Abstract
Neonicotinoid insecticides (NEOs), one of the most widely used pesticide classes worldwide, have raised concerns due to potential neurotoxic effects. Yet evidence on human exposure and health outcomes, particularly in preschool children, remains limited. In this study, 506 children aged 3–6 years from Shenzhen, China, were assessed. Neurobehavioral development was evaluated with the Strengths and Difficulties Questionnaire (SDQ), and urinary concentrations of 11 NEOs were measured, including imidacloprid (IMI), clothianidin (CLO), thiamethoxam (THM), dinotefuran (DNT), nitenpyram (NIT), sulfoxaflor (SFX), acetamiprid (ACE), thiacloprid (THD), flonicamid (FLO), 6-chloronicotinic acid (6-CINA), N-desmethyl-acetamiprid (NACE), and N-desmethyl-thiamethoxam (NTHM). Seven compounds showed high detection rates, including IMI (97.4%), CLO (100%), THM (100%), DNT (99.8%), NIT (99.8%), NACE (100%), and NTHM (99.8%). The mean urinary concentration was 234.145 μg/g creatinine, exceeding levels in earlier studies and indicating widespread exposure. IMI, NTHM, and NACE showed significant positive dose–response relationships with emotional symptoms, hyperactivity, and total difficulties and were major contributors in mixture models; sex-stratified analyses suggested effect modification for NTHM and NACE. These findings provide new epidemiological evidence to inform public health risk assessment and regulatory action on NEOs.
1. Introduction
Neurobehavioral development during early childhood lays the foundation for later cognitive functioning and social adaptation. This developmental process depends heavily on tightly regulated neurobiological events such as neuronal differentiation, migration, myelination, and synaptic plasticity [1,2]. The preschool period (ages 3–6) is a particularly sensitive window, characterized by rapid structural and functional maturation of the central nervous system [3]. While neurobehavioral disorders have traditionally been attributed to genetic, psychosocial, and nutritional factors, growing evidence indicates that environmental pollutants represent an important and often underrecognized contributor, particularly when exposure occurs during early life [4,5]. During this stage, the brain is highly susceptible to external insults, and adverse environmental exposures may result in long-term or even irreversible disruptions to neurodevelopment [6]. Such exposures may disrupt neurodevelopment through mechanisms including interference with neurotransmitter systems, induction of oxidative stress, and modulation of synaptic function [7,8].
At present, several academic studies have reported associations between environmental factors such as heavy metals, organic pollutants, and air pollution with neurobehavioral abnormalities in children [9,10]. However, neonicotinoid insecticides (NEOs), as a class of emerging environmental contaminants, although their widespread presence in the environment has been well documented and animal as well as in vitro studies have demonstrated various adverse health effects including neurotoxicity, their neurotoxic impacts on human populations remain insufficiently understood and require further systematic investigation [11,12]. NEOs are synthetic insecticides structurally derived from nicotine and have been widely applied in agriculture, horticulture, and animal husbandry since the 1990s [13]. Due to their high water solubility, strong systemic activity, and environmental persistence, NEOs are widely present in surface water, groundwater, soil, agricultural products, and even the atmosphere, thereby posing potential exposure risks to humans [14,15].
Existing studies have shown that at least one neonicotinoid compound was detected in over 90% of surface water samples collected from major water bodies in China [16,17]. Moreover, neonicotinoids can be bioaccumulated in aquatic vertebrates (such as fish and amphibians) as well as invertebrates (including mayflies and water fleas), suggesting their potential transmission through the food chain and ecological threat [18]. More importantly, urine biomonitoring in some regions has shown detection rates of neonicotinoids and their metabolites approaching 100%. This indicates long-term, low-level exposure in the general population of China, with particularly elevated risks in agriculturally intensive and downstream urban areas [19]. Previous studies have demonstrated that NEOs can activate mammalian nicotinic acetylcholine receptors (nAChRs), leading to abnormal neuronal excitability and impaired synaptic formation, potentially leading to deficits in learning, memory, and behavioral regulation in animal models [20,21]. Regarding the neurotoxic mechanism of NEOs, some studies reported that exposure to NEOs may induce oxidative stress, mitochondrial dysfunction, and apoptosis, all of which are key mechanisms implicated in neurodevelopmental impairment [22,23]. Although an increasing body of evidence suggests that NEOs may exert neurotoxic effects on non-target organisms, including mammals, most of this evidence comes from laboratory studies using cell cultures or animal models. Furthermore, the NEO concentrations applied in such experiments are often several orders of magnitude higher than those encountered under real-world environmental exposure conditions [24]. Therefore, the impact of NEO exposure on human neurobehavioral development, particularly in children, requires further investigation under environmentally relevant conditions through population-based epidemiological study.
The Pearl River Delta (PRD) region in southern China provides a representative setting for such research. Due to highly intensive forestry and protected agriculture in the upper and middle reaches of the Pearl River, coupled with substantial pesticide application, large quantities of NEOs are introduced into water bodies through atmospheric deposition and surface runoff, resulting in downstream migration of contamination to urban areas [25]. Consequently, residents of PRD cities with high water utilization—such as Guangzhou, Shenzhen, and Foshan—are at increased risk of NEO pollution. Environmental monitoring has also revealed that residual NEO concentrations in the Pearl River rank among the highest in China, corroborating that urban residents in this region—particularly children and other vulnerable populations—are at considerable exposure risk [26]. These regional characteristics provide an ideal context for assessing the health impacts of chronic, low-level, mixed NEO exposure under real-world environmental conditions.
Previous research indicates that urinary neonicotinoids and their metabolites can serve as biomarkers of total exposure from multiple sources based on human metabolic characteristics [27]. Therefore, this study aimed to assess the associations between real-life mixed exposures to NEOs and their urinary metabolites and neurobehavioral development in preschool children living in Shenzhen in the Pearl River Delta of China. Our findings are expected to provide epidemiological evidence and scientific insights to support the identification of environmental health risks during critical windows of childhood neurodevelopment and inform future policy interventions at the national and global levels.
2. Materials and Methods
2.1. Research Design and Study Participants
This cross-sectional study was conducted in Shenzhen, Guangdong Province, from June to September 2024, using a random cluster sampling approach to recruit participants. Shenzhen consists of ten administrative districts. In this study, one district was randomly selected from the list of all districts using a random number generator, and Bao’an District was determined as the study site. The expected sample size was proportionally allocated across subdistricts within Bao’an District according to the number of preschool-aged children. Finally, one to two kindergartens were randomly selected from each subdistrict using a random number table, and children attending these kindergartens were invited to participate in the study. The inclusion criteria were (1) residence within the study area and (2) no diagnosis of major diseases (e.g., severe cardiovascular conditions or psychiatric disorders). Exclusion criteria included (1) residence outside the study area or recent migration into the area within the past year; (2) presence of major illnesses as described above, including diagnosed neurodevelopmental or psychiatric disorders such as Autism Spectrum Disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), or other clinically recognized behavioral abnormalities; and (3) failure to complete all study questionnaires. A total of 743 questionnaires were collected, and 512 participants also provided urine samples. After data validation, 6 participants were excluded due to missing key questionnaire information. The final sample included 506 preschool-aged children who met both the inclusion and exclusion criteria and were included in subsequent analyses. The study design and participant selection process are illustrated in Figure 1.
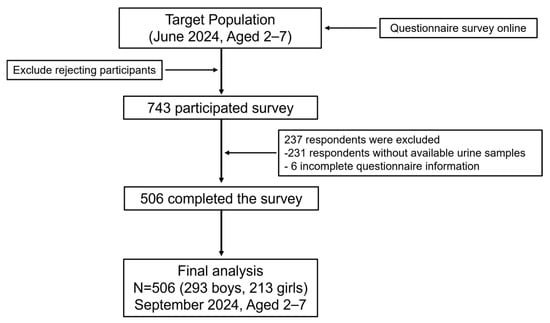
Figure 1.
Flowchart of population included in final analysis (n = 506).
All participants (with written consent provided by legal guardians for those under 18 years of age) gave informed consent prior to participation. The study protocol was approved by the Ethics Committee of the School of Public Health, Sun Yat-sen University (Approval No. IRB-SYSU-2024-104).
