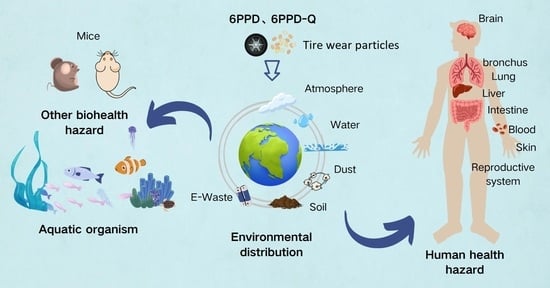
Environmental and Human Health Risks of 6PPD and 6PPDQ: Assessment and Implications
Abstract
1. Introduction
2. Methodology
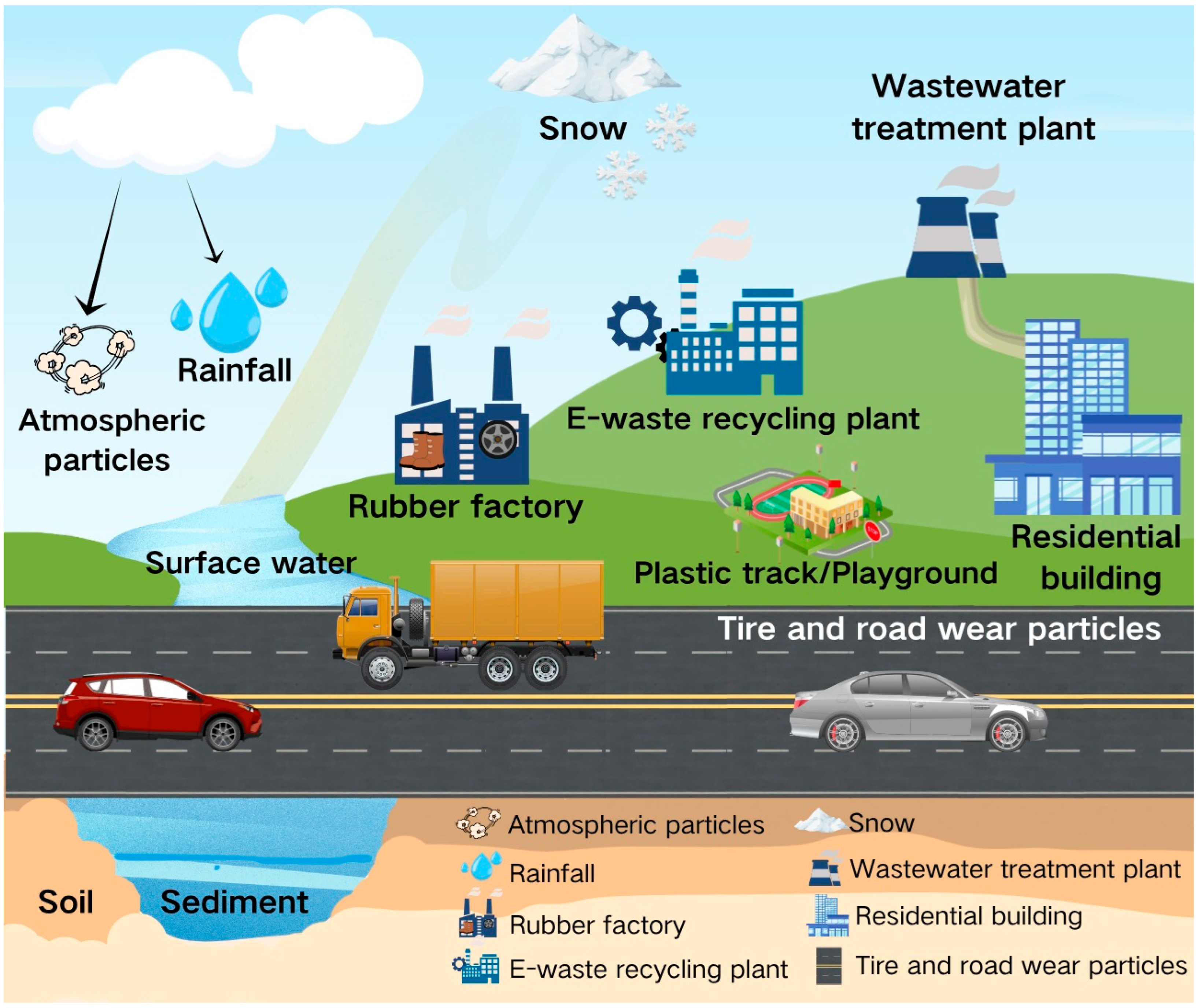
3. Environmental Distribution of 6PPD and 6PPDQ
3.1. Atmosphere
3.1.1. Atmospheric Distribution and Dynamics of 6PPD and 6PPDQ
3.1.2. Dust
| Environmental Media | Characteristics | 6PPDQ | 6PPD | References |
|---|---|---|---|---|
| Air | City | 1.18 (0.54–13.8) pg/m3 | 1.78 (0.82–6.30) pg/m3 | [27] |
| City | 0.85 (NQ–1.75) pg/m3 | (NQ–<LOQ) pg/m3 | [28] | |
| Guangzhou | 1.7 (0.1–15) pg/m3 | 0.9 (0.3–10) pg/m3 | [29] | |
| Hangzhou | 6.7 (0.8–26) pg/m3 | 4.6 (0.1–6.0) pg/m3 | ||
| Nangjing | 2.3 (1.1–68) pg/m3 | 2.1 (0.4–75) pg/m3 | ||
| Shanghai | 5.9 (0.3–39) pg/m3 | 4.4 (0.5–135) pg/m3 | ||
| Taiyuan | 3.3 (1.1–84) pg/m3 | 6.9 (0.02–487) pg/m3 | ||
| Zhengzhou | 2.9 (0.3–32) pg/m3 | 8.4 (1.2–109) pg/m3 | ||
| Guangzhou | 1100 (3.04–2350) pg/m3 | 1820 (22.2–6050) pg/m3 | [20] | |
| Roadside in Guangzhou | 2810 (2.96–7250) pg/m3 | 4040 (2.23–9340) pg/m3 | ||
| Taiyuan | 744 (2.44–1780) pg/m3 | 81 (1.02–3190) pg/m3 | ||
| Dust | E-waste recycling workshops | 375 (87.1–2850) ng/g | 113 (13.8–1020) ng/g | [30] |
| Playground | / | 30.4 (<MQL–685) ng/g | [21] | |
| Indoor dust | / | 16.4 (<MQL–180) ng/g | ||
| Air conditioner filters—male dormitories | 4.76 ± 2.81 (1.95–13.4) ng/g | / | [23] | |
| Air conditioner filters—female dormitories | 6.78 ± 2.98 (2.85–12.6) ng/g | / | ||
| Air conditioner filters—residential houses | 11.4 ± 8.11 (0.62–31.7) ng/g | / | ||
| Settled dust—residential bedrooms | 10.7 ± 7.58 (0.97–26.1) ng/g | / | ||
| Settled dust—buses | 43.0 ± 12.9 (19.7–71.4) ng/g | / | ||
| Settled dust—shopping malls | 23.5 ± 23.4 (3.92–106) ng/g | / | ||
| Vehicle dust | 80.9 (17.9–146) ng/g | 19.3 (5.0–41.9) ng/g | [31] | |
| House dust | <LOQ (<LOQ–0.4) ng/g | 0.3 (<LOQ–6.1) ng/g | ||
| E-waste dust | / | 15.4 (7.31–37.7) ng/g | [32] | |
| House dust (Canada) | / | 0.083 (<MDL–6.65) ng/g | ||
| House dust (United States) | / | 1.84 (<MDL–23.7) ng/g | ||
| indoor dust | 9.5 (0.33–82) ng/g | 10 (0.48–135) ng/g | [16] | |
| E-waste community indoor dust | 3.2 ng/g | / | [33] | |
| E-waste kindergarten indoor dust | 7.5 ng/g | / | ||
| Haojiang—house dust | 1.4 ng/g | / | ||
| Haojiang—kindergarten dust | 1.3 ng/g | / |
3.2. Water Environment
3.3. Soil Environment
3.4. Special Exposure Scenarios of 6PPD and 6PPDQ Derived from E-Waste
4. Human Exposure Routes
5. Biotoxicity Studies of 6PPD and 6PPDQ
5.1. Aquatic Organisms
5.1.1. Coho Salmon
5.1.2. Zebrafish
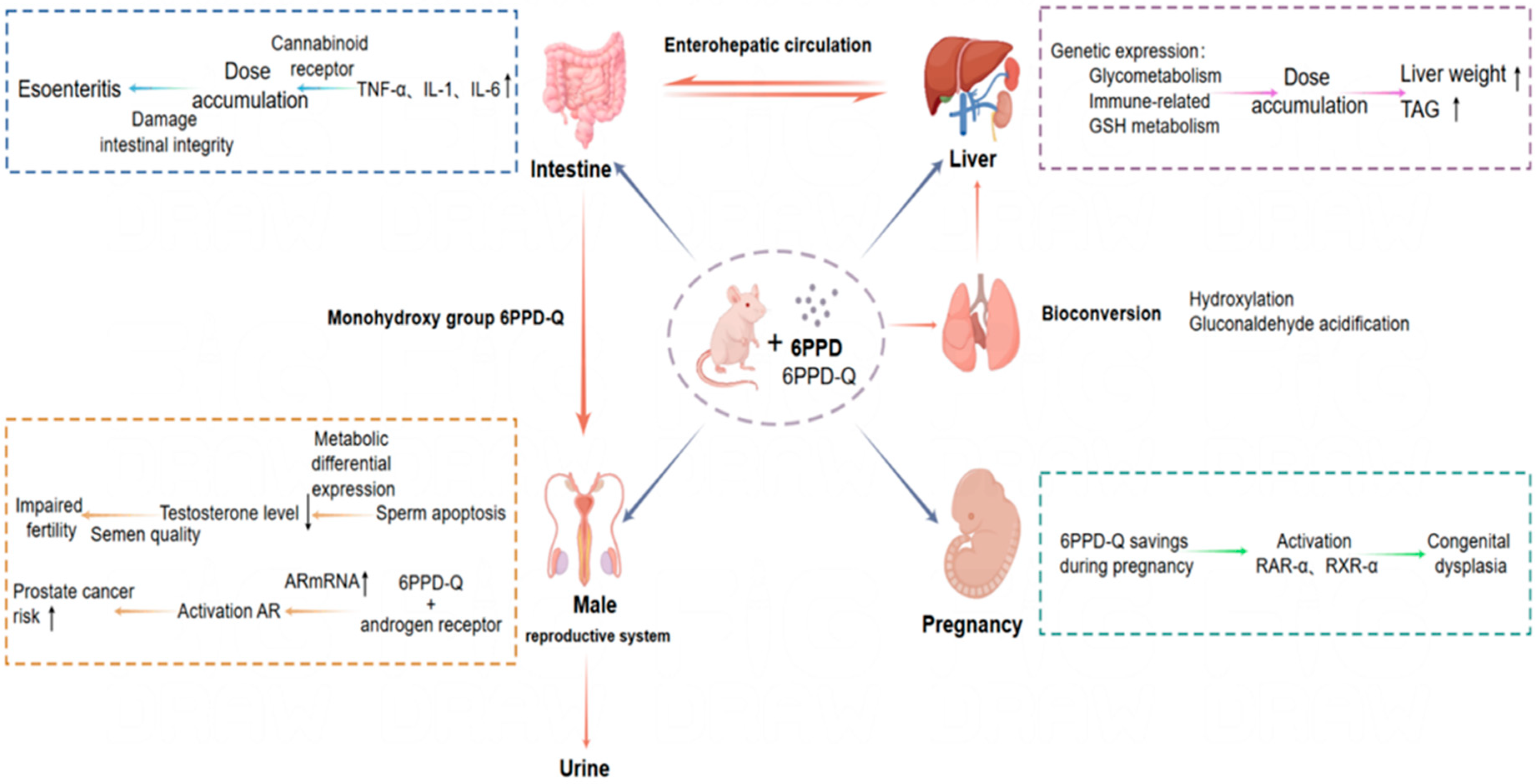
5.2. Mice
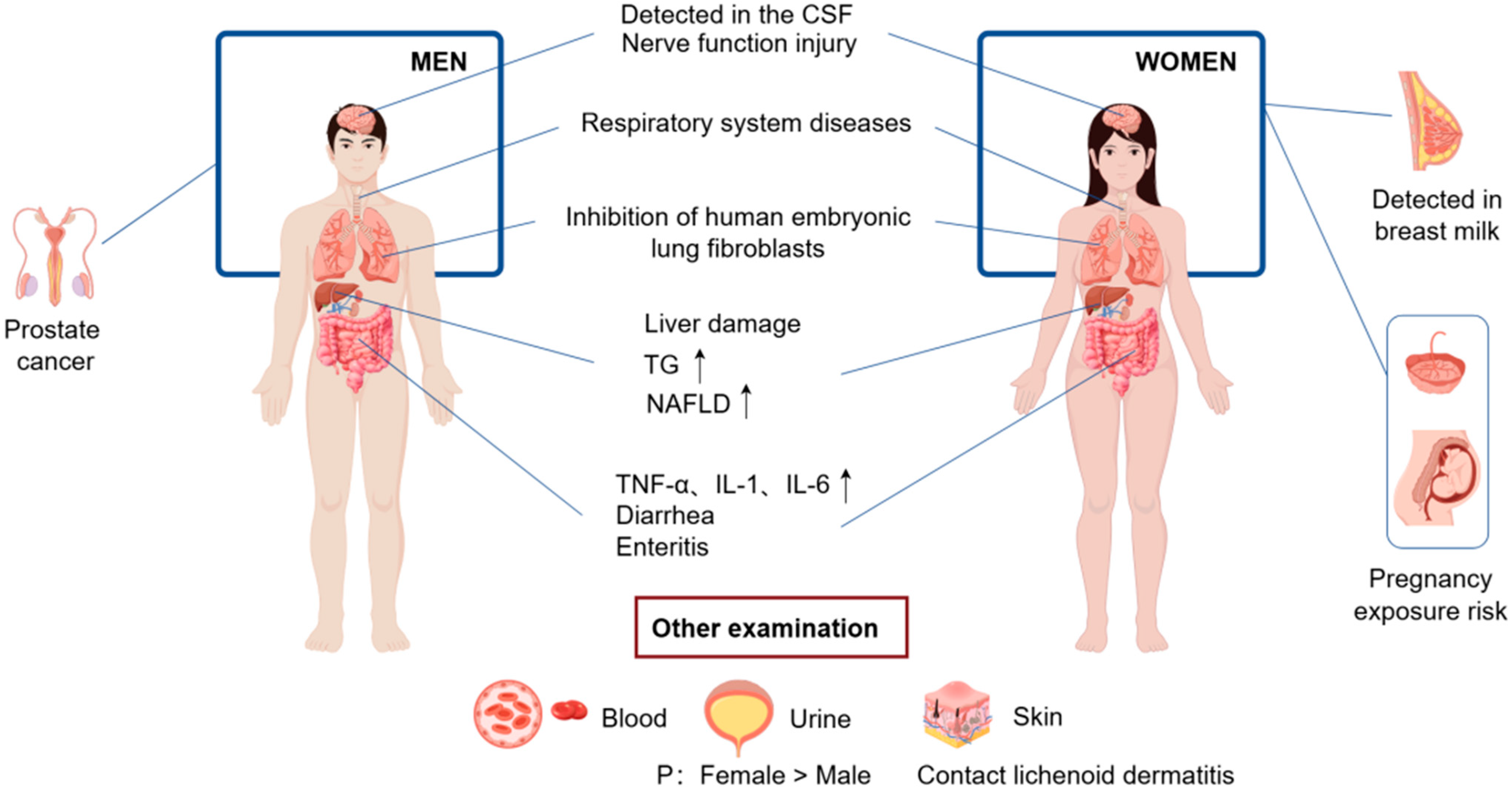
5.3. The Human Body
6. Health Risk Assessment for Human Exposure to 6PPD and 6PPDQ
7. Prevention and Intervention
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Wang, C.; Ma, L.; Gao, T.; Wang, Y. Environmental profiles, hazard identification, and toxicological hallmarks of emerging tire rubber-related contaminants 6ppd and 6ppd-quinone. Environ. Int. 2024, 187, 108677. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R.; et al. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185–189. [Google Scholar] [CrossRef]
- Mott, P.H.; Roland, C.M. Ozone detection by crack-induced opacity in rubber. Rubber Chem. Technol. 1999, 72, 769–778. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Guo, X.; Wei, Y.; Liao, S. Synergistic effect of thermal oxygen and uv aging on natural rubber. e-Polymers 2023, 23. [Google Scholar] [CrossRef]
- Yan, X.; Xiao, J.; Kiki, C.; Zhang, Y.; Manzi, H.P.; Zhao, G.; Wang, S.; Sun, Q. Unraveling the fate of 6ppd-q in aquatic environment: Insights into formation, dissipation, and transformation under natural conditions. Environ. Int. 2024, 191, 109004. [Google Scholar] [CrossRef]
- Klöckner, P.; Seiwert, B.; Weyrauch, S.; Escher, B.I.; Reemtsma, T.; Wagner, S. Comprehensive characterization of tire and road wear particles in highway tunnel road dust by use of size and density fractionation. Chemosphere 2021, 279, 130530. [Google Scholar] [CrossRef]
- Rossomme, E.; Hart-Cooper, W.M.; Orts, W.J.; McMahan, C.M.; Head-Gordon, M. Computational studies of rubber ozonation explain the effectiveness of 6ppd as an antidegradant and the mechanism of its quinone formation. Environ. Sci. Technol. 2023, 57, 5216–5230. [Google Scholar] [CrossRef]
- Thodhal Yoganandham, S.; Daeho, K.; Heewon, J.; Shen, K.; Jeon, J. Unveiling the environmental impact of tire wear particles and the associated contaminants: A comprehensive review of environmental and health risk. J. Hazard. Mater. 2024, 480, 136155. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wang, Q.; Zhang, J.; Zhang, Z.; Cao, G.; Zeng, Z.; Tan, H.; Xu, X.; Wang, W.; Lei, B. Child exposure to N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6ppd) and its derived quinone (6ppdq) in e-waste areas: Urinary concentrations, sources, and health effect assessment. J. Environ. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cao, G.; Zhang, J. P-phenylenediamine-derived quinones as new contributors to the oxidative potential of fine particulate matter. Environ. Sci. Technol. Lett. 2022, 9, 712–717. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, H.N.; Tian, Z.; Peter, K.T.; Dodd, M.C.; Kolodziej, E.P. Transformation product formation upon heterogeneous ozonation of the tire rubber antioxidant 6ppd (N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine). Environ. Sci. Technol. Lett. 2022, 9, 413–419. [Google Scholar] [CrossRef]
