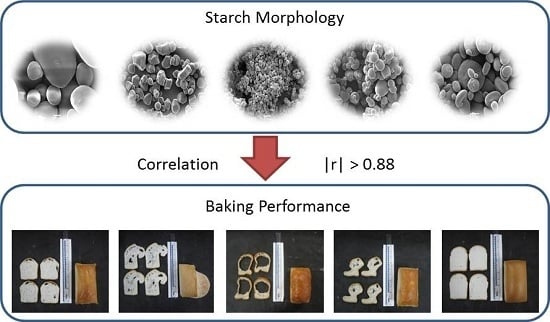
Fundamental Study on the Impact of Gluten-Free Starches on the Quality of Gluten-Free Model Breads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microscopy
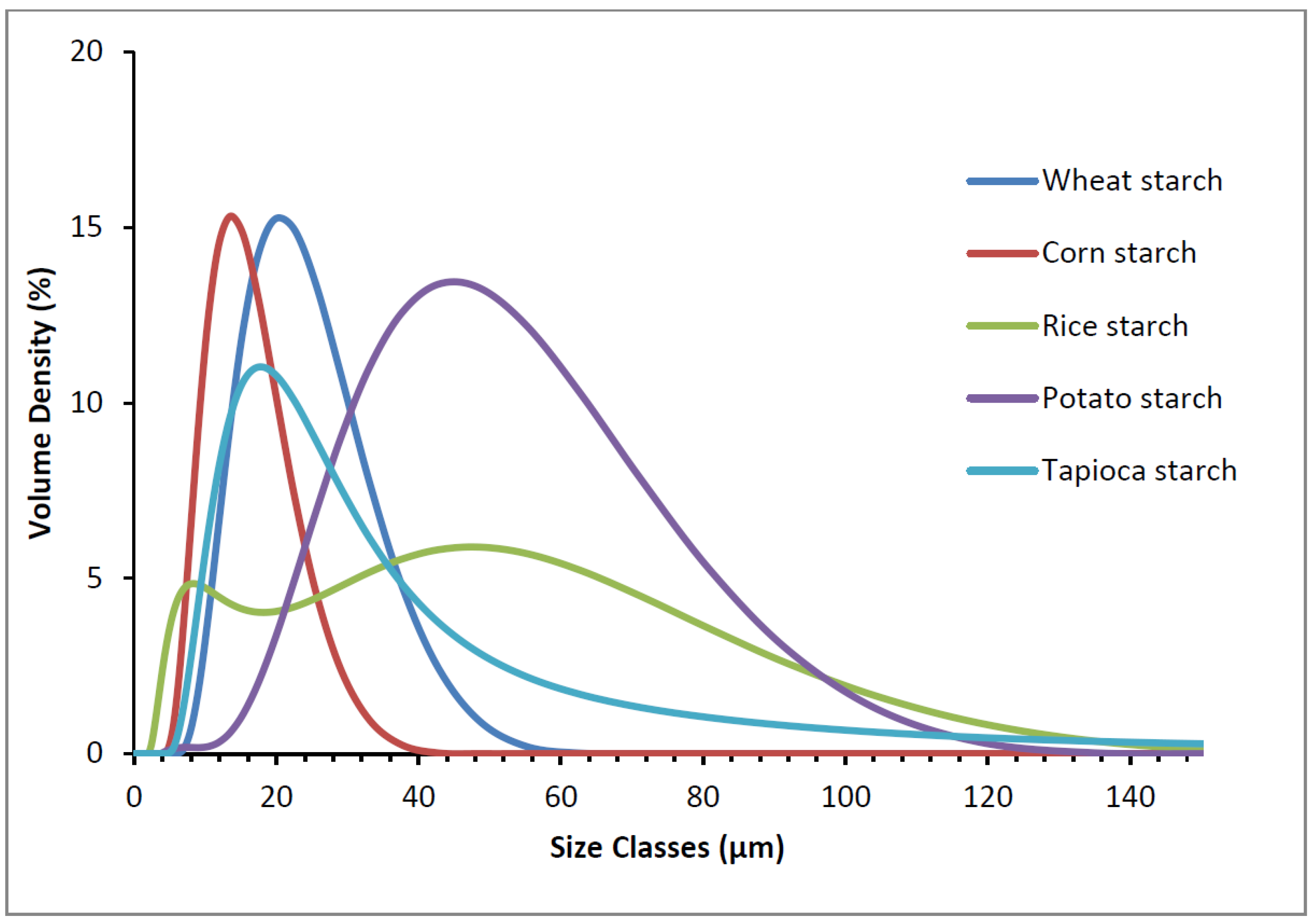
2.3. Particle Size
2.4. Chemical Characterisation of the Starches
2.5. Rapid Visco Analysis
2.6. Bread Making Procedure
2.7. Bread Characteristics
2.8. Statistical Analysis
3. Results and Discussion
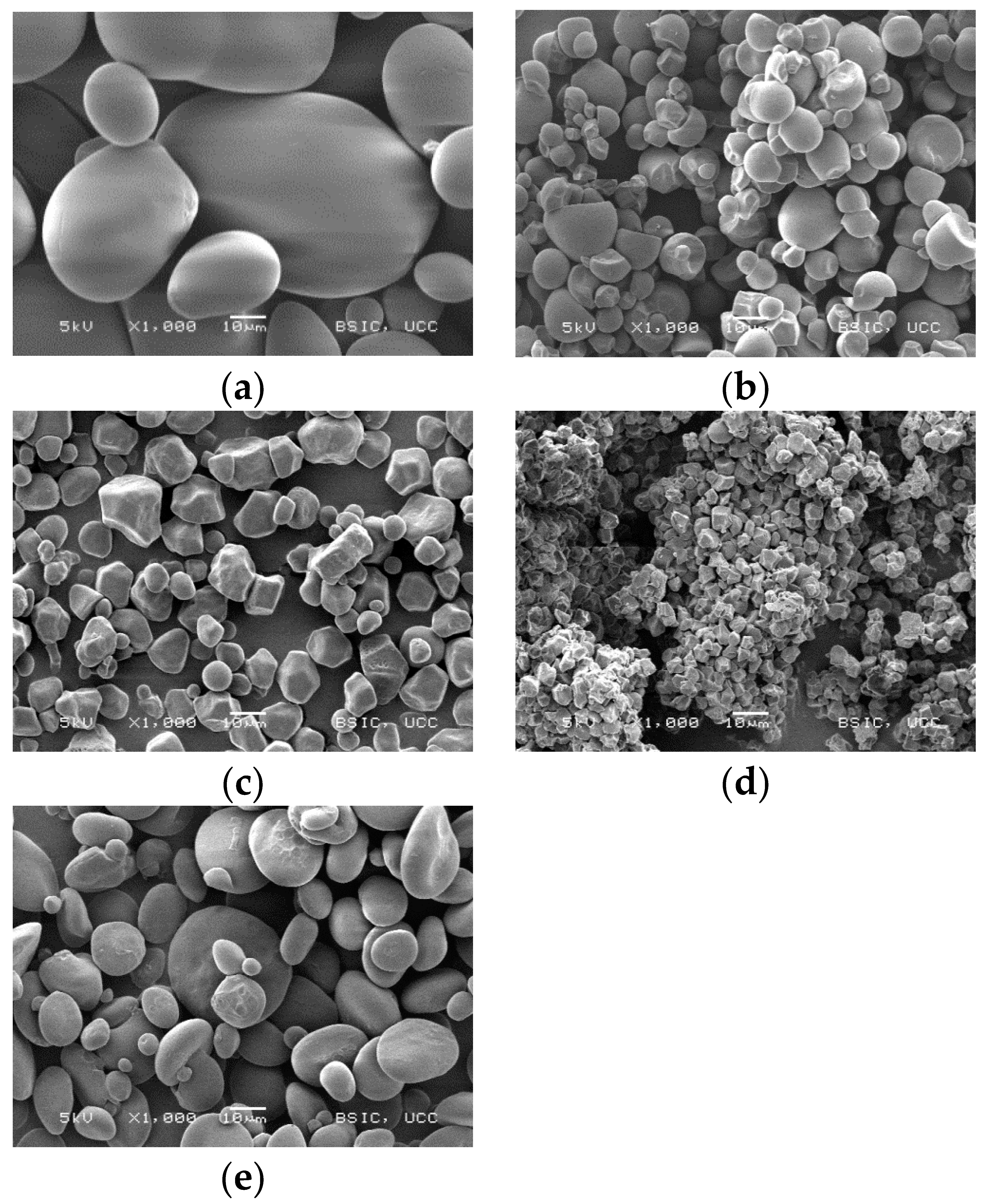
3.1. Starch Granule Morphology
3.2. Chemical Characterisation of the Starches
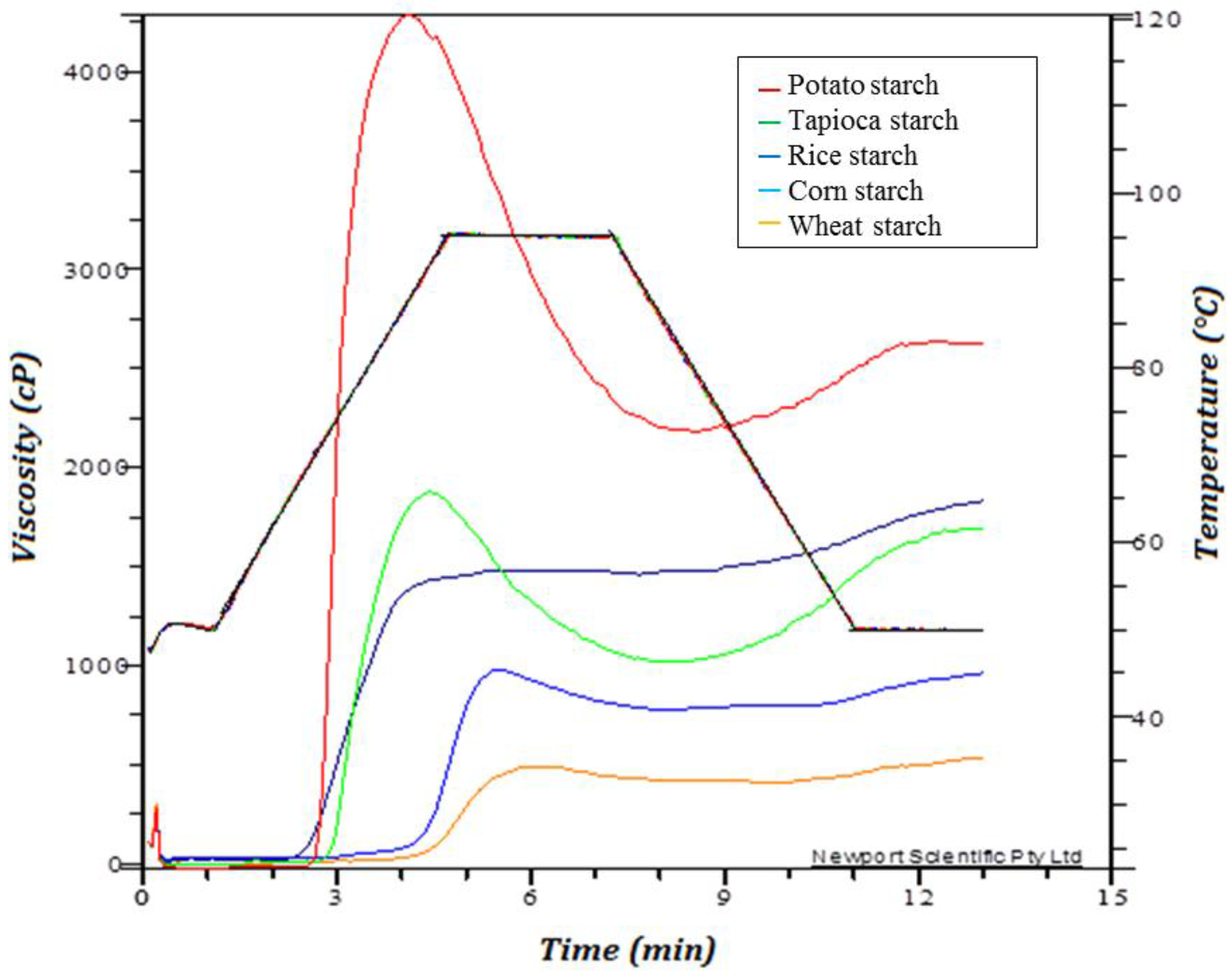
3.3. Pasting Properties
3.4. Model Bread Systems
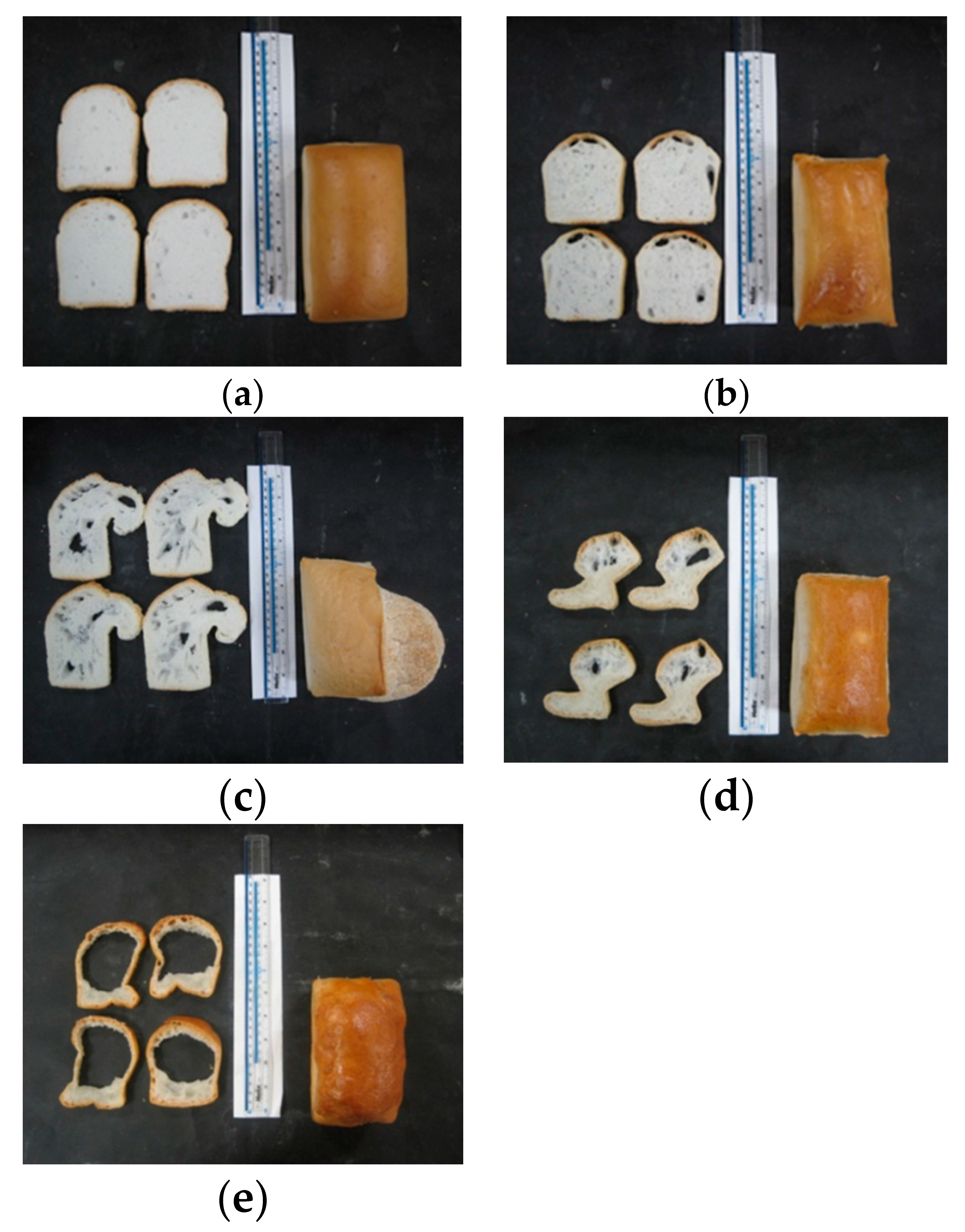
3.4.1. Bread Structure
3.4.2. Bread Texture
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, D.J.; Atwell, W.A. Starches. In Eagan Press Handbook; Amer Assn of Cereal Chemists: St. Paul, MN, USA, 1999. [Google Scholar]
- Abdel-Aal, E.-S.M. Functionality of Starches and Hydrocolloids in Gluten-Free Foods. In Gluten-Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Oxford, UK, 2009; p. 200. [Google Scholar]
- Arendt, E.K.; Renzetti, S.; Dal Bello, F. Dough Microstructure and Textural Aspects of Gluten-Free Yeast Bread and Biscuits. In Gluten-Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 107–129. [Google Scholar]
- Miyazaki, M.R.; Hung, P.V.; Maeda, T.; Morita, N. Recent advances in application of modified starches for breadmaking. Trends Food Sci. Technol. 2006, 17, 591–599. [Google Scholar] [CrossRef]
