Growth Performance, Immuno-Oxidant Status, Intestinal Health, Gene Expression, and Histomorphology of Growing Quails Fed Diets Supplemented with Essential Oils and Probiotics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigated Growth Promoters
2.1.1. Orego-Stim-Powder Compounds
2.1.2. Probiotic Compound (Strong-G)
2.2. Experimental Site
2.3. Quails, Treatments, and Experimental Design
2.4. Growth Performance and Carcass Traits
2.5. Serum Biochemical Indices
2.6. Quantitative Real-Time PCR
2.7. Intestinal Histomorphology
2.8. Statistical Analysis
3. Results
3.1. Performance Indices
3.2. Characteristics of the Carcass and Internal Organs
3.3. Blood Constituents
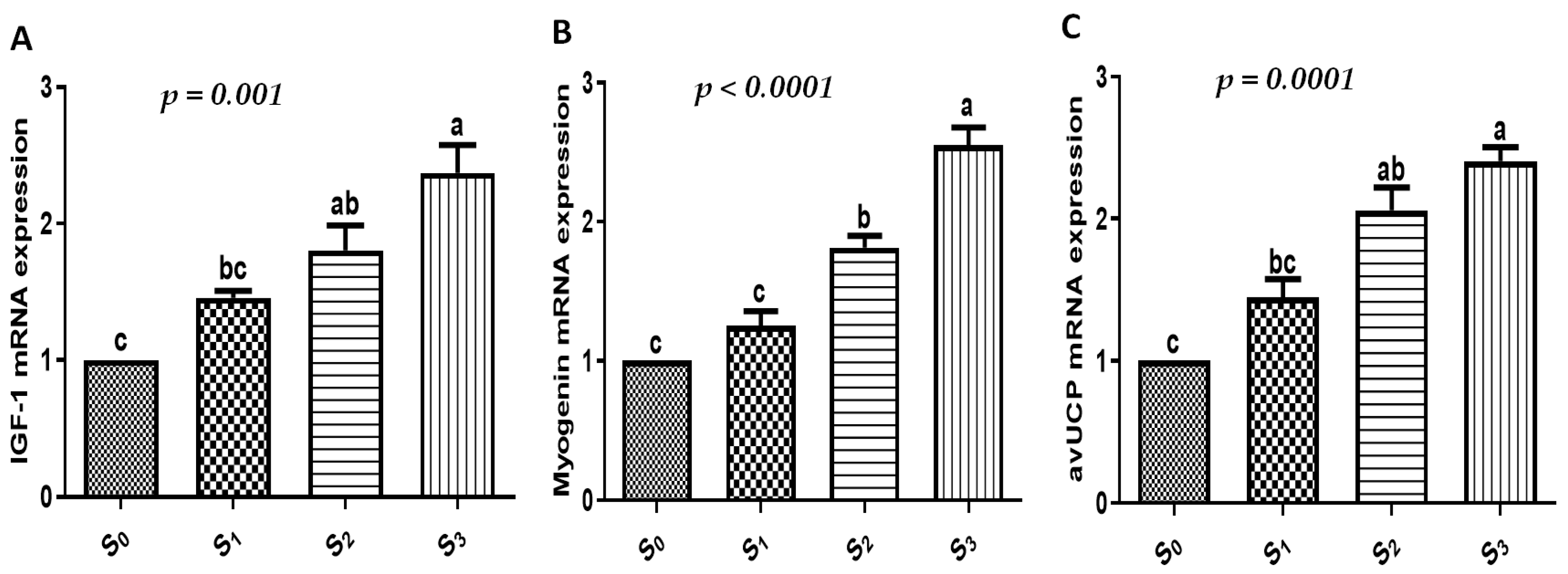
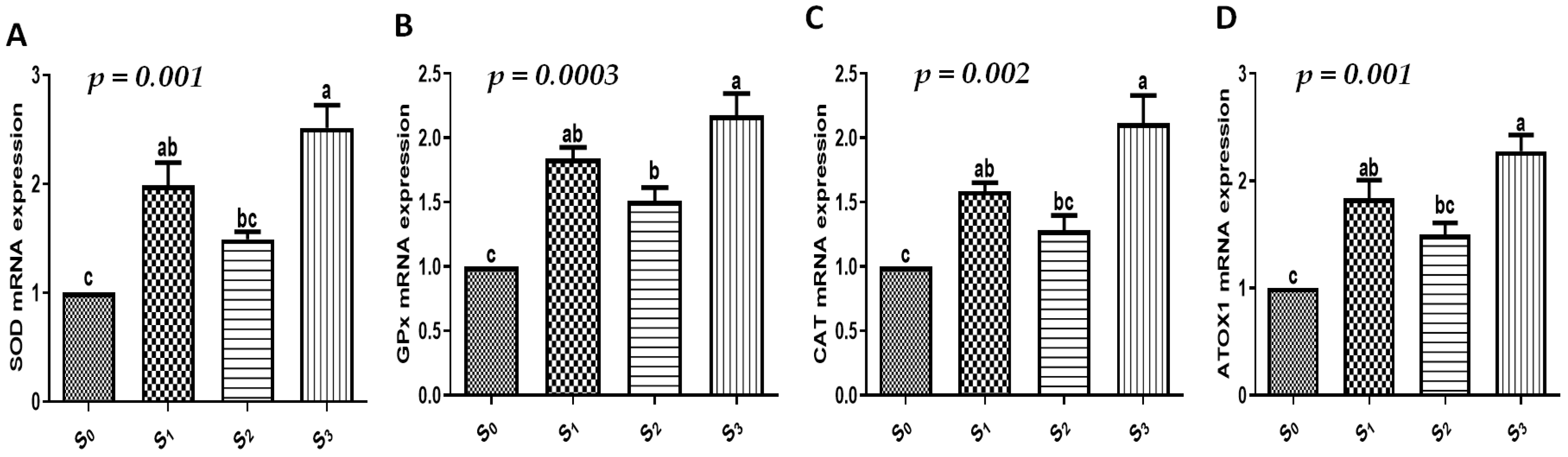
3.4. Growth, Immune, Antioxidant, and Intestinal Absorption Marker Gene Expression Profiles
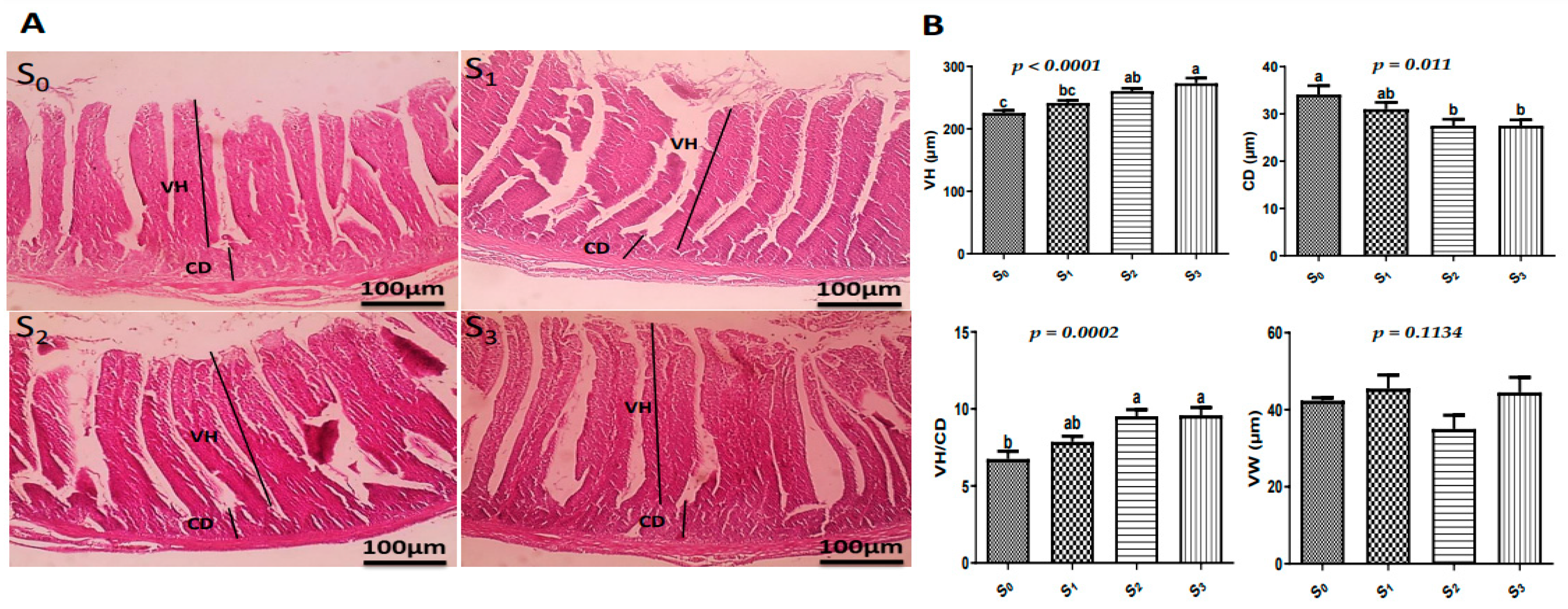
3.5. Histological Examinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGPs | Antibiotic growth promoters |
| ALB | Albumin |
| ATOX1 | Antioxidant protein 1 |
| AVBD | Avian beta defensin |
| AvUCP | Avian uncoupling protein |
| BUN | Blood urea nitrogen |
| BWG | Body weight gain |
| CAT | Catalase |
| CD | Crypt depth |
| cDNA | Complementary DNA |
| Eos | Essential oils |
| FABP6 | Fatty acid binding protein 6 |
| FBW | Final body weight |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| GLB | Calculated globulin |
| GLUT2 | Glucose transporter 2 |
| GPx | Glutathione peroxidase |
| HDL | High-density lipoprotein |
| IBW | Initial body weight |
| IgY | Immunoglobulins |
| IgM | Immunoglobulins |
| IGF-I | Insulin-like Growth Factor 1 |
| IL-1β | Interleukin-1β |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| LBW | Live body weight |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| MUC2 | Mucin 2 |
| NRC | National research council |
| SOD | Superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| TC | Total cholesterol |
| TG | Triglycerides |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor-α |
| TP | Total protein |
| VEGF | Vascular endothelial growth factor |
| VH | Villous height |
| VW | Villous width |
References
- Curry, B.B. Animal Models Used in Identifying Gender-Related Differences. Int. J. Toxicol. 2009, 20, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.E.; McDevitt, R.M.; Hillman, K.; Acamovic, T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [PubMed]
- Collett, S.R. Controlling gastrointestinal disease to improve absorptive membrane integrity and optimize digestion efficiency. In Interfacing Immunity, Gut Health and Performance; Context Products: Packington, UK, 2004; pp. 77–92. [Google Scholar]
- Van der Aar, P.J.; Molist, F.V.; Van Der Klis, J.D. The central role of intestinal health on the effect of feed additives on feed intake in swine and poultry. Anim. Feed. Sci. Technol. 2017, 233, 64–75. [Google Scholar]
- Pandey, A.K.; Kumar, P.; Saxena, M.J. Feed additives in animal health. In Nutraceuticals Veterinary Medicine; Springer: Berlin/Heidelberg, Germany, 2019; pp. 345–362. [Google Scholar]
- Upadhayay, U.P.P.D.D.; Vishwa, P.C.V. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: The trends and advances—A review. Int. J. Pharmacol. 2014, 10, 129–159. [Google Scholar]
