Effect of 6-Gingerol on Oxidation and Structure of Beef Myofibrillar Protein During Heating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical
2.2. Sample Preparation
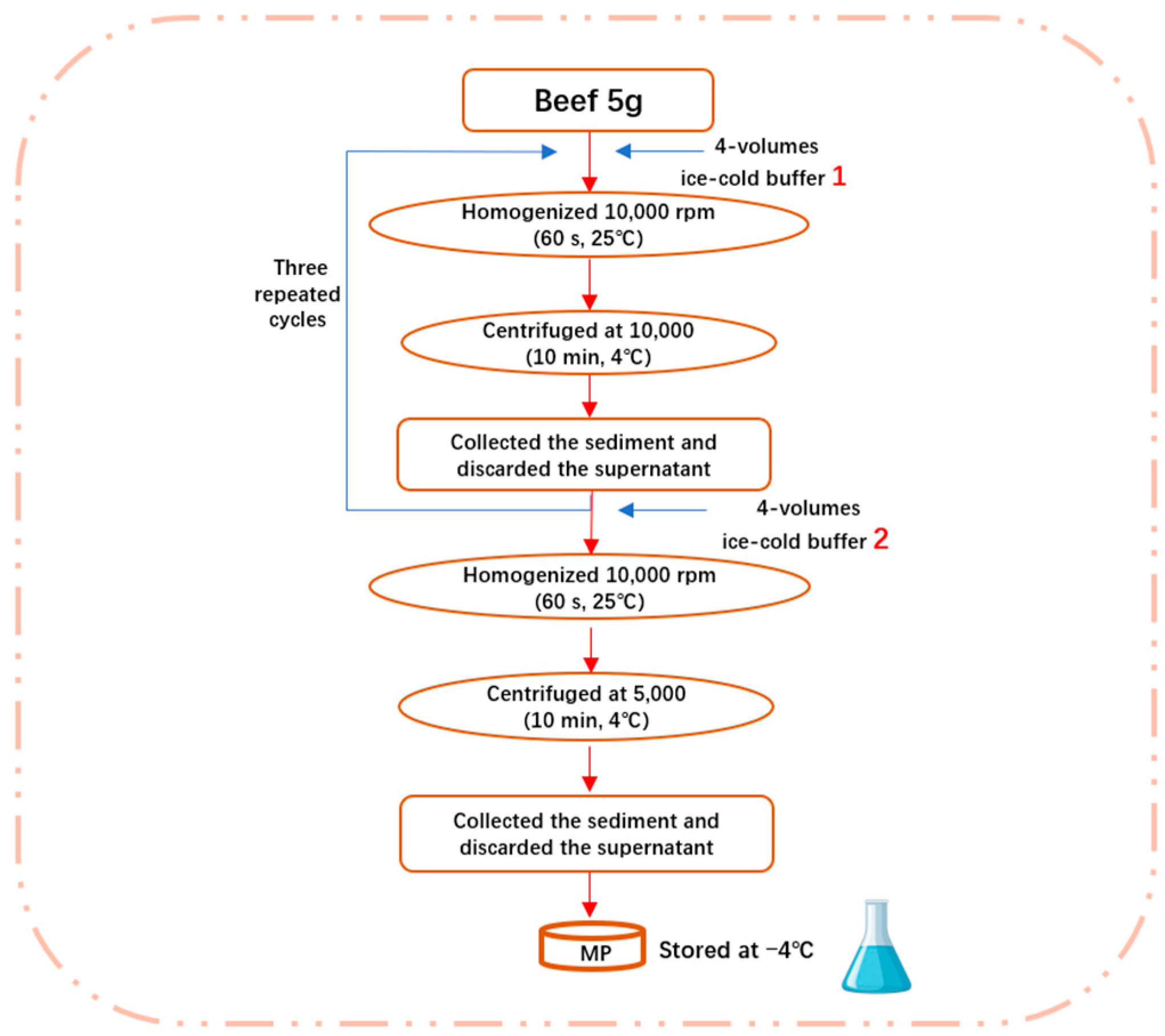
2.3. Extraction of Myofibrillar Protein
2.4. Determination of Surface Hydrophobicity
2.5. Determination of Total Sulfhydryl Content
2.6. Determination of Carbonyl Content
2.7. Determination of Protein Solubility
2.8. Determination of Zeta Potential and Particle Size
2.9. Determination of Intermolecular Force
2.10. Determination of Intrinsic Fluorescence Spectra
2.11. Determination of FT-IR Spectra
2.12. Microstructure Observation
2.13. Molecular Docking Simulation
2.14. Statistical Analysis
3. Results and Discussion
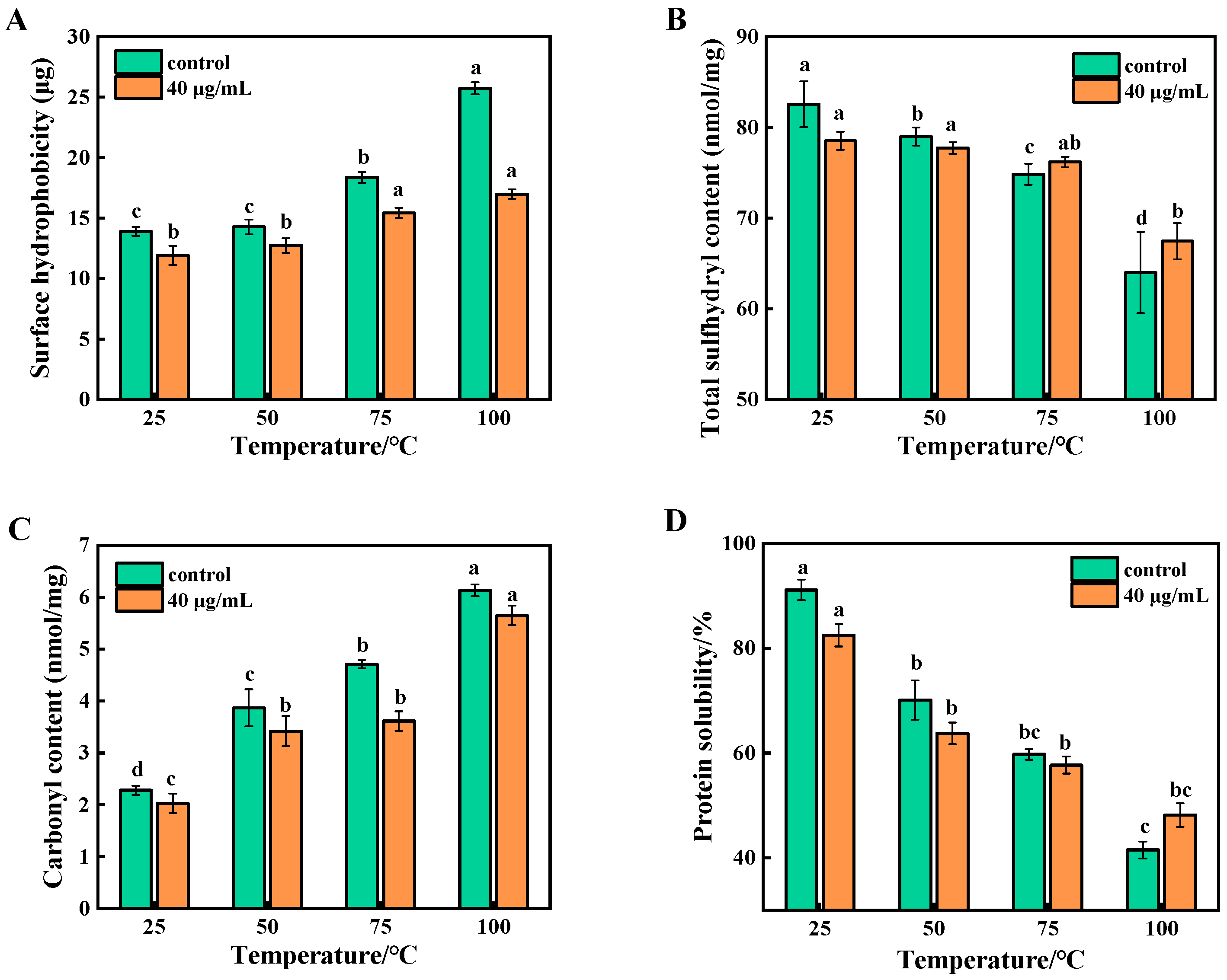
3.1. Analysis of Surface Hydrophobicity of Beef MP During Cooking
3.2. Analysis of Total Sulfhydryl Content of Beef MP During Cooking
3.3. Analysis of Carbonyl Content of Beef MP During Cooking
3.4. Analysis of Protein Solubility of Beef MP During Cooking
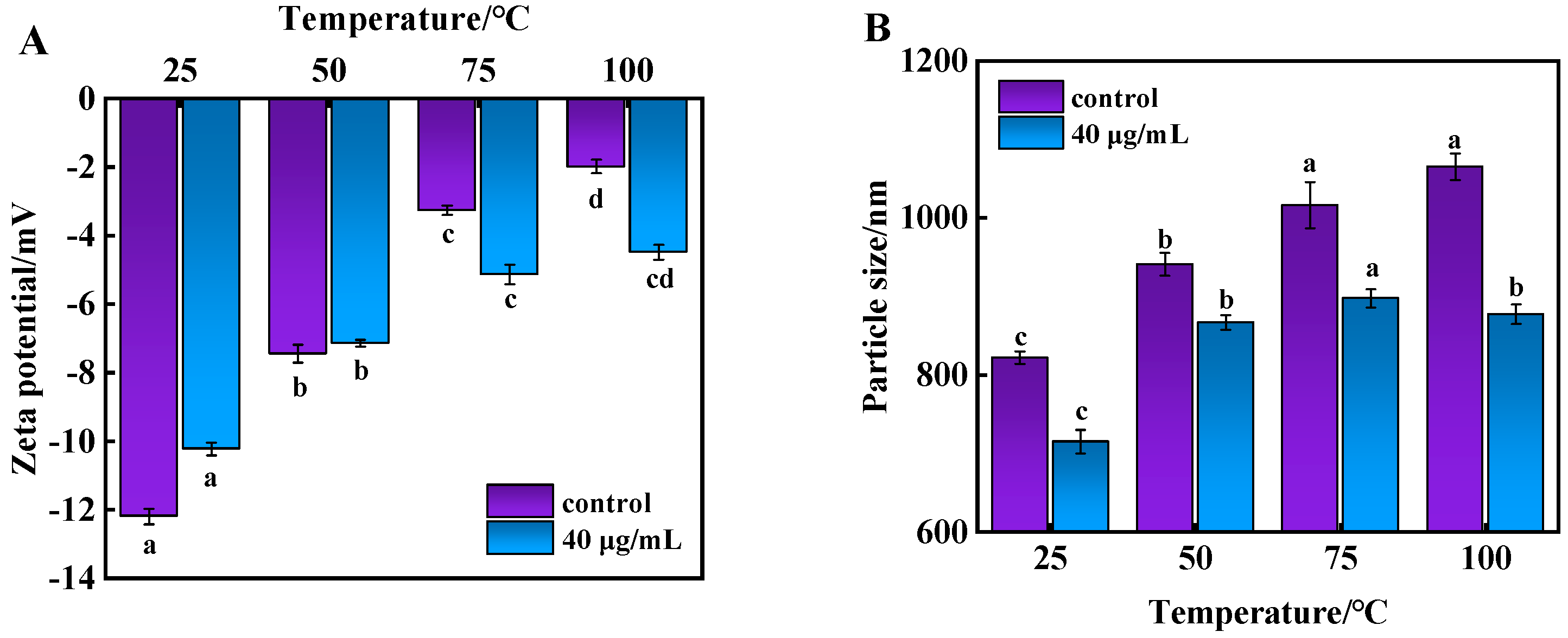
3.5. Analysis of Zeta Potential and Particle Size of Beef MP During Cooking
3.6. Analysis of Intermolecular Force of Beef MP During Cooking
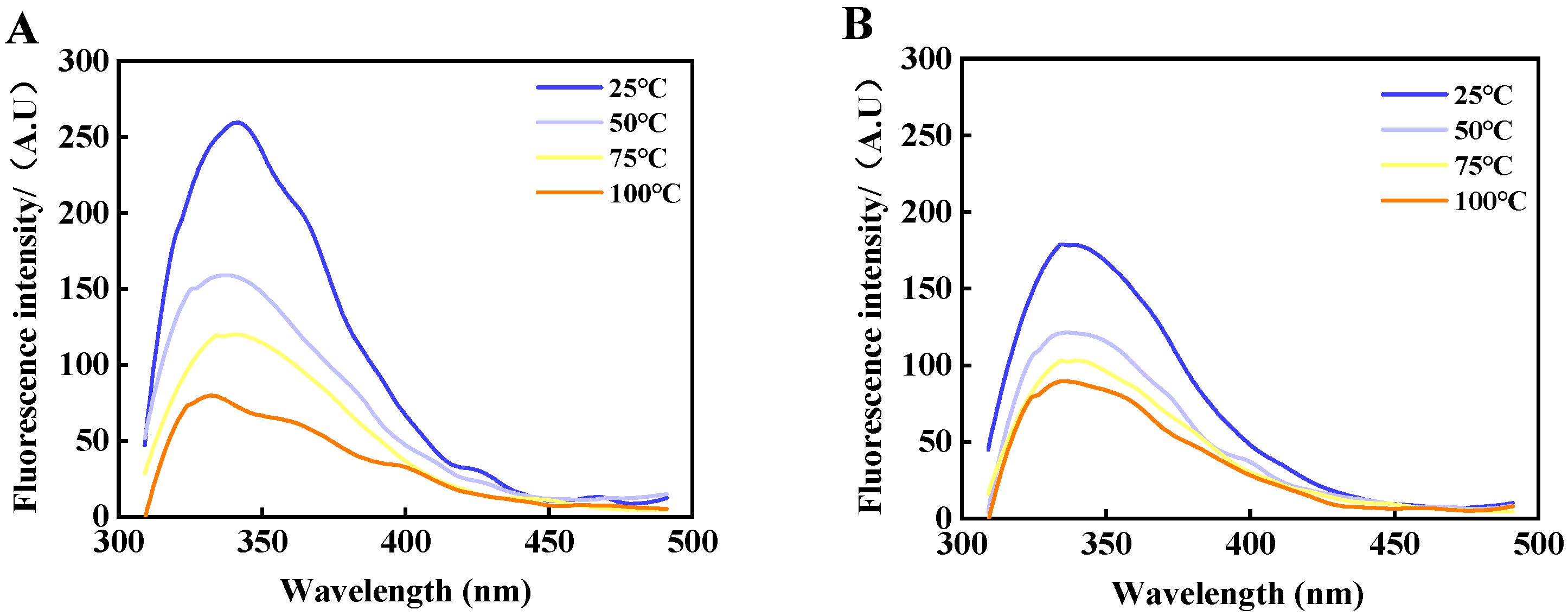
3.7. Intrinsic Fluorescence Spectra Analysis
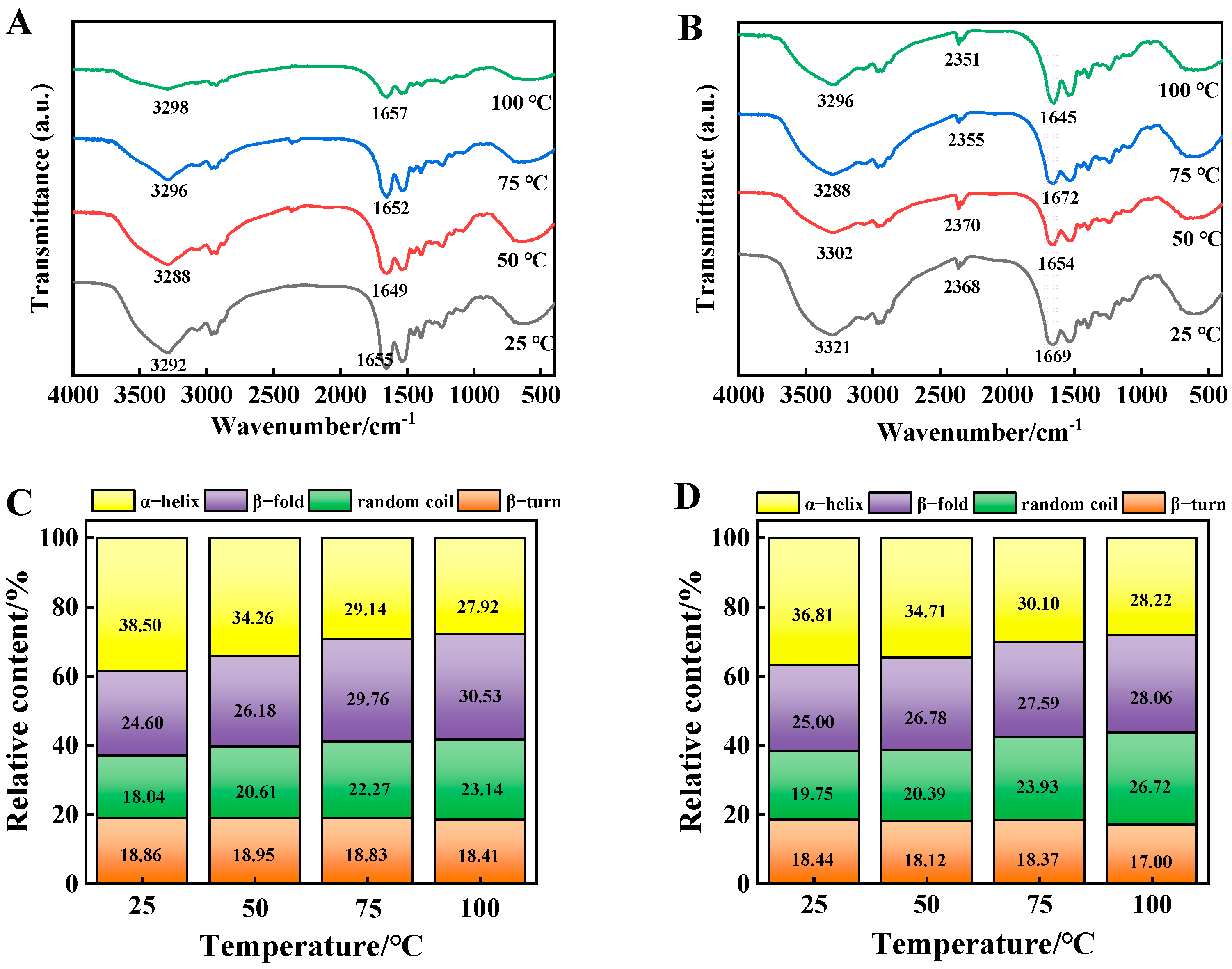
3.8. FT-IR Spectra Analysis
3.9. Secondary Structure Analysis
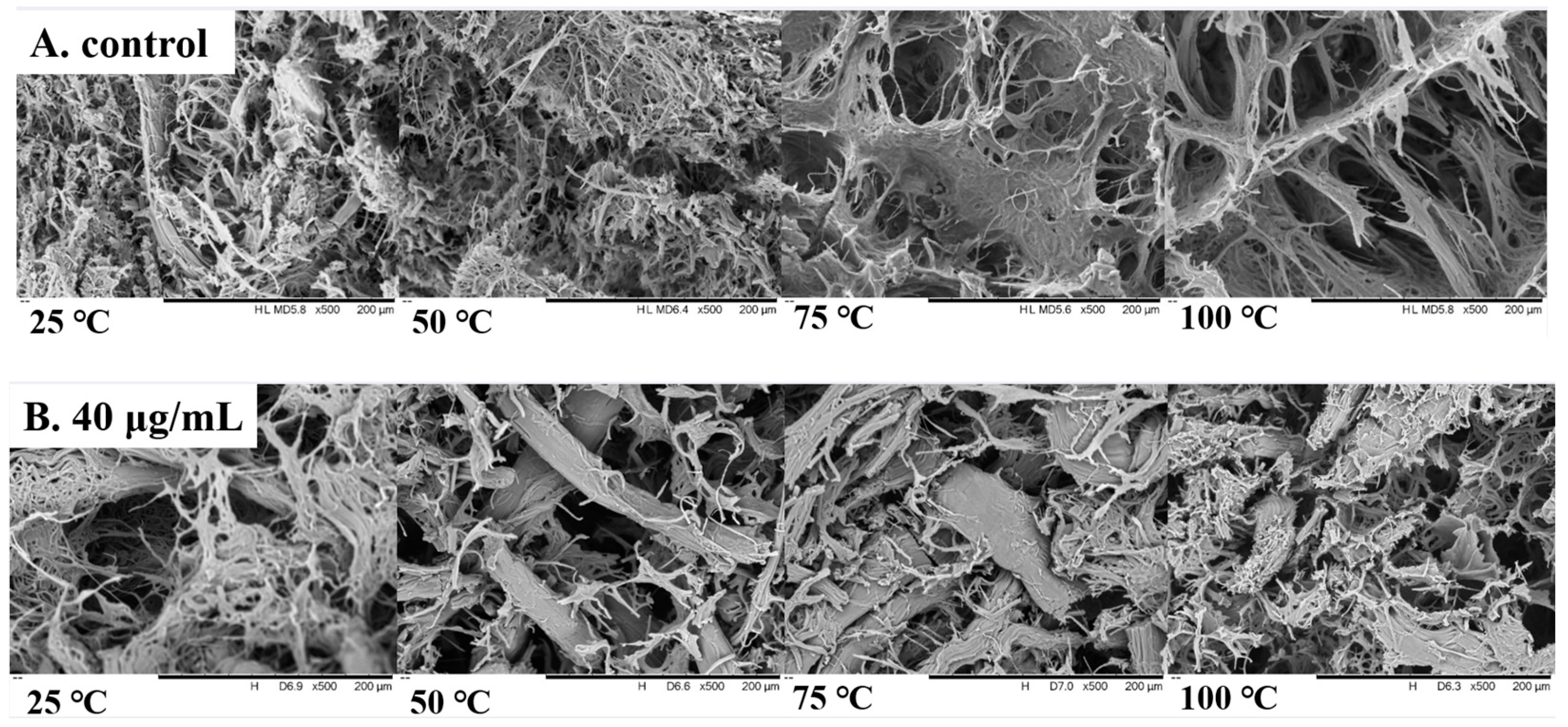
3.10. SEM Analysis
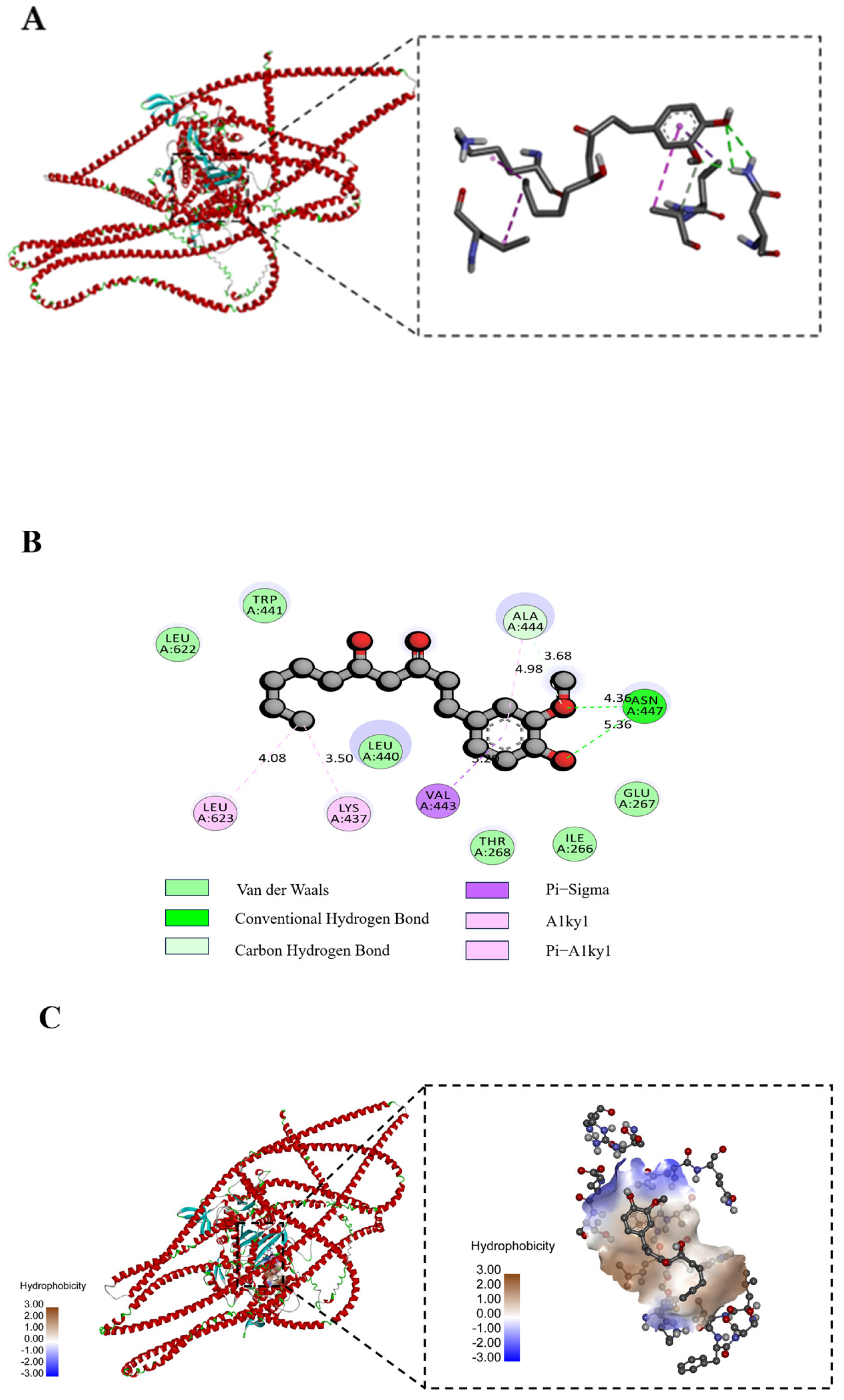
