Abstract
In recent years, dietary intervention has garnered significant attention for T2DM prevention and adjunctive treatment. Lentinula edodes (commonly known as shiitake mushroom), a common edible fungus, has been demonstrated to improve T2DM, primarily attributed to its main bioactive components like peptides and polysaccharides, while their synergistic characteristics are still not fully explained. Therefore, this study investigated the anti-T2DM molecular mechanisms of L. edodes peptides and polysaccharides by integrating network pharmacology and molecular docking. First, systematic searches of the PubMed and HERB databases using keywords such as “Lentinula edodes peptides”, “Lentinula edodes polysaccharides” and “T2DM” and “Lentinula edodes/shiitake mushroom” yielded 25 peptides and 14 polysaccharides. Second, network pharmacology analysis revealed 541 common interaction targets between these peptides/polysaccharides and T2DM. Topological analysis further identified nine core targets: ESR1, MAPK1, AKT1, SRC, EGFR, STAT3, JUN, PIK3CA, and PIK3R1. Third, pathway enrichment analysis showed that these core targets were significantly enriched within the PI3K-Akt signaling pathway and the AGE-RAGE signaling pathway in diabetic complications, suggesting potential anti-T2DM effects through regulation of these key pathways. Finally, molecular docking validation ensured strong binding affinities between peptides/polysaccharides and some core targets, with particularly prominent binding capacities observed for peptides VF and LDELEK with EGFR; peptides KIGSRSRFDVT, LDYGKL, and EDLRLP along with polysaccharides D-glucan and β-glucan with PIK3CA; and peptide DVFAHF with PIK3R1. In summary, this study revealed that L. edodes peptides and polysaccharides may exert synergistic anti-T2DM effects via the regulation of key signaling pathways, including the PI3K-Akt signaling pathway, EGFR tyrosine kinase inhibitor resistance, and the AGE-RAGE signaling pathway in diabetic complications, through their actions on critical targets such as ESR1, PIK3CA, and PIK3R1. These results offer a synergistic mechanism for the anti-T2DM effect of L. edodes, which could be helpful for the development of functional foods and drugs derived from L. edodes.
Keywords:
Lentinula edodes; peptides; polysaccharides; T2DM; network pharmacology; molecular docking 1. Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder, contributing to about 90% of the diabetic cases globally, and their prevalence is steadily increasing annually [1,2]. Its chief pathologic characteristic is defined by absolute or relative deficiency of endogenous insulin and disturbed utilization of insulin by the body [3,4]. Chronic hyperglycemia may trigger a wide array of severe and life-threatening complications, including non-alcoholic fatty liver disease (NAFLD) [1], cardiovascular disorders, and dyslipidemia [5]. The first-line clinical treatments for T2DM at present primarily include metformin [6], sulfonylureas, and glucagon-like peptide-1 receptor agonists [7]. However, the abovementioned drugs often possess a significant assemblage of side effects, like gastrointestinal discomfort, weight gain, and hypoglycemia [8,9,10,11], which seriously reduce patients’ treatment compliance and limit long-term efficacy in glycemic control. Thus, an effective and safe intervention for T2DM has become an urgent need. In recent years, dietary interventions have attracted researchers’ attention for T2DM prevention and/or treatment due to their extensive availability and fewer side effects [12]. It is well known that the adoption of healthy dietary habits could significantly decrease the risk of developing T2DM and promote glycemic control and metabolic health among diabetic patients [2,13,14]. Among the various functional foods or natural products, different bioactive components, including peptides, polysaccharides, and polyphenols, have shown the ability to improve glucose homeostasis. Their functions are chiefly the enhancement of insulin sensitivity, the modulation of inflammation, and the balance of the gut microbiota [15,16,17,18,19]. Hence, it is stated that an in-depth understanding of natural food ingredients with hypoglycemic potential and their mechanisms of action may not only illuminate the scientific explanation for dietary intervention but also provide new insights into developing safe and effective nutritional intervention strategies for T2DM.
As one of the most popular edible fungi with rich nutritional value, Lentinula edodes has been reported to possess significant natural medicinal potential [20]. It serves as a rich source of various bioactive substances and nutritional factors [21]. Many studies have discovered that the main constituents of L. edodes, such as peptides and polysaccharides, possess various physiological functions like immunomodulation, anti-inflammatory [22] and anticancer activities [23,24]. Moreover, both L. edodes peptides and polysaccharides have been found to possess blood glucose-lowering potential according to some studies [25,26,27]. However, there have been limited studies conducted on the synergistic effect between these two bioactive components against T2DM and their complex molecular mechanisms. Therefore, more research is required on this topic.
Traditional experimental approaches such as immunohistochemistry and Western blots are often time-consuming and labor-intensive, with many technical difficulties in the study of complex biological mechanisms. In this respect, network pharmacology, as an emerging systems biology approach, has become an essential tool in drug target identification and in determining the interaction between drugs and proteins [28]. It integrates systems biology, genomics, proteomics, and big data analysis to construct multidimensional “drug-component-target-disease” networks through computational modeling. This enables the prediction of active compound components and their potential disease targets at the systems level [29,30]. The network pharmacology methodology not only helps further understand the synergy mechanism of L. edodes peptides and polysaccharides in combating T2DM but also offers a promising perspective for further development of new hypoglycemic drugs based on these compounds. Such an approach provides new strategies for the long-term management of T2DM.
Therefore, the current study incorporated network pharmacology and molecular docking techniques to elucidate the mechanisms of L. edodes peptides and polysaccharides against T2DM (Figure 1). The possible action targets of L. edodes peptides and polysaccharides against the T2DM-related targets were firstly identified based on searches from multiple databases. Subsequently, network pharmacology analysis identified 541 common interaction targets, which were further refined through topological analysis to select nine core targets: ESR1, MAPK1, AKT1, SRC, EGFR, STAT3, JUN, PIK3CA, and PIK3R1. Enrichment analysis indicated that these targets were significantly enriched with pathways that were pivotal to insulin resistance and glucose metabolism, notably the PI3K-Akt signaling pathway, the AGE-RAGE signaling pathway in diabetic complications, and EGFR tyrosine kinase inhibitor resistance. Finally, molecular docking was employed to confirm the binding ability or modes of interaction between these bioactive components (peptides and polysaccharides) and these nine core targets, thereby elucidating their possible mechanisms for improving T2DM.
