Abstract
As the global population continues to expand and demand for protein increases, alternative proteins (e.g., edible insect proteins, microalgae proteins, fungal or bacterial proteins) have emerged as a significant area of research interest due to their high nutritional value and sustainability. However, these novel protein sources may contain allergenic components, such as tropomyosin and arginine kinase in insects, phycocyanin in microalgae, and ribosomal proteins in fungi, which may trigger allergic reactions and cross-reactivity with traditional allergens. In this review, we systematically retrieved published studies from databases including PubMed and Web of Science, employing keywords such as microbial proteins, edible insects, and allergenicity. Articles were screened based on their relevance to allergenic properties and processing effects, with selected studies subjected to thematic analysis. The present paper reviews the allergenic properties of edible Insects, microalgae, and microorganisms’ proteins and their molecular mechanisms, and explores the effects of various processing techniques (e.g., heat treatment, enzymatic hydrolysis, high-pressure treatment, and glycosylation) on the reduction of allergenic activity. It was determined that the impact of processing methodologies is contingent on protein structure, with certain techniques having the potential to augment sensitization through epitope exposure. Furthermore, there are still gaps in the current research on the reduction in allergenicity of microbial and algal allergens, and future research should focus on the in-depth characterization of allergenic protein structures and the development of novel sensitization reduction techniques. This review provides a significant reference point for the safe development and rational application of edible insects, microalgae, and microorganisms proteins, which is of great importance for the development of sustainable food systems.
1. Introduction
Protein is the main bearer of life activities in the human body and is essential for growth, development, repair and maintenance of immune function. As the world’s population increases year by year, the demand for protein is growing. According to reports, the global population is projected to increase to 9.1 billion by 2050, with per capita daily calorie intake expected to rise from 2772 kcal to 3070 kcal [1].Currently, proteins can be divided into five categories according to their origin: proteins of animal origin (milk, eggs, fish, meat and animal offal), proteins of plant origin (pulses, cereals, oilseeds, etc.), edible insects, micro-algae and unicellular proteins (fungi, yeasts and bacteria). Of these, plant-derived proteins play an important role in the protein supply, with animal-derived proteins being the most expensive due to, inter alia, long economic cycles [2,3].Traditional protein production, which relies mainly on cultivation and farming, is highly land- and water-dependent, making it difficult to meet the growing demand for protein. Given the current shortage of plant and animal proteins, the development of alternative sources of protein has become a priority [4].
To date, a variety of alternative protein sources have been developed, such as: edible insect proteins, microalgae proteins, and single cell proteins (SCPs). Edible insects, with a protein content comparable to that of animal-origin protein sources and with all the essential amino acids, are now being used as an ideal alternative or supplement to traditional protein sources [5]. Unicellular organisms and microalgae are not only comparable to traditional protein sources in terms of amino acid composition and protein content, but also require significantly less water and cultivated area [6,7]. Thus, single-cell proteins and microalgae proteins are also important components of novel alternative proteins. Although, consuming enough protein is very important for the human body, consuming proteins along with allergenic proteins that can cause allergies can be fatal [8].
Food allergy is a condition that occurs when the body’s immune system mistakes a normally harmless food component (usually a protein) for a harmful substance, triggering a series of abnormal immune responses (Figure 1). It is estimated that food allergies affect approximately 5% of adults and 8% of children worldwide [9]. Symptoms of food allergy may involve the respiratory tract, gastrointestinal tract, skin and cardiovascular system [8]. Currently, the World Health Organization (WHO) and members of the International Union of Immunological Societies (IUIS) Allergen Nomenclature Sub-Committee have identified about 390 foods as allergenic (https://www.allergen.org). Eggs, milk, peanuts, nuts, wheat, and fish and shellfish are the most common foods that cause allergic reactions [10]. Although alternative proteins are not as popular in the daily diet nowadays compared to the food proteins mentioned above, allergic reactions caused by allergens in alternative proteins should be taken seriously.
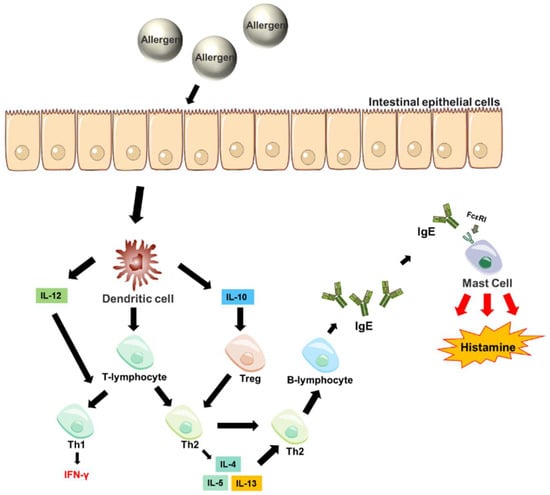
Figure 1.
Causes of food allergy. After an allergen enters the body, it is recognized by effector T-cells and differentiates into immune B-cells that can release IgE. The IgE secreted by the B-cells binds to receptors on mast cells and is activated on a second exposure to the antigen. The activated mast cells release substances such as histamine leading to the development of an allergic reaction [11].
Food allergies can somewhat impair the body’s absorption and utilization of a particular type of protein, which can only be supplemented by other protein sources. Fortunately, some forms of protein processing or modification can make allergenic proteins less allergenic, such as heat treatment, high pressure, glycosylation and fermentation [12,13,14]. In this review, we will focus on the allergenic proteins in these edible insects, microalgae, and microorganisms’ proteins and methods to reduce the allergenicity of the allergenic proteins, to provide guidance for consumers in selecting as well as protein products to reduce or avoid the occurrence of allergic reactions.
1.1. Edible Insect Proteins
Edible insects may refer to whole, dried and minimally processed insects, but may also refer to heavily processed powders, bars, artificial meat and fortified foods [15]. Edible insects are nutrient-dense, providing high-quality protein (up to 60% dry weight), essential amino acids, fats, and micronutrients (e.g., iron, zinc, B-vitamins) [16,17]. Insects consumed as food belong to diverse taxonomic orders (Figure 2), including Orthoptera (crickets, grasshoppers), Coleoptera (mealworms), and Lepidoptera (silkworms) [18]. Edible insects have a low dependence on land and water compared to traditional livestock, and a richer amino acid profile and higher protein content than plant protein sources, as well as simultaneous nutrients such as vitamins and minerals [4,19].
Beyond their nutritional profile, insect proteins also exhibit functional properties that enhance their potential as sustainable food ingredients. For example, edible insect protein has good water-binding, oil-binding, foaming and emulsifying properties, and is able to form functional peptides with health benefits (such as antioxidants, etc.) [20,21,22].
While insect protein is physiologically more acceptable than whole insects to some consumers, safety concerns remain. Heavy metals [23] (e.g., from contaminated environments) and natural toxins [24] (e.g., cyanide in certain species) may pose risks, as can pesticide residues if insects are harvested from treated areas. However, these hazards can be minimized through controlled breeding, feed regulation, and post-harvest processing.

Figure 2.
Fifteen Common Edible Insects and Their Scientific Names. The species represented include Coleoptera (e.g., Rhynchophorus ferrugineus), Lepidoptera (e.g., Bombyx mori), Orthoptera (e.g., Gryllus bimaculatus), and Hymenoptera (e.g., Oecophylla smaragdina).
1.2. Single-Cell Proteins
Cellular agriculture is an emerging field of biotechnology dedicated to the development of a variety of alternatives for commercial agricultural production, including food and feed that utilize microorganisms and microalgae as a source of high protein, collectively referred to as SCP. The development of microbial proteins is both a way to fill possible protein resource shortages and a green and sustainable way to grow. We can produce microbial proteins from by-products of the fermentation industry or agro-industry, e.g., waste brewer’s yeast recycled from the fermentation industry and photosynthetic bacteria (Rhodopseudomonas sp.) from biogas treatment processes [25,26]. However, issues such as high nucleic acid content and cost constraints have prevented large-scale production of microbial proteins for applications [27,28,29].
1.2.1. Microalgae Proteins
Microalgae have been shown to contain a protein content that exceeds 50%, which is indicative of their high nutritional value. This has led to microalgae being identified as a promising source of non-animal proteins [30]. Phycocyanin from microalgae is a natural food colorant. Natural food coloring not only provides vibrant colors, but also has potential health benefits, such as: antioxidant, antibacterial, anticancer, hypolipidemic, etc. [31,32]. Spirulina is a significant source of phycocyanin for human consumption, attributable to its high phycocyanin content [33]. In addition to its high production of phycocyanin, Spirulina has become an important source of dietary supplements due to its nutrient-rich nature and is widely added to snacks, pasta, cookies, and breads, accounting for 99.5% of the total global production of microalgae [34]. Spirulina is rich in protein, up to 60–70% of dry weight, which is much higher than the protein content of meat (15–25%) and beans (35%) [35]. In addition to its high protein content, Spirulina is also high in macronutrients and micronutrients [36]. According to United Nations Educational, Scientific and Cultural Organization (UNESCO), “Spirulina is the perfect food for tomorrow” and the US Food and Drug Administration (USFDA) has called it “one of the best sources of protein” [36]. Despite the evident advantages of microalgae proteins in terms of sustainability and high nutritional value, there are still some disadvantages and shortcomings associated with their development as an alternative protein source. These include higher production costs, organoleptic properties and flavour, innovation in extraction and refining processes, and economic feasibility issues [37,38,39].
1.2.2. Fungal, Bacterial and Yeast Proteins
Fungal or bacterial proteins are another great source of single-cell proteins. The feasibility, tolerance and metabolic effects of consuming fungal proteins were experimentally determined as early as the 1970s [40]. In addition to nutrients such as lipids and cell wall glucans, unicellular organisms have high protein content. In Saccharomyces cerevisiae, for instance, the protein content of yeast extracts can reach 50–70% of the dry weight [41]. Yeast proteins are highly bioavailable to humans and animals, with studies showing that yeast proteins are 96% digestible and 59% net utilizable [42]. Yeast cell proteins have a higher protein efficiency ratio (PER) compared to proteins from conventional sources. In addition, yeast proteins are a rich source of bioactive peptides, which can exert positive effects on human health [41,43]. Another widely used fungus is Fusarium venenatum. F. venenatum has a protein content of 45–54% dry weight and is now often used to produce meat substitutes because of its meat-like structure [42,44,45]. The proteins of F. venenatum are highly similar to yeast proteins in composition and characterization, with digestibility-corrected amino acid scores comparable to milk proteins and higher than those of chicken versus beef, making it a very promising alternative protein source [46]. Bacterial proteins are also a commonly used single-cell proteins and are mostly used in animal feed. Bacterial proteins make up 50–80% of the dry weight and bacteria have shorter growth times and faster growth rates compared to fungi and yeast [45]. Moreover, it has been demonstrated that bacteria have the capacity to proliferate on a wide range of substrates, including gaseous substrates [47]. Photosynthetic Bacteria (PSB) are ancient microorganisms that are widely distributed in a variety of environments [48,49,50]. PSB is rich in microbial proteins and contains high value carotenoids, bacterial chlorophyll and CoQ10 [51,52,53]. Some studies have shown that photosynthetic bacteria can treat a variety of wastewater under the right conditions [54]. It has been demonstrated that PSBs are capable of producing proteins in a highly efficient manner from these wastewaters. Nevertheless, there are numerous challenges associated with the cultivation of PSB using wastewater. The primary issue that must be addressed pertains to the potential presence of pollutants in the wastewater, including heavy metal ions in industrial wastewater, disease-causing microorganisms in municipal wastewater, and pesticide residues in agricultural wastewater. This has a considerable impact on the production of PSB. Moreover, the question of consumer acceptability of wastewater farming represents a significant challenge that impacts the competitiveness of the PSB market. The large-scale development of microbial protein production has the potential to address two significant challenges: the scarcity of protein resources and the environmental impact of intensive livestock farming.
Despite the numerous advantages exhibited by single-cell proteins, which are analogous to microalgae proteins, their elevated nucleic acid content constitutes a pivotal concern. This high nucleic acid content exerts a substantial influence on the organoleptic properties and flavor of the proteins. Consequently, further processing is imperative prior to the extraction of the proteins. SCPs possess considerable potential as a sustainable protein source, exhibiting both sustainability and environmental friendliness. Nevertheless, further process and technology innovations are required in order to address issues such as sensory properties and cost. Should these challenges be surmounted in the future, SCP will indubitably play an important role in the food industry.
1.3. Applications of Insect Protein and Microbial Protein in Foods
As emerging protein sources, insect protein and microbial protein are gradually gaining attention within the food industry. Table 1 summarizes the relevant applications of insect protein and microbial protein in food products. Insect protein is rich in high-quality amino acids and offers advantages such as rapid reproduction and high feed conversion efficiency. Microbial protein, on the other hand, exhibits benefits including short growth cycles and the ability to be produced using waste materials. Furthermore, microbial protein can be processed through cell autolysis or enzymatic hydrolysis to obtain functional peptides (e.g., ACE inhibitory peptides, immunomodulatory peptides), thereby providing potential for the development of high-value food ingredients. These unique characteristics endow them with significant potential to meet future global protein demands, and their application in food systems has garnered increasing interest. In addition, the incorporation of edible insects or microbial protein into food products can enhance both the nutritional profile and physicochemical properties of the final food items.

Table 1.
Applications of Insect Protein and Microbial Protein in Food Products.
2. Allergens
With the widespread use of alternative proteins, especially the continued development of new alternative protein sources such as insect proteins, microalgae proteins, and fungal or bacterial proteins, allergic reactions to these alternative proteins have attracted increasing attention from consumers. Despite the significant environmental and health benefits of these alternative proteins, they also exhibit unique characteristics in terms of the types of allergens they contain. Understanding the types of these allergens and the immune responses they elicit is critical to safeguarding consumer food safety and health. Allergenic proteins in common edible insects, microalgae, and microorganism proteins are shown in Table 2.
2.1. Edible Insect Allergens
In addition to the toxicity and heavy metal exceedances previously mentioned, which render insects less acceptable to consumers, allergic reactions caused by edible insect protein allergens represent a significant issue with regard to the consumption of insects [77]. Tropomyosin, arginine kinase have been identified as major allergens in insects [78]. In economically underdeveloped regions or countries, such as Africa, exposure to or consumption of insects is common, as insects provide the body with the protein and energy it needs on a daily basis. However, studies and reported cases of insect allergy in Africa are uncommon. This may be due to the fact that most studies have focused more on the nutritional value of insects to address problems such as malnutrition in Africa [79]. Whereas in China, there are frequently reported cases of allergy to edible insects [80,81].
2.1.1. Bombyx mori
Bombyx mori and its derivatives are known for their high nutritional, medicinal and economic values [82]. The total protein, lipid content and ash (dry weight) of silkworm pupae were 71.9%, 20.1% and 4.0%, respectively [83]. In China, silkworm pupae are the most commonly consumed insect. However, it is estimated that more than a thousand people become allergic to silkworm pupae each year. Symptoms of silkworm pupa allergy include vomiting, dizziness and asthma [83].
