Physiological and Transcriptomic Analyses of IAA-Induced Inhibition of Chlorophyll Formation in Potato Tubers Post-Harvest
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimal Conditions for IAA Preprocessing
2.3. Experimental Treatment Method
2.4. Evaluation of Potato Surface Color
2.5. Chl Extraction and Measurement
2.6. Microscopic Observation
2.7. Determination of SGAs Content
2.8. Quality Parameters
2.8.1. Fruit Firmness Test
2.8.2. Measurement of Weight Loss
2.8.3. Starch Determination
2.8.4. Determination of Reducing Sugar Content
2.8.5. Determination of Dry Matter Content
2.9. RNA Extraction, Library Preparation, Sequencing, and Data Analysis
2.10. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.11. Quantification of Phytohormones (ABA, JA, and GA)
2.12. Statistical Analysis
3. Results and Discussion
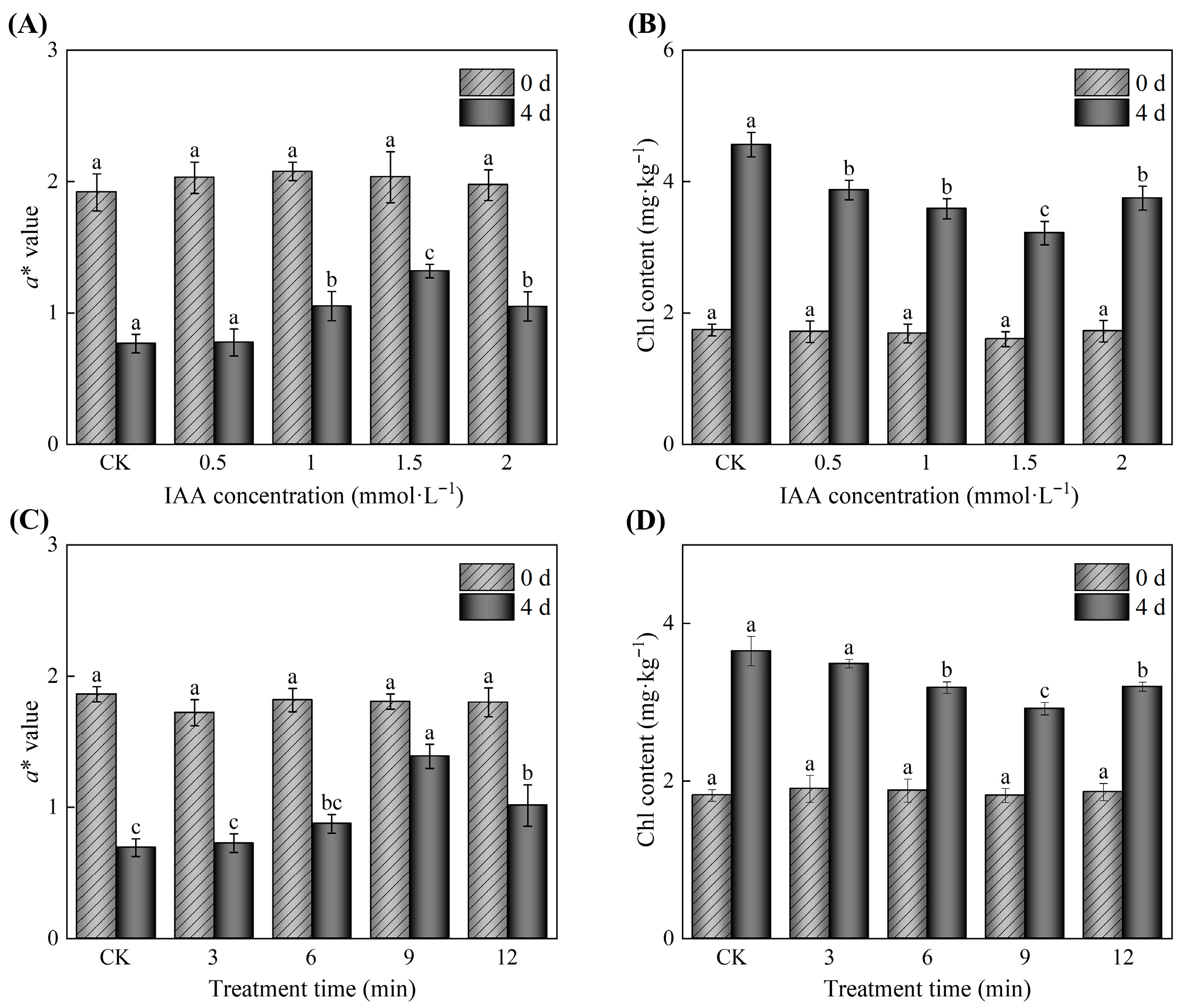
3.1. Determination of the Optimal Conditions for IAA Treatment
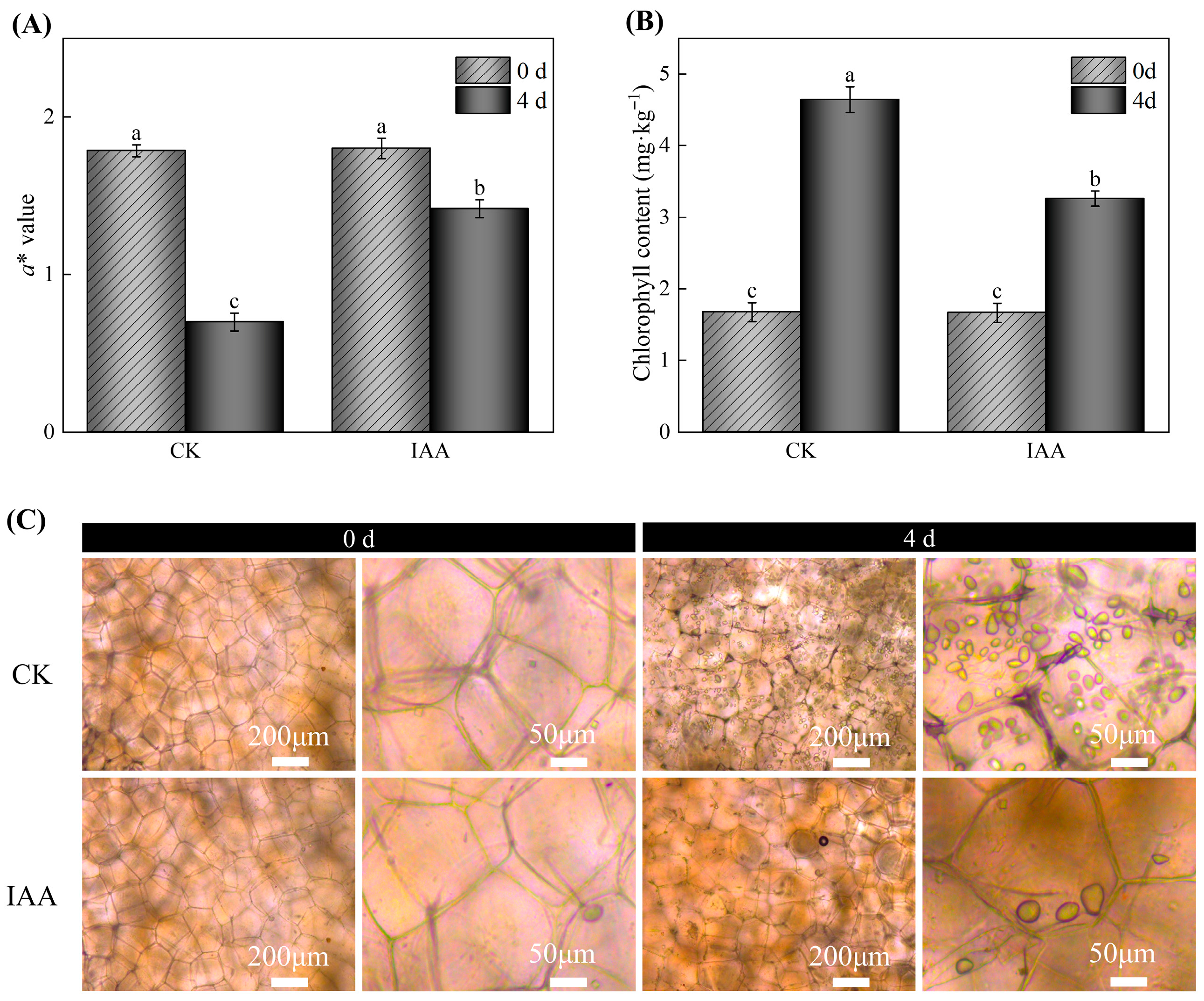
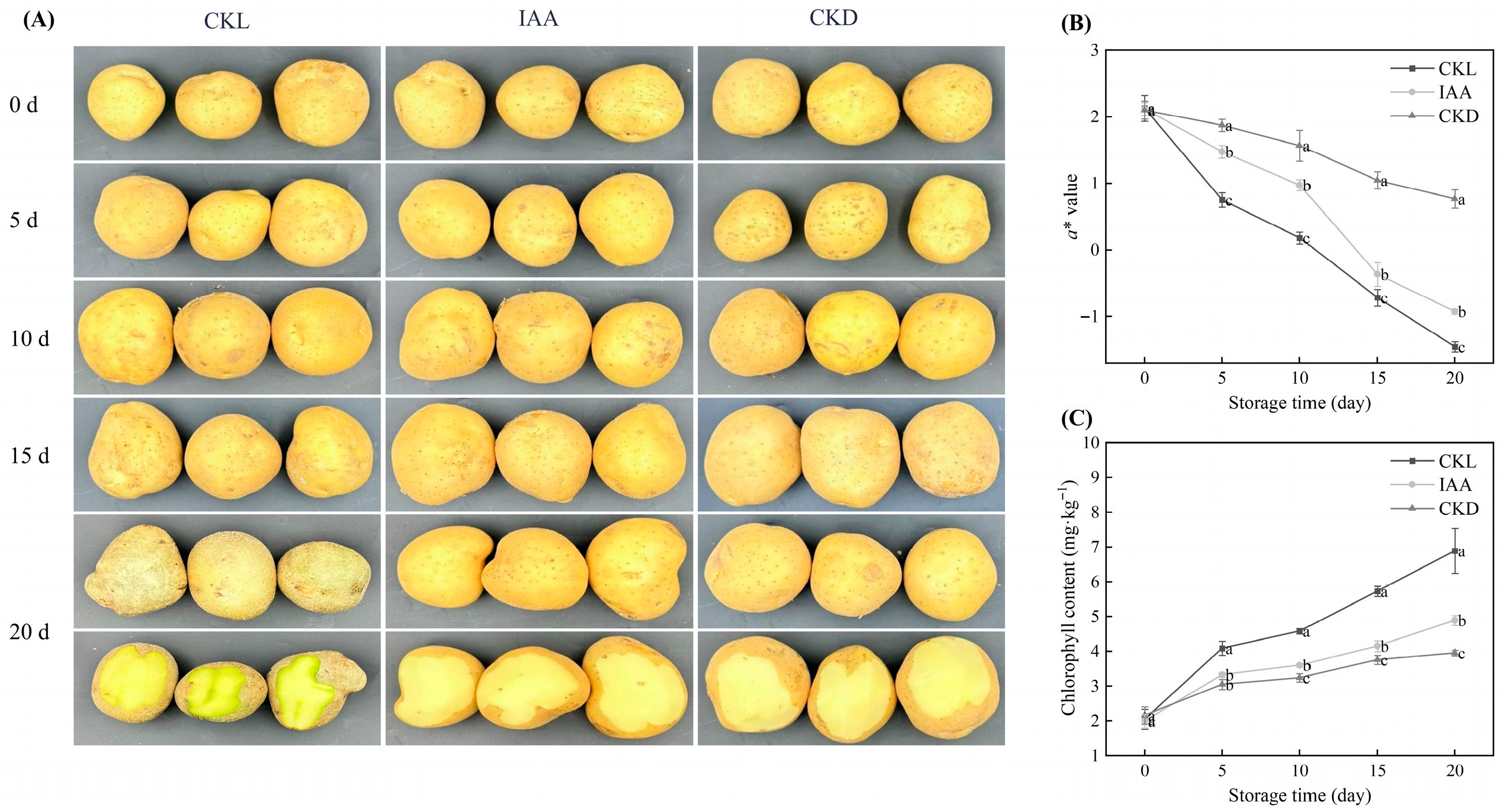
3.2. Effects of Different Treatments on the Overall Appearance, a* Value, and Epidermal Chl Content of Potato Tubers During Storage
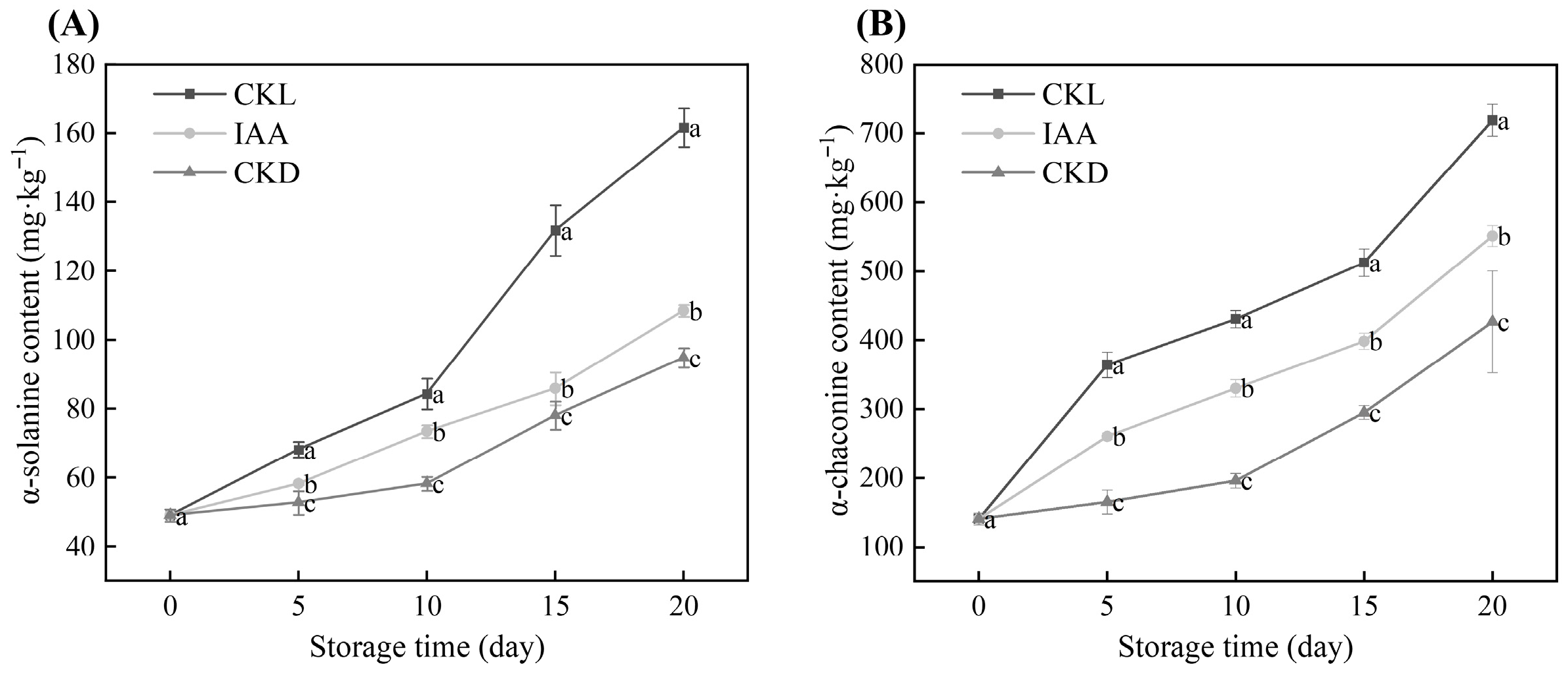
3.3. Effects of IAA Treatment on the SGAs Content of Whole Potato Tubers During Dark Storage at Different Temperatures
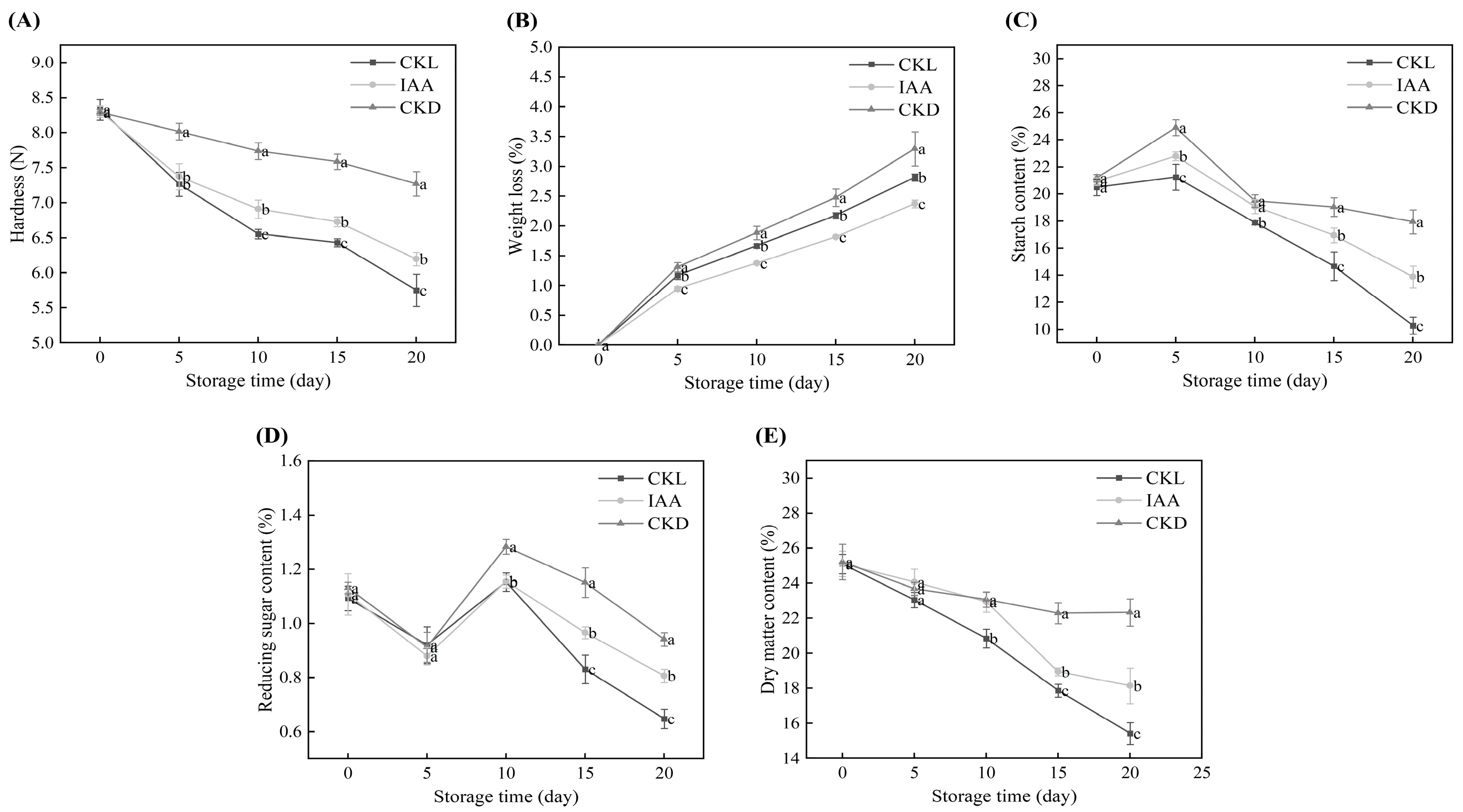
3.4. Exogenous IAA-Induced Quality Changes in Potato Tubers
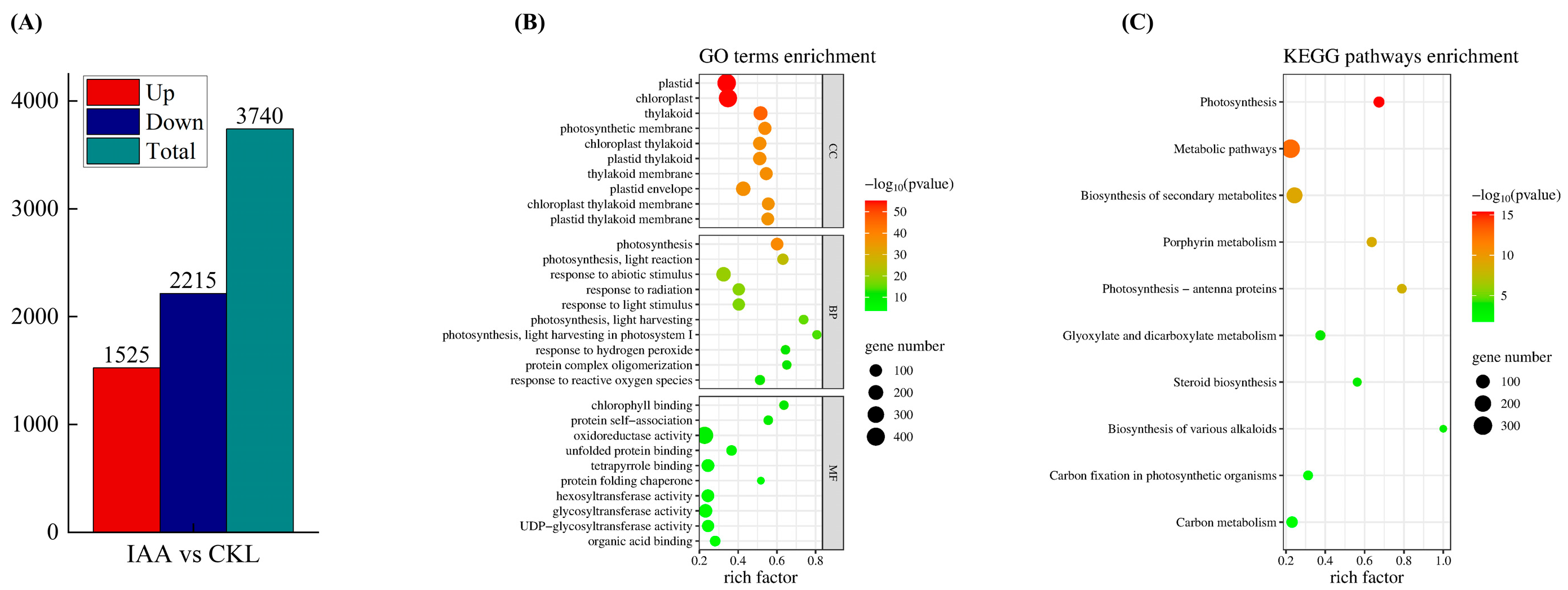
3.5. Analysis of the Transcriptome Profiles and DEGs of Potato Under IAA Treatment
3.6. Gene Ontology (GO) Enrichment Analysis and KEGG Pathway Analysis of DEGs in Potato Tubers
3.7. Chl Biosynthesis Pathway in Potato Greening Process
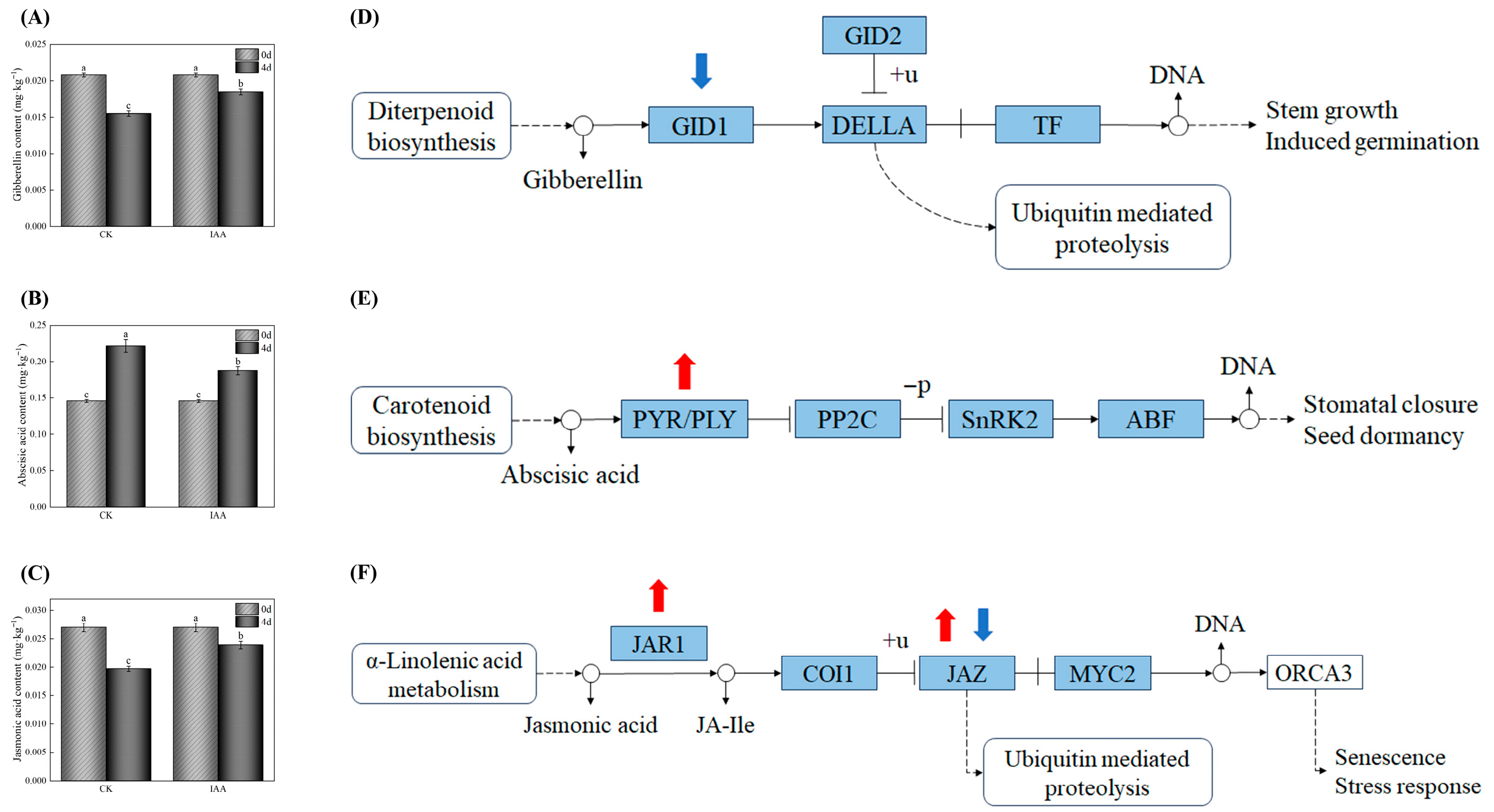
3.8. Exogenous IAA Suppresses Chl Biosynthesis Through a Coordinated Multi-Hormonal Regulatory Network
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardigan, M.A.; Laimbeer, F.P.E.; Newton, L.; Crisovan, E.; Hamilton, J.P.; Vaillancourt, B.; Wiegert-Rininger, K.; Wood, J.C.; Douches, D.S.; Farré, E.M.; et al. Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proc. Natl. Acad. Sci. USA 2017, 114, E9999–E10008. [Google Scholar] [CrossRef]
- Zaheer, K.; Akhtar, M.H. Potato Production, Usage, and Nutrition—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar] [CrossRef]
- Olsen, N.L.; Brandt, T.; Price, W.J. The Impact of Retail Light Source on Greening of Russet Burbank Potato Tubers. Am. J. Potato Res. 2018, 95, 123–129. [Google Scholar] [CrossRef]