- Ward, F.M.; Andon, S.A. Hydrocolloids as film formers, adhesives and gelling agents for bakery and cereal products. Cereal Food World 2002, 47, 52–55. [Google Scholar]
- Hug-Iten, S.; Conde-Petit, B.; Echer, F. Structural properties of starch in bread and bread model systems—Influence of an antistaling α-amylose. Cereal Chem. 2001, 78, 421–428. [Google Scholar] [CrossRef]
- Arendt, E.; Dal Bello, F. Gluten-Free Cereal Products and Beverages; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Zannini, E.; Jones, J.M.; Renzetti, S.; Arendt, E.K. Functional replacements for gluten. Food Sci. Technol. 2012, 3, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Kreisz, S.; Arendt, E.; Hübner, F.; Zarnkov, M. Cereal-Based Gluten-Free Functional Drinks. In Food Science and Technology; Academic Press: Amsterdam, The Netherlands, 2008; pp. 373–391. [Google Scholar]
- Hager, A.-S.; Arendt, E.K. Influence of hydroxypropylmethylcellulose (hpmc), xanthan gum and their combination on loaf specific volume, crumb hardness and crumb grain characteristics of gluten-free breads based on rice, maize, teff and buckwheat. Food Hydrocoll. 2013, 32, 195–203. [Google Scholar] [CrossRef]
