddin, S.G.; Lu, Z.; Namula, Z.; Chen, Z.; Chen, J.J. The Role of Intestinal Microbiota in Chicken Health, Intestinal Physiology and Immunity. JAPS J. Anim. Plant Sci. 2020, 31, 342–351. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, T.; Luo, Z.; Xiao, H.; Wang, D.; Wu, C.; Fang, X.; Li, J.; Zhou, J.; Miao, J.; et al. Impact of Gut Microbial Diversity on Egg Production Performance in Chickens. Microbiol. Spectr. 2025, 13, e01927-24. [Google Scholar] [CrossRef]
- Bhat, A.H.; Malik, I.M.; Tak, H.; Ganai, B.A.; Bharti, P. Host, Parasite, and Microbiome Interaction: Trichuris Ovis and Its Effect on Sheep Gut Microbiota. Vet. Parasitol. 2025, 333, 110356. [Google Scholar] [CrossRef]
- Ghosh, S.; Padalia, J.; Moonah, S. Tissue Destruction Caused by Entamoeba Histolytica Parasite: Cell Death, Inflammation, Invasion, and the Gut Microbiome. Curr. Clin. Microbiol. Rep. 2019, 6, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.M.; Miska, K.B.; Jenkins, M.C.; Yan, X.; Proszkowiec-Weglarz, M. Effects of Eimeria acervulina Infection on the Luminal and Mucosal Microbiota of the Duodenum and Jejunum in Broiler Chickens. Front. Microbiol. 2023, 14, 1147579. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.C.; Katarzyna, B.M.; Stanislaw, K.; Mark, C.J.; Jonathan, S. Effects of Eimeria tenella on Cecal Luminal and Mucosal Microbiota in Broiler Chickens. Avian Dis. 2022, 66, 39–52. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhao, X.Y.; Zhao, G.X.; Huang, H.B.; Li, H.R.; Shi, C.W.; Yang, W.T.; Jiang, Y.L.; Wang, J.Z.; Ye, L.P.; et al. Dissection of the Cecal Microbial Community in Chickens after Eimeria tenella Infection. Parasites Vectors 2020, 13, 56. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Van Diemen, P.M.; Martineau, H.; Stevens, M.P.; Tomley, F.M.; Stabler, R.A.; Blake, D.P. Impact of Eimeria tenella Coinfection on Campylobacter jejuni Colonization of the Chicken. Infect. Immun. 2019, 87, e00772-18. [Google Scholar] [CrossRef]
- Hess, M.; Kolbe, T.; Grabensteiner, E.; Prosl, H. Clonal Cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and a Blastocystis sp. Established through Micromanipulation. Parasitology 2006, 133, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; McDougald, L.R. Blackhead Disease (Histomonas meleagridis) Aggravated in Broiler Chickens by Concurrent Infection with Cecal Coccidiosis (Eimeria tenella). Avian Dis. 2001, 45, 307–312. [Google Scholar] [CrossRef]
- Hu, J.; Fuller, L.; McDougald, L.R. Infection of Turkeys with Histomonas meleagridis by the Cloacal Drop Method. Avian Dis. 2004, 48, 746–750. [Google Scholar] [CrossRef]
- Clark, S.; Kimminau, E. Critical Review: Future Control of Blackhead Disease (Histomoniasis) in Poultry. Avian Dis. 2017, 61, 281–288. [Google Scholar] [CrossRef]
- Liu, C.; Zhuang, W.; Liu, L.; Chen, Y.; Yang, C.; Chen, H.; Yao, Y.; Sun, X.; Hu, W. Research Progress in High-Throughput DNA Synthesis and Its Applications. J. Mater. Chem. B 2025, 13, 7973–8004. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Orouji, S.; Esufali, J.; Mallick, A.I.; Parvizi, P.; Shewen, P.E.; Sharif, S. Oral Treatment of Chickens with Lactobacilli Influences Elicitation of Immune Responses. Clin. Vaccine Immunol. 2011, 18, 1447–1455. [Google Scholar] [CrossRef]