- Reuben, R.C.; Sarkar, S.L.; Roy, P.C.; Anwar, A.; Hossain, M.A.; Jahid, I.K. Prebiotics, probiotics and postbiotics for sustainable poultry production. World’s Poult. Sci. J. 2021, 77, 825–882. [Google Scholar]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Ijaz, A.; Sohail, A.; Shabbir, M.Z.; Rehman, H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar]
- Voon, H.C.; Bhat, R.; Rusul, G. Flower Extracts and Their Essential Oils as Potential Antimicrobial Agents for Food Uses and Pharmaceutical Applications. Compr. Rev. Food Sci. Food Saf. 2011, 11, 34–55. [Google Scholar]
- Dar, R.A.; Shahnawaz, M.; Ahanger, M.A.; Majid, I. Exploring the Diverse Bioactive Compounds from Medicinal Plants: A Review. J. Phytopharm. 2023, 12, 189–195. [Google Scholar] [CrossRef]
- Gholami-Ahangaran, M.; Ahmadi-Dastgerdi, A.; Azizi, S.; Basiratpour, A.; Zokaei, M.; Derakhshan, M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022, 8, 267–288. [Google Scholar] [CrossRef]
- Peng, Q.Y.; Li, J.D.; Li, Z.; Duan, Z.Y.; Wu, Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed. Sci. Technol. 2016, 214, 148–153. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Abdel-Moneim, A.-M.E.; Shehata, A.M.; Mohamed, N.G.; Elbaz, A.M.; Ibrahim, N.S. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol. Trace Elem. Res. 2022, 200, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Piao, X.S.; Kim, S.W.; Wang, L.; Liu, P.; Yoon, I.; Zhen, Y.G. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs1. J. Anim. Sci. 2009, 87, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Al-Fataftah, A.-R.; Abdelqader, A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim. Feed. Sci. Technol. 2014, 198, 279–285. [Google Scholar] [CrossRef]
- Santos, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential Feed Additives as Antibiotic Alternatives in Broiler Production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar]
- Kürekci, C.; Özsoy, B.; Hassan, E.; Özkan, H.; Gundoğdu, A.; Özsoy, Ş.Y.; Yakan, A. Effect of essential oil supplementation to diet on meat quality, fatty acid composition, performance parameters and intestinal microbiota of Japanese quails. J. Anim. Physiol. Anim. Nutr. 2020, 105, 927–937. [Google Scholar] [CrossRef]
- Cetin, İ.; Yesilbag, D.; Cengiz, S.S.; Belenli, D. Bıldırcın Rasyonuna Biberiye Uçucu Yağ İlavesinin Büyüme Performansı, Et MDA Düzeyi ve Bazı Plazma Antioksidan Parametreleri Üzerine Etkisi; Kafkas Universitesi Veteriner Fakultesi Dergisi: Kars, Türkiye, 2017; Volume 23, pp. 283–288. [Google Scholar]
- Saeed, M.; Afzal, Z.; Afzal, F.; Khan, R.U.; Elnesr, S.S.; Alagawany, M.; Chen, H. Use of postbiotic as growth promoter in poultry industry: A review of current knowledge and future prospects. Food Sci. Anim. Resour. 2023, 43, 1111. [Google Scholar] [CrossRef]
- Al-Younes, W.M.; Abdelqader, A.M.; Abuajamieh, M.K.; Nassar, K.O. Efficacy of Probiotics and Essential Oils as Alternatives to Antibiotic Growth Promoters in Broiler Chickens. Iraqi J. Agric. Sci. 2024, 55, 633–643. [Google Scholar] [CrossRef]
- El-Sayed, Y.; Khalil, W.; Fayez, N.; Mohamed Abdel-Fattah, A.-F. Enhancing effect of oregano essential oil and Bacillus subtilis on broiler immune function, intestinal morphology and growth performance. BMC Vet. Res. 2024, 20, 112. [Google Scholar] [CrossRef]