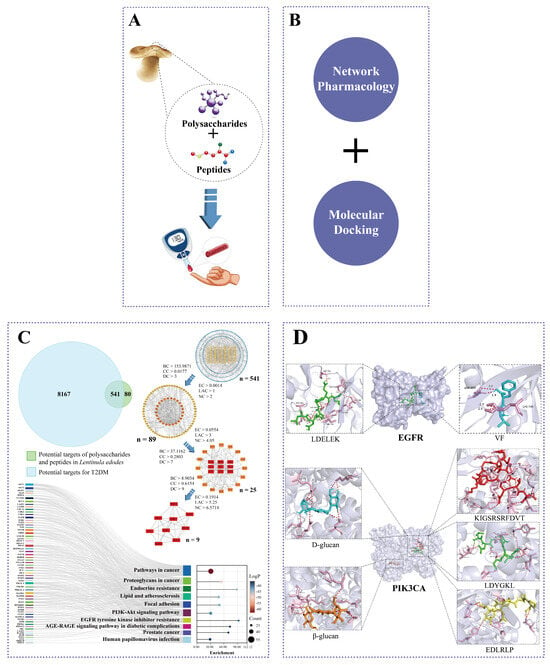
Figure 1.
Framework of the synergistic mechanistic study on anti-T2DM benefits of L. edodes. (A) L. edodes peptides and polysaccharides improve T2DM. (B) Network pharmacology and molecular docking were used in this study. (C) Target screening and KEGG analysis. (D) Visual analysis of docking results between both L. edodes peptides and polysaccharides and core target molecules.
2. Materials and Methods
2.1. Screening of L. edodes Peptides and Polysaccharides
To obtain information related to L. edodes peptides and polysaccharides, this study systematically searched multiple databases. In PubMed, comprehensive searches were conducted using the keywords “Lentinula edodes/shiitake mushroom + diabetes”, “Lentinula edodes/shiitake mushroom + peptides”, and “Lentinula edodes/shiitake mushroom + polysaccharides”. Additionally, a supplementary search was performed in the HERB database using the keyword “Lentinula edodes/shiitake mushroom”.
2.2. Predicting Targets of L. edodes Peptides and Polysaccharides
Firstly, the 2D structure of an L. edodes peptide was drawn using the ChemDraw software, while the 2D structure of an L. edodes polysaccharide was retrieved from the PubChem database. Next, the L. edodes peptide structure was submitted to both the PharmMapper database (Z′-score ≥ 0.5) and the SwissTargetPrediction database (probability > 0) for target prediction. At the same time, the L. edodes polysaccharide structures were submitted to the SwissTargetPrediction database (Probability > 0) for analysis. Finally, the predicted targets were assessed against the UniProt ID database, and redundant targets were removed to ensure target specificity.
2.3. Identification of Therapeutic Targets for T2DM
To extensively identify the disease targets associated with T2DM, this study searched a number of disease databases. Keyword searches were conducted using “T2DM”, “type 2 diabetes mellitus”, and “Diabetes Mellitus, Type 2” in the Online Mendelian Inheritance in Man (OMIM), Therapeutic Target Database (TTD), DrugBank, and GeneCards (relevance score ≥10) databases. All the target data collected was consolidated, and duplicates were removed, yielding a non-redundant set of potential therapeutic targets for subsequent analysis.
2.4. Protein–Protein Interaction Network Construction and Analysis
A cross-analysis was conducted by comparing the predicted targets of L. edodes peptides and polysaccharides with the disease targets of T2DM. Their common targets were visualized using a Venn diagram to find the possible targets for the anti-T2DM effects of L. edodes peptides and polysaccharides. The common targets were imported into the STRING database for the construction of a protein–protein interaction (PPI) network. The minimum required interaction score was assigned as the highest confidence level of 0.900, and isolated nodes were removed. Then, the obtained network was exported as a TSV file and was further loaded into the Cytoscape 3.10.3 software using the CytoNCA 2.1.6 plugin for the purpose of topological analysis. By iterative screening, only nodes surpassing the median value in all six topological metrics, including Betweenness Centrality (BC), Closeness Centrality (CC), Degree Centrality (DC), Eigenvector Centrality (EC), the Local Average Connectivity-based method (LAC), and Network Centrality (NC), were retained to identify the core targets.
2.5. Functional Enrichment and Network Analysis
In order to elucidate the underlying mechanisms of synergy between the L. edodes peptides and polysaccharides in improving T2DM, we carried out a systematic enrichment analysis with the Metascape platform based on 89 cross-targets identified from the initial screening process. The analysis encompassed Gene Ontology (GO) enrichment involving the biological process (BP), molecular function (MF), and cellular component (CC) categories, as well as a Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. The species was generalized as “Homo sapiens” for all analyses. To comprehensively elucidate the mechanism of action, we further constructed an integrated “peptide/polysaccharide-pathway-disease” network so as to systematically reveal the multi-target, multi-pathway synergistic mechanism by which L. edodes peptides and polysaccharides combat T2DM.
2.6. Molecular Docking Analysis
For analyzing the interactions between both L. edodes peptides and polysaccharides and the core targets identified through screening, we conducted molecular docking analysis between the nine core targets previously identified and 25 L. edodes peptides, as well as 14 L. edodes polysaccharides. The 2D structures of L. edodes peptides were constructed using ChemDraw, while the 2D structures of L. edodes polysaccharides were retrieved from the PubChem database. All molecular structures were converted to 3D structures via Chem3D and optimized through energy minimization. The structures of the core targets’ proteins were obtained from the PDB database. After removing water molecules and native organic molecules using the PyMOL 2.4.0a0 software, their structures were hydrogenated and optimized using the AutoDock 1.5.7 software. The processed peptides and polysaccharides served as ligands, while the target proteins acted as receptors when carrying out molecular docking by the AutoDock Vina 1.1.2. And the specific parameters for molecular docking are briefly outlined in Supplementary Table S1. Finally, docking results were visualized with PyMOL 2.4.0a0, and a binding affinity heatmap was prepared using the GraphPad Prism software.
3. Results
3.1. Retrieval and Acquisition of L. edodes Peptides and Polysaccharides
Through systematic searches of the PubMed database, we initially screened 579 potential publications related to L. edodes peptides or polysaccharides. Following rigorous abstract screening and full-text evaluations, combined with relevant information from the HERB database, we ultimately compiled 25 L. edodes peptide sequences and 14 L. edodes polysaccharides from 66 publications and the HERB database (detailed information shown in Table 1). This provides a reliable data foundation for subsequent network pharmacology analysis and mechanism-of-action research. The number of amino acids in these peptides varied from 2 to 11, and there were 24 peptides having 2 to 7 amino acids, while 1 peptide contained 11 amino acids. The polysaccharide components primarily included D-galactose, D-glucose, D-xylose, D-mannose, L-arabinose, L-rhamnose, and β-glucan.

Table 1.
The detailed information of L. edodes peptides and polysaccharides.
