Abstract
This study employed a multi-technique approach to investigate the structural and conformational changes in proteins in Meretrix lyrata (M. lyrata) adductor, foot, and siphon tissues during boiling. Data-independent acquisition (DIA) quantitative proteomics was utilized to identify differentially expressed proteins (DEPs) in six temporal comparison groups (20–0 s, 40–20 s, 60–40 s, 80–60 s, 100–80 s, and 120–100 s). The results showed that key myofibrillar proteins, including myosin heavy chain, paramyosin, and actin, exhibited tissue-specific expression patterns, while low-molecular-weight degradation fragments (<17 kDa) appeared with prolonged heating. Turbidity measurements peaked in adductor and siphon tissues at 60 s and in foot tissue at 80 s. Heating resulted in a narrowed particle size distribution (100–1000 nm), and a decreased zeta potential, indicating a reduction in protein surface charge. Fourier transform infrared spectroscopy revealed hydrogen bond disruption and secondary structure transitions, marked by a reduction in α-helix content with a corresponding increase in β-sheet and random coil structures. In total, 6527 proteins were identified, and Gene Ontology (GO) enrichment analysis highlighted the DEPs’ involvement in biological regulation and metabolic processes. Collectively, these results provide comprehensive characterization of protein denaturation, degradation, and structural reorganization in M. lyrata tissues during the boiling process.
1. Introduction
Hard clam proteins exhibit high digestibility and biological value due to their essential amino acid (EAA) composition, which closely aligns with the EAA requirement pattern recommended by the World Health Organization (WHO) [1]. Meretrix lyrata (M. lyrata) is an edible clam distributed throughout the Indo-West Pacific region, including the intertidal flats of China, Indonesia, and the Philippines. This species is of considerable commercial importance due to its favorable nutritional profile, with muscle protein content of 34.15% [2]. As such, M. lyrata represents a valuable and significant source of dietary protein.
Clam muscle tissues exhibit structural and functional heterogeneity, comprising striated, obliquely striated, and smooth muscle types [3]. In the Asian hard clam (Meretrix lusoria), myofibrillar proteins constitute the predominant protein fraction in the foot and mantle tissues [4]. The amino acid profile of Paphia undulata, which includes 21 amino acids, was reported to be comparable to those of grass carp (Ctenopharyngodon idella) and shrimp (Metapenaeus ensis), suggesting compositional parallels among aquatic species [5].
Boiling, a common thermal processing method, is known to induce extensive protein denaturation and degradation. For instance, heating surf clam (Spisula sachalinensis) at 80 °C would alter its microstructure and modify the proteins’ secondary and tertiary structures [6,7]. Additionally, boiling could enhance the nutritional value, digestibility, and sensory characteristics of aquatic products. High water content promotes heat penetration and facilitates protein denaturation during thermal processing [8]. As heating progresses, the hydrogen bonds and electrostatic interactions weaken, exposing the hydrophobic groups in proteins [7], and thereby altering their functional properties. However, there is a lack of research investigating on the protein structural dynamics in M. lyrata during cooking.
Data-independent acquisition (DIA) quantitative proteomics is a proteomic technology that focuses on comprehensive protein detection and quantification. It is a powerful method that has been used extensively for proteomic studies in aquatic animals, including silver carp (Hypophthalmichthys molitrix), Antarctic krill (Euphausia superba), and razor clam (Sinonovacula constricta) [9,10,11]. Hence, DIA quantitative proteomics provides an effective platform for characterizing protein changes in aquatic animals during processing.
In this study, we aim to investigate the protein dynamics in M. lyrata during boiling. Specimens were boiled for 20, 40, 60, 80, 100, and 120 s to obtain samples at different time points. Protein structural characterization was performed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), turbidity, surface hydrophobicity, particle size, zeta potential, and Fourier transform infrared spectroscopy (FTIR). Subsequently, DIA quantitative proteomics was employed to identify differentially expressed proteins (DEPs) and to elucidate changes in protein composition throughout the boiling process. The integration of structural and proteomic analyses provides a comprehensive understanding of heat-induced protein dynamics in M. lyrata. This study not only addresses the current knowledge gap regarding the effects of boiling on M. lyrata proteins, but also provides essential insights into optimizing thermal processing strategies in shellfish, with the aim of preserving nutritional and sensory quality.
2. Materials and Methods
2.1. Materials and Chemicals
Live clams (M. lyrata) with a mean weight of 13.90 ± 1.79 g were purchased from the Nanxin Farmers’ Market in Sanya, China. The reagents 8-Anilino-1-naphthalenesulfonic acid (ANS) and potassium bromide (spectroscopic-grade) were obtained from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). HPLC-grade formic acid and acetonitrile were obatined from Sigma-Aldrich (St. Louis, MO, USA). The Bradford protein assay kit was purchased from Takara Biomedical Technology (Beijing) Co., Ltd. (Beijing, China). The BeyoGel™ Elite pre-cast PAGE gels (Tris-Gly, 12%) and 5× SDS-PAGE sample loading buffer were purchiased from Beyotime Institute of Biotechnology (Shanghai, China). The ColorMixed Protein Marker (11–245 kDa) was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). All other chemicals were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Sample Preparation
Live M. lyrata clams were boiled for 20 s, 40 s, 60 s, 80 s, 100 s, and 120 s in boiling water and then immediately transferred to an ice-water bath. Adductor muscle, foot, and siphon tissues were collected from both live and boiled clam samples for comparative analyses. All tissue samples were subsequently lyophilized. For each treatment group, tissues from six clams were pooled, mixed, and homogenized to obtain a composite experimental sample.
2.3. Determination of SDS-PAGE Pattern
Protein profiling was carried out based on a modified method described by Shang et al. [12]. In brief, 0.5 g of lyophilized tissue powder was mixed with 9 mL of solubilization buffer containing 0.1 M Tris-HCl (pH 8.0), 8 M urea, 4% SDS, 2% mercaptoethanol, and 20% glycerol. The mixture was boiled for 10 min and sonicated for 2 min to disrupt tissue structure. After centrifugation at 16,000× g for 10 min, an aliquot of 8 μL of the supernatant was subjected to SDS-PAGE using a 4% (w/v) stacking gel and a 12% (w/v) separating gel on a Bio-Rad electrophoresis system (Bio-Rad, Hercules, CA, USA). After electrophoresis, the gel was stained overnight at room temperature with 0.05% Coomassie Brilliant Blue R-250 in 50% ethanol and 7.5% acetic acid. The gel was then destained with a solution of 50% ethanol and 9% acetic acid, rinsed thoroughly with distilled water, and imaged immediately.
2.4. Determination of Turbidity
Turbidity was measured according to a modified method reported by Cai et al. [13]. Protein samples were suspended in 0.2 mol/L phosphate buffer (pH 7.0) and centrifuged at 4000× g for 15 min at 4 °C. The protein concentration in the supernatant was quantified using a Bradford Protein Assay Kit, and all samples were subsequently adjusted to an equivalent protein concentration. For turbidity measurements, a 3 mL aliquot of each solution was transferred to a quartz cuvette with a 1 cm path length. Absorbance was recorded at 660 nm using a UV-Vis spectrophotometer (Hitachi, U-2700, Tokyo, Japan) with phosphate-buffered saline (PBS) serving as the blank control.
2.5. Determination of Surface Hydrophobicity (H0)
Surface hydrophobicity was determined as described by Shang et al. [12]. Protein solutions (0.0002–0.0005 mg/mL) were prepared by dissolving samples in 10 mM phosphate buffer (pH 7.0). To each solution, 20 μL of 8 mmol/L ANS in 10 mmol/L phosphate buffer (pH 7.0) was added. Fluorescence intensity was then measured using a spectrofluorometer (Cary Eclipse, Agilent, Santa Clara, CA, USA) at excitation and emission wavelengths of 390 nm and 470 nm, respectively. The H0 was calculated from the slope of the fluorescence intensity versus protein concentration plot (R2 > 0.95).
2.6. Determination of Particle Size and Zeta Potential
Particle size and zeta potential were measured according to the method by Liu et al. [14] with minor modifications. Briefly, 0.2 g of lyophilized protein powder was dissolved in 20 mL of 10 mM phosphate buffer (pH 7.0). The mixture was stirred for 1 h and centrifuged at 4000× g for 15 min at 4 °C. The resulting supernatant was diluted to a protein concentration of 0.5 mg/mL, and a 1 mL aliquot was transferred to a measurement cuvette. Particle size and zeta potential were then determined using a particle size analyzer (Zetasizer Pro, Malvern, Worcestershire, UK) at 25 °C following a 120 s equilibration time.
2.7. Determination of Secondary Structure
The FTIR spectra of M. lyrata adductor, foot, and siphon proteins were recorded using a modified method described by Agbaje et al. [15]. Briefly, 2 mg of lyophilized tissue powder was ground thoroughly with 200 mg of spectroscopic-grade KBr. The resulting mixture was then compressed into a pellet and analyzed using an FTIR spectrometer (ALPHA II, Bruker, Billerica, MA, USA). Spectra processing included Fourier self-deconvolution and second-derivative analysis within the Amide I region (1700–1600 cm−1). PeakFit software (version 4.12, Systat Software, Inc., Richmond, VA, USA) was used to quantify the relative proportions of α-helix, β-sheet, β-turn, and random coil structures based on peak area integration [16].
