An Optimized Chickpea Protein Hydrolysate Exerts Long-Term Antihypertensive Effects and Upregulates ACE2 and Mas1 Gene Expression in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Protein Concentration
2.2. Chickpea Protein Hydrolysis with Alcalase
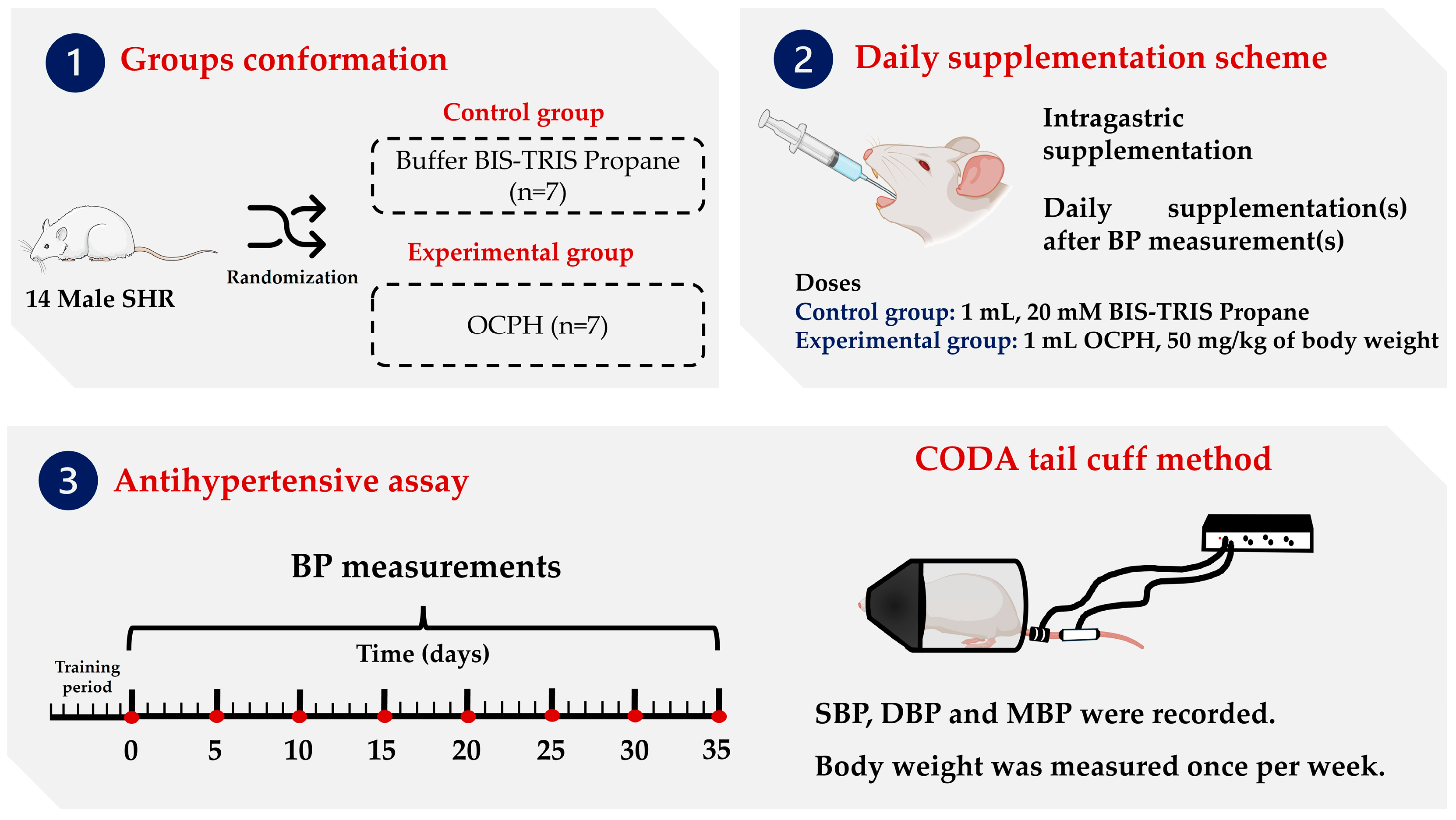
2.3. Animals
2.4. Blood Pressure Assessment
2.5. RNA Extraction and Quantification
2.6. Relative Gene Expression Analysis
2.7. Statistical Analysis and Ethical Aspects
3. Results and Discussion
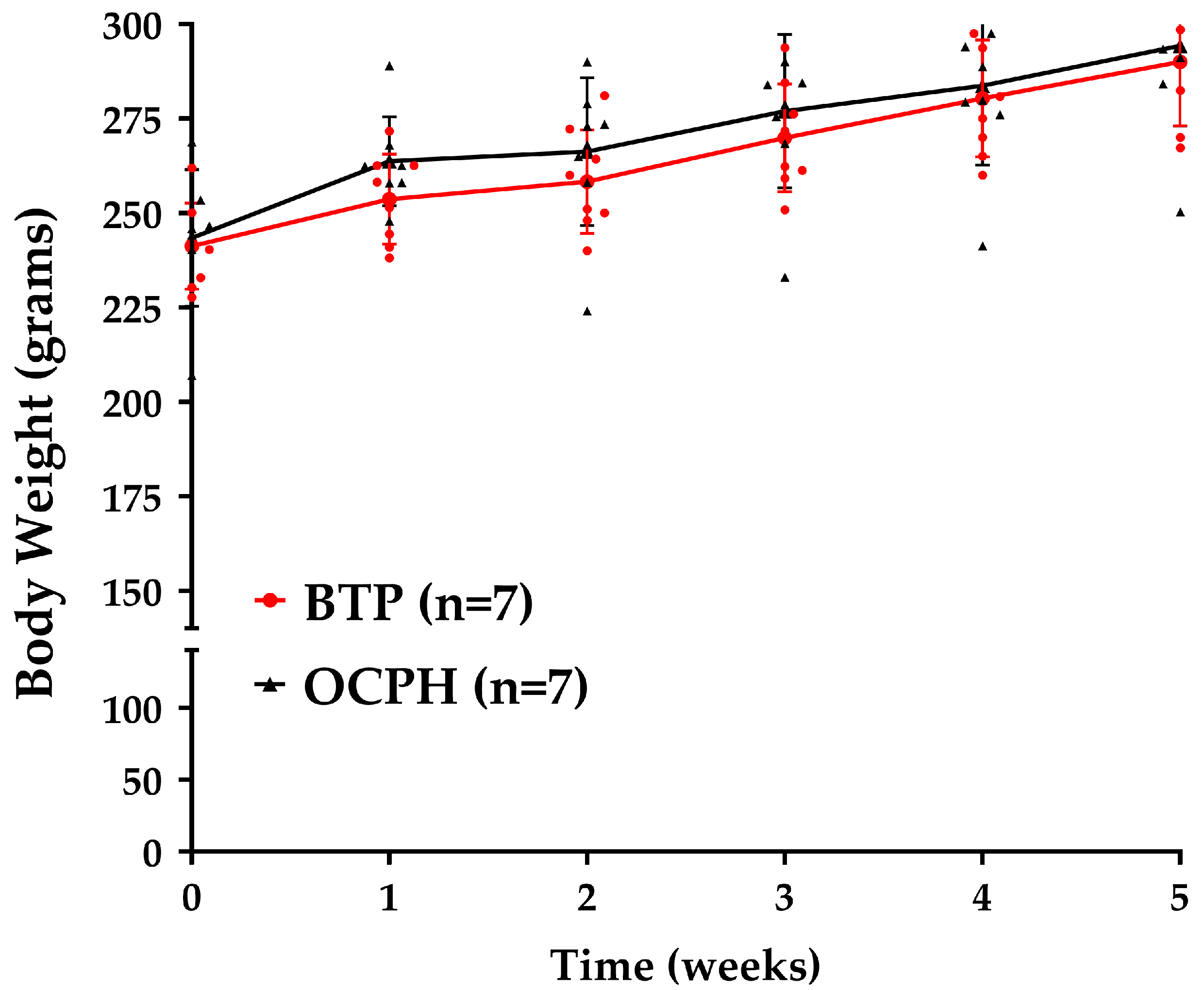
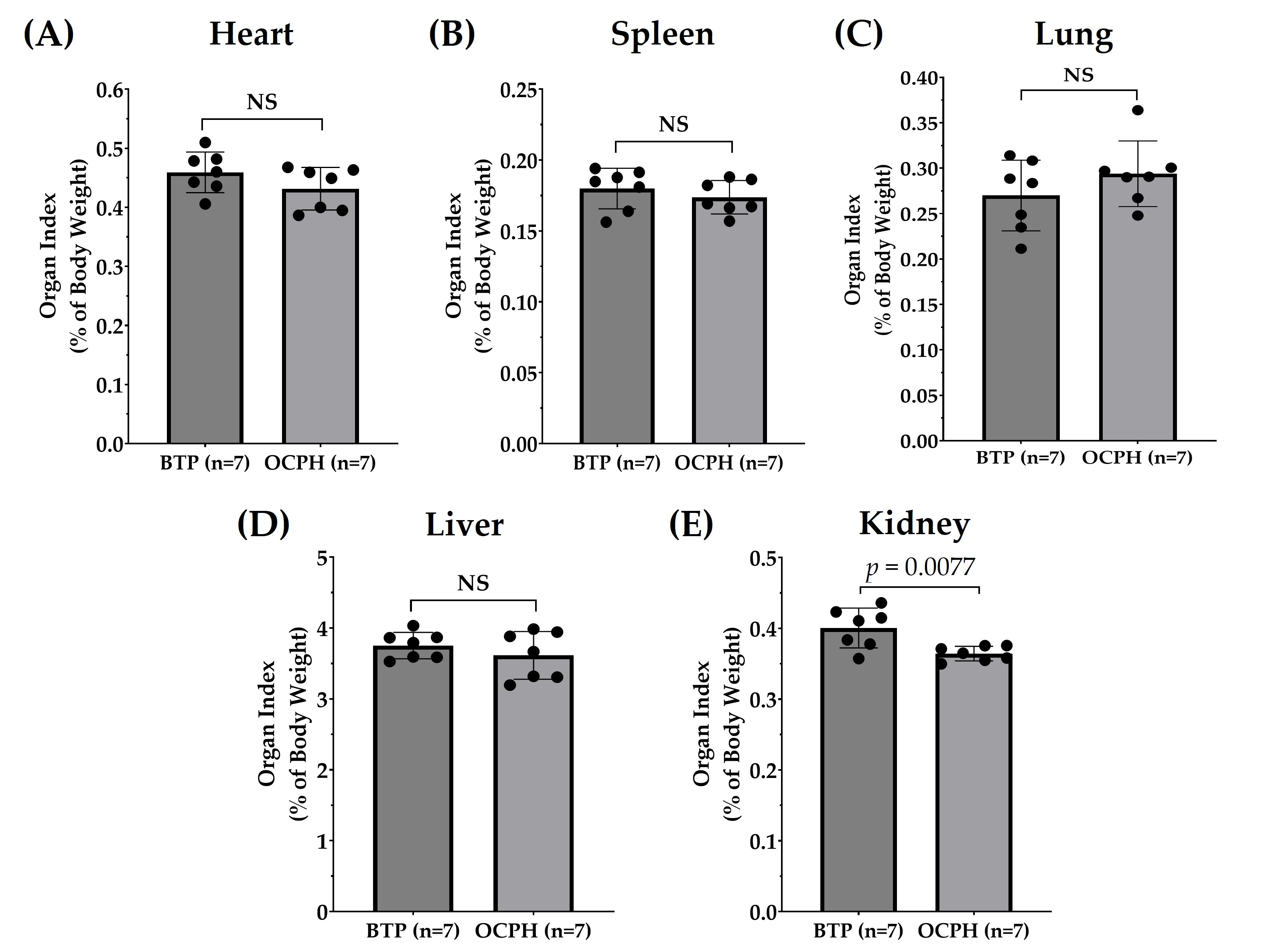
3.1. Optimized Chickpea Protein Hydrolysate Chronic Supplementation Has No Impact on Body Weight but Reduces Kidney Weight
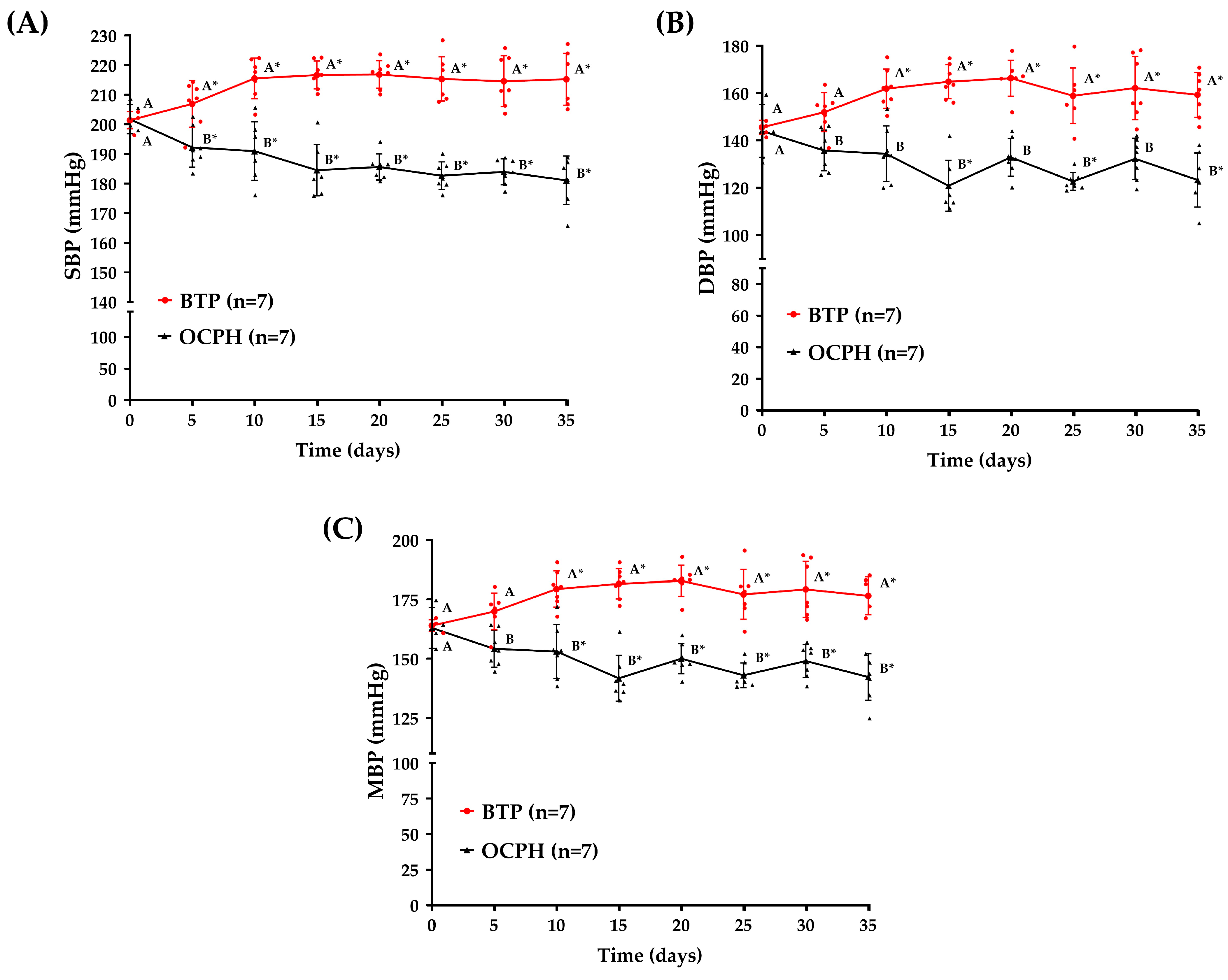
3.2. Optimized Chickpea Protein Hydrolysate Supplementation Triggers a Sustained Antihypertensive Effect
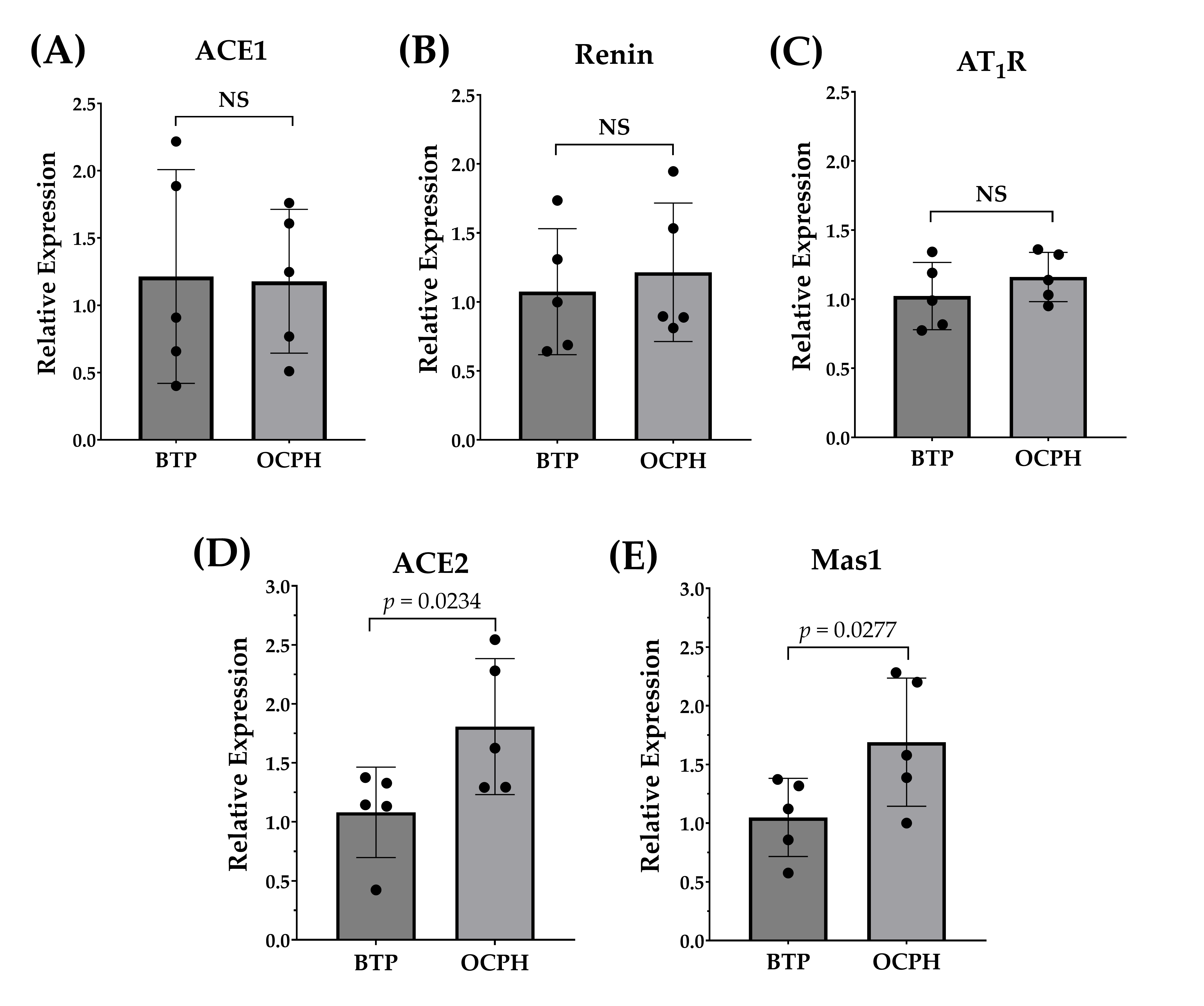
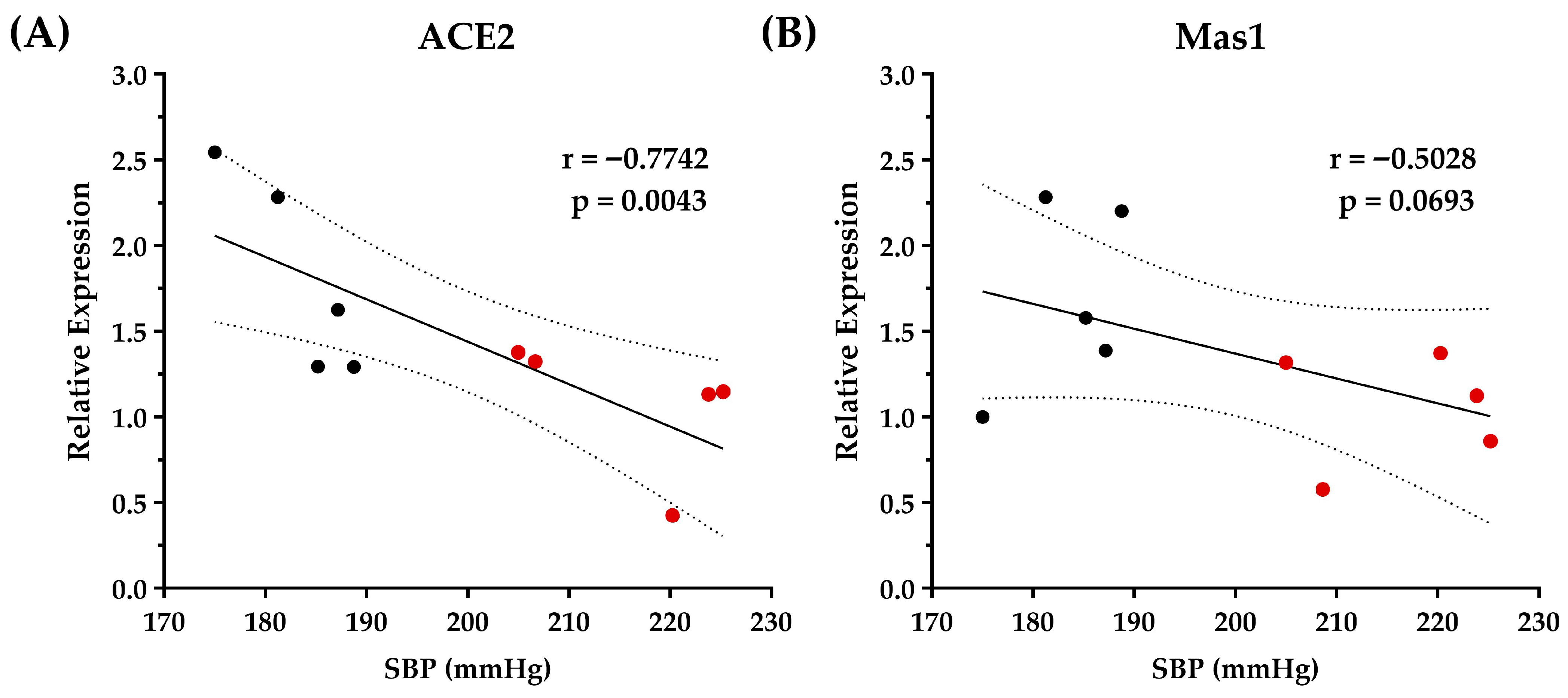
3.3. Optimized Chickpea Protein Hydrolysate Supplementation Increases ACE2/Ang-(1–7)/Mas1 Pathway Expression in the Kidneys
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE1 | Angiotensin-I-converting enzyme |
| BP | Blood pressure |
| SHRs | Spontaneously hypertensive rats |
| OCPH | Optimized chickpea protein hydrolysate |
| RAAS | Renin–angiotensin–aldosterone system |
| AngII | Angiotensin II |
| AT1R | Angiotensin type 1 receptor |
| Mas1 | G protein-coupled Mas receptor |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| MBP | Mean blood pressure |
References
- Mills, K.T.; Stefanescu, A.; He, J. The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, S.; Sudano, I.; Kokubo, Y.; Sulaica, E.M. Arterial Hypertension. Lancet 2021, 398, 249–261. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Carrera-Bastos, P.; Gálvez, B.G.; Ruiz-Hurtado, G.; Ordovas, J.M.; Ruilope, L.M.; Lucia, A. Lifestyle Interventions for the Prevention and Treatment of Hypertension. Nat. Rev. Cardiol. 2021, 18, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Moran, A.E.; Whelton, P.K. Treatment of Hypertension: A Review. JAMA 2022, 328, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Albasri, A.; Hattle, M.; Koshiaris, C.; Dunnigan, A.; Paxton, B.; Fox, S.E.; Smith, M.; Archer, L.; Levis, B.; Payne, R.A.; et al. Association between Antihypertensive Treatment and Adverse Events: Systematic Review and Meta-Analysis. BMJ 2021, 372, n189. [Google Scholar] [CrossRef]
- Na Takuathung, M.; Sakuludomkan, W.; Khatsri, R.; Dukaew, N.; Kraivisitkul, N.; Ahmadmusa, B.; Mahakkanukrauh, C.; Wangthaweesap, K.; Onin, J.; Srichai, S.; et al. Adverse Effects of Angiotensin-Converting Enzyme Inhibitors in Humans: A Systematic Review and Meta-Analysis of 378 Randomized Controlled Trials. Int. J. Environ. Res. Public. Health 2022, 19, 8373. [Google Scholar] [CrossRef]
- Kaur, A.; Kehinde, B.A.; Sharma, P.; Sharma, D.; Kaur, S. Recently Isolated Food-Derived Antihypertensive Hydrolysates and Peptides: A Review. Food Chem. 2021, 346, 128719. [Google Scholar] [CrossRef]
- Olalere, O.A.; Yap, P.-G.; Gan, C.-Y. Comprehensive Review on Some Food-Derived Bioactive Peptides with Anti-Hypertension Therapeutic Potential for Angiotensin-Converting Enzyme (ACE) Inhibition. J. Proteins Proteom. 2023, 14, 129–161. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Legume Proteins and Peptides as Compounds in Nutraceuticals: A Structural Basis for Dietary Health Effects. Nutrients 2022, 14, 1188. [Google Scholar] [CrossRef]
