Impact of Different Types of Lipids on the Quality of Frozen Dough and Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
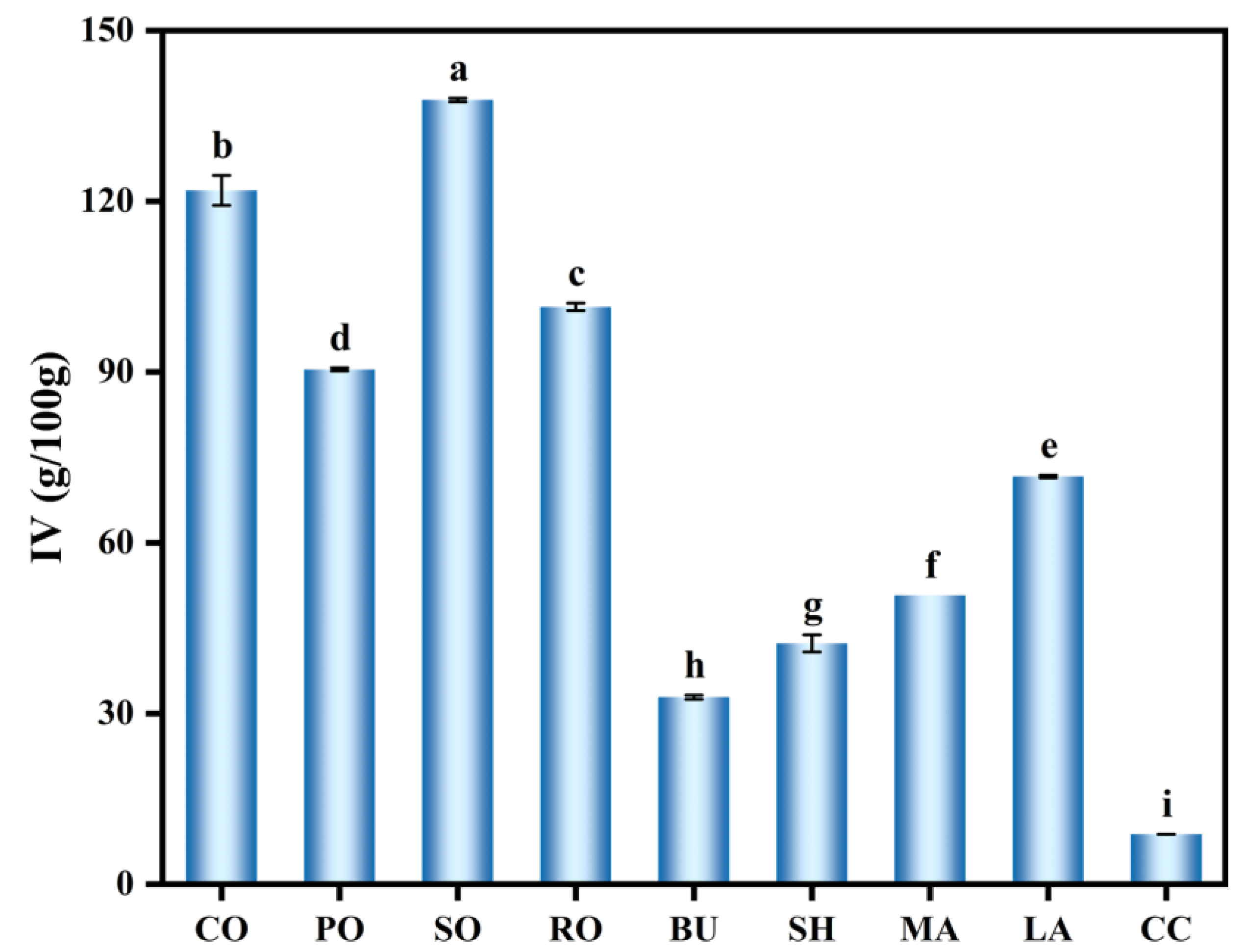
2.2. Iodine Value (IV)
2.3. Preparation of the Frozen Dough
2.4. Texture Properties (TPA)
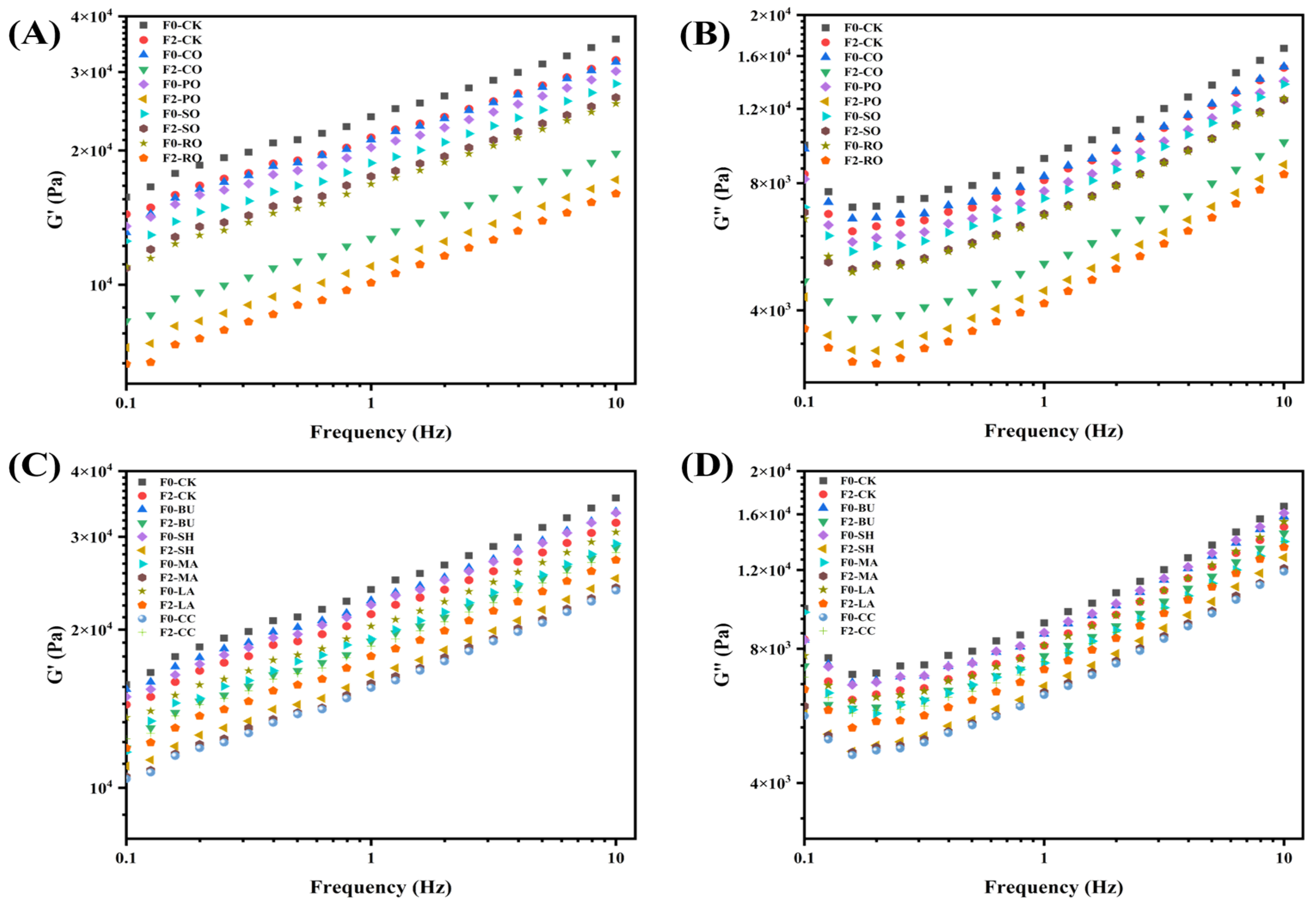
2.5. Dynamic Rheological Rroperties
2.6. Water Distribution
2.7. Differential Scanning Calorimetry
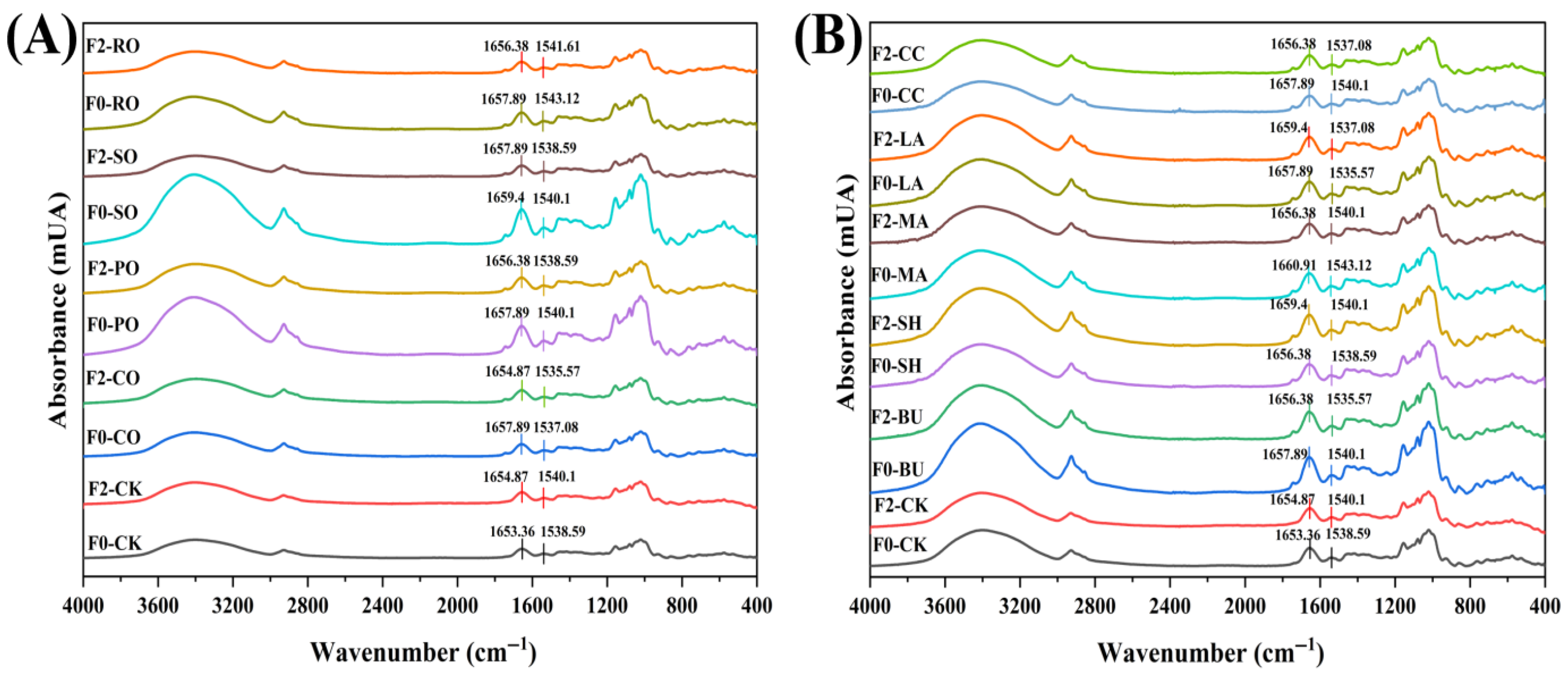
2.8. Fourier Transform Infrared Spectrometry (FT-IR)
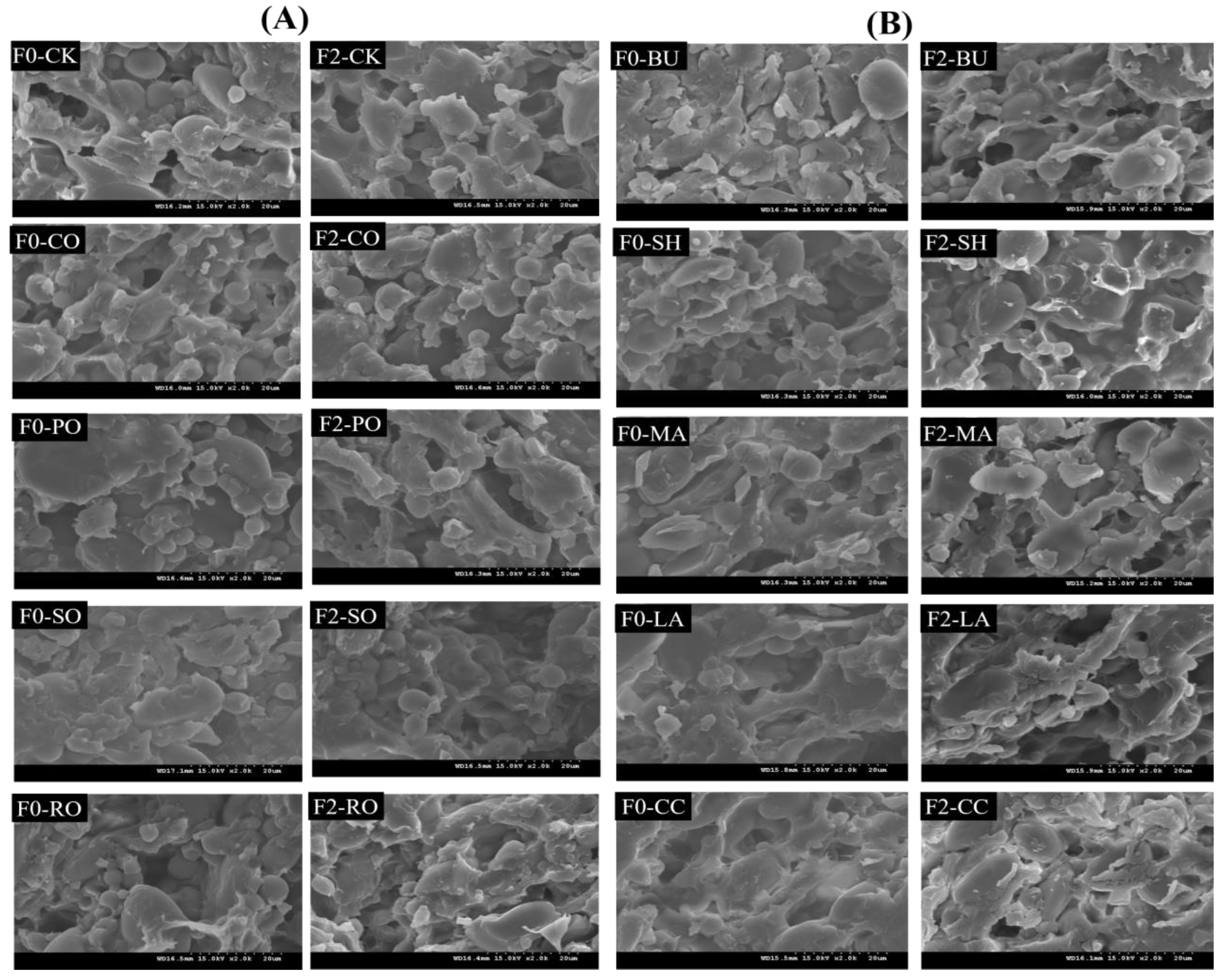
2.9. SEM
2.10. Pasting Properties
2.10.1. Preparation of Bread from Frozen Dough
2.10.2. Textural Properties
2.10.3. Specific Volume (SV)
2.10.4. Sensory Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. IV of Different Lipid Types
3.2. Textural Properties of Frozen Dough
3.3. Dynamic Rheological Properties
3.4. Moisture Migration of Frozen Dough
3.5. DSC
3.6. FT-IR
3.7. Microstructure of Frozen Dough
3.8. Bread Baking Properties
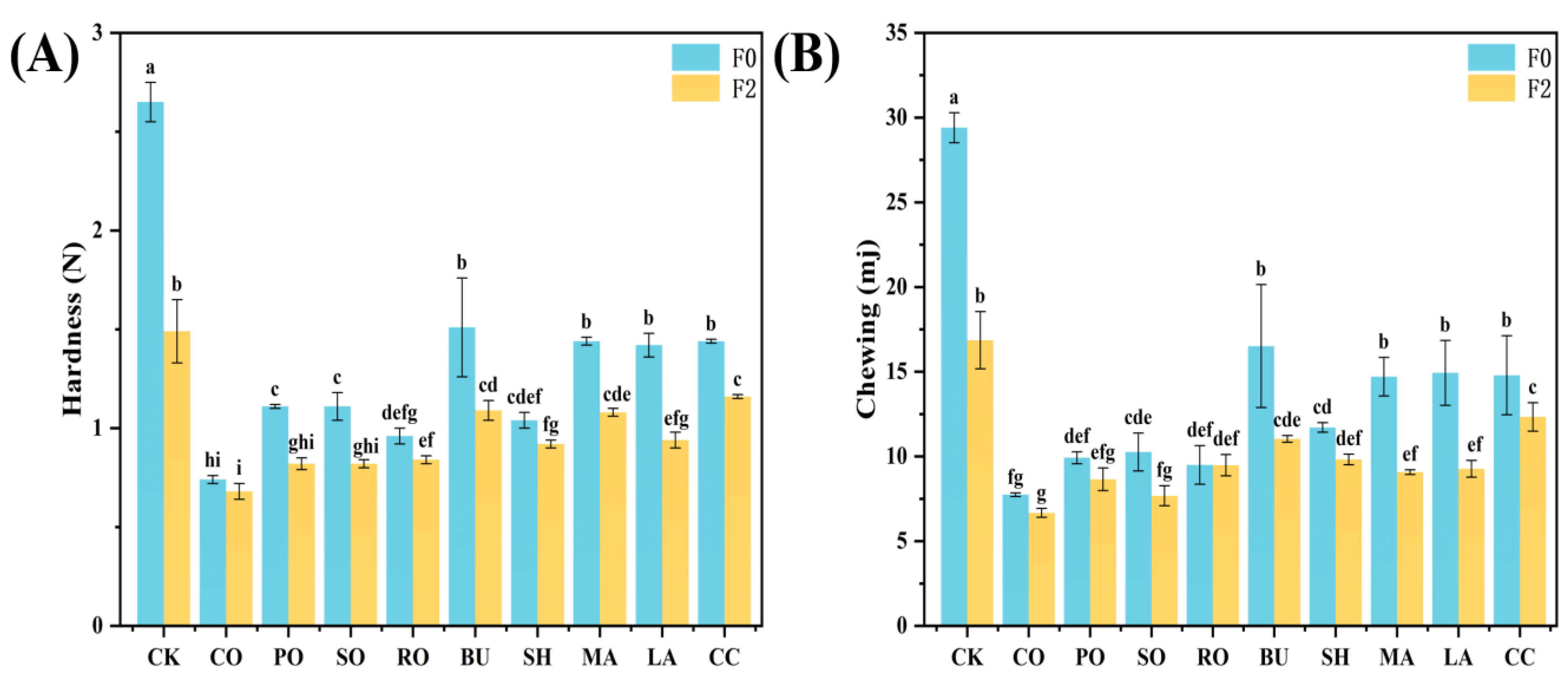
3.8.1. Textural Properties
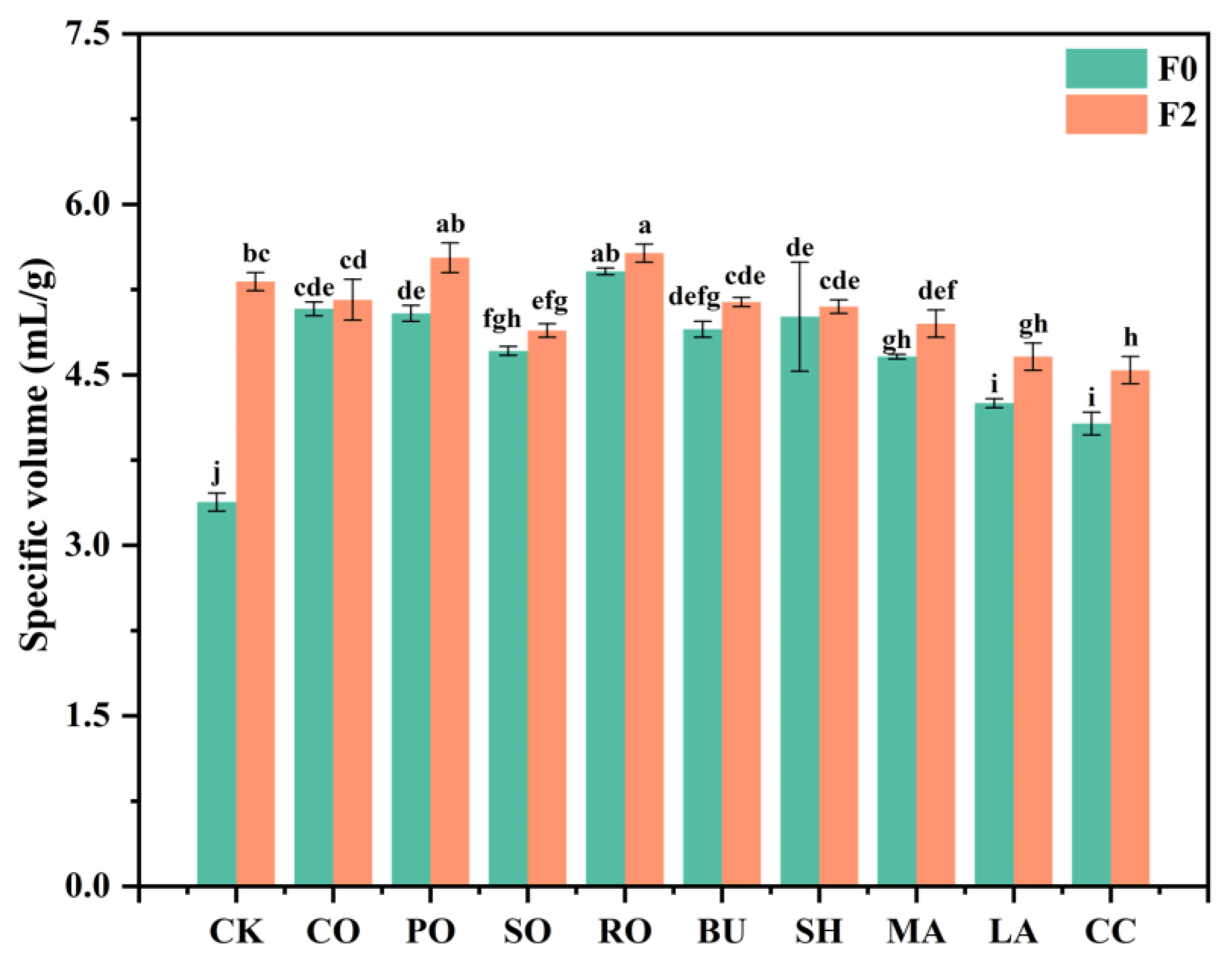
3.8.2. Specific Volume of Frozen Bread
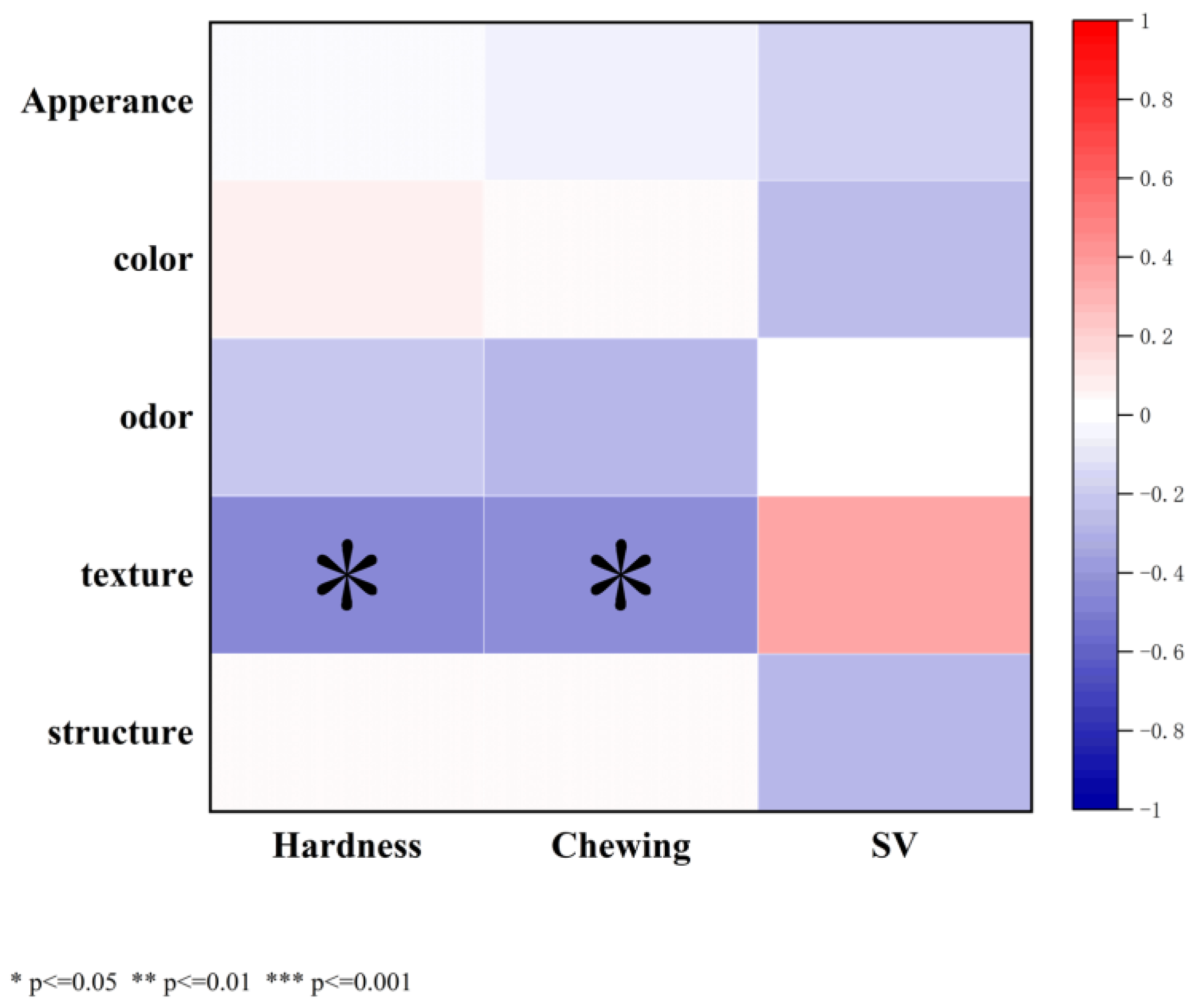
3.8.3. Sensory Evaluation of Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, T.; Sun, M.; Cao, S.; Hao, J.; Rao, J. Enhancing water retention and mechanisms of citrus and soya bean dietary fibres in pre-fermented frozen dough. Food Chem. X 2024, 22, 101269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Liu, Y.; Zhang, H. Effects of multiple freeze—Thaw cycles on the quality of frozen dough. Cereal Chem. 2018, 95, 499–507. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Tu, J.; Yu, L.; Niu, L. The alleviative effect of sweet potato protein hydrolysates on the quality deterioration of frozen dough bread in comparison to trehalose. LWT 2023, 175, 114505. [Google Scholar] [CrossRef]
- Zhang, T.; Guan, E.; Yang, Y.; Liu, F.; Zhang, L. Fatty acid profiles of vegetable oils from four different plant sources and their effects on dough rheology and Chinese steamed bread quality. Int. J. Food Sci. Technol. 2021, 56, 2407–2414. [Google Scholar] [CrossRef]
- Ropciuc, S.; Apostol, L.C.; Damian, C.; Prisacaru, A.E. Effect of hemp seed oil addition on the rheological properties of dough and bread. Appl. Sci. 2022, 12, 2764. [Google Scholar] [CrossRef]