- Wang, B.; Sun, W.; Ye, X.; Liu, Z.; Zhang, H. Occurrence, analytical methods, and ecotoxicological effects of 6ppd-quinone in aquatic environments: A review. TrAC Trends Anal. Chem. 2025, 193, 118449. [Google Scholar] [CrossRef]
- Yi, J.; Ruan, J.; Yu, H.; Wu, B.; Zhao, J.; Wang, H.; Chen, R.; Yang, Q.; Chen, J.; Sun, D. Environmental fate, toxicity, and mitigation of 6ppd and 6ppd-quinone: Current understanding and future directions. Environ. Pollut. 2025, 375, 126352. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, Z.; Wang, X.; Jiang, L.; Sun, H.; Li, H.; Yan, Z.; Wang, Y.; Yao, X.; Zhang, C.; et al. 6ppd-quinone exposure induces oxidative damage and physiological disruption in eisenia fetida: An integrated analysis of phenotypes, multi-omics, and intestinal microbiota. J. Hazard. Mater. 2025, 493, 138334. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Lao, J.; Cao, Y.; Hong, P.; Chen, C.; Lam, E.Y.; Fang, J.K.; Lee, S.; Leung, K.M.Y. Typical tire additives in river water: Leaching, transformation, and environmental risk assessment. Environ. Sci. Technol. 2024, 58, 18940–18949. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, R.; Jiang, S.; Wu, P.; Jin, H. Occurrence of p-phenylenediamine antioxidants (ppds) and ppds-derived quinones in indoor dust. Sci. Total Environ. 2024, 912, 169325. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Gora, A.H.; Siriyappagouder, P.; Kiron, V.; Olsvik, P.A. Toxicological effects of 6ppd and 6ppd quinone in zebrafish larvae. J. Hazard. Mater. 2022, 424, 127623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, Z.; Liu, Y.; Hu, L.; He, L.; Liu, Y.; Zhao, J.; Ying, G. Occurrence and risks of 23 tire additives and their transformation products in an urban water system. Environ. Int. 2023, 171, 107715. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D. Tire-rubber related pollutant 6-ppd quinone: A review of its transformation, environmental distribution, bioavailability, and toxicity. J. Hazard. Mater. 2023, 132265. [Google Scholar] [CrossRef]
- Wang, W.; Cao, G.; Zhang, J.; Wu, P.; Chen, Y.; Chen, Z.; Qi, Z.; Li, R.; Dong, C.; Cai, Z. Beyond substituted p-phenylenediamine antioxidants: Prevalence of their quinone derivatives in pm2. 5. Environ. Sci. Technol. 2022, 56, 10629–10637. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.S.; Saldiva, P.H.; Lavigne, E.; Matus, P. Ambient particulate air pollution and daily mortality in 652 cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- Venkatesan, G.; Dancik, Y.; Sinha, A.; Kyaw, H.M.; Srinivas, R.; Dawson, T.L.J.; Bigliardi, M.; Bigliardi, P.; Pastorin, G. Development of novel alternative hair dyes to hazardous para-phenylenediamine. J. Hazard. Mater. 2021, 402, 123712. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, T.; Ye, D.; Lin, Z.; Wang, F.; Guo, Y. Widespreadn N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine quinone in size-fractioned atmospheric particles and dust of different indoor environments. Environ. Sci. Technol. Lett. 2022, 9, 420–425. [Google Scholar] [CrossRef]
- Xing, F.; Huang, H.; Zhan, Z.; Zhai, X.; Ou, C.; Sze, N.N.; Hon, K.K. Hourly associations between weather factors and traffic crashes: Non-linear and lag effects. Anal. Methods Accid. Res. 2019, 24, 100109. [Google Scholar] [CrossRef]
- Li, R.; Kou, X.; Geng, H.; Dong, C.; Cai, Z. Pollution characteristics of ambient pm2.5-bound pahs and npahs in a typical winter time period in taiyuan. Chin. Chem. Lett. 2014, 25, 663–666. [Google Scholar] [CrossRef]
- Wagner, S.; Klöckner, P.; Reemtsma, T. Aging of tire and road wear particles in terrestrial and freshwater environments–a review on processes, testing, analysis and impact. Chemosphere 2022, 288, 132467. [Google Scholar] [CrossRef]
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Zhao, X.; Yang, Z.; Hu, D.; Cai, Z. New evidence of rubber-derived quinones in water, air, and soil. Environ. Sci. Technol. 2022, 56, 4142–4150. [Google Scholar] [CrossRef]
- Johannessen, C.; Helm, P.; Lashuk, B.; Yargeau, V.; Metcalfe, C.D. The tire wear compounds 6ppd-quinone and 1,3-diphenylguanidine in an urban watershed. Arch. Environ. Contam. Toxicol. 2022, 82, 171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.; Zhang, W.; Qi, Z.; Song, Y.; Zhu, L.; Dong, C.; Chen, J.; Cai, Z. P-phenylenediamine antioxidants in pm2.5: The underestimated urban air pollutants. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, J.; Du, B.; Pan, Z.; Liu, L.; Zeng, L. E-waste recycling emits large quantities of emerging aromatic amines and organophosphites: A poorly recognized source for another two classes of synthetic antioxidants. Environ. Sci. Technol. Lett. 2022, 9, 625–631. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.M.; Huang, J.L.; Deng, C.L.; Tang, S.Q.; Liu, X.T.; Chen, D. Occurrence of substituted p-phenylenediamine antioxidants in dusts. Environ. Sci. Technol. Lett. 2021, 8, 381. [Google Scholar] [CrossRef]
