The Effect of Metronidazole versus a Synbiotic on Clinical Course and Core Intestinal Microbiota in Dogs with Acute Diarrhea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
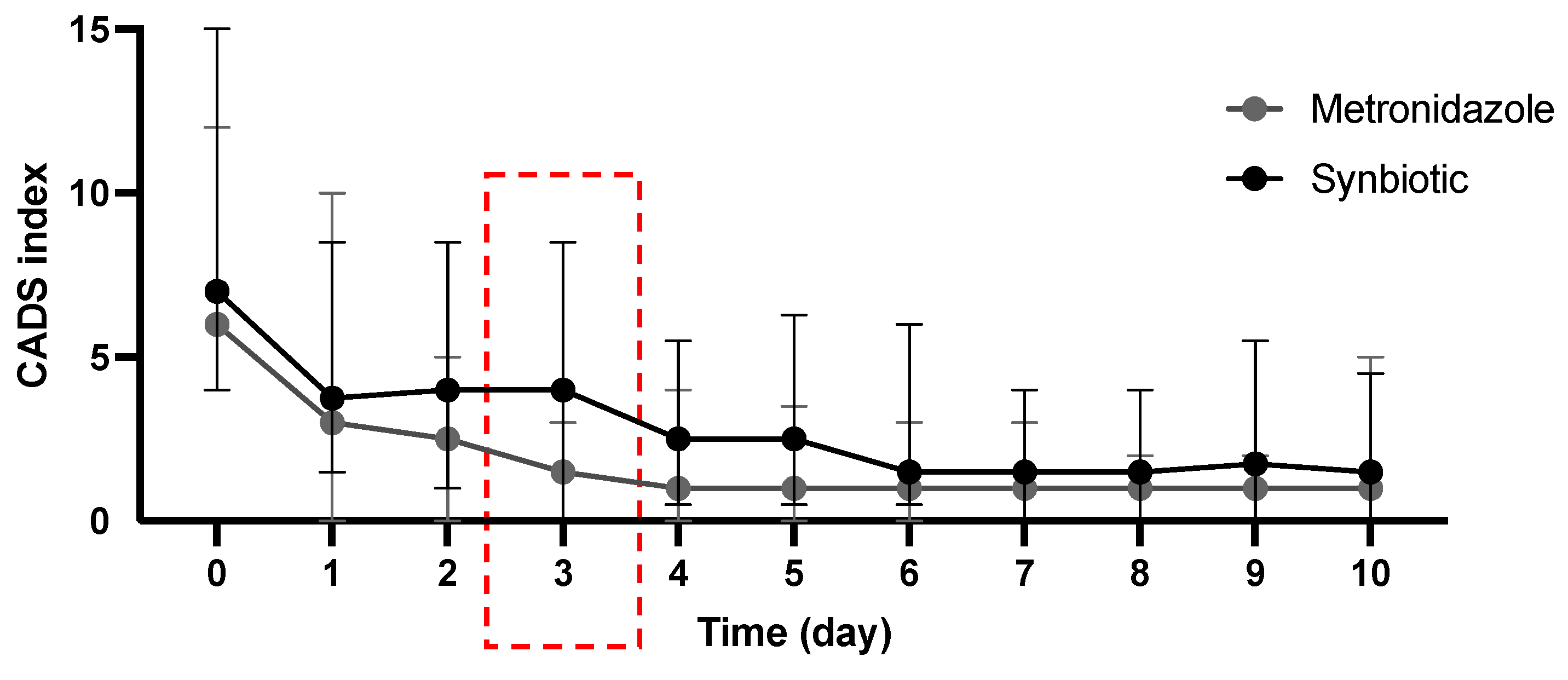
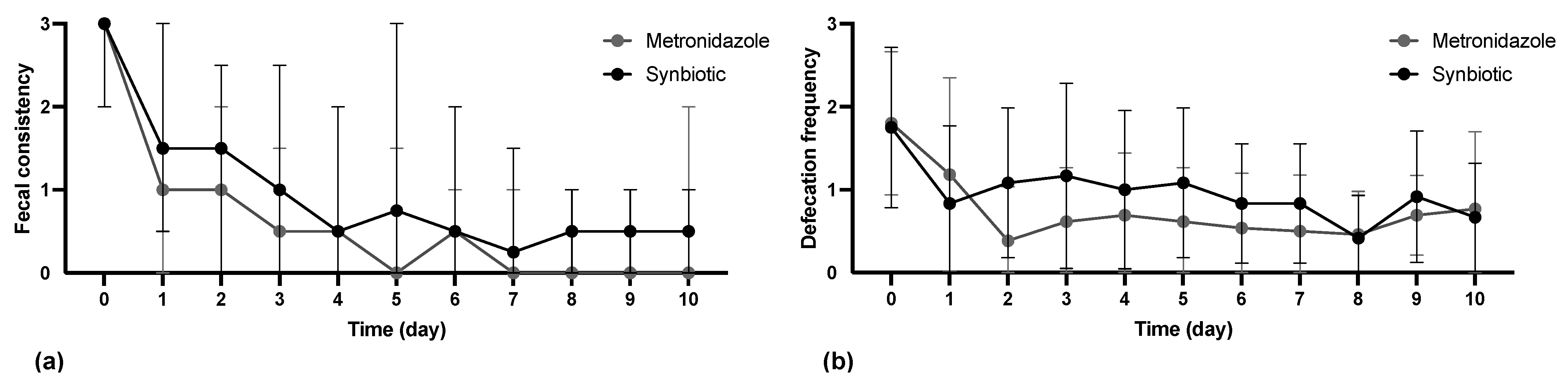
3.2. Treatment Efficacy
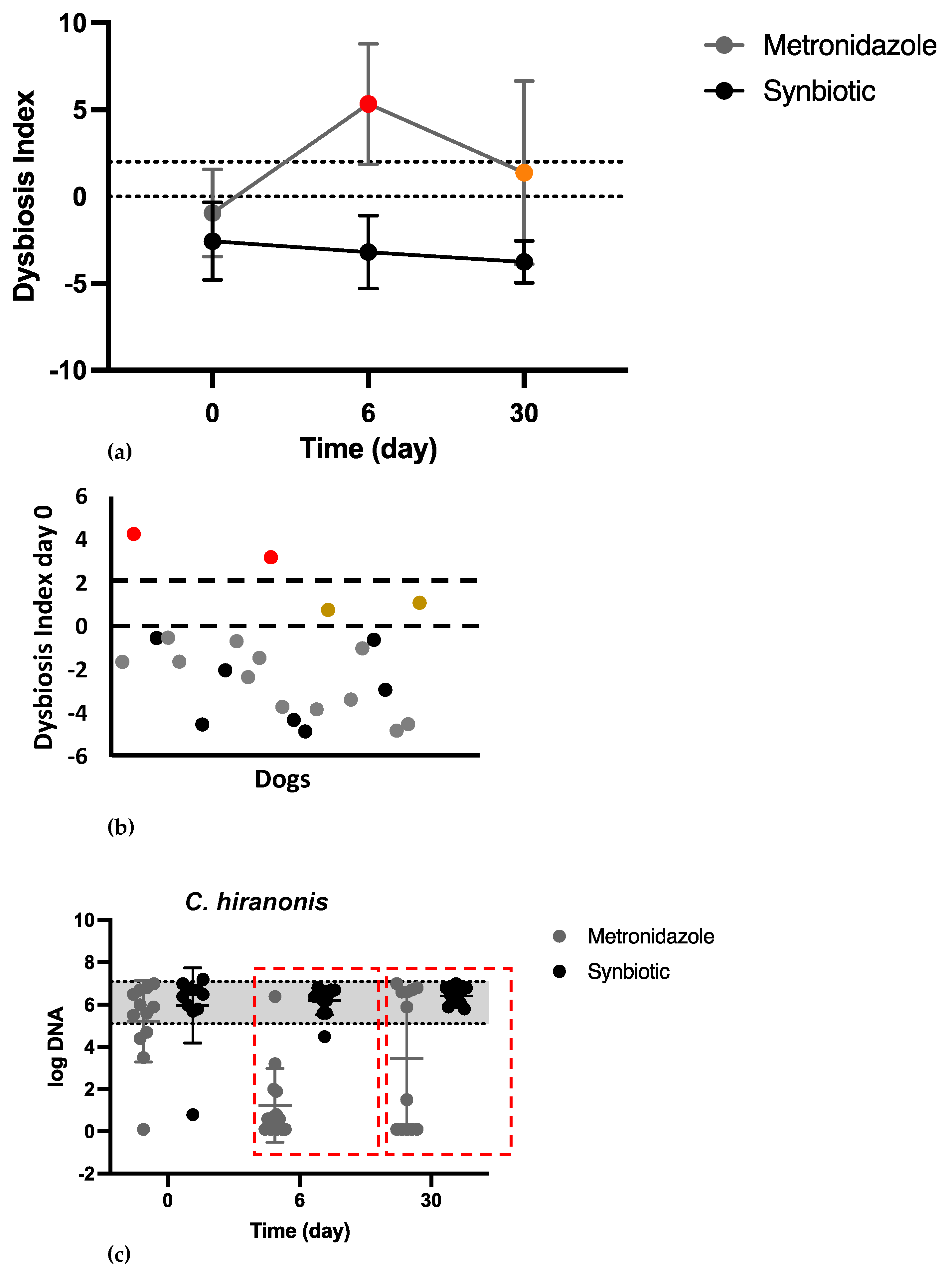
3.3. Microbiome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pugh, C.A.; Bronsvoort, B.M.C.; Handel, I.G.; Querry, D.; Rose, E.; Summers, K.M.; Clements, D.N. Incidence rates and risk factor analyses for owner reported vomiting and diarrhoea in Labrador Retrievers—Findings from the Dogslife Cohort. Prev. Vet. Med. 2017, 140, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.; Skelly, B.J.; McKelvie, J.; Wood, J.L. Risk of vomiting and diarrhoea in dogs. Vet. Rec. 2007, 161, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.; Odunayo, A.; Tolbert, M.K. The use of metronidazole in acute diarrhea in dogs: A narrative review. Top. Companion Anim. Med. 2023, 56–57, 100824. [Google Scholar] [CrossRef] [PubMed]
- Shmalberg, J.; Montalbano, C.; Morelli, G.; Buckley, G.J. A Randomized Double Blinded Placebo-Controlled Clinical Trial of a Probiotic or Metronidazole for Acute Canine Diarrhea. Front. Vet. Sci. 2019, 6, 163. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Suchodolski, J.S.; Straubinger, R.K.; Wolf, G.; Steiner, J.M.; Lidbury, J.A.; Neuerer, F.; Hartmann, K.; Unterer, S. Effect of amoxicillin-clavulanic acid on clinical scores, intestinal microbiome, and amoxicillin-resistant Escherichia coli in dogs with uncomplicated acute diarrhea. J. Vet. Intern. Med. 2020, 34, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Langlois, D.K.; Koenigshof, A.M.; Mani, R. Metronidazole treatment of acute diarrhea in dogs: A randomized double blinded placebo-controlled clinical trial. J. Vet. Intern. Med. 2020, 34, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, G.; Di Prinzio, R.; Crisi, P.E.; Bela, B.; Fusaro, I.; Trevisan, C.; De Acetis, L.; Gramenzi, A. Comparison of the Therapeutic Effect of Treatment with Antibiotics or Nutraceuticals on Clinical Activity and the