Influence of Fermentation Temperature and Metschnikowia pulcherrima/Saccharomyces cerevisiae Multi-Starter Cultures on the Volatile Compounds of Lugana Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Enzymatic Assays
2.3. Microvinification Trials
- (1)
- control S. cerevisiae EC 1118 pure culture (S1), inoculated at time 0;
- (2)
- control S. cerevisiae Zymaflore X5 pure culture (S2), inoculated at time 0;
- (3)
- inoculation of M. pulcherrima Flavia followed by S. cerevisiae EC 1118 after 48 h (M1S1);
- (4)
- inoculation of M. pulcherrima Flavia followed by S. cerevisiae Zymaflore X5 after 48 h (M1S2);
- (5)
- inoculation of M. pulcherrima Initia followed by S. cerevisiae EC 1118 after 48 h (M2S1);
- (6)
- inoculation of M. pulcherrima Initia followed by S. cerevisiae Zymaflore X5 after 48 h (M2S2).
2.4. Fermentation Kinetics and Microbiological Analysis
2.5. Chemical Analysis of Must and Wine
2.6. Quantification of Volatile Compounds
2.7. Sensory Evaluation
2.8. Statistical Data Analysis
3. Results and Discussion
3.1. Enzymatic Activity
3.2. Fermentation Kinetics and Performances
3.3. Dominance of the Inoculated Strains
3.4. Chemical Analysis
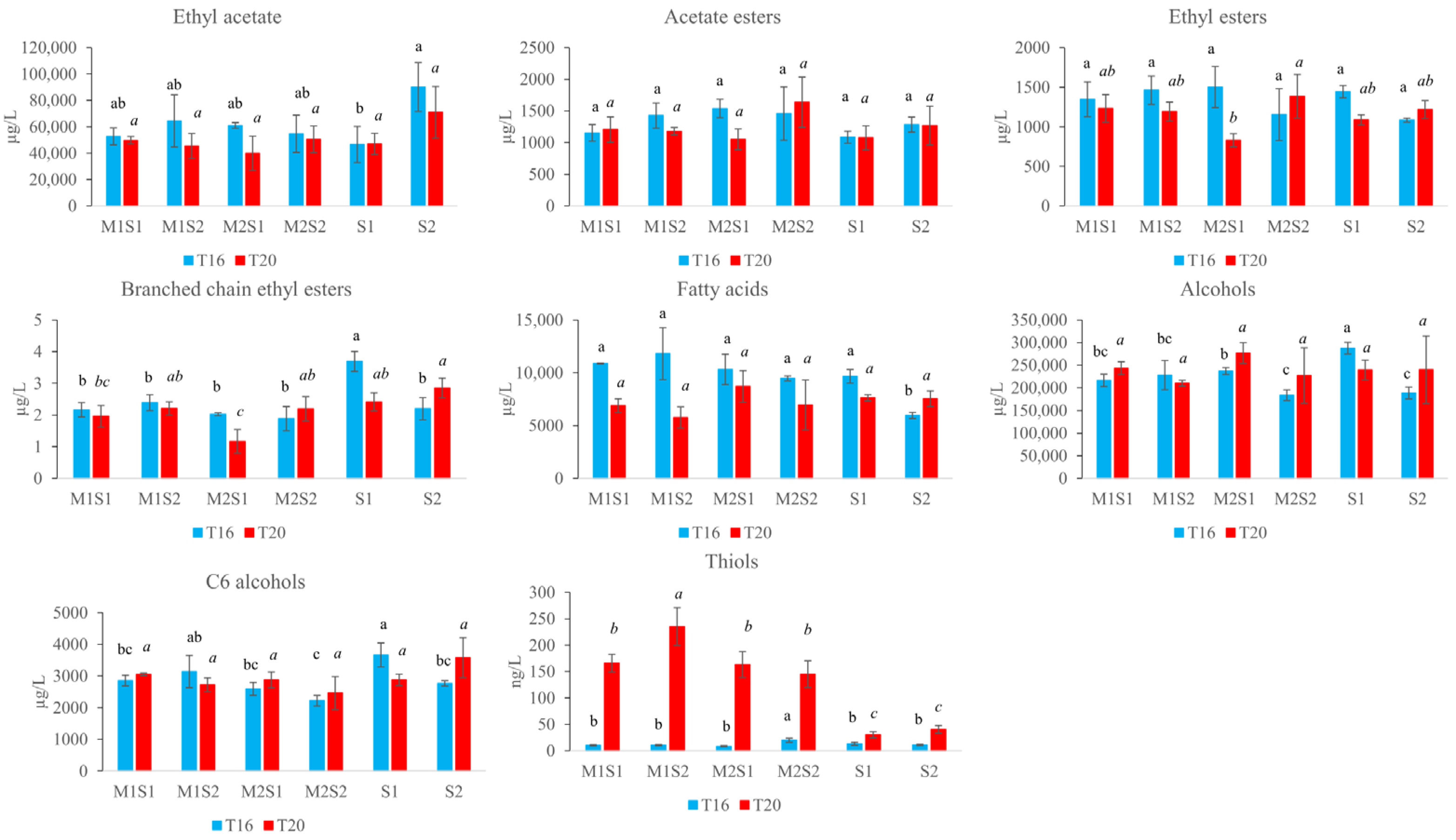
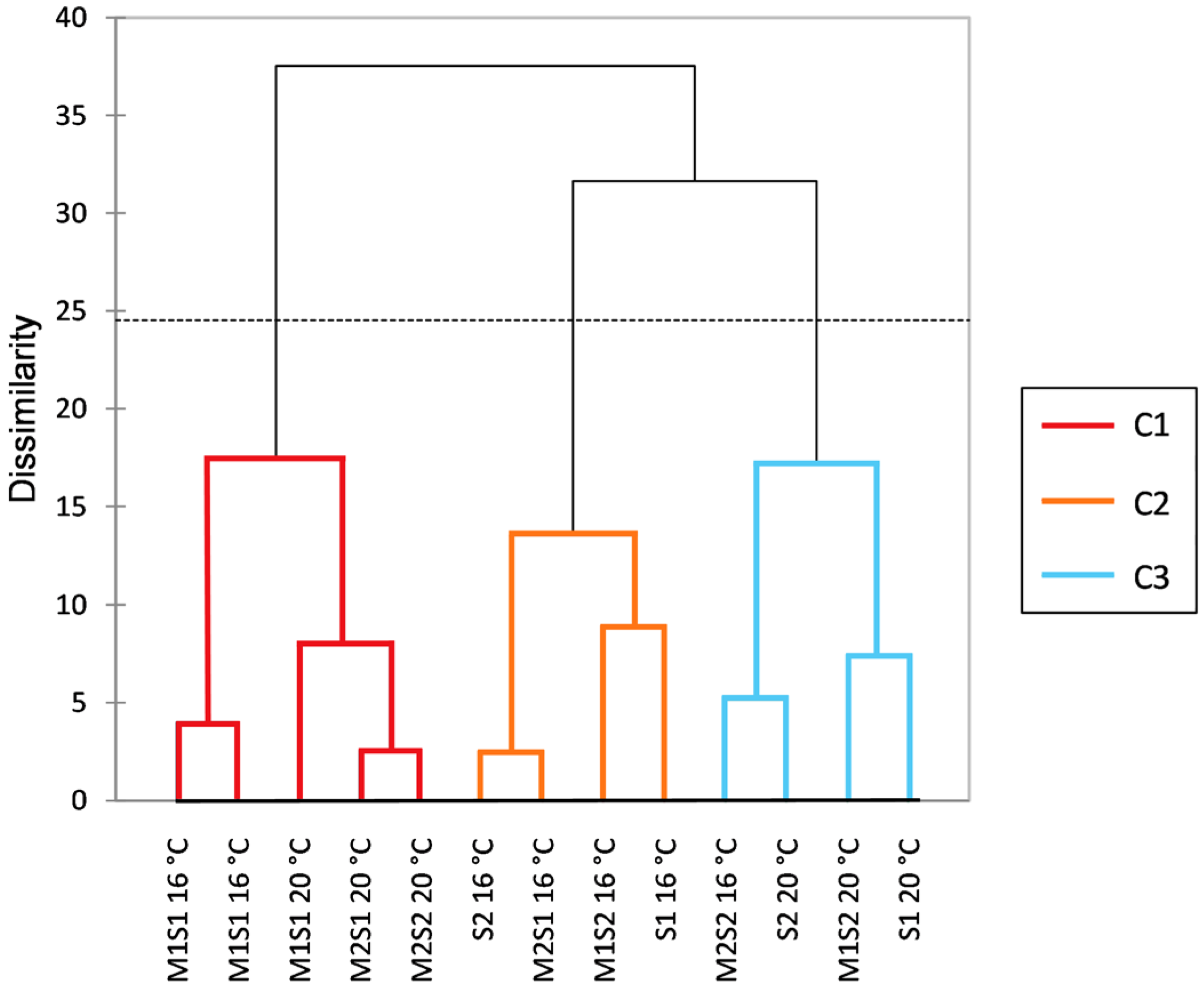
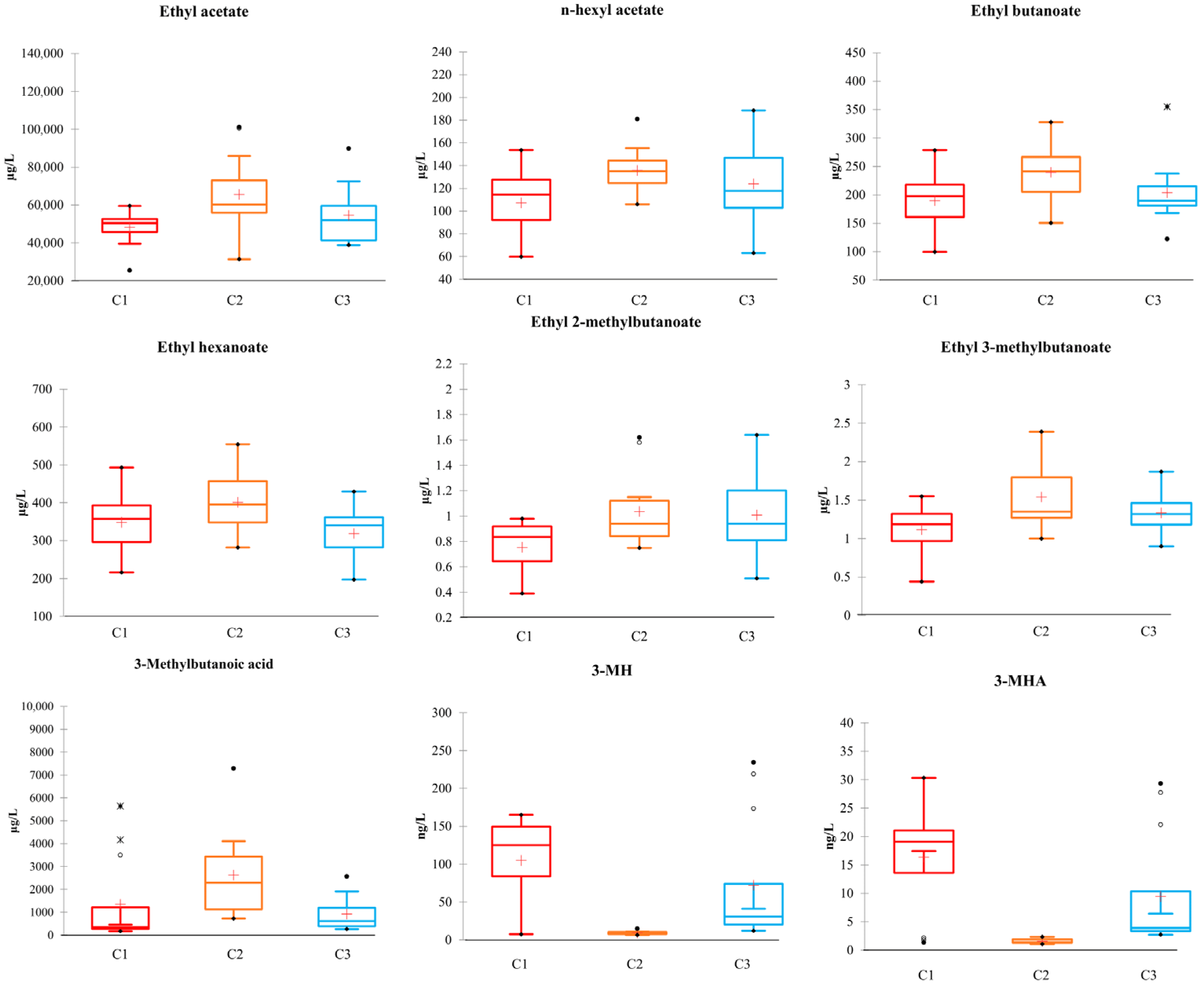
3.5. Quantification of Volatile Compounds
3.6. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Nardi, T. Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms 2020, 8, 507. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Giovenzana, V.; Beghi, R.; Vagnoli, P.; Iacono, F.; Guidetti, R.; Nardi, T. Evaluation of Energy Saving Using a New Yeast Combined with Temperature Management in Sparkling Base Wine Fermentation. Am. J. Enol. Vitic. 2016, 67, 308–314. [Google Scholar] [CrossRef]
- Brito, C.; Pereira, S.; Martins, S.; Monteiro, A.; Moutinho-Pereira, J.M.; Dinis, L. Strategies for Achieving the Sustainable Development Goals across the Wine Chain: A Review. Front. Sustain. Food Syst. 2024, 8, 1437872. [Google Scholar] [CrossRef]
- Giovenzana, V.; Beghi, R.; Guidetti, R.; Luison, M.; Nardi, T. Evaluation of Energy Savings in White Winemaking: Impact of Temperature Management Combined with Specific Yeasts Choice on Required Heat Dissipation during Industrial-Scale Fermentation. J. Agric. Eng. 2023, 54, 1–9. [Google Scholar] [CrossRef]
- Abarca-Rivas, C.; Martín-Garcia, A.; Riu-Aumatell, M.; Bidon-Chanal, A.; López-Tamames, E. Effect of Fermentation Temperature on Oenological Parameters and Volatile Compounds in Wine. BIO Web Conf. 2023, 56, 02034. [Google Scholar] [CrossRef]
