Non-Destructive Luminescence-Based Screening Tool for Listeria monocytogenes Growth on Ham
Abstract
1. Introduction
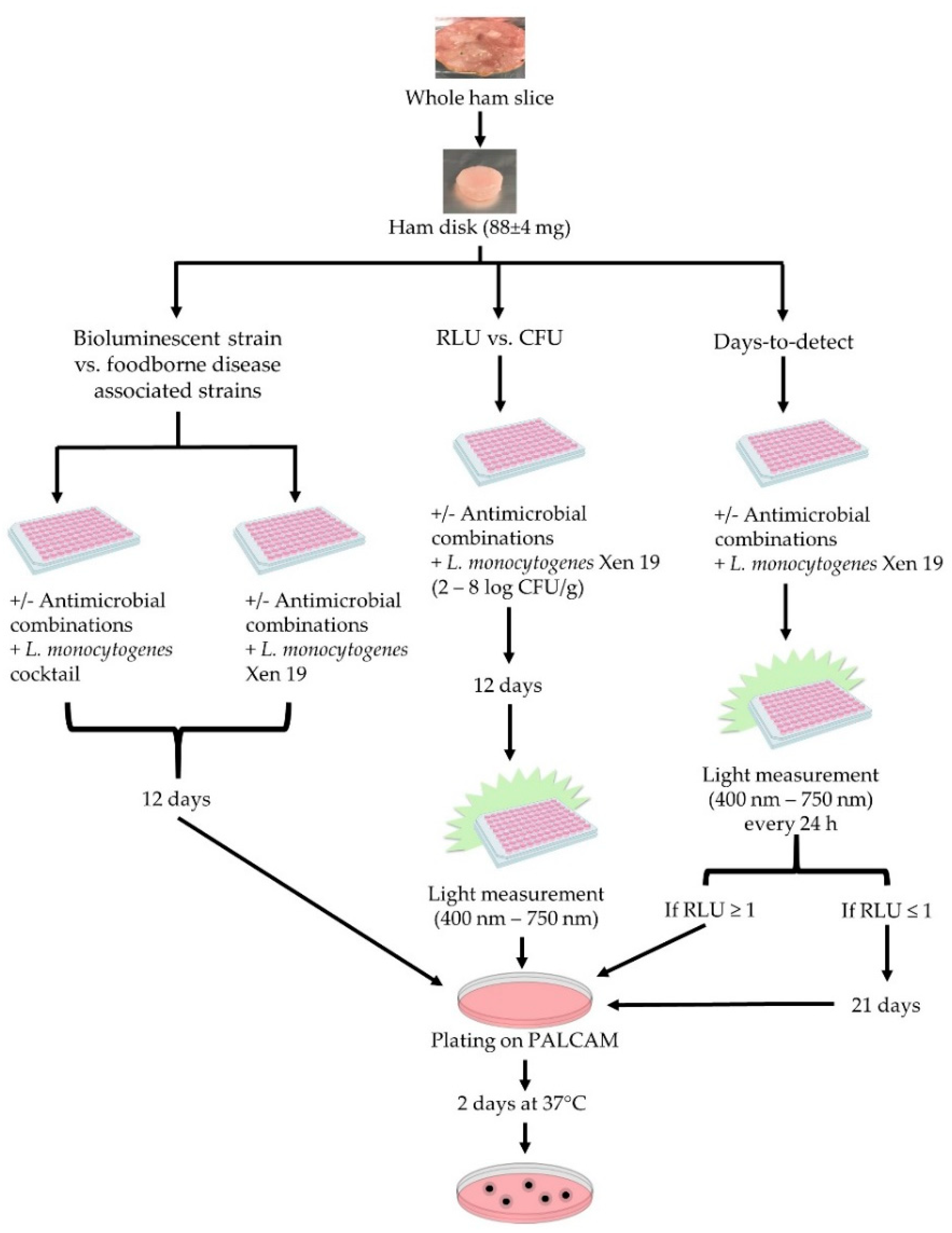
2. Materials and Methods
2.1. Sample Preparation
2.2. Microorganisms and Growth Conditions
2.3. Description of Ham Model
2.4. Antimicrobials Used
2.5. Determination of Growth at the End Point
2.6. Data Analysis
3. Results
3.1. Growth Comparison of the Bioluminescent Strain to Foodborne Disease Associated Strains
