Bioavailability, Human Metabolism, and Dietary Interventions of Glucosinolates and Isothiocyanates: Critical Insights and Future Perspectives
Abstract
1. Introduction
2. Methodology
3. Metabolism, Bioavailability, and Bioaccessibility of GSLs and ITCs
3.1. Metabolism and Myrosinase Activity
3.2. Importance of the Gut Microbiota in GSL Metabolism
| Type of Study | Food Source | Aim of the Study | Experimental Plan | Main Findings | Reference |
|---|---|---|---|---|---|
| Human | Microgreens of Beta vulgaris and Brassica oleracea var capitata | Feasibility, tolerability and human health effects of daily consumption | 2 cups of B. vulgaris or B. oleracea consumed daily for 2 weeks by 26 healthy adults, randomly separated into 2 groups. 2 weeks wash-out period | Gastrointestinal tolerability: daily consumption is tolerable, no changes in GMB composition and abundance | [50] |
| SFN from broccoli | Understand SFN metabolism and excretion | Administration of 100 μmol of SFN from fresh broccoli sprouts to 55 healthy people | Microbiome composition: positive correlation of SFN metabolites with bacteria of Roseburia, Bifidobacterium, Bacteroides vulgatus and Ruminococcus torques, and Dorea longicatena genera; negative correlation of SFN metabolites with Alistipes and Blautia genera | [48] | |
| In vitro | SFN from broccoli (Brassica oleracea var. italica) seeds | Effects of SFN on gut microbiota composition and production of short-chain fatty acids | Simulated gastrointestinal digestion; in vitro fermentation (using fresh human faeces from obese, antibiotic-free volunteers) for 6, 12, and 24 h | Microbiome composition: Higher after 24 h in SFN-treated group compared to the SFN-free; higher abundance of Firmmicutes, Weissella, Leuconostoc, Lactobacillus, Algiphilus and Faecalibacterium; reduction of Proteobacteria, Escherichia-Shigella, Klebsiella, Clostridium_sensu_stricto_1, Sutterella, Megamonas, and Proteus in SFN-treated group | [49] |
| SNG, GTP, GNT and their corresponding DS-GSLs (synthetic GSLs) | GSLs and their DS-GSLs metabolic fate after in vitro processing with Lactobacillus agilis R16, Escherichia coli VL8, and Escherichia casseliflavus CP1 | 24 h incubation with the compounds. GS-MS analysis | Products obtained from GSLs consumption: From SNG = AITC and ANIT (all 3 bacteria); From GTP = BZITC and BNIT (all 3 bacteria); From GNT = PEITC (all 3 bacteria) and PNIT (except L. agilis) Products obtained from DS-GSLs consumption: L. agilis: ANIT from DS-SNG; BNIT from DS-GNT; E. casseliflavus and E.coli: ANIT form DS-SNG; BNIT from DS-GTP; sPNIT form DS-GNT | [51] | |
| GTP (synthetic GSL) | Identification of activation of GSLs by Bacteroides thetaiotaomicron (Bt) | Bt strains VPI-5482, 8736, 7330, and 3731 cultured in a medium containing GTP. LC-MS/MS analysis | Identification of operon BT2159-BT2156 for GSLs conversion. Genes BT2158 and either BT2156 or BT2157 are required to get ITCs formation | [18] | |
| AITC, BZITC from the green parts of Brassica carinata and Sinapis alba | Study the formation of AITC and BZITC after the digestive process; effects of colonic fermentation on the formation of ITCs and their consequent impact on intestinal population | 5 g of fresh samples or 2 g of lyophilised samples analysed after simulated gastrointestinal digestion and in vitro colonic fermentation (using faecal samples of three healthy donors); DNA extraction and sequencing | Final AITC and BZITC concentration after digestion and colonic fermentation: AITC reduction to 0.01 from fresh samples; 0.02 mg/g for lyophilised samples BZITC reduction to 0.1 Microbiome composition: Increase of Bifidobacterium, Faecalibacterium, Blautia, Ruminocaccus; slight increase of Lactobacillaceae in Sinapis alba; reduction of Enterobacter and Klepsia | [52] |
3.3. Food Matrix
4. Intervention Studies on Glucosinolates and Isothiocyanates
4.1. Studies Based on Vegetable and Derivative Interventions
| Dietary Source | Sulphur-Nitrogen-Based Compounds | Biological Samples | Subject Characteristics | Study Record Identifier (NCT Number) | Metabolites and Conjugates–Concentration Range | Main Findings | Analytical Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| Cruciferous vegetables | GSL, ITCs (e.g., SFN) | Blood, urine, tissue (preclinical and human) | Mixed: human (adults 30–60 yrs, both sexes, BMI 25–30 kg/m2). | N.A. | SFN metabolites: SFN-GSH, SFN-Cys, SFN-NAC, SFN-SULF. Plasma levels up to 0.5–2.0 µM; urinary excretion up to 70–80% of dose within 24 h. | Evidence of anti-inflammatory and anti-carcinogenic activity in preclinical models; some human trials support role in chronic disease prevention | HPLC, mass spectrometry, biomarker assays | [60] |