- Ziobro, R.; Witczak, T.; Juszczak, L.; Korus, J. Supplementation of gluten-free bread with non-gluten proteins. Effect on dough rheological properties and bread characteristic. Food Hydrocoll. 2013, 32, 213–220. [Google Scholar] [CrossRef]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014, 59, 41–47. [Google Scholar] [CrossRef]
- Ziobro, R.; Korus, J.; Witczak, M.; Juszczak, L. Influence of modified starches on properties of gluten-free dough and bread. Part II: Quality and staling of gluten-free bread. Food Hydrocoll. 2012, 29, 68–74. [Google Scholar] [CrossRef]
- Schirmer, M.; Höchstötter, A.; Jekle, M.; Arendt, E.; Becker, T. Physicochemical and morphological characterization of different starches with variable amylose/amylopectin ratio. Food Hydrocoll. 2013, 32, 52–63. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Starch: Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: A review. Starch-Stärke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Chung, O.; Ohm, J.-B.; Ram, M.; Park, S.H.; Howitt, C.; Khan, K.; Shewry, P. Wheat lipids. In Wheat: Chemistry and technology; Amer Assn of Cereal Chemists: St. Paul, MN, USA, 2009; pp. 363–399. [Google Scholar]
- Pareyt, B.; Finnie, S.M.; Putseys, J.A.; Delcour, J.A. Lipids in bread making: Sources, interactions, and impact on bread quality. J. Cereal Sci. 2011, 54, 266–279. [Google Scholar] [CrossRef]
- Van der Maarel, M.J.; van der Veen, B.; Uitdehaag, J.C.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef]
