The Establishment of a Novel γ-Interferon In Vitro Release Assay for the Differentiation of Mycobacterial Bovis-Infected and BCG-Vaccinated Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animals
2.3. Sample Collection
2.4. BCG Growth
2.5. BCG Vaccination Procedures
2.6. Commercial PPD-IGRA
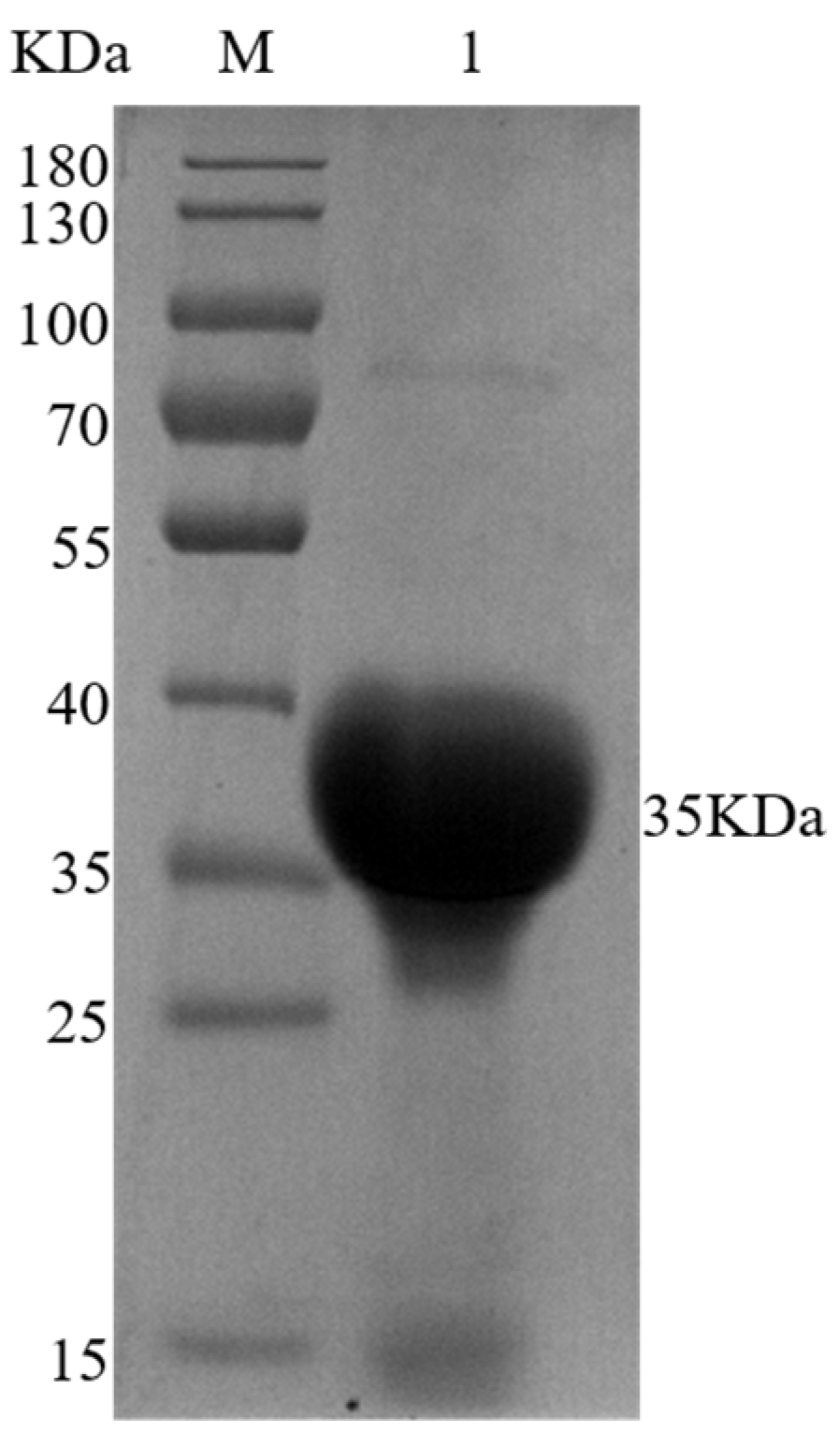
2.7. Expression and Purification of RCE
2.8. Establishment of RCE-DIVA IGRA
2.9. Coincidence Rate Evaluation and Correlation Analysis
2.10. Statistical Analysis
3. Results
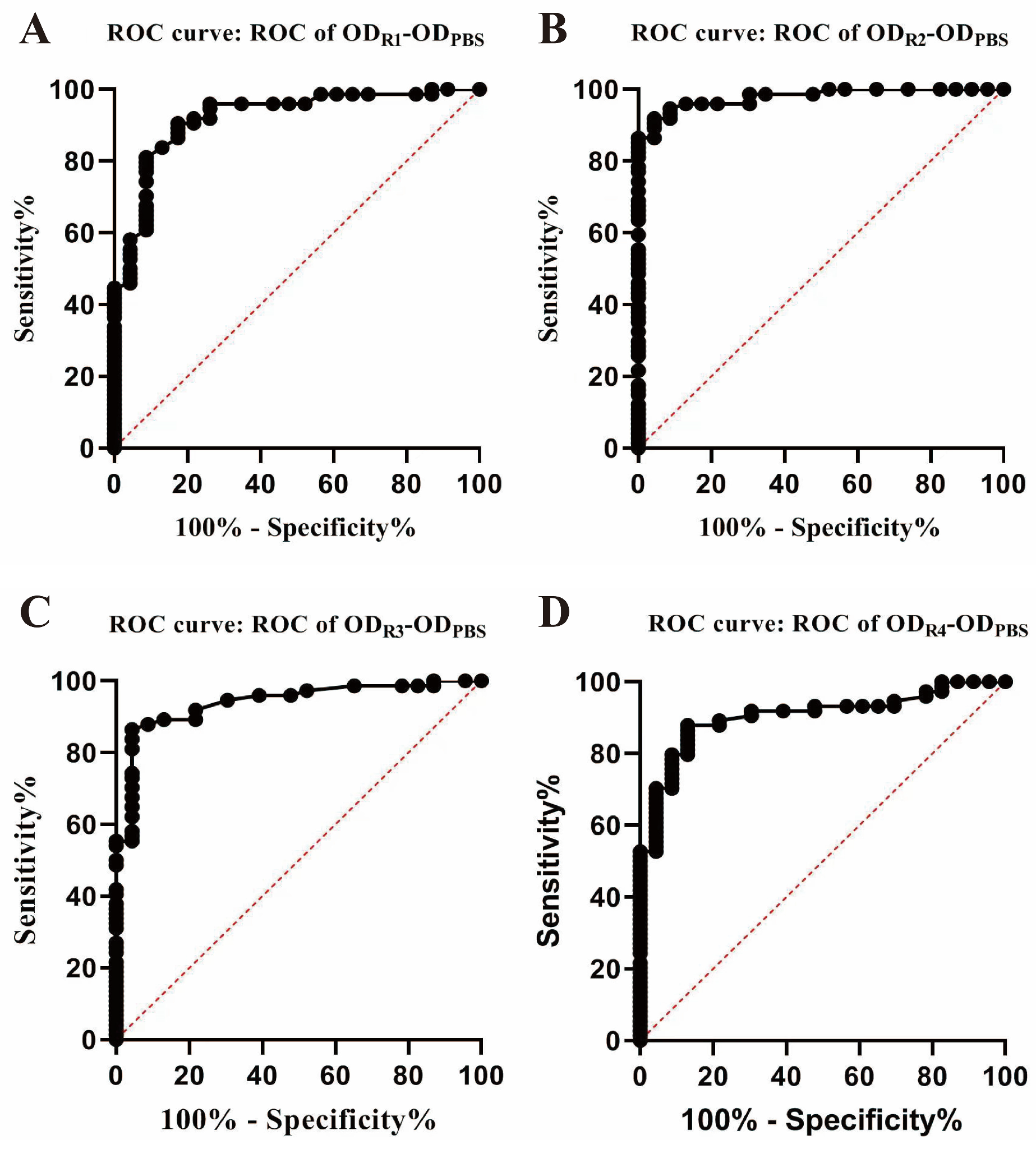
3.1. Determination of RCE Optimal Concentration and Cut-off Value for DIVA RCE-IGRA
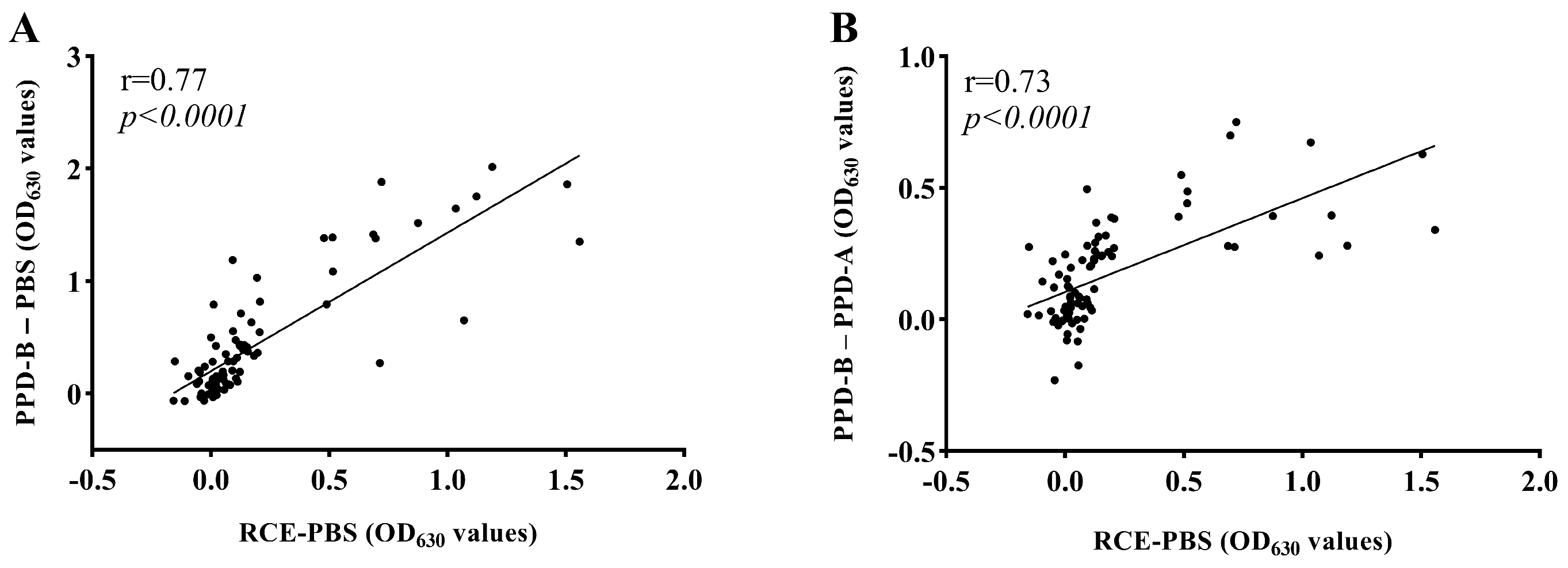
3.2. Coincidence Rate Evaluation and Correlation Analysis
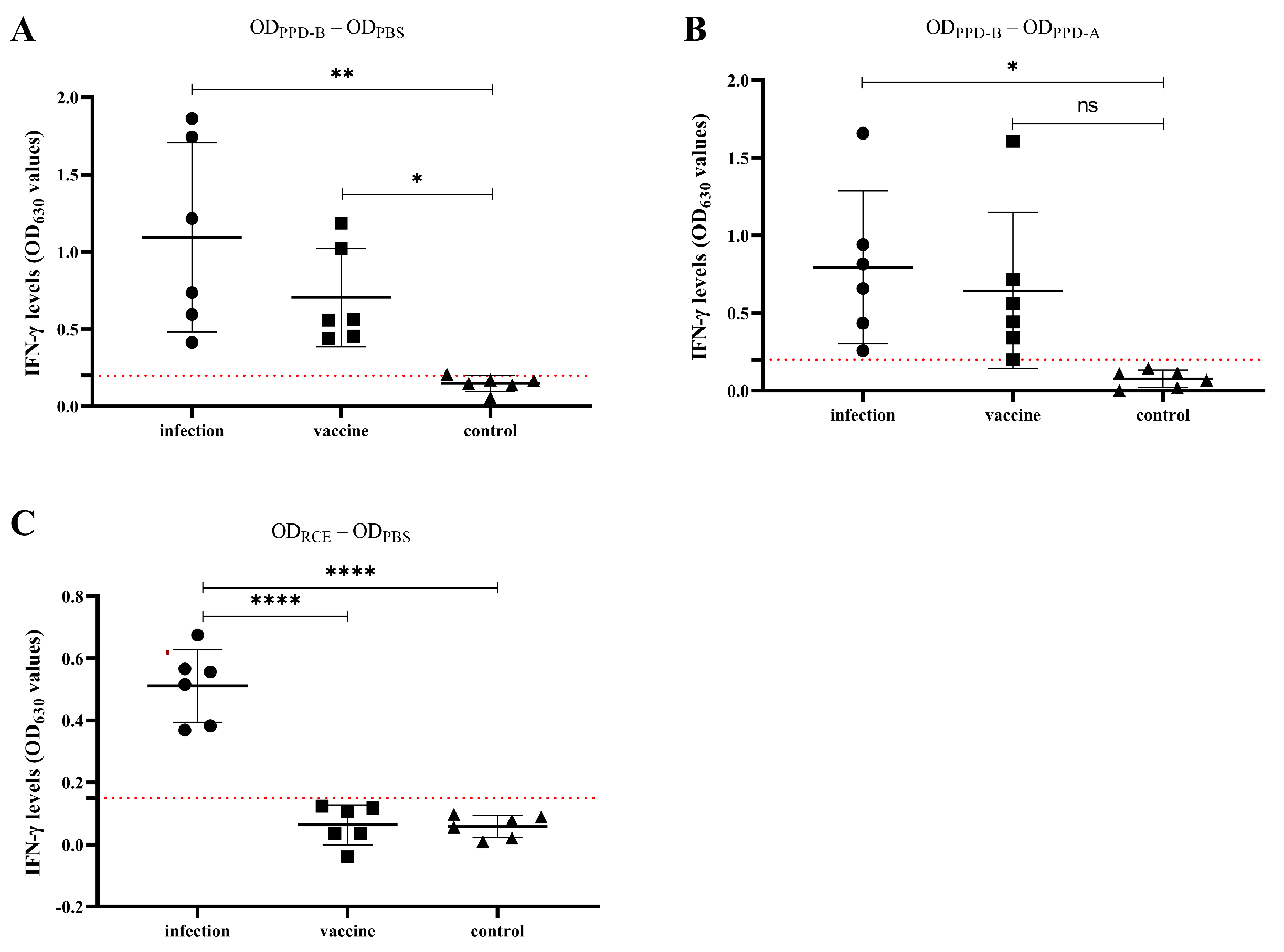
3.3. Differential Diagnosis of BCG-Vaccinated and Naturally Infected Calves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- de Macedo Couto, R.; Santana, G.O.; Ranzani, O.T.; Waldman, E.A. One Health and surveillance of zoonotic tuberculosis in selected low-income, middle-income and high-income countries: A systematic review. PLoS Neglected Trop. Dis. 2022, 16, e0010428. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Y.; Yi, P.; Yang, L.; Yan, Y.; Zhang, K.; Zeng, Q.; Guo, A. Serological detection of Mycobacterium Tuberculosis complex infection in multiple hosts by One Universal ELISA. PLoS ONE 2021, 16, e0257920. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Campos, S.; Smith, N.H.; Boniotti, M.B.; Aranaz, A. Overview and phylogeny of Mycobacterium tuberculosis complex organisms: Implications for diagnostics and legislation of bovine tuberculosis. Res. Vet. Sci. 2014, 97, S5–S19. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Conlan, A.J.K.; Easterling, L.A.; Herrera, C.; Dandapat, P.; Veerasami, M.; Ameni, G.; Jindal, N.; Raj, G.D.; Wood, J.; et al. A Meta-Analysis of the Effect of Bacillus Calmette-Guérin Vaccination Against Bovine Tuberculosis: Is Perfect the Enemy of Good? Front. Vet. Sci. 2021, 8, 637580. [Google Scholar] [CrossRef] [PubMed]