2.2. Analysis of Neonicotinoids in Urine
2.2.1. Sample Preparation
According to the preprocessing and computer application methods reported in relevant literature, the urine samples were processed according to the following operation steps after the optimization of experimental conditions [28,29,30]. One night in advance, transfer the urine sample from the −80 refrigerator to the 4 °C refrigerator for dissolution, vortex for 20 s, transfer 1 mL of urine to a 15 mL glass tube, add 0.3 mL of ammonium acetate buffer solution (prepared as 7.7 g ammonium acetate, 6 mL glacial acetic acid, 100 μL β—glucuronidase, and adding water for the volume to reach 100 mL), add 20 μL of 100 ppb internal standard mixture, and incubate at 37 °C for 6 h. Add 0.5 mL of acetonitrile, vortex for 10 s and mix well. Add 7 mL ethyl acetate, sonicate for 30 min, shake for 30 min, centrifuge at 3400× g for 5 min, and take the supernatant (repeat twice). Add 10 mL n-hexane, sonicate for 30 min, shake for 30 min, and centrifuge at 3400× g for 5 min. Combine the supernatant, blow dry with nitrogen, add 1 mL n-hexane, shake for 5 min, blow dry with nitrogen, then redissolve with 200 μL methanol water, filter through a 0.22 μm organic filter membrane, and finally transfer to the injection vial for sample injection. A pure water blank with pretreatment steps completely consistent with the above operations is set for every 20 samples.
2.2.2. HPLC-MS/MS Analysis and Ion Source Optimization
HPLC-MS/MS was a Xevo TQ-XS (Milford, MA, USA) from Waters. The chromatographic column was a BEH C18 column (2.1 mm × 100 mm, particle size 5 μm) from Waters. After multiple experiments, a mobile phase composed of 0.1% Fa + water (A) and methanol (B) was selected as optimum. The initial mobile phase ratio was 95% A, then changed to 100% B within 4 min, and then continued for 5 min, then changed to 95% A within 1.1 min and maintained for 3.9 min. Mass spectrometry analysis was performed on a mass spectrometry detector analyzer equipped with an ESI source. The ion source temperature was set at 400 °C. The data were processed and exported using Waters software masslynx4.2. See Table A1 for specific mass spectrometric parameter information of each substance.
2.2.3. Urinary Creatinine Assay
Urinary creatinine concentration was determined using a creatinine assay kit (Nanjing Jincheng Bioengineering Inst., Nanjing, China) based on the sarcosine oxidase method. Creatinine is converted to creatine by creatinine; creatine is then converted to sarcosine and urea by creatines; sarcosine is subsequently oxidized by sarcosine oxidase to generate hydrogen peroxide, which reacts with a chromogenic reagent to form a colored product. Absorbance was measured at 546 nm. For the analysis, urine samples were diluted 1:10 with deionized water, and creatinine concentrations were calculated using the formula provided in the kit instructions.
2.3. Assessment of Neurobehavioral Problems in Preschool Children
Neurobehavioral problems in preschool-aged children were assessed using the Strengths and Difficulties Questionnaire (SDQ). The SDQ is a parent-reported instrument comprising 25 items divided into five subscales: emotional symptoms, conduct problems, hyperactivity, peer relationship problems, and prosocial behavior. Each subscale contains five items, and responses are scored on a three-point Likert scale (0 = not true, 1 = somewhat true, 2 = certainly true), yielding subscale scores ranging from 0 to 10. Higher scores on the first four subscales indicate more severe symptoms, while higher scores on the prosocial behavior subscale indicate more positive behavior. In addition to subscale scores, a total difficulties score was calculated by summing the scores of the four problem-oriented subscales (emotional symptoms, conduct problems, hyperactivity/inattention, and peer problems), providing a composite index of children’s overall behavioral and emotional difficulties [31]. Based on established cutoffs in the previous literature, threshold values of 5, 4, 7, 4, and 17 were used for emotional symptoms, conduct problems, hyperactivity, peer problems, and total difficulties, respectively [32,33]. Due to its brevity (25 items), the SDQ is generally well accepted by parents and is suitable for use in large-scale epidemiological studies. The validity and reliability of the SDQ have been well established in diverse populations [34]. In the present study, the Cronbach’s alpha coefficients indicated acceptable internal consistency (α = 0.769), supporting the instrument’s reliability for assessing neurobehavioral outcomes in this population.
2.4. Statistical Analysis
Continuous variables were summarized using means and standard deviations or medians and interquartile ranges, as appropriate. Categorical variables were described using counts and percentages. To account for differences in the toxicological potency of individual NEOs on human health and to facilitate comparability across studies, we applied the Relative Potency Factor (RPF) approach to normalize each NEO to an imidacloprid equivalent (IMIeq), denoted as IMIRPF. The reference doses (RfDs) used for this calculation were derived from the 2021 Human Health Benchmarks for Pesticides, published by the U.S. Environmental Protection Agency (USEPA) (available at: https://www.epa.gov/sdwa/2021-human-health-benchmarks-pesticides (accessed on 15 January 2025) 2021 Human Health Benchmarks for Pesticides—US EPA), which reflect the relative human health toxicity of each compound (Table A2) [35,36]. For neonicotinoids without available EPA cRfD data, RPFs were estimated based on structurally similar compounds following previously reported approaches [37]. RPFs were calculated using the following formula: RPFi = RfDIMI/RfDi, where i denotes an individual NEO. Accordingly, the imidacloprid-equivalent concentration (IMIeq) for each NEO was calculated by multiplying its measured concentration by the corresponding RPFi. The final formula for IMIeq (ng/mL) was as follows:
IMIeq (ng/mL) (NEOi×RPFi) = IMI + 0.82 × CLO + 6.67 × THM + 0.08 × DNT + 0.11 × NIT + 1.6 × SFX + 1.13 × NACE + 6.67 × THM + 1.13 × ACE + 20 × THD.
Concentrations below the limit of quantification (LOQ) were substituted with LOQ divided by the square root of 2 (LOQ/√2) for statistical analysis. All subsequent statistical analyses were conducted using creatinine-adjusted urinary concentrations (μg/g Cr) to account for urine dilution. Prior to statistical analysis, the concentrations of neonicotinoid insecticides were log-transformed. A Directed Acyclic Graph (DAG) was constructed to select covariates (Figure A1), based on existing literature and expert knowledge [38,39]. The selected covariates included sex (boy, girl), child’s age (continuous, years), educational level of the primary caregiver (primary school or below, junior high school, senior high school, college or above), and monthly household income (<2500 CNY, 2500–7500 CNY, 7500–15,000 CNY, >15,000 CNY). Parenting style of the primary caregiver and sleep conditions of the child were also included as confounding factors due to their influences on neurobehavioral development [40,41].
Multiple linear regression was first used to examine the association between neonicotinoid insecticides and neurobehavioral development. Based on the outcome dimensions that showed potential associations in the regression results, binary logistic regression was further conducted to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for abnormal versus normal neurobehavioral outcomes. Given evidence from previous literature suggesting that neonicotinoid insecticides may have sex-specific effects, stratified analyses by sex were also performed.
The Weighted Quantile Sum (WQS) regression model was used to assess the effect of mixed exposure to neonicotinoid compounds. The WQS model constructs a weighted index by combining the concentrations of multiple chemicals and assigning weights based on the strength of their associations with the health outcome, thereby estimating the overall effect of the mixture. Specifically, WQS regression transforms the concentrations of each neonicotinoid into quartiles to build the index and assigns weights according to their associations with neurobehavioral development to evaluate the joint exposure effects [42].
The Bayesian Kernel Machine Regression (BKMR) model was further applied to assess the effects of mixed exposure to neonicotinoid compounds. BKMR is a flexible Bayesian method capable of accounting for nonlinear and interactive effects among exposure variables. Unlike WQS regression, BKMR estimates the overall effect of multiple exposures on health outcomes by simulating changes in the mixture and modeling the exposure–response relationship through 10,000 iterations. The model provides posterior inclusion probabilities (PIPs) for each compound, with a PIP > 0.50 indicating a significant contribution to the outcome. This approach allows for the identification of key contributors within the exposure mixture and the evaluation of both independent and interactive effects [43].
