1. Introduction
Lactic acid (2-hydroxypropanoic acid) is a versatile organic acid with many industrial applications, including in the food, pharmaceutical, and biodegradable plastic industries [
1]. The increasing demand for lactic acid, particularly in the production of poly(lactic acid) (PLA), which is a promising bio-based material that is biocompatible, transparent, and thermoplastic, making it a strong candidate for replacing conventional plastics, has led to a significant need for cost-effective and sustainable production methods [
2,
3]. Notably, the LA structure features a chiral carbon atom, and the (L)-(+)-enantiomer with an (S)-configuration is widely used in the production of renewable materials that have replaced a significant proportion of conventional plastics in food packaging. The biodegradability of PLA is negatively affected by the crystallinity of the polymer, which is highest in stereohomogeneous polymers composed of a sequence of PLA molecules with the same absolute configuration [
4].
The global PLA market is expected to grow rapidly due to increased environmental awareness and regulatory pressure [
2]. However, the high cost of producing lactic acid, particularly when derived from refined sugars and food-based substrates, remains a significant barrier to market expansion [
3]. Conventional lactic acid fermentation often relies on starch or glucose derived from corn or sugarcane, raising concerns about food security, raw material cost volatility, and ethical concerns [
1]. Consequently, agro-industrial residues have attracted growing attention as low-cost, non-food feedstocks for bioproduction processes [
5,
6].
Among these, sugarcane molasses, a viscous byproduct of sugar refining, is particularly promising due to its high fermentable sugar content, year-round availability, and low market value [
7,
8,
9]. Several studies have explored its use as a substrate for the microbial production of value-added products such as lactic acid [
1,
10,
11,
12], bioethanol [
13], succinic acid [
14], biosurfactants [
15], and enzymes [
7]. Its richness in sugars (typically 40–60%
w/
v) and micronutrients (e.g., potassium, calcium, and nitrogenous compounds) makes it an ideal candidate for microbial fermentation without requiring extensive supplementation [
8,
16]. Moreover, molasses can support the growth of a wide variety of microorganisms, including
Lactobacillus spp.,
Saccharomyces cerevisiae, and
Bacillus spp. [
10,
11,
17]. Valorising molasses aligns with circular economy principles by converting waste streams into high-value chemicals while mitigating environmental disposal issues.
Recent advances highlight the capacity of lactic acid bacteria (LAB) to metabolise a variety of agro-industrial residues for the production of lactic acid and other value-added biochemicals. Select strains of
Bifidobacterium and
Lactobacillus have demonstrated efficient carbohydrate utilisation and biopolymer synthesis when cultivated on lignocellulosic or sugar-rich agricultural waste streams, supporting their role in low-cost, substrate-flexible fermentation systems [
18,
19]. Balasubramanian et al. [
19] further emphasised the role of renewable substrates in microbial lactic acid production for bioplastic synthesis, while Hussein et al. [
18] demonstrated the capacity of local
Bifidobacterium strains to produce exopolysaccharides from low-cost media, reinforcing the broader biotechnological potential of LAB and related microorganisms in agro-waste valorisation.
In Côte d’Ivoire, the sugar industry produces approximately 76,000 tonnes of molasses annually as a by-product of 200,000 tonnes of raw sugar production [
8]. Despite this abundance, molasses remains underutilised, often discarded or sold for low-value purposes such as animal feed. Given its local abundance and inherent nutrient content, molasses could serve as a carbon and micronutrient source, reducing reliance on expensive fermentation additives such as yeast extract and peptone [
20]. Recent local research efforts have explored the valorisation of molasses and other local agro-wastes. Studies have examined the chemical characterisation of Ivorian molasses, its use in ethanol production, and its combination with cashew apple juice to improve lactic acid yields [
8,
21]. Parallel research has also investigated alternative agricultural wastes such as cocoa pod husks for lactic acid production [
22], indicating growing national interest in bioresource conversion. However, these studies are mostly preliminary, often lacking process optimisation, enantiomeric characterisation, or economic assessment.
This fragmented research landscape and continued disposal of molasses and agro-waste hampers waste management and compromises alignment with global sustainability goals. Without integrated bioprocessing strategies, valuable resources are lost and pollution increases. Hybrid approaches that combine chemical pretreatment with microbial conversion have shown promise for valorising complex substrates, as demonstrated in a study coupling catalytic and microbial degradation for waste transformation [
23]. Similarly, developing a scalable, environmentally friendly fermentation process using sugarcane molasses in Côte d’Ivoire is a scientific and socio-environmental imperative.