2.8. Proteomics of Proteins in M. lyrata Clam During the Boiling Process
2.8.1. Protein Digestion
Protein digestion was performed in accordance with the Filter-Aided Sample Preparation (FASP) method described by Wiśniewski et al. [17]. First, samples were mixed with an equal volume of 100 mM dithiothreitol (DTT) and boiled for 5 min. After cooling to room temperature, the mixtures were transferred to 10 kDa ultrafiltration centrifuge tubes (Millipore Amicon Ultra-0.5, Billerica, MA, USA) and were washed twice with 200 μL of uric acid (UA) buffer (8 M urea, 150 mM Tris-HCl, pH 8.0) by centrifugation at 12,000× g for 15 min, with the supernatant discarded after each wash. The retentate was then incubated with 100 μL of 50 mM iodoacetamide (IAA) in UA buffer for 20 min in the dark with gentle shaking (600 rpm), before being centrifuged at 12,000× g for 30 min. Subsequently, the retentate was washed twice with 100 μL of UA buffer and centrifuged at 12,000× g for 10 min each time. The retentate was then resuspended in 100 μL of 50 mM NH4HCO3 buffer and centrifuged at 14,000× g for 10 min, and this wash step was repeated twice.
Protein hydrolysis was performed by resuspending the retentate in 40 μL of trypsin solution (6 μg trypsin in 40 μL of 50 mM NH4HCO3 buffer) with shaking at 600 rpm for 1 min, followed by incubation at 37 °C for 16–18 h. The resulting peptide supernatant was collected by centrifugation at 12,000× g for 10 min at 25 °C. The supernatant was acidified with trifluoroacetic acid (TFA) to a final concentration of 0.1%, desalted using a Sep-Pak C18 cartridge (Waters, Milford, MA, USA), and dried in a vacuum concentrator. The resulting peptide powder was reconstituted in 20 μL of 0.1% formic acid (FA) for Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) analysis.
2.8.2. LC-MS/MS Analysis for DIA
Peptide samples were analyzed according to Jiao et al. [9] with minor modifications. Separation was performed on a Vanquish Neo Ultra-High Performance Liquid Chromatography (UHPLC) system coupled to an Orbitrap Astral mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). Peptides were loaded onto a 50 cm µPAC™ Neo column (Thermo Fisher Scientific, Bremen, Germany) at a flow rate of 2.2 μL/min. The reversed-phase High-Performance Liquid Chromatography (RP-HPLC) mobile phases consisted of: Phase A, 0.1% FA; and Phase B, 0.1% FA in 80% acetonitrile. The linear gradient program was as follows: 0–0.1 min, 4–6% B; 0.1–1.1 min, 6–12% B; 1.1–4.3 min, 12–25% B; 4.3–6.1 min, 25–45% B; 6.1–6.5 min, 45–99% B; 6.5–8.0 min, 99% B.
Data acquisition was performed in DIA mode using Xcalibur 7.5.1 (Thermo Fisher Scientific, Bremen, Germany). The acquisition parameters were: total run time, 8 min; spray voltage, 2.2 kV; polarity, positive; MS1 scan range, 380–980 m/z. MS1 resolution was set to 240,000 at m/z 200 with an automatic gain control (AGC) target of 500% and a maximum injection time of 3 ms. The MS2 resolution was set to 80,000 with an AGC target of 500%, maximum injection time 0.6 s, RF lens at 40%, higher-energy collisional dissociation (HCD) activation, a 2 Th isolation window, and normalized collision energy of 25%. Both MS1 and MS2 spectra were acquired in profile mode.
2.8.3. DIA Sequence Database Searching
The DIA data processing and protein quantification were performed using DIA-NN 1.8.1 according to approaches as described by Demichev et al. [18] and Barkovits et al. [19]. The MS data were searched against the verified protein sequences of M. lyrata in the UniProtKB/Swiss-Prot database (https://www.expasy.org/resources/uniprotkb-swiss-prot; accessed on 1 March 2025). The database search parameters were as follows: (1) a maximum of one missed cleavage site; (2) precursor mass tolerance of 10 ppm; (3) fragment mass tolerance of 10 ppm. Trypsin was specified as the digestion enzyme. carbamidomethylation (C) was set as a fixed modification, while N-terminal acetylation and oxidation (M) were set as variable modifications with a maximum of 1 variable modification allowed per peptide. Peptide length was set to 7–30 amino acids, and charge states +1 to +4 were considered. Fragment ion m/z range was set to 150–2000 in centroid mode. Search results were filtered to a 1% false discovery rate (FDR)at both peptide-spectrum match (PSM) and protein levels. All procedures followed established proteomics protocols without specific technical standard citations.
2.9. Bioinformatic and Statistical Analysis
Six comparison groups (20–0 s, 40–20 s, 60–40 s, 80–60 s, 100–80 s and 120–100 s) were analyzed for protein quantification. The DEPs were defined as those showing a fold change (FC) > 1.5 or <0.67 combined with Student’s t-test p < 0.05 [9]. Statistical analyses were performed using R version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria) and Microsoft Excel 2019 (Microsoft Corporation, Redmond, WA, USA). Hierarchical clustering and volcano plots were generated using R software. Protein sequences were annotated against the UniProtKB/Swiss-Prot database (https://www.expasy.org/resources/uniprotkb-swiss-prot; accessed on 1 March 2025) and Gene Ontology (GO) (http://geneontology.org; accessed on 1 March 2025) resource [20]. The GO enrichment analysis was performed using Fisher’s exact test with the entire identified proteome as background, followed by Benjamini–Hochberg false discovery rate (FDR) correction. Enriched GO terms were considered significant at an FDR-adjusted p < 0.01.
All experiments were performed using three biological replicates, and the results are expressed as mean ± standard deviation (SD). Statistical significance was determined by one-way ANOVA followed by Duncan’s multiple range test (p < 0.05) using IBM SPSS Statistics version 27.0.0 (IBM Corporation, Armonk, NY, USA).
3. Results and Discussion
3.1. Changes in SDS-PAGE Pattern
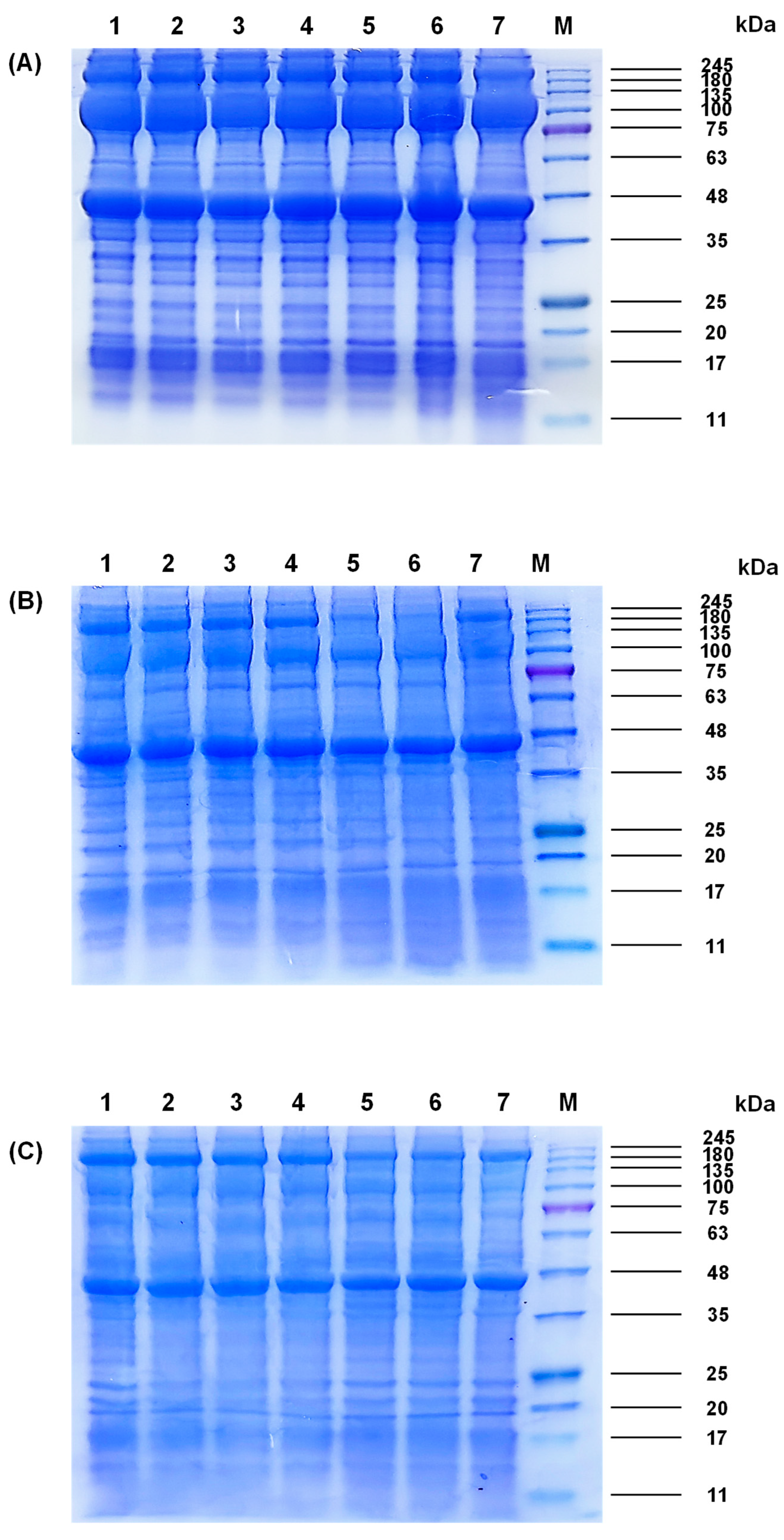
Protein profiles of M. lyrata adductor, foot, and siphon tissues are shown in Figure 1. Myosin heavy chain (MHC, ~200 kDa) and actin (~42 kDa) were ubiquitously expressed across all three tissues, whereas PA (~100 kDa) exhibited tissue-specific distribution, being abundant in adductor and foot but barely detectable in the siphon tissues (Figure 1).