3.11. Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puolanne, E.; Halonen, M. Theoretical aspects of water-holding in meat. Meat Sci. 2010, 86, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Cercel, F.; Stroiu, M.; Alexe, P.; Ianiţchi, D. Characterization of Myofibrillar Chicken Breast Proteins for Obtain Protein Films and Biodegradable Coatings Generation. Agric. Agric. Sci. Procedia 2015, 6, 197–205. [Google Scholar] [CrossRef]
- Qiu, D.; Duan, R.; Wang, Y.; He, Y.; Li, C.; Shen, X.; LI, Y. Effects of different drying temperatures on the profile and sources of flavor in semi-dried golden pompano (Trachinotus ovatus). Food Chem. 2023, 401, 134112. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Li, X.; Hou, C.; Hussain, Z.; Zhang, D. Role of Heat-Shock Proteins in the Determination of Postmortem Metabolism and Meat Quality Development of DFD Meat. Foods 2024, 13, 2965. [Google Scholar] [CrossRef]
- Cullere, M.; Hoffman, L.C.; Dalle, Z.A. First evaluation of unfermented and fermented rooibos (Aspalathus linearis) in preventing lipid oxidation in meat products. Meat Sci. 2013, 95, 72–77. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Y.; Yu, J.; Cui, H.; Hayat, K.; Zhang, X.; Ho, C.T. Evolution of lean meat tenderness stimulated by coordinated variation of water status, protein structure and tissue histology during cooking of braised pork. Food Res. Int. 2023, 171, 113081. [Google Scholar] [CrossRef]
- Pang, B.; Yu, X.; Bowker, B.; Zhang, J.; Yang, Y.; Zhuang, H. Effect of meat temperature on moisture loss, water properties, and protein profiles of broiler pectoralis major with the woody breast condition. Poult. Sci. 2021, 100, 1283–1290. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Haniadka, R.; Saldanha, E.; Sunita, V.; Palatty, P.L.; Fayad, R.; Baliga, M.S. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe). Food Funct. 2013, 4, 845–855. [Google Scholar] [CrossRef]