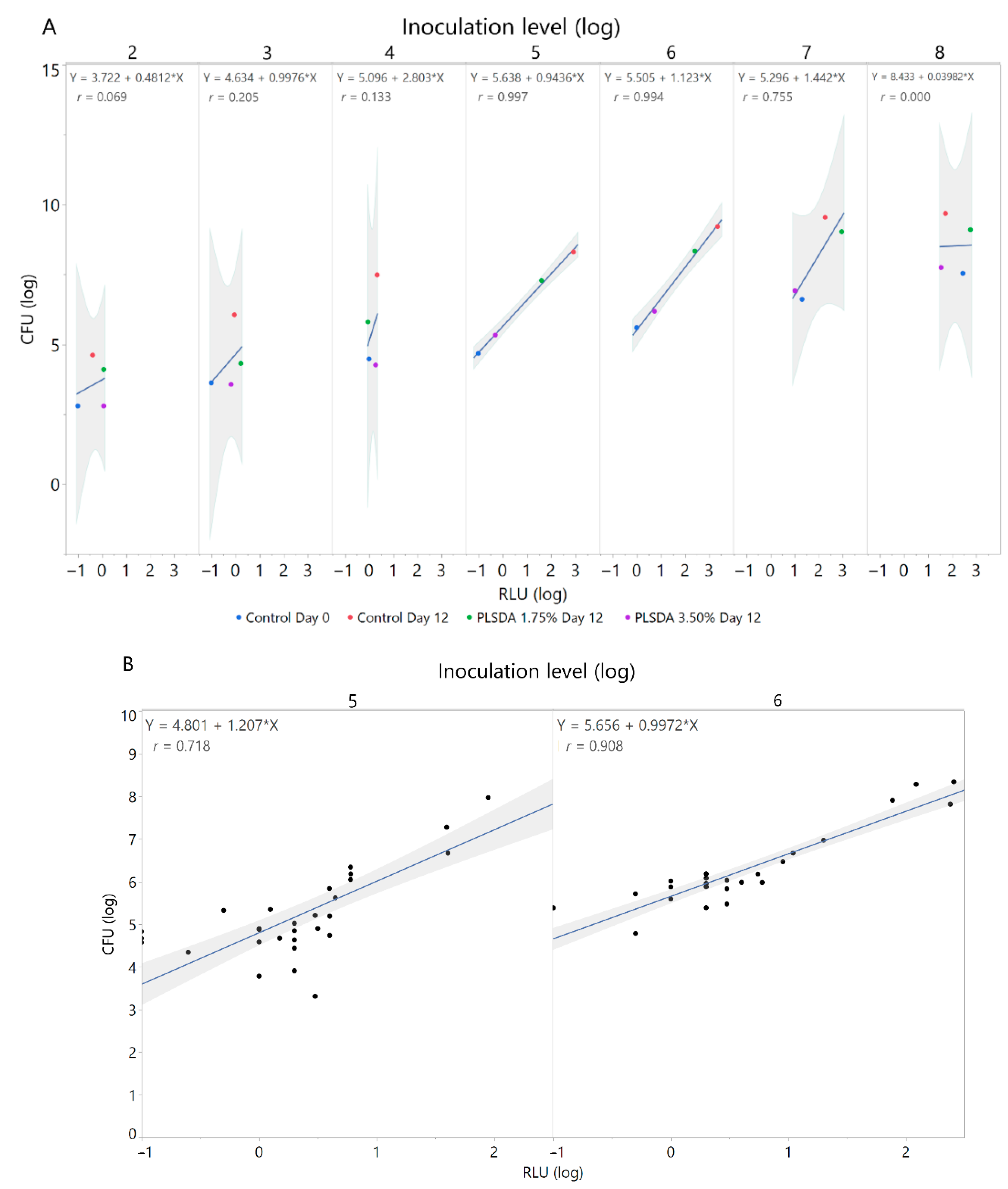
3.2. Correlation of Colony Forming Units and Relative Light Units
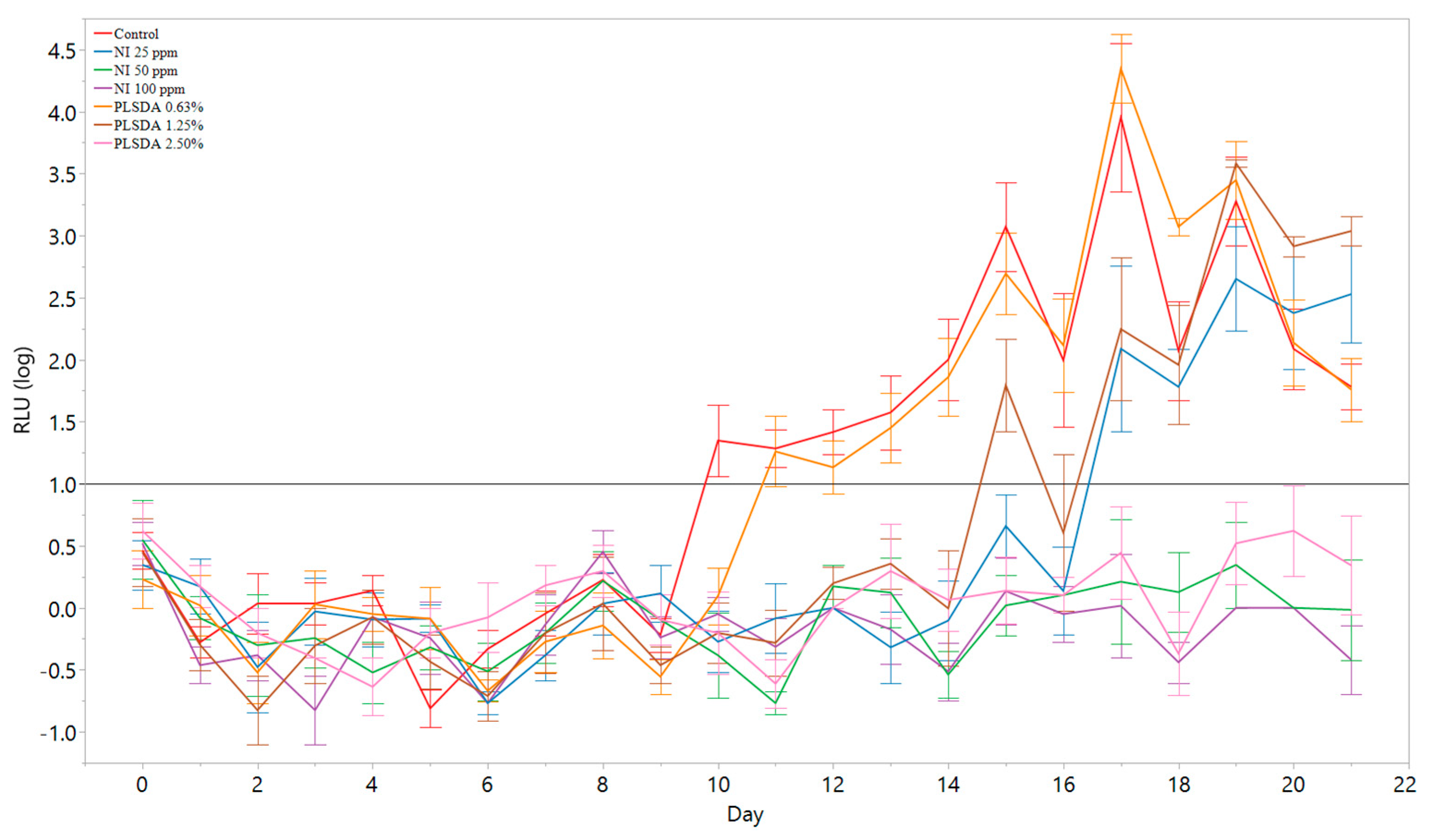
3.3. Preliminary Relative Light Unit Threshold Determination with Limited Antimicrobial Treatments
3.4. Application of the Model to Antimicrobial Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tack, D.M. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Pouillot, R.; Gallagher, D.; Tang, J.; Hoelzer, K.; Kause, J.; Dennis, S.B. Listeria monocytogenes in Retail Delicatessens: An Interagency Risk Assessment—Model and Baseline Results. J. Food Prot. 2015, 78, 134–145. [Google Scholar] [CrossRef] [PubMed]
- CDC Outbreak of Listeria Infections Linked to Deli-Sliced Meats and Cheeses | Outbreak of Listeria Infections Linked to Deli-Sliced Products | April 2019 | Listeria | CDC. Available online: https://www.cdc.gov/listeria/outbreaks/deliproducts-04-19/index.html (accessed on 5 October 2020).