- Percival, G.; Dixon, G.R. Glycoalkaloid Concentrations in Aerial Tubers of Potato (Solanum tuberosum L.). J. Sci. Food Agric. 1996, 70, 439–448. [Google Scholar] [CrossRef]
- Baur, S.; Bellé, N.; Hausladen, H.; Wurzer, S.; Brehm, L.; Stark, T.D.; Hücklhoven, R.; Hofmann, T.; Dawid, C. Quantitation of Toxic Steroidal Glycoalkaloids and Newly Identified Saponins in Post-Harvest Light-Stressed Potato (Solanum tuberosum L.) Varieties. J. Agric. Food Chem. 2022, 70, 8300–8308. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Chen, W.; Yang, Z.; Li, X.; Wang, L.; Cao, S.; Shi, L. The changes in chlorophyll, solanine, and phytohormones during light-induced greening in postharvest potatoes. Postharvest Biol. Technol. 2025, 219, 113291. [Google Scholar] [CrossRef]
- Zao, X.; Li, W.; Cheng, L.; Yu, B.; Sa, G. Physiological and Molecular Mechanisms of Light-Induced Greening in Potatoes: A Path to Food Safety. Foods 2025, 14, 1798. [Google Scholar] [CrossRef]
- Sa, G.; Zao, X.; Yuan, J.; Cheng, L.; Yu, B. Light-Quality-Dependent Greening and Steroidal Glycoalkaloid Accumulation in Potato Tubers: Regulatory Mechanisms and Postharvest Strategies to Reduce Food Safety Risks. Foods 2025, 14, 3394. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Ducreux, L.J.M.; Allwood, J.W.; Hedley, P.E.; Wright, A.; Gururajan, V.; Terry, M.J.; Taylor, M.A. Light Regulation of Chlorophyll and Glycoalkaloid Biosynthesis During Tuber Greening of Potato S. tuberosum. Front. Plant Sci. 2020, 11, 753. [Google Scholar] [CrossRef]
- Larsen, H.; Molteberg, E.L. Discolouration of Potato Tubers Under Retail Light: Cultivar Variations and Effect of Different Packaging Materials for Folva Potatoes Stored at 20 and 6 °C. Potato Res. 2023, 66, 507–523. [Google Scholar] [CrossRef]
- Dong, T.; Meng, W.; Shi, J.; Jiang, C.-Z.; Wang, Q. Ethanol fumigation combined with and without nitrogen gas delays potato greening and inhibits glycoalkaloids generation under light. Postharvest Biol. Technol. 2017, 134, 31–37. [Google Scholar] [CrossRef]
- Banks, N.H. Coating and modified atmosphere effects on potato tuber greening. J. Agric. Sci. 1985, 105, 59–62. [Google Scholar] [CrossRef]
- Nyankanga, R.O.; Murigi, W.W.; Shibairo, S.I. Effect of Packaging Material on Shelf Life and Quality of Ware Potato Tubers Stored at Ambient Tropical Temperatures. Potato Res. 2018, 61, 283–296. [Google Scholar] [CrossRef]