3.2. Target Prediction Analysis of L. edodes Peptides and Polysaccharides
In order to enhance the coverage and reliability of target prediction, a collaborative prediction based on a multi-database strategy was employed to systematically identify potential functional targets for L. edodes peptides and polysaccharides (Supplementary Figure S1). Specifically, a target prediction of L. edodes peptides using PharmMapper initially resulted in 3080 human protein targets. After elimination of duplicates, 322 targets were retained. Simultaneously, the SwissTargetPrediction database was applied to the same set of peptides, yielding 1870 targets. After removing duplicates, 343 targets remained. Lastly, target prediction of L. edodes polysaccharides using the SwissTargetPrediction database initially resulted in 267 targets. After removing duplicates, 47 targets were retained. Integrating and further deduplicating the L. edodes peptide and polysaccharide targets from these databases yielded 621 valid, unique human protein targets. They serve as potential targets for L. edodes peptides and polysaccharides to exert their anti-T2DM effects, which will be elucidated in subsequent studies.
3.3. Systematic Identification of Therapeutic Targets for T2DM
A joint search approach using multiple databases resulted in the procurement of disease targets relevant to T2DM. Searches were conducted using the keywords “T2DM”, “type 2 diabetes mellitus”, and “Diabetes Mellitus, Type 2” in the OMIM, TTD, DrugBank, and GeneCards (relevance score ≥10) databases. Preliminary search results yielded 184, 99, 107, and 8606 targets from OMIM, TTD, DrugBank, and GeneCards, respectively. All initially identified targets were integrated and systematically deduplicated, ultimately yielding 8708 independent T2DM-related targets. This provides the basis for subsequent analysis.
3.4. PPI Network Analysis on the Common Targets of L. edodes Peptides and Polysaccharides Against T2DM
The 621 targets related to the L. edodes peptides and polysaccharides, identified through the abovementioned screening steps, were intersected with the 8708 T2DM-related nodes, obtaining 541 common targets (Figure 2A). The PPI network was created by submitting the intersecting target nodes to the STRING database, with the species restricted to “Homo sapiens” and a minimum required interaction score of 0.900, which is the highest value. Then the result of the STRING analysis was exported in the form of a TSV file and then imported into the Cytoscape 3.10.3 software. Iterative topological analysis was performed using the CytoNCA 2.1.6 plugin. Six topological parameters, namely BC, CC, DC, EC, LAC, and ND, were measured for each target, and key targets with all metrics above the median were picked (Figure 2B). Ultimately, we identified nine core targets—ESR1, MAPK1, AKT1, SRC, EGFR, STAT3, JUN, PIK3CA, and PIK3R1—for subsequent in-depth investigation into their underlying molecular mechanisms.
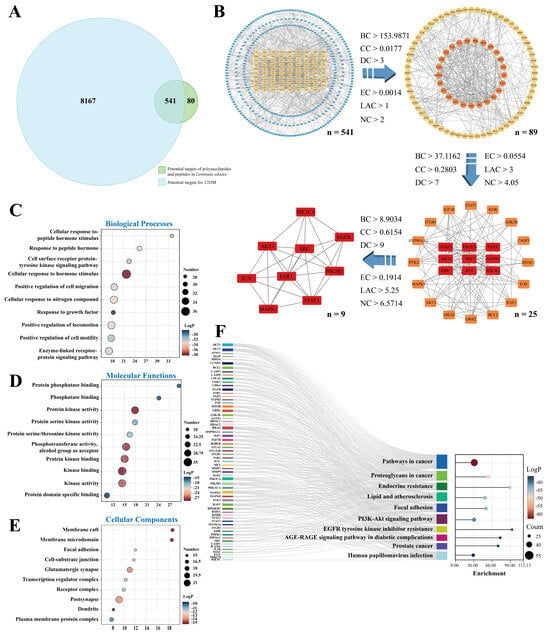
Figure 2.
Network pharmacology analysis for the mechanism of L. edodes peptides and polysaccharides in treating T2DM. (A) Venn diagram showing the intersection of target proteins for L. edodes peptides and polysaccharides with T2DM disease targets. Among the 621 L. edodes peptide- and polysaccharide-related targets screened from databases and the 8707 T2DM-related targets, 541 common targets were identified. (B) Screening process and criteria for identifying core targets in the interaction network between L. edodes peptides/polysaccharides and T2DM. (C–E): Gene Ontology (GO) enrichment analysis of L. edodes peptides and polysaccharides for potential targets against T2DM. (F): KEGG enrichment analysis of potential anti-T2DM targets for L. edodes peptides and polysaccharides.
3.5. Functional Enrichment Analysis of Core Targets
Based on the 89 targets identified through initial screening, the GO enrichment analysis revealed significant enrichment entries across all three ontology categories: BP, MF, and CC. Within the BP category, targets were predominantly enriched in positive regulation of biological processes, responses to hormone stimulation, and multiple signal transduction pathways (Figure 2C). In the MF category, significantly enriched functions included protein kinase activity, kinase activity, protein serine/threonine kinase activity, and protein phosphatase binding (Figure 2D). In CC analysis (Figure 2E), targets were significantly enriched in structures such as the membrane microdomain, membrane raft, glutamatergic synapse, and postsynapse. Further KEGG pathway analysis (p < 0.05, Figure 2F) revealed significant enrichment of these targets in the PI3K-Akt signaling pathway, EGFR tyrosine kinase inhibitor resistance, and the AGE-RAGE signaling pathway in diabetic complications. These pathways are important for glucose and insulin resistance regulation. As shown in Figure 3, the enrichment results suggest that L. edodes peptides and polysaccharides may improve T2DM through multiple pathways, primarily by regulating insulin signaling (PI3K-Akt) and inflammatory responses (AGE-RAGE).
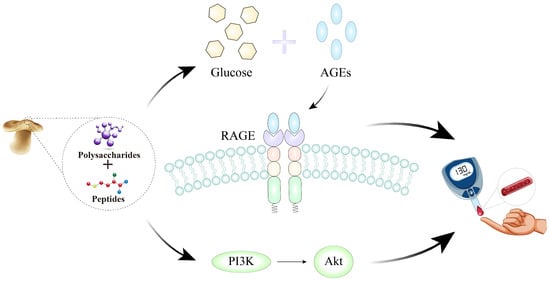
Figure 3.
Possible mechanisms of L. edodes peptides and polysaccharides in improving T2DM complications via the PI3K-Akt signaling pathway and the AGE-RAGE signaling pathway in diabetic complications.