- Jane, J.L.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J.F. Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke 1994, 46, 121–129. [Google Scholar] [CrossRef]
- Houben, A.; Höchstötter, A.; Becker, T. Possibilities to increase the quality in gluten-free bread production: An overview. Eur. Food Res. Technol. 2012, 235, 195–208. [Google Scholar] [CrossRef]
- Sánchez, T.; Dufour, D.; Moreno, I.X.; Ceballos, H.N. Comparison of pasting and gel stabilities of waxy and normal starches from potato, maize, and rice with those of a novel waxy cassava starch under thermal, chemical, and mechanical stress. J. Agric. Food Chem. 2010, 58, 5093–5099. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.N.; Bustos, M.C.; Iturriaga, L.; Flores, S.K.; León, A.E.; Ribotta, P.D. Effect of damaged starch on the rheological properties of wheat starch suspensions. J. Food Eng. 2013, 116, 233–239. [Google Scholar]
- Park, S.; Wilson, J.; Chung, O.; Seib, P. Size distribution and properties of wheat starch granules in relation to crumb grain score of pup-loaf bread. Cereal Chem. 2004, 81, 699–704. [Google Scholar] [CrossRef]
- Chiotellis, E.; Campbell, G.M. Proving of bread dough I: Modelling the evolution of the bubble size distribution. Food Bioprod. Process. 2003, 81, 194–206. [Google Scholar] [CrossRef]
- Kim, S.; D’Appolonia, B. Bread staling studies. I. Effect of protein content on staling rate and bread crumb pasting properties. Cereal Chem. 1977, 54, 207–215. [Google Scholar]