- Dhalsamant, K.; Singh, C.B.; Lankapalli, R. A Review on Greening and Glycoalkaloids in Potato Tubers: Potential Solutions. J. Agric. Food Chem. 2022, 70, 13819–13831. [Google Scholar] [CrossRef]
- Mwelase, S.; Adeyemi, J.O.; Fawole, O.A. Recent Advances in Postharvest Application of Exogenous Phytohormones for Quality Preservation of Fruits and Vegetables. Plants 2024, 13, 3255. [Google Scholar] [CrossRef]
- Qian, C.; Sun, Y.; Zhang, B.; Shao, Y.; Liu, J.; Kan, J.; Zhang, M.; Xiao, L.; Jin, C.; Qi, X. Effects of melatonin on inhibiting quality deterioration of postharvest water bamboo shoots. Food Chem. Mol. Sci. 2024, 8, 100208. [Google Scholar] [CrossRef]
- Yu, Y.; Bao, Z.; Zhou, Q.; Wu, W.; Chen, W.; Yang, Z.; Wang, L.; Li, X.; Cao, S.; Shi, L. EjWRKY6 Is Involved in the ABA-Induced Carotenoid Biosynthesis in Loquat Fruit during Ripening. Foods 2024, 13, 2829. [Google Scholar] [CrossRef]
- Di, H.; Zhang, C.; Zhou, A.; Huang, H.; Tang, Y.; Li, H.; Huang, Z.; Zhang, F.; Sun, B. Transcriptome Analysis Reveals the Mechanism by Which Exogenous Melatonin Treatment Delays Leaf Senescence of Postharvest Chinese Kale (Brassica oleracea var. alboglabra). Int. J. Mol. Sci. 2024, 25, 2250. [Google Scholar] [CrossRef]
- Liu, P.-Z.; Wang, Y.-H.; Wang, L.-X.; Wei, Y.-J.; Sun, Y.-H.; Sun, X.; Chen, C.; Li, Y.-P.; Xiong, A.-S. Integrated transcriptomic and metabolomic revealed the effect of salicylic acid treatment on improving the storability of ‘Ventura’ celery. Postharvest Biol. Technol. 2025, 228, 113656. [Google Scholar] [CrossRef]
- Keawmanee, N.; Ma, G.; Zhang, L.; Yahata, M.; Murakami, K.; Yamamoto, M.; Kojima, N.; Kato, M. Exogenous gibberellin induced regreening through the regulation of chlorophyll and carotenoid metabolism in Valencia oranges. Plant Physiol. Biochem. 2022, 173, 14–24. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Bacterial indole-3-acetic acid: A key regulator for plant growth, plant-microbe interactions, and agricultural adaptive resilience. Microbiol. Res. 2024, 281, 127602. [Google Scholar] [CrossRef]
- Zhou, Q.; Bao, Z.; Yu, Y.; Chen, W.; Yang, Z.; Cao, S.; Shi, L. IAA regulated levels of endogenous phytohormones in relation to chilling tolerance in cold-stored peaches after harvest. Postharvest Biol. Technol. 2023, 205, 112490. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, L.; Liu, S.; Zhao, M.; Liu, J.; Lin, L.; Liu, K. Physiological and transcriptomic analysis of IAA-induced antioxidant defense and cell wall metabolism in postharvest mango fruit. Food Res. Int. 2023, 174, 113504. [Google Scholar] [CrossRef]