3. Results
3.1. SDQ Scores by Demographic Characteristics
This study included 506 preschool children, aged from 2 to 7 years. Of these, 293 were boys (57.9%) and 213 were girls (42.1%). In terms of household characteristics, the largest proportion of families (33.6%) reported a monthly income exceeding 15,000 CNY, and most primary caregivers had completed high school education (66.2%). The mean scores for the SDQ subscales were as follows: emotional symptoms, 1.74 (M = 1.78); conduct problems, 1.86 (M = 1.28); hyperactivity, 3.70 (M = 2.19); peer problems, 2.33 (M = 1.54); and total difficulties, 9.63 (M = 4.82). Participant characteristics are presented in Table 1.

Table 1.
Sociodemographic, psychological, and behavioral characteristics of participants.
3.2. Urinary Concentrations of Neonicotinoids
Urinary concentrations and detection rates of 11 neonicotinoid compounds among the 506 preschool children are presented in Table 2. High detection rates were noted for imidacloprid (IMI, 97.4%), clothianidin (CLO, 100%), thiamethoxam (THM, 100%), dinotefuran (DNT, 99.8%), nitenpyram (NIT, 99.8%), nitenpyram-N-desmethyl (NACE, 100%), and thiacloprid-N-desmethyl (NTHM, 99.8%). The mean concentrations of these compounds were as follows: 0.452 μg/g Cr (IMI), 14.165 μg/g Cr (CLO), 10.471 μg/g Cr (THM), 23.925 μg/g Cr (DNT), 7.767 μg/g Cr (NIT), 5.456 μg/g Cr (NACE), and 21.328 μg/g Cr (NTHM). The detection rates for flonicamid (FLO, 82.2%), sulfoxaflor (SFX, 69.4%), and thiacloprid (THD, 67.6%) were comparatively lower, with mean concentrations of 0.069 μg/g Cr, 0.382 μg/g Cr, and 0.018 μg/g Cr, respectively. However, 6-chloronicotinic acid (6-CINA) showed a low detection rate (6.10%), leading to the exclusion of its concentration data from subsequent analyses to maintain analytical validity and reliability. The overall mean concentration of neonicotinoid compounds in the study population was 234.145 μg/g Cr.

Table 2.
Concentrations of neonicotinoids in the urine of preschool children (n = 506).
3.3. Association Between Neonicotinoid Concentrations and Neurobehavioral Problems, and the Effect Modification by Gender
3.3.1. Generalized Linear Regression Model
In this study, we first used multiple linear regression models to evaluate the associations between urinary concentrations of NEOs and neurobehavioral issues in preschool children, including emotional symptoms, conduct problems, hyperactivity, peer relationship problems, and total difficulties. The regression results are illustrated in Figure 2.
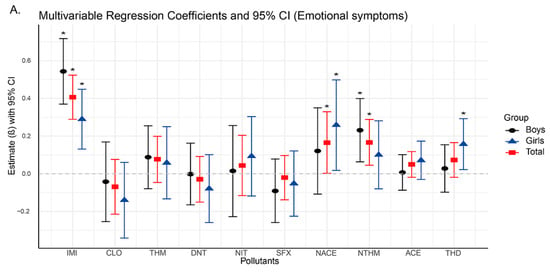
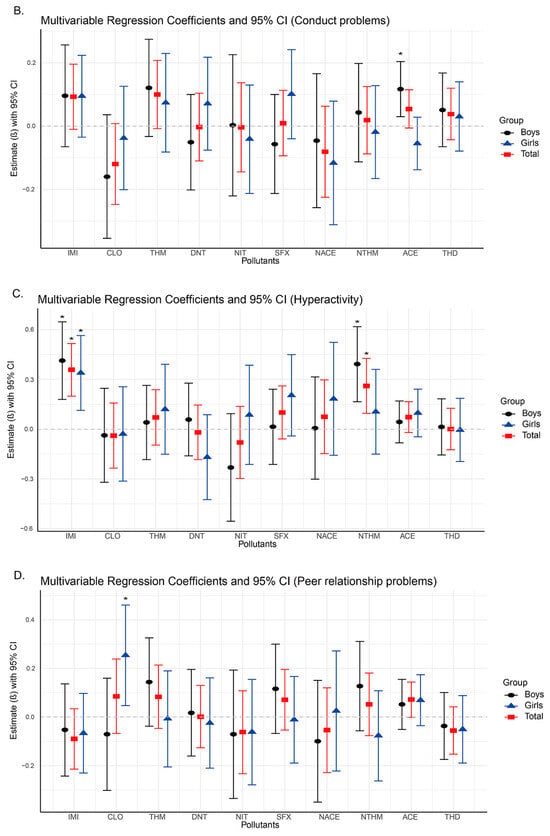
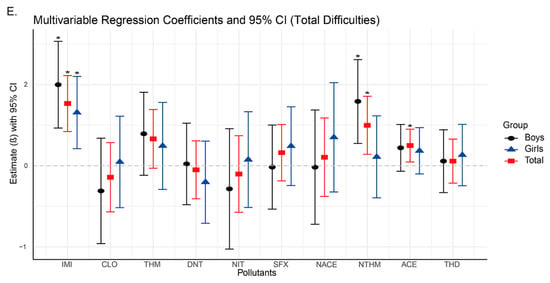
Figure 2.
Changes in neurobehavioral symptoms associated with neonicotinoid use based on linear regression models (95% confidence intervals). Asterisks indicate significant differences with p < 0.05.
In our study, the analysis results showed that significant positive associations were found between emotional symptoms and the urinary concentrations of IMI (β = 0.406, 95% CI: 0.289, 0.523), NACE (β = 0.165, 95% CI: 0.002, 0.329), and NTHM (β = 0.406, 95% CI: 0.045, 0.288) in the total population (Figure 2A,C,E), indicating that higher exposure levels were associated with a greater likelihood of emotional problems. In terms of hyperactivity, significant positive associations were observed for both IMI (β = 0.358, 95% CI: 0.199, 0.516) and NTHM (β = 0.261, 95% CI: 0.096, 0.426). Regarding total difficulties, which is a composite measure derived from the four problem subscales, significant associations were identified for IMI (β = 0.767, 95% CI: 0.422, 1.111), NTHM (β = 0.498, 95% CI: 0.141, 0.856), and ACE (β = 0.249, 95% CI: 0.047, 0.451). No significant associations were observed for conduct problems or peer relationship problems in the total sample (Figure 2B,D).
Sex-stratified analyses revealed differences in the associations of NACE and NTHM with neurobehavioral outcomes. For emotional symptoms (Figure 2A), NACE was significantly associated with the total population and girls (β = 0.258, 95% CI: 0.018, 0.498), but not with boys. In contrast, NTHM showed significant positive associations with the total population and boys (β = 0.231, 95% CI: 0.063, 0.399), but not with girls. Similarly, for hyperactivity and total difficulties, NTHM was significantly associated with both the total population and boys (hyperactivity: β = 0.392, 95% CI: 0.166, 0.618; total difficulties: β = 0.793, 95% CI: 0.276, 1.309), with no significant associations found in girls.
To further explore the impact of neonicotinoid exposure on neurobehavioral problems, we constructed binary logistic regression models for outcome dimensions that demonstrated potential associations in the multiple linear regression: emotional symptoms, conduct problems, and total difficulties. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) to estimate the association between each neonicotinoid compound and neurobehavioral abnormalities (Table 3, Table 4 and Table 5). The results indicated that IMI exhibited significant positive associations across all three dimensions in the total population: emotional symptoms (OR = 2.194, 95% CI: 1.490, 3.229), hyperactivity (OR = 1.692, 95% CI: 1.197, 2.392), and total difficulties (OR = 1.616, 95% CI: 1.124, 2.323). This result suggests that increased IMI exposure may elevate the risk of neurobehavioral problems. In sex-stratified analyses, significant positive associations were observed in both boys and girls for emotional symptoms and hyperactivity. For boys, the odds ratios were as follows: emotional symptoms (OR = 2.743, 95% CI: 1.532, 4.912); hyperactivity (OR = 1.834, 95% CI: 1.139, 2.954). For girls, the odds ratios were as follows: emotional symptoms (OR = 2.727, 95% CI: 1.200, 6.200); hyperactivity (OR = 1.961, 95% CI: 1.070, 3.593). However, a significant positive association for total difficulties was observed only among girls (OR = 2.223, 95% CI: 1.101, 4.488). Furthermore, after sex stratification, the results for NACE and NTHM revealed distinct sex-specific differences. Among girls, NACE exhibited significant positive associations across all three dimensions: emotional symptoms (OR = 5.692, 95% CI: 1.910, 16.958), hyperactivity (OR = 2.567, 95% CI: 1.189, 5.542), and total difficulties (OR = 3.512, 95% CI: 1.402, 8.796). No significant associations were observed for NACE in boys. In contrast, NTHM was significantly associated with hyperactivity (OR = 1.513, 95% CI: 1.042, 2.196) and total difficulties (OR = 1.605, 95% CI: 1.078, 2.390) exclusively in boys, with no corresponding associations identified in girls. These findings further support the potential sex-specific heterogeneity in the neurobehavioral effects of NACE and NTHM exposure.

Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) of urinary NEO concentrations and emotional problem dimensions in preschool children.

Table 4.
Odds ratios (ORs) and 95% confidence intervals (CIs) of urinary NEO concentrations and hyperactivity dimensions in preschool children.

Table 5.
Odds ratios (ORs) and 95% confidence intervals (CIs) of urinary NEO concentrations and total difficulties dimensions in preschool children.
3.3.2. WQS Regression Model
To further investigate the combined effects of neonicotinoid compounds on neurobehavioral problems in preschool children, Weighted Quantile Sum (WQS) regression models were applied to assess their joint impact. Based on the outcome dimensions that demonstrated significant associations in prior regression analyses, WQS models were constructed for emotional symptoms, hyperactivity, and total difficulties, followed by sex-stratified analyses. After adjusting for all covariates in the total population, the WQS index showed statistical significance for emotional symptoms (p < 0.001), hyperactivity (p < 0.001), and total difficulties (p < 0.001) (Table A3). In the emotional symptoms model, the primary contributors with weights exceeding 0.1 were IMI (0.464), NTHM (0.160), and NACE (0.123) (Figure 3A, Table A4). In the hyperactivity model, the main contributors included IMI (0.349), NACE (0.220), and NTHM (0.136) (Figure 3D, Table A5). In the total difficulties model, the primary contributors included IMI (0.416), ACE (0.136), NTHM (0.124), and NACE (0.118) (Figure 3G, Table A6).
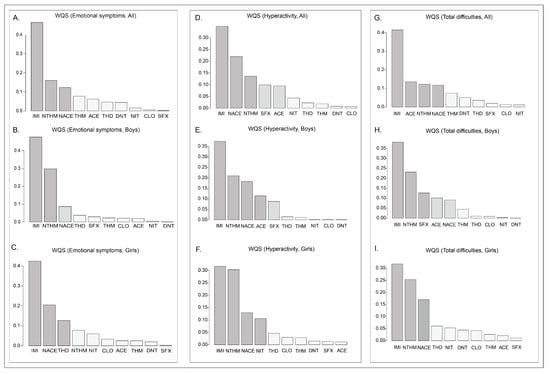
Figure 3.
WQS regression weights between the concentrations of neonicotinoid substances in the urine of preschool children and various dimensions of neurobehavior.
In addition, we conducted sex-stratified WQS regression models. The WQS indices displayed statistically significant positive effects in both boys and girls (p < 0.001). For boys, the primary contributors (weights > 0.1) in the emotional symptoms model were IMI (weight = 0.478) and NTHM (0.298); the main contributors in the hyperactivity model included IMI (0.374), NTHM (0.210), NACE (0.183), and ACE (0.114) (Figure 3B,E, Table A5). The major contributors in the model of total difficulties were IMI (0.382), NTHM (0.232), SFX (0.127), and ACE (0.102) (Figure 3H, Table A6). For girls, the key contributors to emotional symptoms were IMI (0.424), NACE (0.205), and THD (0.127) (Figure 3C, Table A4); the major contributors in the hyperactivity model included IMI (0.316), NTHM (0.303), NACE (0.130), and NIT (0.106) (Figure 3F, Table A5); and the primary contributors in the model of total difficulties were IMI (0.318), NTHM (0.253), and NACE (0.170) (Figure 3I, Table A6).
In summary, the results of the WQS model demonstrated significant combined effects of NEOs exposure on emotional symptoms, hyperactivity, and total difficulties, mainly driven by IMI, NTHM, and NACE.
3.3.3. BKMR Model
Due to the limitations of the Weighted Quantile Sum (WQS) regression model in accounting for nonlinear relationships among exposure variables, we further applied Bayesian Kernel Machine Regression (BKMR) to assess the nonlinear joint effects of exposure to ten NEOs on neurobehavioral issues in preschool children. The BKMR model concentrated on three SDQ dimensions (emotional symptoms, hyperactivity, and total difficulties), which were positively correlated with the exposure to NEOs in the aforementioned models. To investigate potential effect modification by gender, sex-stratified analyses were conducted. After adjusting for all covariates, the BKMR model estimated the overall mixture effect of the ten neonicotinoid compounds (Figure 4).
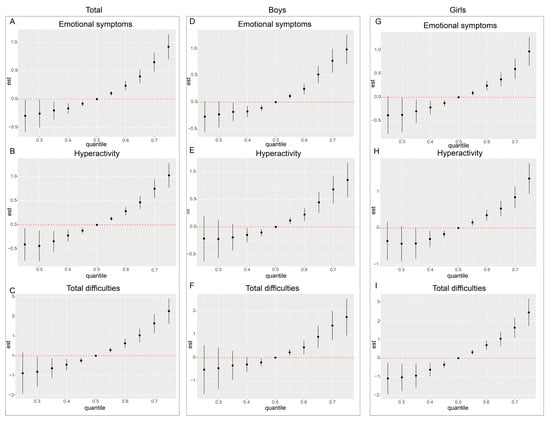
Figure 4.
Cumulative effects (95% CI) of the neonicotinoid mixture on the emotional problems dimension (A–C), the hyperactivity problems dimension (D–F), and the total difficulties dimension (G–I) for the whole population and for boys versus girls when all specific percentiles of the analytes were compared to all 50th percentiles of the analytes.
The results indicated that when the concentrations of all compounds exceeded the median exposure level (50th percentile), the estimated values for emotional symptoms, hyperactivity, and total difficulties exhibited a monotonic increasing trend. This trend was statistically significant at multiple percentile points, suggesting that exposure to NEOs may have a cumulative adverse effect on neurobehavioral outcomes. Additionally, sex-stratified analyses revealed that the positive joint effects of NEO mixture exposure were evident in both boys and girls, with no significant sex-specific differences observed. These findings indicate a consistent association between NEO exposure and neurobehavioral outcomes across sexes. In all three outcome dimensions and across sex-stratified subgroups, the estimated values of neurobehavioral problems were lower when all compound concentrations were below the 50th percentile. Notably, within the 40th to 50th percentile range, several subgroups displayed statistically significant differences compared to the 50th percentile.
In subsequent analyses, the Posterior Inclusion Probability (PIP) was used to evaluate the relative importance of each exposure variable in the model (Figure 5). IMI, NTHM, and NACE exhibited higher PIP values in the emotional symptoms and total difficulties models. Conversely, compounds such as THD and ACE demonstrated relatively lower PIP values across multiple dimensions.
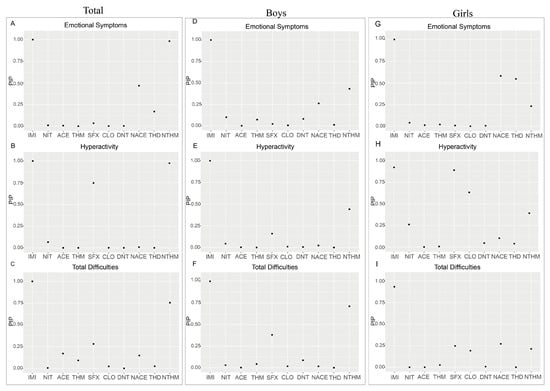
Figure 5.
PIP analysis of different neonicotinoids on emotional symptoms (A–C), hyperactivity (D–F), and total difficulties (G–I) dimensions among the total preschool population, boys, and girls using the BKMR model.