- Khan, S.; McWhorter, A.R.; Willson, N.L.; Andrews, D.M.; Underwood, G.J.; Moore, R.J.; Hao Van, T.T.; Chousalkar, K.K. Vaccine Protection of Broilers against Various Doses of Wild-Type Salmonella Typhimurium and Changes in Gut Microbiota. Vet. Q. 2025, 45, 1–14. [Google Scholar] [CrossRef]
- Hauck, R.; Hafez, H.M. Experimental Infections with the Protozoan Parasite Histomonas meleagridis: A Review. Parasitol. Res. 2013, 112, 19–34. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Rong, J.; Chen, C.; Wang, S.; Wang, J.; Li, Z.; Hou, Z.; Liu, D.; Tao, J.; et al. MicroRNA Expression Profile of Chicken Liver at Different Times after Histomonas meleagridis Infection. Vet. Parasitol. 2024, 329, 110200. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, Z.; Broadwater, C.; Tobin, I.; Liu, J.; Whitmore, M.; Zhang, G. Is Intestinal Microbiota Fully Restored After Chickens Have Recovered from Coccidiosis? Pathogens 2025, 14, 81. [Google Scholar] [CrossRef]
- Memon, F.U.; Yang, Y.; Leghari, I.H.; Lv, F.; Soliman, A.M.; Zhang, W.; Si, H. Transcriptome Analysis Revealed Ameliorative Effects of Bacillus Based Probiotic on Immunity, Gut Barrier System, and Metabolism of Chicken under an Experimentally Induced Eimeria tenella Infection. Genes 2021, 12, 536. [Google Scholar] [CrossRef]
- Dorbek-Kolin, E.; Husso, A.; Niku, M.; Loch, M.; Pessa-Morikawa, T.; Niine, T.; Kaart, T.; Iivanainen, A.; Orro, T. Faecal Microbiota in Two-Week-Old Female Dairy Calves during Acute Cryptosporidiosis Outbreak–Association with Systemic Inflammatory Response. Res. Vet. Sci. 2022, 151, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Mammeri, M.; Obregón, D.A.; Chevillot, A.; Polack, B.; Julien, C.; Pollet, T.; Cabezas-Cruz, A.; Adjou, K.T. Cryptosporidium Parvum Infection Depletes Butyrate Producer Bacteria in Goat Kid Microbiome. Front. Microbiol. 2020, 11, 548737. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.; Xiao, K.; Jiang, F.; Wang, H.; Tang, D.; Liu, D.; Liu, B.; Liu, Y.; He, X.; et al. The Chicken Gut Metagenome and the Modulatory Effects of Plant-Derived Benzylisoquinoline Alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ji, Z.; Shen, Z.; Wu, Y.; Zhang, B.; Tang, J.; Hou, S.; Xie, M. Effects of Total Dietary Fiber on Cecal Microbial Community and Intestinal Morphology of Growing White Pekin Duck. Front. Microbiol. 2021, 12, 727200. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Xu, X.; Wang, L.; Yan, J.; Yu, Y.; Shan, X.; Zhang, R.; Xing, H.; Zhang, T.; et al. Alterations of Intestinal Mucosal Barrier, Cecal Microbiota Diversity, Composition, and Metabolites of Yellow-Feathered Broilers under Chronic Corticosterone-Induced Stress: A Possible Mechanism Underlying the Anti-Growth Performance and Glycolipid Metabolism Disorder. Microbiol. Spectr. 2024, 12, e03473-23. [Google Scholar] [CrossRef]
- Umeda, S.; Sujino, T.; Miyamoto, K.; Yoshimatsu, Y.; Harada, Y.; Nishiyama, K.; Aoto, Y.; Adachi, K.; Hayashi, N.; Amafuji, K.; et al. D-Amino Acids Ameliorate Experimental Colitis and Cholangitis by Inhibiting Growth of Proteobacteria: Potential Therapeutic Role in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 1011–1031. [Google Scholar] [CrossRef]
- Yang, M.; Shi, L.; Ge, Y.; Leng, D.; Zeng, B.; Wang, T.; Jie, H.; Li, D. Dynamic Changes in the Gut Microbial Community and Function during Broiler Growth. Microbiol. Spectr. 2022, 10, e01005-22. [Google Scholar] [CrossRef] [PubMed]
- Halder, N.; Sunder, J.; De, A.K.; Bhattacharya, D.; Joardar, S.N. Probiotics in Poultry: A Comprehensive Review. J. Basic Appl. Zool. 2024, 85, 23. [Google Scholar] [CrossRef]