| Cruciferous vegetables | GR and SFN | Blood and urine | 67 adults with mildly elevated BP (randomised crossover design) | ACTRN12618001010246 (VESSEL study) | SFN metabolites: SFN and metabolites: SFN-Cys, SFN-NAC, SFN-GSH, SFN-SULF quantified in plasma and urine | Significant BP reduction compared to control; improved vascular function markers | LC-MS/MS, BP monitoring | [61] |
| Bitter brassica vegetables (wild/traditional cultivars) | GSLs (higher levels due to cultivars) | Blood and urine | Type 2 diabetic patients, randomised control trial | N.A. | Not quantified | Improved glycemic control, insulin sensitivity, reduced inflammation markers | Clinical biochemistry assays, HOMA-IR | [62] |
4.2. Studies Based on Sprout Interventions
| Dietary Source | Sulphur-Nitrogen-Based Compounds | Biological Samples | Subject Characteristics | Study Record Identifier (NCT Number) | Metabolites and Conjugates–Concentration Range | Main Findings | Analytical Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| Broccoli sprout powder (200 mg per capsule, 2 capsules daily during 12 weeks) | GR | Blood (plasma/serum) | 288 adults (18–60 years) in India with at least one metabolic risk factor | CTRI/2016/05/006977 (Indian Clinical Trials Registry) | N.A. | Significant reductions in fasting glucose, glycated haemoglobin, and triglycerides in the broccoli sprout group compared to placebo; improved metabolic profiles observed over 90 days | Standard clinical biochemistry assays for glucose, lipids, glycated haemoglobin | [64] |
| Broccoli sprout extract (12 weeks) | SFN ~150 µmol daily | Fasting blood; stool for gut microbiota | 74 drug-naive adults (35–75 yr) with prediabetes, overweight/obese | NCT03763240 | Serum SFN measured in subset; concentration correlated with gut BT2160 gene abundance | Modest glucose reduction overall (−0.2 mmol/L); greater effect (−0.4 mmol/L) in responders with mild obesity and low insulin resistance. Gut microbiota profile predicted response and was linked to higher SFN levels. | Standard biochemistry for fasting glucose and clinical biomarkers; microbiome sequencing; targeted assay for sulforaphane in blood | [67] |
| 2 bottles of sprout juice per day (75 g of sprouts) | GR | Skeletal muscle biopsies; blood (plasma/serum) | 9 healthy adults in combination with daily intense exercise | Not registered | N.A. | Broccoli sprouts combined with training lowered muscle oxidation and blood myeloperoxidase, reduced lactate in exercise, lessened night-time hypoglycaemia, and enhanced performance versus placebo | • Protein carbonylation in muscle: ELISA or spectrophotometric detection • Blood myeloperoxidase: immunoassay • Lactate during sub-maximal exercise: enzymatic assay • Continuous glucose monitoring | [68] |
| Broccoli sprout extract (200 µmol daily) | SFN 100 µmol/capsule | Blood (plasma/urine); prostate tissue biopsies | 98 men scheduled for prostate biopsy | NCT01265953 | SFN-GSH: 0.03/0.0002 µM (plasma/urine) SFN-CysGly: 0.04/0.005 µM SFN-Cys: 0.02/1.2 µM SFN-NAC: 0.03/2.9 µM | BSE supplementation increased SFN metabolites but did not significantly affect HDAC activity or prostate tissue biomarkers | QTRAP LC-MS/MS for SFN metabolites; RNA-Seq for gene expression; immunohistochemistry for tissue biomarkers | [69] |
| Broccoli sprout extract | ITC 200 µmol/day | Plasma, urine, breast tissue biopsies | 30 postmenopausal women with stage I–II breast cancer | NCT01753908 | Urinary ITC metabolites in BSE group: Pre-intervention: 15.9 ± 28.3 µmol/g creatinine Post-intervention: 228.0 ± 152.0 µmol/g creatinine Net change range: 6.6 to 598.7 µmol/g creatinine | BSE raised SFN metabolites and showed trends for increased tumour cell death markers and immune infiltration, with reduced proliferation markers | LC-MS/MS for SFN metabolites; immunohistochemistry for Ki-67, cleaved caspase-3, ER-α; proteomic analysis of morning urine | [70] |