- Kaur, I.P.; Deol, P.K.; Kondepudi, K.K.; Bishnoi, M. Anticancer Potential of Ginger: Mechanistic and Pharmaceutical Aspects. Curr. Pharm. Des. 2016, 22, 4160–4172. [Google Scholar] [CrossRef]
- Travičić, V.; Cvanić, T.; Vidović, S.; Pezo, L.; Hidalgo, A.; Šovljanski, O.; Ćetković, G. Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction. Foods 2024, 13, 2863. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gao, J.; Sun, X.; Du, J.; Wu, Z.; Liang, D.; Ling, C.; Fang, B. Immunomodulatory Mechanisms of Tea Leaf Polysaccharide in Mice with Cyclophosphamide-Induced Immunosuppression Based on Gut Flora and Metabolomics. Foods 2024, 13, 2994. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Zhao, B.; Wang, C.; Yi, S.; Xu, Y.; Li, J. Effect of 6-gingerol on physicochemical properties of grass carp (Ctenopharyngodon idellus) surimi fortified with perilla oil during refrigerated storage. J. Sci. Food Agric. 2017, 97, 4807–4814. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Mu, H.; Ren, G.; Ge, M.; Dong, J.; Wang, Q.; Sun, J. Effect of 6-gingerol on oxidative stability and quality characteristics of mutton meatballs during refrigerated storage. Food Chem. 2024, 24, 101865. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Q.; Long, P.; Wen, M.; Han, Z.; Granato, D.; Qi, J.; Zhang, L.; Zhu, M. Effects of green tea and its polyphenols on the formation of heterocyclic aromatic amines, antioxidant capacity, and quality characteristics of roasted pork patties. Food Chem. 2024, 4, 100606. [Google Scholar] [CrossRef]
- Zhang, M.; He, Z.; Qin, F.; Tao, G.; Zhang, S.; Gao, Y.; Chen, J. Inhibitory profiles of chilli pepper and capsaicin on heterocyclic amine formation in roast beef patties. Food Chem. 2016, 221, 404–411. [Google Scholar]