- Tawalbeh, D.; Al-U’datt, M.H.; Wan Ahmad, W.A.N.; Ahmad, F.; Sarbon, N.M. Recent Advances in in Vitro and in Vivo Studies of Antioxidant, Ace-Inhibitory and Anti-Inflammatory Peptides from Legume Protein Hydrolysates. Molecules 2023, 28, 2423. [Google Scholar] [CrossRef]
- Arámburo-Gálvez, J.G.; Arvizu-Flores, A.A.; Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Ramírez-Torres, G.I.; Flores-Mendoza, L.K.; Gastelum-Acosta, P.E.; Figueroa-Salcido, O.G.; Ontiveros, N. Prediction of ACE-I Inhibitory Peptides Derived from Chickpea (Cicer arietinum L.): In Silico Assessments Using Simulated Enzymatic Hydrolysis, Molecular Docking and ADMET Evaluation. Foods 2022, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Ontiveros, J.; Reyes-Moreno, C.; Ramírez-Torres, G.I.; Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Montoya-Rodríguez, A.; Ontiveros, N.; Cuevas-Rodríguez, E.O. Extrusion Improves the Antihypertensive Potential of a Kabuli Chickpea (Cicer arietinum L.) Protein Hydrolysate. Foods 2022, 11, 2562. [Google Scholar] [CrossRef]
- Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Mora-Melgem, J.A.; Camacho-Cervantes, D.L.; Gracia-Valenzuela, M.H.; Cuevas-Rodríguez, E.O.; Ontiveros, N. Alcalase-Based Chickpea (Cicer arietinum L.) Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Foods 2024, 13, 1216. [Google Scholar] [CrossRef]
- Mora-Melgem, J.A.; Arámburo-Gálvez, J.G.; Cárdenas-Torres, F.I.; Gonzalez-Santamaria, J.; Ramírez-Torres, G.I.; Arvizu-Flores, A.A.; Figueroa-Salcido, O.G.; Ontiveros, N. Dipeptidyl Peptidase IV Inhibitory Peptides from Chickpea Proteins (Cicer arietinum L.): Pharmacokinetics, Molecular Interactions, and Multi-Bioactivities. Pharmaceuticals 2023, 16, 1109. [Google Scholar] [CrossRef]
- Triebel, H.; Castrop, H. The Renin Angiotensin Aldosterone System. Pflüg. Arch.-Eur. J. Physiol. 2024, 476, 705–713. [Google Scholar] [CrossRef]