- Rayas-Duarte, P.; Mulvaney, S. Bread Staling. In Breadmaking: Improving Quality; Woodhead Publishing: Camebridge, UK, 2012; pp. 580–596. [Google Scholar]
- Hoseney, R.C.; Lineback, D.R.; Seib, P.A. Role of starch in baked foods. Bak. Dig. 1978, 52, 11–16. [Google Scholar]
- Le-Bail, A.; Agrane, S.; Queveau, D. Impact of the baking duration on bread staling kinetics. Food Bioprocess Technol. 2012, 5, 2323–2330. [Google Scholar] [CrossRef]
- Mäkinen, O.; Zannini, E.; Arendt, E. Germination of oat and quinoa and evaluation of the malts as gluten free baking ingredients. Plant Foods Hum. Nutr. 2013, 68, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Bulkin, B.J.; Kwak, Y.; Dea, I. Retrogradation kinetics of waxy-corn and potato starches; a rapid, raman-spectroscopic study. Carbohydr. Res. 1987, 160, 95–112. [Google Scholar] [CrossRef]
- Maurer, H.W. Starch in the Paper Industry. In Food Science and Technology, 3rd ed.; Elsevier: New York, NY, USA, 2009; pp. 658–706. [Google Scholar]
- Keetels, C.J.A.M.; Visser, K.A.; van Vliet, T.; Jurgens, A.; Walstra, P. Structure and mechanics of starch bread. J. Cereal Sci. 1996, 24, 15–26. [Google Scholar] [CrossRef]
- Bárcenas, M.E.; O-Keller, J.D.L.; Rosell, C.M. Influence of different hydrocolloids on major wheat dough components (gluten and starch). J. Food Eng. 2009, 94, 241–247. [Google Scholar] [CrossRef]