3.6. Molecular Docking of L. edodes Peptides and Polysaccharides with Target Molecules
To further investigate the interaction mechanisms between both L. edodes peptides and polysaccharides and their core targets, we conducted molecular docking analyses between the nine core targets identified in previous screening and 25 L. edodes peptides along with 14 L. edodes polysaccharides (Figure 4). The results revealed strong binding affinities between peptides VF and LDELEK and the target EGFR, corresponding to binding energies of −8.2 kcal/mol and −8.0 kcal/mol, respectively. Additionally, peptides KIGSRSRFDVT, LDYGKL, and EDLRLP, along with polysaccharides D-glucan and β-glucan, exhibited significant interactions with the target PIK3CA, with binding energies of −8.1 kcal/mol, −8.3 kcal/mol, −8.2 kcal/mol, −8.3 kcal/mol, and −8.8 kcal/mol, respectively. Simultaneously, the peptide DVFAHF also exhibited significant binding with the target PIK3R1, with a binding energy of −8.4 kcal/mol (Figure 5).
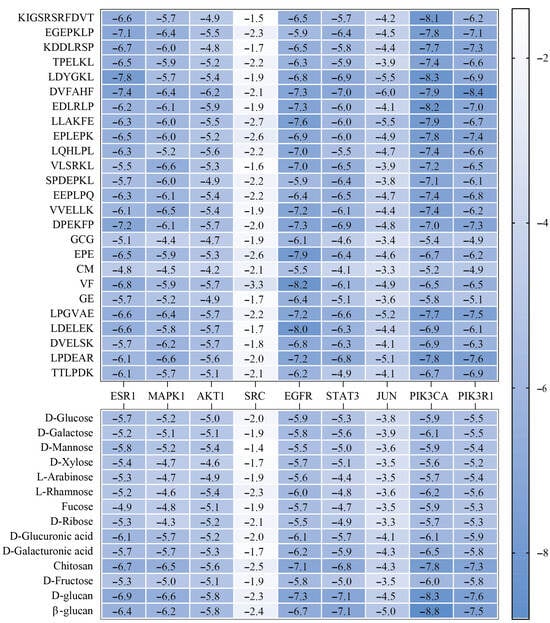
Figure 4.
Binding affinity between L. edodes peptides and polysaccharides (vertical axis) with core target proteins (horizontal axis). Color intensity corresponds to binding energy scores (kcal/mol), ranging from −1.4 (weak binding, white) to −8.8 (strong binding, blue).
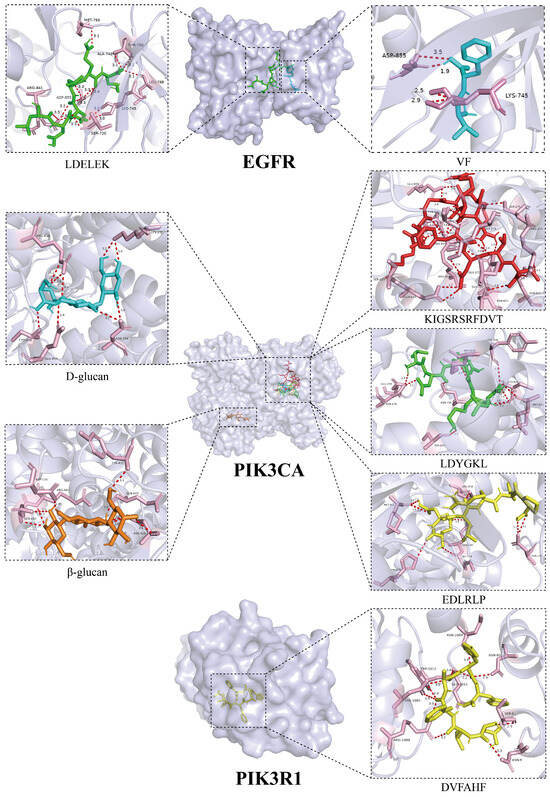
Figure 5.
Binding modes of peptides VF and LDELEK with EGFR; binding modes of peptides KIGSRSRFDVT, LDYGKL, and EDLRLP, as well as polysaccharides D-glucan and β-glucan, with PIK3CA; binding mode of peptide DVFAHF with PIK3R1 (The light purple regions indicate target proteins, while the pink regions represent amino acid residues within the target proteins that interact with ligands (L. edodes peptides and polysaccharides). Ligands are displayed in multiple colors: green, blue, red, orange, and yellow structures represent different polysaccharides or peptides, respectively. The red dashed lines in the figure indicate hydrogen bond interactions formed between ligands and target residues).
4. Discussion
With the continuous rise in global incidence and mortality rates, T2DM has become a major public health challenge [62,63,64,65]. Although first-line clinical drugs like metformin and sulfonylureas are widely used, they still carry significant side effects [66]. Therefore, developing novel therapeutic strategies holds urgent clinical significance. Recently, the application of diet intervention in the prevention and/or management of T2DM has been steadily increasing [67], gradually become an important component of comprehensive T2DM management.
As a kind of large edible and medicinal fungus, L. edodes exhibits diverse therapeutic potential [20,49,68,69]. Accumulating evidence shows that both the peptides and polysaccharides of L. edodes have potential efficacy for blood glucose regulation and for improving T2DM [26,70,71]. However, the synergistic mechanism between these two classes of bioactive components remains unexplored, necessitating systematic research for in-depth investigation.
In order to investigate the synergistic mechanisms of L. edodes peptides and polysaccharides against T2DM, this study first retrieved L. edodes peptides and polysaccharides from databases and predicted their potential targets. The analysis revealed 541 common interaction targets with T2DM targets. Among these overlapping targets, the majority are involved in biological processes including insulin signaling pathways and inflammatory responses. Second, we built a protein interaction network for the common targets through the STRING database and performed topological analysis using the Cytoscape 3.10.3 software. This finally identified nine core target genes: ESR1, MAPK1, AKT1, SRC, EGFR, STAT3, JUN, PIK3CA, and PIK3R1. Extensive research shows that these core genes mainly participate in two ways in the pathogenesis of T2DM.
- (i)
- Regulation of insulin signaling: The PI3K/Akt signaling pathway, as a crucial downstream signaling hub of the insulin receptor, has been identified to play significant roles in regulating glucose metabolism and glycogen synthesis [72]. Among these, PIK3CA and PIK3R1, as members of the PI3K family, can activate AKT (e.g., AKT1), which in turn modulates various downstream substrates through serine and/or threonine phosphorylation, thereby influencing cellular metabolic functions [73]. ESR1 (encoding ERα) not only activates insulin signaling independently of IRS1 and IRS2 via the E2-ERα pathway but also upregulates endogenous IRS1 expression in breast cancer cells, thereby modulating glucose homeostasis and insulin sensitivity [17]. SRC, as a non-receptor tyrosine kinase, can directly modulate the function of the PI3K-Akt signaling pathway, contributing to the promotion of glucose metabolism [74,75]. Likewise, EGFR activation enhances the PI3K/Akt signaling pathway, hence stimulating glucose flow and glucose flow-dependent uptake [75]. Experiments further indicate that STAT3 phosphorylation significantly upregulates mRNA levels of cytokine-regulated inhibitor 3 (SOCS3). As a kind of negative regulator, SOCS3 has the ability to efficiently suppress the phosphorylation of the PI3K/Akt signaling pathway, thus causing insulin resistance in the liver by reducing the sensitivity to insulin [76].