- Colafella, K.M.M.; Bovée, D.M.; Danser, A.J. The Renin-Angiotensin-Aldosterone System and Its Therapeutic Targets. Exp. Eye Res. 2019, 186, 107680. [Google Scholar] [CrossRef]
- Povlsen, A.L.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. The Vasoactive Mas Receptor in Essential Hypertension. J. Clin. Med. 2020, 9, 267. [Google Scholar] [CrossRef]
- Xiang, L.; Zheng, Z.; Guo, X.; Bai, R.; Zhao, R.; Chen, H.; Qiu, Z.; Qiao, X. Two Novel Angiotensin I-Converting Enzyme Inhibitory Peptides from Garlic Protein: In Silico Screening, Stability, Antihypertensive Effects in Vivo and Underlying Mechanisms. Food Chem. 2024, 435, 137537. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yang, Y.-J.; Wang, Z.; Xing, C.; Yuan, J.; Wang, L.-F.; Udenigwe, C.; Ju, X.-R. Rapeseed Protein-Derived Peptides, LY, RALP, and GHS, Modulates Key Enzymes and Intermediate Products of Renin–Angiotensin System Pathway in Spontaneously Hypertensive Rat. NPJ Sci. Food 2019, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; She, X.; Wu, H.; Ma, J.; Ren, D.; Lu, J. Long-Term Regulation of the Local Renin–Angiotensin System in the Myocardium of Spontaneously Hypertensive Rats by Feeding Bioactive Peptides Derived from Spirulina Platensis. J. Agric. Food Chem. 2015, 63, 7765–7774. [Google Scholar] [CrossRef]
- Lu, J.; Sawano, Y.; Miyakawa, T.; Xue, Y.-L.; Cai, M.-Y.; Egashira, Y.; Ren, D.-F.; Tanokura, M. One-Week Antihypertensive Effect of Ile-Gln-Pro in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2011, 59, 559–563. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Ofosu, F.K.; Chelliah, R.; Park, M.H.; Kim, J.-H.; Oh, D.-H. Development of a Soy Protein Hydrolysate with an Antihypertensive Effect. Int. J. Mol. Sci. 2019, 20, 1496. [Google Scholar] [CrossRef]