- Chen, D.; Yan, H.; Xing, Q.; Xiao, X.; Yu, B.; He, J.; Mao, X.; Yu, J.; Zheng, P.; Luo, Y.; et al. Effect of Saccharomyces cerevisiae Postbiotics and Essential Oil on Growth Performance and Intestinal Health of Weanling Pigs During K88 ETEC Infection. J. Anim. Sci. 2024, 102, skae007. [Google Scholar]
- Yang, H. Exploring the combined effects of probiotics and phytogenics on livestock growth. Anim. Nutr. Welf. 2023, 15, 45–60. [Google Scholar]
- Hussein, E.O.S.; Ahmed, S.H.; Abudabos, A.M.; Aljumaah, M.R.; Alkhlulaifi, M.M.; Nassan, M.A.; Suliman, G.M.; Naiel, M.A.; Swelum, A.A. Effect of Antibiotic, Phytobiotic and Probiotic Supplementation on Growth, Blood Indices and Intestine Health in Broiler Chicks Challenged with Clostridium perfringens. Animals 2020, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of Feeding Different Postbiotics Produced by Lactobacillus plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Rodrigues, E.A.; Marques, R.H.; Gravena, R.A.; Guandolini, G.C.; Moraes, V.M.B. Performance and morphology of intestinal mucosa of broilers fed mannan-oligosaccharides and enzymes. Arq. Bras. Med. Veterinária Zootec. 2008, 60, 442–448. [Google Scholar]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar]
- Heydarian, M.; Ebrahimnezhad, Y.; Meimandipour, A.; Hosseini, S.A.; Banabazi, M.H. Effects of dietary inclusion of the encapsulated thyme and oregano essential oils mixture and probiotic on growth performance, immune response and intestinal morphology of broiler chickens. Poult. Sci. J. 2020, 8, 17–25. [Google Scholar]
- Pipaliya, G.; Yadav, A.S.; Sindhoora, K.; Gopi, M.; Rokade, J.; Tiwari, A.K. Individual and combined effects of dietary supplementation of probiotic and essential oil on the growth performance, immunity status, gut health and jejunal histomorphology of broiler chickens. Indian J. Anim. Sci. 2022, 92, 986–990. [Google Scholar]
- Gogoi, S.; Kolluri, G.; Tyagi, J.S.; Marappan, G.; Manickam, K.; Narayan, R. Impact of heat stress on broilers with varying body weights: Elucidating their interactive role through physiological signatures. J. Therm. Biol. 2021, 97, 102840. [Google Scholar]
- Oleforuh-Okoleh, V.U.; Ndofor-Foleng, H.M.; Olorunleke, S.O.; Uguru, J.O. Evaluation of Growth Performance, Haematological and Serum Biochemical Response of Broiler Chickens to Aqueous Extract of Ginger and Garlic. J. Agric. Sci. 2015, 7, 167. [Google Scholar]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb. Cell Fact. 2019, 18, 112. [Google Scholar] [CrossRef]
- Islam, Z.; Sultan, A.; Khan, S.; Khan, K.; Jan, A.U.; Aziz, T.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. Effects of an organic acids blend and coated essential oils on broiler growth performance, blood biochemical profile, gut health, and nutrient digestibility. Ital. J. Anim. Sci. 2024, 23, 152–163. [Google Scholar]
- Obianwuna, U.E.; Qiu, K.; Chang, X.-Y.; Zhang, H.-J.; Wang, J.; Qi, G.-H.; Sun, T.H.; Su, Y.B.; Wu, S.G. Enhancing egg production and quality by the supplementation of probiotic strains (Clostridium and Brevibacillus) via improved amino acid digestibility, intestinal health, immune response, and antioxidant activity. Front. Microbiol. 2022, 13, 987241. [Google Scholar] [CrossRef] [PubMed]
- Gopi, M. Essential Oils as a Feed Additive in Poultry Nutrition. Adv. Anim. Vet. Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Paryad, A.; Mahmoudi, M. Effect of different levels of supplemental yeast (Saccharomyces cerevisiae) on performance, blood constituents and carcass characteristics of broiler chicks. Afr. J. Agric. Res. 2008, 3, 835–842. [Google Scholar]
