Natural Hematite and Siderite as Heterogeneous Catalysts for an Effective Degradation of 4-Chlorophenol via Photo-Fenton Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Physicochemical Characterization
2.3. Photo-Fenton Experiments and Analytical Procedures
3. Results and Discussion
3.1. Catalysts Characterization
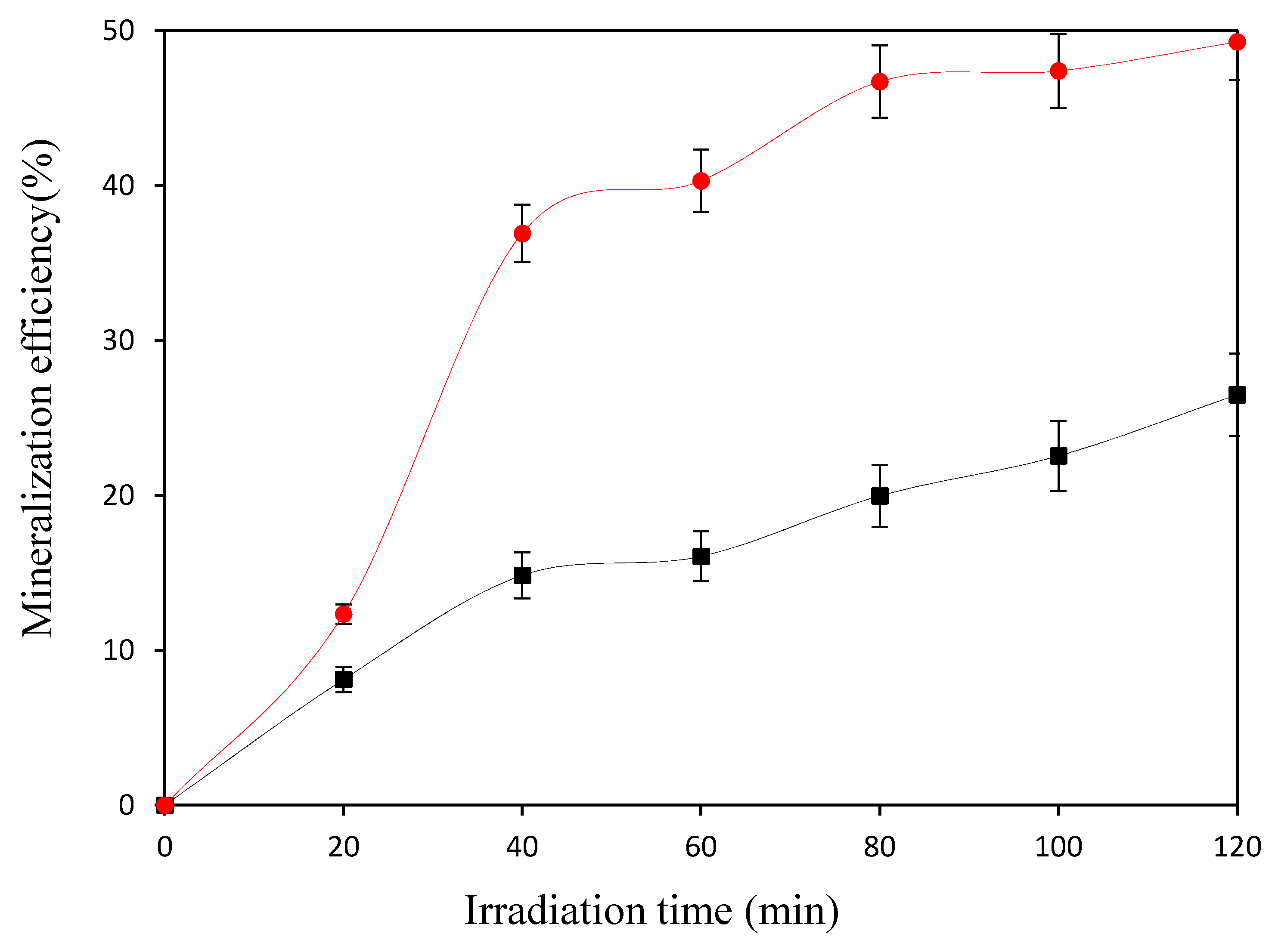
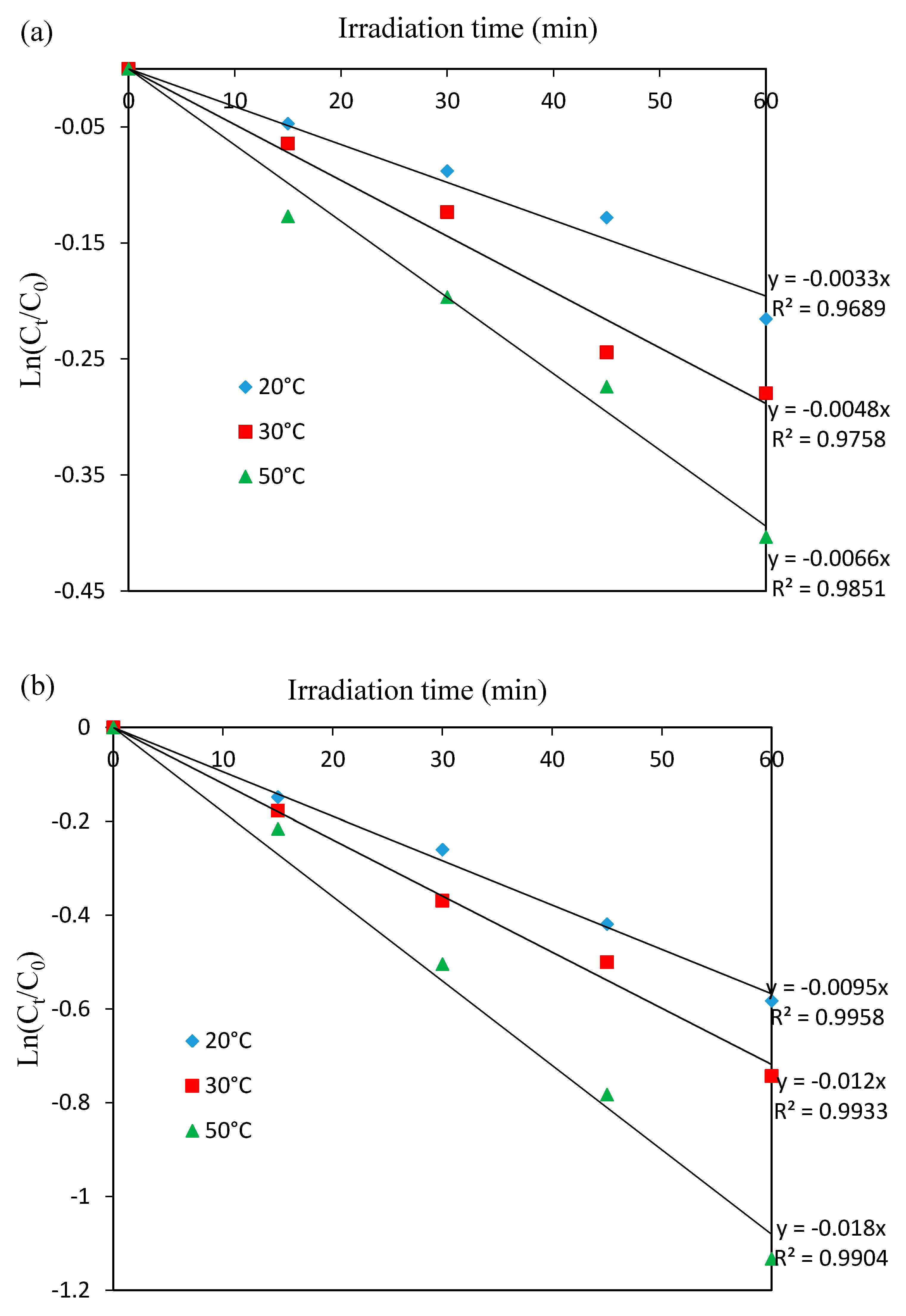
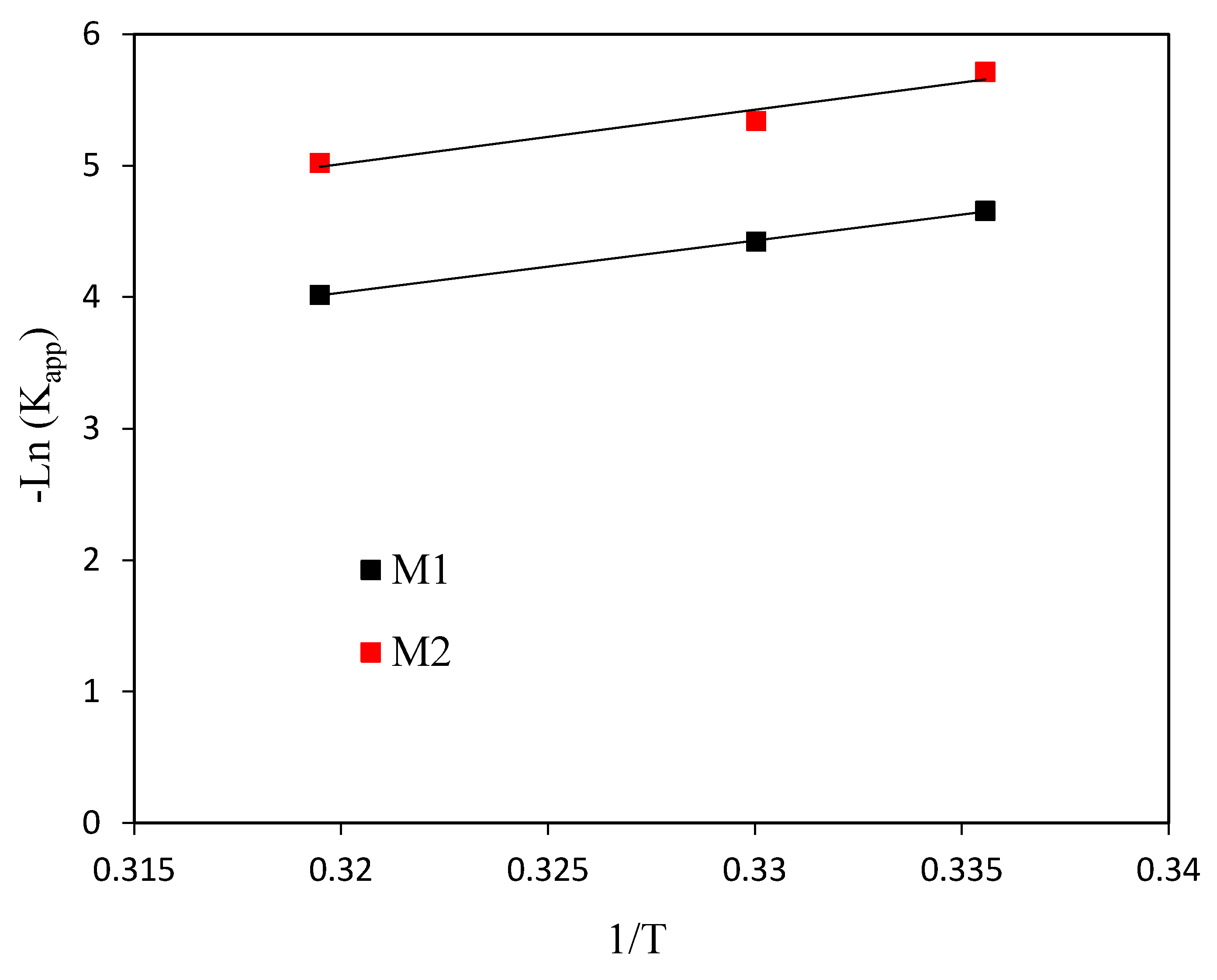
3.2. Catalytic Performance in the Photo-Fenton Oxidation of 4-Chlorophenol
3.3. Comparison of Efficiency of Reaction with Previous Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Z.; Wen, M.; Wu, Q.; Zhang, Y.; Fang, H.; Chen, H. Fabrication of Cu@AgCl nanocables for their enhanced activity toward the catalytic degradation of 4-chlorophenol. J. Colloid Interface Sci. 2015, 460, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Okoth, O.K.; Yan, K.; Zhang, J. A highly selective electrochemical sensor for 4-chlorophenol determination based on molecularly imprinted polymer and PDDA-functionalized graphene. Sens. Actuators B Chem. 2016, 236, 294–303. [Google Scholar] [CrossRef]
- Chen, X.; Bian, W.; Song, X.; Liu, D.; Zhang, J. Degradation of 4-chlorophenol in a dielectric barrier discharge system. Sep. Purif. Technol. 2013, 120, 102–109. [Google Scholar] [CrossRef]