- Deed, R.C.; Fedrizzi, B.; Gardner, R.C. Influence of Fermentation Temperature, Yeast Strain, and Grape Juice on the Aroma Chemistry and Sensory Profile of Sauvignon Blanc Wines. J. Agric. Food Chem. 2017, 65, 8902–8912. [Google Scholar] [CrossRef]
- Deed, R.C.; Deed, N.K.; Gardner, R.C. Transcriptional Response of Saccharomyces cerevisiae to Low Temperature during Wine Fermentation. Antonie Van Leeuwenhoek 2015, 107, 1029–1048. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of Wine Fermentation Temperature on the Synthesis of Yeast-Derived Volatile Aroma Compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast Fermentation at Low Temperatures: Adaptation to Changing Environmental Conditions and Formation of Volatile Compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef]
- Alonso-del-Real, J.; Lairón-Peris, M.; Barrio, E.; Querol, A. Effect of Temperature on the Prevalence of Saccharomyces Non-cerevisiae Species against a S. cerevisiae Wine Strain in Wine Fermentation: Competition, Physiological Fitness, and Influence in Final Wine Composition. Front. Microbiol. 2017, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Muhl, J.R.; Pilkington, L.I.; Fedrizzi, B.; Deed, R.C. Insights into the Relative Contribution of Four Precursors to 3-Sulfanylhexan-1-Ol and 3-Sulfanylhexylacetate Biogenesis during Fermentation. Food Chem. 2024, 449, 139193. [Google Scholar] [CrossRef]
- Fazio, N.A.; Russo, N.; Foti, P.; Pino, A.; Caggia, C.; Randazzo, C.L. Inside Current Winemaking Challenges: Exploiting the Potential of Conventional and Unconventional Yeasts. Microorganisms 2023, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Oenological Potential of Non-Saccharomyces Yeasts to Mitigate Effects of Climate Change in Winemaking: Impact on Aroma and Sensory Profiles of Treixadura Wines. FEMS Yeast Res. 2019, 19, foz065. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Mateo, J.; Maicas, S. Application of Non-Saccharomyces Yeasts to Wine-Making Process. Fermentation 2016, 2, 14. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiololy 2016, 7, 411. [Google Scholar] [CrossRef]