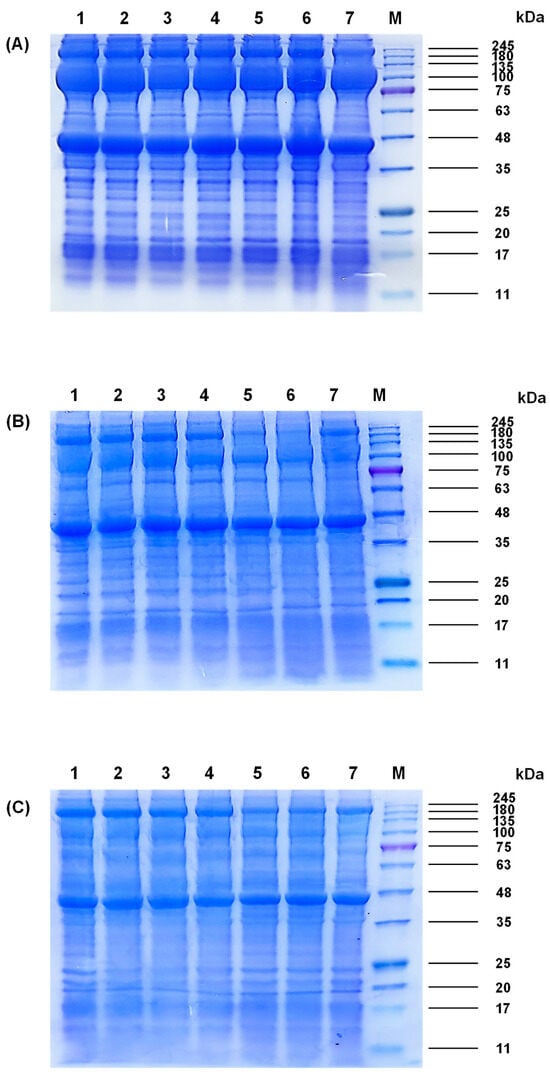
Figure 1.
SDS-PAGE patterns of different fractions from the (A) adductor, (B) foot, and (C) siphon of M. lyrata. Samples in lanes 1, 2, 3, 4, 5, 6, and 7 represented boiling times of 0 s, 20 s, 40 s, 60 s, 80 s, 100 s and 120 s, respectively. M denoted the molecular weight standard.
SDS-PAGE analysis revealed that MHC from M. lyrata adductor, foot, and siphon migrated at ~200 kDa (Figure 1), consistent with the reported MHC molecular weight of ~210 kDa in baby clam (Paphia undulata) [4]. Paramyosin (PA) was detected at ~100 kDa in all tissues, which is aligned with observations in the Asian hard clam (Meretrix lusoria) where PA similarly migrates at ~100 kDa [4]. Actin (~45 kDa) was ubiquitously expressed across all tissues (Figure 1), consistent with results reported in Mactra chinensis tissue [21].
Quantitative densitometry further revealed tissue-specific expression levels: band intensities of MHC and actin were highest in the adductor, followed by the foot, and were least abundant in the siphon (Figure 1). Low-molecular-weight fragments (<17 kDa) emerged after boiling the adductor for 100–120 s (Figure 1A) and after 80–120 s for the foot and siphon (Figure 1B,C), indicating a progressive protein degradation.
Shellfish proteins generally consist of water-soluble, salt-soluble, and insoluble fractions. Salt-soluble proteins such as MHC, myosin light chain (MLC), actin, and tropomyosin typically contribute to muscle contraction. Previous studies on limpet (Patella vulgata) and M. lusoria have revealed variations in water-soluble and salt-soluble protein composition [22,23], which may be attributed to muscle functions and structural organization. A well-balanced distribution of protein types is essential for optimal contraction and metabolism in molluscan muscles [24]. Furthermore, the major water-soluble proteins in the scallop adductor muscle are reported to be mainly distributed in the range between 10 and 250 kDa [25]. During boiling, thermal-induced protein denaturation in M. lyrata tissues led to decreased protein solubility associated with structural alterations. Similar reductions in tissue solubility caused by leaching of water-soluble proteins have been documented in Asiatic hard clam (Meretrix meretrix) during heat treatment [26].
According to Tang et al. [23], the insoluble protein fractions in M. lusoria exhibit molecular weights ranging from 44.3 to 200 kDu, which affects their solubility in the loading buffer. Therefore, the bands observed in lanes 5 and 6 of Figure 1B, migrating at approximately 200 kDa, are most likely attributable to these insoluble proteins. This interpretation is further supported by their reduced band intensities, which suggested low protein solubilities. These findings indicate that the thermal treatment has altered the protein composition. Taken together, boiling-induced protein degradation and denaturation contributed to structural changes in M. lyrata proteins.
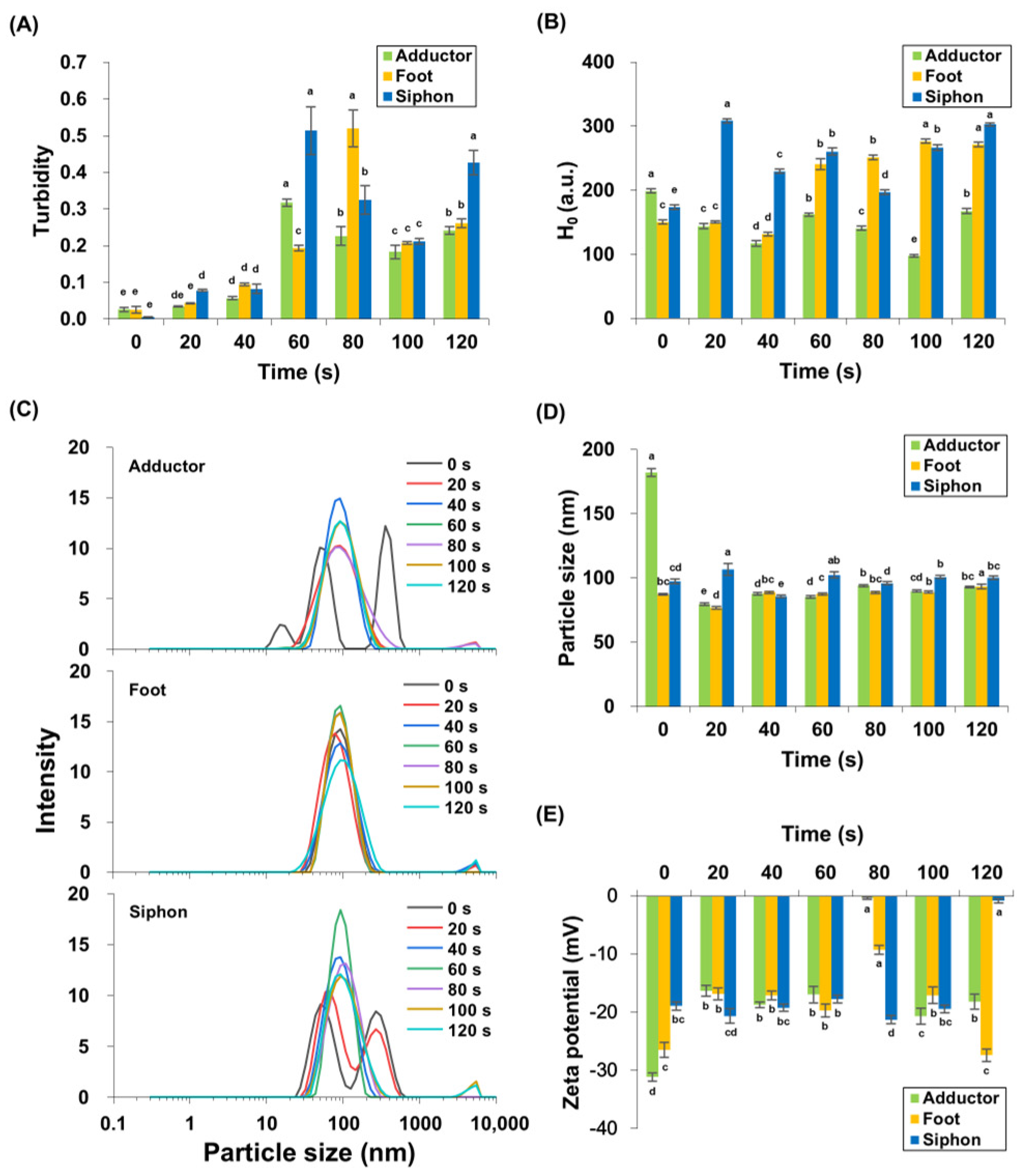
3.2. Changes in Protein Turbidity
Protein turbidity of M. lyrata adductor, foot, and siphon tissues varied during boiling (Figure 2A). Throughout the boiling process, turbidity exhibited a significant (p < 0.05) triphasic pattern, characterized by an initial increase, followed by a decrease, and a subsequent rise. Baseline turbidity was lowest in unheated samples, reflecting minimal protein aggregation. After 60 s of boiling, the adductor and siphon tissues achieved peak protein turbidity values of 0.32 ± 0.01 and 0.51 ± 0.07, respectively, with no significant difference in siphon turbidity between 60 s and 120 s (p > 0.05). In contrast, the foot tissues exhibited a maximum protein turbidity of 0.52 ± 0.05 after 80 s of boiling. These findings are consistent with observations by Dong et al. [27], who found that the turbidity of scallop (Patinopecten yessoensis) actomyosin solution increased upon heating from 20 to 90 °C. Similarly, Reed and Park [28] reported that the turbidity of tilapia myosin increased at 39 °C, plateaued, and stabilized between 70 and 90 °C. In M. lyrata, the initial turbidity increase likely reflects heat-induced protein unfolding and aggregation, while the subsequent decrease after 60–80 s (Figure 2A) may result from flocculation and coalescence of protein aggregates [28]. Results from SDS-PAGE analysis confirmed thermal degradation across all tissues (Figure 1), with protein bands ranging from 25–210 kDa contributing to the sample turbidity (Figure 1). The formation of high-molecular-weight aggregates during boiling further increased the turbidity. Akihiro et al. [29] identified a 33 kDa protein in the boiled soup of bloody clam (Anadara broughtonii), Asiatic hard clam (Meretrix lusoria), Mediterranean mussel (Mytilus galloprovincialis), and other similar species, supporting the notion that protein aggregation during thermal processing is a common phenomenon in bivalve tissues.