- Khanikar, N.; Bhattacharyya, K.G. Cu(II)-kaolinite and Cu(II)-montmorillonite as catalysts for wet oxidative degradation of 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol. Chem. Eng. J. 2013, 233, 88–97. [Google Scholar] [CrossRef]
- Chen, C.; Geng, X.; Huang, W. Adsorption of 4-chlorophenol and aniline by nanosized activated carbons. Chem. Eng. J. 2017, 327, 941–952. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, M.; Cao, H.; Zhang, S.; Zhao, H. Laccase immobilized on magnetic nanoparticles by dopamine polymerization for 4-chlorophenol removal. Green Energy Environ. 2017, 2, 393–400. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.Y.; Kang, C.L.; Peng, F.; Liu, H.F.; Yang, T.; Shi, L.; Wang, H.L. Photo-fenton effect of 4-chlorophenol in ice. J. Hazard. Mater. 2013, 261, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, E.M.; Romero, R.; Roa, G.; Peralta-Reyes, E.; Espino-Valencia, J.; Natividad, R. Photo-Fenton oxidation of phenolic compounds catalyzed by iron-PILC. Fuel 2014, 138, 149–155. [Google Scholar] [CrossRef]
- Fida, H.; Zhang, G.; Guo, S.; Naeem, A. Heterogeneous Fenton degradation of organic dyes in batch and fixed bed using La-Fe montmorillonite as catalyst. J. Colloid Interface Sci. 2017, 490, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Hadjltaief, H.B.; Sdiri, A.; Ltaief, W.; da Costa, P.; Gálvez, M.E.; Zina, M.B. Efficient removal of cadmium and 2-chlorophenol in aqueous systems by natural clay: Adsorption and photo-Fenton degradation processes. C. R. Chim. 2018, 21, 253–262. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; da Costa, P.; Beaunier, P.; Gálvez, M.E.; Zina, M.B. Fe-clay-plate as a heterogeneous catalyst in photo-Fenton oxidation of phenol as probe molecule for water treatment. Appl. Clay Sci. 2014, 91–92, 46–54. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Wang, J. Photo-Fenton degradation of rhodamine B using Fe2O3-Kaolin as heterogeneous catalyst: Characterization, process optimization and mechanism. J. Colloid Interface Sci. 2014, 433, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Liotta, L.F.; Gruttadauria, M.; di Carlo, G.; Perrini, G.; Librando, V. Heterogeneous catalytic degradation of phenolic substrates: Catalysts activity. J. Hazard. Mater. 2009, 162, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramírez, E.G.; Theng, B.K.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Ltaïef, A.H.; Pastrana-Martínez, L.M.; Ammar, S.; Gadri, A.; Faria, J.L.; Silva, A.M.T. Mined pyrite and chalcopyrite as catalysts for spontaneous acidic pH adjustment in Fenton and LED photo-Fenton-like processes. J. Chem. Technol. Biotechnol. 2017, 93, 1137–1146. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Dhir, A. Natural soil mediated photo Fenton-like processes in treatment of pharmaceuticals: Batch and continuous approach. Chemosphere 2017, 188, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Minella, M.; Marchetti, G.; de Laurentiis, E.; Malandrino, M.; Maurino, V.; Minero, C.; Vione, D.; Hanna, K. Photo-Fenton oxidation of phenol with magnetite as iron source. Appl. Catal. B Environ. 2014, 154–155, 102–109. [Google Scholar] [CrossRef]
- El Mehdi Benacherine, M.; Debbache, N.; Ghoul, I.; Mameri, Y. Heterogeneous photoinduced degradation of amoxicillin by Goethite under artificial and natural irradiation. J. Photochem. Photobiol. A Chem. 2017, 335, 70–77. [Google Scholar] [CrossRef]
- Djeffal, L.; Abderrahmane, S.; Benzina, M.; Fourmentin, M.; Siffert, S.; Fourmentin, S. Efficient degradation of phenol using natural clay as heterogeneous Fenton-like catalyst. Environ. Sci. Pollut. Res. Int. 2013, 21, 3331–3338. [Google Scholar] [CrossRef] [PubMed]
- Bel Hadjltaief, H.; Zina, M.B.; Galvez, M.E.; da Costa, P. Photo-Fenton oxidation of phenol over a Cu-doped Fe-pillared clay. C. R. Chim. 2015, 18, 1161–1169. [Google Scholar] [CrossRef]
- Bel Hadjltaief, H.; da Costa, P.; Galvez, M.E.; Zina, M.B. Influence of operational parameters in the heterogeneous photo-fenton discoloration of wastewaters in the presence of an iron-pillared clay. Ind. Eng. Chem. Res. 2013, 52, 16656–16665. [Google Scholar] [CrossRef]