- Li, Z.-X.; Yang, S.; Wang, X.; Liao, Q.-H.; Zhang, W.-L.; Liu, J.; Liu, G.-H.; Tang, J.-M. Widely targeted metabolomics analysis reveals the effect of exogenous auxin on postharvest resistance to Botrytis cinerea in kiwifruit (Actinidia chinensis L.). Postharvest Biol. Technol. 2023, 195, 112129. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kudaka, R.; Inaba, H.; Murakami, K.; Yamamoto, M.; Kojima, N.; Yahata, M.; Matsumoto, H.; Kato, M. Auxin induced carotenoid accumulation in GA and PDJ-treated citrus fruit after harvest. Postharvest Biol. Technol. 2021, 181, 111676. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Zhang, Y.; Xu, Y.; Chen, X.; Huang, W.; Li, P. The MYB transcriptional factor BrMYB108 regulates Auxin-mediated delayed leaf senescence in postharvest Pak Choi. Postharvest Biol. Technol. 2025, 219, 113181. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Song, B.; Du, P.; Liu, X. Light-induced ultrastructure changes of amyloplasts and effect of nitrogen fertilization on greening in potato tubers (Solanum tuberosum L.). Postharvest Biol. Technol. 2020, 168, 111275. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, K.; Shi, B.; Wang, J.; Yang, F.; Li, G.; Zhang, P.; Wu, Y.; Erihemu. Mechanism of exogenous MeJA inhibition of potato tuber greening based on transcriptomic analysis: Regulation of chlorophyll biosynthesis enzymes. Food Chem. 2025, 496, 146689. [Google Scholar] [CrossRef]
- Emragi, E.; Kalita, D.; Jayanty, S.S. Effect of edible coating on physical and chemical properties of potato tubers under different storage conditions. LWT—Food Sci. Technol. 2022, 153, 112580. [Google Scholar] [CrossRef]
- Nourian, F.; Ramaswamy, H.S.; Kushalappa, A.C. Kinetics of quality change associated with potatoes stored at different temperatures. LWT—Food Sci. Technol. 2003, 36, 49–65. [Google Scholar] [CrossRef]
- Sidauruk, L.; Sembiring, T.; Susilawati; Humaidi, S.; Rianna, M.; Sianturi, H.A. Comprehensive Analysis of Dry Material Content, Moisture Content, and FTIR Spectroscopy in Durian Peel. J. Phys. Conf. Ser. 2024, 2733, 012018. [Google Scholar] [CrossRef]