- Nugent, G.; Yockney, I.J.; Whitford, J.; Aldwell, F.E.; Buddle, B.M. Efficacy of oral BCG vaccination in protecting free-ranging cattle from natural infection by Mycobacterium bovis. Vet. Microbiol. 2017, 208, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, A.; Pelone, F.; LaTorre, G.; De Giusti, M.; Saulle, R.; Mannocci, A.; Sala, M.; Della Marta, U.; Scaramozzino, P. Control and eradication of tuberculosis in cattle: A systematic review of economic evidence. Vet. Rec. 2016, 179, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.A.; Woodroffe, R.; Cox, D.R.; Bourne, J.; Gettinby, G.; Le Fevre, A.M.; McInerney, J.P.; Morrison, W.I. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature 2003, 426, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Buddle, B.M.; Vordermeier, H.M.; Chambers, M.A.; de Klerk-Lorist, L.M. Efficacy and Safety of BCG Vaccine for Control of Tuberculosis in Domestic Livestock and Wildlife. Front. Vet. Sci. 2018, 5, 259. [Google Scholar] [CrossRef]
- Hope, J.C.; Thom, M.L.; McAulay, M.; Mead, E.; Vordermeier, H.M.; Clifford, D.; Hewinson, R.G.; Villarreal-Ramos, B. Identification of surrogates and correlates of protection in protective immunity against Mycobacterium bovis infection induced in neonatal calves by vaccination with M. bovis BCG Pasteur and M. bovis BCG Danish. Clin. Vaccine Immunol. 2011, 18, 373–379. [Google Scholar] [CrossRef]
- Ameni, G.; Vordermeier, M.; Aseffa, A.; Young, D.B.; Hewinson, R.G. Field evaluation of the efficacy of Mycobacterium bovis bacillus Calmette-Guerin against bovine tuberculosis in neonatal calves in Ethiopia. Clin. Vaccine Immunol. 2010, 17, 1533–1538. [Google Scholar] [CrossRef]