- Khalil, S.; Shourbela, R.; Zidan, E.; Hassan, S.; Sleim, A.; Hawarry, W. Effect of Stocking Density and Diets Supplemented with Essential oils and or Probiotic on the Performance of Juveniles Nile tilapia (Oreochromis niloticus). Alex. J. Vet. Sci. 2024, 81, 142. [Google Scholar] [CrossRef]
- Yalçın, S.; Eser, H.; Yalçın, S.; Cengiz, S.; Eltan, Ö. Effects of dietary yeast autolysate (Saccharomyces cerevisiae) on performance, carcass and gut characteristics, blood profile, and antibody production to sheep red blood cells in broilers. J. Appl. Poult. Res. 2013, 22, 55–61. [Google Scholar]
- Chung, M.J.; Kang, A.-Y.; Park, S.-O.; Park, K.-W.; Jun, H.-J.; Lee, S.-J. The effect of essential oils of dietary wormwood (Artemisia princeps), with and without added vitamin E, on oxidative stress and some genes involved in cholesterol metabolism. Food Chem. Toxicol. 2007, 45, 1400–1409. [Google Scholar]
- Mahboobi, S.; Iraj, B.; Maghsoudi, Z.; Feizi, A.; Ghiasvand, R.; Askari, G.; Maayeshi, N. The effects of probiotic supplementation on markers of blood lipids, and blood pressure in patients with prediabetes: A randomized clinical trial. Int. J. Prev. Med. 2014, 5, 1239. [Google Scholar]
- Wang, L.; Guo, M.-J.; Gao, Q.; Yang, J.-F.; Yang, L.; Pang, X.-L.; Jiang, X.-J. The effects of probiotics on total cholesterol: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e9679. [Google Scholar] [CrossRef]
- Al-Saad, S.; Abbod, M.; Yones, A.A. Effects of some Growth Promoters on Blood Hematology and Serum Composition of Broiler Chickens. Int. J. Agric. Res. 2014, 9, 265–270. [Google Scholar]
- Haghighi, H.R.; Gong, J.; Gyles, C.L.; Hayes, M.A.; Zhou, H.; Sanei, B.; Chambers, J.R.; Sharif, S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006, 13, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections With Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Meng, Q.W.; Kim, I.H. Effect of an herb extract mixture on growth performance, nutrient digestibility, blood characteristics, and fecal microbial shedding in weanling pigs. Livest. Sci. 2012, 145, 189–195. [Google Scholar] [CrossRef]
- El-Shenway, A.M.; Ali, G.I.E. Effect of Some Organic Acids and Essential Oils as Feed Additives on Growth Performance, Immune Response and Carcass Quality of Japanese Quail. Alex. J. Vet. Sci. 2016, 51, 68–77. [Google Scholar]
- Manzanilla, E.G.; Perez, J.F.; Martin, M.; Kamel, C.; Baucells, F.; Gasa, J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs1. J. Anim. Sci. 2004, 82, 3210–3218. [Google Scholar] [CrossRef]
- Namkung, H.; Li, J.; Gong, M.; Yu, H.; Cottrill, M.; de Lange, C.F.M. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can. J. Anim. Sci. 2004, 84, 697–704. [Google Scholar] [CrossRef]
- Turner, J.L.; Dritz, S.S.; Higgins, J.J.; Minton, J.E. Effects of Ascophyllum nodosum extract on growth performance and immune function of young pigs challenged with Salmonella typhimurium1. J. Anim. Sci. 2002, 80, 1947–1953. [Google Scholar] [CrossRef]
- Oke, O.E.; Akosile, O.A.; Oni, A.I.; Opowoye, I.O.; Ishola, C.A.; Adebiyi, J.O.; Odeyemi, A.J.; Adjei-Mensah, B.; Uyanga, V.A.; Abioja, M.O. Oxidative stress in poultry production. Poult. Sci. 2024, 103, 104003. [Google Scholar] [CrossRef]