- Li, G.; Zhou, Y.; Zhao, Y.; Liu, Y.; Ke, Y.; Jin, X.; Ma, H. Internal Reference Gene Selection for Quantitative Real-Time RT-PCR Normalization in Potato Tissues. Phyton—Int. J. Exp. Bot. 2020, 89, 329–344. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, Z.; Zhang, J.; Chen, X.; Cui, J.; Xu, W.; Liu, H.-y. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, W.; Li, Z. Mediating effect of indole-3-acetic acid on chilling injury and fruit softening during cold storage of tomato fruit. Postharvest Biol. Technol. 2025, 230, 113725. [Google Scholar] [CrossRef]
- Guan, W.; Cao, M.; Chen, W.; Yang, Z.; Li, X.; Wang, L.; Shi, L. Indole-3-acetic acid treatment promotes postharvest kiwifruit softening by regulating starch and cell wall metabolism. Front. Plant Sci. 2024, 15, 1485678. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Xu, X.; Jiang, F.; Li, Q.; Zhang, H.; Zhang, A.; Li, J. IAA treatment accelerates post-harvest softening in ‘Docteur Jules Guyot’ pear via activation of pectinase-encoding genes. Sci. Hortic. 2025, 341, 113965. [Google Scholar] [CrossRef]
- Grunenfelder, L.A.; Knowles, L.O.; Hiller, L.K.; Knowles, N.R. Glycoalkaloid Development during Greening of Fresh Market Potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2006, 54, 5847–5854. [Google Scholar] [CrossRef]
- Bamberg, J.; Moehninsi; Navarre, R.; Suriano, J. Variation for Tuber Greening in the Diploid Wild Potato Solanum Microdontum. Am. J. Potato Res. 2015, 92, 435–443. [Google Scholar] [CrossRef]
- Shen, D.-D.; Hua, Y.-P.; Huang, J.-Y.; Yu, S.-T.; Wu, T.-B.; Zhang, Y.; Chen, H.-L.; Yue, C.-P. Multiomic Analysis Reveals Core Regulatory Mechanisms underlying Steroidal Glycoalkaloid Metabolism in Potato Tubers. J. Agric. Food Chem. 2022, 70, 415–426. [Google Scholar] [CrossRef]
- Rymuza, K.; Gugała, M.; Zarzecka, K.; Sikorska, A.; Findura, P.; Malaga-Toboła, U.; Kapela, K.; Radzka, E. The Effect of Light Exposures on the Content of Harmful Substances in Edible Potato Tuber. Agriculture 2020, 10, 139. [Google Scholar] [CrossRef]
- Liu, T.; Kawochar, M.A.; Begum, S.; Wang, E.; Zhou, T.; Jing, S.; Liu, T.; Yu, L.; Nie, B.; Song, B. Potato tonoplast sugar transporter 1 controls tuber sugar accumulation during postharvest cold storage. Sci. Hortic. 2023, 10, uhad035. [Google Scholar] [CrossRef]
- Qu, L.; Huang, X.; Su, X.; Zhu, G.; Zheng, L.; Lin, J.; Wang, J.; Xue, H. Potato: From functional genomics to genetic improvement. Mol. Hortic. 2024, 4, 34. [Google Scholar] [CrossRef]