- Zhu, M.; Du, M.; Cordray, J.; Ahn, D.U. Control of Listeria monocytogenes contamination in ready-to-eat meat products. Compr. Rev. Food Sci. Food Saf. 2005, 4, 34–42. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Available online: https://www.hindawi.com/journals/tswj/2014/248935/ (accessed on 22 March 2019).
- Kozak, S.M.; Margison, K.M.; D’amico, D.J. Synergistic antimicrobial combinations inhibit and inactivate Listeria monocytogenes in neutral and acidic broth systems. J. Food Prot. 2017, 80, 1266–1272. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef]
- Terjung, N.; Holzwarth, S.; Loeffler, M.; Gibis, M.; Herrmann, K.; Hinrichs, J.; Weiss, J. Antimicrobial efficacy of a spice ferment in emulsion type sausages and restructured ham. Food Control 2016, 59, 139–147. [Google Scholar] [CrossRef]
- Lavieri, N.A.; Sebranek, J.G.; Brehm-Stecher, B.F.; Cordray, J.C.; Dickson, J.S.; Horsch, A.M.; Jung, S.; Larson, E.M.; Manu, D.K.; Mendonça, A.F. Investigating the control of Listeria monocytogenes on a ready-to-eat ham product using natural antimicrobial ingredients and postlethality interventions. Foodborne Pathog. Dis. 2014, 11, 462–467. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, H.; Zhou, H.; Jin, J.; Xie, Y. Synergistic effect of plantaricin BM-1 combined with physicochemical treatments on the control of Listeria monocytogenes in cooked ham. J. Food Prot. 2017, 80, 976–981. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.D.; Lacroix, M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Technologies and Mechanisms for Safety Control of Ready-to-eat Muscle Foods: An Updated Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1886–1901. [Google Scholar] [CrossRef] [PubMed]
- Asperger, H.; Heistinger, H.; Wagner, M.; Lehner, A.; Brandl, E. A contribution of Listeria enrichment methodology-growth of Listeria monocytogenes under varying conditions concerning enrichment broth composition, cheese matrices and competing microbial flora. Food Microbiol. 1999, 16, 419–431. [Google Scholar] [CrossRef]
- Huq, T.; Vu, K.D.; Riedl, B.; Bouchard, J.; Lacroix, M. Synergistic effect of gamma (γ)-irradiation and microencapsulated antimicrobials against Listeria monocytogenes on ready-to-eat (RTE) meat. Food Microbiol. 2015, 46, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, M.L.; Ibarra-Sánchez, L.A.; Takhar, S.R.; Amaya-Llano, S.L.; Miller, M.J. Use of a miniature laboratory fresh cheese model for investigating antimicrobial activities. J. Dairy Sci. 2015, 98, 8515–8524. [Google Scholar] [CrossRef] [PubMed]
- Baños, A.; Ananou, S.; Martínez-Bueno, M.; Gálvez, A.; Maqueda, M.; Valdivia, E. Prevention of spoilage by enterocin AS-48 combined with chemical preservatives, under vacuum, or modified atmosphere in a cooked ham model. Food Control 2012, 24, 15–22. [Google Scholar] [CrossRef]
- Tassou, C.C.; Panagou, E.Z.; Samaras, F.J.; Galiatsatou, P.; Mallidis, C.G. Temperature-assisted high hydrostatic pressure inactivation of Staphylococcus aureus in a ham model system: Evaluation in selective and nonselective medium. J. Appl. Microbiol. 2008, 104, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Tassou, C.C.; Galiatsatou, P.; Samaras, F.J.; Mallidis, C.G. Inactivation kinetics of a piezotolerant Staphylococcus aureus isolated from high-pressure-treated sliced ham by high pressure in buffer and in a ham model system: Evaluation in selective and non-selective medium. Innov. Food Sci. Emerg. Technol. 2007, 8, 478–484. [Google Scholar] [CrossRef]