- Lopresti, F.; Capuana, E.; Serio, G.; Gentile, C.; Botta, L. Polylactic Acid/Bamboo Leaf Extract Electrospun Mats with Antioxidant Activity for Food Packaging Applications. Antioxidants 2024, 13, 1555. [Google Scholar] [CrossRef]
- Park, D.; Xiong, Y.L.; Alderton, A.L. Concentration effects of hydroxyl radical oxidizing systems on biochemical properties of porcine muscle myofibrillar protein. Food Chem. 2007, 101, 1239–1246. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Yongsawatdigul, J.; Park, J.W. Thermal denaturation and aggregation of threadfin bream actomyosin. Food Chem. 2003, 83, 409–416. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, R.; Wang, H.; Hua, C.; Song, S.; Zhou, G.; Zhang, W. Effects of Oxidation In Vitro on Structures and Functions of Myofibrillar Protein from Beef Muscles. J. Agric. Food Chem. 2019, 67, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.T.; Kauffman, R.G.; Kim, B.C.; Park, G.B. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999, 52, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Beliciu, C.M.; Moraru, C.I. The effect of protein concentration and heat treatment temperature on micellar casein-soy protein mixtures. Food Hydrocoll. 2011, 25, 1448–1460. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.M.; Xie, B.J.; Xiong, S.B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; Xiong, S.B.; Liu, Y.; Yin, T.; Hu, Y.; You, J. Effect of Mild Ozone Oxidation on Structural Changes of Silver Carp (Hypophthalmichthys molitrix) Myosin. Food Bioproc. Technol. 2017, 10, 370–378. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, Y.; Guo, J.; Feng, X.; Wang, X.; Wang, L.; Ma, J.; Sun, W. Low frequency magnetic field plus high pH promote the quality of pork myofibrillar protein gel: A novel study combined with low field NMR and Raman spectroscopy. Food Chem. 2020, 326, 126896. [Google Scholar] [CrossRef]