- Kim, H.-S.; Patel, B.; BeMiller, J.N. Effects of the amylose-amylopectin ratio on starch-hydrocolloid interactions. Carbohydr. Polym. 2013, 98, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
| Starch Sample | Moisture (%) | Total Starch (db) (%) | Amylose (%) | Damaged Starch (db) (%) | Protein (db) (%) | Lipids (db) (%) | Beta-Amylase (U/g) |
|---|---|---|---|---|---|---|---|
| Potato | 18.0 ± 0.1 a | 92.2 ± 2.1 a | 54.2 ± 0.6 a | 1.0 ± 0.1 a | 0.02 ± 0.03 a | n.d. | 0.18 ± 0.01 b |
| Tapioca | 13.7 ± 0.0 b | 94.2 ± 0.1 b | 36.0 ± 0.3 b | 0.7 ± 0.0 b | 0.03 ± 0.04 a,b | n.d. | 0.25 ± 0.01 b |
| Corn | 12.2 ± 0.1 c | 92.5 ± 0.9 b | 28.9 ± 0.2 c | 1.0 ± 0.1 a | 0.04 ± 0.05 a,b | 0.26 ± 0.01 a | 0.32 ± 0.0 b |
| Rice | 12.5 ± 0.1 d | 83.4 ± 2.5 a | 46.4 ± 0.4 d | 7.4 ± 0.2 c | 0.04 ± 0.06 b | 0.71 ± 0.02 b | 2.33 ± 0.15 a |
| Wheat | 12.8 ± 0.2 e | 97.4 ± 5.7 b | 25.1 ± 0.3 e | 2.5 ± 0.2 d | 0.10 ± 0.14 c | 0.50 ± 0.01 c | 0.33 ± 0.0 b |
| Starch | Bake Loss (%) | Specific Volume (mL/g) | Crumb Moisture (%) | C-Cell | Texture Profile Analysis | |||
|---|---|---|---|---|---|---|---|---|
| Area of Holes (%) | Wall Thickness (mm) | Day of Analysis | Hardness (N) | Cohesiveness (-) | ||||
| Potato starch | 18.3 ± 2.7 a | 3.3 ± 0.1 a | 49.2 ± 1.8 a | 4.1 ± 1.6 a | 0.5 ± 0.1 a | Day 0 | 4.2 ± 0.5 a | 0.71 ± 0.02 a |
| Day 2 | 24.2 ± 2.0 a | 0.55 ± 0.05 a | ||||||
| Day 5 | 28.8 ± 2.0 a | 0.53 ± 0.04 a | ||||||