- Wu, Y.; Venier, M.; Hites, R.A. Broad exposure of the north american environment to phenolic and amino antioxidants and to ultraviolet filters. Environ. Sci. Technol. 2020, 54, 9345–9355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dai, C.; Chen, S.; Hu, H.; Kang, R.; Xu, X.; Huo, X. Spatiotemporal variation of 6ppd and 6ppdq in dust and soil from e-waste recycling areas. Sci. Total Environ. 2024, 923, 171495. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.; Ragas, A.M. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Deng, C.; Huang, J.; Qi, Y.; Chen, D.; Huang, W. Distribution patterns of rubber tire-related chemicals with particle size in road and indoor parking lot dust. Sci. Total Environ. 2022, 844, 157144. [Google Scholar] [CrossRef]
- Redondo-Hasselerharm, P.E.; de Ruijter, V.N.; Mintenig, S.M.; Verschoor, A.; Koelmans, A.A. Ingestion and chronic effects of car tire tread particles on freshwater benthic macroinvertebrates. Environ. Sci. Technol. 2018, 52, 13986–13994. [Google Scholar] [CrossRef]
- Alhelou, R.; Seiwert, B.; Reemtsma, T. Hexamethoxymethylmelamine—A precursor of persistent and mobile contaminants in municipal wastewater and the water cycle. Water Res. 2019, 165, 114973. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.R.; Halle, L.L.; Palmqvist, A. Acute and long-term toxicity of micronized car tire wear particles to hyalella azteca. Aquat. Toxicol. 2019, 213, 105216. [Google Scholar] [CrossRef]
- Halle, L.L.; Palmqvist, A.; Kampmann, K.; Khan, F.R. Ecotoxicology of micronized tire rubber: Past, present and future considerations. Sci. Total Environ. 2020, 706, 135694. [Google Scholar] [CrossRef]
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Barber, L.B.; Burden, D.S.; Foreman, W.T.; Forshay, K.J.; Furlong, E.T.; Groves, J.F.; Hladik, M.L. Urban stormwater: An overlooked pathway of extensive mixed contaminants to surface and groundwaters in the united states. Environ. Sci. Technol. 2019, 53, 10070–10081. [Google Scholar] [CrossRef]
- Bohara, K.; Timilsina, A.; Adhikari, K.; Kafle, A.; Basyal, S.; Joshi, P.; Yadav, A.K. A mini review on 6ppd quinone: A new threat to aquaculture and fisheries. Environ. Pollut. 2024, 340, 122828. [Google Scholar] [CrossRef]
- Lo, B.P.; Marlatt, V.L.; Liao, X.; Reger, S.; Gallilee, C.; Ross, A.R.; Brown, T.M. Acute toxicity of 6ppd-quinone to early life stage juvenile chinook (oncorhynchus tshawytscha) and coho (oncorhynchus kisutch) salmon. Environ. Toxicol. Chem. 2023, 42, 815–822. [Google Scholar] [CrossRef]
- Greer, J.B.; Dalsky, E.M.; Lane, R.F.; Hansen, J.D. Establishing an in vitro model to assess the toxicity of 6ppd-quinone and other tire wear transformation products. Environ. Sci. Technol. Lett. 2023, 10, 533–537. [Google Scholar] [CrossRef]
- Hiki, K.; Yamamoto, H. Concentration and leachability of N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6ppd) and its quinone transformation product (6ppd-q) in road dust collected in tokyo, japan. Environ. Pollut. 2022, 302, 119082. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.; Montgomery, D.; Selinger, S.; Miller, J.G.; Stock, E.; Alcaraz, A.J.; Challis, J.K.; Weber, L.; Janz, D.; Hecker, M. Acute toxicity of the tire rubber-derived chemical 6ppd-quinone to four fishes of commercial, cultural, and ecological importance. Environ. Sci. Technol. Lett. 2022, 9, 333–338. [Google Scholar] [CrossRef]
- Liao, X.; Chen, Z.; Ou, S.; Liu, Q.; Lin, S.; Zhou, J.; Wang, Y.; Cai, Z. Neurological impairment is crucial for tire rubber-derived contaminant 6ppdq-induced acute toxicity to rainbow trout. Sci. Bull. 2024, 69, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Hiki, K.; Asahina, K.; Kato, K.; Yamagishi, T.; Omagari, R.; Iwasaki, Y.; Watanabe, H.; Yamamoto, H. Acute toxicity of a tire rubber-derived chemical, 6ppd quinone, to freshwater fish and crustacean species. Environ. Sci. Technol. Lett. 2021, 8, 779–784. [Google Scholar] [CrossRef]
- Xu, Q.; Li, G.; Fang, L.; Sun, Q.; Han, R.; Zhu, Z.; Zhu, Y. Enhanced formation of 6ppd-q during the aging of tire wear particles in anaerobic flooded soils: The role of iron reduction and environmentally persistent free radicals. Environ. Sci. Technol. 2023, 57, 5978–5987. [Google Scholar] [CrossRef]
- Hua, X.; Feng, X.; Liang, G.; Chao, J.; Wang, D. Exposure to 6-ppd quinone at environmentally relevant concentrations causes abnormal locomotion behaviors and neurodegeneration in caenorhabditis elegans. Environ. Sci. Technol. 2023, 57, 4940–4950. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Wang, M.; Wu, N.; Xu, R.; Wei, L.; Xu, X.; Zhao, J.; Xing, P.; Li, H. Nationwide occurrence and prioritization of tire additives and their transformation products in lake sediments of china. Environ. Int. 2024, 109139. [Google Scholar] [CrossRef]
- Johannessen, C.; Helm, P.; Metcalfe, C.D. Detection of selected tire wear compounds in urban receiving waters. Environ. Pollut. 2021, 287, 117659. [Google Scholar] [CrossRef]
- Zhou, Y.; Yixi, L.; Kong, Q.; Peng, J.; Pan, Y.; Qiu, J.; Yang, X. Sunlight-induced transformation of tire rubber antioxidant N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6ppd) to 6ppd-quinone in water. Environ. Sci. Technol. Lett. 2023, 10, 798–803. [Google Scholar] [CrossRef]