- Tang, L.; Hatab, S.; Yan, J.; Miao, W.; Nyaisaba, B.M.; Piao, X.; Zheng, B.; Deng, S. Changes in Biochemical Properties and Activity of Trypsin-like Protease (Litopenaeus vannamei) Treated by Atmospheric Cold Plasma (ACP). Foods 2022, 11, 1277. [Google Scholar] [CrossRef]
- He, Y.; Zhou, C.; Li, C.; Zhou, G. Effect of incubation temperature on the binding capacity of flavor compounds to myosin. Food Chem. 2021, 346, 128976. [Google Scholar] [CrossRef]
- Pacifici, R.E.; Kono, Y.; Davies, K.J. Hydrophobicity as the signal for selective degradation of hydroxyl radical-modified hemoglobin by the multicatalytic proteinase complex, proteasome. J. Biol. Chem. 1993, 268, 15405–15411. [Google Scholar] [CrossRef]
- Feng, X.; Wu, D.; Yang, K.; Wang, L.; Wang, X.; Ma, J.; Zhang, Y.; Wang, C.; Zhou, Y.; Sun, W. Effect of sarcoplasmic proteins oxidation on the gel properties of myofibrillar proteins from pork muscles. J. Food Sci. 2021, 86, 1835–1844. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Zhou, S.; Qian, H.; Zhang, H.; Qi, X.; Fan, M. Interaction between Vaccinium bracteatum Thunb. leaf pigment and rice proteins. Food Chem. 2016, 194, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein carbonyls in meat system: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Liu, L.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Study on the mechanism of mulberry polyphenols inhibiting oxidation of beef myofibrillar protein. Food Chem. 2022, 372, 131241. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhao, X.; Li, R.; Bassey, A.; Bai, Y.; Ye, K.; Deng, S.; Zhou, G. Synergistic effects of polysaccharide addition-ultrasound treatment on the emulsified properties of low-salt myofibrillar protein. Food Hydrocoll. 2022, 123, 107143. [Google Scholar] [CrossRef]
- Li, F.; Du, X.; Ren, Y.; Kong, B.; Wang, B.; Xia, X.; Bao, Y. Impact of ice structuring protein on myofibrillar protein aggregation behaviors and structural property of quick-frozen patty during frozen storage. Int. J. Biol. Macromol. 2021, 178, 136–142. [Google Scholar] [CrossRef]
- Wang, W.; Ma, S.; Shao, Q.; Yi, S. Effects of Soy Protein Isolate and Inulin Conjugate on Gel Properties and Molecular Conformation of Spanish Mackerel Myofibrillar Protein. Foods 2024, 13, 2920. [Google Scholar] [CrossRef]
- You, G.; Niu, G.; Gao, K.; Liu, X. Effects of hsian-tsao polysaccharide on myosin gel structure and its binding capacity to flavor compounds. Int. J. Biol. Macromol. 2024, 260, 129492. [Google Scholar] [CrossRef]
- Day, R.; García, A.E. Water penetration in the low and high pressure native states of ubiquitin. Proteins 2008, 70, 1175–1184. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Friess, W.; Lee, G. Basic thermoanalytical studies of insoluble collagen matrices. Biomaterials 1996, 17, 2289–2294. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Lv, Y.; Su, Y.; Chang, C.; Gu, L.; Yang, Y.; Li, J. Influence of konjac glucomannan on the emulsion-filled/non-filled chicken gel: Study on intermolecular forces, microstructure and gelling properties. Food Hydrocoll. 2021, 124, 107269. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, R.; Shui, S.; Yan, H.; Song, J.; Ying, X.; Benjakul, S.; Zhang, B. Comparative Analyses of Muscle Quality in Hooked, Trawl-Net, and Radar-Net Hairtail (Trichiurus haumela) During Thermal Processing. Foods 2024, 13, 3005. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.W.; Song, D.H.; Ham, Y.K.; Kim, T.K.; Choi, Y.S.; Kim, H.W. Interaction of Porcine Myofibrillar Proteins and Various Gelatins: Impacts on Gel Properties. Food Sci. Anim. Resour. 2019, 39, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Mallepally, R.R.; Chintakuntla, N.; Putta, V.R.; K, N.; Vuradi, R.K.; P, M.; Chitumalla, R.K.; Jang, J.; Penumaka, N.; Sirasani, S. Synthesis, spectral properties and DFT calculations of new ruthenium (II) polypyridyl complexes; DNA binding affinity and in vitro cytotoxicity activity. J. Fluoresc. 2017, 27, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Liu, X.; Xie, P.; Han, A.; Lei, Y.; Yang, X.; Liu, Y.; Zhang, S.; Sun, B. Investigating the interactions between selected heterocyclic flavor compounds and beef myofibrillar proteins using SPME-GC–MS, spectroscopic, and molecular docking approaches. J. Mol. Liq. 2024, 403, 124878. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, C.; Qi, J.; Zhao, X.; Yang, H.; Ye, G.; Zhang, M.; Liu, D. Effect of ultrasound treatment on porcine myofibrillar protein binding furan flavor compounds at different salt concentrations. Food Chem. 2024, 443, 138427. [Google Scholar] [CrossRef]
- Cao, Y.; True, A.D.; Chen, J.; Xiong, Y.L. Dual Role (Anti- and Pro-Oxidant) of Gallic Acid in Mediating Myofibrillar Protein Gelation and Gel In Vitro Digestion. J. Agric. Food Chem. 2016, 64, 3054–3061. [Google Scholar] [CrossRef]
| Temperature/°C | Ionic Bonds/% | Hydrogen Bonds/% | Disulfide Bonds/% | Hydrophobic Interactions/% |