4. Discussion
This study is an epidemiological investigation to systematically assess urinary concentrations of NEOs and their associations with neurobehavioral development in a preschool-age population. Among the 11 neonicotinoid substances and their metabolites measured, five neonicotinoid prototypes, including IMI, CLO, THM, DNT, and FLO, and two of their metabolites (NACE and NTHM) were detected in over 90% of samples. The mean total urinary concentration of NEOs reached 234.145 μg/g Cr, which exceeds the exposure levels reported in previous studies, suggesting that preschool children in China may be facing substantial exposure risks to neonicotinoids [44]. The detection rates observed in this study are consistent with the results reported from recent population studies in China, in which most of the neonicotinoids tested showed high detection frequencies [26]. However, in earlier population studies, the reported detection rates were generally lower [45,46]. In addition, the overall human exposure to neonicotinoids and the detection rates of individual compounds reported in the literature have shown an upward trend over time. For instance, a 2020 epidemiological survey among children in Shanghai reported detection rates ranging from 0.6% to 62.6% [46], whereas a 2016 survey conducted in Japan reported detection rates of ≤57.8%. This upward trend in detection metrics suggests that preschool children and the general population may now be facing higher exposure risks [45].
Notably, this study observed considerable inter-individual differences in urinary neonicotinoid concentrations. Such variations may reflect disparities in the recent living environments of the preschool participants, including the use of household insecticides and the presence of pesticide residues in fruits, vegetables, and drinking water. Only a very small proportion of participants exhibited extremely high concentrations, which are likely to represent short-term, episodic exposure events rather than chronic accumulation. Nevertheless, these abnormally elevated levels merit future attention, as such episodic exposures may pose potential health risks to sensitive populations, particularly preschool children.
Subsequent regression analyses revealed significant positive associations between urinary concentrations of IMI, NTHM, and NACE and neurobehavioral problems in preschoolers, particularly in the domains of emotional symptoms, hyperactivity, and total difficulties. As the concentrations of IMI, NTHM, and NACE increased, the risk of neurobehavioral abnormalities also appeared to rise. These compounds may exert adverse neurodevelopmental effects by activating nicotinic acetylcholine receptors (nAChRs) in the nervous system [21]. Early studies suggested that the neurotoxicity of neonicotinoids was low because mammals, especially humans, have relatively few nAChRs in the brain with low binding affinity [47]. However, more recent animal and mechanistic studies have challenged this view [24]. Several commonly used insecticides, including neonicotinoids, have demonstrated neurotoxic effects on the developing nervous system to varying degrees. IMI has been shown in brainstem slices from neonatal mice to induce depolarizing inward currents, increase neuronal excitability, and provide direct physiological evidence of neurotoxicity [21]. In a mouse study, low-dose exposure to neonicotinoids such as sulfoxaflor impaired performance in elevated plus maze tests and motor coordination in juvenile mice [48]. Mechanistic data suggested that nAChRs and other mediators of neurodevelopment play crucial roles during fetal and early postnatal brain development, with particularly high expression during infancy [49]. Exposure to certain neonicotinoids during this developmental window may disrupt nAChR expression and regulation, interfere with neuronal migration and synaptogenesis, and affect other developmental processes, potentially yielding adverse neurobehavioral outcomes [49]. Collectively, the above evidence indicates that neonicotinoid insecticides or their metabolites can act on the nervous system of mammals, including humans, altering neuronal signaling and inducing toxic or oxidative stress–related effects in developing neurons. Therefore, the neurotoxic potential of neonicotinoids in mammals may have been underestimated, and continued scientific and regulatory attention to their safety is warranted.
The findings of this study suggest that among the detected neonicotinoid insecticides, IMI, NTHM, and NACE are the most influential compounds associated with neurobehavioral outcomes in preschoolers. Notably, IMI showed consistent and statistically significant positive associations across all strata (overall population, boys, and girls). Together with the WQS regression results, IMI emerged as a key contributor within the mixture. To date, no epidemiological studies have directly examined IMI exposure and neurobehavioral outcomes in humans; however, experimental evidence suggests that IMI’s potent effects may arise from interference with neurotransmitter systems and the induction of oxidative stress [50]. For instance, in a prior study using cultured cerebellar neurons from neonatal rats, IMI significantly enhanced the activity of nAChR subunits α3, α4, and α7, an effect not observed with other neonicotinoids [21]. In a mouse low-dose IMI exposure reduced SOX2-positive neural stem cells and GFAP-positive astrocytes, indicating suppressed neurogenesis and glial development in the dentate gyrus (DG) [51]. Behavioral impairments were observed in the elevated plus maze (EPM) and fear conditioning (FC) tests, suggesting anxiety-like behavior and deficits in learning and memory [48]. Collectively, these mechanistic studies suggest that IMI may exert a more pronounced neurotoxic impact than other neonicotinoids and threaten neurobehavioral development in preschool-aged children during early life. Therefore, given the consistently observed adverse effects of IMI across different subgroups, targeted mitigation strategies addressing IMI exposure may reduce health risks from neonicotinoids in young children and the general population.
In this study, both NTHM (a metabolite of THM) and NACE (a metabolite of ACE) showed significant positive associations with neurobehavioral outcomes, whereas the parent compounds (THM and ACE) showed no such associations. Prior toxicokinetic studies have shown that demethylated metabolites such as NTHM and NACE are more hydrophilic and possess slower metabolic clearance and longer biological half-lives, resulting in more persistent internal exposure and potentially stronger biological effects than their parent compounds [52]. For example, a 2022 study reported that NACE could be consistently detected in the cerebrospinal fluid (CSF) of children, whereas its parent compound was less frequently detected, and that CSF NACE concentrations correlated linearly with those in blood and urine. These findings suggest that these NEO metabolites can remain in the human body for a longer time and penetrate the central nervous system [53]. Mechanistic studies further revealed that demethylated THM has 28–3600 times higher receptor affinity than THM, and THM itself resembles a pro-pesticide, with its neuroactivity primarily mediated by its transformation products [52].
In addition, NTHM and NACE show notable sex-specific differences in stratified analyses. Among boys, NTHM is positively associated with hyperactivity and total difficulty scores, whereas no such associations are observed among girls. These findings may partly reflect sex-related differences in the metabolism of neonicotinoids [54,55]. However, the mechanisms underlying the observed sex differences in NACE and NTHM effects remain poorly understood and require further investigation to elucidate how these compounds may differentially influence neurobehavioral development in boys and girls through distinct physiological pathways.
Subsequent BKMR analysis shows that urinary neonicotinoid concentrations are positively associated with neurobehavioral problem scores across all population subgroups, with prominent effects on emotional symptoms, hyperactivity, and overall difficulties. Notably, effect estimates rose with exposure and remained statistically significant across all percentile levels. These results suggest that even low levels of neonicotinoid exposure may have adverse effects on the neurobehavioral development of preschool children. This finding aligns with prior in vivo and in vitro studies showing that low-dose neonicotinoid exposure can induce nicotine-like neurotoxicity [56]. Additional experimental work indicates that neonicotinoids, even at environmentally relevant concentrations, can activate nAChRs, induce oxidative stress, and damage synaptic structure, thereby disrupting neuroplasticity and regulatory function in the developing nervous system [57]. Taken together, IMI, NTHM, and NACE showed significant positive associations in the regression models, whereas the contributions of other neonicotinoids were relatively limited. This suggests that particular attention should be paid to the potential health risks of IMI, THM (the parent compound of NTHM), and ACE (the parent compound of NACE). From a public health perspective, reducing the use of these compounds and exploring safer alternatives may help mitigate their potential adverse effects on neurobehavioral development in preschool children.
Nevertheless, this study has several limitations that warrant acknowledgment. First, because this is a cross-sectional study, it cannot establish causal relationships between neonicotinoid exposure and neurobehavioral outcomes. Second, most neonicotinoids have relatively short biological half-lives in humans, and the spot urine samples collected in this study may not accurately reflect long-term cumulative exposure. In addition, because urine collection did not occur immediately after potential exposure events, transient peak concentrations may have been missed, meaning that the actual short-term exposure levels could have been underestimated. Meanwhile, the total IMIeq concentrations calculated using RPFs based on EPA reference doses primarily serve to reflect overall exposure levels for comparability across studies, rather than to precisely represent differences in neurotoxic potency, as the underlying RfDs were derived from heterogeneous toxicological endpoints. Third, neurodevelopmental outcomes were assessed with the SDQ, which may be affected by recall bias and other reporting inaccuracies. Finally, the sample size of this study was relatively modest (n = 506), and all participants were recruited from a single city (Shenzhen), which may limit generalizability and robustness of the findings. Future prospective cohort studies and experimental investigations are warranted to provide more comprehensive evidence on the impact of neonicotinoid exposure on human neurobehavioral development.