- Vicente, J.; Ruiz, J.; Belda, I.; Benito-Vázquez, I.; Marquina, D.; Calderón, F.; Santos, A.; Benito, S. The Genus Metschnikowia in Enology. Microorganisms 2020, 8, 1038. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical Impact of Metschnikowia pulcherrima in the Volatile Profile of Verdejo White Wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of Non-Saccharomyces Yeasts to Wine Volatile and Sensory Diversity: A Study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris Strains Isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; Del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Navascués, E.; Marquina, D.; Santos, A. Improvement of Aromatic Thiol Release through the Selection of Yeasts with Increased β-Lyase Activity. Int. J. Food Microbiol. 2016, 225, 1–8. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; González-Arenzana, L.; Garijo, P.; López, R.; Santamaría, P.; Gutiérrez, A.R. Selection Process of a Mixed Inoculum of Non-Saccharomyces Yeasts Isolated in the D.O.Ca. Rioja. Fermentation 2021, 7, 148. [Google Scholar] [CrossRef]
- Huang, J.; Li, H.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Gao, Z. Evaluation of the Quality of Fermented Kiwi Wines Made from Different Kiwifruit Cultivars. Food Biosci. 2021, 42, 101051. [Google Scholar] [CrossRef]
- Troiano, E. Biodiversity of Metschnikowia pulcherrima as a Resource for Innovation in Fermented Beverages. Ph.D. Thesis, University of Verona, Verona, Italy, 2022. [Google Scholar]
- Cooper, A.J.L.; Krasnikov, B.F.; Pinto, J.T.; Bruschi, S.A. Measurement of Cysteine S -Conjugate β-Lyase Activity. Curr. Protoc. ToxicolToxicol. 2010, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, C.; Du Toit, W.J. A Comprehensive Review on Sauvignon Blanc Aroma with a Focus on Certain Positive Volatile Thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- Murat, M.-L.; Masneuf, I.; Darriet, P.; Lavigne, V.; Tominaga, T.; Dubourdieu, D. Effect of Saccharomyces cerevisiae Yeast Strains on the Liberation of Volatile Thiols in Sauvignon Blanc Wine. Am. J. Enol. Vitic. 2001, 52, 136–139. [Google Scholar] [CrossRef]
- Holt, S.; Cordente, A.G.; Curtin, C. Saccharomyces cerevisiae STR3 and Yeast Cystathionine β-Lyase Enzymes: The Potential for Engineering Increased Flavor Release. Bioengineered 2012, 3, 180–182. [Google Scholar] [CrossRef]
- Seguinot, P.; Bloem, A.; Brial, P.; Meudec, E.; Ortiz-Julien, A.; Camarasa, C. Analysing the Impact of the Nature of the Nitrogen Source on the Formation of Volatile Compounds to Unravel the Aroma Metabolism of Two Non-Saccharomyces Strains. Int. J. Food Microbiol. 2020, 316, 108441. [Google Scholar] [CrossRef]