- Hendriks, J.H.M.; Kolbe, A.; Gibon, Y.; Stitt, M.; Geigenberger, P. ADP-Glucose Pyrophosphorylase Is Activated by Posttranslational Redox-Modification in Response to Light and to Sugars in Leaves of Arabidopsis and Other Plant Species. Plant Physiol. 2003, 133, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Naznin, S.; Naznin, A.; Uddin, M.N.; Amin, M.N.; Rahman, M.M.; Tipu, M.M.H.; Alsuhaibani, A.M.; Gaber, A.; Ahmed, S. Dry Matter, Starch Content, Reducing Sugar, Color and Crispiness Are Key Parameters of Potatoes Required for Chip Processing. Horticulturae 2022, 8, 362. [Google Scholar] [CrossRef]
- Gachango, E.; Shibairo, S.I.; Kabira, J.N.; Chemining’wa, G.N.; Demo, P. Effects of light intensity on quality of potato seed tubers. Glob. J. Food Agribus. Manag. 2019, 10, 53–60. [Google Scholar]
- Zhang, J.; Sui, C.; Liu, H.; Chen, J.; Han, Z.; Yan, Q.; Liu, S.; Liu, H. Effect of chlorophyll biosynthesis-related genes on the leaf color in Hosta (Hosta plantaginea Aschers) and tobacco (Nicotiana tabacum L.). BMC Plant Biol. 2021, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhao, S.; Zhang, F.; Zhao, A.; Zhang, W.; Zhang, M.; Liu, L. The Arabidopsis glutamyl-tRNA reductase (GluTR) forms a ternary complex with FLU and GluTR-binding protein. Sci. Rep. 2016, 6, 19756. [Google Scholar] [CrossRef]
- Wang, P.; Richter, A.S.; Kleeberg, J.R.W.; Geimer, S.; Grimm, B. Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat. Commun. 2020, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Shih, T.-H.; Lin, S.-H.; Lin, J.-W.; Nguyen, H.C.; Yang, Z.-W.; Yang, C.-M. Transcription Profile Analysis of Chlorophyll Biosynthesis in Leaves of Wild-Type and Chlorophyll b-Deficient Rice (Oryza sativa L.). Agriculture 2021, 11, 401. [Google Scholar] [CrossRef]
- Liang, M.; Gu, D.; Lie, Z.; Yang, Y.; Lu, L.; Dai, G.; Peng, T.; Deng, L.; Zheng, F.; Liu, X. Regulation of chlorophyll biosynthesis by light-dependent acetylation of NADPH:protochlorophyll oxidoreductase A in Arabidopsis. Plant Sci. 2023, 330, 111641. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, S.; Jiao, B.; Duan, M.; Meng, Q.; Ma, N.; Lv, W. SlSGRL, a tomato SGR-like protein, promotes chlorophyll degradation downstream of the ABA signaling pathway. Plant Physiol. Biochem. 2020, 157, 316–327. [Google Scholar] [CrossRef]