- Un-Nisa, A.; Khan, A.; Zakria, M.; Siraj, S.; Ullah, S.; Tipu, M.K.; Ikram, M.; Kim, M.O. Updates on the Role of Probiotics against Different Health Issues: Focus on Lactobacillus. Int. J. Mol. Sci. 2022, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, X.; Liu, X.; Zhao, X.; Liu, S.; Li, Y.; Zhang, Y. Growth, Health, Rumen Fermentation, and Bacterial Community of Holstein Calves Fed Lactobacillus Rhamnosus GG during the Preweaning Stage1. J. Anim. Sci. 2019, 97, 2598–2608. [Google Scholar] [CrossRef]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The Use of Lactic Acid Bacteria as a Probiotic in Swine Diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Tsen, H.Y.; Lin, C.L.; Yu, B.; Chen, C.S. Oral Administration of a Combination of Select Lactic Acid Bacteria Strains to Reduce the Salmonella Invasion and Inflammation of Broiler Chicks. Poult. Sci. 2012, 91, 2139–2147. [Google Scholar] [CrossRef]
- Joo, S.S.; Yoon, J.H.; Jung, J.Y.; Joo, S.Y.; An, S.H.; Ban, B.C.; Kong, C.; Kim, M. The Modulatory Effects of Lacticaseibacillus paracasei Strain NSMJ56 on Gut Immunity and Microbiome in Early-Age Broiler Chickens. Animals 2022, 12, 3413. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Mortada, M.; Cosby, D.E.; Singh, M.; Applegate, T.J.; Syed, B.; Pender, C.M.; Curry, S.; Murugesan, G.R.; Selvaraj, R.K. Synbiotic Supplementation to Decrease Salmonella Colonization in the Intestine and Carcass Contamination in Broiler Birds. PLoS ONE 2019, 14, e0223577. [Google Scholar] [CrossRef]
- Yang, J.; Qian, K.; Wang, C.; Wu, Y. Roles of Probiotic Lactobacilli Inclusion in Helping Piglets Establish Healthy Intestinal Inter-environment for Pathogen Defense. Probiotics Antimicro. 2018, 10, 243–250. [Google Scholar] [CrossRef]
- Al, K.F.; Parris, J.; Engelbrecht, K.; Reid, G.; Burton, J.P. Interconnected Microbiomes—Insights and Innovations in Female Urogenital Health. FEBS J. 2025, 292, 1378–1396. [Google Scholar] [CrossRef]
- Kobierecka, P.A.; Wyszyńska, A.K.; Aleksandrzak-Piekarczyk, T.; Kuczkowski, M.; Tuzimek, A.; Piotrowska, W.; Górecki, A.; Adamska, I.; Wieliczko, A.; Bardowski, J.; et al. In Vitro Characteristics of Lactobacillus spp. Strains Isolated from the Chicken Digestive Tract and Their Role in the Inhibition of Campylobacter Colonization. MicrobiologyOpen 2017, 6, e00512. [Google Scholar] [CrossRef] [PubMed]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of Organic Acids by Probiotic Lactobacilli Can Be Used to Reduce Pathogen Load in Poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef] [PubMed]
- Chavan, N.; Gupta, M.; Bahiram, K.B.; Korde, J.P.; Kadam, M.; Dhok, A.; Kumar, S. Ligilactobacillus salivarius and Limosilactobacillus reuteri Improve Growth and Intestinal Health in Broilers Via Modulating Gut Microbiota and Immune Response. Res. Vet. Sci. 2024, 194, 105837. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Nadeem, A.; Shi, J. Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob. Proteins 2019, 11, 1132–1142. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important Metabolic Pathways and Biological Processes Expressed by Chicken Cecal Microbiota. Appl. Environ. Microbiol. 2016, 82, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.-T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From Microbiology to Diagnostics and Prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Liu, M.