- Ma, R.; Han, S.; Song, J.; Bai, Z.; Yue, C. Rheology, Moisture Distribution, and Retrogradation Characteristics of Dough Containing Peony Seed Oil and Quality of Corresponding Steamed Bread. Foods 2025, 14, 1505. [Google Scholar] [CrossRef]
- Culetu, A.; Stoica-Guzun, A.; Duta, D.E. Impact of fat types on the rheological and textural properties of gluten-free oat dough and cookie. Int. J. Food Sci. Technol. 2021, 56, 126–137. [Google Scholar] [CrossRef]
- Sun, H.; Yan, S.; Jiang, W.; Li, G.; MacRitchie, F. Contribution of lipid to physicochemical properties and Mantou-making quality of wheat flour. Food Chem. 2010, 121, 332–337. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Su, Y.; Fu, M.; Kan, J. Effects of oil and heating on the physicochemical and microstructural properties of gluten-starch dough. Food Chem. 2024, 436, 137571. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yang, T.; Ma, Y.; McClements, D.; Ren, F.; Tian, Y.; Jin, Z. A review of structural transformations and properties changes in starch during thermal processing of foods. Food Hydrocoll. 2021, 113, 106543. [Google Scholar] [CrossRef]
- Srikanlaya, C.; Therdthai, N.; Ritthiruangdej, P.; Zhou, W. Effect of butter content and baking condition on characteristics of the gluten-free dough and bread. Int. J. Food Sci. Technol. 2017, 52, 1904–1913. [Google Scholar] [CrossRef]
- He, T.; Zhang, B.; Cai, K.; Tao, H.; Xu, X. Application of magnetic field for improving the frozen deterioration of wheat dough. LWT 2023, 186, 115227. [Google Scholar] [CrossRef]
- Luo, D.; Liang, X.; Xu, B.; Kou, X.; Li, P. Effect of inulin with different degree of polymerization on plain wheat dough rheology and the quality of steamed bread. J. Cereal Sci. 2017, 75, 205–212. [Google Scholar] [CrossRef]
- GB/T 20981-2021; General Quality of Bread. Bakery Products. Standards Press of China: Beijing, China, 2021.
- Geng, L.; Zhou, W.; Qu, X.; Sa, R.; Liang, J. Iodine values, peroxide values and acid values of Bohai algae oil compared with other oils during the cooking. Heliyon 2023, 9, 15088. [Google Scholar] [CrossRef]