Liu et al. cloned and expressed arginine kinase (Bomb m 1) from silkworm larvae and confirmed its allergenicity by methods such as ELISA and Western blotting [84]. It was found that silkworm arginine kinase binds to serum IgE from silkworm-allergic patients and that it is cross-reactive with arginine kinase from cockroaches. Tropomyosin is a recognised invertebrate pan-allergen and is no exception in the Bombyx mori. Tropomyosin (Bomb m 3) is an important allergen in silkworm pupae. By comparison with other known allergens, Tropomyosin from silkworm pupae showed 73.5% to 92.3% amino acid sequence homology, with particularly high similarity to Tropomyosin from cockroaches and dust mites [85]. Bombyx mori lipoprotein 3 (Bmlp3) is a 27.6 kDa allergenic protein defined as Bomb m 6 that elicits a strong IgE response in the sera of domestic silkworm allergy sufferers [86]. Bmlp3 is a major allergen in the domestic silkworm and is highly stable to heat, acid and enzyme digestion, meaning that it can still trigger allergic reactions in a wide range of food processing conditions. In addition, another major allergen in the silkworm is Hemolymph lipoprotein (Bomb m 4), which Jeong et al. identified by proteomic analysis and confirmed to be allergenic by ELISA [87]. Bomb m 9 is a structural protein present in the haemolymph of the silkworm, Bombyx mori, belonging to the 30k family, which binds to IgE in the serum of silkworm pupa-allergic individuals to cause allergic asthma [88]. However, unlike silkworm pupa arginine kinase, Bomb m 9 has no cross-reactivity with allergenic proteins in cockroaches. In addition to the allergenic proteins mentioned above, there are two other allergenic proteins, lipoprotein (Bomb m 5) and lipoprotein 3 (Bomb m 6), in the domestic silkworm (Table 2).
2.1.2. Tenebrio molitor
Tenebrio molitor is an emerging edible insect. Freeze-dried T. molitor larvae contain about 33% fat, 51% crude protein and 43% true protein (on a dry matter basis) and are often added to pasta, energy bars and biscuits [89,90]. T. molitor protein extracts contain a variety of possible allergens including Actin, HSP70, α-amylase, Myosin light chain, Arginine kinase and Tropomyosin [78,91]. Among them, Tropomyosin and Arginine Kinase are known to be the main allergens of T. molitor [78]. Multiple allergens in T. molitor cross-react with other known arthropod (e.g., shrimp, crab, etc.) allergens [91]. The Allermatch website (https://allermatch.org/) allows comparative analysis of the sequences of target proteins and allergenic proteins. T. molitor Tropomyosin has more than 35% identity with known Tropomyosin that can cause allergies, such as shrimp Tropomyosin [91]. Therefore, people who are allergic to shrimp may also be allergic to T. molitor and should be vigilant when choosing edible insect foods [78,92].
2.1.3. Hermetia illucens
Hermetia illucens is a potential sustainable and nutritious edible insect. The protein content of H. illucens can reach 40–60%, with a relatively low fat content of 10–30%, and they are rich in essential amino acids, fatty acids, and minerals such as calcium, iron, and zinc, making them an ideal raw material for healthy food [93]. However, at present, the utilization of H. illucens is mainly limited to animal feed, and it has not been approved by regulations as a food ingredient. This is because they mostly grow in garbage, and the decomposition of garbage and waste may cause H. illucens to accumulate a large amount of heavy metals and pollutants in their bodies. Eating H. illucens is bound to have an impact on human health. Nevertheless, it cannot be denied that if waste and other waste materials can be used reasonably to breed H. illucens as a food ingredient, it will be a green, sustainable and nutritious food source. The main allergens in H. illucens include Tropomyosin, Arginine Kinase, and Myosin light chain. Studies have shown that the Tropomyosin in H. illucens has a high degree of similarity (about 75–80% sequence similarity) with that in shrimp (such as Penaeus monodon), which means that people allergic to shrimp may also have allergic reactions to H. illucens [94]. In addition, through immunoblotting experiments, Tropomyosin is widely recognized in H. illucens extracts and shows strong IgE binding ability, especially for people allergic to seafood [94]. In addition, other allergenic proteins such as Troponin, Triosephosphate isomerase, Hemocyanin, Cuticle proteins, and Odorant-binding proteins have also been identified in H. illucens.
2.1.4. Periplaneta americana & Blattella germanica
Periplaneta americana and Blattella germanica are two common cockroach species. Periplaneta americana is larger in size and prefers hot and humid environments, while Blattella germanica is smaller and more commonly found in dry and cool areas [95,96]. According to the WHO/IUIS website (https://www.allergen.org), both P. americana and B. germanica have been identified to possess over ten allergenic proteins (Table 2). Notably, there is a considerable degree of homology and cross-reactivity between the allergens of B. germanica and P. americana [97]. Studies have shown that Bla g 1 and Bla g 2 are the major allergens of B. germanica. These allergenic proteins bind to IgE in the serum of allergic patients, triggering allergic asthma, which is commonly observed in regions such as the United States [98,99]. Among them, Bla g 2 can also serve as a key biomarker for B. germanica exposure and allergy. In addition, Bla g 5, a Glutathione S-transferase (GST), is one of the most prevalent cockroach allergens in the US population [100]. Research has found that GST from different cockroach species exhibit low cross-reactivity, making this protein highly useful for accurate allergen diagnosis [97]. Tropomyosin in cockroaches (Bla g 7 and Per a 7) is a “pan-allergen” present in the muscle tissues of various organisms (mollusks, arthropods, parasites, etc.) and can induce IgE cross-reactivity [101]. Arginine kinase (Bla g 9 and Per a 9) is also a major cockroach allergen that binds strongly to IgE in patient serum [95]. As a cross-reactive allergen between shrimp and arthropods, Arginine Kinase in cockroaches also exhibits IgE cross-reactivity [102]. Studies have indicated a close association between cockroaches and allergic respiratory diseases, with a more severe situation observed among low-income urban populations [103]. In Taiwan, 58% of asthma patients are allergic to the Per a 2 allergen of cockroaches, and in Poland, approximately 25% of asthmatic children are sensitive to cockroaches [104,105]. Kangfuxin liquid (KFX) is a traditional Chinese medicine for promoting wound healing in China. It can be applied externally by soaking gauze in the medicinal liquid and applied to the affected area, or taken orally. The main component of KFX is an extract from P. americana. This may be one of the reasons why KFX is prohibited for use in individuals with asthma. For allergic asthma caused by cockroaches, immunotherapy for cockroach allergy is currently available, but the most effective approach remains avoiding exposure to cockroach-infested environments [106]. In addition to the aforementioned allergenic proteins, cockroaches also contain various other allergens such as hemolymph proteins, Troponin, Myosin Light Chain, serine proteases, α-Amylases, and chitinases (Table 2).
2.1.5. Other Edible Insects
Acheta domesticus is another popular edible insect commonly used as a food ingredient in barbecues. The protein content of A. domesticus is 47%, with a fat content of 25%, and it is also rich in minerals and vitamins [107]. To date, various allergenic proteins have been identified in A. domesticus, including tropomyosin, Myosin heavy chain isoform, Myosin heavy chain, and Myosin Light Chain [108]. Tropomyosin is the major allergen in A. domesticus. Studies have shown that the actin in crickets shares significant amino acid sequence similarity with that in shrimp, particularly in the binding epitopes [109]. This similarity may lead to allergic reactions to crickets in individuals allergic to shrimp. A real-world prevalence study on IgE reactivity to crickets, locusts, and mealworms involving 2000 participants revealed that 9.7% (195 individuals) were sensitive to insects. Among these 195 individuals, 34% had common recognition of tropomyosin, and 18.5% tested positive for arginine kinase [110].
Locusta migratoria, belonging to the order Orthoptera and family Acrididae, comprise 6787 known species [111]. The protein content of locusts can reach 18–29%, comparable to or even higher than that of meat [112]. In addition to their high protein content, locusts are also rich in vitamins, minerals, amino acids, as well as high levels of sterols and unsaturated fatty acids [112]. Locusts have become a traditional food in some regions as part of the diet [113,114]. However, for certain populations, consuming locusts may trigger acute allergic reactions and even lead to anaphylactic shock [115]. Ji et al. reported 27 cases of anaphylactic shock caused by locust consumption over the twenty-year period from 1980 to 2008 [116]. The main allergenic proteins in locusts include arginine kinase and hemocyanin [114,117]. These two proteins are commonly found in various insects and crustaceans, thus potentially causing cross-reactions similar to seafood allergies. Wang et al. identified a hexamerin-2 protein in locusts that binds strongly to IgE in the serum of locust-allergic patients [118]. Nevertheless, when processing locusts, removing body parts such as wings, legs, and antennae and employing processing methods like grinding can reduce the risk of allergies caused by locusts [112].
2.1.6. Prevention and Control of Allergy Risks from Edible Insects
Edible insects, as an emerging sustainable protein source, possess high nutritional value and environmental friendliness; however, their potential allergenicity cannot be overlooked. Tropomyosin and Arginine Kinase are the most prevalent “pan-allergens” in edible insects, widely present in B. mori, T. molitor, H. illucens, A. domesticus and L. migratoria (Figure 3). These allergens not only have the potential to trigger local or systemic allergic reactions (such as rashes, vomiting, asthma, and even anaphylactic shock) but also exhibit significant cross-reactivity with allergens from crustaceans (e.g., shrimp, crab), mites, and cockroaches, thereby increasing the allergenic risk for individuals with seafood allergies or asthma. Therefore, when edible insects are used as food ingredients, potential allergenic risks should be clearly indicated on the packaging to inform consumers. For edible insect proteins, further development of low-allergenicity processing technologies is essential in the future to mitigate the risk of allergic reactions in sensitized individuals following consumption.
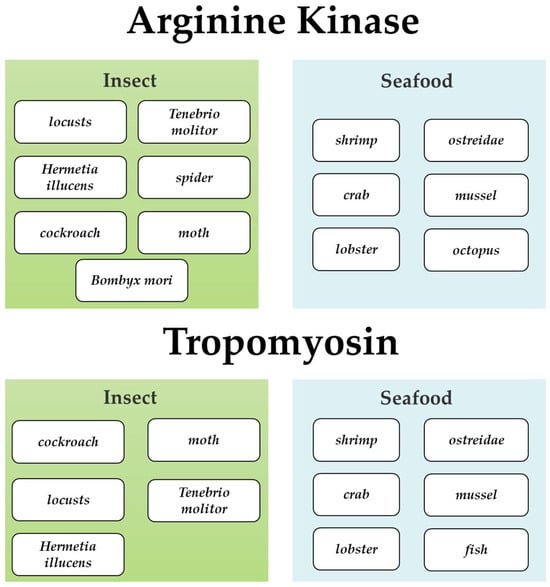
Figure 3.
Distribution of allergenic proteins arginine kinase and tropomyosin in insect and seafood sources. The upper figure shows arginine kinase present in various insects and seafood. The lower figure displays tropomyosin in different insects and seafood products.
2.2. Microbial Allergens
The increasing integration of microorganisms into food production, pharmaceuticals, and environmental applications has revealed an underappreciated public health concern: microbial allergenicity. Unlike conventional allergens derived from pollen or animal dander, microbial allergens exhibit unique molecular features—including intrinsic thermostability, conserved functional domains, and frequent cross-reactivity across phylogenetically distant species—that complicate their detection and clinical management. These characteristics stem from the evolutionary conservation of proteins essential for microbial survival, which paradoxically render them both biotechnologically valuable and immunologically reactive. Despite the increasing evidence for the existence of microbial allergens, systematic studies on their molecular mechanisms and allergenic pathways are still limited, posing challenges to risk assessment and regulatory frameworks.
2.2.1. Microalgae Allergens
As a highly diverse group of photosynthetic organisms, microalgae contain intracellular proteins such as phycocyanin that have been confirmed to induce allergic symptoms through IgE-mediated immune responses, clinically manifesting as respiratory inflammation, skin reactions, and even systemic anaphylaxis. Studies have demonstrated that phycocyanin is the primary allergen responsible for Spirulina-induced allergies [119,120]. As the main pigment protein in Spirulina, phycocyanin exhibits various biological effects, including antioxidant and anti-inflammatory properties. In addition to phycocyanin, six proteins in Spirulina extracts have been detected with sequence homology to allergenic proteins from other sources [34]. Computational and proteomic analyses have revealed that these proteins share sequence homology with food allergens such as corn, fish, and shrimp [121]. Therefore, individuals allergic to fish and shellfish should exercise caution when consuming Spirulina-enriched foods due to potential allergic risks.
Another microalga frequently reported to cause allergies is Chlorella, likely because it is more commonly used in health supplements alongside Spirulina. Allergenic proteins with molecular weights of 13, 17, 19, 25–26, 46–50, and 72 kDa have been identified in Chlorella [122,123]. Similarly, Mariachiara et al. conducted a computational assessment of sequence homology between Chlorella proteins and known allergens [120]. Four proteins were found to exhibit sequence identity with other allergenic proteins, among which fructose-bisphosphate aldolase was identified as the Chlorella protein most likely to cause cross-reactivity upon ingestion due to its significant sequence homology with the corresponding protein in edible fish and crustaceans. Consequently, individuals allergic to fish and crustaceans should be cautious about consuming health supplements containing algal additives to avoid potential allergic reactions.
2.2.2. Fungal Allergens
The widespread application of microorganisms in environmental, food, and pharmaceutical fields has increasingly drawn attention to their potential allergenicity. Fungi, bacteria, and yeasts can produce various allergenic proteins capable of inducing specific IgE antibody production in the host immune system, thereby triggering allergic reactions. Alternaria alternata, a common outdoor fungus, is one of the major airborne allergens whose spores are significant contributors to allergic sensitization. It shows a strong association with allergic respiratory diseases, particularly asthma [124,125]. To date, numerous A. alternata allergenic proteins have been identified and purified (Table 2) [126]. Alt a 1 is the major allergen of A. alternata, with approximately 80% of patients allergic to this fungus showing sensitivity to this allergen [127,128]. Ribosomal protein P2 (Alt a 5) represents a minor allergen of A. alternata and shares sequence homology with the Ribosomal protein P2 found in Fusarium culmorum [129]. It is well established that cross-reactivity between proteins can arise from shared amino acid or carbohydrate epitopes [130]. Given the presence of Ribosomal protein P2 in both A. alternata and F. culmorum, cross-reactivity between these two species is highly plausible. In addition to Ribosomal protein P2 (Alt a 5), other allergens in A. alternata such as Heat shock protein 70 (Alt a 3), Enolase (Alt a 6), Glutathione-S-transferase (Alt a 13), and Manganese superoxide dismutase (Alt a 14) also exhibit potential for cross-reactivity with other known allergenic proteins.
Saccharomyces cerevisiae, an essential microorganism in food processing widely used in bread and beer production, may still induce allergic reactions despite inactivation during processing [131]. Among its allergenic components, enolase from S. cerevisiae has drawn particular attention as a major sensitizer due to its high secretory level and conserved structure. Baldo et al. evaluated the sensitization profile of 47 fungal inhalation allergy patients to S. cerevisiae through specific allergy testing [131]. The study revealed that 35 patients exhibited positive reactions to S. cerevisiae extracts, with immunoblot analysis confirming yeast enolase as the primary allergenic protein. RAST inhibition assays further demonstrated significant cross-reactivity between S. cerevisiae enolase and Candida albicans extracts. Clinically, a case of anaphylactic shock was reported in a fungal allergy patient following consumption of yeast-containing food products. Skin prick tests (SPT) and serum IgE measurements confirmed cross-reactive IgE antibodies against multiple fungi including S. cerevisiae. Additionally, allergen databases have identified several other potential allergens in S. cerevisiae, including glucosidase, β-fructofuranosidase, manganese superoxide dismutase (Mn-SOD), profilin, and ribosomal proteins (Table 2).