- Monaghan, J.; Jaeger, A.; Agua, A.R.; Stanton, R.S.; Pirrung, M.; Gill, C.G.; Krogh, E.T. A direct mass spectrometry method for the rapid analysis of ubiquitous tire-derived toxin N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine quinone (6-ppdq). Environ. Sci. Technol. Lett. 2021, 8, 1051–1056. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, S.; Liu, X.; Thomes, M.W.; Bong, C.W.; Samaraweera, D.N.D.; Priyadarshana, T.; Zhong, G.; Li, J.; Zhang, G. Fates of benzotriazoles, benzothiazoles, and p-phenylenediamines in wastewater treatment plants in malaysia and sri lanka. ACS ES T Water 2023, 3, 1630–1640. [Google Scholar] [CrossRef]
- Rauert, C.; Charlton, N.; Okoffo, E.D.; Stanton, R.S.; Agua, A.R.; Pirrung, M.C.; Thomas, K.V. Concentrations of tire additive chemicals and tire road wear particles in an australian urban tributary. Environ. Sci. Technol. 2022, 56, 2421–2431. [Google Scholar] [CrossRef]
- Kryuchkov, F.; Foldvik, A.; Sandodden, R.; Uhlig, S. Presence of 6ppd-quinone in runoff water samples from norway using a new lc–ms/ms method. Front. Environ. Chem. 2023, 4, 1194664. [Google Scholar] [CrossRef]
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Qiao, H.; Li, H.; Huang, G.; Yang, Z.; Cai, Z. Occurrence and fate of substituted p-phenylenediamine-derived quinones in hong kong wastewater treatment plants. Environ. Sci. Technol. 2023, 57, 15635–15643. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.; Carmona, E.; Machate, O.; Schulze, T.; Krauss, M.; Brack, W. Contamination pattern and risk assessment of polar compounds in snow melt: An integrative proxy of road runoffs. Environ. Sci. Technol. 2023, 57, 4143–4152. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, S.; Liu, X.; Tian, L.; Mo, Y.; Yi, X.; Liu, S.; Liu, J.; Li, J.; Zhang, G. Aquatic environmental fates and risks of benzotriazoles, benzothiazoles, and p-phenylenediamines in a catchment providing water to a megacity of China. Environ. Res. 2023, 216, 114721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, X.; Shen, B.; Jiang, J.; Shen, H.; Lei, Y.; Liang, Q.; Bai, C.; Huang, C.; Wu, W.; et al. 6ppd and its metabolite 6ppdq induce different developmental toxicities and phenotypes in embryonic zebrafish. J. Hazard. Mater. 2023, 455, 131601. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, B.; Nihemaiti, M.; Troussier, M.; Weyrauch, S.; Reemtsma, T. Abiotic oxidative transformation of 6-ppd and 6-ppd quinone from tires and occurrence of their products in snow from urban roads and in municipal wastewater. Water Res. 2022, 212, 118122. [Google Scholar] [CrossRef] [PubMed]
- Marques Dos Santos, M.; Snyder, S.A. Occurrence of polymer additives 1,3-diphenylguanidine (dpg), N-(1,3-dimethylbutyl)-N′-phenyl-1,4-benzenediamine (6ppd), and chlorinated byproducts in drinking water: Contribution from plumbing polymer materials. Environ. Sci. Technol. Lett. 2023, 10, 885–890. [Google Scholar] [CrossRef]
- Challis, J.K.; Popick, H.; Prajapati, S.; Harder, P.; Giesy, J.P.; McPhedran, K.; Brinkmann, M. Occurrences of tire rubber-derived contaminants in cold-climate urban runoff. Environ. Sci. Technol. Lett. 2021, 8, 961. [Google Scholar] [CrossRef]
- Ji, J.; Li, C.; Zhang, B.; Wu, W.; Wang, J.; Zhu, J.; Liu, D.; Gao, R.; Ma, Y.; Pang, S. Exploration of emerging environmental pollutants 6ppd and 6ppdq in honey and fish samples. Food Chem. 2022, 396, 133640. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Fang, C.; Di, S.; Yu, Y.; Wang, C.; Wang, X.; Jin, Y. Oral exposure to tire rubber-derived contaminant 6ppd and 6ppd-quinone induce hepatotoxicity in mice. Sci. Total Environ. 2023, 869, 161836. [Google Scholar] [CrossRef]
- Nair, P.; Sun, J.; Xie, L.; Kennedy, L.; Kozakiewicz, D.; Kleywegt, S.M.; Hao, C.; Byun, H.; Barrett, H.; Baker, J.; et al. Synthesis and toxicity evaluation of p-phenylenediamine-quinones. Environ. Sci. Technol. 2025, 59, 7485–7494. [Google Scholar] [CrossRef]
- Hägg, F.; Herzke, D.; Nikiforov, V.A.; Booth, A.M.; Sperre, K.H.; Sørensen, L.; Creese, M.E.; Halsband, C. Ingestion of car tire crumb rubber and uptake of associated chemicals by lumpfish (cyclopterus lumpus). Front. Environ. Sci. 2023, 11, 1219248. [Google Scholar] [CrossRef]
- Grasse, N.; Seiwert, B.; Massei, R.; Scholz, S.; Fu, Q.; Reemtsma, T. Uptake and biotransformation of the tire rubber-derived contaminants 6-ppd and 6-ppd quinone in the zebrafish embryo (danio rerio). Environ. Sci. Technol. 2023, 57, 15598–15607. [Google Scholar] [CrossRef]
- Castan, S.; Sherman, A.; Peng, R.; Zumstein, M.T.; Wanek, W.; Hüffer, T.; Hofmann, T. Uptake, metabolism, and accumulation of tire wear particle-derived compounds in lettuce. Environ. Sci. Technol. 2022, 57, 168–178. [Google Scholar] [CrossRef]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment-a review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef]
- Ding, J.; Lv, M.; Zhu, D.; Leifheit, E.F.; Chen, Q.; Wang, Y.; Chen, L.; Rillig, M.C.; Zhu, Y. Tire wear particles: An emerging threat to soil health. Crit. Rev. Environ. Sci. Technol. 2023, 53, 239–257. [Google Scholar] [CrossRef]
- Wu, W.; Xu, Q.; Li, J.; Wang, Z.; Li, G. The spatio-temporal accumulation of 6 ppd-q in greenbelt soils and its effects on soil microbial communities. Environ. Pollut. 2024, 358, 124477. [Google Scholar] [CrossRef]