- Aruchunan, U.; Henry, C.J.; Sim, S.Y.J. Role of protein-lipid interactions for food and food-based applications. Food Hydrocoll. 2025, 160, 110715. [Google Scholar] [CrossRef]
- Pan, Z.; Bai, Y.; Xu, L.; Zhang, Y.; Lei, M. The Effect of Freeze–Thaw Cycles on the Microscopic Properties of Dumpling Wrappers. Foods 2023, 12, 3388. [Google Scholar] [CrossRef]
- Jung, D.; Oh, I.; Lee, J.H.; Lee, S. Utilization of butter and oleogel blends in sweet pan bread for saturated fat reduction: Dough rheology and baking performance. LWT 2020, 125, 109194. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Chen, K.; Wang, Z.; Fu, M. Effect of oleic acid-rich rapeseed oil on the physicochemical, rheological, and structural characteristics of wheat dough. Food Chem. 2024, 458, 140227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, D.; Zhou, H.; Wu, F.; Xu, X. Rheological, microstructure and mixing behaviors of frozen dough reconstituted by wheat starch and gluten. Int. J. Biol. Macromol. 2022, 212, 517–526. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D.; Sajeevkumar, V.A.; Sharma, G. Melting, crystallization and storage stability of virgin coconut oil and its blends by differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR). J. Food Sci. Technol. 2017, 54, 45–54. [Google Scholar] [CrossRef]
- Li, Z.; Deng, C.; Li, H.; Liu, C.; Bian, K. Characteristics of remixed fermentation dough and its influence on the quality of steamed bread. Food Chem. 2015, 179, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, L.; Zhao, R.; Liu, Q.; Liu, W. Effects of particle size distribution of potato starch granules on rheological properties of model dough underwent multiple freezing-thawing cycles. Food Res. Int. 2022, 156, 111112. [Google Scholar] [CrossRef]
- Duong, Q.; Purgianto, A.; Maleky, F. Dynamics of moisture diffusivity in solid triacylglycerol matrices. Food Res. Int. 2015, 75, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Huang, Q.; Li, H.; Wang, Z.; Fu, X. Controlled gelatinization of potato parenchyma cells under excess water condition: Structural and in vitro digestion properties of starch. Food Funct. 2019, 10, 5312–5322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, X.; Li, B.; Wu, H. Effect of interesterified blend-based fast-frozen special fat on the physical properties and microstructure of frozen dough. Food Chem. 2019, 272, 76–83. [Google Scholar] [CrossRef]