- (ii)
- Modulation of inflammatory responses: Many studies have implicated chronic inflammation in the etiology and progression of T2DM [77,78,79]. For instance, the MAPK family includes a critical component, MAPK1, which plays a pivotal role in inflammatory development. Oxidative stress and advanced glycation end products (AGEs) activate MAPK signaling during diabetic states, leading to the secretion of inflammatory mediators and promoting T2DM [80]. In addition, JUN promotes the production of inflammatory factors, reducing hepatic insulin sensitivity and thereby acting to promote insulin resistance in T2DM [77,81].
Then, 89 targets identified from initial screening were subjected to GO and KEGG enrichment analyses. The outcome of the KEGG pathway enrichment analysis indicated that these targets were found to be significantly enriched in endocrine resistance, lipid and atherosclerosis, the PI3K-Akt signaling pathway, EGFR tyrosine kinase inhibitor resistance, and the AGE-RAGE signaling pathway in diabetic complications. The PI3K-Akt signaling pathway is essential for normal metabolism in target tissues, with key functions including regulation of glucose metabolism. Impairment of this pathway results in developing insulin resistance, which subsequently progresses into T2DM [82]. Firstly, this pathway triggers downstream serine/threonine kinases, promoting the translocation of GLUT4 transporters to the cell membrane from the cytoplasm. Thus, it promotes a significant increase in glucose uptake by cells, thereby lowering blood glucose concentration [72]. Second, it also alleviates T2DM by modulating the activity of GSK-3β to improve glycogen accumulation [83]. Third, the PI3K-Akt signaling pathway upregulates the expression of PFKFB3 to promote glycolysis [84,85], playing a positive role in T2DM [75]. Additionally, elevated circulating glucose levels associated with insulin resistance tend to bind with proteins and lipids to form AGEs. These substances have also been cited to be among the major factors responsible for the evolution and continuous development of diabetic pathologies [70]. By binding to RAGE (receptor for AGEs), they can further modulate the NF-κB and PI3K/Akt signaling pathways, influencing T2DM progression through both inflammatory and glucose metabolic pathways [86,87].
Next, the molecular docking method was applied to systematically analyze the binding characteristics of 25 peptides and 14 polysaccharides with nine core targets to illustrate the interaction mechanisms of L. edodes peptides and polysaccharides with the core targets. The results indicated that there were strong binding affinities between the peptides VF (−8.2 kcal/mol) and LDELEK (−8.0 kcal/mol) with the EGFR target; peptides KIGSRSRFDVT (−8.1 kcal/mol), LDYGKL (−8.3 kcal/mol), and EDLRLP (−8.2 kcal/mol), along with polysaccharides D-glucan (−8.3 kcal/mol) and β-glucan (−8.8 kcal/mol), showed significant interaction with the PIK3CA target. The peptide DVFAHF also exhibited high binding affinity with the PIK3R1 target, with a binding energy of −8.4 kcal/mol.
Therefore, based on the results of integrating molecular docking and KEGG enrichment analysis, this study suggests that L. edodes peptides and polysaccharides may improve T2DM by acting on multiple targets and pathways. Specifically, the strong binding of peptides KIGSRSRFDVT, LDYGKL, and EDLRLP, along with polysaccharides D-glucan and β-glucan, to PIK3CA and the high binding affinity of peptide DVFAHF to target PIK3R1 suggest that these components may exert anti-T2DM effects by regulating the PI3K-Akt signaling pathway. Meanwhile, the AGE-RAGE pathway can modulate the PI3K/Akt signaling pathway and the NF-κB pathway, thus jointly affecting T2DM through glucose metabolism and inflammation.
Several limitations exist for the current study. Firstly, many L. edodes peptide and polysaccharide components may remain inadequately explored and identified at present, preventing this research from covering these potentially relevant constituents. Secondly, although multiple databases were integrated for target prediction to increase the coverage and reliability of the results, incomplete information on L. edodes peptides, polysaccharides, and T2DM-related targets in existing databases may have resulted in the exclusion of certain key targets or pathways from analysis [29,88]. Finally, the potential targets and pathways derived from network pharmacology analysis remains to be verified by subsequent experiments [28,89].
5. Conclusions
This study comprehensively leveraged network pharmacology and molecular docking methods to elaborate the potential mechanisms of L. edodes peptides and polysaccharides in alleviating T2DM through multi-target and multi-pathway synergistic effects. Results indicate that L. edodes peptides and polysaccharides exhibit strong binding affinities with the core targets, including EGFR, PIK3CA, and PIK3R1. This suggests that these peptides and polysaccharides may exert a synergistic effect to enhance insulin sensitivity and mitigate glucose metabolic disorders by regulating key pathways like PI3K-Akt and inflammation mediated by AGE-RAGE. This study provides theoretical support for the use of L. edodes peptides and polysaccharides as a potential intervention strategy against T2DM while also guiding future L. edodes-based drug and functional food development research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/foods15030453/s1, Table S1: The specific parameters for molecular docking; Figure S1: Summary of target sites for L. edodes peptides and polysaccharides.