The success of such a strategy depends on the ability of robust lactic acid bacteria (LAB) strains to tolerate variable substrate quality while maintaining high yields. Lactic acid bacteria, especially
Lactobacillus strains, are widely used in bioprocessing for their ability to convert sugars into lactic acid under mild conditions [
10,
24,
25,
26].
Limosilactobacillus fermentum ATCC 9338, a heterofermentative strain, is known for its adaptability to diverse sugar profiles and moderate fermentation conditions [
22,
27,
28]. This makes it suitable for molasses-based fermentation systems in regions with limited processing infrastructure.
This study investigates the feasibility of using Ivorian sugarcane molasses as a cost-effective carbon source for lactic acid production. This by-product, originating from a semi-industrial sugar sector characterised by localised agro-ecological practices and partially mechanised processing, exhibits a distinctive biochemical profile, with a particularly high sucrose content and relatively low concentrations of fermentation inhibitors. Unlike many molasses types from Asia, Australia, or South America, which often contain higher levels of inhibitors due to more intensive refining processes [
29,
30,
31], these less intensive practices likely confer a composition that is highly desirable for lactic acid production.
Therefore, the objective of this study was to develop and optimise a fermentation process for lactic acid production using Ivorian sugarcane molasses as the primary substrate. The fermentation performance of L. fermentum ATCC 9338 was evaluated on both raw and pretreated molasses, with process conditions optimised via Box–Behnken design under response surface methodology. The specific rotational power of the lactic acid was assessed due to its importance in industrial applications, and comparative cost analysis was conducted to assess the economic feasibility of this process relative to conventional fermentation substrates. By targeting both process performance and local resource valorisation, this study contributes to sustainable biomanufacturing in West Africa.
2. Materials and Methods
2.1. Raw Material
The sugarcane molasses (SCM) used in this study was obtained from the Société Sucrière Africaine (SUCAF) sugar factory in Ferkessédougou, northern Côte d’Ivoire (9°32′ N, 6°29′ W). The molasses had a viscosity of 4798 cP and a density of 1.38 g/mL. Analytical grade sulphuric acid (H
2SO
4) and sodium hydroxide (NaOH) used for pretreatment and pH adjustment were supplied by Fisher Scientific (Waltham, MA, USA). The materials used for the fermentation experiments are shown in