Fecal Microbiome of Dogs with Acute Diarrhea. Animals 2021, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Stavisky, J.; Radford, A.D.; Gaskell, R.; Dawson, S.; German, A.; Parsons, B.; Clegg, S.; Newman, J.; Pinchbeck, G. A case-control study of pathogen and lifestyle risk factors for diarrhoea in dogs. Prev. Vet. Med. 2011, 99, 185–192. [Google Scholar] [CrossRef]
- Saevik, B.K.; Skancke, E.M.; Trangerud, C. A longitudinal study on diarrhoea and vomiting in young dogs of four large breeds. Acta Vet. Scand. 2012, 54, 8. [Google Scholar] [CrossRef]
- Gomez-Gallego, C.; Junnila, J.; Mannikko, S.; Hameenoja, P.; Valtonen, E.; Salminen, S.; Beasley, S. A canine-specific probiotic product in treating acute or intermittent diarrhea in dogs: A double-blind placebo-controlled efficacy study. Vet. Microbiol. 2016, 197, 122–128. [Google Scholar] [CrossRef]
- Minamoto, Y.; Dhanani, N.; Markel, M.E.; Steiner, J.M.; Suchodolski, J.S. Prevalence of Clostridium perfringens, Clostridium perfringens enterotoxin and dysbiosis in fecal samples of dogs with diarrhea. Vet. Microbiol. 2014, 174, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.Y.; Ali, M.S.; Kwon, D.H.; Heo, Y.E.; Hwang, Y.J.; Kim, J.I.; Lee, Y.J.; Yoon, S.S.; Moon, D.C.; Lim, S.K. Antimicrobial Resistance in Escherichia coli Isolated from Healthy Dogs and Cats in South Korea, 2020–2022. Antibiotics 2023, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Singleton, D.A.; Noble, P.J.M.; Sanchez-Vizcaino, F.; Dawson, S.; Pinchbeck, G.L.; Williams, N.J.; Radford, A.D.; Jones, P.H. Pharmaceutical Prescription in Canine Acute Diarrhoea: A Longitudinal Electronic Health Record Analysis of First Opinion Veterinary Practices. Front. Vet. Sci. 2019, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Halladay, L.J.; Noble, P.J. First-choice therapy for dogs presenting with diarrhoea in clinical practice. Vet. Rec. 2010, 167, 810–814. [Google Scholar] [CrossRef]