Author Contributions
Conceptualization, L.S. and H.-F.J.; methodology, H.-K.M. and L.S.; formal analysis, H.-K.M., L.M., L.S. and H.-F.J.; writing—original draft preparation, H.-K.M.; writing—review and editing, L.S., H.-F.J. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Funds from Ludong University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, T.; Yan, W.; Cui, X.; Liu, N.; Wei, X.; Sun, Y.; Fan, K.; Liu, J.; Zhu, Y.; Wang, Z.; et al. Liraglutide attenuates type 2 diabetes mellitus-associated non-alcoholic fatty liver disease by activating AMPK/ACC signaling and inhibiting ferroptosis. Mol. Med. 2023, 29, 132. [Google Scholar] [CrossRef]
- Toi, P.L.; Anothaisintawee, T.; Chaikledkaew, U.; Briones, J.R.; Reutrakul, S.; Thakkinstian, A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients 2020, 12, 2722. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, H.; Tang, W.; Nie, S. Plant non-starch polysaccharides that inhibit key enzymes linked to type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1401, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Liu, X.; Chen, X.; Zhang, S.; Chen, Y.; Chen, J.; Chen, J.; Wu, F.; Chen, G.Q. 3-Hydroxybutyrate ameliorates insulin resistance by inhibiting PPARγ Ser273 phosphorylation in type 2 diabetic mice. Signal Transduct. Target. Ther. 2023, 8, 190. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- Kirpichnikov, D.; McFarlane, S.I.; Sowers, J.R. Metformin: An update. Ann. Intern. Med. 2002, 137, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Flory, J.; Lipska, K. Metformin in 2019. JAMA 2019, 321, 1926–1927. [Google Scholar] [CrossRef]
- Lv, W.; Wang, X.; Xu, Q.; Lu, W. Mechanisms and Characteristics of Sulfonylureas and Glinides. Curr. Top. Med. Chem. 2020, 20, 37–56. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat. Med. 2025, 31, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Jing, T.; Zhang, S.; Bai, M.; Chen, Z.; Gao, S.; Li, S.; Zhang, J. Effect of Dietary Approaches on Glycemic Control in Patients with Type 2 Diabetes: A Systematic Review with Network Meta-Analysis of Randomized Trials. Nutrients 2023, 15, 3156. [Google Scholar] [CrossRef]
- Rein, M.; Ben-Yacov, O.; Godneva, A.; Shilo, S.; Zmora, N.; Kolobkov, D.; Cohen-Dolev, N.; Wolf, B.C.; Kosower, N.; Lotan-Pompan, M.; et al. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: A randomized dietary intervention pilot trial. BMC Med. 2022, 20, 56. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Fu, X.; Wang, P.; Chen, C. Fructus mori polysaccharide alleviates diabetic symptoms by regulating intestinal microbiota and intestinal barrier against TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2023, 249, 126038. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Wang, F.; Wang, L.; Xiong, L.; Shen, X.; Song, H. Polysaccharide from Momordica charantia L. Alleviates Type 2 Diabetes Mellitus in Mice by Activating the IRS1/PI3K/Akt and AMPK Signaling Pathways and Regulating the Gut Microbiota. J. Agric. Food Chem. 2025, 73, 7298–7309. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, W.; Liao, W.; Yan, H.; Ai, W.; Pan, Q.; Brashear, W.A.; Xu, Y.; He, L.; Guo, S. An estrogen receptor α-derived peptide improves glucose homeostasis during obesity. Nat. Commun. 2024, 15, 3410. [Google Scholar] [CrossRef]
- Li, W.; Fu, X.; Zhang, T.; Li, H.; Chen, T.; Liu, X. Isolation and identification of an α-glucosidase inhibitory peptide from extruded soybean protein and its hypoglycemic activity in T2DM mice. Food Funct. 2023, 14, 4288–4301. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Antonelli, A.; Balercia, G.; Sabatelli, S.; Maggi, F.; Caprioli, G.; Giacchetti, G.; Micucci, M. Antioxidant, Anti-Inflammatory, Anti-Diabetic, and Pro-Osteogenic Activities of Polyphenols for the Treatment of Two Different Chronic Diseases: Type 2 Diabetes Mellitus and Osteoporosis. Biomolecules 2024, 14, 836. [Google Scholar] [CrossRef]
- Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory Properties of Polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef] [PubMed]
- Bugajewski, M.; Angerhoefer, N.; Pączek, L.; Kaleta, B. Lentinula edodes as a Source of Bioactive Compounds with Therapeutical Potential in Intestinal Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 3320. [Google Scholar] [CrossRef]
- Alagbaoso, C.A.; Mizuno, M. Lentinula edodes Polysaccharides Suppressed Pro-Inflammatory Cytokines Expression and Colitis in Mice. Arq. Gastroenterol. 2022, 59, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Din, S.R.U.; Nisar, M.A.; Ramzan, M.N.; Saleem, M.Z.; Ghayas, H.; Ahmad, B.; Batool, S.; Kifayat, K.; Guo, X.; Huang, M.; et al. Latcripin-7A from Lentinula edodes C(91-3) induces apoptosis, autophagy, and cell cycle arrest at G1 phase in human gastric cancer cells via inhibiting PI3K/Akt/mTOR signaling. Eur. J. Pharmacol. 2021, 907, 174305. [Google Scholar] [CrossRef] [PubMed]
- Din, S.R.U.; Zhong, M.; Nisar, M.A.; Saleem, M.Z.; Hussain, A.; Khinsar, K.H.; Alam, S.; Ayub, G.; Kanwal, S.; Li, X.; et al. Latcripin-7A, derivative of Lentinula edodes C(91-3), reduces migration and induces apoptosis, autophagy, and cell cycle arrest at G(1) phase in breast cancer cells. Appl. Microbiol. Biotechnol. 2020, 104, 10165–10179. [Google Scholar] [CrossRef]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Liu, X.; Wang, W.; Wang, J.; Li, X.; Sun, S. Preparation and Identification of Peptides with α-Glucosidase Inhibitory Activity from Shiitake Mushroom (Lentinus edodes) Protein. Foods 2023, 12, 2534. [Google Scholar] [CrossRef]
- Yehia, R.S. Evaluation of the biological activities of β-glucan isolated from Lentinula edodes. Lett. Appl. Microbiol. 2022, 75, 317–329. [Google Scholar] [CrossRef]
- Luo, W.; Deng, J.; He, J.; Yin, L.; You, R.; Zhang, L.; Shen, J.; Han, Z.; Xie, F.; He, J.; et al. Integration of molecular docking, molecular dynamics and network pharmacology to explore the multi-target pharmacology of fenugreek against diabetes. J. Cell. Mol. Med. 2023, 27, 1959–1974. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef]
- Ye, J.; Li, L.; Hu, Z. Exploring the Molecular Mechanism of Action of Yinchen Wuling Powder for the Treatment of Hyperlipidemia, Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Biomed Res. Int. 2021, 2021, 9965906. [Google Scholar] [CrossRef]