- López-Moreno, M.; Jiménez-Moreno, E.; Márquez Gallego, A.; Vera Pasamontes, G.; Uranga Ocio, J.A.; Garcés-Rimón, M.; Miguel-Castro, M. Red Quinoa Hydrolysates with Antioxidant Properties Improve Cardiovascular Health in Spontaneously Hypertensive Rats. Antioxidants 2023, 12, 1291. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.; Xu, C.; Wang, S.; Li, L.; Zou, S.; Yu, J.; Wei, Y. Positive Effect of a Pea–Clam Two-Peptide Composite on Hypertension and Organ Protection in Spontaneously Hypertensive Rats. Nutrients 2022, 14, 4069. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, J.; Guo, Y.; Quaisie, J.; Hong, C.; Ma, H. Antihypertensive and Immunomodulatory Effects of Defatted Corn Germ Hydrolysates: An in Vivo Study. Front. Nutr. 2021, 8, 679583. [Google Scholar] [CrossRef]
- Fan, H.; Liao, W.; Spaans, F.; Pasha, M.; Davidge, S.T.; Wu, J. Chicken Muscle Hydrolysate Reduces Blood Pressure in Spontaneously Hypertensive Rats, Upregulates ACE2, and Ameliorates Vascular Inflammation, Fibrosis, and Oxidative Stress. J. Food Sci. 2022, 87, 1292–1305. [Google Scholar] [CrossRef]
- Hultström, M. Development of Structural Kidney Damage in Spontaneously Hypertensive Rats. J. Hypertens. 2012, 30, 1087–1091. [Google Scholar] [CrossRef]
- Bianchi, G.; Fox, U.; Di Francesco, G.; Giovanetti, A.; Pagetti, D. Blood Pressure Changes Produced by Kidney Cross-Transplantation between Spontaneously Hypertensive Rats and Normotensive Rats. Clin. Sci. 1974, 47, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Canoy, D.; Nazarzadeh, M.; Copland, E.; Bidel, Z.; Rao, S.; Li, Y.; Rahimi, K. How Much Lowering of Blood Pressure Is Required to Prevent Cardiovascular Disease in Patients with and without Previous Cardiovascular Disease? Curr. Cardiol. Rep. 2022, 24, 851–860. [Google Scholar]