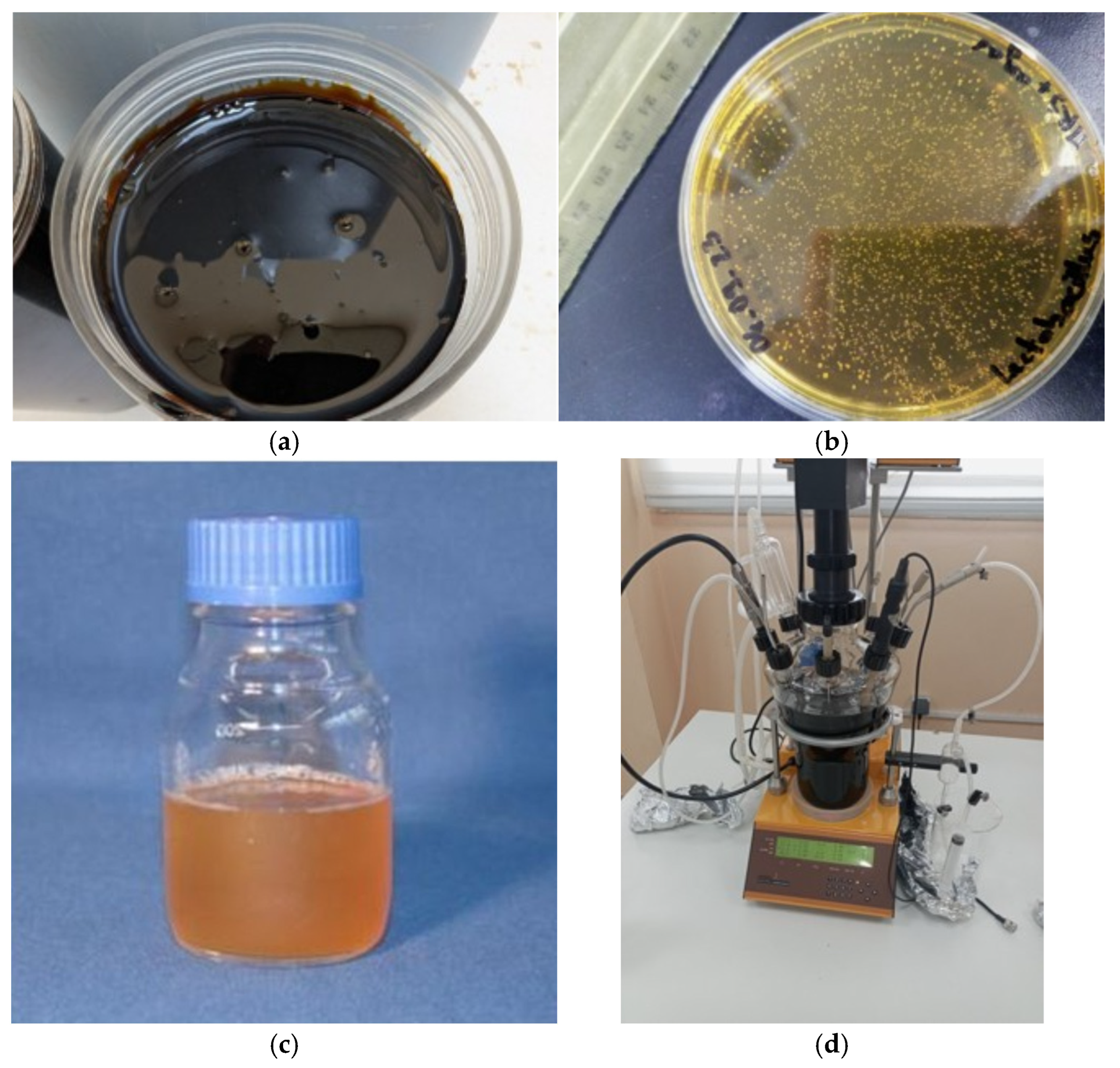
Figure 1, including raw sugarcane molasses, the
Limosilactobacillus fermentum ATCC 9338 strain, the prepared inoculum, and the LAMBDA MINIFOR biofermentor.
2.2. Pretreatment of Sugarcane Molasses
To improve fermentability, SCM was subjected to a dilution and acidolysis pretreatment protocol with modification [
32]. The molasses was diluted with distilled water in a ratio of 1:5 (
w/
v) and heated at 80 °C for 30 min in the presence of 1 N H
2SO
4. The pH was adjusted to 8.5 throughout the process with 4 M NaOH to prevent sugar degradation. After heating, the solution was cooled and neutralised to pH 5.5 for optimal fermentation conditions. This pretreatment aimed to hydrolyse sucrose into fermentable monosaccharides (glucose and fructose) and reduce the viscosity and inhibitory mineral content.
2.3. Determination of the SCM Composition
The composition of both raw and pretreated molasses was analysed to determine the concentrations of key carbohydrates (sucrose, fructose, and glucose) and other components. Carbohydrate concentrations were measured using spectrophotometric analysis following the phenol–sulfuric acid method described in [
33], with absorbance measured at 490 nm using a Jenway 75862 spectrophotometer (Cole-Parmer Ltd., Stone, UK). Calibration curves were prepared using standard solutions of each sugar. Mineral concentrations (Ca, Mg, Fe, Cu, Mn, Na, K, Zn) were quantified by inductively coupled plasma atomic emission spectroscopy (ICP-AES) 20 type VARIAN, following the AOAC 984.27 method as improved by Poitevin et al. [
34], with minor adaptations for the sugarcane molasses matrix. Samples were digested in nitric acid using microwave-assisted digestion prior to analysis. The crude protein content was calculated from the total nitrogen, determined by the Kjeldahl digestion method following ISO 1871:2009 [
35], using a conversion factor of 6.25. These compositional data supported the selection of suitable fermentation conditions and helped to evaluate the effect of pretreatment on molasses quality.
2.4. Microorganism and Inoculum Preparation
Limosilactobacillus fermentum ATCC 9338, a heterofermentative LAB, was used as the production strain. It was purchased from Industrial Analytical (PTY, Johannesburg, South Africa) and maintained at −80 °C in MRS broth supplemented with 20% (v/v) glycerol to ensure long-term viability.
For activation, the strain was cultured in MRS broth at 37 °C with shaking at 200 rpm for 72 h according to the procedure described in [
22]. Optical density (OD
600) was monitored using a Jenway 75862 spectrophotometer.