- Cheminant, S.; Wild, M.; Bouvier, F.; Pelletier, S.; Renou, J.-P.; Erhardt, M.; Hayes, S.; Terry, M.J.; Genschik, P.; Achard, P. DELLAs Regulate Chlorophyll and Carotenoid Biosynthesis to Prevent Photooxidative Damage during Seedling Deetiolation in Arabidopsis. The Plant Cell 2011, 23, 1849–1860. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Huang, M.; Jin, Y.; Sun, J.; Tao, X.; Zhang, G.; He, K.; Zhao, Y.; Zhao, H. Uniconazole-induced starch accumulation in the bioenergy crop duckweed (Landoltia punctata) II: Transcriptome alterations of pathways involved in carbohydrate metabolism and endogenous hormone crosstalk. Biotechnol. Biofuels 2015, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.A.U.; Guan, X.; Zhang, Y.; Zhou, L.; Bartas, M.; Ullah, N.; Zhou, W.; Cheng, F. Nitrogen Deficiency Accelerates Rice Leaf Senescence Through ABA Signaling and Sugar Metabolic Shifts. Physiol. Plant. 2025, 177, e70124. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kundu, S.; Dutta, S.K. Proteomic analysis of JAZ interacting proteins under methyl jasmonate treatment in finger millet. Plant Physiol. Biochem. 2016, 108, 79–89. [Google Scholar] [CrossRef]
- Yang, M.; Yang, S.; Wang, W.; Wei, X.; Lou, F.; He, G.; He, T. Multiomics Combined with Expression Pattern Analysis Reveals the Regulatory Response of Key Genes in Potato Jasmonic Acid Signaling Pathways to Cadmium Stress. J. Agric. Food Chem. 2024, 72, 22369–22384. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Zhang, S.; Ge, C.; Liu, S.; Ma, H.; Pang, C.; Shen, Q. Integrating physiological and transcriptomic analyses explored the regulatory mechanism of cold tolerance at seedling emergence stage in upland cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2024, 217, 109297. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ma, Z.; Zhou, K.; Niu, Q.; Luo, Q.; Yang, X.; Chu, X.; Shan, G. Elucidating the Underlying Allelopathy Effects of Euphorbia jolkinii on Arundinella hookeri Using Metabolomics Profiling. Plants 2025, 14, 123. [Google Scholar] [CrossRef]
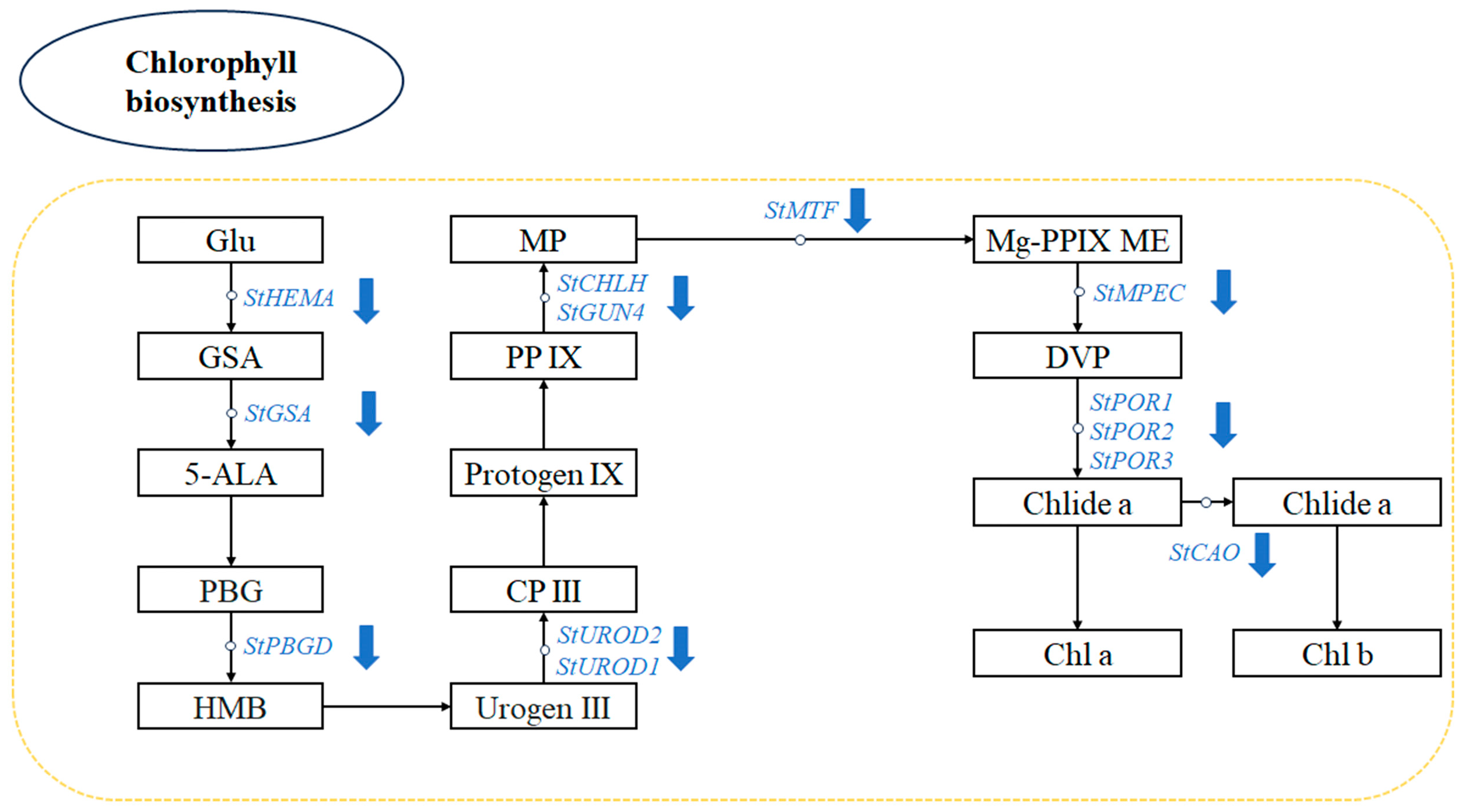
| Gene ID | Gene Name | Log2(FC) | Description |
|---|---|---|---|
| PGSC0003DMG400006381 | StHEMA | −4.3692 | Glutamyl-tRNA reductase |
| PGSC0003DMG400032546 | StGSA | −2.183 | Glutamate-1-semialdehyde 2,1-aminomutase, chloroplastic |
| PGSC0003DMG400022126 | StPBGD | −1.9418 | Porphobilinogen deaminase |
| PGSC0003DMG400027602 | StUROD1 | −2.755 | Uroporphyrinogen decarboxylase, chloroplastic |
| PGSC0003DMG402021263 | StUROD2 | −2.1133 | Uroporphyrinogen decarboxylase |
| PGSC0003DMG400027276 | StCHLH | −7.8366 | Mg protoporphyrin IX chelatase |