| Broccoli sprout (15 caps/day) | Caps content: 90 mg SFN + 180 mg GR | Urine | 40 patients with advanced, surgically non-resectable pancreatic ductal adenocarcinoma receiving palliative chemotherapy | NCT01879878 (POUDER pilot study) | N.A. | Feasibility confirmed despite high dropout and side effects; survival trend favoured treatment but not statistically significant | N.A. | [71] |
| White cabbage or pak choi sprouts beverage | 1-cyano-2,3-epithiopropane (CETP), 1-cyano-3,4-epithiobutane (CETB), 1-cyano-4,5-epithiopentane (CETPent) | Blood and urine | 9 (7 women and 2 men) Healthy adults aged 18−70 years with a BMI between 18 and 30 kg m−2 | Not registered | CETP metabolite reached peak blood concentrations after 3 h of 70 nmol/L after cabbage and 12 nmol/L after pak choi sprout consumption. | CETP and CETPent rapidly absorbed and metabolised via the mercapturic acid pathway; CETB metabolite undetectable | UHPLC-TOF-MS | [72] |
4.3. Studies Based on Enriched Food and Extract Interventions
| Dietary Source | Sulphur-Nitrogen-Based Compounds | Biological Samples | Subject Characteristics | Study Record Identifier (NCT Number) | Metabolites and Conjugates–Concentration Range | Main Findings | Analytical Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| Broccoli sprout supplements | GR | Blood | Healthy middle-aged adults, high-normal liver markers | N.A. | No direct measurement of circulating metabolites; significant reduction in γ-GTP compared to control, with favourable trends in ALT and AST | Improved liver enzyme levels post-intervention | Clinical blood chemistry analysis | [75] |
| Freeze-dried cabbage bar | GSLs | Blood | T2D patients, double-blinded RCT | N.A. | No direct measurement of GSLs or metabolite concentrations in blood; clinical outcomes include reduced HbA1c, body weight, and calorie intake | Improved glucose control and insulin response | Biochemical clinical assays | [76] |
| Descurainia sophia seed extract | GSLs (assumed) | Blood (thyroid-related markers) | Hyperthyroid patients, pilot RCT | N.A. | No direct measurement of GSLs or metabolite concentrations in blood. D. sophia extract results in increase TSH compared to placebo | Reduction in thyroid hormone levels (T3, T4) | Hormonal assays (ELISA, RIA) | [77] |
| SFN + Alliin (broccoli + garlic) | SFN, Alliin | Prostate tissue (biopsy) | Humans undergoing prostate biopsy | N.A. | Tissue concentration measured: Total ITC metabolites 1.1 nmol/g tissue. SFN: 0.4 nmol/g. SFN-NAC: 0.6 nmol/g | SFN and alliin detected in prostate tissue after diet | Tissue LC-MS/MS analysis | [78] |
| Nasturtium officinale extract | GSLs | Blood (oxidative/inflammatory markers) | People with physical disability, double-blind RCT | N.A. | No specific metabolite concentrations reported | Reduced oxidative stress and inflammation biomarkers | ELISA, biochemical markers | [79] |
| Cruciferous vegetables (general intake) | GSLs | Urine (2/16α-hydroxyestrone ratio) | Premenopausal women | N.A. | Urinary 2/16α-hydroxyestrone ratio measured; no significant changes observed after cruciferous vegetable intake | No significant effect on estrogen metabolite ratio | Urine LC-MS analysis | [80] |
| Maca extract (Lepidium meyenii) | Benzyl glucosinolate | Blood, fatigue questionnaires | Women, fatigue symptoms, RCT | N.A. | No specific metabolite concentrations reported | Significant reduction in fatigue with maca extract | Subjective fatigue scales + blood analysis | [81] |
| Supplement with GSLs + flavonoids | GSLs, phytosterols, flavonoids | Blood (safety, tolerance) | Adult women, Phase I RCT | N.A. | No specific metabolite concentrations reported | Safe and well tolerated across doses | Clinical safety and tolerability assays | [82] |
5. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| AITC | Allyl-isothiocyanate |
| ALT | Alanine aminotransferase |
| AMACR | α-methylacyl-CoA-racemase |
| ARLNC1 | Androgen receptor-regulated long noncoding RNA 1 |
| AST | Aspartate aminotransferase |
| BF | Bioaccessible fraction |
| BMI | Body mass index |
| BP | Pea protein isolate |
| BSE | Broccoli seed extract |
| BSP | Broccoli sprout powder |
| BST | Bitter and strong-tasting vegetable |
| BW | Whey protein isolate |
| CETB | 1-cyano-3,4-epithiobutane |
| CETP | 1-cyano-2,3-epithiopropane |
| CETPent | 1-cyano-4,5-epithiopentane |
| CRP | C-reactive protein |