In addition to S. cerevisiae, another well-known commercial fungus is Fusarium culmorum, which is commonly utilized in the production of meat substitutes, specifically plant-based meats. F. culmorum contains three identified allergenic proteins: Ribosomal protein P2, Thioredoxin reductase, and transaldolase. Research has demonstrated that Ribosomal protein P2 (Fus c 1) present in the mycoprotein (Quorn) produced by F. culmorum serves as the primary allergen [132]. Fus c 1 exhibits high sequence homology with Ribosomal proteins from various molds, including Alt a 5 mentioned previously, which may lead to IgE cross-reactivity [129,132]. Furthermore, both F. culmorum and S. cerevisiae possess allergenic proteins of the same types found in algal proteins, suggesting potential sequence homology among these proteins. Consequently, individuals allergic to these fungal proteins may also be at risk of allergic reactions to algal proteins.
2.2.3. Prevention and Control of Microbial Allergy Risks
The widespread application of fungi, bacteria, and yeasts in environmental, food, and pharmaceutical fields necessitates attention to their potential allergenicity. Microorganisms such as A. alternata, S. cerevisiae, and F. culmorum can produce various allergenic proteins that induce IgE-mediated allergic reactions and are closely associated with diseases such as asthma, allergic rhinitis, and even anaphylactic shock. Notably, homologous proteins across different microorganisms (e.g., Alt a 5 and Fus c 1, S. cerevisiae enolase and C. albicans protein extracts) may trigger cross-reactivity, thereby increasing the risk of allergic sensitization. This cross-sensitization is not only observed among fungi but may also extend to cross-reactivity with algal proteins.

Table 2.
Allergenic proteins in edible insects and microalgae (“*” denotes formally registered allergenic proteins in the allergen nomenclature database, “**” denotes non-formally registered allergenic proteins in the allergen nomenclature database, Allergen Nomenclature Database Website: www.allergen.org).
Table 2.
Allergenic proteins in edible insects and microalgae (“*” denotes formally registered allergenic proteins in the allergen nomenclature database, “**” denotes non-formally registered allergenic proteins in the allergen nomenclature database, Allergen Nomenclature Database Website: www.allergen.org).
| Type | Source | Protein Type | Allergen | MW (kDa) | Reference |
|---|---|---|---|---|---|
| Edible insects | Bombyx mori | Arginine kinase | Bomb m 1 * | 42 | Yue et al. [86] |
| Bombyx mori | Tropomyosin | Bomb m 3 * | 40 | Yue et al. [86] | |
| Bombyx mori | Hemolymph lipoprotein | Bomb m 4 * | 30 | Yue et al. [86] | |
| Bombyx mori | lipoprotein | Bomb m 5 * | 30 | Yue et al. [86] | |
| Bombyx mori | Apolipophorin-III | Bomb m 6 * | 25–33 | Yue et al. [86] | |
| Bombyx mori | 30K Protein | Bomb m 9 ** | 30 | Zuo et al. [88] | |
| Acheta domesticus | Tropomyosin | Unknown ** | 32–4 | Lamberti et al. [108] | |
| Acheta domesticus | Myosin heavy chain isoforms | Unknown ** | 224.7 | Kamemura et al. [133] | |
| Acheta domesticus | Myosin heavy chain | Unknown ** | 135 | Lamberti et al. [108] | |
| Acheta domesticus | Myosin Light Chain | Unknown ** | 2 | Lamberti et al. [108] | |
| Acheta domesticus | Troponin T | Unknown ** | 46.7 | Lamberti et al. [108] | |
| Acheta domesticus | Hexamerin-like protein 2 | Unknown ** | 75 | de las Marinas et al. [134] | |
| Pygmy grasshoppers | Cuticle proteins | Unknown ** | 18.9 | Lamberti et al. [108] | |
| Pygmy grasshoppers | Myosin heavy chain | Unknown ** | 222.8 | Lamberti et al. [108] | |
| Pygmy grasshoppers | Myosin Light Chain | Unknown ** | 22.5 | Lamberti et al. [108] | |
| Pygmy grasshoppers | β-Actin | Unknown ** | 41.7 | Lamberti et al. [108] | |
| Pygmy grasshoppers | Troponin T | Unknown ** | 46.7 | Lamberti et al. [108] | |
| Pygmy grasshoppers | Lysozyme | Unknown ** | 80.0 | Lamberti et al. [108] | |
| Pygmy grasshoppers | Tropomyosin | Mec e 7 ** | 38 | Leung et al. [135] | |
| Locusta migratoria | Hexamerins | Unknown ** | 78 | Pharima et al. [136] | |
| Locusta migratoria | Arginine kinase | Unknown ** | ≈40 | Egonyu et al. [112] | |
| Locusta migratoria | Hemocyanin | Unknown ** | ≈75 | Egonyu et al. [112] | |
| Locusta migratoria | Cuticle proteins | Unknown ** | 12–17 | Lamberti et al. [108] | |
| Locusta migratoria | Myosin Light Chain | Unknown ** | 23 | Lamberti et al. [108] | |
| Tenebrio molitor | Tropomyosin | Ten m 7 ** | 35 | Barre et al. [78] | |
| Tenebrio molitor | α-Amylases | Unknown ** | ≈50 | Barre et al. [78] | |
| Tenebrio molitor | Arginine kinase | Unknown ** | ≈40 | Barre et al. [78] | |
| Tenebrio molitor | Hexamerins | Unknown ** | 70 | Barre et al. [78] | |
| Tenebrio molitor | α-tubulin | Unknown ** | Unknown | Ribeiro et al. [91] | |
| Tenebrio molitor | β-tubulin | Unknown ** | Unknown | Ribeiro et al. [91] | |
| Tenebrio molitor | Actin | Unknown ** | 41.9 | van Broekhoven et al. [137] | |
| Tenebrio molitor | Fructose biphosphate aldolase | Unknown ** | Unknown | Ribeiro et al. [91] | |
| Tenebrio molitor | Myosin Light Chain | Unknown ** | 21.7 | Lamberti et al. [108] | |
| Tenebrio molitor | Troponin T | Unknown ** | 45.8 | Lamberti et al. [108] | |
| Hermetia illucens | Hemocyanin | Unknown ** | ≈75 | Karnaneedi et al. [109] | |
| Hermetia illucens | Tropomyosin | Unknown ** | ≈33 | Karnaneedi et al. [109] | |
| Hermetia illucens | Cuticle proteins | Unknown ** | ≈24 | Karnaneedi et al. [109] | |
| Hermetia illucens | Odorant-binding proteins | Unknown ** | ≈14 | Karnaneedi et al. [109] | |
| Hermetia illucens | Arginine kinase | Unknown ** | Unknown | Broekman et al. [138] | |
| Hermetia illucens | Troponin | Unknown ** | Unknown | Broekman et al. [138] | |
| Hermetia illucens | Triosephosphate Isomerase | Unknown ** | Unknown | Broekman et al. [138] | |
| Blattella germanica | Bd 90 K | Bla g 1 * | 45.8 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Blattella germanica | Inactive Aspartic Proteases | Bla g 2 * | 38.5 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Blattella germanica | Hemocyanin | Bla g 3 * | 78.7 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Blattella germanica | Calycin, lipocalin | Bla g 4 * | 20.0 | Chuang et al. [139] | |
| Blattella germanica | Glutathione S-transferase | Bla g 5 * | 22.9 | Chuang et al. [139] | |
| Blattella germanica | Troponin | Bla g 6 * | 17.2 | Hindley et al. [140] | |
| Blattella germanica | Tropomyosin | Bla g 7 * | 32.8 | Chuang et al. [139] | |
| Blattella germanica | Myosins | Bla g 8 * | 22. | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Blattella germanica | Arginine Kinase | Bla g 9 * | 39.7 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Blattella germanica | Serine protease | Bla g 10 * | 26.3 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Blattella germanica | α-Amylase | Bla g 11 * | 54.0 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Blattella germ anica | Chitinase | Bla g 12 * | 58.0 | Pomés et al. [141] | |
| Blattella germanica | Enolase | Bla g Enolase ** | 47.1 | Chuang et al. [139] | |
| Blattella germanica | Vitellogenin | Bla g Vitellogenin ** | 213.5 | Chuang et al. [139] | |
| Blattella germanica | Triosephosphate Isomerase | Bla g TPI ** | 26.8 | Chuang et al. [139] | |
| Blattella germanica | Receptors for Activated Protein Kinase, RACK1 | Bla g RACK1 ** | 35.7 | Chuang et al. [139] | |
| Periplaneta americana | Cr-PII | Per a 1 | 44.5 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Periplaneta americana | Aspartic Protease | Per a 2 * | 36.0 | Gustchina et al. [142] | |
| Periplaneta americana | Hemocyanin | Per a 3 * | 78.6 | Wu et al. [143] | |
| Periplaneta americana | Lipocalin | Per a 4 * | 17.0 | Wangorsch et al. [144] | |
| Periplaneta americana | Glutathione-S-transferases | Per a 5 * | 23.0 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Periplaneta americana | Troponin | Per a 6 * | 17.1 | Hindley et al. [140] | |
| Periplaneta americana | Tropomyosin | Per a 7 * | 33.0 | Asturias et al. [145] | |
| Periplaneta americana | Myosin | Per a 8 * | 28. | Wangorsch et al. [144] | |
| Periplaneta americana | Arginine Kinase | Per a 9 * | 43.0 | Sookrung et al. [95] | |
| Periplaneta americana | Serine protease | Per a 10 * | 28.0 | Sudha et al. [146] | |
| Periplaneta americana | alpha-Amylase | Per a 11 * | 55. | Fang et al. [147] | |
| Periplaneta americana | Chitinase | Per a 12 * | 45.0 | Fang et al. [147] | |
| Periplaneta americana | GAPDH | Per a 13 * | 36.0 | Xu et al. [148] | |
| Periplaneta americana | Glyceraldehyde-3-phosphate dehydrogenase | Per a 13 * | 36.0 | Xu et al. [148] | |
| Periplaneta americana | Enolase | Per a 14 * | 50.0 | Wang et al. [149] | |
| Periplaneta americana | Cytochrome C | Per a 15 * | 15.0 | Wang et al. [149] | |
| Periplaneta americana | Cofilin | Per a 16 * | 20.0 | Wang et al. [149] | |
| Periplaneta americana | Alpha-tubulin | Per a 17 * | 53.0 | Wang et al. [149] | |
| Periplaneta americana | Peptidyl-prolyl-cis-trans isomerase; Cyclophilin | Per a 18 * | 24.0 | Wang et al. [149] | |
| Periplaneta americana | Porin 3 | Per a 19 * | 7.4 | Wang et al. [149] | |
| Periplaneta americana | Peroxiredoxin-6 (Prx6) | Per a 20 * | 24.0 | Wang et al. [149] | |
| Periplaneta americana | Fructose bisphosphate aldolase | Per a 21 * | 39 kDa, 160 kDa | www.allergen.org | |
| Periplaneta americana | Pyruvate kinase | Per a 22 * | 58 kDa, ~220 kDa | www.allergen.org | |
| Mic roalgae | Spirulina | Superoxide dismutase (C3V3P3) | Unknown ** | Unknown | Gromek et al. [34] |
| Spirulina | GAPDH | Unknown ** | Unknown | Gromek et al. [34] | |
| Spirulina | Triosephosphate Isomerase (D5A635) | Unknown ** | Unknown | Gromek et al. [34] | |
| Spirulina | Thioredoxin reductase (D4ZSU6) | Unknown ** | Unknown | Bianco et al. [120] | |
| Spirulina | Thioredoxin reductase (K1VP15) | Unknown ** | Unknown | Bianco et al. [120] | |
| Spirulina | C-Phycocyanin-β-Subunit | Art pl beta_Phycocyanin ** | 18.0 | Bianco et al. [120] | |
| Chlorella | Calmodulin proteins | Unknown ** | Unknown | Bianco et al. [120] | |
| Chlorella | Troponin C | Unknown ** | Unknown | Bianco et al. [120] | |
| Chlorella | Triosephosphate Isomerase | Unknown ** | Unknown | Hamzelou et al. [150] | |
| Chlorella | Heat Shock Proteins | Unknown ** | Unknown | Hamzelou et al. [150] | |
| Chlorella | Cyclic protein | Unknown ** | Unknown | Hamzelou et al. [150] | |
| Chlorella | Fructose biphosphate aldolase | Unknown ** | Unknown | Mariachiara et al. [120] | |
| Bacteria bacillota | Nattokinase (subtilisin-like serine protease) | Bac s 1 * | 30.0 | Suzuki et al. [151] | |
| Bacte ria and fungi | Saccharomyces cerevisiae | Transaldolase | Sac c 14 ** | 37.0 | Chou et al. [152] |
| Saccharomyces cerevisiae | Carboxypeptidase | Sac c Carboxypeptidase Y ** | 59.8 | WHO/IUIS (https://www.allergen.org) | |
| Saccharomyces cerevisiae | Cyclophilin | Sac c CyP ** | ≈18 | Flückiger et al. [153] | |
| Saccharomyces cerevisiae | Enolase | Sac c Enolase ** | 46.8 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Saccharomyces cerevisiae | α-Glucosidase | Sac c Glucosidase ** | 68.1 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Saccharomyces cerevisiae | β-fructofuranosidase | Sac c Invertase ** | 60.0 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Saccharomyces cerevisiae | Manganese SO dismutase | Sac c MnSOD ** | 25.7 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Saccharomyces cerevisiae | Profiling | Sac c Profilin ** | 13.6 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Saccharomyces cerevisiae | Ribosomal Proteins | Sac c P2 ** | 10.7 | WHO/IUIS (https://www.allergen.org) Allergome (https://www.allergome.org/index.php, accessed on 15 November 2025) Uniport (https://www.uniprot.org/) | |
| Fusarium spp. | Ribosomal Proteins P2 | Fus c 1 * | 11.0 | Weber et al. [154] | |
| Fusarium spp. | Thioredoxin reductase | Fus c 2 * | 13.0. | Weber et al. [154] | |
| Fusarium spp. | Transaldolase | Fus p 4 * | 37.5 | Weber et al. [154] | |
| Fusarium spp. | Vacuolar serine protease | Fus p 9 * | 36.5 | Yeh et al. [155] | |
| Alternaria alternata | Unknown | Alt a 1 * | 16.4, 15.3 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Heat shock protein 70 | Alt a 3 * | 85.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Disulfide isomerase | Alt a 4 * | 57.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Ribosomal protein P2 | Alt a 5 * | 11.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Enolase | Alt a 6 * | 45.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Flavodoxin, YCP4 protein | Alt a 7 * | 22.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Mannitol dehydrogenase | Alt a 8 * | 29.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Aldehyde dehydrogenase | Alt a 10 * | 53.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Aid ribosomal protein P1 | Alt a 12 * | 11.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Glutathione-transferase | Alt a 13 * | 26.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Manganese SO dismutase | Alt a 14 * | 24.0 | Abel-Fernández et al. [126] | |
| Alternaria alternata | Vacuolar serine protease | Alt a 15 * | 58.0 | Abel-Fernández et al. [126] |
3. Influence of Food Processing Techniques on Protein Sensitization
Food allergies typically arise from abnormal immune system responses to specific proteins, triggering a spectrum of clinical symptoms ranging from mild discomfort to severe anaphylactic reactions that can be life-threatening. The mechanisms underlying protein allergenicity are complex and involve antigen recognition, immune activation, and the subsequent inflammatory responses mediated by these processes. Recent studies have demonstrated that appropriate food processing techniques-including thermal treatment, enzymatic modification, fermentation, high-pressure processing, and plant polyphenol conjugation-can significantly reduce the cross-reactivity and allergenic potential of allergenic proteins (Figure 4) [131,137,138,156,157,158]. These processing methods can alter the structural conformation of allergenic proteins, thereby reducing the availability of IgE-binding epitopes. Since the allergenicity of a protein is largely determined by its capacity to bind IgE antibodies, diminishing the number of accessible IgE-binding sites through structural modification can effectively decrease the protein’s immunoglobulin E affinity, ultimately mitigating its allergenic potential.