- Francillon, W.B.; Winston, J.A.; Schreeg, M.E.; Lilly, M.L.; Parker, V.J.; Rudinsky, A.J. Clinician prescribing practices for managing canine idiopathic acute diarrhea are not evidence based. J. Am. Vet. Med. Assoc. 2023, 261, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alauzet, C.; Lozniewski, A.; Marchandin, H. Metronidazole resistance and nim genes in anaerobes: A review. Anaerobe 2019, 55, 40–53. [Google Scholar] [CrossRef]
- Hernandez Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; Lopez Contreras, L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Steagall, P.V.; Pelligand, L.; Page, S.; Granick, J.L.; Allerton, F.; Beczkowski, P.M.; Weese, J.S.; Hrcek, A.K.; Queiroga, F.; Guardabassi, L. The 2023 World Small Animal Veterinary Association (WSAVA): List of essential medicines for cats and dogs. J. Small Anim. Pract. 2023, 64, 731–748. [Google Scholar] [CrossRef]
- Pilla, R.; Gaschen, F.P.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; AlShawaqfeh, M.K.; et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef]
- Chaitman, J.; Ziese, A.L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs With Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef]
- Boekhoud, I.M.; Hornung, B.V.H.; Sevilla, E.; Harmanus, C.; Bos-Sanders, I.; Terveer, E.M.; Bolea, R.; Corver, J.; Kuijper, E.J.; Smits, W.K. Plasmid-mediated metronidazole resistance in Clostridioides difficile. Nat. Commun. 2020, 11, 598. [Google Scholar] [CrossRef]
- Scahill, K.; Jessen, L.R.; Prior, C.; Singleton, D.; Foroutan, F.; Ferran, A.A.; Arenas, C.; Bjornvad, C.R.; Lavy, E.; Allerton, F.; et al. Efficacy of antimicrobial and nutraceutical treatment for canine acute diarrhoea: A systematic review and meta-analysis for European Network for Optimization of Antimicrobial Therapy (ENOVAT) guidelines. Vet. J. 2023, 303, 106054. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Dosa, P.I.; DeWinter, E.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Khoruts, A.; Sadowsky, M.J. Changes in Colonic Bile Acid Composition following Fecal Microbiota Transplantation Are Sufficient to Control Clostridium difficile Germination and Growth. PLoS ONE 2016, 11, e0147210. [Google Scholar] [CrossRef]