| DEXA | dual-energy X-ray absorptiometry |
| DNMTs | DNA methyltransferases |
| DW | dry weight |
| ER-α | oestrogen receptor alpha |
| FBG | fasting blood glucose |
| γ-GTP | γ-glutamyl transferase |
| GSLs | Glucosinolates |
| GMB | Gut microbiota |
| GR | Glucoraphanin |
| GRS | Glucosinolate-rich broccoli sprout |
| GSH | Glutathione |
| HDAC | Histone deacetylase |
| HDL | High-density lipoprotein |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| I3C | Indole-3-carbinol |
| IL | Interleukin |
| Keap1 | Kelch Like ECH Associated Protein 1 |
| ITCs | Isothiocyanates |
| LDL | Low-density lipoprotein |
| MAPK | Mitogen-activated protein kinase |
| MeJA | Methyl jasmonate |
| MITC-1 | Moringin |
| MRTFB | Myocardin-related transcription factor B |
| MSP | Mustard seed powder |
| MST | Mild and sweet-tasting vegetable |
| NAC | N-acetylcysteine |
| NF-κB | Nuclear factor kappa B |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OGTT | Oral glucose tolerance test |
| Papp | Apparent permeability coefficient |
| PCS | Physical Component Summary |
| PEITC | Phenyl-ITC |
| PPBG | Postprandial blood glucose |
| SBP | Systolic blood pressure |
| SCFA | Short-chain fatty acid |
| SENO | Standardised extract of Nasturtium officinale |
| SF-NAC | Sulforaphane-N-acetyl-L-cysteine |
| SFN | Sulforaphane |
| SMCSO | S-methylcysteine sulfoxide |
| SRF | Serum response factor |
| TILs | Tumour-infiltrating lymphocytes |
| TNF-α | Tumour necrosis factor-α |
| TSH | Thyroid-stimulating hormone |
| VAS | Visual analogue scale |
| VLDL | Very low-density lipoprotein |
References
- Tarar, A.; Peng, S.; Cheema, S.; Peng, C.-A. Anticancer Activity, Mechanism, and Delivery of Allyl Isothiocyanate. Bioengineering 2022, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate Structural Diversity, Identification, Chemical Synthesis and Metabolism in Plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Chen, S.; Li, Y.; Hamouda, H.I.; Balah, M.A.; Xue, C.; Mao, X. Strategy for Preparing of Glucosinolate Derivatives with Outstanding Functional Activities Based on Myrosinase. Food Chem. 2025, 479, 143778. [Google Scholar] [CrossRef]
- Mitreiter, S.; Gigolashvili, T. Regulation of Glucosinolate Biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Esteve, M. Mechanisms Underlying Biological Effects of Cruciferous Glucosinolate-Derived Isothiocyanates/Indoles: A Focus on Metabolic Syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Song, F.; Verheust, Y.; Sampers, I.; Raes, K. The Stability of Isothiocyanates in Broccoli Extract: Oxidation from Erucin to Sulforaphane Was Discovered. Food Chem. 2025, 480, 143872. [Google Scholar] [CrossRef]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of Long-Term Consumption of Broccoli Sprouts on Inflammatory Markers in Overweight Subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane Reduces Hepatic Glucose Production and Improves Glucose Control in Patients with Type 2 Diabetes. Sci. Transl. Med. 2017, 9, aah4477. [Google Scholar] [CrossRef]
- Prieto, L.A.; Khiar-Fernández, N.; Calderón-Montaño, J.M.; López-Lázaro, M.; Lucía-Tamudo, J.; Nogueira, J.J.; León, R.; Moreno, N.; Valdivia, V.; Recio, R.; et al. Exploring the Broad-Spectrum Activity of Carbohydrate-Based Iberin Analogues: From Anticancer Effect to Antioxidant Properties. Eur. J. Med. Chem. 2025, 289, 117469. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Dinkova-Kostova, A.T.; Georgiev, M.I. Obesity and NRF2-Mediated Cytoprotection: Where Is the Missing Link? Pharmacol. Res. 2020, 156, 104760. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, J.; Zou, T.; Teng, Z.; Qin, Y.; Wu, R.; Yan, Y.; Fu, K.; Jiang, W.; Ju, Y.; et al. Effects of Sulforaphane on Negative Symptoms and Cognitive Impairments in Chronic Schizophrenia Patients: A Randomized Double-Blind Trial. J. Psychiatr. Res. 2025, 184, 464–472. [Google Scholar] [CrossRef]
- Hamad, H.T. The Anti-Cancer Effectiveness of Some Heterocyclic Compounds Containing Sulfur Atom. Results Chem. 2025, 15, 102182. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds. Mol. Nutr. Food Res. 2018, 62, e1700990. [Google Scholar] [CrossRef]