- Bustamante, M.; Giménez, P.; Just-Borràs, A.; Solé-Clua, I.; Gombau, J.; Heras, J.M.; Sieczkowski, N.; Gil, M.; Canals, J.M.; Zamora, F. Inoculation with a Selected Strain of Metschnikowia pulcherrima as a Bioprotective Alternative to Sulphites for Preventing Browning of White Grape Must. OENO One 2024, 58, 1–10. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-Sulfanylhexan-1-Ol Production in Sequential Mixed Fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae Reveals a Situation of Synergistic Interaction between Two Industrial Strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef]
- González Flores, M.; Rodríguez, M.E.; Oteiza, J.M.; Barbagelata, R.J.; Lopes, C.A. Physiological Characterization of Saccharomyces uvarum and Saccharomyces eubayanus from Patagonia and Their Potential for Cidermaking. Int. J. Food Microbiol. 2017, 249, 9–17. [Google Scholar] [CrossRef]
- Cavazza, A.; Grando, M.S.; Zini, C. Rilevazione Della Flora Microbica Di Mosti e Vini. Vignevini 1992, 9, 17–20. [Google Scholar]
- Legras, J.-L.; Karst, F. Optimisation of Interdelta Analysis for Saccharomyces cerevisiae Strain Characterisation. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef]
- Luzzini, G.; Slaghenaufi, D.; Ugliano, M. Volatile Compounds in Monovarietal Wines of Two Amarone Della Valpolicella Terroirs: Chemical and Sensory Impact of Grape Variety and Origin, Yeast Strain and Spontaneous Fermentation. Foods 2021, 10, 2474. [Google Scholar] [CrossRef] [PubMed]
- Carlin, S.; Piergiovanni, M.; Pittari, E.; Tiziana Lisanti, M.; Moio, L.; Piombino, P.; Marangon, M.; Curioni, A.; Rolle, L.; Río Segade, S.; et al. The Contribution of Varietal Thiols in the Diverse Aroma of Italian Monovarietal White Wines. Food Res. Int. 2022, 157, 111404. [Google Scholar] [CrossRef]
- Alegre, Y.; Sáenz-Navajas, M.-P.; Ferreira, V.; García, D.; Razquin, I.; Hernández-Orte, P. Rapid Strategies for the Determination of Sensory and Chemical Differences between a Wealth of Similar Wines. Eur. Food Res. Technol. 2017, 243, 1295–1309. [Google Scholar] [CrossRef]
- Parr, W.V.; Heatherbell, D.; White, K.G. Demystifying Wine Expertise: Olfactory Threshold, Perceptual Skill and Semantic Memory in Expert and Novice Wine Judges. Chem. Senses 2002, 27, 747–755. [Google Scholar] [CrossRef]
- ISO 3591:1977; Sensory Analysis—Apparatus—Wine-Tasting Glass. American National Standards Institute: Washington, DC, USA, 1977. Available online: https://www.iso.org/standard/9002.html (accessed on 29 November 2024).