- Blake, A.B.; Cigarroa, A.; Klein, H.L.; Khattab, M.R.; Keating, T.; Van De Coevering, P.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Developmental stages in microbiota, bile acids, and clostridial species in healthy puppies. J. Vet. Intern. Med. 2020, 34, 2345–2356. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. S2), S1–S63. [Google Scholar] [CrossRef]
- Rioux, K.P.; Madsen, K.L.; Fedorak, R.N. The role of enteric microflora in inflammatory bowel disease: Human and animal studies with probiotics and prebiotics. Gastroenterol. Clin. N. Am. 2005, 34, 465–482. [Google Scholar] [CrossRef]
- Bengmark, S. Bioecologic control of the gastrointestinal tract: The role of flora and supplemented probiotics and synbiotics. Gastroenterol. Clin. N. Am. 2005, 34, 413–436. [Google Scholar] [CrossRef]
- Mortier, F.; Strohmeyer, K.; Hartmann, K.; Unterer, S. Acute haemorrhagic diarrhoea syndrome in dogs: 108 cases. Vet. Rec. 2015, 176, 627. [Google Scholar] [CrossRef]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Sung, C.H.; Pilla, R.; Chen, C.C.; Ishii, P.E.; Toresson, L.; Allenspach-Jorn, K.; Jergens, A.E.; Summers, S.; Swanson, K.S.; Volk, H.; et al. Correlation between Targeted qPCR Assays and Untargeted DNA Shotgun Metagenomic Sequencing for Assessing the Fecal Microbiota in Dogs. Animals 2023, 13, 2597. [Google Scholar] [CrossRef]
- Li, Q.; Larouche-Lebel, E.; Loughran, K.A.; Huh, T.P.; Suchodolski, J.S.; Oyama, M.A. Gut Dysbiosis and Its Associations with Gut Microbiota-Derived Metabolites in Dogs with Myxomatous Mitral Valve Disease. mSystems 2021, 6, e00111-21. [Google Scholar] [CrossRef]
- Ziese, A.L.; Suchodolski, J.S.; Hartmann, K.; Busch, K.; Anderson, A.; Sarwar, F.; Sindern, N.; Unterer, S. Effect of probiotic treatment on the clinical course, intestinal microbiome, and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea. PLoS ONE 2018, 13, e0204691. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef]
- Werner, M.; Ishii, P.E.; Pilla, R.; Lidbury, J.A.; Steiner, J.M.; Busch-Hahn, K.; Unterer, S.; Suchodolski, J.S. Prevalence of Clostridioides difficile in Canine Feces and Its Association with Intestinal Dysbiosis. Animals 2023, 13, 2441. [Google Scholar] [CrossRef]