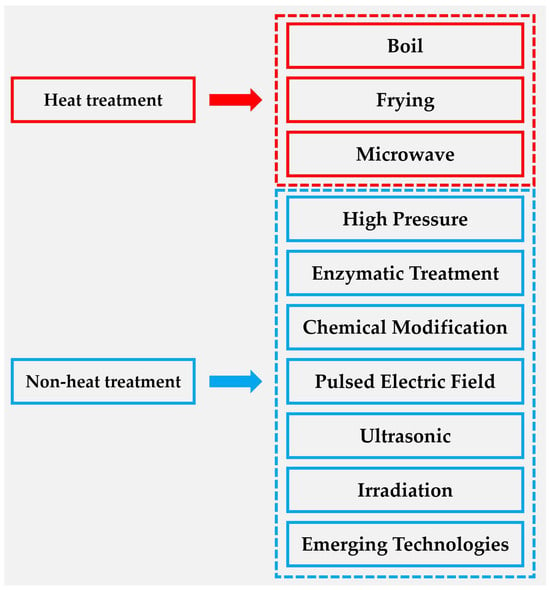
Figure 4.
Food processing methods can be classified according to the heat effect: heat treatment methods include boiling, frying and microwave; non-heat treatment methods include high pressure, enzyme treatment, chemical modification, pulsed electric field, ultrasound, irradiation and emerging methods.
3.1. Heat Treatment
3.1.1. Boiling and Frying
Thermal processing represents a commonly employed method for reducing the allergenicity of allergenic proteins, as high-temperature treatments induce conformational changes in protein spatial structures that may either mask or destroy IgE-binding epitopes (thereby reducing allergenicity) or conversely expose new epitopes (thereby increasing allergenicity) (Table 3) [159,160,161]. He et al. investigated the effects of heating on the allergenicity of silkworm pupa protein extracts [162], demonstrating that protein bands corresponding to allergens in the 43–90 kDa range disappeared after heating above 80 °C, while bands at 25–33 kDa remained clearly visible, indicating that heating reduces but does not completely eliminate the allergenicity of silkworm pupa protein extracts. This phenomenon was further corroborated by Lamberti et al. [108]. He et al. also observed that heating induced alterations in the higher-order structures of silkworm pupa protein extracts, such as reduced α-helix and β-sheet contents compared to non-heated extracts, which exposed hydrophobic regions that could either mask or expose epitopes, thereby affecting IgE-binding capacity. Moreover, protein degradation and aggregation occurring at heating temperatures above 80 °C further diminished allergenicity. Broekman et al. examined the effects of boiling (100 °C for 1 or 10 min), electric stove roasting (1000 W for 3.5 min), and frying (180 °C for 30 s) on mealworm allergenicity [138], finding that none of these treatments significantly altered IgE-binding or cross-linking capacities, but merely changed the solubility of mealworm proteins without affecting allergenicity. This solubility change likely resulted from heat-induced structural modifications; tropomyosin (the primary mealworm allergen), as a muscle protein composed of highly α-helical monomers assembled into rod-shaped coiled-coil dimers, possesses a complex structure conferring strong thermal stability that resists heat-induced structural disruption and consequent changes in allergenicity, explaining why thermal processing failed to significantly affect mealworm allergen allergenicity [163]. Subsequently, Lamberti et al. treated mealworms with boiling (100 °C for 5 min) and frying (180 °C for 3 min), comparing their allergenicity to untreated controls [108], and found that high-temperature frying partially reduced cross-reactivity of mealworm protein extracts without completely eliminating allergenicity. Compared to Broekman et al.’s shorter frying time (180 °C for 30 s), Lamberti et al.’s longer frying duration (180 °C for 3 min) appeared more effective at reducing mealworm allergen allergenicity, potentially explaining why frying is more commonly applied to mealworm processing. Fried locusts represent another common insect food product; Phiriyangkul et al. found that frying (180 °C for 3 min) significantly reduced allergenicity of arginine kinase and enolase in locusts while paradoxically increasing that of glyceraldehyde-3-phosphate dehydrogenase [136]. Interestingly, different thermal processing methods applied to the same allergenic protein may yield diametrically opposed results. Lamberti et al. treated locusts with both boiling (100 °C for 5 min) and frying (180 °C for 3 min) [108], finding that fried locust proteins exhibited cross-reactivity reduced to 29% of the original level, whereas boiled locust proteins not only failed to reduce but actually increased cross-reactivity. This discrepancy likely arises because frying induces irreversible denaturation of locust tropomyosin, eliminating IgE-binding activity, while boiling causes partial denaturation that exposes previously hidden epitopes, enhancing IgE-binding activity. Zhang et al. subjected peanut allergen Ara h 2 to both boiling and roasting treatments, observing that boiling reduced allergenicity while roasting enhanced allergic reactions [164]. Similarly, Li et al. found that 1 h heating of β-conglycinin (Gly m 5) increased its allergenicity [165]. In summary, while heating generally reduces allergenicity of allergenic proteins, its effectiveness is significantly influenced by protein structure, with instances where allergenicity remains unchanged or even increases post-heating. For edible insects, heating or boiling may be more suitable for processing less stable allergens, whereas more extreme treatments like high-temperature frying effectively disrupt higher-order protein structures, proving particularly effective against thermostable allergens such as myosin and troponin.

Table 3.
Summary of the Effects of Heat Treatment on the Allergenicity of Selected Proteins.
Table 3.
Summary of the Effects of Heat Treatment on the Allergenicity of Selected Proteins.
| Allergen Source | Heat Treatment | Effect on Allergenicity | Key Determining Factor |
|---|---|---|---|
| Silkworm pupa | Heating > 80 °C | Decreased | Protein degradation/aggregation; structural unfolding leading to epitope masking. |
| Silkworm pupa | Heating > 80 °C | Unchanged/Partial decrease | Thermostability of specific protein components. |
| Tenebr io molitor | Boiling (100 °C, 1–10 min), Frying (180 °C, 30 s) | Largely unchanged | High intrinsic thermal stability of coiled-coil structure. |
| Tenebrio molitor | Frying (180 °C, 3 min) | Decreased (Partial) | Longer duration/intensity of heating leading to irreversible denaturation. |
| Locust | Frying (180 °C, 3 min) | Decreased | Heat-labile structure; denaturation destroys epitopes. |
| Locust | Frying (180 °C, 3 min) | Increased | Exposure of previously hidden (cryptic) epitopes due to unfolding. |
| Locust | Frying (180 °C, 3 min) | Decreased | Irreversible denaturation eliminating IgE-binding |
| Locust | Boiling (100 °C, 5 min) | Increased | Partial denaturation exposing hidden epitopes. |
| Peanut | Boiling | Decreased | Leaching of allergens into water; structural changes. |
| Peanut | Roasting | Increased | Maillard reaction creating new or stabilizing existing epitopes. |
| Soybean | Heating (1 h) | Increased | Aggregation or stabilization of conformational epitopes. |
3.1.2. Microwave
Microwave treatment may significantly affect the activity and structural properties of proteins and peptides, including unfolding of tertiary structures and alterations in secondary structures [166,167].Microwave is a commonly employed food processing method, as proteins and peptides possess higher dielectric constants, making them particularly susceptible to structural and functional alterations induced by microwaves [167]. Dong et al. investigated the effects of microwave treatment on the allergenicity of shrimp allergenic proteins [168], finding that the lowest intensity of tropomyosin bands was observed after treatment at 125 °C for 10 min.
3.2. Non-Heat Treatment
3.2.1. High Pressure
High-pressure processing is widely applied in food manufacturing. This treatment may alter the spatial conformation of allergenic proteins, disrupting the integrity of their higher-order structures. This disruption can lead to the complete exposure or masking of antibody-binding epitopes, thereby affecting the allergenicity of these proteins [169,170]. Studies have demonstrated that high-pressure treatment can effectively reduce the allergenicity of edible insect allergenic proteins. Li et al. found that 200 MPa pressure caused deformation of myosin heavy chain while 500 MPa pressure led to significant degradation of troponin [171]. Chen et al. investigated the effects of high-temperature-high-pressure treatment on the allergenicity of tropomyosin in ready-to-eat clam meat [172], showing that extended processing time improved both flavor and texture of the clam meat while significantly reducing the allergenicity of clam tropomyosin. Martínez-Maldonado et al. observed that myosin heavy chain underwent unfolding and aggregation under 600 MPa pressure, which likely resulted in epitope destruction and subsequent changes in allergenicity.
3.2.2. Enzymatic Treatment
Enzymatic treatment represents another common approach for reducing allergen immunogenicity. Enzyme treatment to reduce the allergenicity of allergenic proteins generally involves two processes. The first process is enzymatic hydrolysis, wherein proteases hydrolyze proteins and disrupt their spatial structure and linear epitopes, thereby achieving a reduction in allergenicity [173]. Another method is enzyme cross-linking, which involves intramolecular or intermolecular cross-linking reactions induced by enzymes that alter protein molecular weight. This induces protein aggregation and disrupts secondary structures and IgG/IgE binding epitopes [174]. He et al. investigated the effects of pepsin on the allergenicity of silkworm pupa proteins [162], demonstrating that pepsin treatment reduced the allergenicity of silkworm pupa proteins to a certain extent, although 25–33 kDa allergenic proteins still exhibited resistance to pepsin digestion, indicating that these allergens in silkworm pupa protein extracts possess both thermal stability and partial resistance to enzymatic degradation. Notably, low-molecular-weight fragments generated from silkworm proteolysis may retain allergenic potential, particularly proteins in the 25–33 kDa range. Enzymatic hydrolysis also shows some efficacy in attenuating allergenicity of mealworm and cricket allergens. Hall et al. examined the impact of alkaline protease treatment on cricket allergenicity [175], finding that enzymatic hydrolysis significantly affected the allergenicity of cricket proteins, especially tropomyosin. Untreated cricket proteins and protein hydrolysates (CPH) with 15–50% degree of hydrolysis exhibited strong reactivity with anti-tropomyosin antibodies in immunoblotting, whereas CPH with 60–85% hydrolysis showed negligible reactivity, suggesting that higher degrees of hydrolysis significantly reduce protein allergenicity, likely through conformational changes in tropomyosin that diminish or eliminate its antigenicity. Shen et al. conducted similar studies [176], showing that α-chymotrypsin treatment markedly reduced tropomyosin allergenicity, with nearly complete loss of immunoreactivity after 240 min of continuous treatment. Although enzymatic hydrolysis is highly effective in reducing allergenicity, its specificity prevents targeted modification of IgE-binding epitopes, and enzymatic action may even expose additional epitopes [177,178], necessitating the combination of enzymatic hydrolysis with other processing methods. Hall and Liceaga demonstrated that microwave-assisted enzymatic hydrolysis significantly reduced IgE-binding capacity of cricket allergens [179]. Boukil et al. employed high hydrostatic pressure-assisted enzymatic hydrolysis for mealworm powder processing [180], finding that high hydrostatic pressure disrupted protein tertiary structures, facilitated enzyme access to proteins, and enabled more efficient hydrolysis into peptides, thereby reducing allergenicity. However, Clemente et al. observed increased antigenicity in chickpea proteins after flavor protease hydrolysis [181], and Cabanillas et al. noted increased IgE reactivity detected by ELISA at 30 min when using pepsin alone to treat roasted peanut proteins [182]. In another study, Sung et al. found that while enzymatic hydrolysis with bromelain slightly reduced buckwheat protein allergenicity, subsequent treatment with pepsin and GC106 actually increased allergenicity [183]. Moreover, as insect proteins inherently possess earthy flavor profiles, Grossmann et al. examined flavor changes in mealworm proteins before and after enzymatic hydrolysis [184], revealing that enzymatic hydrolysis significantly enhanced both bitterness and umami flavors [161]. Therefore, when applying enzymatic hydrolysis to allergenic proteins, comprehensive consideration of factors including allergenicity and sensory evaluation is essential.
3.2.3. Chemical Modification
Glycosylation of proteins in food refers to the non-enzymatic chemical reaction between amino compounds in proteins (primarily lysine with ε-amino groups) and carbonyl compounds (mainly reducing sugars) [174]. Protein glycosylation, which involves the reaction of proteins with carbohydrate compounds to attach sugar chains to amino acid residues, can lead to the destruction or masking of allergenic epitopes. Zhang et al. investigated the glycosylation reactions of tropomyosin with carbohydrates of varying molecular weights [185], finding that glycosylation with glucose, maltotriose, maltopentaose, and maltulose significantly disrupted allergenic epitopes and reduced allergenicity, whereas glycosylation with maltose showed no significant effect on reducing tropomyosin allergenicity. Han et al. examined the impact of the Maillard reaction on the allergenicity of tropomyosin and arginine kinase [186], demonstrating that the Maillard reaction significantly reduced the allergenicity of both TM and AK, particularly when arabinose was used as the reducing sugar. The Maillard reaction caused a reduction in α-helices and an increase in β-turns and random coils in the structures of tropomyosin and arginine kinase, leading to the masking or destruction of epitopes and consequent reductions in allergenicity.
3.2.4. Pulsed Electric Field
Pulsed electric field (PEF) represents a novel non-thermal food sterilization technology that processes liquid and semi-solid foods using high electric field strengths (10–50 kV/cm), short pulse widths (0–100 μs), and high pulse frequencies (0–2000 Hz). Researchers have begun investigating the effects of PEF technology on the allergenicity of algal-derived nutrients and bioactive substances. Polikovsky et al. compared proteins extracted from Ulva treated with PEF versus those processed using thermochemical methods or without PEF treatment [187]. The study found that PEF selectively prevented the release of calmodulin, a major allergenic protein in algae, whereas non-PEF-treated samples contained identifiable Superoxide dismutase (SOD), and thermochemically treated samples revealed four potential allergens including SOD, Troponin C, Aldolase A, and Thioredoxin reductase. These results suggest that PEF can reduce the release of certain known allergens during extraction, potentially offering a safer method for extracting edible algal proteins.
3.2.5. Ultrasonic
High-intensity ultrasound, an efficient food processing and preservation technique, has been successfully applied in homogenizing emulsions, enzyme inactivation, assisted extraction processes, accelerating dehydration, and expediting aging and maturation procedures [188,189]. Ultrasonic treatment generates intense shear forces and temperature fluctuations through acoustic vibration and cavitation, thereby altering the spatial structure of allergenic proteins. This process disrupts or conceals the antibody-binding epitopes of allergenic proteins, consequently reducing their allergenicity [174]. Li et al. utilized high-intensity ultrasound to treat shrimp allergenic proteins [190], demonstrating that high-intensity ultrasonic treatment significantly reduced the allergenicity of shrimp tropomyosin, with the treated tropomyosin exhibiting markedly diminished IgE-binding capacity compared to untreated controls. Ultrasound treatment induced structural changes in the proteins, including the unfolding of secondary structures that may lead to either exposure or obliteration of allergenic epitopes, thereby influencing immune system recognition and response to these proteins. Furthermore, the study revealed that combining heat treatment with ultrasound produced enhanced effects, potentially attributable to the thermal effects initiated by ultrasonic waves. Under high-temperature conditions, the reversible unfolding of proteins and rearrangement of disulfide bonds may alter or partially inactivate allergenic epitopes, consequently reducing allergenicity.