- Perpetuini, G.; Rossetti, A.P.; Quadrani, L.; Arfelli, G.; Piva, A.; Suzzi, G.; Tofalo, R. Sequential Inoculation of Metschnikowia pulcherrima and Saccharomyces cerevisiae as a Biotechnological Tool to Increase the Terpenes Content of Pecorino White Wines. Fermentation 2023, 9, 785. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Assessment of Spontaneous Fermentation and Non-Saccharomyces Sequential Fermentation in Verdicchio Wine at Winery Scale. Beverages 2022, 8, 49. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alastruey-Izquierdo, A.; Navascués, E.; Marquina, D.; Santos, A. Unraveling the Enzymatic Basis of Wine “Flavorome”: A Phylo-Functional Study of Wine Related Yeast Species. Front. Microbiol. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Thibon, C.; Marullo, P.; Claisse, O.; Cullin, C.; Dubourdieu, D.; Tominaga, T. Nitrogen Catabolic Repression Controls the Release of Volatile Thiols by Saccharomyces cerevisiae during Wine Fermentation. FEMS Yeast Res. 2008, 8, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.S.; Klein, M.; Swiegers, J.H.; Hayasaka, Y.; Elsey, G.M.; Fleet, G.H.; Høj, P.B.; Pretorius, I.S.; de Barros Lopes, M.A. Genetic Determinants of Volatile-Thiol Release by Saccharomyces cerevisiae during Wine Fermentation. Appl. Environ. Microbiol. 2005, 71, 5420–5426. [Google Scholar] [CrossRef]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The Grape Must Non-Saccharomyces Microbial Community: Impact on Volatile Thiol Release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and Diversity of Non-Saccharomyces Yeasts during the Early Stages in Winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Marin, I.K.; González-Hernández, J.C.; Regalado-Gonzalez, C.; Madrigal-Perez, L.A. Saccharomyces cerevisiae Exponential Growth Kinetics in Batch Culture to Analyze Respiratory and Fermentative Metabolism. J. Vis. Exp. JoVE 2018, 139, 58192. [Google Scholar] [CrossRef]
- Charoenchai, C.; Fleet, G.H.; Henschke, P.A. Effects of Temperature, pH, and Sugar Concentration on the Growth Rates and Cell Biomass of Wine Yeasts. Am. J. Enol. Vitic. 1998, 49, 283–288. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on Quality and Composition of Riesling Wines Fermented by Sequential Inoculation with Non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environ. Microbiol. J. 2014, 80, 1670–1678. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The Role and Use of Non-Saccharomyces Yeasts in Wine Production. S. Afr. J. Enol. Vitic. 2006, 27, 15–38. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial Terroir and Food Innovation: The Case of Yeast Biodiversity in Wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Sadoudi, M.; Rousseaux, S.; David, V.; Alexandre, H.; Tourdot-Maréchal, R. Metschnikowia pulcherrima Influences the Expression of Genes Involved in PDH Bypass and Glyceropyruvic Fermentation in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1137. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airen Wines Fermented by Sequential Inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Prior, K.J.; Bauer, F.F.; Divol, B. The Utilisation of Nitrogenous Compounds by Commercial Non-Saccharomyces Yeasts Associated with Wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef]
- Maarse, H. Volatile Compounds in Foods and Beverages, 1st ed.Maarse, H., Ed.; Routledge: New York, NY, USA, 2017; ISBN 978-0-203-73428-5. [Google Scholar]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Wildenradt, H.L.; Christensen, E.N.; Stackler, B.; Caputi, A.; Slinkard, K.; Scutt, K. Volatile Constituents of Grape Leaves. I. Vitis Vinifera Variety “Chenin Blanc”. Am. J. Enol. Vitic. 1975, 26, 148–153. [Google Scholar] [CrossRef]
- Ferreira, L.; Perestrelo, R.; Camara, J. Comparative Analysis of the Volatile Fraction from Annona Cherimola Mill. Cultivars by Solid-Phase Microextraction and Gas Chromatography–Quadrupole Mass Spectrometry Detection. Talanta 2009, 77, 1087–1096. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Tominaga, T.; Baltenweck-Guyot, R.; Gachons, C.P.D.; Dubourdieu, D. Contribution of Volatile Thiols to the Aromas of White Wines Made From Several Vitis Vinifera Grape Varieties. Am. J. Enol. Vitic. 2000, 51, 178–181. [Google Scholar] [CrossRef]
- Beltran, G.; Novo, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effect of Fermentation Temperature and Culture Media on the Yeast Lipid Composition and Wine Volatile Compounds. Int. J. Food Microbiol. 2008, 121, 169–177. [Google Scholar] [CrossRef]
- Masneufpomarede, I.; Mansour, C.; Murat, M.; Tominaga, T.; Dubourdieu, D. Influence of Fermentation Temperature on Volatile Thiols Concentrations in Sauvignon Blanc Wines. Int. J. Food Microbiol. 2006, 108, 385–390. [Google Scholar] [CrossRef]
- Castellari, L.; Magrini, A.; Passarelli, P.; Zambonelli, C. Effect of Must Fermentation Temperature on Minor Products Formed by Cryo and Non-cryotolerant Saccharomyces cerevisiae Strains. Ital. J. Food Sci. 1995, 7, 125–132. [Google Scholar]
- Hu, K.; Jin, G.-J.; Mei, W.-C.; Li, T.; Tao, Y.-S. Increase of Medium-Chain Fatty Acid Ethyl Ester Content in Mixed H. Uvarum/S. cerevisiae Fermentation Leads to Wine Fruity Aroma Enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Eden, A.; Van Nedervelde, L.; Drukker, M.; Benvenisty, N.; Debourg, A. Involvement of Branched-Chain Amino Acid Aminotransferases in the Production of Fusel Alcohols during Fermentation in Yeast. Appl. Microbiol. Biotechnol. 2001, 55, 296–300. [Google Scholar] [CrossRef]