- Whittemore, J.C.; Price, J.M.; Moyers, T.; Suchodolski, J.S. Effects of Synbiotics on the Fecal Microbiome and Metabolomic Profiles of Healthy Research Dogs Administered Antibiotics: A Randomized, Controlled Trial. Front. Vet. Sci. 2021, 8, 665713. [Google Scholar] [CrossRef]
- Gronvold, A.M.; L’Abee-Lund, T.M.; Sorum, H.; Skancke, E.; Yannarell, A.C.; Mackie, R.I. Changes in fecal microbiota of healthy dogs administered amoxicillin. FEMS Microbiol. Ecol. 2010, 71, 313–326. [Google Scholar] [CrossRef]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef]
- Igarashi, H.; Maeda, S.; Ohno, K.; Horigome, A.; Odamaki, T.; Tsujimoto, H. Effect of oral administration of metronidazole or prednisolone on fecal microbiota in dogs. PLoS ONE 2014, 9, e107909. [Google Scholar] [CrossRef] [PubMed]
- Sindern, N.; Suchodolski, J.S.; Leutenegger, C.M.; Mehdizadeh Gohari, I.; Prescott, J.F.; Proksch, A.L.; Mueller, R.S.; Busch, K.; Unterer, S. Prevalence of Clostridium perfringens netE and netF toxin genes in the feces of dogs with acute hemorrhagic diarrhea syndrome. J. Vet. Intern. Med. 2019, 33, 100–105. [Google Scholar] [CrossRef]
- Leipig-Rudolph, M.; Busch, K.; Prescott, J.F.; Mehdizadeh Gohari, I.; Leutenegger, C.M.; Hermanns, W.; Wolf, G.; Hartmann, K.; Verspohl, J.; Unterer, S. Intestinal lesions in dogs with acute hemorrhagic diarrhea syndrome associated with netF-positive Clostridium perfringens type A. J. Vet. Diagn. Investig. 2018, 30, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Belas, A.; Franco, A.; Aboim, C.; Gama, L.T.; Pomba, C. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. J. Antimicrob. Chemother. 2018, 73, 377–384. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Activity | normal | mildly decreased | moderately decreased | severely decreased |
| Appetite | normal | mildly decreased | moderately decreased | severely decreased |
| Vomiting (times/day) | 0 | 1 | 2–3 | >3 |
| Fecal consistency | normal | moist, shaped | pasty | watery diarrhea |
| Defecation frequency (times/day) | 1 | 2–3 | 4–5 | >5 |
| Parameter | Reference Range |
|---|---|
| Rectal temperature | <37.0 and >39.0 °C [<98.6 °F and >102.2 °F] |