- Paisansak, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Angiotensin-I converting enzyme inhibitory peptide derived from the shiitake mushroom (Lentinula edodes). J. Food Sci. Technol. 2021, 58, 85–97. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, L.L.; Zhao, J.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T. Isolation and identification of the umami peptides from shiitake mushroom by consecutive chromatography and LC-Q-TOF-MS. Food Res. Int. 2019, 121, 463–470. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Li, W.; Wen, X.; Wu, D.; Zhang, Z.; Yang, Y. Effects of continuous enzymolysis on the umami characteristics of Lentinula edodes and the flavor formation mechanism of umami peptides. Food Chem. 2023, 420, 136090. [Google Scholar] [CrossRef]
- Wu, H.; Tao, N.; Liu, X.; Li, X.; Tang, J.; Ma, C.; Xu, X.; Shao, H.; Hou, B.; Wang, H.; et al. Polysaccharide from Lentinus edodes inhibits the immunosuppressive function of myeloid-derived suppressor cells. PLoS ONE 2012, 7, e51751. [Google Scholar] [CrossRef]
- Cao, X.; Xia, Y.; Liu, D.; He, Y.; Mu, T.; Huo, Y.; Liu, J. Inhibitory effects of Lentinus edodes mycelia polysaccharide on α-glucosidase, glycation activity and high glucose-induced cell damage. Carbohydr. Polym. 2020, 246, 116659. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Chen, Z.; Gao, X.; Chen, Y.; Xue, Z.; Guo, Q.; Ma, Q.; Chen, H. Physicochemical properties of polysaccharides from Lentinus edodes under high pressure cooking treatment and its enhanced anticancer effects. Int. J. Biol. Macromol. 2018, 115, 994–1001. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Zhang, X. Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes. J. Agric. Food Chem. 2012, 60, 11560–11566. [Google Scholar] [CrossRef]
- Ren, Z.; Li, J.; Song, X.; Zhang, J.; Wang, W.; Wang, X.; Gao, Z.; Jing, H.; Li, S.; Jia, L. The regulation of inflammation and oxidative status against lung injury of residue polysaccharides by Lentinula edodes. Int. J. Biol. Macromol. 2018, 106, 185–192. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Zheng, Q.; Wang, Y.; Hu, M.; Ma, F.; Qin, Z.; Lei, N.; Tao, N. MPSSS impairs the immunosuppressive function of cancer-associated fibroblasts via the TLR4-NF-κB pathway. Biosci. Rep. 2019, 39, BSR20182171. [Google Scholar] [CrossRef]
- Li, J.H.; Gu, F.T.; Yang, Y.; Zhao, Z.C.; Huang, L.X.; Zhu, Y.Y.; Chen, S.; Wu, J.Y. Simulated human digestion and fermentation of a high-molecular weight polysaccharide from Lentinula edodes mushroom and protective effects on intestinal barrier. Carbohydr. Polym. 2024, 343, 122478. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, H.; Liu, X.; Liu, C.; Liang, Y.; Mei, Y. Mycelial polysaccharides of Lentinus edodes (shiitake mushroom) in submerged culture exert immunoenhancing effect on macrophage cells via MAPK pathway. Int. J. Biol. Macromol. 2019, 130, 745–754. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Cheng, J.; Zhao, X.; Ma, B.; Yang, X.; Shao, H. Ultrasound-assisted extraction of polysaccharide from spent Lentinus edodes substrate: Process optimization, precipitation, structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 1038–1045. [Google Scholar] [CrossRef]
- Zhu, H.; Tian, L.; Zhang, L.; Bi, J.; Song, Q.; Yang, H.; Qiao, J. Preparation, characterization and antioxidant activity of polysaccharide from spent Lentinus edodes substrate. Int. J. Biol. Macromol. 2018, 112, 976–984. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Dan, D.; Hu, G. Physicochemical properties and bioactivities of Lentinula edodes polysaccharides at different development stages. Int. J. Biol. Macromol. 2020, 150, 573–577. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Wang, J.; Wu, Z.G.; Yang, J.M.; Li, W.; Shen, L.X. Extraction, purification and anti-proliferative activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2016, 93, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Xu, H. Analysis of chemical components of shiitake polysaccharides and its anti-fatigue effect under vibration. Int. J. Biol. Macromol. 2009, 45, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ren, Z.; Wang, X.; Jia, L.; Zhang, C. Antioxidant, anti-inflammatory and renoprotective effects of acidic-hydrolytic polysaccharides by spent mushroom compost (Lentinula edodes) on LPS-induced kidney injury. Int. J. Biol. Macromol. 2020, 151, 1267–1276. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, Y.; Meng, P.; Tu, D.; Zhao, Y.; Fu, L.; Tian, Y. Impact of combined ultrasonic and microwave vacuum drying on physicochemical properties and structural characteristics of polysaccharides from shiitake mushrooms (Lentinus edodes). Ultrason. Sonochem. 2025, 115, 107283. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef]
- Chen, H.; Ju, Y.; Li, J.; Yu, M. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int. J. Biol. Macromol. 2012, 50, 214–218. [Google Scholar] [CrossRef]
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Unursaikhan, S.; Xu, X.; Zeng, F.; Zhang, L. Antitumor activities of O-sulfonated derivatives of (1 → 3)-alpha-D-glucan from different Lentinus edodes. Biosci. Biotechnol. Biochem. 2006, 70, 38–46. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Yuan, Y.; Wang, Q.; Wang, L.; Wu, J. Lentinan progress in inflammatory diseases and tumor diseases. Eur. J. Med. Res. 2024, 29, 8. [Google Scholar] [CrossRef]
- Nishitani, Y.; Zhang, L.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal anti-inflammatory activity of lentinan: Influence on IL-8 and TNFR1 expression in intestinal epithelial cells. PLoS ONE 2013, 8, e62441. [Google Scholar] [CrossRef] [PubMed]
- Masterson, C.H.; Murphy, E.J.; Gonzalez, H.; Major, I.; McCarthy, S.D.; O’Toole, D.; Laffey, J.G.; Rowan, N.J. Purified β-glucans from the Shiitake mushroom ameliorates antibiotic-resistant Klebsiella pneumoniae-induced pulmonary sepsis. Lett. Appl. Microbiol. 2020, 71, 405–412. [Google Scholar] [CrossRef]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total. Environ. 2020, 732, 139330. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.A.S.; Rasouli, A.; Vahidi, H.; Kobarfard, F. Molecular identification of Shiitake (Lentinula edodes), analysis and production of beta-glucan using beech wood sawdust waste. Int. J. Biol. Macromol. 2024, 280, 135539. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Q.; Zeng, L.; Liu, Y.; Zhang, Y.; Sun, Z.; Guo, Q.; Cui, S.W. Structural Dynamics, Gut Microbiota Modulation, and Immunological Impacts of Shiitake Mushroom β-Glucan during In Vitro Intestinal Fermentation. J. Agric. Food Chem. 2025, 73, 12049–12060. [Google Scholar] [CrossRef]