5. Conclusions
The findings suggest that exposure to neonicotinoid insecticides may be associated with neurobehavioral problems in preschool-aged children. Among the compounds examined, IMI, NTHM, and NACE emerged as the primary contributors and showed significant positive associations with neurobehavioral problems. In addition, NTHM and NACE exhibited sex-specific differences in this population. The BKMR analyses revealed that the combined effects of neonicotinoid mixtures were also positively associated with neurobehavioral difficulties, indicating potential adverse impacts even at low exposure levels. Therefore, future efforts should prioritize reducing the use of IMI, THM, and ACE in agricultural practices and using safer alternatives to mitigate their potential risks to children’s neurodevelopment. Future studies should involve larger sample sizes and complementary in vitro and in vivo experiments to validate and extend these findings, thereby informing early identification of environmental health risks and preventive strategies for vulnerable pediatric populations.
Author Contributions
Conceptualization, Y.Z. and Y.W.; methodology, Y.Z. and L.H.; software, Y.Z., L.H. and Y.W.; validation, Y.Z. and Z.H.; formal analysis, H.G., Y.Z. and W.X.; investigation, L.L., Y.Z., Y.W. and H.G.; resources, Y.W. and Q.W.; data curation, Z.L., J.L. and C.L.; writing—original draft preparation, Y.Z. and Y.H.; writing—review and editing, Q.W. and W.C.; visualization, L.L., Y.Z. and S.A.; supervision, S.A., L.L., J.L. and L.H.; project administration, S.A., L.L., J.L. and L.H.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Shenzhen Bao’an District Public Health Service Center, with a grant of CNY 300,000. (grant numbers: 440306241173100400106).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the School of Public Health, Sun Yat-sen University (Approval No. IRB-SYSU-2024-104, 17 June 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article are not publicly available due to ethical review restrictions and participant confidentiality requirements but can be provided by the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SDQ | The Strengths and Difficulties Questionnaire |
| NEOs | Neonicotinoid Insecticides |
| IMI | Imidacloprid |
| CLO | Clothianidin |
| THM | Thiamethoxam |
| DNT | Dinotefuran |
| NIT | Nitenpyram |
| SFX | Sulfoxaflor |
| ACE | Acetamiprid |
| THD | Thiacloprid |
| FLO | Flonicamid |
| 6-CINA | 6-Chloronicotinic acid |
| NACE | N-Desmethyl-Acetamiprid |
| NTHM | N-Desmethyl-Thiamethoxam |
| WQS | Weighted Quantile Sum |
| BKMR | Bayesian Kernel Machine Regression |
| OR | Odds Ratio |
| CIs | Confidence Intervals |
| DAG | Directed Acyclic Graph |
| Cr | Creatinine |
| RPF | Relative Potency Factor |
| nAChRs | Nicotinic Acetylcholine Receptors |
Appendix A

Table A1.
HPLC-MS/MS analytical parameters for NEOs.
Table A1.
HPLC-MS/MS analytical parameters for NEOs.
| Compound | CAS-Number | Elemental Composition | Diagnostic ion | Transitions (m/z) | Collision Energy (Ev) | Tube Lens Offset Voltage (v) | RT Retention Time (min) |
|---|---|---|---|---|---|---|---|
| IMI | 138261-41-3 | C9H10ClN5O2 | ESI+ | 256.08/209.12 | 12 | 38 | 3.57 |
| 256.08/174.98 | 16 | 38 | 3.57 | ||||
| CLO | 210880-92-5 | C6H8ClN5O2S | ESI+ | 250.03/168.87 | 12 | 4 | 3.46 |
| 250.03/131.87 | 14 | 4 | 3.46 | ||||
| THM | 153719-23-4 | C8H10ClN5O3S | ESI+ | 292.05/210.94 | 14 | 24 | 3.28 |
| 292.05/131.92 | 18 | 24 | 3.28 | ||||
| ACE | 135410-20-7 | C10H11ClN4 | ESI+ | 223.03/125.88 | 24 | 40 | 3.66 |
| 223.03/55.92 | 14 | 42 | 3.66 | ||||
| THD | 111988-49-9 | C10H9ClN4S | ESI+ | 252.99/125.9 | 18 | 52 | 3.84 |
| 252.99/90.07 | 40 | 52 | 3.84 | ||||
| DNT | 165252–70–0 | C7H14N4O3 | ESI+ | 203.07/128.93 | 12 | 4 | 2.76 |
| 203.07/113.26 | 10 | 4 | 2.76 | ||||
| NIT | 150824-47-8 | C11H15ClN4O2 | ESI+ | 271.12/125.97 | 28 | 2 | 3.44 |
| 271.12/129.71 | 12 | 2 | 3.44 | ||||
| SFX | 946578-00-3 | C10H11F3N3OS | ESI+ | 278.01/173.97 | 8 | 28 | 3.85 |
| 278.01/153.94 | 28 | 28 | 3.85 | ||||
| FLO | 158062-67-0 | C9H6F3N3O | ESI+ | 230.07/203.00 | 16 | 22 | 3.32 |
| 230.07/173.98 | 18 | 22 | 3.32 | ||||
| 6-ClNA | 5326-23-8 | C6H4ClNO2 | ESI+ | 158.02/121.92 | 16 | 22 | 3.48 |
| 158.02/77.96 | 22 | 22 | 3.48 | ||||
| N-ACE | 190604-92-3 | C9H9ClN4 | ESI+ | 209.02/125.89 | 18 | 16 | 3.49 |
| 209.02/90.05 | 32 | 26 | 3.49 | ||||
| N-THM | 171103-04-1 | C7H8ClN5O3S | ESI+ | 278.03/173.99 | 10 | 30 | 3.9 |
| 278.03/104.87 | 10 | 30 | 3.9 |
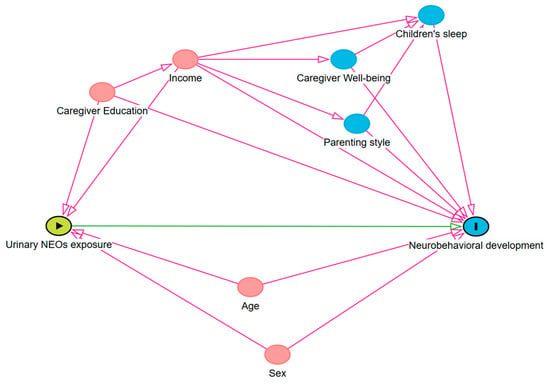
Figure A1.
Directed acyclic graph (DAG) used to select potential covariates in the associations between urinary NEO exposure and measures of neurobehavioral development in preschool children.

Table A2.
The chronic reference dose and relative toxic effect factors of neonicotinoids.
Table A2.
The chronic reference dose and relative toxic effect factors of neonicotinoids.
| NNIs | cRFD (mg/kg/Day) | RPF |
|---|---|---|
| IMI | 0.080 | 1.000 |
| CLO | 0.098 | 0.820 |
| THM | 0.012 | 6.670 |
| DNT | 1.000 | 0.080 |
| NIT | - | 0.110 |
| SFX | 0.050 | 1.600 |
| NACE | - | 1.130 |
| NTHM | - | 6.670 |
| ACE | 0.071 | 1.130 |
| THD | 0.004 | 20.000 |

Table A3.
WQS regression index between urinary neonicotinoid concentrations and various dimensions of neurobehavioral development in preschool children (All people, n = 506).
Table A3.
WQS regression index between urinary neonicotinoid concentrations and various dimensions of neurobehavioral development in preschool children (All people, n = 506).
| Outcomes | β | p |
|---|---|---|
| Emotional problem | 0.970 | p < 0.001 |
| Hyperactivity | 0.961 | p < 0.001 |
| Total difficulties | 2.138 | p < 0.001 |

Table A4.