- Zeng, L.; Li, Y.; Sun, Y.; Liu, L.; Shen, M.; Du, B. Widespread occurrence and transport of p-phenylenediamines and their quinones in sediments across urban rivers, estuaries, coasts, and deep-sea regions. Environ. Sci. Technol. 2023, 57, 2393–2403. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Qian, Z.; Zhong, Q.; Wang, Q.; Hylkema, M.N.; Snieder, H.; Huo, X. Association between 6ppd-quinone exposure and bmi, influenza, and diarrhea in children. Environ. Res. 2024, 247, 118201. [Google Scholar] [CrossRef]
- Botelho, M.T.; Militão, G.G.; Brinkmann, M.; Umbuzeiro, G.D.A. Toxicity and mutagenicity studies of 6ppd-quinone in a marine invertebrate species and bacteria. Environ. Mol. Mutagen. 2023, 64, 335–341. [Google Scholar] [CrossRef]
- Liu, J.; Yu, M.; Shi, R.; Ge, Y.; Li, J.; Zeb, A.; Cheng, Z.; Liu, W. Comparative toxic effect of tire wear particle-derived compounds 6ppd and 6ppd-quinone to chlorella vulgaris. Sci. Total Environ. 2024, 951, 175592. [Google Scholar] [CrossRef]
- Lane, R.F.; Smalling, K.L.; Bradley, P.M.; Greer, J.B.; Gordon, S.E.; Hansen, J.D.; Kolpin, D.W.; Spanjer, A.R.; Masoner, J.R. Tire-derived contaminants 6ppd and 6ppd-q: Analysis, sample handling, and reconnaissance of united states stream exposures. Chemosphere 2024, 363, 142830. [Google Scholar] [CrossRef]
- Shi, C.; Wu, F.; Zhao, Z.; Ye, T.; Luo, X.; Wu, Y.; Liu, Z.; Zhang, H. Effects of environmental concentrations of 6ppd and its quinone metabolite on the growth and reproduction of freshwater cladoceran. Sci. Total Environ. 2024, 948, 175018. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wu, W.; Xiao, Z.; Sun, X.; Ma, J.; Ding, J.; Zhu, Z.; Li, G. Responses of soil and collembolan (folsomia candida) gut microbiomes to 6ppd-q pollution. Sci. Total Environ. 2023, 900, 165810. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, J.; Liang, Y.; Zhao, Y.; Zhang, S.; Chen, Z.; Zhang, J.; Shen, X.; Wang, J.; Zhang, Y.; et al. A review of N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine (6ppd) and its derivative 6ppd-quinone in the environment. Toxics 2024, 12, 394. [Google Scholar] [CrossRef]
- Ihenetu, S.C.; Xu, Q.; Khan, Z.H.; Kazmi, S.S.U.H.; Ding, J.; Sun, Q.; Li, G. Environmental fate of tire-rubber related pollutants 6ppd and 6ppd-q: A review. Environ. Res. 2024, 258, 119492. [Google Scholar] [CrossRef]
- Chen, X.; He, T.; Yang, X.; Gan, Y.; Qing, X.; Wang, J.; Huang, Y. Analysis, environmental occurrence, fate and potential toxicity of tire wear compounds 6ppd and 6ppd-quinone. J. Hazard. Mater. 2023, 452, 131245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhong, Q.; Qian, Z.; Zeng, X.; Zhang, J.; Xu, X.; Hylkema, M.N.; Nolte, I.M.; Snieder, H.; Huo, X. Alterations of gut microbiota and its metabolomics in children with 6ppdq, pbde, pcb, and metal(loid) exposure. J. Hazard. Mater. 2024, 475, 134862. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Zheng, X.; Chung, K.F.; Zhong, N. Impact of air pollution on the burden of chronic respiratory diseases in china: Time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef]
- Chen, R.; Yin, P.; Meng, X.; Wang, L.; Liu, C.; Niu, Y.; Liu, Y.; Liu, J.; Qi, J.; You, J. Associations between coarse particulate matter air pollution and cause-specific mortality: A nationwide analysis in 272 chinese cities. Environ. Health Perspect. 2019, 127, 17008. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, C.Y.; Wang, Z.W. The migration and degradation of N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine from rubber hoses in milk lines. Int. J. Dairy. Technol. 2023, 76, 329–338. [Google Scholar] [CrossRef]
- Hansson, C. Allergic contact dermatitis from N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine and from compounds in polymerized 2,2,4-trimethyl-1,2-dihydroquinoline. Contact Dermat. 1994, 30. [Google Scholar] [CrossRef]
- Nishioka, K.; Murata, M.; Ishikawa, T.; Kaniwa, M. Contact dermatitis due to rubber boots worn by japanese farmers, with special attention to 6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline (etmdq) sensitivity. Occup. Health Ind. Med. 1997, 2, 81. [Google Scholar]
- Sathiakumar, N.; Graff, J.; Macaluso, M.; Maldonado, G.; Matthews, R.; Delzell, E. An updated study of mortality among north american synthetic rubber industry workers. Occup. Environ. Med. 2005, 62, 822–829. [Google Scholar] [CrossRef]
- Lyu, Y.; Guo, H.; Cheng, T.; Li, X. Particle size distributions of oxidative potential of lung-deposited particles: Assessing contributions from quinones and water-soluble metals. Environ. Sci. Technol. 2018, 52, 6592–6600. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, G.; Zhang, F.; Cai, Z. A new toxicity mechanism of N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine quinone: Formation of dna adducts in mammalian cells and aqueous organisms. Sci. Total Environ. 2023, 866, 161373. [Google Scholar] [CrossRef]