- Ábalos, P.; Valdivieso, N.; Pérez de Val, B.; Vordermeier, M.; Benavides, M.B.; Alegría-Morán, R.; Saadi, K.; Wistuba, M.; Ortega, C.; Sánchez, N.; et al. Vaccination of Calves with the Mycobacterium bovis BCG Strain Induces Protection against Bovine Tuberculosis in Dairy Herds under a Natural Transmission Setting. Animals 2022, 12, 1083. [Google Scholar] [CrossRef] [PubMed]
- Buddle, B.M.; Parlane, N.A.; Keen, D.L.; Aldwell, F.E.; Pollock, J.M.; Lightbody, K.; Andersen, P. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 1999, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Subramanian, S.; Shankar Balakrishnan, S.; Ramaiyan Selvaraju, K.; Manomohan, V.; Selladurai, S.; Jothivelu, M.; Kandasamy, S.; Gopal, D.R.; Kathaperumal, K.; et al. A Defined Antigen Skin Test That Enables Implementation of BCG Vaccination for Control of Bovine Tuberculosis: Proof of Concept. Front. Vet. Sci. 2020, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Jones, G.; Veerasami, M.; Steinbach, S.; Holder, T.; Zewude, A.; Fromsa, A.; Ameni, G.; Easterling, L.; Bakker, D.; et al. A defined antigen skin test for the diagnosis of bovine tuberculosis. Sci. Adv. 2019, 5, eaax4899. [Google Scholar] [CrossRef]
- Whelan, A.O.; Clifford, D.; Upadhyay, B.; Breadon, E.L.; McNair, J.; Hewinson, G.R.; Vordermeier, M.H. Development of a skin test for bovine tuberculosis for differentiating infected from vaccinated animals. J. Clin. Microbiol. 2010, 48, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Buddle, B.M.; Ryan, T.J.; Pollock, J.M.; Andersen, P.; de Lisle, G.W. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 2001, 80, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ruhwald, M.; Bjerregaard-Andersen, M.; Rabna, P.; Kofoed, K.; Eugen-Olsen, J.; Ravn, P. CXCL10/IP-10 release is induced by incubation of whole blood from tuberculosis patients with ESAT-6, CFP10 and TB7.7. Microbes Infect. 