- Loessner, M.J.; Rudolf, M.; Scherer, S. Evaluation of luciferase reporter bacteriophage A511::luxAB for detection of Listeria monocytogenes in contaminated foods. Appl. Environ. Microbiol. 1997, 63, 2961–2965. [Google Scholar] [CrossRef]
- Walker, A.J.; Holah, J.T.; Denyer, S.P.; Stewart, G.S.A.B. The antibacterial activity of Virkon measured by colony growth and bioluminescence of lux recombinant Listeria monocytogenes. Lett. Appl. Microbiol. 1992, 15, 80–82. [Google Scholar] [CrossRef]
- Morrissey, R.; Hill, C.; Begley, M. Shining light on food microbiology; applications of Lux-tagged microorganisms in the food industry. Trends Food Sci. Technol. 2013, 32, 4–15. [Google Scholar] [CrossRef]
- Riedel, C.U.; Monk, I.R.; Casey, P.G.; Morrissey, D.; O’Sullivan, G.C.; Tangney, M.; Hill, C.; Gahan, C.G.M. Improved luciferase tagging system for Listeria monocytogenes allows real-time monitoring in vivo and in vitro. Appl. Environ. Microbiol. 2007, 73, 3091–3094. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Basu, U.; Miller, P.; McMullen, L.M. Stress response and adaptation of Listeria monocytogenes 08-5923 exposed to a sublethal dose of carnocyclin A. Appl. Environ. Microbiol. 2014, 80, 3835–3841. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.Y.; Hanson, R.E.; Johnson, N.R.; Chappa, K.; Berrang, M.E. Combining organic acid treatment with steam pasteurization to eliminate Listeria monocytogenes on fully cooked frankfurters. J. Food Prot. 2006, 69, 47–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lianou, A.; Geornaras, I.; Kendall, P.A.; Belk, K.E.; Scanga, J.A.; Smith, G.C.; Sofos, J.N. Fate of Listeria monocytogenes in Commercial Ham, Formulated with or without Antimicrobials, under Conditions Simulating Contamination in the Processing or Retail Environment and during Home Storage. J. Food Prot. 2007, 70, 378–385. [Google Scholar] [CrossRef]
- Mellefont, L.A.; Ross, T. Effect of Potassium Lactate and a Potassium Lactate–Sodium Diacetate Blend on Listeria monocytogenes Growth in Modified Atmosphere Packaged Sliced Ham. J. Food Prot. 2007, 70, 2297–2305. [Google Scholar] [CrossRef]
- Seman, D.L.; Borger, A.C.; Meyer, J.D.; Hall, P.A.; Milkowski, A.L. Modeling the Growth of Listeria monocytogenes in Cured Ready-to-Eat Processed Meat Products by Manipulation of Sodium Chloride, Sodium Diacetate, Potassium Lactate, and Product Moisture Content. J. Food Prot. 2002, 65, 651–658. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef]
- Loessner, M.J.; Rees, C.E.; Stewart, G.S.; Scherer, S. Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable Listeria cells. Appl. Environ. Microbiol. 1996, 62, 1133–1140. [Google Scholar] [CrossRef]
- Bhatti, I.A.; Kim, B.K.; Kim, M.Y.; Lee, J.; Kim, H.K.; Kwon, J.H. The screening and/or identification of different types of irradiated eggs by analyzing photo-stimulated luminescence and thermoluminescence. Food Control 2008, 19, 587–591. [Google Scholar] [CrossRef]