- Hoch, C.C.; Shoykhet, M.; Weiser, T.; Griesbaum, L.; Petry, J.; Hachani, K.; Multhoff, G.; Bashiri Dezfouli, A.; Wollenberg, B. Isothiocyanates in Medicine: A Comprehensive Review on Phenylethyl-, Allyl-, and Benzyl-Isothiocyanates. Pharmacol. Res. 2024, 201, 107107. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.-Y.; Fu, T.-M.; Chen, H.; Ishikawa, T.; Chiang, S.-Y.; Katon, J.; et al. Reactivation of PTEN Tumor Suppressor for Cancer Treatment through Inhibition of a MYC-WWP1 Inhibitory Pathway. Science 2019, 364, eaau0159. [Google Scholar] [CrossRef]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.C.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Erez, A.; Donia, M.S.; Sonnenburg, J.L.; Sattely, E.S. A Metabolic Pathway for Activation of Dietary Glucosinolates by a Human Gut Symbiont. Cell 2020, 180, 717–728.e19. [Google Scholar] [CrossRef]
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae Microgreens: A Novel and Promissory Source of Sustainable Bioactive Compounds. Curr. Res. Food Sci. 2023, 6, 100480. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kusznierewicz, B. Young Shoots of White and Red Headed Cabbages Like Novel Sources of Glucosinolates as Well as Antioxidative Substances. Antioxidants 2021, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Olayanju, J.B.; Bozic, D.; Naidoo, U.; Sadik, O.A. A Comparative Review of Key Isothiocyanates and Their Health Benefits. Nutrients 2024, 16, 757. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, H.-J.; Sheng, J.; Su, L.-Y.; Tian, Y. Extraction, Structural Properties, and Bioactivities of Moringa (Moringa oleifera Lam.) Isothiocyanates: A Review. Food Biosci. 2024, 57, 103447. [Google Scholar] [CrossRef]
- Fahey, J.W.; Olson, M.E.; Stephenson, K.K.; Wade, K.L.; Chodur, G.M.; Odee, D.; Nouman, W.; Massiah, M.; Alt, J.; Egner, P.A.; et al. The Diversity of Chemoprotective Glucosinolates in Moringaceae (Moringa Spp.). Sci. Rep. 2018, 8, 7994. [Google Scholar] [CrossRef] [PubMed]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in Food. In Living Reference Work Entry; Springer: Cham, Switzerland, 2017; pp. 87–132. [Google Scholar]
- Costa-Pérez, A.; Núñez-Gómez, V.; Baenas, N.; Di Pede, G.; Achour, M.; Manach, C.; Mena, P.; Del Rio, D.; García-Viguera, C.; Moreno, D.A.; et al. Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health. Nutrients 2023, 15, 1424. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cui, S.; Liu, J.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. The Recent Advances of Glucosinolates and Their Metabolites: Metabolism, Physiological Functions and Potential Application Strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 4217–4234. [Google Scholar] [CrossRef]
- Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Na, G.; He, C.; Zhang, S.; Tian, S.; Bao, Y.; Shan, Y. Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 1962. [Google Scholar] [CrossRef] [PubMed]
- Bones, A.M.; Rossiter, J.T. The Enzymic and Chemically Induced Decomposition of Glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Ooms, V.; Weemaes, C.; Hendrickx, M. Kinetic Study of the Irreversible Thermal and Pressure Inactivation of Myrosinase from Broccoli (Brassica oleracea L. Cv. Italica). J. Agric. Food Chem. 1999, 47, 1794–1800. [Google Scholar] [CrossRef]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. The Impact of Domestic Cooking Methods on Myrosinase Stability, Glucosinolates and Their Hydrolysis Products in Different Cabbage (Brassica oleracea) Accessions. Foods 2021, 10, 2908. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 213183. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.B.; Patel, J.K. Chemopreventive Aspects, Investigational Anticancer Applications and Current Perspectives on Allyl Isothiocyanate (AITC): A Review. Mol. Cell. Biochem. 2023, 478, 2763–2777. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Han, X.; Oh, G.; Im, J.-H.; Lim, J.S.; Cho, G.H.; Choi, S.-I.; Lee, O.-H. Plant Sources, Extraction Techniques, Analytical Methods, Bioactivity, and Bioavailability of Sulforaphane: A Review. Food Sci. Biotechnol. 2024, 33, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mao, J.; You, Y.; Liu, S. Study on Degradation Kinetics of Sulforaphane in Broccoli Extract. Food Chem. 2014, 155, 235–239. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Obregón-Cano, S.; de Haro-Bailón, A. Glucosinolates, Ca, Se Contents, and Bioaccessibility in Brassica Rapa Vegetables Obtained by Organic and Conventional Cropping Systems. Foods 2022, 11, 350. [Google Scholar] [CrossRef]