WQS regression weights between urinary neonicotinoid concentrations and emotional symptoms in preschool children.
Table A4.
WQS regression weights between urinary neonicotinoid concentrations and emotional symptoms in preschool children.
| Mix Name | Mean Weight (Emotional Symptoms) | ||
|---|---|---|---|
| Total | Boys | Girls | |
| IMI | 46.44% | 47.67% | 42.42% |
| CLO | 0.55% | 2.14% | 3.34% |
| THM | 7.75% | 2.30% | 2.51% |
| DNT | 4.43% | 0.14% | 1.97% |
| NIT | 1.57% | 0.50% | 5.98% |
| SFX | 0.14% | 2.93% | 0.26% |
| NACE | 12.27% | 8.70% | 20.50% |
| NTHM | 16.02% | 29.84% | 7.81% |
| ACE | 6.19% | 2.01% | 2.53% |
| THD | 4.63% | 3.77% | 12.67% |

Table A5.
WQS regression weights between urinary neonicotinoid concentrations and hyperactivity in preschool children.
Table A5.
WQS regression weights between urinary neonicotinoid concentrations and hyperactivity in preschool children.
| Mix Name | Mean Weight (Hyperactivity) | ||
|---|---|---|---|
| Total | Boys | Girls | |
| IMI | 34.88% | 37.43% | 31.64% |
| CLO | 0.71% | 0.14% | 3.00% |
| THM | 1.87% | 1.19% | 2.91% |
| DNT | 0.83% | 0.11% | 1.47% |
| NIT | 4.34% | 0.16% | 10.61% |
| SFX | 9.97% | 8.81% | 1.31% |
| NACE | 21.98% | 18.27% | 12.97% |
| NTHM | 13.64% | 21.00% | 30.29% |
| ACE | 9.50% | 11.42% | 1.16% |
| THD | 2.28% | 1.47% | 4.64% |

Table A6.
WQS regression weights between urinary neonicotinoid concentrations and total difficulties in preschool children.
Table A6.
WQS regression weights between urinary neonicotinoid concentrations and total difficulties in preschool children.
| Mix Name | Mean Weight (Total Difficulties) | ||
|---|---|---|---|
| Total | Boys | Girls | |
| IMI | 41.57% | 38.17% | 31.77% |
| CLO | 1.36% | 0.91% | 4.21% |
| THM | 7.46% | 4.46% | 2.60% |
| DNT | 5.10% | 0.06% | 4.40% |
| NIT | 1.18% | 0.32% | 5.31% |
| SFX | 1.97% | 12.66% | 1.13% |
| NACE | 11.79% | 9.11% | 17.03% |
| NTHM | 12.35% | 23.16% | 25.37% |
| ACE | 13.61% | 10.15% | 2.11% |
| THD | 3.60% | 1.00% | 6.07% |
References
- Fox, G. From Neurons to Neighborhoods: The Science of Early Childhood Development. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 625–626. [Google Scholar] [CrossRef]
- Schneider, N.; Greenstreet, E.; Deoni, S.C.L. Connecting inside out: Development of the social brain in infants and toddlers with a focus on myelination as a marker of brain maturation. Child Dev. 2022, 93, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Benischek, A.; Dewey, D.; Lebel, C. Age-related functional brain changes in young children. NeuroImage 2017, 155, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Payne-Sturges, D.C.; Taiwo, T.K.; Ellickson, K.; Mullen, H.; Tchangalova, N.; Anderko, L.; Chen, A.; Swanson, M. Disparities in Toxic Chemical Exposures and Associated Neurodevelopmental Outcomes: A Scoping Review and Systematic Evidence Map of the Epidemiological Literature. Environ. Health Perspect. 2023, 131, 096001. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef]
- Rock, K.D.; Patisaul, H.B. Environmental Mechanisms of Neurodevelopmental Toxicity. Curr. Environ. Health Rep. 2018, 5, 145–157. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Garrick, J.M. Developmental impact of air pollution on brain function. Neurochem. Int. 2019, 131, 104580. [Google Scholar] [CrossRef]
- Roy, R.; D’Angiulli, A. Air pollution and neurological diseases, current state highlights. Front. Neurosci. 2024, 18, 1351721. [Google Scholar] [CrossRef]
- Cartier, C.; Warembourg, C.; Le Maner-Idrissi, G.; Lacroix, A.; Rouget, F.; Monfort, C.; Limon, G.; Durand, G.; Saint-Amour, D.; Cordier, S.; et al. Organophosphate Insecticide Metabolites in Prenatal and Childhood Urine Samples and Intelligence Scores at 6 Years of Age: Results from the Mother–Child PELAGIE Cohort (France). Environ. Health Perspect. 2016, 124, 674–680. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Jain, C.D.; Madhavan, B.L.; Singh, V.; Prasad, P.; Sai Krishnaveni, A.; Ravi Kiran, V.; Venkat Ratnam, M. Phase-wise analysis of the COVID-19 lockdown impact on aerosol, radiation and trace gases and associated chemistry in a tropical rural environment. Environ. Res. 2021, 194, 110665. [Google Scholar] [CrossRef]
- Song, S.; Yue, Y.; Jiang, Q.; Li, C.; Zhang, H. Perspective on neonicotinoid insecticides in human exposure and risk assessment. J. Environ. Expo. Assess. 2025, 4, 4. [Google Scholar] [CrossRef]
- Kawata, M.; Umetsu, N.; Goto, T.; Fukuto, T.R. Synthesis and Biological Activity of Alkoxysulfenyl Derivatives of Methylcarbamate Insecticides. J. Pestic. Sci. 1988, 13, 595–603. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Mahai, G.; Wan, Y.; Xia, W.; Yang, S.; He, Z.; Xu, S. Neonicotinoid insecticides in surface water from the central Yangtze River, China. Chemosphere 2019, 229, 452–460. [Google Scholar] [CrossRef]
- Qin, R.; Wei, Z.; Huang, Y.; Bai, X.; Zhang, Z.; Zhong, Z.; Gui, D.; Wang, L.; Sun, H.; Zhang, T. Large Geographical Scale Study on the Concentrations, Distribution, and Source Analysis of Neonicotinoid Insecticides in Surface Waters of South China. ACS EST Water 2024, 4, 68–78. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, Y.; Li, S.; He, Z.; Xu, S.; Xia, W. Occurrence, spatial variation, seasonal difference, and risk assessment of neonicotinoid insecticides, selected agriculture fungicides, and their transformation products in the Yangtze River, China: From the upper to lower reaches. Water Res. 2023, 247, 120724. [Google Scholar] [CrossRef]
- Burke, A.P.; Niibori, Y.; Terayama, H.; Ito, M.; Pidgeon, C.; Arsenault, J.; Camarero, P.R.; Cummins, C.L.; Mateo, R.; Sakabe, K.; et al. Mammalian Susceptibility to a Neonicotinoid Insecticide after Fetal and Early Postnatal Exposure. Sci. Rep. 2018, 8, 16639. [Google Scholar] [CrossRef]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats. PLoS ONE 2012, 7, e32432. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. 2020, 27, 24799–24814. [Google Scholar] [CrossRef]
- Wei, F.; Cheng, F.; Li, H.; You, J. Imidacloprid affects human cells through mitochondrial dysfunction and oxidative stress. Sci. Total Environ. 2024, 951, 175422. [Google Scholar] [CrossRef] [PubMed]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dionysiou, D.D.; Wen, R.; Zhang, H.; Wan, X.; Wang, X.; Li, F.; Li, Y.; Zhou, Q.; Ying, G.-G.; et al. Inference of emission history of neonicotinoid pesticides from marine sediment cores impacted by riverine runoff of a developed agricultural region: The Pearl River Basin, China. Water Res. 2022, 218, 118475. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.; Xiao, Q.; Li, Z.; Jia, X.; Hu, W.; Liu, K.; Lu, S. Urinary neonicotinoid insecticides in children from South China: Concentrations, profiles and influencing factors. Chemosphere 2022, 291, 132937. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, J.; Nomura, H.; Kondo, T.; Saito, I.; Ito, Y.; Osaka, A.; Kamijima, M. Biological Monitoring Method for Urinary Neonicotinoid Insecticides Using LC-MS/MS and Its Application to Japanese Adults. J. Occup. Health 2014, 56, 461–468. [Google Scholar] [CrossRef]