- Anishchenko, A.; Aliev, O.; Sidekhmenova, A.; Shamanaev, A.Y.; Plotnikov, M. Dynamics of Blood Pressure Elevation and Endothelial Dysfunction in SHR Rats during the Development of Arterial Hypertension. Bull. Exp. Biol. Med. 2015, 159, 591–593. [Google Scholar]
- Huang, J.; Liu, Q.; Xue, B.; Chen, L.; Wang, Y.; Ou, S.; Peng, X. Angiotensin-i-Converting Enzyme Inhibitory Activities and in Vivo Antihypertensive Effects of Sardine Protein Hydrolysate. J. Food Sci. 2016, 81, H2831–H2840. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prairie, N.; Udenigwe, C.C.; Adebiyi, A.P.; Tappia, P.S.; Aukema, H.M.; Jones, P.J.; Aluko, R.E. Blood Pressure Lowering Effect of a Pea Protein Hydrolysate in Hypertensive Rats and Humans. J. Agric. Food Chem. 2011, 59, 9854–9860. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Pons, Z.; Aleixandre, A.; Bravo, F.I.; Muguerza, B. Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats. Nutrients 2018, 10. [Google Scholar]
- Fritz, M.; Vecchi, B.; Rinaldi, G.; Añón, M.C. Amaranth Seed Protein Hydrolysates Have in Vivo and in Vitro Antihyperten-sive Activity. Food Chem. 2011, 126, 878–884. [Google Scholar]
- Liao, W.; Fan, H.; Davidge, S.T.; Wu, J. Egg White–Derived Antihypertensive Peptide IRW (Ile-Arg-Trp) Reduces Blood Pressure in Spontaneously Hypertensive Rats via the ACE2/Ang (1-7)/Mas Receptor Axis. Mol. Nutr. Food Res. 2019, 63, 1900063. [Google Scholar] [CrossRef]
- Jahandideh, F.; Chakrabarti, S.; Majumder, K.; Li, Q.; Panahi, S.; Morton, J.S.; Davidge, S.T.; Wu, J. Egg White Protein Hydrolysate Reduces Blood Pressure, Improves Vascular Relaxation and Modifies Aortic Angiotensin II Receptors Expression in Spontaneously Hypertensive Rats. J. Funct. Foods 2016, 27, 667–673. [Google Scholar]