- Zou, X.; Chen, T.; Liu, H.; Zhang, P.; Chen, D.; Zhu, C. Catalytic cracking of toluene over hematite derived from thermally treated natural limonite. Fuel 2016, 177, 180–189. [Google Scholar] [CrossRef]
- Acisli, O.; Khataee, A.; Soltani, R.D.C.; Karaca, S. Ultrasound-assisted Fenton process using siderite nanoparticles prepared via planetary ball milling for removal of reactive yellow 81 in aqueous phase. Ultrason. Sonochem. 2017, 35, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Guo, H.; Zhou, X. Adsorption and heterogeneous oxidation of arsenite on modified granular natural siderite: Characterization and behaviors. Appl. Geochem. 2014, 48, 184–192. [Google Scholar] [CrossRef]
- Selmani, S.; Essaidi, N.; Gouny, F.; Bouaziz, S.; Joussein, E.; Driss, A.; Sdiri, A.; Rossignol, S. Physical-chemical characterization of Tunisian clays for the synthesis of geopolymers materials. J. Afr. Earth Sci. 2015, 103, 113–120. [Google Scholar] [CrossRef]
- Eloussaief, M.; Benzina, M. Efficiency of natural and acid-activated clays in the removal of Pb(II) from aqueous solutions. J. Hazard. Mater. 2010, 178, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, A.; Higashi, T.; Hatta, T.; Jamoussi, F.; Tase, N. Mineralogical and spectroscopic characterization, and potential environmental use of limestone from the Abiod formation, Tunisia. Environ. Earth Sci. 2010, 61, 1275–1287. [Google Scholar] [CrossRef]