2.5. Lactic Acid Production
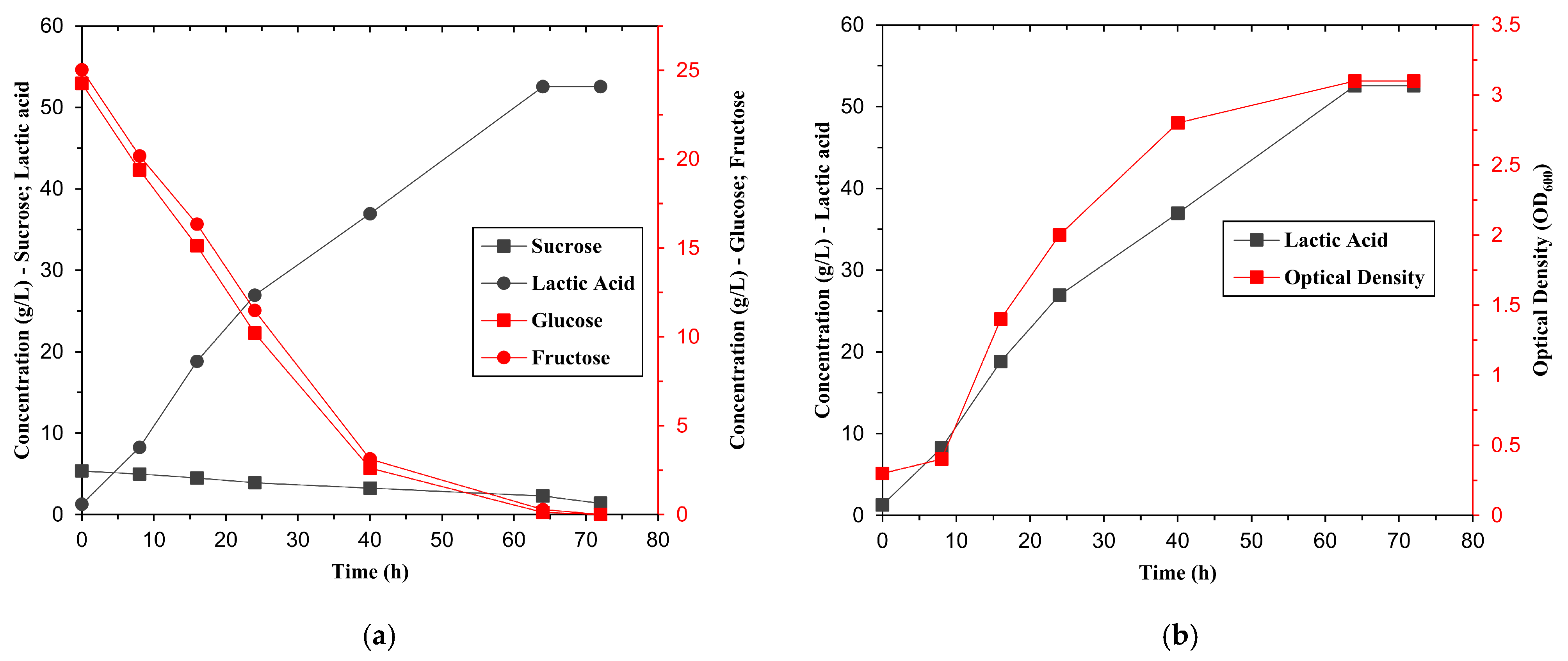
Batch fermentations were initially carried out using raw and pretreated molasses to assess the substrate suitability. The diluted molasses was adjusted to an initial sugar concentration of 40 g/L for each experiment. The fermentations were carried out in a 3 L biofermentor (LAMBDA MINIFOR, LAMBDA Laboratory Instruments, Baar, Switzerland) with a 2 L working volume, operated at 37 °C, 200 rpm and an inoculation rate of 10% (v/v) using a cell density of approximately 2.38 × 106 CFU/mL. The pH was maintained at 5.5 by automatic titration with 4 M NaOH.
Only pretreated molasses was used for optimisation. A Box–Behnken design (BBD) under response surface methodology (RSM) was used to investigate the effects of three independent variables: initial sugar concentration (g/L), inoculation rate (%), and stirring speed (rpm). The factor choice was based on preliminary experiments. The three independent factors used were investigated at three different levels, as shown in
Table 1.