- Chang, X.; Liu, H.; Zhuang, K.; Chen, L.; Zhang, Q. Study on the Quality Variation and Internal Mechanisms of Frozen Oatmeal Cooked Noodles during Freeze–Thaw Cycles. Foods 2024, 13, 541. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ding, H.; Zhang, W.; Dai, Z. Effect of Washing Times on the Quality Characteristics and Protein Oxidation of Silver Carp Surimi. Foods 2022, 11, 2397. [Google Scholar] [CrossRef]
- Jia, S.; Cao, J.; Dai, Y.; Cui, B.; Yuan, C. Effects of soybean oil on rheological characteristics of dough under high hydrostatic pressure. J. Texture Stud. 2022, 53, 684–692. [Google Scholar] [CrossRef]
- Gutierrez, A.L.; Rico, D.; Ronda, F.; Caballero, P.A.; Martin-Diana, A.B. The Application of High-Hydrostatic-Pressure Processing to Improve the Quality of Baked Products: A Review. Foods 2023, 13, 130. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, X.; Liu, Y. Microstructural and crystallinity changes in starch-gluten model dough with varying palm oil solid fat content. Carbohydr. Polym. 2025, 368, 124103. [Google Scholar] [CrossRef]
- Devi, A.; Khatkar, B.S. Physicochemical, rheological and functional properties of fats and oils in relation to cookie quality: A review. J. Food Sci. Technol. 2016, 53, 3633–3641. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Yang, Z.; Wang, M.; Shang, S. Effect of acetylated distarch adipate on fermentation properties of freeze-thawed post-fermented frozen dough and bread characteristics. J. Cereal Sci. 2025, 121, 104094. [Google Scholar] [CrossRef]
- Zhao, B.; Fu, S.; Li, H.; Li, H.; Liu, C. Effect of storage conditions on the quality of frozen steamed bread. Int. J. Food Sci. Technol. 2022, 57, 695–704. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Wu, C.; Hou, L.; Liu, X. Cryoprotective mechanism of enzymatically interesterified rapeseed oil-based plastic fats on frozen dough: Interaction with water, gluten and starch. Int. J. Biol. Macromol. 2025, 319, 145689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pi, X.; Zhang, R.; Zhang, B. Evaluation of moisture migration and microstructure of quick-frozen wet rice flour after freeze—Thaw cycles and changes in texture, cooking, and sensory properties. J. Food Sci. 2024, 89, 8420–8430. [Google Scholar] [CrossRef]