2007, 9, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Cockle, P.J.; Gordon, S.V.; Lalvani, A.; Buddle, B.M.; Hewinson, R.G.; Vordermeier, H.M. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 2002, 70, 6996–7003. [Google Scholar] [CrossRef] [PubMed]
- Cockle, P.J.; Gordon, S.V.; Hewinson, R.G.; Vordermeier, H.M. Field evaluation of a novel differential diagnostic reagent for detection of Mycobacterium bovis in cattle. Clin. Vaccine Immunol. 2006, 13, 1119–1124. [Google Scholar] [CrossRef]
- Brodin, P.; Majlessi, L.; Marsollier, L.; de Jonge, M.I.; Bottai, D.; Demangel, C.; Hinds, J.; Neyrolles, O.; Butcher, P.D.; Leclerc, C.; et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect. Immun. 2006, 74, 88–98. [Google Scholar] [CrossRef]
- Mukherjee, F. Comparative prevalence of tuberculosis in two dairy herds in India. Rev. Sci. Tech. 2006, 25, 1125–1130. [Google Scholar] [CrossRef]
- Rivas, S.; Mucyn, T.; Van Den Burg, H.A.; Vervoort, J.; Jones, J.D. Retraction: ‘An ~400 kDa membrane-associated complex that contains one molecule of the resistance protein Cf-4’. Plant J. 2017, 90, 1214. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Dutta, M.; Datta, P.; Dasgupta, A.; Pradhan, R.; Pradhan, M.; Kundu, M.; Basu, J.; Chakrabarti, P. The RD1-encoded antigen Rv3872 of Mycobacterium tuberculosis as a potential candidate for serodiagnosis of tuberculosis. Clin. Microbiol. Infect. 2007, 13, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Dong Liu, J.L.; Yu, Q.; Chen, Y.; Chen, H.; Guo, A. Preparation of Fusion Protein Rv3872/CFP-10/ESAT-6 and Primary Application in Detection of Bovine Tuberculosis. China Dairy. Cattle 2009, 10, 5. [Google Scholar]
- Dong, L. Preparation of New Fusion Proteins and Application in Antibody Detection for Diaganosis of Bovine Tuberculosis; Huazhong Agricultural University: Wuhan, China, 2013. [Google Scholar]