3.2.6. Irradiation
Irradiation technology is not only an environmentally friendly, low-carbon, and highly efficient method for eliminating allergenicity, but also effectively preserves the flavor and quality of food [191]. Irradiation generates free radicals through radiolysis, which can disrupt the spatial conformation and epitope structure of allergenic proteins, leading to their denaturation, aggregation, or fragmentation [192]. This conformational change may mask or disrupt antigenic epitopes in allergenic proteins that are recognized by the immune system, thereby reducing their allergenicity. Ji-Hyun Seo et al. irradiated Ovalbumin with gamma rays at doses of 50 kGy and 100 kGy, respectively. They found that compared to groups attacking non-irradiated ovalbumin, mice treated with irradiated ovalbumin exhibited significantly reduced total IgE levels in serum [192]. This indicates irradiation effectively diminishes ovalbumin’s sensitizing capacity. Meng et al. measured antibody binding capacity and histamine release capacity after gamma irradiation of α-lactalbumin [193]. The study showed that after 10 kGy irradiation, the antibody binding capacity of α-lactalbumin decreased by approximately 95%, histamine release capacity decreased by approximately 26%, and sensitization potential was significantly reduced. Zhu et al. measured antibody binding capacity and immunoblotting signals in porcine serum albumin after gamma irradiation at 1 kGy and 8 kGy, respectively [194]. Results indicated that antibody binding capacity of irradiated pig serum albumin decreased by over 90% compared to the untreated group, while Western blot analysis showed progressively lighter signal band coloration with increasing irradiation intensity. This demonstrates a significant reduction in protein allergenicity. Additionally, Liu et al. observed that high-dose electron beam irradiation (10 kGy) of frozen shrimp allergenic proteins significantly reduced protein allergenicity [195]. However, allergenicity increased after low-dose irradiation, likely due to structural changes that increased antibody-binding epitopes. Low-dose irradiation may enhance allergenicity, while higher doses effectively reduce allergens but may adversely affect food flavor and quality. Therefore, controlling irradiation dose is a critical step in using irradiation for desensitization processes [174].
3.2.7. Emerging Technologies
Beyond traditional processing methods such as boiling, frying, and high-pressure treatment, numerous emerging technologies have been developed to reduce the allergenicity of allergenic proteins. Cold atmospheric pressure plasma (CAPP) technology represents a non-thermal method for food preservation [196]. CAPP can reduce allergenicity by disrupting the structural conformation of allergenic proteins, particularly through the reduction of IgE-binding epitopes. Research has demonstrated CAPP’s effectiveness in attenuating allergenicity; for instance, direct dielectric barrier discharge plasma treatment reduced the allergenicity of shrimp tropomyosin by 60% [160,197]. Furthermore, studies have shown that algal allergens exhibit significant resistance to conventional heat treatment and enzymatic hydrolysis methods for reducing allergenicity [198], suggesting that CAPP may hold particular promise for mitigating algal allergenicity. However, research on the application of CAPP specifically targeting protein allergenicity remains limited and requires further in-depth investigation. Beyond its potential for allergen reduction, CAPP has also been utilized to produce high-quality insect powders [199]. If this technology were to be more comprehensively studied, it is conceivable that it could simultaneously enhance the quality of insect powders while reducing the allergenicity of insect allergenic proteins, which would have significant positive implications for sustainable development and food safety.
Li et al. developed a magnetic nanocomposite with photochemical synergistic capabilities to reduce the allergenicity of phospholipase A2 (PLA2) [200]. The photochemical dynamic interactions induced structural alterations in PLA2, specifically transforming its α-helical conformation into β-sheet structures, thereby diminishing its IgE-binding capacity and mitigating the risk of allergic reactions. Furthermore, owing to the magnetic properties of the nanocomposite, the treated PLA2 could be effectively adsorbed and separated using an external magnetic field, ensuring the safety of the processed product.
The contents of Table 4 are summarized from the preceding sections.

Table 4.
Advantages and limitations of processing technology.
3.3. Challenges and Future Directions of Processing Technology Applications
Current research predominantly focuses on how processing methods influence the allergenicity of edible insect allergens, whereas studies investigating the impact of processing on the allergenicity of algal and microbial (bacterial and fungal) allergens remain limited. Yeast proteins are generally considered hypoallergenic [201]; however, there have been documented cases of yeast proteins inducing allergic reactions. Regarding S. cerevisiae, thermal processing appears to be a potentially effective method for reducing allergenicity. A clinical case report described a patient who experienced severe allergic reactions to S. cerevisiae extracts yet tolerated S. cerevisiae present in baked goods such as bread [202]. This phenomenon was hypothesized to result from thermal denaturation of allergenic proteins during baking, which reduced the allergenic potential. Additionally, research on processing modifications of algal allergenic proteins remains limited, primarily due to the pivotal role of phycocyanin as the principal allergen in algae; processing methods that compromise the structural integrity and functional properties of phycocyanin would significantly diminish the nutritional value of algal proteins. Therefore, against the backdrop of security challenges in sustainable protein resource development, systematically evaluating the impact of processing on sensitization to algal and microbial allergens represents a critical scientific issue that demands urgent priority.
To address these challenges, future research should focus on several key areas. First, comprehensive identification and characterization of allergens in microalgae and microorganisms should be conducted using proteomics and bioinformatics technologies. Standardized IgE panels should be established using serum samples from sensitized individuals to lay the foundation for allergy risk assessment. Building on this, for algal allergens, a comprehensive investigation of the structure-function relationships of key sensitizing components like phycocyanin is needed. The focus should be on exploring non-thermal processing technologies (such as cold plasma or pulsed electric fields) or targeted enzymatic hydrolysis techniques to achieve precise destruction of IgE-binding epitopes while preserving nutritional value. For microbial protein sources like yeast, strategies may include: developing hypoallergenic strains through genetic engineering or transgenic techniques, or employing targeted protein extraction during processing to selectively degrade allergenic proteins and reduce sensitization risks. Finally, any effective desensitization processing technology must undergo validation through clinical or animal trials. Therefore, individuals with a history of allergies to algae or fungi should continue to avoid consuming related products as a precautionary measure to safeguard health until more comprehensive safety solutions are established.
Food processing technologies exhibit diverse potential in modulating the allergenicity of allergenic proteins, yet their efficacy is highly contingent upon the intrinsic properties of the allergens (such as structural stability and epitope distribution) and the optimization of processing parameters. Thermal processing, while capable of disrupting certain IgE-binding epitopes by altering protein conformation, demonstrates limited effectiveness against thermally stable allergens (e.g., tropomyosin) and may even enhance allergenicity through epitope exposure under certain conditions. Enzymatic hydrolysis requires precise control of hydrolysis degree or integration with complementary treatments (such as high pressure or microwave) to achieve effective allergen degradation; however, the inherent specificity of enzymes and the potential risk of epitope exposure necessitate careful evaluation. Physical methods including high pressure, microwave, and ultrasound disrupt protein tertiary structures to reduce allergenicity, while emerging technologies such as pulsed electric fields and cold atmospheric plasma offer novel approaches by selectively inhibiting allergen release through non-thermal mechanisms, thereby providing innovative pathways for developing hypoallergenic foods. Notably, different processing methods may exert opposite effects on the same allergen (e.g., the contrasting impacts of boiling versus frying on grasshopper allergenicity), underscoring the necessity of tailoring processing strategies based on allergen-specific characteristics. Future research should prioritize elucidating the structure-processing response relationships of allergens, developing integrated multi-technology solutions, and extending these strategies to emerging protein sources such as algae and microorganisms, while simultaneously balancing the comprehensive impacts on nutritional quality, sensory attributes, and safety. Such advancements will provide critical scientific support for the development of sustainable, low-allergenic protein resources.
4. Conclusions and Prospects
As global population growth and resource pressures intensify, the development of sustainable alternative protein sources has become a key strategy to address protein supply issues. This paper systematically reviews the nutritional value, potential allergens and their sensitising mechanisms of alternative proteins such as edible insects, microalgae and microorganisms (fungi, bacteria, yeasts) and explores the role of different processing technologies in reducing the risk of allergens.
Edible insects, microalgae, and microorganism proteins, with their high nutritional density and low environmental dependence, are gradually emerging as a crucial supplement to traditional animal proteins. Edible insects such as T. molitor and B. mori contain protein levels of 50–70%, along with abundant essential amino acids and minerals; microalgae like A. platensis produce phycocyanin with both nutritional and functional properties; and microorganisms including S. cerevisiae and F. venenatum manufacture proteins with high bioavailability through efficient fermentation. This diversity of protein sources provides the food industry with abundant options, yet their widespread application remains restricted by allergenic risks. Allergenic proteins commonly present in edible insects, microalgae, and microorganism proteins include Tropomyosin and Arginine Kinase in insects, phycocyanin in microalgae, and Ribosomal Protein P2 in fungi. These allergens can not only trigger local or systemic allergic reactions (such as asthma and anaphylactic shock) but also exhibit significant cross-reactivity with traditional allergens like crustaceans and mites. For example, the Tropomyosin in H. illucens shares 75–80% sequence homology with shrimp allergens, posing high risks for seafood-allergic individuals.
The application of processing technologies such as thermal treatment, enzymatic hydrolysis, high-pressure processing, and glycosylation can partially reduce the IgE-binding capacity of food allergens. Various processing techniques demonstrate significant potential in reducing the allergenicity of microbial and edible insect proteins. For example, high-temperature deep-frying at 180 °C significantly reduces the allergenicity of T. molitor and L. migratoria allergens by destroying the advanced protein structure, and enzymatic hydrolysis (such as treatment with alkaline protease) can degrade the epitopes of Tropo-myosin in A. domesticus, while non-thermal technologies (such as cold atmospheric plasma) show potential by selectively inhibiting the release of allergens. However, the effectiveness of these methods is highly dependent on the inherent properties of specific allergens. The key challenge in heat treatment lies in the thermal stability of proteins such as actin, which may reduce the effectiveness of heat processing. In certain cases, it can even expose hidden epitopes, thereby enhancing allergenicity. Additionally, various desensitization processing techniques may be accompanied by nutrient loss or deterioration of sensory characteristics. For instance, processes like enzymatic hydrolysis may generate bitter peptides or compromise sensory properties.
In summary, future research should prioritize expanding allergen protein databases by comprehensively identifying sensitizing proteins through proteomics and bioinformatics tools. Secondly, novel desensitization techniques or optimized composite treatment methods should be developed to achieve efficient desensitization while preserving the nutritional and sensory qualities of food. Additionally, targeted processing techniques for allergen reduction should be developed, aiming to minimize the impact on sensory characteristics or nutritional value while reducing the allergenicity of allergenic proteins.
Author Contributions
F.X.: Conceptualization, Investigation, Visualization, Writing—Original Draft; Y.Z.: Investigation, Visualization, Writing—Original Draft; Z.H.: Investigation, Visualization, Writing—Original Draft; X.Z. (Xiaoyue Zhang): Investigation, Writing—Original Draft; B.C.: Writing—Review and Editing; X.Z. (Xuchun Zhu): Writing—Review and Editing; H.L.: Supervision, Project administration, Writing—Review and Editing, Funding acquisition. F.X. and Y.Z. are the co-first authors of this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Natural Science Foundation of China (32472269), Guizhou Provincial Science and Technology Department (KXJZ [2024]021) and Beijing High-level Talent Project (19008024075).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author Fei Xu was employed by the company Heilongjiang Feihe Dairy Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alexandros, N.; Bruinsma, J.; Bodeker, G.; Schmidhuber, J.; Broca, S.; Shetty, P.; Ottaviani, M.G. World Agriculture Towards 2030/2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Dolganyuk, V.; Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Kashirskikh, E.; Prosekov, A.; Michaud, P.; Babich, O. Food proteins: Potential resources. Sustainability 2023, 15, 5863. [Google Scholar] [CrossRef]