- Wolfgang, G.; Jolly, R.A.; Donarski, W.J.; Petry, T.W. Inhibition of diquat-induced lipid peroxidation and toxicity in precision-cut rat liver slices by novel antioxidants. Toxicol. Appl. Pharmacol. 1991, 108, 321–329. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.; Guo, L.; Yu, Q.; Zhang, W.; Peng, Z.; Gao, Y.; Gong, X.; Li, P.; Jiao, H. Emerging N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6ppd) and 6ppd quinone in paired human plasma and urine from tianjin, china: Preliminary assessment with demographic factors. J. Hazard. Mater. 2024, 476, 134818. [Google Scholar] [CrossRef]
- Huang, Y.; Hung, W.; Kang, W.; Chen, W.; Chai, C. P-phenylenediamine induced dna damage in sv-40 immortalized human uroepithelial cells and expression of mutant p53 and cox-2 proteins. Toxicol. Lett. 2007, 170, 116–123. [Google Scholar] [CrossRef]
- Peng, W.; Liu, C.; Chen, D.; Duan, X.; Zhong, L. Exposure to N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6ppd) affects the growth and development of zebrafish embryos/larvae. Ecotoxicol. Environ. Saf. 2022, 232, 113221. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zeng, Z.; Xu, R.; Liang, W.; Guo, Y.; Huo, X. Risk assessment of pbdes and pcbs in dust from an e-waste recycling area of china. Sci. Total Environ. 2022, 803, 150016. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Kang, Q.; Liu, W.; Chen, D.; Wang, L.; Li, S. Chronic exposure to tire rubber-derived contaminant 6ppd-quinone impairs sperm quality and induces the damage of reproductive capacity in male mice. J. Hazard. Mater. 2024, 470, 134165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.N.; Thomas, S.P.; Zylka, M.J.; Dorrestein, P.C.; Hu, W. Urine excretion, organ distribution, and placental transfer of 6ppd and 6ppd-quinone in mice and potential developmental toxicity through nuclear receptor pathways. Environ. Sci. Technol. 2023, 57, 13429–13438. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, N.; Lv, J.; Chen, H.; Wang, H.; Xu, J.; Hu, J.; Tao, L.; Fang, M.; Huang, Y. Environmentally realistic dose of tire-derived metabolite 6ppd-q exposure causes intestinal jejunum and ileum damage in mice via cannabinoid receptor-activated inflammation. Sci. Total Environ. 2024, 918, 170679. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Cao, G.; Wang, F.; Ru, Y.; Wang, B.; Zhang, Y.; Zhang, D.; Yan, J.; Xu, J.; et al. 6ppd-quinone exposure induces neuronal mitochondrial dysfunction to exacerbate lewy neurites formation induced by α-synuclein preformed fibrils seeding. J. Hazard. Mater. 2024, 465, 133312. [Google Scholar] [CrossRef]
- Yong, Z.; Lu, D.; Yan, X. A comparative study of toxicity of rubber antioxidant 4020 and 4010na on human embryonic lung fibroblast cells. Occup. Health 2016, 32, 1991–1992. [Google Scholar] [CrossRef]
- Ancona, A.; Monroy, F.; Fernández-Diez, J. Occupational dermatitis from ippd in tyres. Contact Dermat. 1982, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Herve-Bazin, B.; Gradiski, D.; Duprat, P.; Marignac, B.; Foussereau, J.; Cavelier, C.; Bieber, P. Occupational eczema from n-isopropyl -n’-phenylparaphenylenediamine (ippd) and n-dimethy-1,3 butyl-n’-phenylparaphenylenediamine (dmppd) in tyres. Contact Dermat. 1977, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, F.; Zhang, L.; Shan, C.; Bai, Z.; Sun, Z.; Liu, L.; Shen, B. A comprehensive assessment of human exposure to phthalates from environmental media and food in Tianjin, China. J. Hazard. Mater. 2014, 279, 133–140. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wu, L.; Wang, T.; Zhao, J.; Zhang, Y.; Men, Z.; Mao, H. Occurrence of benzothiazole and its derivates in tire wear, road dust, and roadside soil. Chemosphere 2018, 201, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Zetterlund, P.B.; Yamada, B. Prevention of rubber degradation by use of microencapsulated antioxidants. Rubber Chem. Technol. 2003, 76, 948–956. [Google Scholar] [CrossRef]
- El Wakil, A.E.A.A.; El Mogy, S.; Halim, S.F.; Abdel Hakim, A. Enhancement of aging resistance of epdm rubber by natural rubber-g-n (4-phenylenediamine) maleimide as a grafted antioxidant. J. Vinyl Addit. Technol. 2022, 28, 367–378. [Google Scholar] [CrossRef]
- Kruger, R.H.; Boissière, C.; Klein-Hartwig, K.; Kretzschmar, H. New phenylenediamine antiozonants for commodities based on natural and synthetic rubber. Food Addit. Contam. 2005, 22, 968–974. [Google Scholar] [CrossRef]
- Huntink, N.M.; Datta, R.N.; Talma, A.; Noordermeer, J.W. Ozonolysis of model olefins—Efficiency of antiozonants. J. Appl. Polym. Sci. 2006, 100, 853–866. [Google Scholar] [CrossRef]
| Species | Endpoint | Pollutants | LC50 (μg/L) | Test Duration | References | |
|---|---|---|---|---|---|---|
| Salvelinus leucomaenis pluvius | Death | 6PPDQ | 0.51 | 24 h | [41] | |
| Oncorhynchus kisutch | Infancy | Death | 6PPDQ | 0.95 | 24 h | [42] |
| Adult | Death | 6PPDQ | 0.41 | 24 h | ||
| Oncorhynchus kisutch | Death | 6PPDQ | 0.804 | 24 h | [43] | |
| Oncorhynchus masou | Death | 6PPDQ | <3.8 | 24 h | [41] | |
| Brachymystax lenok | Death | 6PPDQ | <3.8 | 24 h | [41] | |
| Salvelinus leucomaenis | Death | 6PPDQ | 5.1 | 24 h | [44] | |
| Salvelinus fontinalis | Death | 6PPDQ | 5.9 | 24 h | [45] | |
| Oncorhynchus mykiss | Death | 6PPDQ | 19.6 | 24 h | [45] | |
| Death | 6PPDQ | 9.0 | 24 h | [46] | ||
| Oncorhynchus masou | Death | 6PPDQ | >100 | 24 h | [44] | |
| Salvelinus alpinus | Death | 6PPDQ | >127 | 24 h | [45] | |
| Psephurus gladius | Death | 6PPDQ | >127 | 24 h | [45] | |
