Microarray Gene Expression Analysis of Lesional Skin in Canine Pemphigus Foliaceus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Inclusion
2.2. Tissue Samples and RNA Extraction
2.3. Microarray Analysis Using the NanoString Canine IO Panel
2.4. Differential Expression Analysis and Cell Type Profiling
2.5. Cell Type Profiling
2.6. Enrichment Analysis
3. Results
3.1. Patient Characteristics and Histopathological Examination
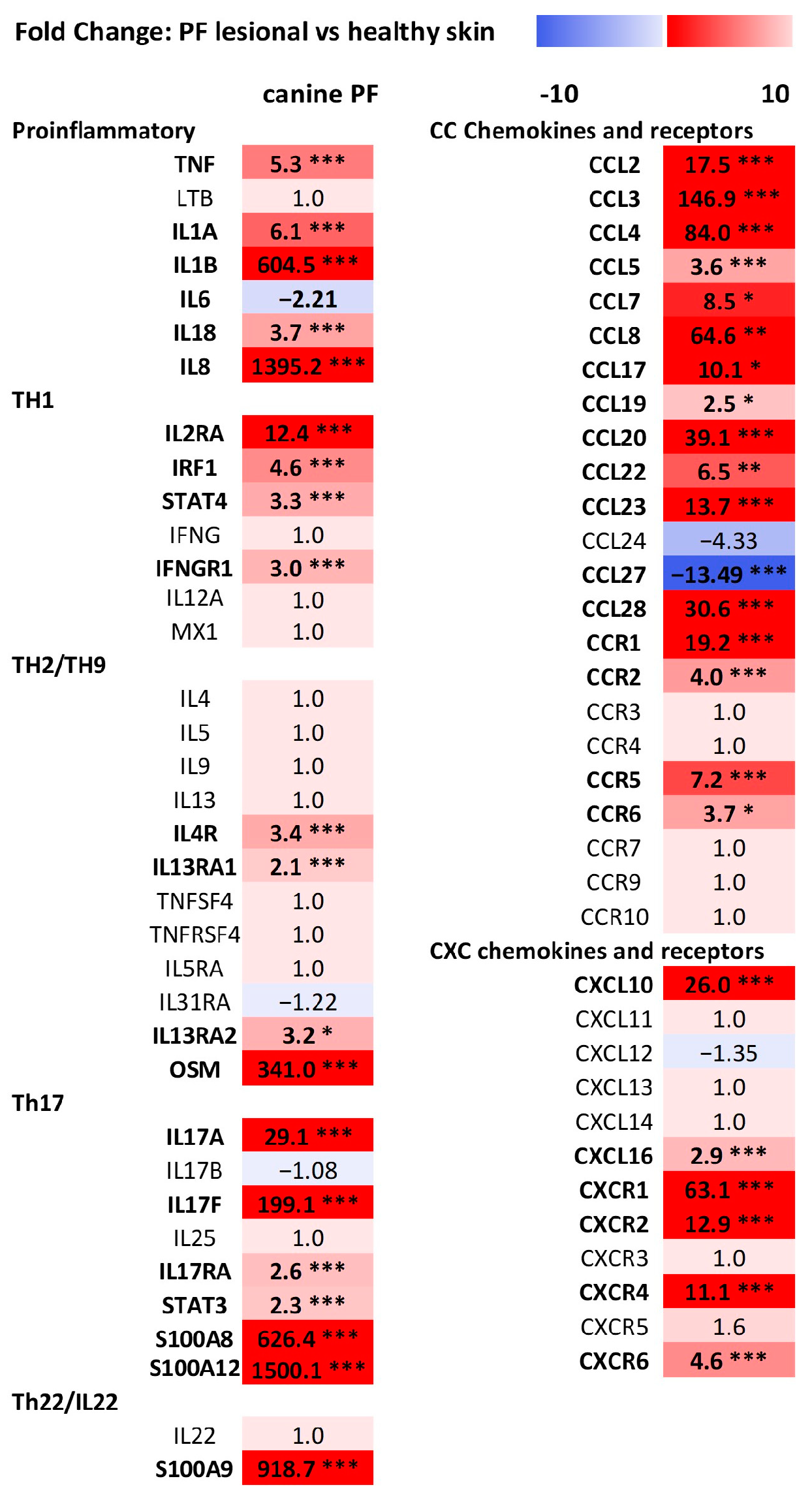
3.2. Differential Gene Expression (DEGs) Analysis
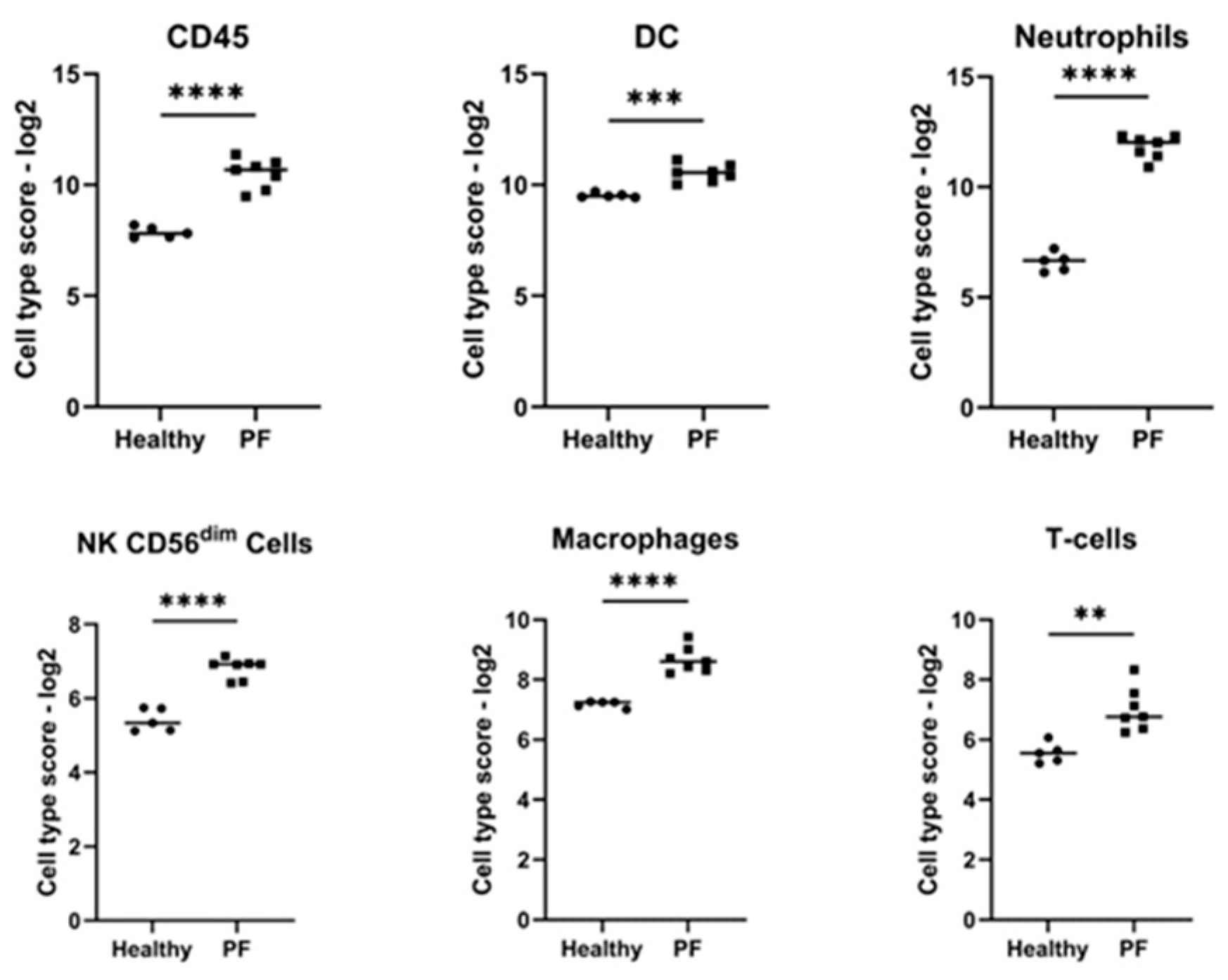
3.3. Cell Type Profiling
3.4. Enrichment Analysis Using Metacore Process Networks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olivry, T. A review of autoimmune skin diseases in domestic animals: I—Superficial pemphigus. Vet. Dermatol. 2006, 17, 291–305. [Google Scholar] [CrossRef]
- Ellebrecht, C.T.; Maseda, D.; Payne, A.S. Pemphigus and Pemphigoid: From Disease Mechanisms to Druggable Pathways. J. Investig. Dermatol. 2022, 142, 907–914. [Google Scholar] [CrossRef]
- Holstein, J.; Solimani, F.; Baum, C.; Meier, K.; Pollmann, R.; Didona, D.; Tekath, T.; Dugas, M.; Casadei, N.; Hudemann, C.; et al. Immunophenotyping in pemphigus reveals a T(H)17/T(FH)17 cell-dominated immune response promoting desmoglein1/3-specific autoantibody production. J. Allergy Clin. Immunol. 2021, 147, 2358–2369. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.; Waschke, J. Autoantibody-Specific Signalling in Pemphigus. Front. Med. 2021, 8, 701809. [Google Scholar] [CrossRef]
- Egu, D.T.; Kugelmann, D.; Waschke, J. Role of PKC and ERK Signaling in Epidermal Blistering and Desmosome Regulation in Pemphigus. Front. Immunol. 2019, 10, 2883. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, A.; Orfanidou, T.; Liaskos, C.; Smyk, D.S.; Billinis, C.; Blank, M.; Rigopoulou, E.I.; Bogdanos, D.P. p38 mitogen-activated protein kinase (p38 MAPK)-mediated autoimmunity: Lessons to learn from ANCA vasculitis and pemphigus vulgaris. Autoimmun. Rev. 2013, 12, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Bizikova, P.; Linder, K.E.; Olivry, T. Immunomapping of desmosomal and nondesmosomal adhesion molecules in healthy canine footpad, haired skin and buccal mucosal epithelia: Comparison with canine pemphigus foliaceus serum immunoglobulin G staining patterns. Vet. Dermatol. 2011, 22, 132–142. [Google Scholar] [CrossRef]