- Volden, J. ‘A wing in the throat’: Negotiating edibility in everyday insect consumption. Geoforum 2024, 157, 104132. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, S.; Kuang, H.; Tang, C.; Song, J. Edible insects as ingredients in food products: Nutrition, functional properties, allergenicity of insect proteins, and processing modifications. Crit. Rev. Food Sci. Nutr. 2024, 64, 10361–10383. [Google Scholar] [CrossRef]
- Meng, T.; Zhu, X.; He, S.; Liu, X.; Mitra, P.; Liu, H. Advances in extraction, structural design, functional activity and application of yeast protein peptides. Food Biosci. 2024, 62, 105026. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Z.; Zhu, X.; Chen, B.; Zhou, L.; Liu, X.; Liu, H. Yeast proteins: Proteomics, extraction, modification, functional characterization, and structure: A Review. J. Agric. Food Chem. 2024, 72, 18774–18793. [Google Scholar] [CrossRef]
- Patel, B.Y.; Volcheck, G.W. Food allergy: Common causes, diagnosis, and treatment. Mayo Clin. Proc. 2015, 90, 1411–1419. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307.e5. [Google Scholar] [CrossRef]
- Zhou, F.; He, S.; Sun, H.; Wang, Y.; Zhang, Y. Advances in epitope mapping technologies for food protein allergens: A review. Trends Food Sci. Technol. 2021, 107, 226–239. [Google Scholar] [CrossRef]
- van Wijk, F.; Knippels, L. Initiating mechanisms of food allergy: Oral tolerance versus allergic sensitization. Biomed. Pharmacother. 2007, 61, 8–20. [Google Scholar] [CrossRef]
- Yang, A.; Zuo, L.; Cheng, Y.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Huang, H.-W.; Yang, B.B.; Wang, C.-Y. Effects of high pressure processing on immunoreactivity and microbiological safety of crushed peanuts. Food Control 2014, 42, 290–295. [Google Scholar] [CrossRef]
- Cabanillas, B.; Cuadrado, C.; Rodriguez, J.; Hart, J.; Burbano, C.; Crespo, J.F.; Novak, N. Potential changes in the allergenicity of three forms of peanut after thermal processing. Food Chem. 2015, 183, 18–25. [Google Scholar] [CrossRef] [PubMed]
- House, J. Insects as food in the Netherlands: Production networks and the geographies of edibility. Geoforum 2018, 94, 82–93. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Ohara, A.; Aguilar, J.G.d.S.; Domingues, M.A.F. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Arp, C.G.; Pasini, G. Exploring edible insects: From sustainable nutrition to pasta and noodle applications—A Critical Review. Foods 2024, 13, 3587. [Google Scholar] [CrossRef]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat Alternatives: Life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Vanqa, N.; Mshayisa, V.V.; Basitere, M. Proximate, Physicochemical, techno-functional and antioxidant properties of three edible insect (Gonimbrasia belina, Hermetia illucens and Macrotermes subhylanus) Flours. Foods 2022, 11, 976. [Google Scholar] [CrossRef]
- Aguilera, Y.; Pastrana, I.; Rebollo-Hernanz, M.; Benitez, V.; Álvarez-Rivera, G.; Viejo, J.L.; Martín-Cabrejas, M.A. Investigating edible insects as a sustainable food source: Nutritional value and techno-functional and physiological properties. Food Funct. 2021, 12, 6309–6322. [Google Scholar] [CrossRef]
- Oliveira, L.A.; Dias, K.A.; Pereira, S.M.S.; Vicente, L.C.O.S.; Barnabé, M.L.F.; Lucia, C.M.D. Digestibility and quality of edible insect proteins: A systematic review ofin vivo studies. J. Insects Food Feed. 2023, 9, 1333–1344. [Google Scholar] [CrossRef]
- Handley, M.A.; Hall, C.; Sanford, E.; Diaz, E.; Gonzalez-Mendez, E.; Drace, K.; Wilson, R.; Villalobos, M.; Croughan, M. Globalization, binational communities, and imported food risks: Results of an outbreak investigation of lead poisoning in monterey county, California. Am. J. Public Health 2007, 97, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.S. The limits of entomophagy: A discretionary gourmand in a world of toxic insects. Food Insects Newsl. 1994, 7, 1–6. [Google Scholar]
- Yang, A.; Zhang, G.; Meng, F.; Lu, P.; Wang, X.; Peng, M. Enhancing protein to extremely high content in photosynthetic bacteria during biogas slurry treatment. Bioresour. Technol. 2017, 245, 1277–1281. [Google Scholar] [CrossRef]
- Vieira, E.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Characterization of a potential bioactive food ingredient from inner cellular content of brewer’s spent yeast. Waste Biomass Valorization 2019, 10, 3235–3242. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Otero, M.A.; Guerrero, I.; Wagner, J.R.; Cabello, A.J.; Sceni, P.; García, R.; Soriano, J.; Tomasini, A.; Saura, G.; Almazán, O.J.B.A. Yeast and its derivatives as ingredients in the food industry. Biotecnol. Apl. 2011, 28, 273–275. [Google Scholar]
- Goldberg, I. Single Cell Protein; Springer Science & Business Media: Berlin, Germany, 2013; Volume 1. [Google Scholar]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Afraz, M.T.; Yılmaz, B.B.; Adil, M.; Arshad, N.; Goksen, G.; Ali, M.; Zeng, X.-A. Recent progress in natural seaweed pigments: Green extraction, health-promoting activities, techno-functional properties and role in intelligent food packaging. J. Agric. Food Res. 2024, 15, 100991. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Wang, J.; Cheng, K.; Liu, J.; He, Y.; Zhang, Y.; Mou, H.; Sun, H. Microalgal protein for sustainable and nutritious foods: A joint analysis of environmental impacts, health benefits and consumer’s acceptance. Trends Food Sci. Technol. 2024, 143, 104278. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, W.; Sun, H.; Mou, H.; Liu, J.; Yu, H.; Dai, L.; Kong, Q.; Yang, S. Phycocyanin from microalgae: A comprehensive review covering microalgal culture, phycocyanin sources and stability. Food Res. Int. 2024, 186, 114362. [Google Scholar] [CrossRef] [PubMed]
- Gromek, W.; Kołdej, N.; Kurowski, M.; Majsiak, E. Spirulina (Arthrospira platensis): Antiallergic agent or hidden allergen? A literature review. Foods 2024, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.; Phan Phuc, A.; Dubacq, J.P. Nutritional value of the alga Spirulina. World Rev. Nutr. Diet. 1995, 77, 32–46. [Google Scholar] [PubMed]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Debnath, C.; Bandyopadhyay, T.K.; Bhunia, B.; Mishra, U.; Narayanasamy, S.; Muthuraj, M. Microalgae: Sustainable resource of carbohydrates in third-generation biofuel production. Renew. Sustain. Energy Rev. 2021, 150, 111464. [Google Scholar] [CrossRef]
- Wicker, R.J.; Kumar, G.; Khan, E.; Bhatnagar, A. Emergent green technologies for cost-effective valorization of microalgal biomass to renewable fuel products under a biorefinery scheme. Chem. Eng. J. 2021, 415, 128932. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Coelho, M.O.C.; Monteyne, A.J.; Dunlop, M.V.; Harris, H.C.; Morrison, D.J.; Stephens, F.B.; Wall, B.T. Mycoprotein as a possible alternative source of dietary protein to support muscle and metabolic health. Nutr. Rev. 2020, 78, 486–497. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M. Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: Purification and molecular docking. J. Food Drug Anal. 2018, 26, 696–705. [Google Scholar] [CrossRef]
- Wang, B.; Shi, Y.; Lu, H.; Chen, Q. A critical review of fungal proteins: Emerging preparation technology, active efficacy and food application. Trends Food Sci. Technol. 2023, 141, 104178. [Google Scholar] [CrossRef]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single cell protein: A potential substitute in human and animal nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Li, D.; Tong, S.; Zeng, Y.; Yang, J.; Qi, X.; Wang, Q.; Sun, Y. [Low carbon biomanufacturing for future food]. Sheng Wu Gong Cheng Xue Bao 2022, 38, 4311–4328. [Google Scholar] [CrossRef]
- Cardoso Alves, S.; Díaz-Ruiz, E.; Lisboa, B.; Sharma, M.; Mussatto, S.I.; Thakur, V.K.; Kalaskar, D.M.; Gupta, V.K.; Chandel, A.K. Microbial meat: A sustainable vegan protein source produced from agri-waste to feed the world. Food Res. Int. 2023, 166, 112596. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.J.; Delange, J. Fungal Protein—What Is It and What Is the Health Evidence? A Systematic Review Focusing on Mycoprotein. Front. Sustain. Food Syst. 2021, 5, 581682. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Yamakawa, C.K.; van der Maas, L.; Dragone, G. New trends in bioprocesses for lignocellulosic biomass and CO2 utilization. Renew. Sustain. Energy Rev. 2021, 152, 111620. [Google Scholar] [CrossRef]
- Prasertsan, P.; Choorit, W.; Suwanno, S. Isolation, identification and growth conditions of photosynthetic bacteria found in seafood processing wastewater. World J. Microbiol. Biotechnol. 1993, 9, 590–592. [Google Scholar] [CrossRef]
- Kobayashi, M. Mass culture and cell utilization of photosynthetic bacteria. Process Biochem. 1978, 13, 27–30. [Google Scholar]
- Hülsen, T.; Hsieh, K.; Lu, Y.; Tait, S.; Batstone, D.J. Simultaneous treatment and single cell protein production from agri-industrial wastewaters using purple phototrophic bacteria or microalgae—A comparison. Bioresour. Technol. 2018, 254, 214–223. [Google Scholar] [CrossRef]
- Fradinho, J.C.; Domingos, J.M.; Carvalho, G.; Oehmen, A.; Reis, M.A. Polyhydroxyalkanoates production by a mixed photosynthetic consortium of bacteria and algae. Bioresour. Technol. 2013, 132, 146–153. [Google Scholar] [CrossRef]
- Sasaki, K.; Watanabe, M.; Suda, Y.; Ishizuka, A.; Noparatnaraporn, N. Applications of photosynthetic bacteria for medical fields. J. Biosci. Bioeng. 2005, 100, 481–488. [Google Scholar] [CrossRef]
- Hülsen, T.; Barry, E.M.; Lu, Y.; Puyol, D.; Keller, J.; Batstone, D.J. Domestic wastewater treatment with purple phototrophic bacteria using a novel continuous photo anaerobic membrane bioreactor. Water Res. 2016, 100, 486–495. [Google Scholar] [CrossRef]
- Cao, K.; Zhi, R.; Li, Q.; Zhang, G.; Wang, H. Photosynthetic bacterial protein production from wastewater: Effects of C/N and light-oxygen condition. J. Water Process Eng. 2021, 44, 102361. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, T.-K.; Choi, H.-D.; Park, J.-D.; Sung, J.-M.; Jeon, K.-H.; Paik, H.-D.; Kim, Y.-B. Optimization of replacing pork meat with yellow worm (Tenebrio molitor L.) for Frankfurters. Food Sci. Anim. Resour. 2017, 37, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Choi, Y.-S.; Hwang, K.-E.; Kim, T.-K.; Lee, C.-W.; Shin, D.-M.; Han, S.G. Physicochemical properties of meat batter added with edible silkworm pupae (Bombyx mori) and transglutaminase. Food Sci. Anim. Resour. 2017, 37, 351–359. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, T.-K.; Cha, J.Y.; Lee, J.H.; Kang, M.-C.; Jung, S.; Choi, Y.-S. Effects of edible insect extracts on the antioxidant, physiochemical, and microbial properties of Tteokgalbi during refrigerated storage. Food Biosci. 2023, 52, 102377. [Google Scholar] [CrossRef]
- Stephan, A.; Ahlborn, J.; Zajul, M.; Zorn, H. Edible mushroom mycelia of Pleurotus sapidus as novel protein sources in a vegan boiled sausage analog system: Functionality and sensory tests in comparison to commercial proteins and meat sausages. Eur. Food Res. Technol. 2018, 244, 913–924. [Google Scholar] [CrossRef]
- Pancrazio, G.; Cunha, S.C.; de Pinho, P.G.; Loureiro, M.; Meireles, S.; Ferreira, I.M.; Pinho, O. Spent brewer’s yeast extract as an ingredient in cooked hams. Meat Sci. 2016, 121, 382–389. [Google Scholar] [CrossRef]
- Bertolo, A.P.; Kempka, A.P.; Rigo, E.; Sehn, G.A.R.; Cavalheiro, D. Incorporation of natural and mechanically ruptured brewing yeast cells in beef burger to replace textured soy protein. J. Food Sci. Technol. 2022, 59, 935–943. [Google Scholar] [CrossRef]
- Grasso, S.; Smith, G.; Bowers, S.; Ajayi, O.M.; Swainson, M. Effect of texturised soy protein and yeast on the instrumental and sensory quality of hybrid beef meatballs. J. Food Sci. Technol. 2019, 56, 3126–3135. [Google Scholar] [CrossRef]
- Sriprablom, J.; Kitthawee, S.; Suphantharika, M. Functional and physicochemical properties of cookies enriched with edible insect (Tenebrio molitor and Zophobas atratus) powders. J. Food Meas. Charact. 2022, 16, 2181–2190. [Google Scholar] [CrossRef]
- dos Santos Richards, N.S.P.; Freiberg, J.A.; Schardong, I.S.; Pedroso, M.A.P.; Jiménez, M.S.E. Chrysodeixis includens as a potential source of protein and acceptance of cookies containing Tenebrio molitor. J. Food Meas. Charact. 2025, 19, 3278–3287. [Google Scholar] [CrossRef]
- Ochieng, B.O.; Anyango, J.O.; Nduko, J.M.; Mudalungu, C.M.; Cheseto, X.; Tanga, C.M. Aroma characterization and consumer acceptance of four cookie products enriched with insect (Ruspolia differens) meal. Sci. Rep. 2023, 13, 11145. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Marcia, J.; Pournaki, S.K.; Borrás-Linares, I.; Lozano-Sanchez, J.; Fernandez, I.M. Formulation of protein-rich chocolate chip cookies using cricket (Acheta domesticus) powder. Foods 2022, 11, 3275. [Google Scholar] [CrossRef] [PubMed]
- Mashau, M.E.; Ramalisa, T.; Ramashia, S.E.; Mshayisa, V.V. Development of high-protein biscuits by the enrichment with mopane worm (Gonimbrasia belina) flour. Food Sci. Technol. Int. 2024. [Google Scholar] [CrossRef]
- Gomes, N.G.; Schirmann, G.d.S.; de los Santos, M.L.P.; Zago, A.C.; Bortolini, V.M.d.S.; Rockenbach, R.; Bragança, G.C.M. Development and sensory evaluation of a hyperprotein bakery product using brewer’s yeast as the main innovative ingredient. Braz. J. Dev. 2021, 7, 9396–9413. [Google Scholar] [CrossRef]
- Çabuk, B. Influence of grasshopper (Locusta migratoria) and mealworm (Tenebrio molitor) powders on the quality characteristics of protein rich muffins: Nutritional, physicochemical, textural and sensory aspects. J. Food Meas. Charact. 2021, 15, 3862–3872. [Google Scholar] [CrossRef]
- Gumul, D.; Oracz, J.; Kowalski, S.; Mikulec, A.; Skotnicka, M.; Karwowska, K.; Areczuk, A. Bioactive Compounds and antioxidant composition of nut bars with addition of various edible insect flours. Molecules 2023, 28, 3556. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Lima, R.C.; Maia, M.R.G.; Almeida, A.A.; Fonseca, A.J.M.; Cabrita, A.R.J.; Cunha, L.M. Impact of defatting freeze-dried edible crickets (Acheta domesticus and Gryllodes sigillatus) on the nutritive value, overall liking and sensory profile of cereal bars. LWT 2019, 113, 108335. [Google Scholar] [CrossRef]
- Çabuk, B.; Yılmaz, B. Fortification of traditional egg pasta (erişte) with edible insects: Nutritional quality, cooking properties and sensory characteristics evaluation. J. Food Sci. Technol. 2020, 57, 2750–2757. [Google Scholar] [CrossRef]