| Heart rate | >140/min |
| Hematocrit | >58% |
| WBC | <5 × 109/L or >20 × 109/L |
| Banded neutrophils | >1.5 × 109/L |
| METg (n = 15) | SYNg (n = 12) | p Value | |
|---|---|---|---|
| Sex | 7 male, 8 female | 4 male, 8 female | 0.69 |
| Breeds | Mixed breed (3), Miniature Australian Shepherd (1), Giant Schnauzer (1), Maltese dog (1), Poodle (1), Pug (2), American Bulldog (1), Bichon Frise (1), Border Collie (1), Labrador (1), Yorkshire Terrier (1), French Bulldog (1) | Mixed breed (5), Vizsla (2), Golden Retriever (1), Chihuahua (1), Australian Shepherd (1), Shi Tzu (1), Yorkshire Terrier (1) | 0.27 |
| METg (n = 15) | SYNg (n = 12) | p Value | |
|---|---|---|---|
| Median (Range) | Median (Range) | ||
| Bodyweight (kg) | 12.7 (5–36.3) | 17.2 (5–35.5) | 0.31 |
| Age (years) | 4.7 (1–13) | 5.2 (2–12) | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stübing, H.; Suchodolski, J.S.; Reisinger, A.; Werner, M.; Hartmann, K.; Unterer, S.; Busch, K. The Effect of Metronidazole versus a Synbiotic on Clinical Course and Core Intestinal Microbiota in Dogs with Acute Diarrhea. Vet. Sci. 2024, 11, 197. https://doi.org/10.3390/vetsci11050197
Stübing H, Suchodolski JS, Reisinger A, Werner M, Hartmann K, Unterer S, Busch K. The Effect of Metronidazole versus a Synbiotic on Clinical Course and Core Intestinal Microbiota in Dogs with Acute Diarrhea. Veterinary Sciences. 2024; 11(5):197. https://doi.org/10.3390/vetsci11050197
Chicago/Turabian StyleStübing, Helene, Jan S. Suchodolski, Andrea Reisinger, Melanie Werner, Katrin Hartmann, Stefan Unterer, and Kathrin Busch. 2024. "The Effect of Metronidazole versus a Synbiotic on Clinical Course and Core Intestinal Microbiota in Dogs with Acute Diarrhea" Veterinary Sciences 11, no. 5: 197. https://doi.org/10.3390/vetsci11050197
APA StyleStübing, H., Suchodolski, J. S., Reisinger, A., Werner, M., Hartmann, K., Unterer, S., & Busch, K. (2024). The Effect of Metronidazole versus a Synbiotic on Clinical Course and Core Intestinal Microbiota in Dogs with Acute Diarrhea. Veterinary Sciences, 11(5), 197. https://doi.org/10.3390/vetsci11050197