| Oryzias latipes | Death | 6PPDQ | >340 | 96 h | [47] | |
| Danio rerio larva | Death | 6PPD | 1384.93 | 24 h | [17] | |
| 442.62 | 96 h | |||||
| 6PPDQ | 308.67 | 24 h | ||||
| 132.92 | 96 h | |||||
| Danio rerio | Death | 6PPDQ | >540 | 96 h | [47] | |
| Death | 6PPDQ | 3086.7 | 96 h | [17] | ||
| Oncorhynchus tshawytscha | Death | 6PPDQ | >673.06 | 24 h | [42] | |
| Death | 6PPDQ | >800 | 24 h | [43] | ||
| Collembola | Death | 6PPDQ | 16.31 (μg/kg) in soil | 28 d | [48] | |
| Caenorhabditis elegans | Death | 6PPDQ | >100 | 4.5 d | [49] | |
| Environmental Media | 6PPDQ | 6PPD | Countries and References |
|---|---|---|---|
| Surface water | |||
| City surface water | 96–112 ng/L | / | Canada [53] |
| Zhujiang River | 1.51 (0.26–11.3) ng/L | 0.48 (0.31–1.07) ng/L | China [54] |
| Dongjiang River | 0.91 (0.29–8.12) ng/L | 0.36 (0.27–1.29) ng/L | |
| Liuxi River | 0.18 (/–0.75) ng/L | / | |
| Brisbane River | 17.5 (0.38–88) ng/L | / | Australia [55] |
| Jiaojiang | 6.1 (<LOD–21) ng/L | 10 (4.0–72) ng/L | China [16] |
| Stormwater | |||
| A city with heavy traffic | 1.12 (0.21–2.43) µg/L | 0.32 (0.21–2.71) µg/L | China [27] |
| Tunnel wash runoff | 49.5–143 ng/L | / | Norway [56] |
| Artificial turf runoff | 159 ng/L | / | |
| City stormwater | 48–5580 ng/L | / | Canada [53] |
| Roadway runoff | 576 (38.5–1562) ng/L | 3.05 (0.41–7.52) ng/L | China [54] |
| Courtyard runoff | 51.6 (6.03–875) ng/L | 0.89 (0.19–1.10) ng/L | |
| Farmland runoff | 0.73 (0.53–5.58) ng/L | / | |
| Groundwater | |||
| Guangzhou | 0.11 (/–0.70) ng/L | / | China [54] |
| Waste water | |||
| Influent (raw) | 53 (1.9–470) ng/L | 12 (1.1–59) ng/L | China [57] |
| Effluent (treated) | 3.4 (1.1–37) ng/L | 0.30 (<LOQ–15) ng/L | |
| Influent (raw) | 64.8 ± 5.3–145.7 ± 46.7 ng | / | Canada [51] |
| Effluent (treated) | <LOD–446.5 ± 37.7 ng | / | |
| Influent (raw) | 777 (592–1100) ng/L | / | Germany [58] |
| Effluent (treated) | 50 (41–66) ng/L | / | |
| Malaysia WWTP influent (raw) | / | / | Malaysia and Sri Lanka [59] |
| Malaysia WWTP effluent (treated) | /(/–0.11) ng/L | / | |
| Sri Lanka WWTP influent (raw) | /(/–0.37) ng/L | / | |
| Sri Lanka WWTP effluent (treated) | /(/–0.37) ng/L | / | |
| WWTP influent (raw) | 14.2 ± 0.80 to 69.8 ± 2.40 ng/L | / | China [60] |
| WWTP effluent (treated) | /–2.09 ± 0.16 ng/L | / | |
| Snowmelt period WWTP influent (raw) | 0.105 ± 0.037 µg/L | 4.4 µg/L | Germany [61] |
| Snowmelt period WWTP Effluent (treated) | / | 2.4 µg/L | |
| Rainfall period WWTP influent (raw) | 0.052 ± 0.022 | 14.3 | |
| Rainfall period WWTP effluent (treated) | / | 11.2 | |
| Dry weather WWTP influent (raw) | / | 0.9 | |
| Dry weather WWTP effluent (treated) | / | 0.3 | |
| Drinking water | |||
| Singapore | / | <10 ng/L | Singapore [62] |
| Snowmelt | |||
| City | 2019: 367 (74–756) ng/L | / | Canada [63] |
| City | 2020: 81 (15–172) ng/L | / | |
| Snow | |||
| Roadside | 259 (110–428) ng/L | 329 (/–784) ng/L | Germany [58] |
| Species | 6PPDQ | 6PPD | Countries/Regions and References | |
|---|---|---|---|---|
| Aquatic organism | Snakehead | / | 0.669 μg/kg | China [64] |
| Weever | / | 0.481 μg/kg | ||
| Spanish mackerel | <LOQ | / | ||
| Zebrafish | / | 351 ng/g | China [65] | |
| Rainbow trout | BCFs of 6PPDQ were calculated as 2.9, 19, 25, and 17.2 | / | Canada [66] | |
| 293 L/kg at the water concentrations of 0.8, 3, 12, and 25 µg/L | ||||
| Rotundipterus | / | 1206 pg/g | Norway [67] | |
| Zebra fish | Max of 225 at 48 h | Max of 3000 at 48 h | Germany [68] | |
| Food | Lettuce-1 mg/L6PPDQ | 2.19 µg/g | 0.78 µg/g | Austria [69] |
| Lettuce-TWP | 0.02 µg/g | 0.4 µg/g | ||
| Honey | / | / | China [64] |
| Environmental Media | Type | 6PPDQ | 6PPD | Countries/Regions and References |
|---|---|---|---|---|
| Soil | Roadside | 234 (9.50–936) ng/g | 309 (31.4–831) ng/g | China [Hong Kong, New Territories and Kowloon] [27] |
| Sediment | Fluvial sediment | 9.03 (1.87–18.2) ng/g | 14.4 (0.585–468) ng/g | China [Pearl River Delta, Pearl River Estuary, South China Sea] [73] |
| Estuarine sediment | 2.00 (<MDL–4.88) ng/g | 3.9 (1.49–5.71) ng/g | ||
| Coast sediment | 1.27 (0.431–2.98) ng/g | 1.82 (1.07–11.1) ng/g | ||
| Abyssal sediment | 2.71 (<MDL–3.02) ng/g | 2.66 (<MDL–2.69) ng/g |
| Environmental Media | Main Content | References |
|---|---|---|
| Water Environment | The occurrence and partitioning of p-phenylenediamine antioxidants and their quinone derivatives in water and sediment. | [16] |
| The toxicological effects of 6PPD and 6PPD quinone on zebrafish larvae. | [17] | |
| 6PPD quinone and its emergence as a new threat to aquaculture and fisheries. | [41] | |
| The detection of specific tire wear compounds in urban receiving waters. | [51] | |
| 6PPD and its metabolite 6PPDQ cause different developmental toxicities and phenotypes in embryonic zebrafish. | [60] | |
| Conducting toxicity and mutagenicity studies of 6PPD quinone in a marine invertebrate species as well as in bacteria. | [75] | |
| Comparing the toxic effects of 6PPD and 6PPD quinone, which are compounds derived from tire wear particles, on Chlorella vulgaris. | [76] | |
| The exposure of 6PPD and 6PPDQ in rivers in the United States. | [77] | |