- Martínez-Castro, J.; de Haro-Bailón, A.; Obregón-Cano, S.; García Magdaleno, I.M.; Moreno Ortega, A.; Cámara-Martos, F. Bioaccessibility of Glucosinolates, Isothiocyanates and Inorganic Micronutrients in Cruciferous Vegetables through INFOGEST Static In Vitro Digestion Model. Food Res. Int. 2023, 166, 112598. [Google Scholar] [CrossRef]
- García-Pérez, P.; Tomas, M.; Rivera-Pérez, A.; Patrone, V.; Giuberti, G.; Capanoglu, E.; Lucini, L. Exploring the Bioaccessibility of Polyphenols and Glucosinolates from Brassicaceae Microgreens by Combining Metabolomics Profiling and Computational Chemometrics. Food Chem. 2024, 452, 139565. [Google Scholar] [CrossRef]
- Salas-Millán, J.Á.; Aguayo, E. Bioaccessibility and Unravelling of Polyphenols, Sulforaphane, and Indoles Biotransformation after In Vitro Gastrointestinal Digestion of a Novel Lactofermented Broccoli Beverage. Food Funct. 2024, 15, 11949–11960. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Sánchez-Bravo, P.; Medina, S.; Domínguez-Perles, R.; García-Viguera, C. Bioaccessible Organosulfur Compounds in Broccoli Stalks Modulate the Inflammatory Mediators Involved in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 800. [Google Scholar] [CrossRef]
- Egner, P.A.; Chen, J.G.; Wang, J.B.; Wu, Y.; Sun, Y.; Lu, J.H.; Zhu, J.; Zhang, Y.H.; Chen, Y.S.; Friesen, M.D.; et al. Bioavailability of Sulforaphane from Two Broccoli Sprout Beverages: Results of a Short-Term, Cross-over Clinical Trial in Qidong, China. Cancer Prev. Res. 2011, 4, 384–395. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Moreno, D.A.; Periago, P.M.; García-Viguera, C.; Domínguez-Perles, R. A New Food Ingredient Rich in Bioaccessible (Poly) Phenols (and Glucosinolates) Obtained from Stabilized Broccoli Stalks. Foods 2022, 11, 1734. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Doniec, J.; Kusznierewicz, B.; Dera, T.; Filipiak-Florkiewicz, A.; Florkiewicz, A. The Influence of Different Hydrothermal Processes Used in the Preparation of Brussels Sprouts on the Availability of Glucosinolates to Humans. Foods 2024, 13, 2988. [Google Scholar] [CrossRef]
- Vancoillie, F.; Verkempinck, S.H.E.; Sluys, L.; De Mazière, S.; Van Poucke, C.; Hendrickx, M.E.; Van Loey, A.M.; Grauwet, T. Stability and Bioaccessibility of Micronutrients and Phytochemicals Present in Processed Leek and Brussels Sprouts during Static In Vitro Digestion. Food Chem. 2024, 445, 138644. [Google Scholar] [CrossRef]
- Pagliari, S.; Domínguez-Rodríguez, G.; Cifuentes, A.; Ibáñez, E.; Labra, M.; Campone, L. Pressurized Liquid Extraction of Glucosinolates from Camelina sativa (L.) Crantz by-Products: Process Optimization and Biological Activities of Green Extract. Food Chem. X 2024, 22, 101324. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef]
- Bouranis, J.A.; Beaver, L.M.; Wong, C.P.; Choi, J.; Hamer, S.; Davis, E.W.; Brown, K.S.; Jiang, D.; Sharpton, T.J.; Stevens, J.F.; et al. Sulforaphane and Sulforaphane-Nitrile Metabolism in Humans Following Broccoli Sprout Consumption: Inter-Individual Variation, Association with Gut Microbiome Composition, and Differential Bioactivity. Mol. Nutr. Food Res. 2024, 68, 2300286. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, Z.; Hao, T.; Qiu, Z.; Zhang, B. Simulated Digestion and Fermentation In Vitro by Obese Human Gut Microbiota of Sulforaphane from Broccoli Seeds. Foods 2022, 11, 4016. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Michell, K.A.; Butler, M.M.; Smith, B.T.; Woolf, E.K.; Holmes, S.C.; Grabos, L.E.; Vazquez, A.R.; Isweiri, H.; Bunning, M.; et al. Feasibility and Tolerability of Daily Microgreen Consumption in Healthy Middle-Aged/Older Adults: A Randomized, Open-Label, Controlled Crossover Trial. Nutrients 2025, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Albaser, A.A.; Nueno-Palop, C.; Bennett, M.H.; Narbad, A.; Rossiter, J.T. Glucosinolate and Desulfo-Glucosinolate Metabolism by a Selection of Human Gut Bacteria. Curr. Microbiol. 2016, 73, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Martos, F.; Bolívar, A.; Rabasco-Vílchez, L.; Lafont-Déniz, F.; Luque-Ojeda, J.L.; Pérez-Rodríguez, F. Exploring the Bioaccessibility, In Vitro Colonic Fermentation, and the Impact on the Intestinal Microbiota of Allyl-and Benzyl-Isothiocyanate from White and Ethiopian Mustard. Food Res. Int. 2025, 203, 115781. [Google Scholar] [CrossRef]