- Byrne, A.W.; Barrett, D.; Breslin, P.; Fanning, J.; Casey, M.; Madden, J.M.; Lesellier, S.; Gormley, E. Bovine tuberculosis in youngstock cattle: A narrative review. Front. Vet. Sci. 2022, 9, 1000124. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, H.; Thiruvengadam, K.; Singaraj, R.; Palaniyandi, K. Role of abattoir monitoring in determining the prevalence of bovine tuberculosis: A systematic review and meta-analysis. Transbound. Emerg. Dis. 2022, 69, 958–973. [Google Scholar] [CrossRef] [PubMed]
- Gortázar, C.; de la Fuente, J.; Perelló, A.; Domínguez, L. Will we ever eradicate animal tuberculosis? Ir. Vet. J. 2023, 76, 24. [Google Scholar] [CrossRef] [PubMed]
- Ameni, G.; Tafess, K.; Zewde, A.; Eguale, T.; Tilahun, M.; Hailu, T.; Sirak, A.; Salguero, F.J.; Berg, S.; Aseffa, A.; et al. Vaccination of calves with Mycobacterium bovis Bacillus Calmette-Guerin reduces the frequency and severity of lesions of bovine tuberculosis under a natural transmission setting in Ethiopia. Transbound. Emerg. Dis. 2018, 65, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ameni, G.; Erkihun, A. Bovine tuberculosis on small-scale dairy farms in Adama Town, central Ethiopia, and farmer awareness of the disease. Rev. Sci. Tech. 2007, 26, 711–719. [Google Scholar] [CrossRef]
- Ramanujam, H.; Palaniyandi, K. Bovine tuberculosis in India: The need for One Health approach and the way forward. One Health 2023, 16, 100495. [Google Scholar] [CrossRef]
- Singh, A.V.; Yadav, V.S.; Chauhan, D.S.; Singh, S.V. Mycobacterium bovis induced human tuberculosis in India: Current status, challenges & opportunities. Indian. J. Med. Res. 2022, 156, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.A.; Aldwell, F.; Williams, G.A.; Palmer, S.; Gowtage, S.; Ashford, R.; Dalley, D.J.; Davé, D.; Weyer, U.; Salguero, F.J.; et al. The Effect of Oral Vaccination with Mycobacterium bovis BCG on the Development of Tuberculosis in Captive European Badgers (Meles meles). Front. Cell Infect. Microbiol. 