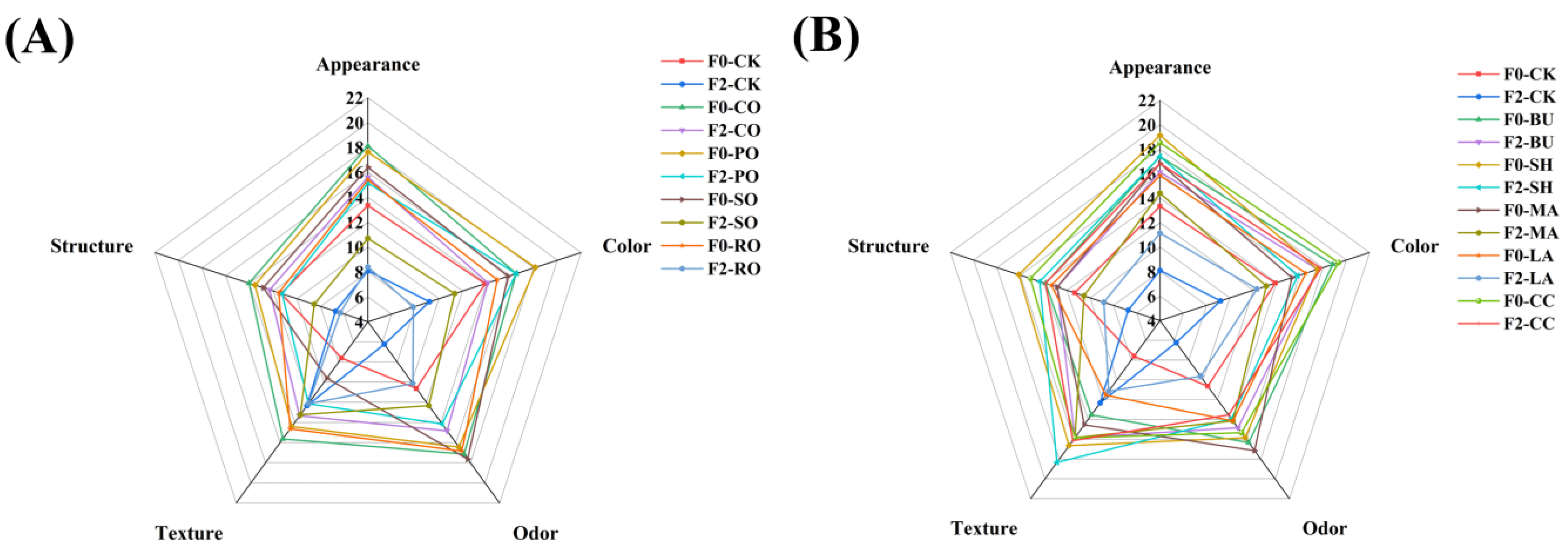
| Sensory Characteristics | Score | Definition |
|---|---|---|
| Appearance | 20 | Smooth surface without cracks. 16–20 points |
| Smooth surface with minor cracks. 11–15 points | ||
| Collapsed with visible cracks. 1–10 points | ||
| Color | 20 | The crust has a uniform color and good sheen. 16–20 points |
| The crust is light golden with a moderate sheen. 11–15 points | ||
| The crust is burnt. 1–10 points | ||
| Odor | 20 | A rich and pleasant oily fragrance. 16–20 points |
| A slightly faint oily fragrance. 11–15 points | ||
| Rancid and putrid oily odors. 1–10 points | ||
| Texture | 20 | Soft, non-sticky, smooth. 16–20 points |
| Slightly sticky/firm. 11–15 points | ||
| Too hard/sticky, hard to swallow. 1–10 points | ||
| Structure | 20 | Even pores, thin intact walls. 16–20 points |
| Few large pores, medium walls. 11–15 points | ||
| Oversized pores, broken loose walls. 1–10 points |
| Lipid Types | Number of Cycles | Hardness (N) | Elasticity (mm) | Chewability (mj) | |
|---|---|---|---|---|---|
| — | F0 | CK | 12.23 ± 0.30 d | 14.32 ± 0.67 b | 132.65 ± 16.71 cde |
| F2 | CK | 15.40 ± 0.71 b | 12.83 ± 0.67 cd | 163.48 ± 4.99 a | |
| Liquid oils | F0 | CO | 10.68 ± 0.14 g | 11.74 ± 0.27 ef | 87.90 ± 3.67 ij |
| F2 | CO | 15.20 ± 0.24 b | 10.77 ± 1.14 g | 118.82 ± 13.87 defgh | |
| F0 | PO | 10.94 ± 0.06 fg | 13.12 ± 0.40 c | 99.34 ± 8.62 hij | |
| F2 | PO | 14.68 ± 0.56 c | 12.65 ± 0.61 cde | 133.93 ± 9.50 cd | |
| F0 | SO | 11.55 ± 0.33 e | 11.06 ± 0.79 fg | 82.68 ± 5.03 j | |
| F2 | SO | 15.66 ± 0.21 b | 12.96 ± 0.17 cd | 157.25 ± 13.57 ab | |
| F0 | RO | 11.33 ± 0.46 ef | 12.15 ± 0.00 cde | 111.11 ± 18.94 fghi | |
| F2 | RO | 14.30 ± 0.41 c | 12.46 ± 1.00 cde | 123.26 ± 17.82 cdefg | |
| Solid fats | F0 | BU | 11.46 ± 0.36 ef | 12.26 ± 0.72 cde | 97.87 ± 10.22 hij |
| F2 | BU | 14.30 ± 0.25 c | 12.46 ± 0.98 cde | 108.78 ± 14.39 efgh | |
| F0 | SH | 12.17 ± 0.13 d | 12.29 ± 0.30 cde | 106.72 ± 3.57 ghi | |
| F2 | SH | 14.35 ± 0.29 c | 12.47 ± 0.24 cde | 140.97 ± 24.96 bcd | |
| F0 | MA | 11.72 ± 0.15 de | 12.06 ± 0.21 de | 102.71 ± 4.02 ghij | |