- Bizikova, P.; Dean, G.A.; Hashimoto, T.; Olivry, T. Cloning and establishment of canine desmocollin-1 as a major autoantigen in canine pemphigus foliaceus. Vet. Immunol. Immunopathol. 2012, 149, 197–207. [Google Scholar] [CrossRef]
- Starr, H.; Howerth, E.; Gogal, R., Jr.; Barber, J.; Leon, R.; Blubaugh, A.; Banovic, F. Characterization of the serum and skin inflammatory profile in canine pemphigus foliaceus using multiplex assay and quantitative real-time polymerase chain reaction (qRT-PCR). Vet. Immunol. Immunopathol. 2023, 262, 110631. [Google Scholar] [CrossRef]
- Qiu, W.; Lee, L.M.-L.; Whitmore, G.A. Sample Size and Power Calculation in Microarray Studies Using the Sizepower Package, R Packag Version 132. 2008. Available online: https://bioconductor.org/packages/release/bioc/vignettes/sizepower/inst/doc/sizepower.pdf (accessed on 1 September 2023).
- Bizikova, P.; Olivry, T. Oral glucocorticoid pulse therapy for induction of treatment of canine pemphigus foliaceus—A comparative study. Vet. Dermatol. 2015, 26, 354–358, e76–e77. [Google Scholar] [CrossRef]
- Chong, E.; Austel, M.; Banovic, F. A Retrospective Evaluation of the Steroid-Sparing Effect of Oral Modified Ciclosporin for Treatment of Canine Pemphigus Foliaceus. Vet. Sci. 2022, 9, 153. [Google Scholar] [CrossRef]
- Putra, A.; Austel, M.; Banovic, F. A retrospective evaluation of the steroid sparing effects of oral mycophenolate mofetil (MMF) as an adjunct immunosuppressant for the treatment of canine pemphigus foliaceus. Vet. Dermatol. 2022, 33, 77-e24. [Google Scholar] [CrossRef]
- Perkins, J.R.; Dawes, J.M.; McMahon, S.B.; Bennett, D.L.; Orengo, C.; Kohl, M. ReadqPCR and NormqPCR: R packages for the reading, quality checking and normalisation of RT-qPCR quantification cycle (Cq) data. BMC Genom. 2012, 13, 296. [Google Scholar] [CrossRef]
- Raef, H.S.; Piedra-Mora, C.; Wong, N.B.; Ma, D.J.; David, C.N.; Robinson, N.A.; Almela, R.M.; Richmond, J.M. Gene Expression Analysis in Four Dogs with Canine Pemphigus Clinical Subtypes Reveals B Cell Signatures and Immune Activation Pathways Similar to Human Disease. Front. Med. 2021, 8, 723982. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Kepczynska, A.; Mleczko, M.; Domerecka, W.; Krasowska, D.; Donica, H. Assessment of Immune Cell Activation in Pemphigus. Cells 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Schinner, J.; Cunha, T.; Mayer, J.U.; Horster, S.; Kind, P.; Didona, D.; Keber, C.; Hertl, M.; Worzfeld, T.; Juratli, H.A. Skin-infiltrating T cells display distinct inflammatory signatures in lichen planus, bullous pemphigoid and pemphigus vulgaris. Front. Immunol. 