| PGSC0003DMG400014243 | StMTF | −3.7492 | S-adenosyl-L-methionine Mg-protoporphyrin IX methyltranserase |
| PGSC0003DMG400007188 | StMPEC | −6.8581 | Desaturase |
| PGSC0003DMG400015356 | StPOR1 | −6.6087 | NADPH:protochlorophyllide oxidoreductase |
| PGSC0003DMG400025007 | StPOR2 | −5.6145 | NADPH:protochlorophyllide oxidoreductase |
| PGSC0003DMG400018351 | StPOR3 | −3.0866 | NADPH:protochlorophyllide oxidoreductase |
| PGSC0003DMG400017570 | StCAO | −2.8109 | Chlorophyll synthase |
| PGSC0003DMG400027013 | StGUN4 | −5.3941 | Tetrapyrrole-binding protein, chloroplast |
| PGSC0003DMG400034947 | StHMGR | −5.5889 | HMGR CoA reductase |
| PGSC0003DMG400003849 | StGID1 | −1.2406 | GID1-like gibberellin receptor |
| PGSC0003DMG400033879 | StJAR1 | 1.484 | Jasmonic acid-amino acid-conjugating enzyme |
| PGSC0003DMG400015667 | StPtoR1 | −2.7824 | Pto-responsive gene 1 protein |
| PGSC0003DMG400004367 | StSRP1 | 4.9798 | Salt responsive protein 1 |
| PGSC0003DMG400032119 | StJAZ3 | 1.506 | Jasmonate ZIM-domain protein 3 |
| PGSC0003DMG400022888 | StSRP1 | −1.2682 | Salt responsive protein 1 |
| PGSC0003DMG400002100 | StPYR1 | 1.0557 | Abscisic acid receptor PYR1 |
| PGSC0003DMG400029194 | StPYL4 | 2.8127 | Abscisic acid receptor PYL4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Yang, F.; Shi, B.; Zhang, C.; Ma, H.; Wang, J.; Shi, K.; Li, G.; Wu, Y.; Zhang, P.; et al. Physiological and Transcriptomic Analyses of IAA-Induced Inhibition of Chlorophyll Formation in Potato Tubers Post-Harvest. Foods 2025, 14, 4031. https://doi.org/10.3390/foods14234031
Lv H, Yang F, Shi B, Zhang C, Ma H, Wang J, Shi K, Li G, Wu Y, Zhang P, et al. Physiological and Transcriptomic Analyses of IAA-Induced Inhibition of Chlorophyll Formation in Potato Tubers Post-Harvest. Foods. 2025; 14(23):4031. https://doi.org/10.3390/foods14234031
Chicago/Turabian StyleLv, Hongze, Fan Yang, Bidan Shi, Chuchu Zhang, Hui Ma, Jing Wang, Ke Shi, Guoqin Li, Yi Wu, Pengfei Zhang, and et al. 2025. "Physiological and Transcriptomic Analyses of IAA-Induced Inhibition of Chlorophyll Formation in Potato Tubers Post-Harvest" Foods 14, no. 23: 4031. https://doi.org/10.3390/foods14234031
APA StyleLv, H., Yang, F., Shi, B., Zhang, C., Ma, H., Wang, J., Shi, K., Li, G., Wu, Y., Zhang, P., & Erihemu. (2025). Physiological and Transcriptomic Analyses of IAA-Induced Inhibition of Chlorophyll Formation in Potato Tubers Post-Harvest. Foods, 14(23), 4031. https://doi.org/10.3390/foods14234031