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Aznar, I.; Frankena, K.; More, S.J.; O’Keeffe, J.; McGrath, G.; de Jong, M.C.M. Quantification of Mycobacterium bovis transmission in a badger vaccine field trial. Prev. Vet. Med. 2018, 149, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Conlan, A.J.; Brooks Pollock, E.; McKinley, T.J.; Mitchell, A.P.; Jones, G.J.; Vordermeier, M.; Wood, J.L. Potential benefits of cattle vaccination as a supplementary control for bovine tuberculosis. PLoS Comput. Biol. 2015, 11, e1004038. [Google Scholar] [CrossRef]
- Mustafa, A.S.; Cockle, P.J.; Shaban, F.; Hewinson, R.G.; Vordermeier, H.M. Immunogenicity of Mycobacterium tuberculosis RD1 region gene products in infected cattle. Clin. Exp. Immunol. 2002, 130, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Vordermeier, M.; Jones, G.J.; Whelan, A.O. DIVA reagents for bovine tuberculosis vaccines in cattle. Expert. Rev. Vaccines 2011, 10, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- More, S.J.; Radunz, B.; Glanville, R.J. Lessons learned during the successful eradication of bovine tuberculosis from Australia. Vet. Rec. 2015, 177, 224–232. [Google Scholar] [CrossRef]
- Lloyd, L.C. Progress in the eradication of contagious bovine pleuropneumonia in Australia. Aust. Vet. J. 1969, 45, 145–149. [Google Scholar] [CrossRef]
- Gaskamp, J.A.; Gee, K.L.; Campbell, T.A.; Silvy, N.J.; Webb, S.L. Pseudorabies Virus and Brucella abortus from an Expanding Wild Pig (Sus scrofa) Population in Southern Oklahoma, USA. J. Wildl. Dis. 2016, 52, 383–386. [Google Scholar] [CrossRef]
| RCE Concentrations (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |||||
| Cut-off value | 0.09 | 0.15 | 0.10 | 0.06 | ||||