- Okunade, O.; Niranjan, K.; Ghawi, S.K.; Kuhnle, G.; Methven, L. Supplementation of the Diet by Exogenous Myrosinase via Mustard Seeds to Increase the Bioavailability of Sulforaphane in Healthy Human Subjects after the Consumption of Cooked Broccoli. Mol. Nutr. Food Res. 2018, 62, 1700980. [Google Scholar] [CrossRef]
- Oliviero, T.; Lamers, S.; Capuano, E.; Dekker, M.; Verkerk, R. Bioavailability of Isothiocyanates From Broccoli Sprouts in Protein, Lipid, and Fiber Gels. Mol. Nutr. Food Res. 2018, 62, 1700837. [Google Scholar] [CrossRef]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Effect of Meal Composition and Cooking Duration on the Fate of Sulforaphane Following Consumption of Broccoli by Healthy Human Subjects. Br. J. Nutr. 2007, 97, 644–652. [Google Scholar] [CrossRef]
- Garcia-Ibañez, P.; Roses, C.; Agudelo, A.; Milagro, F.I.; Barceló, A.M.; Viadel, B.; Nieto, J.A.; Moreno, D.A.; Carvajal, M. The Influence of Red Cabbage Extract Nanoencapsulated with Brassica Plasma Membrane Vesicles on the Gut Microbiome of Obese Volunteers. Foods 2021, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ibañez, P.; Moreno, D.A.; Carvajal, M. Nanoencapsulation of Bimi® Extracts Increases Its Bioaccessibility after In Vitro Digestion and Evaluation of Its Activity in Hepatocyte Metabolism. Food Chem. 2022, 385, 132680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cremonini, E.; Mastaloudis, A.F.; Mitchell, A.E.; Bornhorst, G.M.; Oteiza, P.I. Optimization of Sulforaphane Bioavailability from a Glucoraphanin-Rich Broccoli Seed Extract in a Model of Dynamic Gastric Digestion and Absorption by Caco-2 Cell Monolayers. Food Funct. 2025, 16, 314–328. [Google Scholar] [CrossRef]
- Ali Redha, A.; Torquati, L.; Bows, J.R.; Gidley, M.J.; Cozzolino, D. Microencapsulation of Broccoli Sulforaphane Using Whey and Pea Protein: In Vitro Dynamic Gastrointestinal Digestion and Intestinal Absorption by Caco-2-HT29-MTX-E12 Cells. Food Funct. 2024, 16, 71–86. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates From Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Liu, A.H.; Radavelli-Bagatini, S.; Shafaei, A.; Boyce, M.C.; Wood, L.G.; McCahon, L.; Koch, H.; Sim, M.; Hill, C.R.; et al. Cruciferous Vegetables Lower Blood Pressure in Adults with Mildly Elevated Blood Pressure in a Randomized, Controlled, Crossover Trial: The VEgetableS for VaScular HEaLth (VESSEL) Study. BMC Med. 2024, 22, 353. [Google Scholar] [CrossRef]
- Thorup, A.C.; Kristensen, H.L.; Kidmose, U.; Lambert, M.N.T.; Christensen, L.P.; Fretté, X.; Clausen, M.R.; Hansen, S.M.; Jeppesen, P.B. Strong and Bitter Vegetables from Traditional Cultivars and Cropping Methods Improve the Health Status of Type 2 Diabetics: A Randomized Control Trial. Nutrients 2021, 13, 1813. [Google Scholar] [CrossRef] [PubMed]
- Alaba, T.E.; Holman, J.M.; Ishaq, S.L.; Li, Y. Current Knowledge on the Preparation and Benefits of Cruciferous Vegetables as Relates to In Vitro, In Vivo, and Clinical Models of Inflammatory Bowel Disease. Curr. Dev. Nutr. 2024, 8, 102160. [Google Scholar] [CrossRef]
- Fatima, H.; Radhakrishnan, Y.; Nandakumar, K.S.; Lakshmanan, C.C. Efficacy of Broccoli Sprout Powder in Management of Metabolic Health Risk Factors: A Randomized, Double Blind, Placebo Controlled Clinical Study. Cardiol. Res. Cardiovasc. Med. 2024, 9, 254. [Google Scholar] [CrossRef]
- Gupta, S.; Burman, S.; Nair, A.B.; Chauhan, S.; Sircar, D.; Roy, P.; Dhanwat, M.; Lahiri, D.; Mehta, D.; Das, R.; et al. Brassica oleracea Extracts Prevent Hyperglycemia in Type 2 Diabetes Mellitus. Prev. Nutr. Food Sci. 2022, 27, 50–62. [Google Scholar] [CrossRef]
- Petri, N.; Tannergren, C.; Holst, B.; Mellon, F.A.; Bao, Y.; Plumb, G.W.; Bacon, J.; O’Leary, K.A.; Kroon, P.A.; Knutson, L.; et al. Absorption/Metabolism of Sulforaphane and Quercetin, and Regulation of Phase II Enzymes, in Human Jejunum In Vivo. Drug Metab. Dispos. 2003, 31, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Dwibedi, C.; Axelsson, A.S.; Abrahamsson, B.; Fahey, J.W.; Asplund, O.; Hansson, O.; Ahlqvist, E.; Tremaroli, V.; Bäckhed, F.; Rosengren, A.H. Effect of Broccoli Sprout Extract and Baseline Gut Microbiota on Fasting Blood Glucose in Prediabetes: A Randomized, Placebo-Controlled Trial. Nat. Microbiol. 2025, 10, 681–693. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.C.; Tillqvist, E.N.; Vinge, F.; Millbert, F.; Lännerström, J.; Nilsson, P.H.; Samyn, D.; Apró, W.; Sundqvist, M.L.; et al. Glucosinolate-Rich Broccoli Sprouts Protect against Oxidative Stress and Improve Adaptations to Intense Exercise Training. Redox Biol. 2023, 67, 102873. [Google Scholar] [CrossRef]