| F2 | MA | 14.61 ± 0.22 c | 12.84 ± 0.18 cd | 131.01 ± 18.95 cdef | |
| F0 | LA | 11.48 ± 0.06 ef | 11.94 ± 0.07 def | 119.01 ± 0.77 defgh | |
| F2 | LA | 16.41 ± 0.12 a | 11.97 ± 0.28 def | 141.87 ± 5.27 bc | |
| F0 | CC | 11.84 ± 0.32 de | 12.24 ± 0.11 cde | 103.11 ± 10.68 ghij | |
| F2 | CC | 14.20 ± 0.22 c | 16.79 ± 0.24 a | 142.82 ± 13.89 abc | |
| Lipid Types | Number of Cycles | T21 (ms) | T22 (ms) | T23 (ms) | A21 (%) | A22 (%) | A23 (%) | |
|---|---|---|---|---|---|---|---|---|
| — | F0 | CK | 1.32 ± 0.00 | 16.30 ± 0.00 | 231.01 ± 0.00 | 20.82 ± 0.12 bcde | 78.75 ± 0.11 ab | 0.44 ± 0.03 k |
| F2 | CK | 1.15 ± 0.000 | 16.30 ± 0.00 | 265.61 ± 0.00 | 21.24 ± 0.65 ab | 78.50 ± 0.65 bc | 0.27 ± 0.01 l | |
| Liquid oils | F0 | CO | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 21.22 ± 0.25 ab | 77.00 ± 0.20 k | 1.77 ± 0.05 d |
| F2 | CO | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 20.47 ± 0.23 defg | 77.70 ± 0.24 efghi | 1.84 ± 0.02 cd | |
| F0 | PO | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 20.91 ± 0.07 bcd | 77.25 ± 0.08 jk | 1.84 ± 0.10 cd | |
| F2 | PO | 1.32 ± 0.00 | 16.30 ± 0.00 | 174.75 ± 0.00 | 20.44 ± 0.11 efg | 77.69 ± 0.10 efghi | 1.87 ± 0.05 cd | |
| F0 | SO | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 20.72 ± 0.20 cdef | 77.29 ± 0.19 ijk | 1.99 ± 0.02 b | |
| F2 | SO | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 20.13 ± 0.09 gh | 77.78 ± 0.04 efgh | 2.08 ± 0.06 a | |
| F0 | RO | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 20.72 ± 0.23 cdef | 77.47 ± 0.25 ghij | 1.81 ± 0.06 cd | |
| F2 | RO | 1.32 ± 0.00 | 16.30 ± 0.00 | 174.75 ± 0.00 | 20.31 ± 0.07 fgh | 77.80 ± 0.04 efg | 1.88 ± 0.09 c | |
| Solid fats | F0 | BU | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 21.45 ± 0.06 a | 77.37 ± 0.06 hijk | 1.19 ± 0.05 h |
| F2 | BU | 1.32 ± 0.00 | 16.30 ± 0.00 | 174.75 ± 0.00 | 19.54 ± 0.10 j | 79.09 ± 0.10 a | 1.34 ± 0.03 f | |
| F0 | SH | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 21.41 ± 0.07 a | 77.50 ± 0.09 ghij | 1.09 ± 0.06 i | |
| F2 | SH | 1.32 ± 0.00 | 16.30 ± 0.00 | 174.75 ± 0.00 | 19.99 ± 0.54 hi | 78.72 ± 0.50 ab | 1.30 ± 0.05 fg | |