The experimental matrix consisted of 15 randomised runs, including 3 centres. Design Expert software (version 13, Stat-Ease Inc., Minneapolis, MN, USA) was used for statistical design and data analysis. This design allowed for the efficient exploration of the parameter space and the identification of optimal conditions for maximising lactic acid production. Fermentation experiments were conducted in a 3 L laboratory fermenter LAMBDA MINIFOR model, with a working volume of 2 L, and samples were taken every 8 h. Optical density, sugar concentration, and lactic acid content were monitored. After fermentation, cells were removed by centrifugation (6000 rpm, 15 min, 4 °C, HERMLE Z 326 K, HERMLE Labortechnik GmbH, Wehingen, Germany) and the supernatant was used for analysis.
2.6. Kinetic Parameters of Lactic Acid Production Process
The kinetic parameters of the lactic acid production process were calculated to evaluate fermentation performance. Volumetric lactic acid productivity (Q
p, g/L/h) was calculated as the ratio of lactic acid concentration at the end of fermentation (P
t, g/L) to the total fermentation time (t, h), as shown in Equation (1). The yield of lactic acid based on sugar consumed (Y
P/S, g/g) was determined using Equation (2), where S
0 and S
f (g/L) represent the initial and final sugar concentrations, respectively.
2.7. Cost Analysis
A simplified cost analysis was conducted to evaluate the economic feasibility of lactic acid production using pretreated sugarcane molasses as a cost-effective carbon source in Côte d’Ivoire. Data on the sugar content (%
w/
w), local price (USD/ton), and effective sugar cost (USD/kg) for each substrate were compiled from peer-reviewed scientific articles, local agro-industry reports, and publicly available market databases such as FAO statistics. For molasses, direct pricing was verified with the local industry. Where primary data were unavailable, estimates were derived based on regional biomass collection costs, pretreatment efficiencies, and reported hydrolysis yields. The effective sugar cost was calculated using Equation (3), ensuring a normalised comparison across substrates with different sugar contents. Sugar content values correspond to fermentable (monomeric) sugars after any necessary pretreatment, consistent with the compositional analysis reported in
Section 3.1.
The calculation did not include pretreatment costs (energy, chemicals), which are qualitatively discussed in the economic analysis. To ensure the robustness of the estimates, a range of values was reported based on the compositional variability found in previous studies. This approach provides a realistic and comparable basis for evaluating the economic feasibility of molasses and alternative substrates for lactic acid production under West African industrial conditions.
2.8. Analytical Methods
Batch lactic acid quantification was performed using a spectrophotometric method adapted from [
36] based on the reaction between lactate ions and iron (III) chloride, which forms a coloured complex with an absorbance peak at 390 nm. Calibration curves were prepared using pure D/L-lactic acid (90%). For each condition, samples were centrifuged at 6000 rpm for 15 min, and the supernatant was analysed. A 50 μL aliquot of the supernatant was mixed with 2 mL of 0.2% iron (III) chloride, and the absorbance was measured within 15 min.
The specific rotational power was measured with a Jasco P-2000 polarimeter (JASCO Corporation, Tokyo, Japan), using a Jasco J/39.35 quartz cell (JASCO Corporation, Tokyo, Japan), with an optical path length of 100 mm. Optical rotation was measured in water at a concentration of 2.5 g/100 mL at 20 °C, as reported in the literature and confirmed by Sigma-Aldrich technical data ([α]
D = +2.6°) [
37,
38].