- Duda, A.; Adamczak, J.; Chełmińska, P.; Juszkiewicz, J.; Kowalczewski, P. Quality and nutritional/textural properties of durum wheat pasta enriched with cricket powder. Foods 2019, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Piazza, L.; Ratti, S.; Girotto, F.; Cappellozza, S. Silkworm pupae derivatives as source of high value protein intended for pasta fortification. J. Food Sci. 2023, 88, 341–355. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cullere, M.; Dalle Zotte, A.; Pasini, G. Salt-soluble protein extracts from Hermetia illucens and Bombyx mori for high protein pasta production. LWT 2023, 190, 115507. [Google Scholar] [CrossRef]
- Althwab, S.A.; Alhomaid, R.M.; Ali, R.F.M.; Mohammed El-Anany, A.; Mousa, H.M. Effect of migratory locust (Locusta migratoria) powder incorporation on nutritional and sensorial properties of wheat flour bread. Br. Food J. 2021, 123, 3576–3591. [Google Scholar] [CrossRef]
- Tian, Y.; Li, C.; Xue, W.; Huang, L.; Wang, Z. Natural immunomodulating substances used for alleviating food allergy. Crit. Rev. Food Sci. Nutr. 2023, 63, 2407–2425. [Google Scholar] [CrossRef]
- Barre, A.; Pichereaux, C.; Velazquez, E.; Maudouit, A.; Simplicien, M.; Garnier, L.; Bienvenu, F.; Bienvenu, J.; Burlet-Schiltz, O.; Auriol, C.; et al. Insights into the allergenic potential of the edible yellow mealworm (Tenebrio molitor). Foods 2019, 8, 515. [Google Scholar] [CrossRef]
- Kewuyemi, Y.O.; Kesa, H.; Chinma, C.E.; Adebo, O.A. Fermented edible insects for promoting food security in Africa. Insects 2020, 11, 283. [Google Scholar] [CrossRef]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects; FAO: Rome, Italy, 2013. [Google Scholar]
- Suh, S.-M.; Kim, K.; Yang, S.-M.; Lee, H.; Jun, M.; Byun, J.; Lee, H.; Kim, D.; Lee, D.; Cha, J.-E.; et al. Comparative analysis of LC-MS/MS and real-time PCR assays for efficient detection of potential allergenic silkworm. Food Chem. 2024, 445, 138761. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, M.; Xu, Y.; Mu, L.; Gao, J.; Chen, H.; Wu, Z.; Yang, A.; Wu, Y.; Li, X. Enzymatic hydrolysis of silkworm pupa and its allergenicity evaluation by animal model with different immunization routes. Food Sci. Hum. Wellness 2023, 12, 774–782. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, L.; Wu, Y.; Xia, Q.; Chen, J.; Roux, K.H. Identification and characterization of an arginine kinase as a major allergen from silkworm (Bombyx mori) larvae. Int. Arch. Allergy Immunol. 2009, 150, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Han, I.S.; Lee, J.Y.; Park, K.H.; Lee, J.H.; Park, J.W. Role of tropomyosin in silkworm allergy. Mol. Med. Rep. 2017, 15, 3264–3270. [Google Scholar] [CrossRef]
- Yue, W.; Huang, S.; Lin, S.; He, K.; He, W.; Chen, J.; Li, L.; Chai, W.; Wu, X. Purification, immunological identification, and characterization of the novel silkworm pupae allergen Bombyx mori Lipoprotein 3 (Bomb m 6). J. Agric. Food Chem. 2023, 71, 13527–13534. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Lee, J.S.; Yuk, J.E.; Song, H.; Lee, H.J.; Kim, K.J.; Kim, B.J.; Lim, K.-J.; Park, K.H.; Lee, J.-H.; et al. Allergenic characterization of Bomb m 4, a 30-kDa Bombyx mori lipoprotein 6 from silkworm pupa. Clin. Exp. Allergy 2022, 52, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Lei, M.; Yang, R.; Liu, Z. Bom m 9 from Bombyx mori is a novel protein related to asthma. Microbiol. Immunol. 2015, 59, 410–418. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes—Extraction and functional properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef]
- Efsa Panel on Nutrition, N.F.; Food, A.; Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1700030. [Google Scholar] [CrossRef]
- Emilia, M.; Magdalena, C.; Weronika, G.; Julia, W.; Danuta, K.; Jakub, S.; Bożena, C.; Krzysztof, K. IgE-based analysis of sensitization and cross-reactivity to yellow mealworm and edible insect allergens before their widespread dietary introduction. Sci. Rep. 2025, 15, 1466. [Google Scholar] [CrossRef]
- Salahuddin, M.; Abdel-Wareth, A.A.A.; Hiramatsu, K.; Tomberlin, J.K.; Luza, D.; Lohakare, J. Flight toward sustainability in poultry nutrition with black soldier fly larvae. Animals 2024, 14, 510. [Google Scholar] [CrossRef]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S.; et al. Shotgun proteomics, in-silico evaluation and immunoblotting assays for allergenicity assessment of lesser mealworm, black soldier fly and their protein hydrolysates. Sci. Rep. 2020, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Sookrung, N.; Chaicumpa, W.; Tungtrongchitr, A.; Vichyanond, P.; Bunnag, C.; Ramasoota, P.; Tongtawe, P.; Sakolvaree, Y.; Tapchaisri, P. Periplaneta americana arginine kinase as a major cockroach allergen among Thai patients with major cockroach allergies. Environ. Health Perspect. 2006, 114, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Pomés, A.; Arruda, L.K. Investigating cockroach allergens: Aiming to improve diagnosis and treatment of cockroach allergic patients. Methods 2014, 66, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Pomés, A.; Mueller, G.A.; Randall, T.A.; Chapman, M.D.; Arruda, L.K. New Insights into cockroach allergens. Curr. Allergy Asthma Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Helm, R.; Cockrell, G.; Stanley, J.S.; Brenner, R.J.; Burks, W.; Bannon, G.A. Isolation and characterization of a clone encoding a major allergen (Bla g Bd90K) involved in IgE-mediated cockroach hypersensitivity. J. Allergy Clin. Immunol. 1996, 98, 172–180. [Google Scholar] [CrossRef]
- Arruda, L.K.; Vailes, L.D.; Mann, B.J.; Shannon, J.; Fox, J.W.; Vedvick, T.S.; Hayden, M.L.; Chapman, M.D. Molecular cloning of a major cockroach (Blattella germanica) allergen, Bla g 2: Sequence homology to the aspartic proteases*. J. Biol. Chem. 1995, 270, 19563–19568. [Google Scholar] [CrossRef]
- Arruda, L.K.; Vailes, L.D.; Platts-Mills, T.A.E.; Hayden, M.L.; Chapman, M.D. Induction of IgE antibody responses by glutathione S-Transferase from the german cockroach (Blattella germanica)*. J. Biol. Chem. 1997, 272, 20907–20912. [Google Scholar] [CrossRef]
- Ayuso, R.; Reese, G.; Leong-Kee, S.; Plante, M.; Lehrer, S.B. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int. Arch. Allergy Immunol. 2002, 129, 38–48. [Google Scholar] [CrossRef]
- Pascal, M.; Grishina, G.; Yang, A.C.; Sánchez-García, S.; Lin, J.; Towle, D.; Ibañez, M.D.; Sastre, J.; Sampson, H.A.; Ayuso, R. Molecular diagnosis of shrimp allergy: Efficiency of several allergens to predict Clinical Reactivity. J. Allergy Clin. Immunol. Pract. 2015, 3, 521–529.e510. [Google Scholar] [CrossRef]
- Togias, A.; Fenton, M.J.; Gergen, P.J.; Rotrosen, D.; Fauci, A.S. Asthma in the inner city: The perspective of the National Institute of Allergy and Infectious Diseases. J. Allergy Clin. Immunol. 2010, 125, 540–544. [Google Scholar] [CrossRef]
- Lee, M.-F.; Song, P.-P.; Hwang, G.-Y.; Lin, S.-J.; Chen, Y.-H. Sensitization to per a 2 of the American cockroach correlates with more clinical severity among airway allergic patients in Taiwan. Ann. Allergy Asthma Immunol. 2012, 108, 243–248. [Google Scholar] [CrossRef]
- Stelmach, I.; Jerzynska, J.; Stelmach, W.; Majak, P.; Chew, G.; Gorski, P.; Kuna, P. Cockroach allergy and exposure to cockroach allergen in Polish children with asthma. Allergy 2002, 57, 701–705. [Google Scholar] [CrossRef]
- Pomés, A.; Arruda, L.K. Cockroach allergy: Understanding complex immune responses to develop novel therapies. Mol. Immunol. 2023, 156, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Ayieko, M.A.; Ogola, H.J.; Ayieko, I.A. Introducing rearing crickets (gryllids) at household levels: Adoption, processing and nutritional values. J. Insects Food Feed. 2016, 2, 203–212. [Google Scholar] [CrossRef]
- Lamberti, C.; Nebbia, S.; Cirrincione, S.; Brussino, L.; Giorgis, V.; Romito, A.; Marchese, C.; Manfredi, M.; Marengo, E.; Giuffrida, M.G.; et al. Thermal processing of insect allergens and IgE cross-recognition in Italian patients allergic to shrimp, house dust mite and mealworm. Food Res. Int. 2021, 148, 110567. [Google Scholar] [CrossRef] [PubMed]
- Karnaneedi, S.; Johnston, E.B.; Bose, U.; Juhász, A.; Broadbent, J.A.; Ruethers, T.; Jerry, E.M.; Kamath, S.D.; Limviphuvadh, V.; Stockwell, S.; et al. The allergen profile of two edible insect species—Acheta domesticus and Hermetia illucens. Mol. Nutr. Food Res. 2024, 68, 2300811. [Google Scholar] [CrossRef]
- Scala, E.; Abeni, D.; Villella, V.; ViIlalta, D.; Cecchi, L.; Caprini, E.; Asero, R. Investigating novel food sensitization: A real-life prevalence study of cricket, Locust, and mealworm ige-reactivity in naïve allergic individuals. J. Investig. Allergol. Clin. Immunol. 2024, 35, 197–202. [Google Scholar]
- Cullen, D.A.; Cease, A.J.; Latchininsky, A.V.; Ayali, A.; Berry, K.; Buhl, C.; De Keyser, R.; Foquet, B.; Hadrich, J.C.; Matheson, T.; et al. Chapter Seven—From molecules to management: Mechanisms and consequences of Locust phase polyphenism. In Advances in Insect Physiology; Verlinden, H., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 53, pp. 167–285. [Google Scholar]
- Egonyu, J.P.; Subramanian, S.; Tanga, C.M.; Dubois, T.; Ekesi, S.; Kelemu, S. Global overview of locusts as food, feed and other uses. Glob. Food Secur. 2021, 31, 100574. [Google Scholar] [CrossRef]
- Mitsuhashi, J. Edible Insects of the World; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Meir Paul, P. Allergy to locusts and Acridid Grasshoppers: A Review. J. Orthoptera Res. 2014, 23, 59–67. [Google Scholar] [CrossRef]
- Ji, K.; Chen, J.; Li, M.; Liu, Z.; Wang, C.; Zhan, Z.; Wu, X.; Xia, Q. Anaphylactic shock and lethal anaphylaxis caused by food consumption in China. Trends Food Sci. Technol. 2009, 20, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Brogan, E.N. Protein and Lipid Characterization of Acheta domesticus, Bombyx mori, and Locusta migratoria Dry Flours; West Virginia University: Morgantown, WV, USA, 2018. [Google Scholar]
- Wang, Y.; Zhang, Y.; Lou, H.; Wang, C.; Ni, M.; Yu, D.; Zhang, L.; Kang, L. Hexamerin-2 Protein of locust as a novel allergen in occupational allergy. J. Asthma Allergy 2022, 15, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Petrus, M.; Culerrier, R.; Campistron, M.; Barre, A.; Rougé, P. First case report of anaphylaxis to spirulin: Identification of phycocyanin as responsible allergen. Allergy 2010, 65, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. A new paradigm to search for allergenic proteins in novel foods by integrating proteomics analysis and in silico sequence homology prediction: Focus on spirulina and chlorella microalgae. Talanta 2022, 240, 123188. [Google Scholar] [CrossRef]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Discovery of marker peptides of spirulina microalga proteins for allergen detection in processed foodstuffs. Food Chem. 2022, 393, 133319. [Google Scholar] [CrossRef]
- Tiberg, E.; Einarsson, R. Variability of allergenicity in eight strains of the green algal genus Chlorella. Int. Arch. Allergy Appl. Immunol. 2009, 90, 301–306. [Google Scholar] [CrossRef]
- Tiberg, E.; Rolfsen, W.; Einarsson, R.; Dreborg, S. Detection of Chlorella-specific IgE in mould-sensitized children. Allergy 1990, 45, 481–486. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Denk, U.; Pöll, V.; Rid, R.; Breitenbach, M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008, 145, 58–86. [Google Scholar] [CrossRef]
- Bush, R.K.; Prochnau, J.J. Alternaria-induced asthma. J. Allergy Clin. Immunol. 2004, 113, 227–234. [Google Scholar] [CrossRef]
- Abel-Fernández, E.; Martínez, M.J.; Galán, T.; Pineda, F. Going over Fungal Allergy: Alternaria alternata and Its Allergens. J. Fungi 2023, 9, 582. [Google Scholar] [CrossRef]
- Asturias, J.A.; Ibarrola, I.; Ferrer, A.; Andreu, C.; López-Pascual, E.; Quiralte, J.; Florido, F.; Martínez, A. Diagnosis of Alternaria alternata sensitization with natural and recombinant Alt a 1 allergens. J. Allergy Clin. Immunol. 2005, 115, 1210–1217. [Google Scholar] [CrossRef]
- Paris, S.; Debeaupuis, J.P.; Prévost, M.C.; Casotto, M.; Latgé, J.-P. The 31 kd major allergen, ALT a I1563, of Alternaria alternata. J. Allergy Clin. Immunol. 1991, 88, 902–908. [Google Scholar] [CrossRef]
- De Vouge, M.W.; Thaker, A.J.; Zhang, L.; Muradia, G.; Rode, H.; Vijay, H.M. Molecular cloning of IgE–Binding fragments of Alternaria alternata allergens. Int. Arch. Allergy Immunol. 1998, 116, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Twaroch, T.E.; Curin, M.; Valenta, R.; Swoboda, I. Mold allergens in respiratory allergy: From structure to therapy. Allergy Asthma Immunol. Res. 2015, 7, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Wei, Y.; Shi, J.; Zhang, H.; Xiao, Y.; Gan, Z.; Jia, G.; Qian, X.; Gao, W.; Zhang, Y.; et al. Allergenic risk assessment of enolase leaked from Saccharomyces cerevisiae under pressurization. Food Biosci. 2023, 56, 103399. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Martínez, M.; Sánchez, J.J.; Fernández-Caldas, E. [Legume cross-reactivity]. Allergol. Immunopathol. 2003, 31, 151–161. [Google Scholar]
- Kamemura, N.; Sugimoto, M.; Tamehiro, N.; Adachi, R.; Tomonari, S.; Watanabe, T.; Mito, T. Cross-allergenicity of crustacean and the edible insect Gryllus bimaculatus in patients with shrimp allergy. Mol. Immunol. 2019, 106, 127–134. [Google Scholar] [CrossRef]
- de las Marinas, M.D.; Cerdá, J.C.; López-Matas, M.A.; González-Ruiz, A.; Martorell, C.; Felix, R.; Alvariño, M.; Carnés, J. Hexamerin-like protein 2, a cricket allergen involved in occupational and food allergy. Clin. Exp. Allergy 2021, 51, 858–860. [Google Scholar] [CrossRef]
- Leung, P.S.; Chow, W.K.; Duffey, S.; Kwan, H.S.; Gershwin, M.E.; Chu, K.H. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: Evidence for tropomyosin as the common allergen. J. Allergy Clin. Immunol. 1996, 98, 954–961. [Google Scholar] [CrossRef]
- Phiriyangkul, P.; Srinroch, C.; Srisomsap, C.; Chokchaichamnankit, D.; Punyarit, P. Effect of food thermal processing on allergenicity proteins in Bombay Locust (Patanga succincta). Int. J. Food Eng. 2015, 1, 23–28. [Google Scholar] [CrossRef]
- van Broekhoven, S.; Bastiaan-Net, S.; de Jong, N.W.; Wichers, H.J. Influence of processing and in vitro digestion on the allergic cross-reactivity of three mealworm species. Food Chem. 2016, 196, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Broekman, H.; Knulst, A.; den Hartog Jager, S.; Monteleone, F.; Gaspari, M.; de Jong, G.; Houben, G.; Verhoeckx, K. Effect of thermal processing on mealworm allergenicity. Mol. Nutr. Food Res. 2015, 59, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.-G.; Su, S.-N.; Chiang, B.-L.; Lee, H.-J.; Chow, L.-P. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics 2010, 10, 3854–3867. [Google Scholar] [CrossRef] [PubMed]