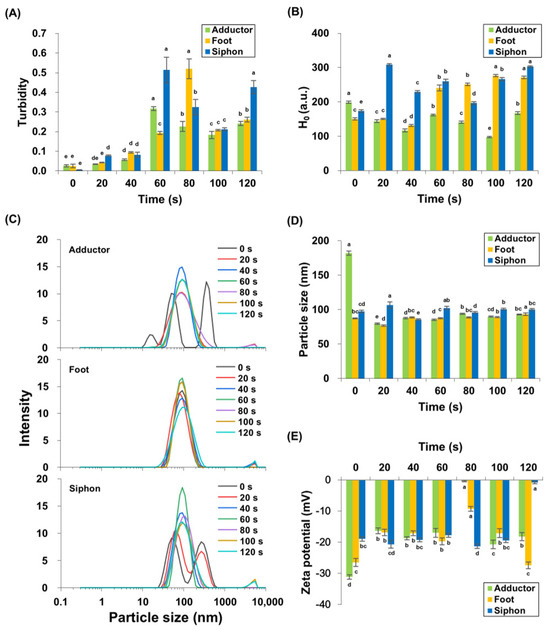
Figure 2.
Turbidity (A), surface hydrophobicity (B), particle size distribution (C,D), and zeta potential (E) were measured in M. lyrata adductor, foot, and siphon tissues during the boiling process. Different letters indicate significant differences (p < 0.05).
3.3. Changes in Surface Hydrophobicity (H0)
Surface hydrophobicity was measured to evaluate protein conformation, as indicated by exposed hydrophobic group content. Figure 2B shows that M. lyrata adductor, foot, and siphon tissues exhibited dynamic changes in surface hydrophobicity during boiling. The H0 in M. lyrata foot tissue significantly increased from 131.70 ± 3.53 to 271.64 ± 2.63 between 40 s and 120 s (p < 0.05). Similarly, H0 in siphon samples rose significantly from 80 to 120 s of boiling (p < 0.05), while adductor H0 showed a significant increase at 100 s and 120 s (p < 0.05). Overall, H0 increased significantly in all tissues by the end of boiling (p < 0.05).
These observations are consistent with trends reported in ready-to-eat shrimp boiled at 121 °C for 0, 2, 4, and 6 min [30]. The distinct protein compositions of M. lyrata adductor, foot, and siphon tissues (Figure 1) likely contributed to the tissue-specific changes in surface hydrophobicity. During boiling, protein unfolding exposed buried hydrophobic groups, increasing H0, whereas self-assembly and aggregation partially buried these groups, reducing H0 [31,32]. As boiling time progressed, conformational alterations and other structural modifications redistributed hydrophobic groups on the protein surface, resulting in the observed dynamic changes in H0.
3.4. Changes in Particle Size and Zeta Potential
Particle size distribution reflected the degree of protein aggregation and cross-linking. Figure 2C,D show that the particle size distributions and volume-weighted mean diameters for boiled M. lyrata adductor, foot, and siphon tissues were mainly distributed in the 100–1000 nm range, while untreated samples of M. lyrata adductor and siphon tissues exhibited a relatively broad particle size distribution. The fresh adductor exhibited a trimodal particle size distribution, peaking at 182.23 ± 3.05 nm, whereas both fresh and 20 s boiled siphon samples showed bimodal distributions. Boiling narrowed the distribution to a single peak at ~150 nm (Figure 2C). These results are in agreement with the findings by Chen et al. [33], which indicated that untreated, aggregated tropomyosin exhibited the largest particle size among samples. Since high-temperature cooking can simultaneously induce both protein aggregation and particle size reduction [34,35], the observed decreases in peak intensity and particle size of M. lyrata adductor and siphon tissues are thus likely to reflect molecular structural changes during boiling, resulting in altered protein dimensions. In contrast, foot tissue displayed a single peak (Figure 2C), indicating relatively stable protein particle size throughout boiling. Low-intensity peaks in the 1000–10,000 nm range were also detected, which may be due to aggregation of small particles and protein crosslinking, as reported for Antarctic krill proteins [32].
Zeta potential describes the distribution of net charge on the protein surface, with higher absolute values indicating stronger electrostatic repulsions between particles [36]. Figure 2D shows the zeta potential variations in M. lyrata adductor, foot, and siphon protein particles during boiling. Our results indicate that the zeta potential of adductor and foot protein particles initially increased and then decreased, reaching minimum absolute zeta potentials of −0.39 ± 0.19 mV and −9.26 ± 0.79 mV, respectively, at 80 s. In siphon tissues, the zeta-potential of the proteins decreased, from approximately −20 mV to −0.8 ± 0.36 mV (Figure 2D) following a 120 s boiling treatment. These trends are similarly reported for bighead carp myosin, whose zeta potential decreases with prolonged boiling time [37]. The negative zeta potential is primarily attributed to the exposure of anionic amino acid residues on its surface [38], and may also reflect the myosin content in M. lyrata tissues. As reported by Sun et al. [39], protein dispersions generally achieve greater stability when the absolute zeta potential value exceeds 20 mV. However, as thermal treatment induces the aggregation of myosin particles, it may obscure the negatively charged amino acid side chains and alter the protein surface charge, ultimately leading to a reduction in the absolute zeta potential value [40,41].
3.5. Changes in Secondary Structure
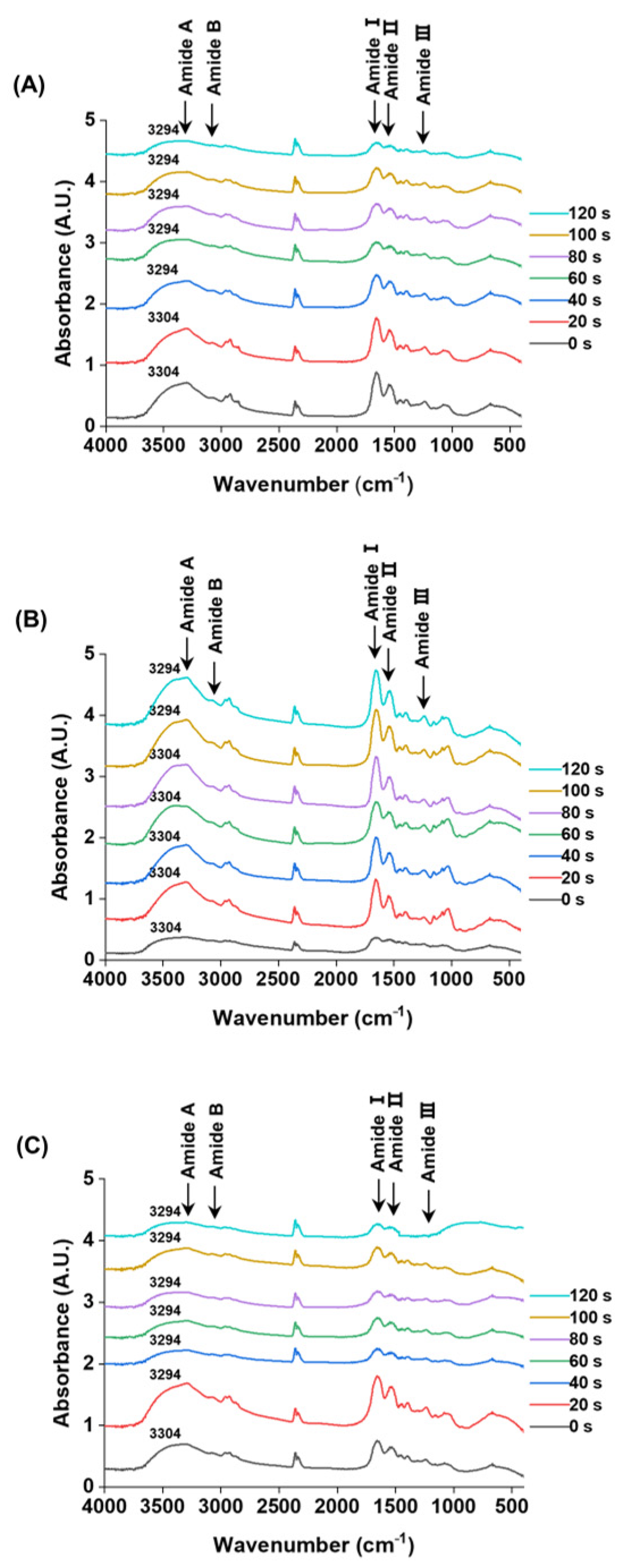
Fourier-transform infrared (FTIR) spectroscopy is a technique that probes molecular vibrations through infrared absorption, allowing the identification of functional groups based on their characteristic vibrational modes [42]. The FTIR spectra of M. lyrata adductor, foot, and siphon tissues are presented in Figure 3, providing insights into their protein secondary structures. Comparison of SDS-PAGE profiles revealed no major changes in protein band patterns between treated and untreated samples (Figure 1).