- Ichikawa, G.; Kuribayashi, R.; Ikenaka, Y.; Ichise, T.; Nakayama, S.M.M.; Ishizuka, M.; Taira, K.; Fujioka, K.; Sairenchi, T.; Kobashi, G.; et al. LC-ESI/MS/MS analysis of neonicotinoids in urine of very low birth weight infants at birth. PLoS ONE 2019, 14, e0219208. [Google Scholar] [CrossRef]
- Sanchis, Y.; Coscollà, C.; Yusà, V. Analysis of four parabens and bisphenols A, F, S in urine, using dilute and shoot and liquid chromatography coupled to mass spectrometry. Talanta 2019, 202, 42–50. [Google Scholar] [CrossRef]
- Venisse, N.; Grignon, C.; Brunet, B.; Thévenot, S.; Bacle, A.; Migeot, V.; Dupuis, A. Reliable quantification of bisphenol A and its chlorinated derivatives in human urine using UPLC–MS/MS method. Talanta 2014, 125, 284–292. [Google Scholar] [CrossRef]
- Guerrini Usubini, A.; Bottacchi, M.; Morelli, G.; Caroli, D.; Marazzi, N.; Castelnuovo, G.; Sartorio, A. The psychosocial functioning in adolescents with severe obesity evaluated throughout the strengths and difficulties questionnaire (SDQ): A preliminary report. Front. Psychol. 2024, 14, 1205113. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R. The Strengths and Difficulties Questionnaire: A Research Note. J. Child Psychol. Psychiatry 1997, 38, 581–586. [Google Scholar] [CrossRef]
- Yan, Z.; Yu, S.; Lin, W. Parents’ perceived social support and children’s mental health: The chain mediating role of parental marital quality and parent—Child relationships. Curr. Psychol. 2024, 43, 4198–4210. [Google Scholar] [CrossRef]
- Fuchs, S.; Klein, A.M.; Otto, Y.; Von Klitzing, K. Prevalence of Emotional and Behavioral Symptoms and their Impact on Daily Life Activities in a Community Sample of 3 to 5-Year-Old Children. Child Psychiatry Hum. Dev. 2013, 44, 493–503. [Google Scholar] [CrossRef]
- Lu, C.; Chang, C.-H.; Palmer, C.; Zhao, M.; Zhang, Q. Neonicotinoid Residues in Fruits and Vegetables: An Integrated Dietary Exposure Assessment Approach. Environ. Sci. Technol. 2018, 52, 3175–3184. [Google Scholar] [CrossRef]
- Nimako, C.; Ichise, T.; Hasegawa, H.; Akoto, O.; Boadi, N.O.; Taira, K.; Fujioka, K.; Isoda, N.; Nakayama, S.M.M.; Ishizuka, M.; et al. Assessment of ameliorative effects of organic dietary interventions on neonicotinoid exposure rates in a Japanese population. Environ. Int. 2022, 162, 107169. [Google Scholar] [CrossRef]
- Wang, L.; Ma, C.; Wei, D.; Wang, M.; Xu, Q.; Wang, J.; Song, Y.; Huo, W.; Jing, T.; Wang, C.; et al. Health risks of neonicotinoids chronic exposure and its association with glucose metabolism: A case-control study in rural China. Environ. Pollut. 2023, 334, 122213. [Google Scholar] [CrossRef]
- Peverill, M.; Dirks, M.A.; Narvaja, T.; Herts, K.L.; Comer, J.S.; McLaughlin, K.A. Socioeconomic status and child psychopathology in the United States: A meta-analysis of population-based studies. Clin. Psychol. Rev. 2021, 83, 101933. [Google Scholar] [CrossRef]
- Reiss, F. Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Soc. Sci. Med. 2013, 90, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.; Lebowitz, E.R. The Scaffold Effect: Raising Resilient, Self-Reliant, and Secure Kids in an Age of Anxiety. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 1535–1536. [Google Scholar] [CrossRef]
- Pinquart, M. Associations of Parenting Styles and Dimensions with Academic Achievement in Children and Adolescents: A Meta-analysis. Educ. Psychol. Rev. 2016, 28, 475–493. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Rustom, S.; Carli, M.; Whitehead, T.P.; Ward, M.H.; Metayer, C. Assessment of Grouped Weighted Quantile Sum Regression for Modeling Chemical Mixtures and Cancer Risk. Int. J. Environ. Res. Public Health 2021, 18, 504. [Google Scholar] [CrossRef]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Wang, A.; Mahai, G.; Wan, Y.; Yang, Z.; He, Z.; Xu, S.; Xia, W. Assessment of imidacloprid related exposure using imidacloprid-olefin and desnitro-imidacloprid: Neonicotinoid insecticides in human urine in Wuhan, China. Environ. Int. 2020, 141, 105785. [Google Scholar] [CrossRef] [PubMed]
- Osaka, A.; Ueyama, J.; Kondo, T.; Nomura, H.; Sugiura, Y.; Saito, I.; Nakane, K.; Takaishi, A.; Ogi, H.; Wakusawa, S.; et al. Exposure characterization of three major insecticide lines in urine of young children in Japan—Neonicotinoids, organophosphates, and pyrethroids. Environ. Res. 2016, 147, 89–96. [Google Scholar] [CrossRef]
- Wang, H.; Yang, D.; Fang, H.; Han, M.; Tang, C.; Wu, J.; Chen, Y.; Jiang, Q. Predictors, sources, and health risk of exposure to neonicotinoids in Chinese school children: A biomonitoring-based study. Environ. Int. 2020, 143, 105918. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Saito, H.; Furukawa, Y.; Sasaki, T.; Kitajima, S.; Kanno, J.; Tanemura, K. Behavioral effects of adult male mice induced by low-level acetamiprid, imidacloprid, and nicotine exposure in early-life. Front. Neurosci. 2023, 17, 1239808. [Google Scholar] [CrossRef]
- Castro, E.M.; Lotfipour, S.; Leslie, F.M. Nicotine on the developing brain. Pharmacol. Res. 2023, 190, 106716. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, U.; Srivastava, M.K.; Bhardwaj, S.; Srivastava, L.P. Effect of Imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive its No Observed Effect Level (NOEL). J. Toxicol. Sci. 2010, 35, 577–581. [Google Scholar] [CrossRef]
- Costas-Ferreira, C.; Faro, L.R.F. Neurotoxic Effects of Neonicotinoids on Mammals: What Is There beyond the Activation of Nicotinic Acetylcholine Receptors?—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8413. [Google Scholar] [CrossRef] [PubMed]
- Moffat, C.; Buckland, S.T.; Samson, A.J.; McArthur, R.; Chamosa Pino, V.; Bollan, K.A.; Huang, J.T.-J.; Connolly, C.N. Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci. Rep. 2016, 6, 24764. [Google Scholar] [CrossRef]
- Laubscher, B.; Diezi, M.; Renella, R.; Mitchell, E.A.D.; Aebi, A.; Mulot, M.; Glauser, G. Multiple neonicotinoids in children’s cerebro-spinal fluid, plasma, and urine. Environ. Health 2022, 21, 10. [Google Scholar] [CrossRef]
- Ndonwi, E.N.; Atogho-Tiedeu, B.; Lontchi-Yimagou, E.; Shinkafi, T.S.; Nanfa, D.; Balti, E.V.; Katte, J.C.; Mbanya, A.; Matsha, T.; Mbanya, J.C.; et al. Metabolic effects of exposure to pesticides during gestation in female Wistar rats and their offspring: A risk factor for diabetes? Toxicol. Res. 2020, 36, 249–256. [Google Scholar] [CrossRef]
- Yang, C.; Liang, J. Associations between neonicotinoids metabolites and hematologic parameters among US adults in NHANES 2015–2016. Environ. Sci. Pollut. Res. 2022, 30, 26327–26337. [Google Scholar] [CrossRef]
- Wang, X.; Anadón, A.; Wu, Q.; Qiao, F.; Ares, I.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Martínez, M.-A. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 471–507. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Zhongyuan, Z.; Wang, J.; Wong, C.-O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Venkatachalam, K.; Perry, T.; Bellen, H.J.; et al. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 25840–25850. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).