| AUCs | 0.92 | 0.98 | 0.94 | 0.90 | ||||
| Results (No.) | +ve | −ve | +ve | −ve | +ve | −ve | +ve | −ve |
| 60 | 21 | 68 | 22 | 64 | 22 | 65 | 20 | |
| Se% (95% CI) | 81.08 (70.71–88.38) | 91.89 (83.42–96.23) | 86.49 (76.88–92.49) | 87.84 (78.47–93.47) | ||||
| Sp% (95% CI) | 91.30 (73.20–98.45) | 95.65 (79.01–99.78) | 95.65 (79.01–99.78) | 86.96 (67.87–95.46) | ||||
| PPD-IGRA | ||||
|---|---|---|---|---|
| +ve | −ve | Total | ||
| RCE-IGRA | +ve | 31 | 2 | 33 |
| -ve | 5 | 48 | 53 | |
| Total | 36 | 50 | 86 | |
| Kappa (95% CI) | 0.83 (0.71, 0.95) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Fei, W.; Yang, L.; Xiang, Z.; Chen, X.; Chen, Y.; Hu, C.; Chen, J.; Guo, A. The Establishment of a Novel γ-Interferon In Vitro Release Assay for the Differentiation of Mycobacterial Bovis-Infected and BCG-Vaccinated Cattle. Vet. Sci. 2024, 11, 198. https://doi.org/10.3390/vetsci11050198
Zhao Y, Fei W, Yang L, Xiang Z, Chen X, Chen Y, Hu C, Chen J, Guo A. The Establishment of a Novel γ-Interferon In Vitro Release Assay for the Differentiation of Mycobacterial Bovis-Infected and BCG-Vaccinated Cattle. Veterinary Sciences. 2024; 11(5):198. https://doi.org/10.3390/vetsci11050198
Chicago/Turabian StyleZhao, Yuhao, Wentao Fei, Li Yang, Zhijie Xiang, Xi Chen, Yingyu Chen, Changmin Hu, Jianguo Chen, and Aizhen Guo. 2024. "The Establishment of a Novel γ-Interferon In Vitro Release Assay for the Differentiation of Mycobacterial Bovis-Infected and BCG-Vaccinated Cattle" Veterinary Sciences 11, no. 5: 198. https://doi.org/10.3390/vetsci11050198
APA StyleZhao, Y., Fei, W., Yang, L., Xiang, Z., Chen, X., Chen, Y., Hu, C., Chen, J., & Guo, A. (2024). The Establishment of a Novel γ-Interferon In Vitro Release Assay for the Differentiation of Mycobacterial Bovis-Infected and BCG-Vaccinated Cattle. Veterinary Sciences, 11(5), 198. https://doi.org/10.3390/vetsci11050198