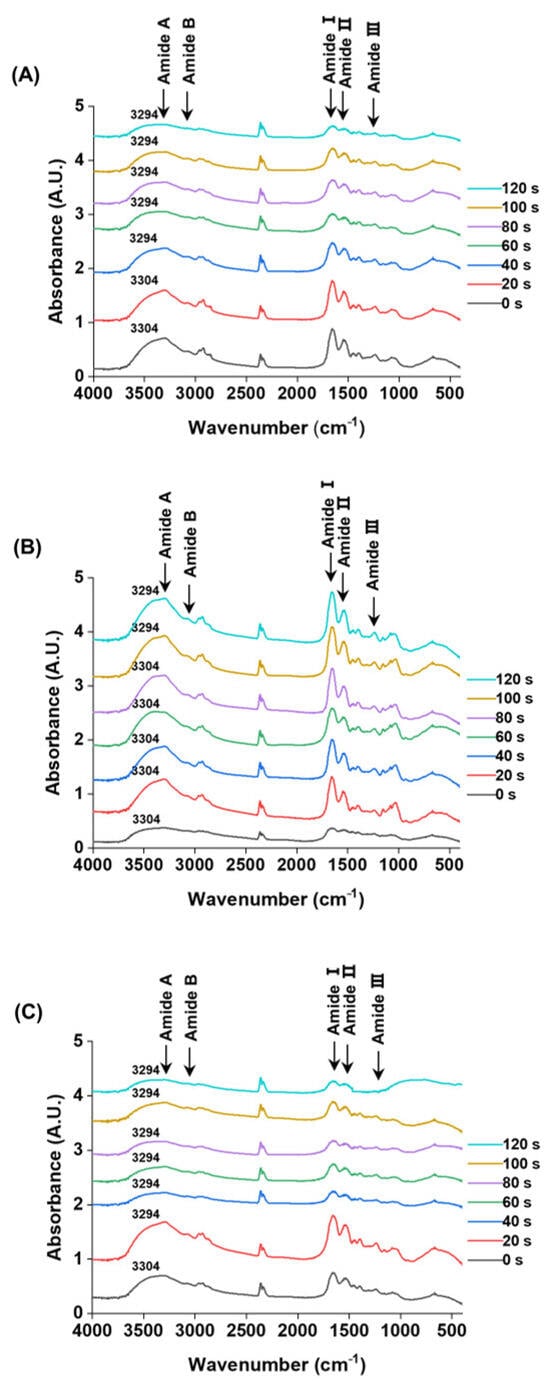
Figure 3.
FTIR spectra of the M. lyrata adductor (A), foot (B), and siphon (C) tissues during the boiling process.
Although all samples exhibited absorption in the amide region (400–4000 cm−1), the three tissue types nevertheless displayed distinct spectral intensities and band shifts during boiling. Specifically, the Amide A band shifted from approximately 3294 to 3304 cm−1, with the timing and extent of this shift varying among tissue types (Figure 3). Such shifts in the Amide A band are indicative of increased hydrogen bonding involving N-H groups [43]. The characteristic Amide B bands appeared in the range of 3076.0–3090.5 cm−1; for instance, the adductor tissue exhibited a band at 3084.3 cm−1. These vibrations are typically associated with the asymmetric stretching of =C-H and -NH3+ groups, as reported for gelatin from the swim bladder of yellowfin tuna [44].
The characteristic Amide I, II, and III bands were detected in the ranges of 1650.2–1658.4 cm−1, 1530.9–1547.3 cm−1, and 1234.6–1238.7 cm−1, respectively (Figure 3). These bands arise from specific atomic displacements: Amide I (~1650 cm−1), Amide II (~1550 cm−1), and Amide III (~1300 cm−1) involve a combination of in-plane (e.g., C=O stretching, C-N stretching, N-H bending) and out-of-plane motions (e.g., C-N torsion, C=O bending) [45,46].
During boiling, a blue shift in the Amide I band was observed for the adductor (from 1650.2 to 1656.4 cm−1) and foot (from 1654.3 to 1656.4 cm−1) tissues. In contrast, the siphon tissue displayed both a blue shift and peak splitting: a single peak at 1652.2 cm−1 resolved into a doublet at 1656.4 cm−1 and 1650.2 cm−1 (Figure 3). This phenomenon is suggestive of protein denaturation, wherein the native peak at ~1651 cm−1 (associated with α-helical structures) diminishes as a new peak at ~1658 cm−1 (corresponding to denatured protein) emerges, as reported in hemoglobin studies [47].
The Amide II bands of the M. lyrata adductor tissue were observed within the range of 1537.0–1547.3 cm−1. In contrast, the foot and siphon tissues each exhibited two distinct peaks at 1547.3 cm−1 and 1537.0 cm−1 after boiling for 20 s and 40 s, respectively (Figure 3). This splitting is consistent with previous findings for egg white proteins, which displayed two peaks at 1545 cm−1 and 1535 cm−1. The ~10 cm−1 red-shift is primarily attributed to the disruption of hydrogen bonds, which restricts protein refolding [48]. Meanwhile, the Amide III bands for all three tissues (adductor, foot, and siphon) were detected in the range of 1234.6–1238.7 cm−1. These vibrations arise from a combination of C–N stretching and N–H deformation in the amide linkage, with additional contributions from CH2 wagging vibrations in the glycine backbone and proline side chains [49].
The secondary structure composition (α-helix, β-sheet, β-turn, and random coil) of proteins in M. lyrata adductor, foot, and siphon tissues was quantified from the infrared spectra in the 1600–1700 cm−1 region using Peak Fit software. As summarized in Table 1, the protein secondary structure was progressively altered with increasing boiling time. Initially, the α-helix was the predominant conformation in all tissues. Over time, thermal denaturation of proteins occurred, as characterized by a marked decrease in α-helix content, coupled with a concurrent increase in β-sheet and random coil structures. This transformation reflects the conversion of ordered, rigid secondary structures into more disordered and flexible conformations upon heating, which can be attributed to thermal disruption of hydrogen bonds, destabilizing native structures while promoting protein unfolding, particularly at exposed hydrophilic residues and flexible loops [50]. Additionally, protein leaching from the tissues may have further contributed to these changes. Similar structural transitions have been reported for tropomyosin from the clam Mactra veneriformis, where combined heat and pressure treatments reduced the α-helix content [33]. Likewise, thermal dissociation of the myosin tail can induce conversion of α-helices into β-sheets [51]. Interestingly, the α-helix content in M. lyrata tissues exhibited an initial decrease, followed by a subsequent increase (Table 1). A comparable non-monotonic trend has been observed in beef myosin, where the α-helix content increased slightly as the temperature rose from 55 °C to 75 °C before decreasing at higher temperatures [51]. The initial increase in α-helix content may be due to the rearrangement of intramolecular hydrogen bonds, leading to a temporary stabilization of helical structures [52].

Table 1.
Effects of heating time on secondary structure of M. lyrata adductor, foot and siphon protein.
Although the adductor, foot, and siphon tissues exhibited distinct absolute secondary structure contents, they exhibited similar trends in their structural changes during boiling (Table 1). These differences are likely to stem from variations in native protein composition and conformation across tissue types. In contrast, the parallel trends in their structural shifts suggest a common underlying protein constitution and denaturation mechanism in M. lyrata tissues.