- Bogolyubova, N.V. Antioxidant Status and Quality of Poultry and Animal Meat under Stress and Its Correction with the Use of Various Adaptogens (review). Sel’skokhozyaistvennaya Biol. 2022, 57, 628–663. [Google Scholar]
- Michiels, J.; Tagliabue, M.M.; Akbarian, A.; Ovyn, A.; De Smet, S. Oxidative Status, Meat Quality and Fatty Acid Profile of Broiler Chickens Reared under Free-range and Severely Feed-restricted Conditions Compared with Conventional Indoor Rearing. Avian Biol. Res. 2014, 7, 74–82. [Google Scholar]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.-M.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Aldhumri, S.A.; Gabr, S.A.; Mesalam, N.M.; Elbaz, A.M.; et al. Nutritional manipulation to combat heat stress in poultry—A comprehensive review. J. Therm. Biol. 2021, 98, 102915. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Shibamoto, T. Antioxidant Activities and Volatile Constituents of Various Essential Oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [PubMed]
- Graßmann, J.; Schneider, D.; Weiser, D.; Elstner, E.F. Antioxidative effects of lemon oil and its components on copper induced oxidation of low density lipoprotein. Arzneimittelforschung 2001, 51, 799–805. [Google Scholar]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2017, 59, 89–101. [Google Scholar] [CrossRef]
- Jarry, A.; Bossard, C.; Bou-Hanna, C.; Masson, D.; Espaze, E.; Denis, M.G.; Laboisse, C.L. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J. Clin. Investig. 2008, 118, 1132–1142. [Google Scholar]
- Hernandez-Rodriguez, J. Tissue production of pro-inflammatory cytokines (IL-1, TNF and IL-6) correlates with the intensity of the systemic inflammatory response and with corticosteroid requirements in giant-cell arteritis. Rheumatology 2003, 43, 294–301. [Google Scholar] [CrossRef]
- Mainardi, G.L.; Saleri, R.; Tamanini, C.; Baratta, M. Effects of Interleukin-1-Beta, Interleukin-6 and Tumor Necrosis Factor-Alpha, Alone or in Association with Hexarelin or Galanin, on Growth Hormone Gene Expression and Growth Hormone Release from Pig Pituitary Cells. Horm. Res. Paediatr. 2002, 58, 180–186. [Google Scholar]
- Zhu, M.; Wang, M.; Shao, Y.; Nan, Y.; Blair, H.T.; Morris, S.T.; Zhao, Z.; Zhang, H. Characterization of muscle development and gene expression in early embryos of chicken, quail, and their hybrids. Gene 2021, 768, 145319. [Google Scholar]
- Ocaña, A.; Reglero, G. Effects of Thyme Extract Oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on Cytokine Production and Gene Expression of oxLDL-Stimulated THP-1-Macrophages. J. Obes. 2012, 2012, 104706. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F. Probiotics in inflammatory bowel disease—Therapeutic rationale and role. Adv. Drug Deliv. Rev. 2004, 56, 809–818. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.-Y.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2019, 104, 335–349. [Google Scholar] [PubMed]
- Wang, T.; Cheng, K.; Li, Q.; Wang, T. Effects of yeast hydrolysate supplementation on intestinal morphology, barrier, and anti-inflammatory functions of broilers. Anim. Biosci. 2022, 35, 858–868. [Google Scholar] [CrossRef]
- Guernec, A.; Berri, C.; Chevalier, B.; Wacrenier-Cere, N.; Le Bihan-Duval, E.; Duclos, M.J. Muscle development, insulin-like growth factor-I and myostatin mRNA levels in chickens selected for increased breast muscle yield. Growth Horm. IGF Res. 2003, 13, 8–18. [Google Scholar]