-J.; Yang, J.-Y.; Yan, Z.-H.; Hu, S.; Li, J.-Q.; Xu, Z.-X.; Jian, Y.-P. Recent Findings in Akkermansia Muciniphila-Regulated Metabolism and Its Role in Intestinal Diseases. Clin. Nutr. 2022, 41, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Maue, A.C.; Mohawk, K.L.; Giles, D.K.; Poly, F.; Ewing, C.P.; Jiao, Y.; Lee, G.; Ma, Z.; Monteiro, M.A.; Hill, C.L.; et al. The Polysaccharide Capsule of Campylobacter jejuni Modulates the Host Immune Response. Infect. Immun. 2013, 81, 665–672. [Google Scholar] [CrossRef]
- Ohkusa, T.; Yoshida, T.; Sato, N.; Watanabe, S.; Tajiri, H.; Okayasu, I. Commensal Bacteria Can Enter Colonic Epithelial Cells and Induce Proinflammatory Cytokine Secretion: A Possible Pathogenic Mechanism of Ulcerative Colitis. J. Med. Microbiol. 2009, 58, 535–545. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kang, M.S.; An, B.K.; Shin, E.G.; Kim, M.J.; Kwon, J.H.; Kwon, Y.K. Isolation and Epidemiological Characterization of Heat-Labile Enterotoxin-Producing Escherichia fergusonii from Healthy Chickens. Vet. Microbiol. 2012, 160, 170–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, P.; Zhao, Y.; Ma, X. Enterotoxigenic Escherichia coli: Intestinal Pathogenesis Mechanisms and Colonization Resistance by Gut Microbiota. Gut Microbes 2022, 14, 2055943. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, X.; Hu, D.; Wang, C.; Chen, Q.; Ni, Y. Comparison of the Effects of Feeding Compound Probiotics and Antibiotics on Growth Performance, Gut Microbiota, and Small Intestine Morphology in Yellow-Feather Broilers. Microorganisms 2023, 11, 2308. [Google Scholar] [CrossRef]
- He, S.; Song, L.; Xiao, Y.; Huang, Y.; Ren, Z. Genomic, Probiotic, and Functional Properties of Bacteroides dorei RX2020 Isolated from Gut Microbiota. Nutrients 2025, 17, 1066. [Google Scholar] [CrossRef] [PubMed]
- Akbuğa-Schön, T.; Suzuki, T.A.; Jakob, D.; Vu, D.L.; Waters, J.L.; Ley, R.E. The Keystone Gut Species Christensenella minuta Boosts Gut Microbial Biomass and Voluntary Physical Activity in Mice. mBio 2024, 15, e02836-23. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Dong, J.; Zhang, W.; Hu, Z.; Tan, X.; Li, H.; Sun, K.; Zhao, A.; Huang, M. Comparison of Fecal Microbiota and Metabolites in Diarrheal Piglets Pre-and Post-Weaning. Front. Vet. Sci. 2025, 12, 1613054. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, Y.; Lao, X. The Role of Akkermansia muciniphila in Disease Regulation. Probiotics Antimicrob. Proteins 2025, 17, 2027–2038. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, J.; Hao, Y.; Zhou, H.; Hu, Y.; Zhang, C.; Zheng, H.; Wang, X.; Zeng, F.; Hu, J.; et al. Akkermansia muciniphila Plays Critical Roles in Host Health. Crit. Rev. Microbiol. 2023, 49, 82–100. [Google Scholar] [CrossRef]
- Niu, J.; Cui, M.; Yang, X.; Li, J.; Yao, Y.; Guo, Q.; Lu, A.; Qi, X.; Zhou, D.; Zhang, C.; et al. Microbiota-Derived Acetate Enhances Host Antiviral Response via NLRP3. Nat. Commun. 2023, 14, 642. [Google Scholar] [CrossRef]