3.6. Proteome Analysis of Proteins in M. lyrata
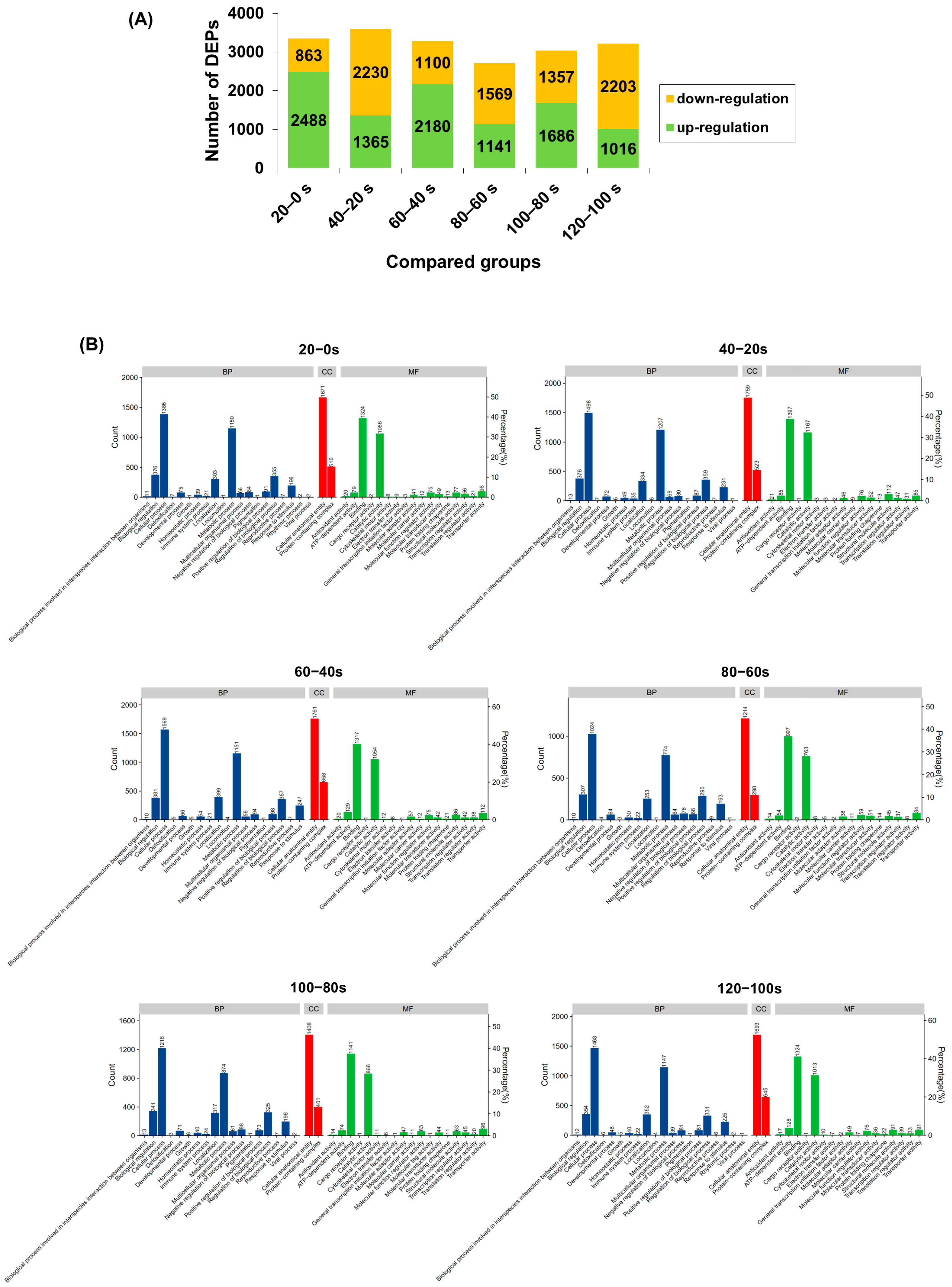
Data-independent acquisition (DIA) quantitative proteomics identified 6527 proteins from M. lyrata tissues. The numbers of differentially expressed proteins (DEPs) in the six pairwise comparisons (20–0 s, 40–20 s, 60–40 s, 80–60 s, 100–80 s and 120–100 s) were 3351, 3595, 3280, 2710, 3043, and 3219, respectively (Figure 4A). Notably, compared with the 20–0 s group, the number of up-regulated DEPs decreased in the subsequent comparisons (60–40 s to 120–100 s), while the number of down-regulated DEPs increased. Given that changes in protein expression during boiling are primarily attributed to thermal denaturation [53], the DEPs identified here provide a comprehensive view of proteins affected by heat treatment in M. lyrata.
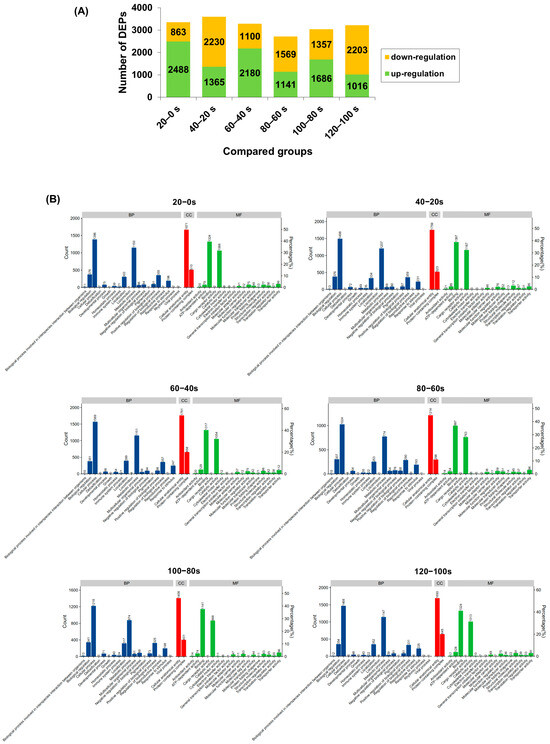
Figure 4.
M. lyrata differential proteomic profile of proteins dealt with boiling process. (A) Count of up- and down-regulated differentially expressed proteins (DEPs) in each pairwise comparison (20–0 s, 40–20 s, 60–40 s, 80–60 s, 100–80 s and 120–100 s). (B) Gene Ontology (GO) classification of differentially expressed proteins (DEPs) in the following pairwise comparisons: 20–0 s, 40–20 s, 60–40 s, 80–60 s, 100–80 s and 120–100 s. BP denoted biological processes; CC, cellular components; and MF, molecular functions. DEPs were considered significantly regulated if they exhibited a fold change (FC) > 1.5 or < 0.67.
Gene Ontology (GO) enrichment analysis was conducted on the DEPs from all six pairwise comparisons (20–0 s, 40–20 s, 60–40 s, 80–60 s, 100–80 s and 120–100 s), categorizing them into biological processes (BP), cellular components (CC), and molecular functions (MF) (Figure 4B). The majority of DEPs across all comparisons were enriched in the BP category. Within BP, the most prominent terms were “biological regulation” and “metabolic process”, which are intrinsically linked as the former can influence the latter at multiple levels [54]. In the MF category, the dominant terms were “binding” and “catalytic activity”, reflecting the interdependence of enzyme-substrate interactions for catalysis [55]. Conversely, the CC category contained the fewest DEPs; among these, “cellular anatomical entity” was the most represented, encompassing a higher number of DEPs than other CC terms, such as “protein-containing complex” (Figure 4B). Collectively, these GO results indicate that boiling significantly affects proteins involved in metabolic and regulatory functions in M. lyrata tissues.
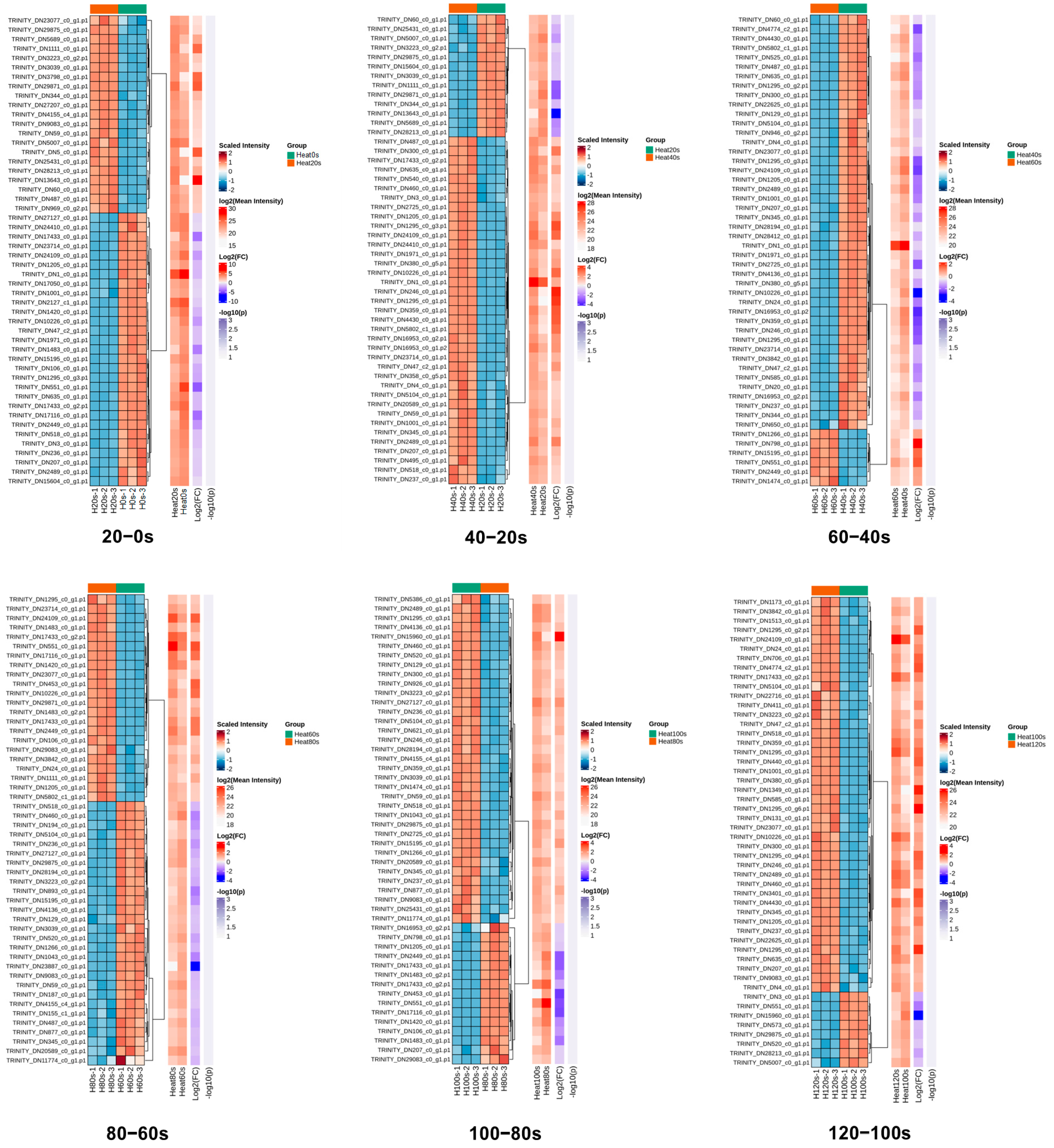
Hierarchical cluster analysis was employed to examine the patterns of DEPs across the six pairwise comparisons (20–0 s, 40–20 s, 60–40 s, 80–60 s, 100–80 s and 120–100 s). A heatmap was constructed to visualize the expression profiles of the top 50 DEPs with the largest fold changes, using Euclidean distance as the clustering metric (Figure 5). The high consistency among biological replicates within each group underscores the reliability of the dataset.