- Yu, Z.; Yin, Y.; Zhao, W.; Chen, F.; Liu, J. Antihypertensive Effect of Angiotensin-Converting Enzyme Inhibitory Peptide RVPSL on Spontaneously Hypertensive Rats by Regulating Gene Expression of the Renin–Angiotensin System. J. Agric. Food Chem. 2014, 62, 912–917. [Google Scholar]
- Janhavi, P.; Divyashree, S.; Sanjailal, K.; Muthukumar, S. DoseCal: A Virtual Calculator for Dosage Conversion between Human and Different Animal Species. Arch. Physiol. Biochem. 2022, 128, 426–430. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, H.; Bao, X.; Wu, J. Angiotensin-Converting Enzyme 2 Activation Is Not a Common Feature of Angiotensin-Converting Enzyme Inhibitory Peptides. J. Agric. Food Chem. 2023, 71, 8867–8876. [Google Scholar] [CrossRef]
- Chappell, M.C. Emerging Evidence for a Functional Angiotensin-Converting Enzyme 2-Angiotensin-(1-7)-MAS Receptor Axis: More than Regulation of Blood Pressure? Hypertension 2007, 50, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Koka, V.; Huang, X.R.; Chung, A.C.; Wang, W.; Truong, L.D.; Lan, H.Y. Angiotensin II Up-Regulates Angiotensin I-Converting Enzyme (ACE), but down-Regulates ACE2 via the AT1-ERK/P38 MAP Kinase Pathway. Am. J. Pathol. 2008, 172, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M. Angiotensin Type-1 Receptor Blockade Increases ACE 2 Expression in the Heart. Hypertension 2004, 43, 943–944. [Google Scholar] [CrossRef]
- Coronado-Cáceres, L.J.; Hernández-Ledesma, B.; Mojica, L.; Quevedo-Corona, L.; Rabadán-Chávez, G.; Castillo-Herrera, G.A.; Lugo Cervantes, E. Cocoa (Theobroma cacao L.) Seed-Derived Peptides Reduce Blood Pressure by Interacting with the Catalytic Site of the Angiotensin-Converting Enzyme. Foods 2021, 10, 2340. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Gan, C.