- Vitetta, L.; Oldfield, D.; Sali, A. Inflammatory Bowel Diseases and the Efficacy of Probiotics as Functional Foods. Front. Biosci. (Elite Ed.) 2024, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Kakita, T.; Lee, K.; Morita, M.; Okuno, M.; Kyan, H.; Okano, S.; Maeshiro, N.; Ishizu, M.; Kudeken, T.; Taira, H.; et al. Isolation and Whole-genome Sequencing Analysis of Escherichia fergusonii Harboring a Heat-labile Enterotoxin Gene from Retail Chicken Meat in Okinawa, Japan. Microbiol. Immunol. 2024, 68, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Okuno, M.; Tsuru, N.; Yoshino, S.; Gotoh, Y.; Yamamoto, T.; Hayashi, T.; Ogura, Y. Isolation and Genomic Characterization of a Heat-Labile Enterotoxin 1-Producing Escherichia fergusonii Strain from a Human. Microbiol. Spectr. 2023, 11, e00491-23. [Google Scholar] [CrossRef]
- Lu, H.P.; Wang, Y.; Huang, S.W.; Lin, C.Y.; Wu, M.; Hsieh, C.; Yu, H.T. Metagenomic Analysis Reveals a Functional Signature for Biomass Degradation by Cecal Microbiota in the Leaf-Eating Flying Squirrel (Petaurista alborufus lena). BMC Genom. 2012, 13, 466. [Google Scholar] [CrossRef]
- Samanta, A.K.; Torok, V.A.; Percy, N.J. Microbial Fingerprinting Detects Unique Bacterial Communities in the Faecal Microbiota of Rats with Experimentally-Induced Colitis. J. Microbiol. 2012, 50, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Yang, L.; Pei, Z. Gut Microbiota, Fusobacteria, and Colorectal Cancer. Diseases 2018, 6, 109. [Google Scholar] [CrossRef]
- Sears, C.L. Enterotoxigenic Bacteroides fragilis: A Rogue among Symbiotes. Clin. Microbiol. Rev. 2009, 22, 349–369. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Khleborodova, A.; Gamboa-Tuz, S.D.; Ramos, M.; Segata, N.; Waldron, L.; Oh, S. Lefser: Implementation of Metagenomic Biomarker Discovery Tool, LEfSe, in R. Bioinformatics 2024, 40, btae707. [Google Scholar] [CrossRef] [PubMed]
| Types | 7 dpi | 14 dpi | 21 dpi |
|---|---|---|---|
| Liver | 0.60 ± 0.54 a | 2.00 ± 1.00 b | 0.80 ± 0.45 a |
| Cecum | 1.60 ± 0.55 a | 2.80 ± 0.84 b | 1.40 ± 0.24 a |
| Group | ACE | Chao1 | Shannon | Simpson | Coverage | Sobs |
|---|---|---|---|---|---|---|
| G1 | 255.8 ± 23.23 a | 263.5 ± 25.10 a | 2.927 ± 0.412 a | 0.1431 ± 0.0563 a | 0.9972 ± 0.00052 a | 215.2 ± 14.62 a |
| G2 | 233.1 ± 28.11 a | 226.4 ± 28.36 a | 2.954 ± 0.410 a | 0.1905 ± 0.0863 a | 0.9972 ± 0.00052 a | 194.6 ± 35.93 a |
| G3 | 310.9 ± 14.98 b | 312.6 ± 18.42 ab | 3.561 ± 0.160 a | 0.0744 ± 0.0075 a | 0.9971 ± 0.00037 a | 273.4 ± 16.62 b |
| G4 | 155.7 ± 28.66 c | 135.4 ± 35.78 c | 2.059 ± 1.059 ab | 0.2875 ± 0.2749 a | 0.9983 ± 0.00018 ab | 104.6 ± 46.45 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Chen, Q.; Liu, Y.; Zhu, W.; Aye, H.; Li, R.; Hou, Z.; Liu, D.; Yin, Y.; Tao, J.; Xu, J. Relationship Between Histomonas meleagridis Infection and Cecal Intestinal Microbiota of Chickens. Vet. Sci. 2026, 13, 118. https://doi.org/10.3390/vetsci13020118
Chen Q, Liu Y, Zhu W, Aye H, Li R, Hou Z, Liu D, Yin Y, Tao J, Xu J. Relationship Between Histomonas meleagridis Infection and Cecal Intestinal Microbiota of Chickens. Veterinary Sciences. 2026; 13(2):118. https://doi.org/10.3390/vetsci13020118
Chicago/Turabian StyleChen, Qiaoguang, Yaxin Liu, Wendi Zhu, HsuPan Aye, Ruting Li, Zhaofeng Hou, Dandan Liu, Yuelan Yin, Jianping Tao, and Jinjun Xu. 2026. "Relationship Between Histomonas meleagridis Infection and Cecal Intestinal Microbiota of Chickens" Veterinary Sciences 13, no. 2: 118. https://doi.org/10.3390/vetsci13020118
APA StyleChen, Q., Liu, Y., Zhu, W., Aye, H., Li, R., Hou, Z., Liu, D., Yin, Y., Tao, J., & Xu, J. (2026). Relationship Between Histomonas meleagridis Infection and Cecal Intestinal Microbiota of Chickens. Veterinary Sciences, 13(2), 118. https://doi.org/10.3390/vetsci13020118