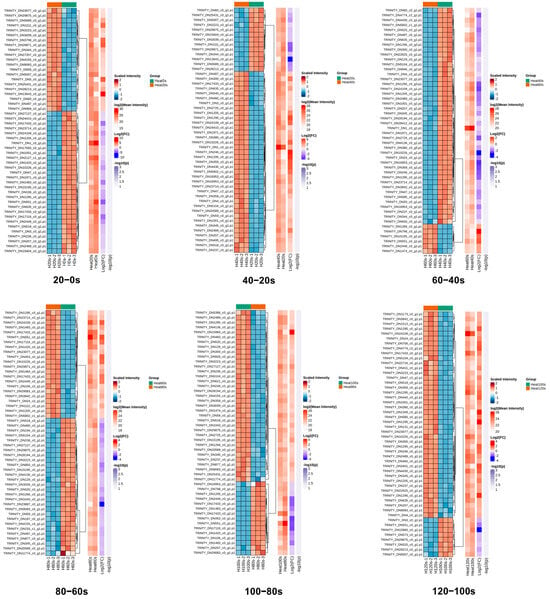
Figure 5.
Hierarchical cluster analysis of the quantified proteins in 20–0 s, 40–20 s, 60–40 s, 80–60 s, 100–80 s and 120–100 s compared groups. Relative expression levels are represented by a color gradient, with red denoting up-regulation and blue denoting down-regulation.
The clustering revealed dynamic changes in protein expression during boiling. The number of up-regulated DEPs initially increased, then decreased, and subsequently increased again across the sequential comparisons. Conversely, the down-regulated DEPs displayed an inverse pattern (Figure 5). These oscillating shifts in protein expression reflect the progressive denaturation and complex transformation of protein characteristics induced by the boiling process. Such dynamic responses of DEPs during thermal processing align with previous findings in boiled abalone [53], highlighting the intricate nature of heat-induced modifications in shellfish proteomes.
4. Conclusions
This study systematically elucidated the structural and proteomic changes in Meretrix lyrata tissues during boiling. Thermal treatment induced tissue-specific protein denaturation and degradation, as evidenced by SDS-PAGE and the emergence of low-molecular-weight fragments. Turbidity, surface hydrophobicity, particle size, and zeta potential analyses revealed distinct aggregation and unfolding kinetics, indicating progressive structural destabilization and diminished electrostatic repulsion. FTIR spectroscopy demonstrated significant secondary structure transitions, including decreases in α-helix content and increases in β-sheet and random coil structures, reflecting disrupted hydrogen bonding and impaired refolding capacity. DIA-based quantitative proteomics identified 6527 proteins, with differentially expressed proteins predominantly involved in metabolic and regulatory processes, highlighting the broad functional impact of boiling. Collectively, these findings provide a comprehensive understanding of heat-induced protein dynamics in M. lyrata and offer a scientific foundation for optimizing thermal processing to preserve the nutritional and textural qualities of shellfish products. Limitations regarding absolute quantification and distinction between intact proteins and degradation products suggest that future studies could employ techniques such as TMT or iTRAQ for more precise protein quantification.
Author Contributions
Conceptualization, X.-R.S. and W.-H.S.; methodology, W.-H.S.; software, Z.-C.C.; validation, Y.-W.W.; formal analysis, Q.L.; investigation, Z.-S.P.; resources, W.-H.S.; data curation, Y.-Y.H.; writing—original draft preparation, W.-H.S.; writing—review and editing, X.-R.S.; visualization, W.-H.S.; supervision, X.-R.S.; project administration, X.-R.S. and W.-H.S.; funding acquisition, X.-R.S. and W.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City, Grant No.: 2021JJLH0028, Hainan Provincial Natural Science Foundation of China, Grant No.: 324QN273 and Scientific Research Foundation of Hainan Tropical Ocean University, Grant No.: RHDRC202314.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Joint WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ. Tech. Rep. Ser. 2007, 935, 1–265. [Google Scholar]
- Hossain, Y.; Uddin, M.; Rahman, A.; Haque, K.; Kormoker, T.; Samad, A.; Tanjin, S.; Rahman, A.; Parvin, F.; Sarmin, S.; et al. Species identification, reproductive biology, and nutritional value of marine shellfish (Meretrix lyrata) in the Bay of Bengal. Mar. Environ. Res. 2023, 192, 106222. [Google Scholar] [CrossRef]
- Walker, W.F.; Warshaw, D.M.; Newsom-Davis, J.M.; Curtin, N.A.; Alexander, R.M.; Wood, B.; Davies, R.E.; Crompton, R.H.; Gergely, J.; Alpert, N.R. Encyclopedia Britannica. Available online: https://www.britannica.com/science/muscle (accessed on 10 August 2025).
- Karnjanapratum, S.; Benjakul, S.; Kishimura, H.; Tsai, Y.H. Chemical compositions and nutritional value of Asian hard clam (Meretrix lusoria) from the coast of Andaman Sea. Food Chem. 2013, 141, 4138–4145. [Google Scholar] [CrossRef]
- Liu, C.W.; Chen, X.L.; Ouyang, Y.; Sun, L.P.; Lu, S.Y. Determination of 21 amino acids in grass carp, clam and shrimp by enzymatic hydrolysis coupled with high-performance liquid chromatography and tandem mass spectrometry. J. Food Compos. Anal. 2025, 142, 107474. [Google Scholar] [CrossRef]
- Wang, S.Q.; Lin, R.; Cheng, S.S.; Tan, M.Q. Water dynamics changes and protein denaturation in surf clam evaluated by two-dimensional LF-NMR T1-T2 relaxation technique during heating process. Food Chem. 2020, 320, 126622. [Google Scholar] [CrossRef]
- Ustunol, Z. Applied Food Protein Chemistry; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Damodaran, S.; Parkin, K.L. Amino acids, peptides, and proteins. In Fennema’s Food Chemistry; CRC press: Boca Raton, FL, USA, 2017; pp. 235–356. [Google Scholar]
- Jiao, X.D.; Li, X.Y.; Zhang, N.N.; Yan, B.W.; Huang, J.L.; Zhao, J.X.; Zhang, H.; Chen, W.; Fan, D.M. Solubilization of fish myofibrillar proteins in NaCl and KCl solutions: A DIA-based proteomics analysis. Food Chem. 2024, 445, 138662. [Google Scholar] [CrossRef]
- Shi, J.C.; Sun, X.; Wang, Y.N.; Yin, S.P.; Liu, Y.F.; Xu, Y.J. Foodomics reveals altered lipid and protein profiles of Antarctic krill (Euphausia superba) under different processing. Food Biosci. 2023, 53, 102565. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Jiang, G.F.; Fan, B.; Hong, F. Protein composition of the foot muscles of the razor clam Sinonovacula constricta based on proteomics analysis. SAJ Biotechnol. 2024, 9, 103. [Google Scholar]
- Shang, W.H.; Yan, J.N.; Du, Y.N.; Cui, X.F.; Su, S.Y.; Han, J.R.; Xu, Y.S.; Xue, C.F.; Zhang, T.T.; Wu, H.T.; et al. Functional properties of gonad protein isolates from three species of sea urchin: A comparative study. J. Food Sci. 2020, 85, 3679–3689. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Y.; Feng, J.H.; Cao, A.L.; Zhang, Y.H.; Lv, Y.F.; Li, J.R. Denaturation kinetics and aggregation mechanism of the sarcoplasmic and myofibril proteins from grass carp during microwave processing. Food Bioprocess Technol. 2018, 11, 417–426. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Z.C.; Lu, Q.Y.; Zhan, S.N.; Jia, R.; Qiao, Z.H.; Wei, H.M.; Huang, T. Glycosylation on the antifreeze and antioxidant capacities of tilapia gelatin hydrolysates. Fishes 2025, 10, 65. [Google Scholar] [CrossRef]
- Agbaje, O.B.A.; Dominguez, J.G.; Jacob, D.E. Organic biopolymers of venus clams: Collagen-related matrix in the bivalve shells with crossed-lamellar ultrastructure. Biochem. Biophys. Rep. 2021, 26, 100939. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Hong, P.Z.; Yang, P.; Zhou, C.X.; Xiao, D.H.; Zhong, T.J. Correlation between the water solubility and secondary structure of tilapia-soybean protein co-precipitates. Molecules 2019, 24, 4337. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef]
- Barkovits, K.; Pacharra, S.; Pfeiffer, K.; Steinbach, S.; Eisenacher, M.; Marcus, K.; Uszkoreit, J. Reproducibility, specificity and accuracy of relative quantification using spectral library-based data-independent acquisition. Mol. Cell. Proteom. 2020, 19, 181–197. [Google Scholar] [CrossRef]
- Bekker-Jensen, D.B.; Bernhardt, O.M.; Hogrebe, A.; Martinez-Val, A.; Verbeke, L.; Gandhi, T.; Kelstrup, C.D.; Reiter, L.; Olsen, J.V. Rapid and site-specific deep phosphoproteome profiling by data-independent acquisition without the need for spectral libraries. Nat. Commun. 2020, 11, 787. [Google Scholar] [CrossRef]
- Zhou, T.Q.; Wang, Y.R.; Wang, Y.Q.; Zhang, L.C.; Wang, C.; Lai, B.; Yan, J.N.; Wu, H.T. Modification of functionalities and in vitro digestibility of clam Mactra chinensis powder upon ultrasound treatment. J. Sci. Food Agric. 2025, 12, 6506–6515. [Google Scholar] [CrossRef]