| The impacts of environmental concentrations of 6PPD and its quinone metabolite on the growth and reproduction of freshwater cladoceran. | [78] | |
| Soil Environment | The enhanced formation of 6PPDQ during the aging of tire wear particles in anaerobic flooded soils and explores the roles of iron reduction and environmentally persistent free radicals in this process. | [48] |
| The nationwide presence and prioritization of tire additives and their transformation products in lake sediments across China. | [50] | |
| The temporal and spatial accumulation of 6PPDQ in green belt soil and its effects on soil microbial community were studied. | [72] | |
| The responses of soil and collembolan (Folsomia candida) gut microbiomes to 6PPDQ pollution. | [79] | |
| Atmospheric Environment | The relevant research content on comprehensively characterizing the tire and road wear particles in the road dust of the highway tunnel by using the size and density fractionation method. | [6] |
| The role of p-phenylenediamine-derived quinones as new contributors to the oxidative potential of fine particulate matter, discussing their presence, distribution, and correlations in PM2. | [10] | |
| The formation of transformation products during the heterogeneous ozonation of the tire rubber antioxidant 6PPD. | [11] | |
| Six PPD-derived quinone compounds and eight PPD antioxidants simultaneously assessed in PM2.5. | [20] | |
| The association between particulate matter pollution (PM2.5) in the atmosphere of 652 cities around the world and daily mortality and found that there were regional differences. | [21] | |
| The development of new hair dyes as alternatives to the hazardous para-phenylenediamine. | [22] | |
| The widespread occurrence and distribution characteristics of 6PPDQ in size-fractioned atmospheric particles and dust from different indoor environments. | [23] | |
| The occurrence of substituted p-phenylenediamine antioxidants in dust. | [31] | |
| The particle size distribution of rubber tire-related chemicals in road and indoor parking lot dust. | [35] | |
| Association between 6PPDQ exposure and body mass index (BMI), influenza, and diarrhea in children. | [74] | |
| Comprehensive | The environmental profiles, hazard identification, and toxicological hallmarks of the emerging tire rubber-related contaminants 6PPD and 6PPD quinone. | [1] |
| The sources of p-phenylenediamine antioxidants (PPDs) and their derived quinone compounds (PPDQs), their distribution in environmental media and the human body, human exposure levels, and health risks. | [9] | |
| The transformation, environmental distribution, bioavailability, and toxicity of the tire-rubber related pollutant 6PPD quinone. | [19] | |
| The article presents new evidence regarding rubber-derived quinones in water, air, and soil. | [27] | |
| E-Waste recycling emits large quantities of emerging aromatic amines and organophosphites, which are a poorly recognized source for two classes of synthetic antioxidants. | [30] | |
| N-(1,3-Dimethylbutyl)-N′-phenyl-p-Phenylenediamine (6PPD) and its derivative 6PPD quinone in the environmental context. | [80] | |
| The environmental fate of tire-rubber related pollutants 6PPD and 6PPDQ. | [81] | |
| The analysis methods, environmental occurrence, fate in the environment, and potential toxicity of tire wear compounds 6PPD and 6PPD quinone. | [82] | |
| E-Waste | Concentration, source, and health effects assessment in urine, involving multiple environmental mediators in e-waste dismantling areas. | [9] |
| Temporal and spatial variations in 6PPD and 6PPDQ in dust and soil of e-waste recycling area were studied, involving the environmental behavior and potential risks of 6PPDQ in various environmental media. | [74] | |
| The changes in gut microbiota and its metabolomics in children who are exposed to 6PPDQ, PBDE, PCB, and metal(loid). | [83] |
| Parameter | Unit | Children | Adult |
|---|---|---|---|
| Ingestion rate (IRing) | mg/d | 200 | 100 |
| Exposure frequency (EF) | d/a | 365 | 365 |
| Exposure duration (ED) | a | 6 | 24 |
| Body weight (BW) | kg | 16.58 | 58.55 |
| Average time during exposure (AT) | d | 365 × 70 | 365 × 70 |
| Conversion factor (CF) | kg/mg | 10−6 | 10−6 |
| Skin surface area available for contact (SA) | cm2 | 1150 | 2145 |
| Soil-to-skin adherence factor (AF) | mg/cm2 | 0.2 | 0.07 |
| Adsorption factor (ABS) | % | 13 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Tang, J.; Qiu, Z.; Huo, X.; Liu, D.; Zeng, X. Environmental and Human Health Risks of 6PPD and 6PPDQ: Assessment and Implications. Toxics 2025, 13, 873. https://doi.org/10.3390/toxics13100873
Zhang S, Tang J, Qiu Z, Huo X, Liu D, Zeng X. Environmental and Human Health Risks of 6PPD and 6PPDQ: Assessment and Implications. Toxics. 2025; 13(10):873. https://doi.org/10.3390/toxics13100873
Chicago/Turabian StyleZhang, Sainan, Jiayue Tang, Zhiying Qiu, Xia Huo, Dongling Liu, and Xiang Zeng. 2025. "Environmental and Human Health Risks of 6PPD and 6PPDQ: Assessment and Implications" Toxics 13, no. 10: 873. https://doi.org/10.3390/toxics13100873
APA StyleZhang, S., Tang, J., Qiu, Z., Huo, X., Liu, D., & Zeng, X. (2025). Environmental and Human Health Risks of 6PPD and 6PPDQ: Assessment and Implications. Toxics, 13(10), 873. https://doi.org/10.3390/toxics13100873