- Ojano-Dirain, C.; Toyomizu, M.; Wing, T.; Cooper, M.; Bottje, W.G. Gene Expression in Breast Muscle and Duodenum from Low and High Feed Efficient Broilers. Poult. Sci. 2007, 86, 372–381. [Google Scholar]
- Wang, Q. Correlation analysis of relationships between polymorphisms of high quality chicken myogenin gene and slaughter and meat quality traits. Hereditas 2007, 29, 1089. [Google Scholar]
- Adedokun, S.A.; Olojede, O.C. Optimizing Gastrointestinal Integrity in Poultry: The Role of Nutrients and Feed Additives. Front. Vet. Sci. 2019, 5, 348. [Google Scholar]
- Chen, J.; Tellez, G.; Richards, J.D.; Escobar, J. Identification of Potential Biomarkers for Gut Barrier Failure in Broiler Chickens. Front. Vet. Sci. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar]
- Grant, A.Q.; Gay, C.G.; Lillehoj, H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018, 47, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, E.R.L.; Souza, P.A.; Souza, H.B.A.; Figueiredo, D.F.; Boiago, M.M.; Carvalho, S.R.; Bordon, V.F. Intestinal mucosa development in broiler chickens fed natural growth promoters. Rev. Bras. Ciência Avícola 2005, 7, 221–229. [Google Scholar] [CrossRef]
- Al-Baadani, H.H.; Abudabos, A.M.; Al-Mufarrej, S.I.; Alzawqari, M. Effects of dietary inclusion of probiotics, prebiotics and synbiotics on intestinal histological changes in challenged broiler chickens. S. Afr. J. Anim. Sci. 2016, 46, 157. [Google Scholar]
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupińska, J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005, 46, 485–493. [Google Scholar]
- Ciftci, M.; Guler, T.; Dalkilic, B.; Ertas, O.N. The Effect of Anise Oil (Pimpinella anisum L.) On Broiler Performance. Int. J. Poult. Sci. 2005, 4, 851–855. [Google Scholar]
- Williams, P.; Losa, R. The use of essential oils and their compounds in poultry nutrition. World Poult. 2001, 17, 14–15. [Google Scholar]
- Lee, K.W.; Everts, H.; Kappert, H.J.; Frehner, M.; Losa, R.; Beynen, A.C. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003, 44, 450–457. [Google Scholar]
- Rocha, R.F.; da Silva Fidelis, P.H.; Massuquetto, A.; Sobrane Filho, S.T.; de Araújo, M.S.; Silva, C.M.; da Costa Lopes, C.; de Medeiros, E.S.; Moreira, R.H.R. Essential oils as a strategy to improve gut histomorphometry and performance of broilers: Systematic review and meta-analysis. J. Agric. Sci. 2024, 162, 404–416. [Google Scholar] [CrossRef]
- Giannenas, I.; Papaneophytou, C.P.; Tsalie, E.; Pappas, I.; Triantafillou, E.; Tontis, D.; Kontopidis, G.A. Dietary Supplementation of Benzoic Acid and Essential Oil Compounds Affects Buffering Capacity of the Feeds, Performance of Turkey Poults and Their Antioxidant Status, pH in the Digestive Tract, Intestinal Microbiota and Morphology. Asian-Australas J. Anim. Sci. 2014, 27, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Gormez, A.; Bozari, S.; Yanmis, D.; Gulluce, M.; Sahin, F.; Agar, G. Chemical composition and antibacterial activity of essential oils of two species of Lamiaceae against phytopathogenic bacteria. Pol. J. Microbiol. 2015, 64, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Roana, J.; Cavallo, L.; Mandras, N. Immune Defences: A View from the Side of the Essential Oils. Molecules 2023, 28, 435. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Amount/kg | ||