- Suomalainen, H. Yeast Esterases and Aroma Esters in Alcoholic Beverages. J. Inst. Brew. 1981, 87, 296–300. [Google Scholar] [CrossRef]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effects of Fermentation Temperature and Saccharomyces Species on the Cell Fatty Acid Composition and Presence of Volatile Compounds in Wine. Int. J. Food Microbiol. 2003, 85, 127–136. [Google Scholar] [CrossRef]
- Du, Q.; Ye, D.; Zang, X.; Nan, H.; Liu, Y. Effect of Low Temperature on the Shaping of Yeast-Derived Metabolite Compositions during Wine Fermentation. Food Res. Int. 2022, 162, 112016. [Google Scholar] [CrossRef]
- Gamero, A.; Tronchoni, J.; Querol, A.; Belloch, C. Production of Aroma Compounds by Cryotolerant Saccharomyces Species and Hybrids at Low and Moderate Fermentation Temperatures. J. Appl. Microbiol. 2013, 114, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Dubourdieu, D.; Tominaga, T. Polyfunctional Thiol Compounds. In Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009; pp. 275–293. [Google Scholar] [CrossRef]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of New Volatile Thiols in the Aroma of Vitis Vinifera L. Var. Sauvignon Blanc Wines. Flavour Fragr. J. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology Volume 1. The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-0-470-01034-1. [Google Scholar]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-Yeast Interactions Revealed by Aromatic Profile Analysis of Sauvignon Blanc Wine Fermented by Single or Co-Culture of Non-Saccharomyces and Saccharomyces Yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Henschke, P.A. Yeasts and Wine Flavour. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 313–392. ISBN 978-0-387-74116-1. [Google Scholar]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and Its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
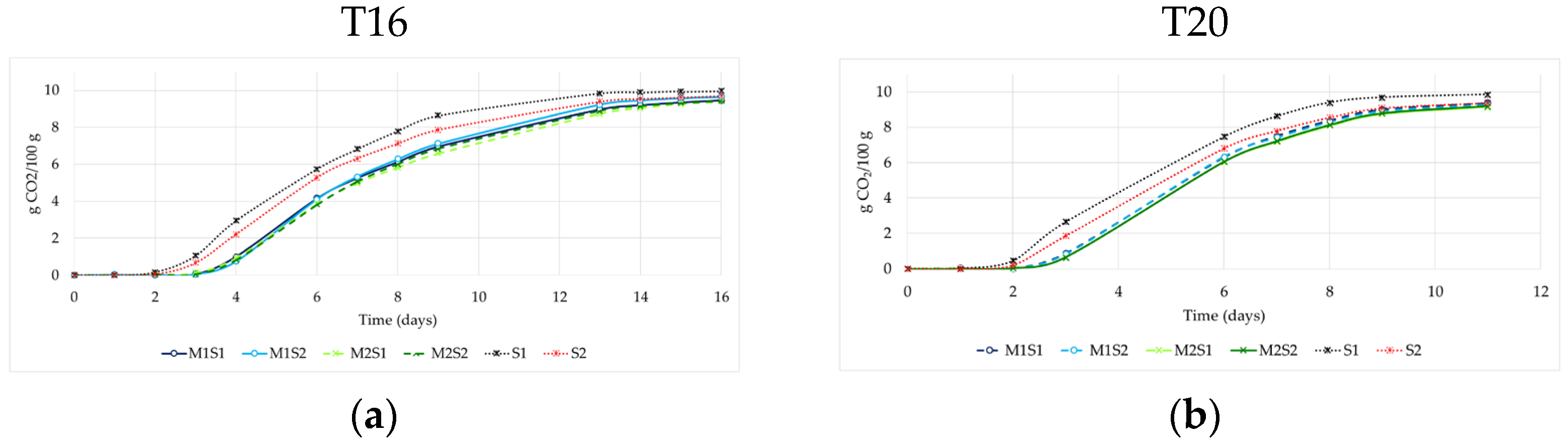
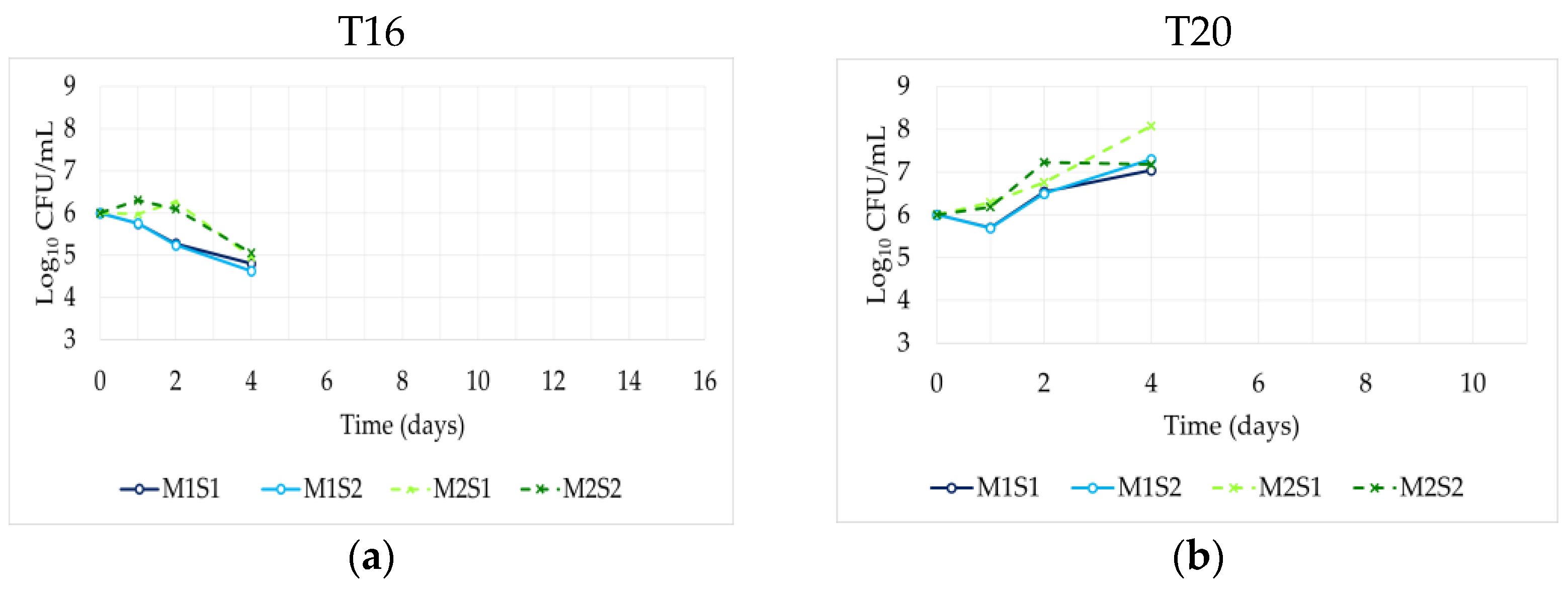
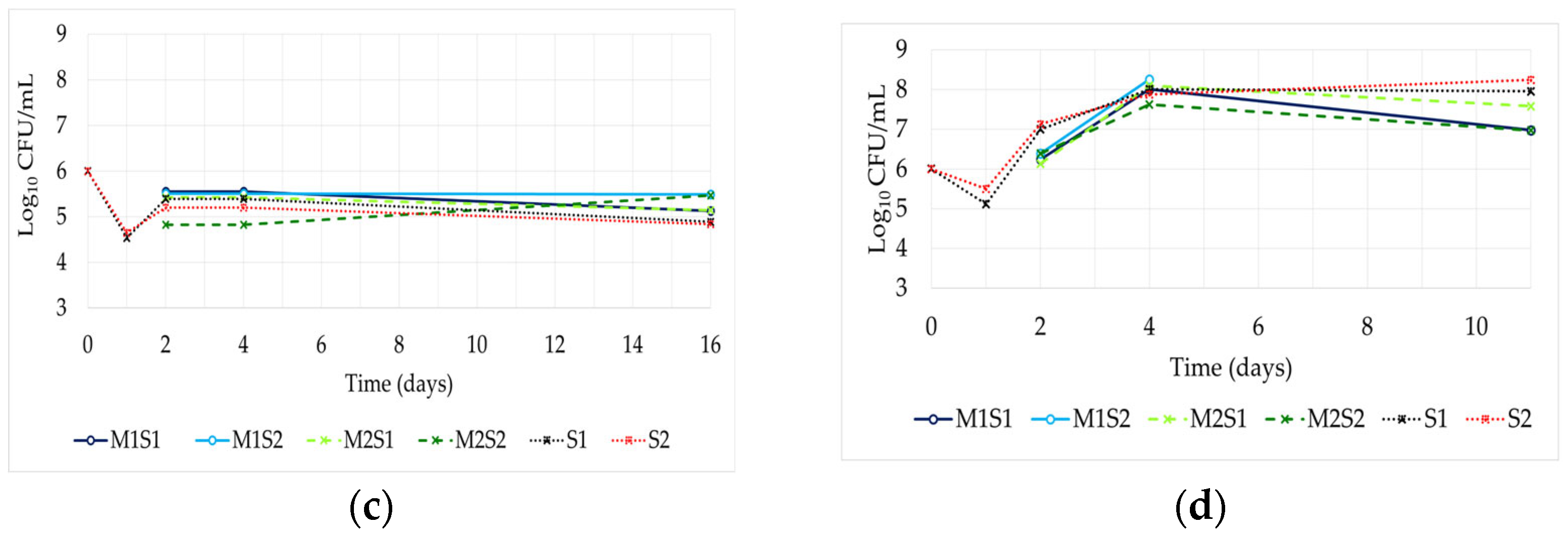

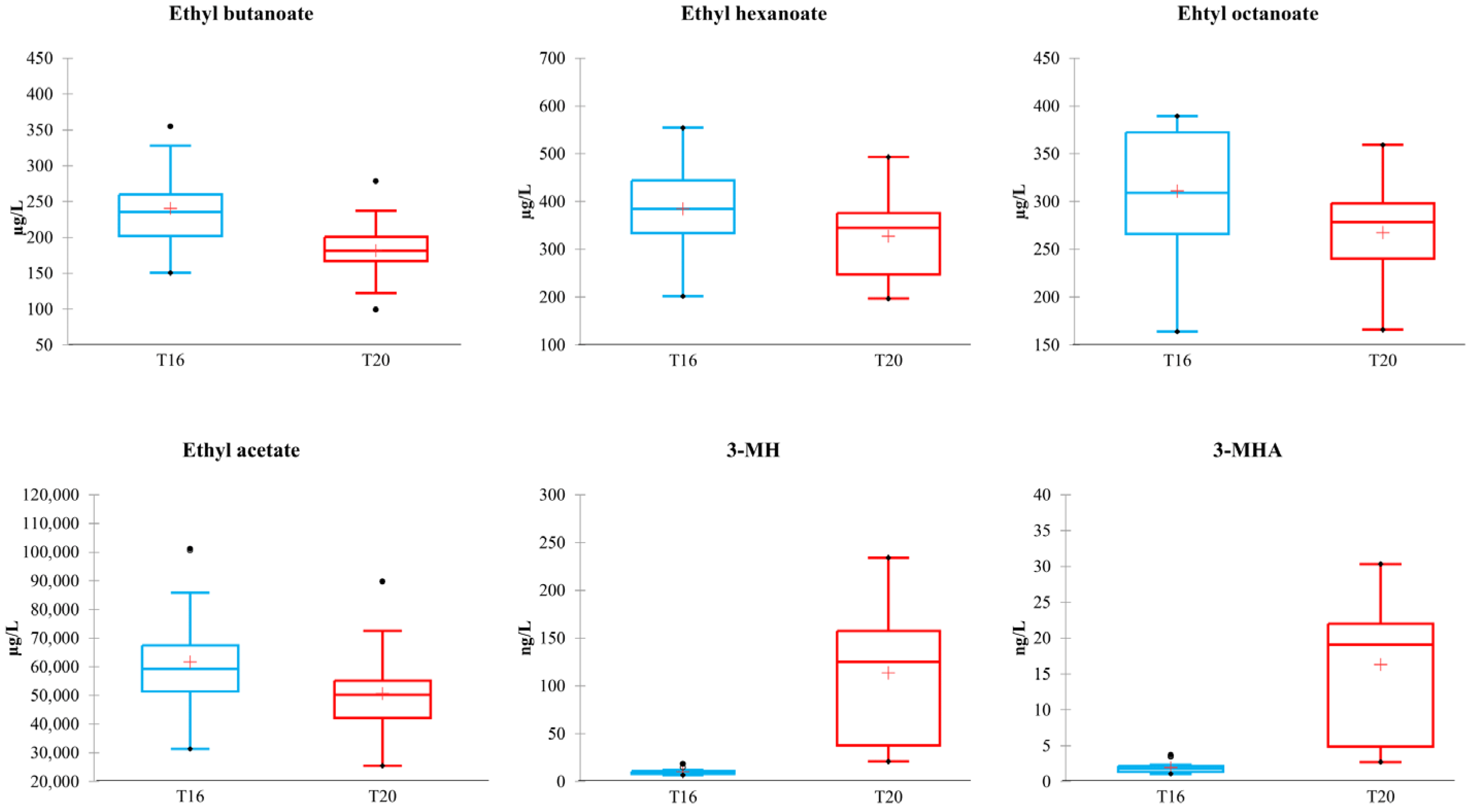
| Code | Species | Commercial Name | Producer | Temperature Range (°C) | β-Lyase Activity a | β-Glucosidase Activity a | Note | Reference |
|---|---|---|---|---|---|---|---|---|
| M1 | M. pulcherrima | Level2 Flavia | Lallemand b | 15–22 | + c | + | Release thiols for fruity aromas and, with S. cerevisiae, boosts wine aromas during fermentation. | [31] |
| M2 | M. pulcherrima | Level2 Initia | Lallemand | 4–18 | + | + | Yeast prevents oxidation, reducing copper, and minimizing SO2 use, preserves aromas. | [32] |
| S1 | S. cerevisiae | EC 1118 | Lallemand | 10–30 | + | -+ d | Reference yeast for base and sparkling wines. | [31] |
| S2 | S. cerevisiae | Zymaflore X5 | Laffort e | 13-nr f | + | -+ | Yeast for white and rosé wines with high aromatic intensity. | [33] |