-Y.; Olalere, O.A.; Farahnaky, A.; Gill, H.; Truong, T. Novel Antihypertensive Peptides from Lupin Protein Hydrolysate: An in-Silico Identification and Molecular Docking Studies. Food Chem. 2023, 407, 135082. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Flores-Mendoza, L.K.; Ramírez-Torres, G.I.; Gracia-Valenzuela, M.H.; Cuevas-Rodríguez, E.O.; Ontiveros, N. An Optimized Chickpea Protein Hydrolysate Exerts Long-Term Antihypertensive Effects and Upregulates ACE2 and Mas1 Gene Expression in Spontaneously Hypertensive Rats. Foods 2025, 14, 3537. https://doi.org/10.3390/foods14203537
Figueroa-Salcido OG, Arámburo-Gálvez JG, Flores-Mendoza LK, Ramírez-Torres GI, Gracia-Valenzuela MH, Cuevas-Rodríguez EO, Ontiveros N. An Optimized Chickpea Protein Hydrolysate Exerts Long-Term Antihypertensive Effects and Upregulates ACE2 and Mas1 Gene Expression in Spontaneously Hypertensive Rats. Foods. 2025; 14(20):3537. https://doi.org/10.3390/foods14203537
Chicago/Turabian StyleFigueroa-Salcido, Oscar Gerardo, Jesús Gilberto Arámburo-Gálvez, Lilian Karem Flores-Mendoza, Giovanni I. Ramírez-Torres, Martina Hilda Gracia-Valenzuela, Edith Oliva Cuevas-Rodríguez, and Noé Ontiveros. 2025. "An Optimized Chickpea Protein Hydrolysate Exerts Long-Term Antihypertensive Effects and Upregulates ACE2 and Mas1 Gene Expression in Spontaneously Hypertensive Rats" Foods 14, no. 20: 3537. https://doi.org/10.3390/foods14203537
APA StyleFigueroa-Salcido, O. G., Arámburo-Gálvez, J. G., Flores-Mendoza, L. K., Ramírez-Torres, G. I., Gracia-Valenzuela, M. H., Cuevas-Rodríguez, E. O., & Ontiveros, N. (2025). An Optimized Chickpea Protein Hydrolysate Exerts Long-Term Antihypertensive Effects and Upregulates ACE2 and Mas1 Gene Expression in Spontaneously Hypertensive Rats. Foods, 14(20), 3537. https://doi.org/10.3390/foods14203537