2.9. Statistical Analysis
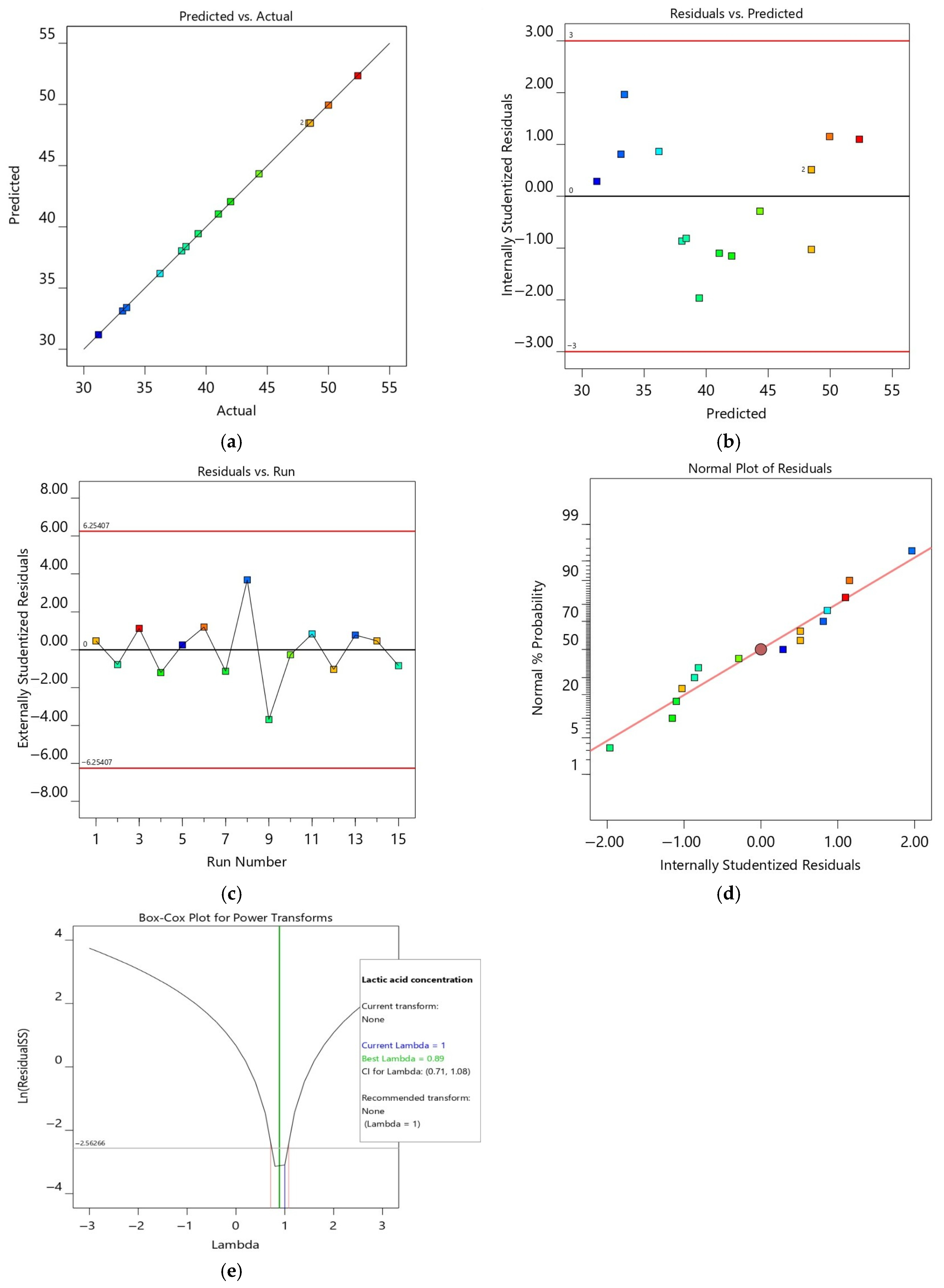
All analyses were performed in triplicate, and the data were expressed as the mean ± standard deviation (SD). Analysis of variance (ANOVA) was performed using Design Expert version 13 to evaluate the significance of model terms, with p-values < 0.05 considered statistically significant. Model adequacy was assessed through R2, adjusted R2, predicted R2, and diagnostic residual plots. Fermentation kinetics plots were produced using OriginPro 2024 (OriginLab Corp., Northampton, MA, USA).
4. Conclusions
This research demonstrates the technical and economic feasibility of producing racemic lactic acid from Ivorian sugarcane molasses using Limosilactobacillus fermentum ATCC 9338 under optimised batch fermentation conditions. Acid hydrolysis pretreatment significantly enhanced the fermentable sugar content of molasses, while optimisation using response surface methodology identified the critical influence of the stirring speed and sugar concentration, leading to maximum lactic acid production.
The findings support the potential of sugarcane molasses as a sustainable and low-cost feedstock for lactic acid fermentation, aligning well with circular bioeconomy objectives by valorising agro-industrial residues. The racemic nature of the lactic acid produced extends its applicability to a wide range of non-stereospecific uses, including pharmaceuticals, cosmetics, and biodegradable materials.
Future work should focus on detailed techno-economic evaluations and the development of cost-effective and eco-friendly downstream recovery methods adapted to local contexts, particularly in resource-limited regions such as Côte d’Ivoire. Such approaches can contribute to reducing import dependency, promoting small-scale bioproduction, and supporting inclusive bioeconomic growth in developing countries like Côte d’Ivoire.