- Hindley, J.; Wünschmann, S.; Satinover, S.M.; Woodfolk, J.A.; Chew, F.T.; Chapman, M.D.; Pomés, A. Bla g 6: A troponin C allergen from Blattella germanica with IgE binding calcium dependence. J. Allergy Clin. Immunol. 2006, 117, 1389–1395. [Google Scholar] [CrossRef]
- Pomés, A.; Schulten, V.; Glesner, J.; da Silva Antunes, R.; Sutherland, A.; Bacharier, L.B.; Beigelman, A.; Busse, P.; Frazier, A.; Sette, A. IgE and T Cell reactivity to a comprehensive panel of cockroach allergens in relation to disease. Front. Immunol. 2020, 11, 621700. [Google Scholar] [CrossRef]
- Gustchina, A.; Li, M.; Wünschmann, S.; Chapman, M.D.; Pomés, A.; Wlodawer, A. Crystal structure of cockroach allergen Bla g 2, an unusual zinc binding aspartic protease with a novel mode of self-inhibition. J. Mol. Biol. 2005, 348, 433–444. [Google Scholar] [CrossRef]
- Wu, C.H.; Lee, M.F.; Liao, S.C.; Luo, S.F. Sequencing analysis of cDNA clones encoding the American cockroach Cr-PI Allergens: Homology with insect hemolymph proteins*. J. Biol. Chem. 1996, 271, 17937–17943. [Google Scholar] [CrossRef]
- Wangorsch, A.; Jamin, A.; Eichhorn, S.; Pablos, I.; Sharma, S.; Schweidler, B.; Kastner, B.; Wildner, S.; Saloga, J.; Führer, F.; et al. Component-resolved diagnosis of American cockroach (Periplaneta americana) allergy in patients from different geographical areas. Front. Allergy 2021, 2, 691627. [Google Scholar] [CrossRef]
- Asturias, J.A.; Gómez-Bayón, N.; Arilla, M.C.; Martínez, A.; Palacios, R.; Sánchez-Gascón, F.; Martínez, J. Molecular characterization of american cockroach tropomyosin (Periplaneta americana allergen 7), a cross-reactive allergen. J. Immunol. 1999, 162, 4342–4348. [Google Scholar] [CrossRef]
- Sudha, V.T.; Arora, N.; Gaur, S.N.; Pasha, S.; Singh, B.P. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy 2008, 63, 768–776. [Google Scholar] [CrossRef]
- Fang, Y.; Long, C.; Bai, X.; Liu, W.; Rong, M.; Lai, R.; An, S. Two new types of allergens from the cockroach, Periplaneta americana. Allergy 2015, 70, 1674–1678. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Zhu, L.-X.; Lu, C.; Jiao, Y.-X.; Zhu, D.-X.; Guo, M.; Yang, Y.-S.; Cao, M.-D.; Zhang, L.-S.; Tian, M.; et al. Identification of Per a 13 as a novel allergen in American cockroach. Mol. Immunol. 2022, 143, 41–49. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Q.; Saelim, N.; Wang, L.; Nong, W.; Wan, A.T.; Shi, M.; Liu, X.; Cao, Q.; Hui, J.H.L.; et al. Genome assembly and annotation of Periplaneta americana reveal a comprehensive cockroach allergen profile. Allergy 2023, 78, 1088–1103. [Google Scholar] [CrossRef]
- Hamzelou, S.; Belobrajdic, D.; Juhász, A.; Brook, H.; Bose, U.; Colgrave, M.L.; Broadbent, J.A. Nutrition, allergenicity and physicochemical qualities of food-grade protein extracts from Nannochloropsis oculata. Food Chem. 2023, 424, 136459. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakamura, M.; Sato, N.; Futamura, K.; Matsunaga, K.; Yagami, A. Nattokinase (Bac s 1), a subtilisin family serine protease, is a novel allergen contained in the traditional Japanese fermented food natto. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2023, 72, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Wu, K.G.; Yeh, C.C.; Tai, H.Y.; Tam, M.F.; Chen, Y.S.; Shen, H.D. The transaldolase, a novel allergen of Fusarium proliferatum, demonstrates IgE cross-reactivity with its human analogue. PLoS ONE 2014, 9, e103488. [Google Scholar] [CrossRef] [PubMed]
- Flückiger, S.; Fijten, H.; Whitley, P.; Blaser, K.; Crameri, R. Cyclophilins, a new family of cross-reactive allergens. Eur. J. Immunol. 2002, 32, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.W.; Levetin, E. Allergen of the Month 2014 Fusarium. Ann. Allergy Asthma Immunol. 2014, 112, A11. [Google Scholar] [CrossRef]
- Yeh, C.C.; Tai, H.Y.; Chou, H.; Wu, K.G.; Shen, H.D. Vacuolar serine protease is a major allergen of Fusarium proliferatum and an IgE-Cross reactive pan-fungal allergen. Allergy Asthma Immunol. Res. 2016, 8, 438–444. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, C.; Pan, F.; Peng, W.; Fang, X.; Che, H.; Tian, W. Allergenicity risk in animal-based food proteins: Source, dietary factors effect, allergen detection and processing modification methods. Trends Food Sci. Technol. 2024, 153, 104726. [Google Scholar] [CrossRef]
- Pi, X.; Sun, Y.; Fu, G.; Wu, Z.; Cheng, J. Effect of processing on soybean allergens and their allergenicity. Trends Food Sci. Technol. 2021, 118, 316–327. [Google Scholar] [CrossRef]
- Pan, T.G.; Wu, Y.N.; He, S.; Wu, Z.; Jin, R. Food allergenic protein conjugation with plant polyphenols for allergenicity reduction. Curr. Opin. Food Sci. 2022, 43, 36–42. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Benedé, S.; Molina, E.; López-Expósito, I. Effect of processing technologies on the allergenicity of food products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- Chizoba Ekezie, F.-G.; Cheng, J.-H.; Sun, D.-W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Pi, X.; Wan, Y.; Yang, Y.; Li, R.; Wu, X.; Xie, M.; Li, X.; Fu, G. Research progress in peanut allergens and their allergenicity reduction. Trends Food Sci. Technol. 2019, 93, 212–220. [Google Scholar] [CrossRef]
- He, W.; He, K.; Sun, F.; Mu, L.; Liao, S.; Li, Q.; Yi, J.; Liu, Z.; Wu, X. Effect of heat, enzymatic hydrolysis and acid-alkali treatment on the allergenicity of silkworm pupa protein extract. Food Chem. 2021, 343, 128461. [Google Scholar] [CrossRef]
- Sodek, J.; Hodges, R.S.; Smillie, L.B.; Jurasek, L. Amino-acid sequence of rabbit skeletal tropomyosin and its coiled-coil structure. Proc. Natl. Acad. Sci. USA 1972, 69, 3800–3804. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Zhao, Y.; Tang, G.; Niu, B.; Chen, Q. Boiling and roasting treatment affecting the peanut allergenicity. Ann. Transl. Med. 2018, 6, 357. [Google Scholar] [CrossRef]
- Li, T.; Bu, G.; Xi, G. Effects of heat treatment on the antigenicity, antigen epitopes, and structural properties of β-conglycinin. Food Chem. 2021, 346, 128962. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Wang, H.; Hu, Y.M.; Wang, X.M.; Chen, H.Q.; Tu, Z.C. Mechanism of the allergenicity reduction of ovalbumin by microwave pretreatment-assisted enzymolysis through biological mass spectrometry. J. Agric. Food Chem. 2023, 71, 15363–15374. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, R.; von Langermann, J.; Kragl, U. Microwave-assisted covalent immobilization of enzymes on inorganic surfaces. Eng. Life Sci. 2014, 14, 493–499. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Impact of microwave processing on the secondary structure, in-vitro protein digestibility and allergenicity of shrimp (Litopenaeus vannamei) proteins. Food Chem. 2021, 337, 127811. [Google Scholar] [CrossRef]
- Liu, K.; Lin, S.; Liu, Y.; Wang, S.; Liu, Q.; Sun, K.; Sun, N. Mechanism of the reduced allergenicity of shrimp (Macrobrachium nipponense) by combined thermal/pressure processing: Insight into variations in protein structure, gastrointestinal digestion and immunodominant linear epitopes. Food Chem. 2023, 405, 134829. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, J.; Yu, N.; He, L.; Chen, Y. Effect of ultrahigh-pressure treatment on the structure and allergenicity of peach allergenic proteins. Food Chem. 2023, 423, 136227. [Google Scholar] [CrossRef]
- Li, D.; Peng, Y.; Tao, Y.; Liu, D.; Zhang, H. Quality changes in high pressure processed tan mutton during storage. Food Sci. Technol. Int. 2021, 27, 517–527. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, X.; Sun, J.; Ma, A. Effects of different high temperature-pressure processing times on the sensory quality, nutrition and allergenicity of ready-to-eat clam meat. Food Res. Int. 2024, 185, 114263. [Google Scholar] [CrossRef]
- Xiong, Z.; Cheng, J.; Hu, Y.; Chen, S.; Qiu, Y.; Yang, A.; Wu, Z.; Li, X.; Chen, H. A composite enzyme derived from papain and chymotrypsin reduces the Allergenicity of Cow’s Milk allergen casein by targeting T and B cell epitopes. Food Chem. 2024, 459, 140315. [Google Scholar] [CrossRef]
- Chen, B.; He, H.; Wang, X.; Wu, S.; Wang, Q.; Zhang, J.; Qiao, Y.; Liu, H. Research rrogress on shrimp allergens and allergenicity reduction methods. Foods 2025, 14, 895. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-W.; Liu, Y.-Y.; Chen, F.; Hu, J.-W.; Cao, M.-J.; Su, W.-J.; Liu, G.-M. Purification, characterization and immunoreactivity of tropomyosin, the allergen in Octopus fangsiao. Process Biochem. 2014, 49, 1747–1756. [Google Scholar] [CrossRef]
- Villas-Boas, M.B.; Benedé, S.; de Lima Zollner, R.; Netto, F.M.; Molina, E. Epitopes resistance to the simulated gastrointestinal digestion of β-lactoglobulin submitted to two-step enzymatic modification. Food Res. Int. 2015, 72, 191–197. [Google Scholar] [CrossRef]
- Golkar, A.; Milani, J.M.; Vasiljevic, T. Altering allergenicity of cow’s milk by food processing for applications in infant formula. Crit. Rev. Food Sci. Nutr. 2019, 59, 159–172. [Google Scholar] [CrossRef]
- Hall, F.; Liceaga, A. Effect of microwave-assisted enzymatic hydrolysis of cricket (Gryllodes sigillatus) protein on ACE and DPP-IV inhibition and tropomyosin-IgG binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Boukil, A.; Perreault, V.; Chamberland, J.; Mezdour, S.; Pouliot, Y.; Doyen, A. High hydrostatic pressure-assisted enzymatic hydrolysis affect mealworm allergenic proteins. Molecules 2020, 25, 2685. [Google Scholar] [CrossRef]
- Clemente, A.; Vioque, J.; Sanchez-Vioque, R.; Pedroche, J.; Millán, F. Production of Extensive Chickpea (Cicer arietinum L.) Protein Hydrolysates with Reduced Antigenic Activity. J. Agric. Food Chem. 1999, 47, 3776–3781. [Google Scholar] [CrossRef]
- Cabanillas, B.; Pedrosa, M.M.; Rodríguez, J.; Muzquiz, M.; Maleki, S.J.; Cuadrado, C.; Burbano, C.; Crespo, J.F. Influence of enzymatic hydrolysis on the allergenicity of roasted peanut protein extract. Int. Arch. Allergy Immunol. 2011, 157, 41–50. [Google Scholar] [CrossRef]
- Sung, D.-E.; Lee, J.; Han, Y.; Shon, D.-H.; Ahn, K.; Oh, S.; Do, J.-R. Effects of enzymatic hydrolysis of buckwheat protein on antigenicity and allergenicity. Nutr. Res. Pract. 2014, 8, 278–283. [Google Scholar] [CrossRef]
- Grossmann, K.K.; Merz, M.; Appel, D.; De Araujo, M.M.; Fischer, L. New insights into the flavoring potential of cricket (Acheta domesticus) and mealworm (Tenebrio molitor) protein hydrolysates and their Maillard products. Food Chem. 2021, 364, 130336. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, H.; Zhou, P. Allergenicity suppression of tropomyosin from exopalaemon modestus by glycation with saccharides of different molecular sizes. Food Chem. 2019, 288, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-Y.; Yang, H.; Rao, S.-T.; Liu, G.-Y.; Hu, M.-J.; Zeng, B.-C.; Cao, M.-J.; Liu, G.-M. The Maillard reaction reduced the sensitization of tropomyosin and arginine kinase from Scylla paramamosain, simultaneously. J. Agric. Food Chem. 2018, 66, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Polikovsky, M.; Fernand, F.; Sack, M.; Frey, W.; Müller, G.; Golberg, A. In silico food allergenic risk evaluation of proteins extracted from macroalgae Ulva sp. with pulsed electric fields. Food Chem. 2019, 276, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.E.; Phillips, M.C. Proteins at liquid interfaces: I. Kinetics of adsorption and surface denaturation. J. Colloid Interface Sci. 1979, 70, 403–414. [Google Scholar] [CrossRef]
- Villamiel, M.; de Jong, P. Influence of High-Intensity Ultrasound and Heat Treatment in Continuous Flow on Fat, Proteins, and Native Enzymes of Milk. J. Agric. Food Chem. 2000, 48, 472–478. [Google Scholar] [CrossRef]
- Zhenxing, L.; Caolimin, L.; Jamil, K. Reduction of allergenic properties of shrimp (Penaeus vannamei) allergens by high intensity ultrasound. Eur. Food Res. Technol. 2006, 223, 639–644. [Google Scholar] [CrossRef]
- Zehi, Z.B.; Afshari, A.; Noori, S.M.A.; Jannat, B.; Hashemi, M. The Effects of X-ray irradiation on safety and nutritional value of food: A Systematic Review Article. Curr. Pharm. Biotechnol. 2020, 21, 919–926. [Google Scholar] [CrossRef]
- Seo, J.-H.; Lee, J.-W.; Kim, J.-H.; Byun, E.-B.; Lee, S.-Y.; Kang, I.-J.; Byun, M.-W. Reduction of allergenicity of irradiated ovalbumin in ovalbumin-allergic mice. Radiat. Phys. Chem. 2007, 76, 1855–1857. [Google Scholar] [CrossRef]
- Meng, X.; Li, X.; Wang, X.; Gao, J.; Yang, H.; Chen, H. Potential allergenicity response to structural modification of irradiated bovine α-lactalbumin. Food Funct. 2016, 7, 3102–3110. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, W.; Shen, J.; Xu, X.; Zhou, G. Influence of gamma irradiation on porcine serum albumin structural properties and allergenicity. J. AOAC Int. 2018, 101, 529–535. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Pavase, T.; Li, Z.; Liu, Y.; Wang, N. Evaluation of electron beam irradiation to reduce the IgE binding capacity of frozen shrimp tropomyosin. Food Agric. Immunol. 2017, 28, 189–201. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, T.; Zhang, Q.; Liu, T.; Lai, J.; Wang, C.; Cao, L.; Liu, Y.; Ruan, R.; Xue, M. Influence of cold atmospheric pressure plasma treatment of Spirulina platensis slurry over biomass characteristics. Bioresour. Technol. 2023, 386, 129480. [Google Scholar] [CrossRef]
- Shriver, S.K. Effect of Selected Nonthermal Processing Methods on the Allergen Reactivity of Atlantic White Shrimp (Litopenaeus setiferus). Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2011. [Google Scholar]
- Davis, C.M.; Gupta, R.S.; Aktas, O.N.; Diaz, V.; Kamath, S.D.; Lopata, A.L. Clinical management of Seafood allergy. J. Allergy Clin. Immunol. Pract. 2020, 8, 37–44. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Fröhling, A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Cold atmospheric pressure plasma processing of insect flour from Tenebrio molitor: Impact on microbial load and quality attributes in comparison to dry heat treatment. Innov. Food Sci. Emerg. Technol. 2016, 36, 277–286. [Google Scholar] [CrossRef]
- Li, F.; Zhou, E.; Wang, M.; Pan, F.; Zhou, J.; Yang, M.; Wang, T.; Li, L.; Wu, L.; Li, Q. A novel sustainable photo/chemical magnetic nanomaterial effectively mitigates the allergenicity of phospholipase A2. Food Chem. 2024, 461, 140851. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Limnaios, A.; Aerakis, E.; Andreou, V.; Taoukis, P. Effect of high pressure on the proteolytic activity and autolysis of yeast Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2021, 74, 102865. [Google Scholar] [CrossRef]
- Sánchez, A.G. Allergy to beer and wine caused by Saccharomyces cerevisiae in a patient sensitized to fungi. J. Investig. Allergol. Clin. Immunol. 2022, 32, 311–313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.