- Rambli, M.M.; Phuah, E.T.; Howell, N.K. Nutritional and functional properties of underutilized shellfish (molluscs), limpet (Patella vulgata). Sci. Rep. 2025, 15, 23130. [Google Scholar] [CrossRef]
- Tang, X.Y.; Zheng, H.N.; Zhang, C.H.; Hao, J.M.; Zhang, J. Protein composition analysis and molecular weight distribution of Meretrix lusoria. Sci. Technol. Food Ind. 2015, 36, 362–366. [Google Scholar] [CrossRef]
- Ochiai, Y.; Ozawa, H. Biochemical and physicochemical characteristics of the major muscle proteins from fish and shellfish. Fish. Sci. 2020, 86, 729–740. [Google Scholar] [CrossRef]
- Wu, X.Z.; Fan, Y.C.; Guo, C.; Liu, Y.X.; Li, D.Y.; Jiang, P.F.; Qin, L.; Bai, Y.H.; Zhou, D.Y. Effects of boiling processing on texture of scallop adductor muscle and its mechanism. Foods 2022, 11, 1947. [Google Scholar] [CrossRef] [PubMed]
- Viji, P.; Binsi, P.K.; Sireesha, S.; Laly, S.J.; Ninanb, G. Nutritional and physicochemical characteristics of Asiatic hard clam powder prepared by different cook-drying processes: A comparative study. J. Sci. Food Agric. 2024, 104, 5104–5113. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Ma, L.L.; Zheng, J.; Wang, J.T.; Wu, Q.; Song, S.; Zhou, D.Y. Effect of pH on the physicochemical and heat-induced gel properties of scallop Patinopecten yessoensis actomyosin. Fish. Sci. 2014, 80, 1073–1082. [Google Scholar] [CrossRef]
- Reed, Z.H.; Park, J.W. Thermophysical characterization of tilapia myosin and its subfragments. J. Food Sci. 2011, 76, C1050–C1055. [Google Scholar] [CrossRef]
- Akihiro, T.; Yasui1, T.; Yasuhira, S.; Matsumoto, K.I.; Tanaka, Y.; Matsuo, Y.; Shimizu, H.; Matsuzaki, T.; Matsumoto, S.; Yoshikiyo, K.; et al. Tropomyosin micelles are the major components contributing to the white colour of boiled shellfish soups. Sci. Rep. 2022, 12, 15253. [Google Scholar] [CrossRef]
- Wang, S.; Lin, S.Y.; Li, S.; Qian, X.X.; Li, C.Q.; Sun, N. Decoding the textural deterioration of ready-to-eat shrimp: Insights from dynamic myofibrillar protein changes during thermal sterilization. Food Res. Int. 2025, 202, 115745. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.D.A.; Kumar, S.; Bhat, H.F. Thermal processing implications on the digestibility of meat, fish and seafood proteins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4511–4548. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lin, S.Y.; Chen, L.; He, X.Q.; Hu, J.H.; Sun, N. Physicochemical properties and metal ion-binding capacity of thermal-induced Antarctic krill protein aggregates under different pH conditions. J. Agric. Food Chem. 2024, 72, 25944–25954. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tian, G.F.; Wang, L.W.; Sang, Y.X.; Sun, J.L. Effects of ultrasound-assisted high temperature-pressure treatment on the structure and allergenicity of tropomyosin from clam (Mactra veneriformis). Food Chem. X 2023, 18, 100740. [Google Scholar] [CrossRef]
- Promeyrat, A.; Bax, M.L.; Traorťe, S.; Aubry, L.; Sante-Lhoutellier, V.; Gatellier, P. Changed dynamics in myofibrillar protein aggregation as a consequence of heating time and temperature. Meat Sci. 2010, 85, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Promeyrat, A.; Gatellier, P.; Lebret, B.; Kajak-Siemaszko, K.; Aubry, L.; Sante-Lhoutellier, V. Evaluation of protein aggregation in cooked meat. Food Chem. 2010, 121, 412–417. [Google Scholar] [CrossRef]
- Ryan, K.N.; Zhong, Q.X.; Foegeding, E.A. Use of whey protein soluble aggregates for thermal stability—A hypothesis paper. J. Food Sci. 2013, 78, R1105–R1115. [Google Scholar] [CrossRef]
- Xu, Y.X.; Yin, Y.M.; Wang, R.; Zhao, H.L.; Li, X.P.; Yi, S.M.; Li, J.R.; Xie, J.C. Effect of deacetylated konjac glucomannan on heat-induced structural changes and flavor binding ability of fish myosin. Food Chem. 2021, 365, 130540. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, W.; Huang, J.; Xiong, Y.L. Effects of sodium pyrophosphate coupled with catechin on the oxidative stability and gelling properties of myofibrillar protein. Food Hydrocoll. 2020, 104, 105722. [Google Scholar] [CrossRef]
- Sun, Z.W.; Xu, S.H.; Dai, G.L.; Li, Y.M.; Lou, L.R.; Liu, Q.S.; Zhu, R.Z. A microscopic approach to studying colloidal stability. J. Chem. Phys. 2003, 119, 2399–2405. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocoll. 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Du, X.; Li, H.J.; Dong, C.H.; Ren, Y.M.; Pan, N.; Kong, B.H.; Liu, H.Y.; Xia, X.F. Effect of ice structuring protein on the microstructure and myofibrillar protein structure of mirror carp (Cyprinus carpio L.) induced by freeze-thaw processes. LWT Food Sci. Technol. 2021, 139, 110570. [Google Scholar] [CrossRef]
- Gong, Y.H.; Chen, X.R.; Wu, W. Application of Fourier transform infrared (FTIR) spectroscopy in sample preparation: Material characterization and mechanism investigation. Adv. Sample Prep. 2024, 11, 100122. [Google Scholar] [CrossRef]
- Wu, J.L.; Guo, X.B.; Liu, H.; Chen, L. Isolation and comparative study on the characterization of guanidine hydrochloride soluble collagen and pepsin soluble collagen from the body of surf clam shell (Coelomactra antiquata). Foods 2019, 8, 11. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S.; Prodpran, T.; Kaewmanee, T.; Kishimura, H. Characteristics of gelatin extracted from the swim bladder of yellowfin tuna (Thunnus albacores) as affected by alkaline pretreatments. J. Aquat. Food Prod. Technol. 2016, 25, 1190–1201. [Google Scholar] [CrossRef]
- Yang, H.Y.; Yang, S.N.; Kong, J.L.; Dong, A.C.; Yu, S.N. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Bandekar, J. Amide modes and protein conformation. Biochim. Biophys. Acta 1992, 1120, 123–143. [Google Scholar] [CrossRef]
- Yan, Y.B.; Wang, Q.; He, H.W.; Zhou, H.M. Protein thermal aggregation involves distinct regions: Sequential events in the heat-induced unfolding and aggregation of hemoglobin. Biophys. J. 2004, 86, 1682–1690. [Google Scholar] [CrossRef]
- Meo, V.D.; Moccia, M.; Sanità, G.; Crescitelli, A.; Lamberti, A.; Galdi, V.; Rendina, I.; Esposito, E. Probing denaturation of protein a via surface-enhanced infrared absorption spectroscopy. Biosensors 2022, 12, 530. [Google Scholar] [CrossRef]
- Jackson, M.; Watson, P.H.; Halliday, W.C.; Mantsch, H.H. Beware of connective tissue proteins: Assignment and implications of collagen absorptions in infrared spectra of human tissues. Biochim. Biophys. Acta Mol. Basis Dis. 1995, 1270, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Zolghadri, S.; Salehi, N.; Ahmad, F.; Haertlé, T.; Rezaei-Ghaleh, N.; Sawyer, L.; Saboury, A.A. Thermal stability enhancement: Fundamental concepts of protein engineering strategies to manipulate the flexible structure. Int. J. Biol. Macromol. 2022, 214, 642–654. [Google Scholar] [CrossRef]
- Yu, C.L.; Chen, L.L.; Xu, M.S.; Ouyang, K.H.; Chen, H.; Lin, S.Y.; Wang, W.J. The effect of pH and heating on the aggregation behavior and gel properties of beef myosin. LWT Food Sci. Technol. 2024, 191, 115615. [Google Scholar] [CrossRef]
- Nakasako, M. Network of hydrogen bonds around proteins. In Hydration Structures of Proteins; Springer: Tokyo, Japan, 2021. [Google Scholar]
- Yu, M.M.; Fan, Y.C.; Zhang, X.R.; Li, D.Y.; Liu, Y.X.; Zhou, D.Y.; Zhu, B.W. Effect of boiling on texture of abalone muscles and its mechanism based on proteomic techniques. Food Chem. 2022, 388, 133014. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Medzhitov, R. Control strategies in systemic metabolism. Nat. Metab. 2019, 1, 947–957. [Google Scholar] [CrossRef]
- Chen, K.; Arnold, F.H. Engineering new catalytic activities in enzymes. Nat. Catal. 2020, 3, 203–213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).