- Pan, W.; Jiang, P.; Zhao, J.; Shi, H.; Zhang, P.; Yang, X.; Biazik, J.; Hu, M.; Hua, H.; Ge, X.; et al. β-Glucan from Lentinula edodes prevents cognitive impairments in high-fat diet-induced obese mice: Involvement of colon-brain axis. J. Transl. Med. 2021, 19, 54. [Google Scholar] [CrossRef]
- Ahn, H.; Jeon, E.; Kim, J.C.; Kang, S.G.; Yoon, S.I.; Ko, H.J.; Kim, P.H.; Lee, G.S. Lentinan from shiitake selectively attenuates AIM2 and non-canonical inflammasome activation while inducing pro-inflammatory cytokine production. Sci. Rep. 2017, 7, 1314. [Google Scholar] [CrossRef]
- Liu, N.; Zou, S.; Xie, C.; Meng, Y.; Xu, X. Effect of the β-glucan from Lentinus edodes on colitis-associated colorectal cancer and gut microbiota. Carbohydr. Polym. 2023, 316, 121069. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Thomas, M.C.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236. [Google Scholar] [CrossRef]
- Spinetti, G.; Mutoli, M.; Greco, S.; Riccio, F.; Ben-Aicha, S.; Kenneweg, F.; Jusic, A.; de Gonzalo-Calvo, D.; Nossent, A.Y.; Novella, S.; et al. Cardiovascular complications of diabetes: Role of non-coding RNAs in the crosstalk between immune and cardiovascular systems. Cardiovasc. Diabetol. 2023, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Xu, Y.; Zhang, G.; Liu, Y.; Li, H.; Chen, L. Natural products with potential hypoglycemic activity in T2DM: 2019–2023. Phytochemistry 2024, 223, 114130. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Nam, M.; Choi, J.Y.; Kim, M.S. Metabolic Profiles, Bioactive Compounds, and Antioxidant Capacity in Lentinula edodes Cultivated on Log versus Sawdust Substrates. Biomolecules 2021, 11, 1654. [Google Scholar] [CrossRef]
- Liu, D.; Mei, X.; Mao, Y.; Li, Y.; Wang, L.; Cao, X. Lentinus edodes mycelium polysaccharide inhibits AGEs-induced HUVECs pyroptosis by regulating LncRNA MALAT1/miR-199b/mTOR axis and NLRP3/Caspase-1/GSDMD pathway. Int. J. Biol. Macromol. 2024, 267, 131387. [Google Scholar] [CrossRef]
- Gong, P.; Wang, X.; Liu, M.; Wang, M.; Wang, S.; Guo, Y.; Chang, X.; Yang, W.; Chen, X.; Chen, F. Hypoglycemic effect of a novel polysaccharide from Lentinus edodes on STZ-induced diabetic mice via metabolomics study and Nrf2/HO-1 pathway. Food Funct. 2022, 13, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, K.; Xia, J.; Qian, D.; Guo, J.; Zhong, L.; Tang, D.; Chen, X.; Peng, W.; Chen, Y.; et al. Natural exosomes-like nanoparticles in mung bean sprouts possesses anti-diabetic effects via activation of PI3K/Akt/GLUT4/GSK-3β signaling pathway. J. Nanobiotechnology 2023, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Ye, C.; Li, Y.; Shi, J.; He, L.; Shi, X.; Yang, W.; Lei, W.; Quan, S.; Lan, X.; Liu, S. Network pharmacology analysis revealed the mechanism and active compounds of jiao tai wan in the treatment of type 2 diabetes mellitus via SRC/PI3K/AKT signaling. J. Ethnopharmacol. 2025, 337, 118898. [Google Scholar] [CrossRef]
- Liu, H.; Ju, A.; Dong, X.; Luo, Z.; Tang, J.; Ma, B.; Fu, Y.; Luo, Y. Young and undamaged recombinant albumin alleviates T2DM by improving hepatic glycolysis through EGFR and protecting islet β cells in mice. J. Transl. Med. 2023, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Yin, L.; Qi, Y.; Sun, H.; Sun, P.; Xu, M.; Tang, Z.; Peng, J. miR-125a-5p ameliorates hepatic glycolipid metabolism disorder in type 2 diabetes mellitus through targeting of STAT3. Theranostics 2018, 8, 5593–5609. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diab. Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Shafiei-Jahani, P.; Yan, S.; Kazemi, M.H.; Li, X.; Akbari, A.; Sakano, K.; Sakano, Y.; Hurrell, B.P.; Akbari, O. CB2 stimulation of adipose resident ILC2s orchestrates immune balance and ameliorates type 2 diabetes mellitus. Cell Rep. 2024, 43, 114434. [Google Scholar] [CrossRef]
- Hu, F.; Xiong, L.; Li, Z.; Li, L.; Wang, L.; Wang, X.; Zhou, X.; Zheng, Y. Deciphering the shared mechanisms of Gegen Qinlian Decoction in treating type 2 diabetes and ulcerative colitis via bioinformatics and machine learning. Front. Med. 2024, 11, 1406149. [Google Scholar] [CrossRef]
- Wen, X.; Lv, C.; Zhou, R.; Wang, Y.; Zhou, X.; Qin, S. The Molecular Mechanism Underlying the Therapeutic Effect of Dihydromyricetin on Type 2 Diabetes Mellitus Based on Network Pharmacology, Molecular Docking, and Transcriptomics. Foods 2024, 13, 344. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Mu, J.; Li, D.; Ma, Y.; Meng, X. Role of Wnt signaling pathways in type 2 diabetes mellitus. Mol. Cell. Biochem. 2021, 476, 2219–2232. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Ren, Y.; Ma, Y.; Liu, J.; Jiang, H.; Liu, C. Artesunate improves glucose and lipid metabolism in db/db mice by regulating the metabolic profile and the MAPK/PI3K/Akt signalling pathway. Phytomedicine 2024, 126, 155382. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, M.; Sun, W.; Li, Q.; Xi, H.; Qiu, Y.; Wang, R.; Ding, Q.; Wang, Z.; Yu, Y.; et al. Hirsutine ameliorates hepatic and cardiac insulin resistance in high-fat diet-induced diabetic mice and in vitro models. Pharmacol. Res. 2022, 177, 105917. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, X.; Li, Q.; Sun, Z.; You, Y.; Zhang, L.; Ji, Z.; Zhou, H.; Zhang, Q.; Wang, L.; et al. Niacin regulates glucose metabolism and osteogenic differentiation via the SIRT2-C/EBPβ-AREG signaling axis. Biomed. Pharmacother. 2024, 180, 117447. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Ramasubbu, K.; Devi Rajeswari, V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: A perspective review. Mol. Cell. Biochem. 2023, 478, 1307–1324. [Google Scholar] [CrossRef]
- Chen, J.; Qu, B.; Yang, D.; Wang, Y.; Zhu, H.; Wang, Z.; Zhang, X.; Ma, H.; Zhao, N.; Zhao, L.; et al. Combined metabolomics and network pharmacology to elucidate the mechanisms of Huiyang Shengji decoction in treating diabetic skin ulcer mice. Phytomedicine 2025, 141, 156569. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, H.; Wang, X.; Kang, J.; Guo, W.; Zhou, L.; Liu, H.; Wang, M.; Jia, R.; Du, X.; et al. Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine 2022, 95, 153837. [Google Scholar] [CrossRef]
- Bisht, A.; Tewari, D.; Kumar, S.; Chandra, S. Network pharmacology, molecular docking, and molecular dynamics simulation to elucidate the mechanism of anti-aging action of Tinospora cordifolia. Mol. Divers. 2024, 28, 1743–1763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.