|---|---|---|---|
| Yellow corn | 54.9 | ||
| Soybean meal | 35.4 | ||
| Corn gluten | 5.8 | ||
| Corn oil | 0.8 | ||
| Limestone | 1.5 | ||
| Dicalcium phosphate | 0.7 | ||
| Premix * | 0.3 | ||
| Salt | 0.3 | ||
| DL. Methionine | 0.1 | ||
| DL. Lysine | 0.2 | ||
| Items | Calculated amount | Analyzed amount | |
| Metabolizable energy (ME, kcal/kg) | 2906.68 | 2895.4 | |
| Crude protein (CP %) | 24.08 | 23.95 | |
| Ca% | 0.83 | 0.81 | |
| Available p, % | 0.54 | 0.52 | |
| Gene | Isolation Source | Primer | Product Length (bp) | Annealing Temperature (°C) | Accession Number |
|---|---|---|---|---|---|
| IGF-I | Liver | F: ACTCAAACTGTCCACACGCA R: AGCATCAGGGAGGTCCTTCT | 155 | 58 | XM_015867574.2 |
| Myogenin | Muscle | F: ATGTCGCTCTCTGACCAACG R: CAATGGCACACAGCAGTCAG | 76 | 60 | XM_065747526.1 |
| AvUCP | Muscle | F: TCCCCTGTCACTTCGTGGCCGC R: GTAGAGACCAGCGACACCGTCCT | 170 | 60 | BAK26782.1 |
| IL-2 | Spleen | F: GTGCAAAGTACTGATCTTCGCC R: CTTGGTGTGTAGAGCTCGAGATG | 195 | 60 | AY613440.1 |
| IL-4 | Spleen | F: GAGAGCATCCGGATAGTGAAG R: TTCGCATAAGAGCTGGGTTC | 168 | 58 | AB559571 |
| IL-6 | Spleen | F: CAACCTCAACCTGCCCAA R: GGAGAGCTTCCTCAGGCATT′ | 85 | 60 | AB5595724 |
| AVBD | Spleen | F: CTATTCGTGCTTGTCGACTTC R: CTTACACAGCAAGAGCTTCTT | 106 | 58 | AB698019.1 |
| SOD | Liver | F: TGGACCTCGTTTAGCTTGTG R: ACACGGAAGAGCAAGTACAGR | 126 | 58 | NM_205064.1 |
| CAT | Liver | F: CCTGACTATGGTGCGCGTAT R: CAGACACACGAGAAGTGGCT | 106 | 58 | XM_015863594.1 |
| GPx | Liver | F: TTGTAAACATCAGGGGCAAA R: TGGGCCAAGATCTTTCTGTAA | 140 | 58 | NM_001163245.1 |
| ATOX1 | Liver | F: TTGTGGACATGACCTGCGAA R: AACTGGACACCTCCCAGTCT | 73 | 60 | XM_015875801.2 |
| VEGF | Intestine | F: TCCGATTTTGAAAGCGGTGG R: CAATGCATCTCTTTCGCCGC | 185 | 58 | XM_015857736.1 |
| MUC2 | Intestine | F: CGACTCCATCAGAGTCACCG R: CGGAGTGGATGAGGATACGC | 104 | 60 | XM_032444897.1 |
| GLUT2 | Intestine | F: TGCATCTCAGACACACGTCC R: CCTGGCTCAGTGTGTTGCTA | 140 | 60 | XM_015872003.1 |
| Calbindin | Intestine | F: CGAGATCTGGCACCACTACG R: TCGGGTGTTAAGTCCAAGCC | 120 | 60 | XM_015855985.2 |
| FABP6 | Intestine | F: TGGCAAGATCTCTTCTAGTCCAC R: GCACCACCTCGGTGACTATT | 187 | 58 | XM_032447429.1 |
| β-Actin | F: CTGGCACCTAGCACAATGAA R: CTGCTTGCTGATCCACATCT | 123 | 58 | AF199488 | |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | p-Value | |
| IBW, g | 49.5 ± 1.57 | 50.5 ± 1.38 | 51 ± 1.25 | 50 ± 1.67 | =0.902 |
| FBW, g | 226 c ± 5.57 | 242 bc ± 5.07 | 249 b ± 5.42 | 270.5 a ± 5.8 | <0.0001 |
| BWG, g | 176.5c ± 4.09 | 191.5 bc ± 3.8 | 198 b ± 4.29 | 220 a± 4.3 | <0.0001 |
| FCR | 4.2 a ± 0.1 | 3.8 b ± 0.07 | 3.59 b ± 0.08 | 3.04 c ± 0.06 | <0.0001 |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | p-Value | |
| Live body weight | 231 c ± 4.4 | 253 bc ± 7.26 | 263ab ± 6.01 | 283 a ± 4.4 | =0.001 |
| Dressed carcass % | 60.98 b ± 1.06 | 63.9 ab ± 0.55 | 64.07ab ± 0.61 | 66.18 a ± 0.49 | =0.006 |
| Heart % | 0.790 ± 0.02 | 0.813 ± 0.01 | 0.946 ± 0.10 | 0.784 ± 0.03 | =0.222 |
| Gizzard % | 3.3 ± 0.27 | 2.8 ± 0.09 | 3.48 ± 0.19 | 3.18 ± 0.16 | =0.117 |
| Liver % | 2.69 ± 0.04 | 2.4 ± 0.53 | 2.66 ± 0.19 | 2.69 ± 0.1 | =0.872 |
| Spleen % | 0.15 ± 0.05 | 0.19 ± 0.03 | 0.14 ± 0.06 | 0.23 ± 0.02 | =0.477 |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | p-Value | |
| Protein profile | |||||