|---|---|---|---|---|
| 25 | 35.12 ± 0.23 a | 29.40 ± 0.77 a | 8.78 ± 0.37 d | 26.70 ± 0.90 b |
| 50 | 32.10 ± 0.39 b | 26.62 ± 0.34 b | 17.09 ± 0.51 c | 25.19 ± 0.17 b,c |
| 75 | 26.58 ± 0.38 c | 24.42 ± 0.73 c | 22.20 ± 0.77 b | 26.80 ± 0.47 b |
| 100 | 20.98 ± 0.16 d | 18.86 ± 0.42 d | 27.50 ± 0.90 a | 32.66 ± 0.76 a |
| Temperature/°C | Ionic Bonds/% | Hydrogen Bonds/% | Disulfide Bonds/% | Hydrophobic Interactions/% |
|---|---|---|---|---|
| 25 | 36.61 ± 0.48 a | 28.58 ± 1.07 a | 9.46 ± 0.5 d | 25.35 ± 1.42 a,b |
| 50 | 34.01 ± 0.16 b | 28.16 ± 0.71 a,b | 11.72 ± 0.52 c | 26.11 ± 0.86 a |
| 75 | 33.80 ± 0.45 b | 25.38 ± 1.51 b | 15.92 ± 0.82 a,b | 24.90 ± 0.92 b |
| 100 | 31.57 ± 0.12 c | 25.19 ± 0.63 b | 16.88 ± 1.48 a | 26.36 ± 0.77 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Xie, A.; Wu, H.; Zhang, K.; Dong, S.; Liu, Y. Effect of 6-Gingerol on Oxidation and Structure of Beef Myofibrillar Protein During Heating. Foods 2025, 14, 1081. https://doi.org/10.3390/foods14071081
Bai R, Xie A, Wu H, Zhang K, Dong S, Liu Y. Effect of 6-Gingerol on Oxidation and Structure of Beef Myofibrillar Protein During Heating. Foods. 2025; 14(7):1081. https://doi.org/10.3390/foods14071081
Chicago/Turabian StyleBai, Ruhong, Anguo Xie, Han Wu, Kun Zhang, Shubei Dong, and Yunhong Liu. 2025. "Effect of 6-Gingerol on Oxidation and Structure of Beef Myofibrillar Protein During Heating" Foods 14, no. 7: 1081. https://doi.org/10.3390/foods14071081
APA StyleBai, R., Xie, A., Wu, H., Zhang, K., Dong, S., & Liu, Y. (2025). Effect of 6-Gingerol on Oxidation and Structure of Beef Myofibrillar Protein During Heating. Foods, 14(7), 1081. https://doi.org/10.3390/foods14071081