2023, 14, 1203776. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, E.A.; Mak, L.L.; Guitart, J.; Woodley, D.T.; Hashimoto, T.; Amagai, M.; Chan, L.S. Induction of keratinocyte IL-8 expression and secretion by IgG autoantibodies as a novel mechanism of epidermal neutrophil recruitment in a pemphigus variant. Clin. Exp. Immunol. 2000, 119, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Riani, M.; Le Jan, S.; Plee, J.; Durlach, A.; Le Naour, R.; Haegeman, G.; Bernard, P.; Antonicelli, F. Bullous pemphigoid outcome is associated with CXCL10-induced matrix metalloproteinase 9 secretion from monocytes and neutrophils but not lymphocytes. J. Allergy Clin. Immunol. 2017, 139, 863–872.e3. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, M.; Dainichi, T.; Yasumoto, S.; Hashimoto, T. Lesional Th17 cells in pemphigus vulgaris and pemphigus foliaceus. J. Dermatol. Sci. 2009, 53, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.X.; Qu, P.; Wang, K.K.; Zheng, J.; Pan, M.; Zhu, H.Q. Transcriptomic profiling of pemphigus lesion infiltrating mononuclear cells reveals a distinct local immune microenvironment and novel lncRNA regulators. J. Transl. Med. 2022, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Singh, P.K.; Dar, S.A.; Rai, G.; Akhter, N.; Pandhi, D.; Gaurav, V.; Bhattacharya, S.N.; Banerjee, B.D.; Ahmad, A.; et al. Deregulated phenotype of autoreactive Th17 and Treg clone cells in pemphigus vulgaris after in-vitro treatment with desmoglein antigen (Dsg-3). Immunobiology 2023, 228, 152340. [Google Scholar] [CrossRef]
- Liu, R.; Lauridsen, H.M.; Amezquita, R.A.; Pierce, R.W.; Jane-Wit, D.; Fang, C.; Pellowe, A.S.; Kirkiles-Smith, N.C.; Gonzalez, A.L.; Pober, J.S. IL-17 Promotes Neutrophil-Mediated Immunity by Activating Microvascular Pericytes and Not Endothelium. J. Immunol. 2016, 197, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Zebrowska, A.; Wozniacka, A.; Juczynska, K.; Ociepa, K.; Waszczykowska, E.; Szymczak, I.; Pawliczak, R. Correlation between IL36alpha and IL17 and Activity of the Disease in Selected Autoimmune Blistering Diseases. Mediat. Inflamm. 2017, 2017, 8980534. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.; Cuppari, C.; Manti, S.; Filippelli, M.; Parisi, G.F.; Borgia, F.; Briuglia, S.; Cannavo, P.; Salpietro, A.; Arrigo, T.; et al. Serum interleukin 17, interleukin 23, and interleukin 10 values in children with atopic eczema/dermatitis syndrome (AEDS): Association with clinical severity and phenotype. Allergy Asthma Proc. 2015, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, C.; Hanni, T.; Yawalkar, N.; Hunger, R.E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2011, 65, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, J.; Simon, J.C.; Kunz, M.; Treudler, R. Possible effect of interleukin-17 blockade in pemphigus foliaceus and neutrophilic diseases. Hautarzt 2019, 70, 641–644. [Google Scholar] [CrossRef]
- Solimani, F.; Meier, K.; Ghoreschi, K. Emerging Topical and Systemic JAK Inhibitors in Dermatology. Front. Immunol. 2019, 10, 2847. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.; McDonald, B.; Crowley, A. A case report of the beneficial effect of oclacitinib in a dog with pemphigus vulgaris. Vet. Dermatol. 2022, 33, 237-e265. [Google Scholar] [CrossRef] [PubMed]
- Denti, D.; Caldin, M.; Ventura, L.; De Lucia, M. Prolonged twice-daily administration of oclacitinib for the control of canine atopic dermatitis: A retrospective study of 53 client-owned atopic dogs. Vet. Dermatol. 2022, 33, 149-e142. [Google Scholar] [CrossRef]
- Harvey, R.G.; Olivri, A.; Lima, T.; Olivry, T. Effective treatment of canine chronic cutaneous lupus erythematosus variants with oclacitinib: Seven cases. Vet. Dermatol. 2023, 34, 53–58. [Google Scholar] [CrossRef]
- Carrasco, I.; Martinez, M.; Albinyana, G. Beneficial effect of oclacitinib in a case of feline pemphigus foliaceus. Vet. Dermatol. 2021, 32, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, G.A.; Park, J.K.; Lavker, R.M.; Petzl-Erler, M.L. Crosstalk between Signaling Pathways in Pemphigus: A Role for Endoplasmic Reticulum Stress in p38 Mitogen-Activated Protein Kinase Activation? Front. Immunol. 2017, 8, 1022. [Google Scholar] [CrossRef] [PubMed]
- Bharathan, N.K.; Giang, W.; Hoffman, C.L.; Aaron, J.S.; Khuon, S.; Chew, T.L.; Preibisch, S.; Trautman, E.T.; Heinrich, L.; Bogovic, J.; et al. Architecture and dynamics of a desmosome-endoplasmic reticulum complex. Nat. Cell Biol. 2023, 25, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.E.; Firestein, G.S. Mitogen activated protein kinase inhibitors: Where are we now and where are we going? Ann. Rheum. Dis. 2006, 65 (Suppl. S3), iii83–iii88. [Google Scholar] [CrossRef]