| TP (g/dL) | 2.08 b ± 0.15 | 3.08 ab ± 0.42 | 3.14 ab ± 0.29 | 3.72 a ± 0.17 | =0.0103 |
| ALB (g/dL) | 1.08 ± 0.11 | 1.33 ± 0.45 | 1.26 ± 0.40 | 1.38 ± 0.38 | =0.942 |
| GLB (g/dL) | 1 ± 0.11 | 1.76 ± 0.07 | 1.88 ± 0.68 | 2.34 ± 0.51 | =0.225 |
| BUN (g/dL) | 0.86 a ± 0.12 | 0.71 ab± 0.12 | 0.65ab ± 0.11 | 0.37 b ± 0.07 | =0.049 |
| Lipid profile | |||||
| TG (mg/dL) | 125.58 a ± 4.27 | 107.42 ab ± 4.93 | 112.29 ab ± 2.46 | 93.89 b ± 5.73 | =0.003 |
| TC (mg/dL) | 228.16 a ± 5.25 | 191.86 bc ± 4.89 | 210.49 ab ± 4 | 179.07 c ± 3.97 | <0.0001 |
| HDL (mg/dL) | 58.76 b ± 4.28 | 65.90 ab ± 4.32 | 64.23 ab ± 5.12 | 78.49 a ± 4.42 | =0.055 |
| LDL (mg/dL) | 114.26 a ± 4.32 | 96.96 ab ± 4.50 | 101.33 ab ± 4.29 | 83.20 b ± 4.44 | =0.003 |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | p-Value | |
| Immunity | |||||
| IgY (mg/mL) | 0.55 c ± 0.02 | 0.68 b ± 0.04 | 0.77 b ± 0.02 | 0.91 a ± 0.01 | <0.0001 |
| IgM (mg/mL) | 0.25 b± 0.008 | 0.28 ab ± 0.01 | 0.28 ab ± 0.01 | 0.33 a ± 0.02 | =0.015 |
| Redox status | |||||
| MDA (nmol/mL) | 4.10 a ± 0.42 | 2.53 ab ± 0.41 | 2.73 ab ± 0.44 | 1.58 b ± 0.31 | =0.006 |
| T-AOC (U/mL) | 7.60 b ± 0.92 | 9.88 ab ± 0.43 | 8.93 b ± 0.63 | 12.26 a ± 0.49 | =0.002 |
| SOD (U/mL) | 40.84 c ± 1.87 | 57.30 b ± 1.96 | 51.09 b ± 2.68 | 70.55 a ± 2.29 | <0.0001 |
| GPx (U/mL) | 64.73 b ± 3.79 | 74.18 ab ± 3.55 | 78.45 ab ± 3.93 | 86.20 a ± 3.33 | =0.001 |
| Inflammatory response | |||||
| IL-1β (Pg/mL) | 54.50 a ± 2.90 | 43.25 ab ± 2.87 | 40.29 b ± 3.24 | 37 b ± 2.58 | =0.006 |
| IL-6 (Pg/mL) | 78 a ± 2.86 | 64.25 ab ± 3.68 | 54.75 bc ± 3.97 | 46.25 c ± 2.53 | =0.0001 |
| TNF-α (Pg/mL) | 269.75 a ± 3.94 | 261 ab ± 2.97 | 255 ab ± 3.92 | 248 b ± 3.63 | =0.008 |
| TGF-β (Pg/mL) | 92.50 c ± 2.02 | 98.50 bc ± 2.25 | 107 b ± 2.35 | 117.75 a ± 1.49 | <0.0001 |
| IL-10 (Pg/mL) | 28 c ± 1.83 | 35.25 bc ± 2.81 | 43.75 ab ± 2.84 | 51.50 a ± 3.88 | =0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, R.E.S.; Ateya, A.; Gadalla, H.; Alharbi, H.M.; Alwutayd, K.M.; Embaby, E.M. Growth Performance, Immuno-Oxidant Status, Intestinal Health, Gene Expression, and Histomorphology of Growing Quails Fed Diets Supplemented with Essential Oils and Probiotics. Vet. Sci. 2025, 12, 341. https://doi.org/10.3390/vetsci12040341
Mahmoud RES, Ateya A, Gadalla H, Alharbi HM, Alwutayd KM, Embaby EM. Growth Performance, Immuno-Oxidant Status, Intestinal Health, Gene Expression, and Histomorphology of Growing Quails Fed Diets Supplemented with Essential Oils and Probiotics. Veterinary Sciences. 2025; 12(4):341. https://doi.org/10.3390/vetsci12040341
Chicago/Turabian StyleMahmoud, Rania El Sayed, Ahmed Ateya, Hossam Gadalla, Hanan M. Alharbi, Khairiah M. Alwutayd, and Eman M. Embaby. 2025. "Growth Performance, Immuno-Oxidant Status, Intestinal Health, Gene Expression, and Histomorphology of Growing Quails Fed Diets Supplemented with Essential Oils and Probiotics" Veterinary Sciences 12, no. 4: 341. https://doi.org/10.3390/vetsci12040341
APA StyleMahmoud, R. E. S., Ateya, A., Gadalla, H., Alharbi, H. M., Alwutayd, K. M., & Embaby, E. M. (2025). Growth Performance, Immuno-Oxidant Status, Intestinal Health, Gene Expression, and Histomorphology of Growing Quails Fed Diets Supplemented with Essential Oils and Probiotics. Veterinary Sciences, 12(4), 341. https://doi.org/10.3390/vetsci12040341