| Corn starch | 20.9 ± 3.6 b | 5.0 ± 0.3 b | 48.2 ± 0.3 a | 12.0 ± 1.0 b | 0.5 ± 0.1 a | Day 0 | 3.2 ± 0.6 b | 0.69 ± 0.02 a |
| Day 2 | 17.7 ± 4.4 b | 0.59 ± 0.06 a | ||||||
| Day 5 | 20.7 ± 3.4 b | 0.54 ± 0.07 a | ||||||
| Wheat starch | 19.1 ± 2.6 a,b | 4.0 ± 0.1 c | 48.3 ± 0.2 a | 2.4 ± 1.7 c | 0.5 ± 0.1 a | Day 0 | 3.0 ± 0.4 b | 0.75 ± 0.01 a |
| Day 2 | 14.9 ± 0.8 b | 0.67 ± 0.05 b | ||||||
| Day 5 | 22.5 ± 0.9 b | 0.57 ± 0.05 a | ||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horstmann, S.W.; Belz, M.C.E.; Heitmann, M.; Zannini, E.; Arendt, E.K. Fundamental Study on the Impact of Gluten-Free Starches on the Quality of Gluten-Free Model Breads. Foods 2016, 5, 30. https://doi.org/10.3390/foods5020030
Horstmann SW, Belz MCE, Heitmann M, Zannini E, Arendt EK. Fundamental Study on the Impact of Gluten-Free Starches on the Quality of Gluten-Free Model Breads. Foods. 2016; 5(2):30. https://doi.org/10.3390/foods5020030
Chicago/Turabian StyleHorstmann, Stefan W., Markus C. E. Belz, Mareile Heitmann, Emanuele Zannini, and Elke K. Arendt. 2016. "Fundamental Study on the Impact of Gluten-Free Starches on the Quality of Gluten-Free Model Breads" Foods 5, no. 2: 30. https://doi.org/10.3390/foods5020030
APA StyleHorstmann, S. W., Belz, M. C. E., Heitmann, M., Zannini, E., & Arendt, E. K. (2016). Fundamental Study on the Impact of Gluten-Free Starches on the Quality of Gluten-Free Model Breads. Foods, 5(2), 30. https://doi.org/10.3390/foods5020030