- Lotti, R.; Shu, E.; Petrachi, T.; Marconi, A.; Palazzo, E.; Quadri, M.; Lin, A.; O’Reilly, L.A.; Pincelli, C. Soluble Fas Ligand Is Essential for Blister Formation in Pemphigus. Front. Immunol. 2018, 9, 370. [Google Scholar] [CrossRef]
- Puviani, M.; Marconi, A.; Cozzani, E.; Pincelli, C. Fas ligand in pemphigus sera induces keratinocyte apoptosis through the activation of caspase-8. J. Investig. Dermatol. 2003, 120, 164–167. [Google Scholar] [CrossRef]
- Lotti, R.; Hundt, J.E.; Ludwig, R.J.; Bennett, B.; Amato, A.; Marconi, A.; Pincelli, C. Blocking soluble Fas Ligand ameliorates pemphigus: PC111 efficacy in ex-vivo human pemphigus models. Front. Immunol. 2023, 14, 1193032. [Google Scholar] [CrossRef]
| Group | Breed | Age (yr) | Sex | Pemphigus Lesion Distribution | Prior Skin Disease | Prior Non-Cutaneous Disease |
|---|---|---|---|---|---|---|
| Healthy | German Shepard Mix | 8.6 | FS | None | None | None |
| American Staffordshire Terrier | 5.6 | M | None | None | None | |
| Mixed | 3 | MC | None | None | None | |
| Golden Retriever | 1.8 | MC | None | None | None | |
| German Shepard | 2.2 | FS | None | None | None | |
| PF | Beagle | 4.6 | MC | Facial and Truncal | None | None |
| Boxer Mix | 7.8 | FS | Facial and Truncal | None | None | |
| Mixed | 7 | FS | Facial and Truncal | None | None | |
| Catahoula Mix | 0.75 | MC | Facial and Truncal | None | None | |
| Labrador Retriever | 7 | FS | Facial and Truncal | None | None | |
| Pitbull Mix | 0.75 | FS | Facial and Truncal | None | None | |
| Mixed | 6 | FS | Facial and Truncal | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starr, H.; Howerth, E.W.; Leon, R.; Gogal, R.M., Jr.; Banovic, F. Microarray Gene Expression Analysis of Lesional Skin in Canine Pemphigus Foliaceus. Vet. Sci. 2024, 11, 89. https://doi.org/10.3390/vetsci11020089
Starr H, Howerth EW, Leon R, Gogal RM Jr., Banovic F. Microarray Gene Expression Analysis of Lesional Skin in Canine Pemphigus Foliaceus. Veterinary Sciences. 2024; 11(2):89. https://doi.org/10.3390/vetsci11020089
Chicago/Turabian StyleStarr, Haley, Elizabeth W. Howerth, Renato Leon, Robert M. Gogal, Jr., and Frane Banovic. 2024. "Microarray Gene Expression Analysis of Lesional Skin in Canine Pemphigus Foliaceus" Veterinary Sciences 11, no. 2: 89. https://doi.org/10.3390/vetsci11020089
APA StyleStarr, H., Howerth, E. W., Leon, R., Gogal, R. M., Jr., & Banovic, F. (2024). Microarray Gene Expression Analysis of Lesional Skin in Canine Pemphigus Foliaceus. Veterinary Sciences, 11(2), 89. https://doi.org/10.3390/vetsci11020089