- Sdiri, A.; Higashi, T.; Jamoussi, F.; Bouaziz, S.; Hatta, T.; Jamoussi, F.; Tase, N.; Bouaziz, S. Effects of impurities on the removal of heavy metals by natural limestones in aqueous systems. J. Environ. Manag. 2012, 93, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, F.; Varona, F.; Villota, N. Changes in solution color during phenol oxidation by fenton reagent. Environ. Sci. Technol. 2006, 40, 5538–5543. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Hao, J. Efficient degradation of organic dyes by titanium dioxide–silicotungstic acid nanocomposite films: Influence of inorganic salts and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 501–507. [Google Scholar] [CrossRef]
- Xu, H.Y.; Prasad, M.; Liu, Y. Schorl: A novel catalyst in mineral-catalyzed Fenton-like system for dyeing wastewater discoloration. J. Hazard. Mater. 2009, 165, 86–92. [Google Scholar]
- Bel Hadjltaief, H.; Zina, M.B.; da Costa, P.; Gálvez, M.E. Heterogeneous TiO2–Fe-plate catalyst for the discoloration and mineralization of aqueous solutions of cationic and anionic dyes. Desalination Water Treat. 2015, 57, 13505–13517. [Google Scholar] [CrossRef]


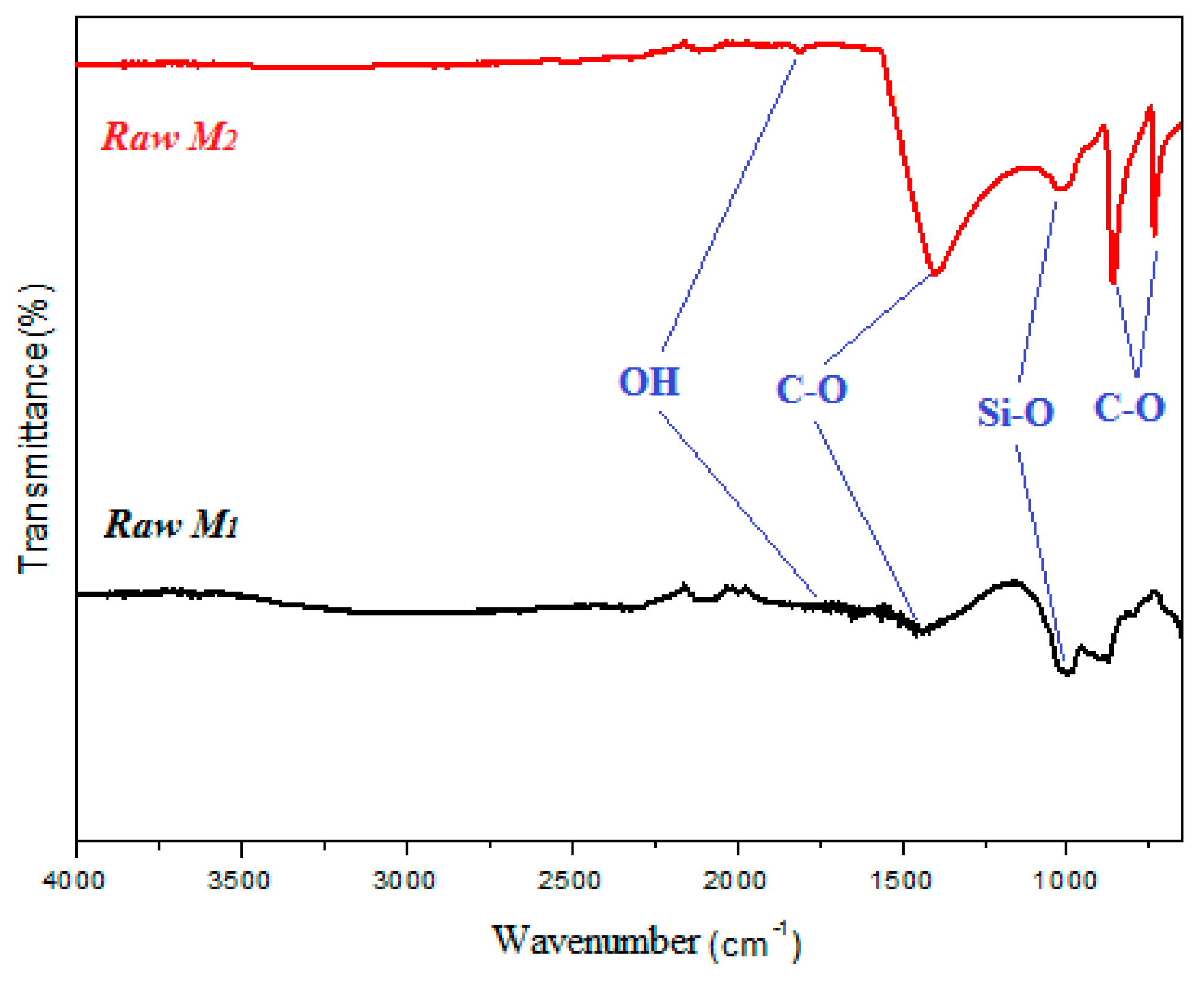

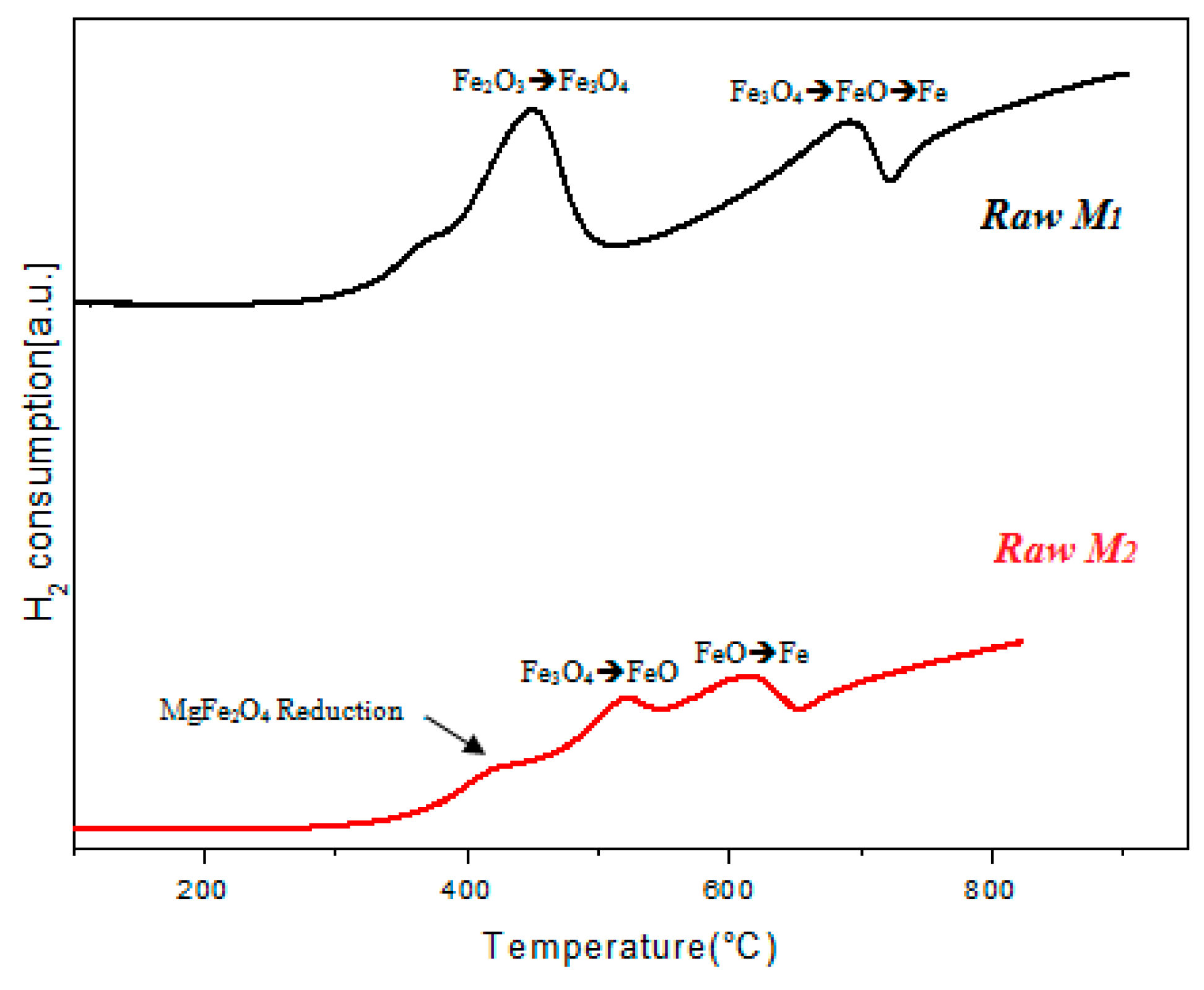
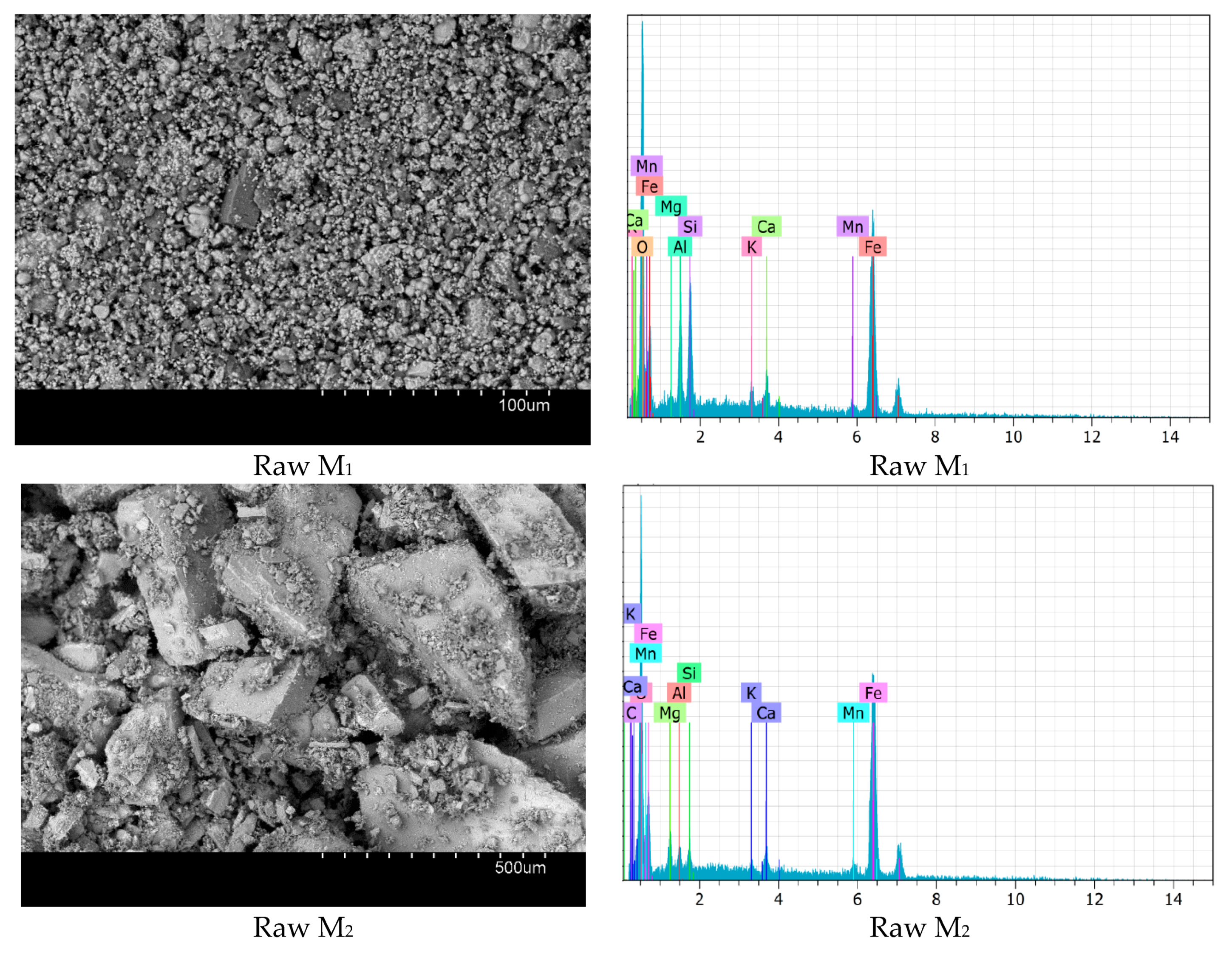
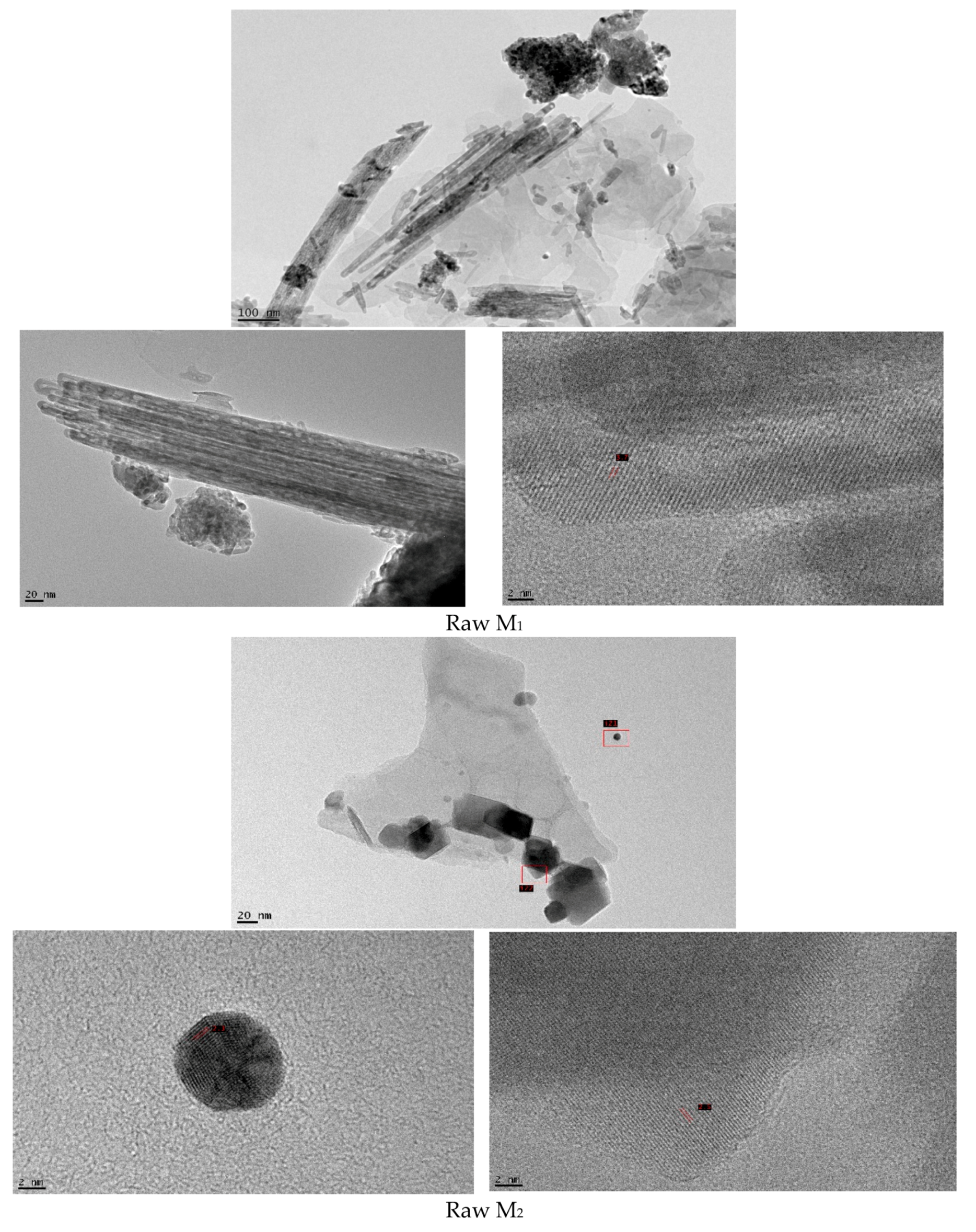

| Samples | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | K2O | PF |
|---|---|---|---|---|---|---|---|---|
| M1 | 9.45 | 2.91 | 46.89 | 5.38 | 4.35 | 0.26 | 0.13 | 31.54 |
| M2 | 7.63 | 0.94 | 57.6 | 2.93 | 2.2 | 0.22 | 0.14 | 28.53 |
| Samples | Surface Area (m2 g−1) | Mean Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| M1 | 8.00 | 16.1 | 0.03 |
| M2 | 37.5 | 8.00 | 0.07 |
| Iron concentration (ppm) | Samples | Time, min | 0 | 20 | 40 | 60 | 80 | 100 | 120 |
| M1 | 0 | 0.035 | 0.052 | 0.060 | 0.065 | 0.077 | 0.080 | 0.081 | |
| M2 | 0 | 0.028 | 0.042 | 0.049 | 0.056 | 0.058 | 0.060 | 0.079 |
| Samples | Run Number | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| M1 | 100 | 100 | 99.87 | 98.66 | 98.07 |
| M2 | 100 | 100 | 100 | 99.01 | 98.24 |
| Method | Reaction Time (min) | Initial Concentration (mg/L) | Degradation Efficiency (%) | Ref. |
|---|---|---|---|---|
| Biodegradation | 120 | 10 | 86 | [6] |
| Adsorption (Activated carbon) | 700 | 100 | 78.5 | [5] |
| Wet oxidation (Cu-Clays) | 480 | 100 | 69 | [4] |
| Photodegradation (Cu@AgCl) | 40 | 20 | 91 | [1] |
| Photo-Fenton | 180 | 100 | 80 | [7] |
| Photo-Fenton (PILC) | 120 | 100 | 94 | [8] |