| Yeast Combination | Enological Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Residual Sugars (g/L) | Ethanol (% v/v) | Glycerol (g/L) | Total Acidity (g/L) | Acetic Acid (g/L) | Total Sulfite (mg/L) | Acetaldehyde (mg/L) | Amino Nitrogen (mg/L) | |
| Trial at 16 °C | ||||||||
| M1S1 | 4.93 ± 0.54 | 11.99 | 4.93 ± 0.38 | 10.54 ± 0.31 | 0.16 ± 0.02 | 81.02 ± 6.97 | 19 ± 1.79 | 6 ± 0.8 |
| M1S2 | 3.49 ± 0.21 | 12.07 | 4.58 ± 0.43 | 10.1 ± 0.22 | 0.13 ± 0.01 | 67.87 ± 5.4 | 19 ± 1.37 | 7 ± 0.7 |
| M2S1 | 4.08 ± 0.4 | 12.09 | 3.4 ± 0.25 | 10.58 ± 0.24 | 0.11 ± 0.02 | 56.53 ± 6.78 | 23 ± 3.43 | 6 ± 0.5 |
| M2S2 | 4.07 ± 0.31 | 11.96 | 4.63 ± 0.39 | 11.66 ± 0.34 | 0.15 ± 0.01 | 53.54 ± 5.71 | 17 ± 1.89 | 8 ± 0.7 |
| S1 | 0.6 ± 0.06 | 12.08 | 4.88 ± 0.37 | 9.73 ± 0.29 | 0.17 ± 0.02 | 67.38 ± 4.04 | 23 ± 1.96 | 8 ± 0.8 |
| S2 | 2.6 ± 0.18 | 12.00 | 4.83 ± 0.3 | 10.22 ± 0.51 | 0.2 ± 0.02 | 86.15 ± 6.66 | 14 ± 1.48 | 9 ± 1.1 |
| Trial at 20 °C | ||||||||
| M1S1 | 5.96 ± 0.76 | 11.81 | 5.33 ± 0.38 | 7.88 ± 0.24 | 0.11 ± 0.01 | 76.1 ± 3.93 | 13 ± 1.24 | 6 ± 0.5 |
| M1S2 | 6.01 ± 0.8 | 11.75 | 5.06 ± 0.45 | 10.21 ± 0.35 | 0.12 ± 0.01 | 74.14 ± 5.78 | 14 ± 1.39 | 6 ± 0.4 |
| M2S1 | 7.95 ± 0.59 | 11.82 | 3.23 ± 0.4 | 11.17 ± 0.44 | 0.11 ± 0.01 | 51.37 ± 5.55 | 9 ± 0.72 | 6 ± 0.7 |
| M2S2 | 7.41 ± 0.84 | 11.88 | 5.08 ± 0.46 | 12.02 ± 0.46 | 0.11 ± 0.01 | 74.99 ± 4.92 | 16 ± 1.84 | 7 ± 0.8 |
| S1 | 1.81 ± 0.17 | 12.18 | 4.07 ± 0.44 | 10.98 ± 0.48 | 0.12 ± 0.02 | 83.74 ± 4.12 | 18 ± 1.26 | 6 ± 0.9 |
| S2 | 6.52 ± 0.37 | 12.08 | 5.09 ± 0.51 | 11.71 ± 0.66 | 0.23 ± 0.02 | 69.59 ± 4.15 | 19 ± 2.68 | 8 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertazzoli, G.; Pelizza, E.; Luzzini, G.; Felis, G.E.; Ugliano, M.; Torriani, S. Influence of Fermentation Temperature and Metschnikowia pulcherrima/Saccharomyces cerevisiae Multi-Starter Cultures on the Volatile Compounds of Lugana Wine. Foods 2025, 14, 3538. https://doi.org/10.3390/foods14203538
Bertazzoli G, Pelizza E, Luzzini G, Felis GE, Ugliano M, Torriani S. Influence of Fermentation Temperature and Metschnikowia pulcherrima/Saccharomyces cerevisiae Multi-Starter Cultures on the Volatile Compounds of Lugana Wine. Foods. 2025; 14(20):3538. https://doi.org/10.3390/foods14203538
Chicago/Turabian StyleBertazzoli, Giulia, Emma Pelizza, Giovanni Luzzini, Giovanna E. Felis, Maurizio Ugliano, and Sandra Torriani. 2025. "Influence of Fermentation Temperature and Metschnikowia pulcherrima/Saccharomyces cerevisiae Multi-Starter Cultures on the Volatile Compounds of Lugana Wine" Foods 14, no. 20: 3538. https://doi.org/10.3390/foods14203538
APA StyleBertazzoli, G., Pelizza, E., Luzzini, G., Felis, G. E., Ugliano, M., & Torriani, S. (2025). Influence of Fermentation Temperature and Metschnikowia pulcherrima/Saccharomyces cerevisiae Multi-Starter Cultures on the Volatile Compounds of Lugana Wine. Foods, 14(20), 3538. https://doi.org/10.3390/foods14203538