| F0 | MA | 1.15 ± 0.00 | 16.30 ± 0.00 | 174.75 ± 0.00 | 20.57 ± 0.17 cdefg | 77.95 ± 0.08 def | 1.49 ± 0.09 e | |
| F2 | MA | 1.15 ± 0.00 | 16.30 ± 0.00 | 174.75 ± 0.00 | 19.69 ± 0.20 ij | 78.67 ± 0.09 b | 1.53 ± 0.03 e | |
| F0 | LA | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 21.40 ± 0.19 a | 77.66 ± 0.21 fghij | 0.94 ± 0.03 j | |
| F2 | LA | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 20.55 ± 0.12 cdefg | 78.22 ± 0.11 cd | 1.23 ± 0.02 gh | |
| F0 | CC | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 21.53 ± 0.18 a | 77.30 ± 0.14 ijk | 1.17 ± 0.04 hi | |
| F2 | CC | 1.32 ± 0.00 | 16.30 ± 0.00 | 200.92 ± 0.00 | 20.96 ± 0.05 bc | 78.09 ± 0.06 de | 0.95 ± 0.02 j | |
| Lipid Types | Number of Cycles | TO (°C) | TP (ms) | TC (ms) | ΔH (J·g) | |
|---|---|---|---|---|---|---|
| — | F0 | CK | 58.09 ± 0.51 f | 64.97 ± 0.21 cdefg | 71.21 ± 0.41 ab | 4.39 ± 0.28 a |
| F2 | CK | 59.86 ± 0.41 bcde | 65.08 ± 0.09 cdef | 70.85 ± 1.31 abc | 4.24 ± 0.36 ab | |
| Liquid oils | F0 | CO | 60.62 ± 0.83 abcd | 65.61 ± 0.18 abc | 71.54 ± 0.93 a | 3.54 ± 0.14 c |
| F2 | CO | 61.06 ± 0.79 ab | 65.29 ± 0.260 bcd | 70.99 ± 0.67 abc | 3.52 ± 0.33 cd | |
| F0 | PO | 61.25 ± 0.95 a | 65.84 ± 0.56 ab | 70.53 ± 0.89 abc | 3.02 ± 0.17 de | |
| F2 | PO | 60.96 ± 0.43 abc | 65.25 ± 0.06 bcde | 70.99 ± 0.14 abc | 3.40 ± 0.13 cd | |
| F0 | SO | 59.55 ± 0.90 de | 64.79 ± 0.30 defg | 70.95 ± 0.68 abc | 3.81 ± 0.11 bc | |
| F2 | SO | 60.06 ± 0.49 abcd | 64.60 ± 0.22 efg | 70.63 ± 0.15 abc | 3.48 ± 0.26 cd | |
| F0 | RO | 59.72 ± 1.45 cde | 64.48 ± 0.35 fg | 69.91 ± 0.48 bc | 2.87 ± 0.21 e | |
| F2 | RO | 61.17 ± 0.61 a | 65.27 ± 0.22 bcde | 71.46 ± 0.96 a | 3.41 ± 0.10 cd | |
| Solid fats | F0 | BU | 58.70 ± 0.35 ef | 64.38 ± 0.00 g | 69.65 ± 0.02 c | 3.44 ± 0.38 cd |
| F2 | BU | 59.56 ± 0.38 de | 65.22 ± 0.24 bcde | 70.85 ± 0.73 abc | 3.80 ± 0.06 bc | |
| F0 | SH | 60.71 ± 0.50 abcd | 65.30 ± 0.31 bcd | 70.86 ± 0.86 abc | 3.58 ± 0.35 c | |
| F2 | SH | 60.95 ± 0.89 abc | 66.13 ± 0.92 a | 71.70 ± 0.27 a | 3.58 ± 0.33 c | |
| F0 | MA | 60.86 ± 0.67 abc | 65.50 ± 0.25 abc | 71.62 ± 0.65 a | 3.70 ± 0.54 c | |
| F2 | MA | 60.63 ± 0.43 abcd | 65.56 ± 0.54 abc | 71.17 ± 0.71 ab | 3.80 ± 0.22 bc | |
| F0 | LA | 60.45 ± 0.63 abcd | 65.19 ± 0.24 bcde | 71.44 ± 1.06 a | 3.71 ± 0.03 c | |