| Photo-Fenton (M1/M2) | 120 | 20 | 100 | this work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bel Hadjltaief, H.; Sdiri, A.; Gálvez, M.E.; Zidi, H.; Da Costa, P.; Ben Zina, M. Natural Hematite and Siderite as Heterogeneous Catalysts for an Effective Degradation of 4-Chlorophenol via Photo-Fenton Process. ChemEngineering 2018, 2, 29. https://doi.org/10.3390/chemengineering2030029
Bel Hadjltaief H, Sdiri A, Gálvez ME, Zidi H, Da Costa P, Ben Zina M. Natural Hematite and Siderite as Heterogeneous Catalysts for an Effective Degradation of 4-Chlorophenol via Photo-Fenton Process. ChemEngineering. 2018; 2(3):29. https://doi.org/10.3390/chemengineering2030029
Chicago/Turabian StyleBel Hadjltaief, Haithem, Ali Sdiri, María Elena Gálvez, Haythem Zidi, Patrick Da Costa, and Mourad Ben Zina. 2018. "Natural Hematite and Siderite as Heterogeneous Catalysts for an Effective Degradation of 4-Chlorophenol via Photo-Fenton Process" ChemEngineering 2, no. 3: 29. https://doi.org/10.3390/chemengineering2030029
APA StyleBel Hadjltaief, H., Sdiri, A., Gálvez, M. E., Zidi, H., Da Costa, P., & Ben Zina, M. (2018). Natural Hematite and Siderite as Heterogeneous Catalysts for an Effective Degradation of 4-Chlorophenol via Photo-Fenton Process. ChemEngineering, 2(3), 29. https://doi.org/10.3390/chemengineering2030029