- Song, B.-S.; Kim, B.-K.; Yoon, Y.-M.; Jung, K.; Park, J.-H.; Kim, J.-K.; Kim, C.-T.; Lee, Y.; Kim, D.-H.; Ryu, S.-R. Identification of red pepper powder irradiated with different types of radiation using luminescence methods: A comparative study. Food Chem. 2016, 200, 293–300. [Google Scholar] [CrossRef]
- El Kheir, S.M.; Cherrat, L.; Awussi, A.A.; Ramia, N.E.; Taha, S.; Rahman, A.; Passerini, D.; Leroi, F.; Petit, J.; Mangavel, C.; et al. High-throughput identification of candidate strains for biopreservation by using bioluminescent Listeria monocytogenes. Front. Microbiol. 2018, 9, 1883. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Griffiths, M.W. Luminescent Salmonella strains as real time reporters of growth and recovery from sublethal injury in food. Int. J. Food Microbiol. 1996, 31, 27–43. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, G.; Doyle, M.P. Green fluorescent protein labeling of Listeria, Salmonella, and Escherichia coli O157:H7 for safety-related studies. PLoS ONE 2011, 6, e18083. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Monk, I.R.; Corr, S.C.; Hill, C.; Gahan, C.G.M. Novel luciferase reporter system for in vitro and organ-specific monitoring of differential gene expression in Listeria monocytogenes. Appl. Environ. Microbiol. 2006, 72, 2876–2884. [Google Scholar] [CrossRef]


| Strain | Serotype | Phenotype/Source of Isolation |
|---|---|---|
| L. monocytogenes Xen19 | 4b | Bioluminescent; Derived from ATCC 23074 |
| L. monocytogenes NRRL B-33419 * | 1/2a | Human, epidemic, sliced turkey |
| L. monocytogenes NRRL B-33424 * | 1/2b | Human, epidemic, chocolate milk |
| L. monocytogenes NRRL B-33420 * | 4b | Food, epidemic, RTE meat products |
| L. monocytogenes NRRL B-33513 * | 4b | Food, epidemic, pate |
| L. monocytogenes NRRL B-33104 * | 4b | Food, epidemic, Jalisco cheese |
| RLU Threshold Status | Antimicrobial Treatment | Plating Days | Average Plate Counts (log CFU/g) |
|---|---|---|---|
| Reached 1 log RLU | Control | 10 | 8.49 ± 0.18 a** |
| PLSDA 0.6% | 11 | 8.37 ± 0.20 a | |
| PLSDA 1.25% | 15 | 8.22 ± 0.29 a | |
| NI 25 ppm | 17 | 8.13 ± 0.23 a | |
| Did not reach 1 log RLU | PLSDA 2.50% | 21 | 7.50 ± 0.39 b |
| NI 50 ppm | 21 | 6.56 ± 0.48 c | |
| NI 100 ppm | 21 | 5.28 ± 0.24 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezac, S.D.; Resendiz-Moctezuma, C.; Boler, D.D.; Stasiewicz, M.J.; Miller, M.J. Non-Destructive Luminescence-Based Screening Tool for Listeria monocytogenes Growth on Ham. Foods 2020, 9, 1700. https://doi.org/10.3390/foods9111700
Rezac SD, Resendiz-Moctezuma C, Boler DD, Stasiewicz MJ, Miller MJ. Non-Destructive Luminescence-Based Screening Tool for Listeria monocytogenes Growth on Ham. Foods. 2020; 9(11):1700. https://doi.org/10.3390/foods9111700
Chicago/Turabian StyleRezac, Shannon D., Cristina Resendiz-Moctezuma, Dustin D. Boler, Matthew J. Stasiewicz, and Michael J. Miller. 2020. "Non-Destructive Luminescence-Based Screening Tool for Listeria monocytogenes Growth on Ham" Foods 9, no. 11: 1700. https://doi.org/10.3390/foods9111700
APA StyleRezac, S. D., Resendiz-Moctezuma, C., Boler, D. D., Stasiewicz, M. J., & Miller, M. J. (2020). Non-Destructive Luminescence-Based Screening Tool for Listeria monocytogenes Growth on Ham. Foods, 9(11), 1700. https://doi.org/10.3390/foods9111700