| F2 | LA | 59.87 ± 0.21 bcde | 64.99 ± 0.20 cdefg | 71.24 ± 0.88 ab | 3.44 ± 0.20 cd | |
| F0 | CC | 60.61 ± 0.25 abcd | 65.23 ± 0.23 bcde | 71.35 ± 0.88 ab | 3.50 ± 0.31 cd | |
| F2 | CC | 60.11 ± 0.19 abcd | 65.09 ± 0.40 cdef | 70.48 ± 0.91 abc | 3.84 ± 0.22 bc | |
| Lipid Types | Number of Cycles | β-Sheet (%) | β-Turn (%) | |
|---|---|---|---|---|
| — | F0 | CK | 25.31 ± 0.54 abc | 25.61 ± 0.26 ef |
| F2 | CK | 24.80 ± 0.89 bcd | 26.88 ± 1.94 cdef | |
| Liquid oils | F0 | CO | 24.69 ± 0.13 bcd | 29.80 ± 0.20 a |
| F2 | CO | 25.95 ± 0.32 ab | 24.71 ± 1.67 f | |
| F0 | PO | 24.80 ± 1.61 bcd | 26.20 ± 0.58 def | |
| F2 | PO | 25.23 ± 0.22 abc | 29.26 ± 0.35 ab | |
| F0 | SO | 24.13 ± 0.83 cd | 28.86 ± 1.93 abc | |
| F2 | SO | 25.88 ± 0.20 ab | 25.77 ± 0.36 def | |
| F0 | RO | 24.87 ± 0.17 bc | 29.77 ± 0.13 def | |
| F2 | RO | 25.76 ± 0.67 ab | 29.11 ± 1.01 ab | |
| Solid fats | F0 | BU | 23.46 ± 0.67 d | 26.44 ± 0.34 def |
| F2 | BU | 23.94 ± 1.14 cd | 27.45 ± 2.21 bcde | |
| F0 | SH | 25.75 ± 0.20 ab | 25.87 ± 0.34 def | |
| F2 | SH | 25.58 ± 0.25 ab | 27.60 ± 1.30 bcde | |
| F0 | MA | 25.70 ± 0.09 ab | 26.04 ± 0.12 d | |
| F2 | MA | 25.80 ± 0.46 ab | 27.38 ± 2.66 bcde | |
| F0 | LA | 26.43 ± 1.34 a | 27.86 ± 1.47 abcd | |
| F2 | LA | 24.83 ± 0.11 bcd | 29.51 ± 0.22 ab | |
| F0 | CC | 25.04 ± 0.58 abc | 26.54 ± 0.53 def | |
| F2 | CC | 25.14 ± 0.77 abc | 25.89 ± 0.51 def | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Yan, K.; Xia, J.; Yang, Z.; Wang, Z. Impact of Different Types of Lipids on the Quality of Frozen Dough and Bread. Foods 2025, 14, 4032. https://doi.org/10.3390/foods14234032
Gao R, Yan K, Xia J, Yang Z, Wang Z. Impact of Different Types of Lipids on the Quality of Frozen Dough and Bread. Foods. 2025; 14(23):4032. https://doi.org/10.3390/foods14234032
Chicago/Turabian StyleGao, Rui, Kai Yan, Jian Xia, Zixuan Yang, and Zhan Wang. 2025. "Impact of Different Types of Lipids on the Quality of Frozen Dough and Bread" Foods 14, no. 23: 4032. https://doi.org/10.3390/foods14234032
APA StyleGao, R., Yan, K., Xia, J., Yang, Z., & Wang, Z. (2025). Impact of Different Types of Lipids on the Quality of Frozen Dough and Bread. Foods, 14(23), 4032. https://doi.org/10.3390/foods14234032