- Zhang, Z.; Garzotto, M.; Davis, E.W.; Mori, M.; Stoller, W.A.; Farris, P.E.; Wong, C.P.; Beaver, L.M.; Thomas, G.V.; Williams, D.E.; et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr. Cancer 2020, 72, 74–87. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, C.; Pratt, R.; Khoury, T.; Qu, J.; Fahey, J.W.; McCann, S.E.; Zhang, Y.; Wu, Y.; Hutson, A.D.; et al. A Presurgical-Window Intervention Trial of Isothiocyanate-Rich Broccoli Sprout Extract in Patients with Breast Cancer. Mol. Nutr. Food Res. 2022, 66, 2101094. [Google Scholar] [CrossRef]
- Lozanovski, V.J.; Polychronidis, G.; Gross, W.; Gharabaghi, N.; Mehrabi, A.; Hackert, T.; Schemmer, P.; Herr, I. Broccoli Sprout Supplementation in Patients with Advanced Pancreatic Cancer Is Difficult despite Positive Effects—Results from the POUDER Pilot Study. Investig. New Drugs 2020, 38, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Baldermann, S.; Wiesner-Reinhold, M.; Bergmann, M.M.; Grune, T.; Hanschen, F.S. Metabolism and Recovery of Epithionitriles from Glucosinolates—A Human Intervention Study. Mol. Nutr. Food Res. 2023, 67, 2200619. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef]
- Nieto, J.A.; Hellín, P.; Valverde, M.; Pérez, B.; Viadel, B.; Agudelo, A. Pak Choi (Brassica rapa ssp. Chinensis) Glucosinolates Profile and Bioaccessibility through In Vitro Dynamic Digestion Model. Eur. Food Res. Technol. 2025, 251, 205–212. [Google Scholar] [CrossRef]
- Satomi, S.; Takahashi, S.; Yoshida, K.; Shimizu, S.; Inoue, T.; Takara, T.; Suganuma, H. Effects of Broccoli Sprout Supplements Enriched in Glucoraphanin on Liver Functions in Healthy Middle-Aged Adults with High-Normal Serum Hepatic Biomarkers: A Randomized Controlled Trial. Front. Nutr. 2022, 9, 1077271. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.; Dorner, A.; Yue, Y.; Poulsen, N.; Andersen, S.; Aalykke, F.; Lambert, M. Beneficial Effects of a Freeze-Dried Kale Bar on Type 2 Diabetes Patients: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 3641. [Google Scholar] [CrossRef]
- Farzameh, F.; Azadbakht, M.; Kashi, Z.; Asgarirad, H.; Salehifar, E.; Mirzaee, F.; Davoodi, A.; Amirkhanloo, S. Evaluation of the Therapeutic Effect of Descurainia sophia (L.) Webb Ex Prantl Seed Extract on Hyperthyroidism: A Double-blind Placebo-controlled Pilot Clinical Trial. Food Sci. Nutr. 2023, 11, 5918–5927. [Google Scholar] [CrossRef]
- Livingstone, T.L.; Saha, S.; Bernuzzi, F.; Savva, G.M.; Troncoso-Rey, P.; Traka, M.H.; Mills, R.D.; Ball, R.Y.; Mithen, R.F. Accumulation of Sulforaphane and Alliin in Human Prostate Tissue. Nutrients 2022, 14, 3263. [Google Scholar] [CrossRef]
- Clemente, M.; Miguel, M.D.; Felipe, K.B.; Gribner, C.; F Moura, P.; R Rigoni, A.A.; B Parisotto, E.; T Piltz, M.; Valdameri, G.; Henneberg, R.; et al. Biomarkers of Oxidative Stress and Inflammation in People Witha Physical Disability Treated with a Standardized Extract of Nasturtium officinale: A Randomized, Double-blind, and Placebo-controlled Trial. Phytother. Res. 2020, 34, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.J.; Arscott, S.A.; Goltz, S.; Muir, C.; Binkley, N.; Tanumihardjo, S.A. Urinary 2- to 16α-Hydroxyestrone Ratio Did Not Change with Cruciferous Vegetable Intake in Premenopausal Women. Int. J. Vitam. Nutr. Res. 2024, 94, 177–186. [Google Scholar] [CrossRef]
- Honma, A.; Fujiwara, Y.; Takei, S.; Kino, T. The Improvement of Daily Fatigue in Women Following the Intake of Maca (Lepidium meyenii) Extract Containing Benzyl Glucosinolate. Funct. Foods Health Dis. 2022, 12, 175. [Google Scholar] [CrossRef]
- Villar-López, M.; Soto-Becerra, P.; Curse Choque, R.; Al-Kassab-Córdova, A.; Bernuy-Barrera, F.; Palomino, H.; Rojas, P.A.; Vera, C.; Lugo-Martínez, G.; Mezones-Holguín, E. Safety and Tolerability of a Natural Supplement Containing Glucosinolates, Phytosterols and Citrus Flavonoids in Adult Women: A Randomized Phase I, Placebo-Controlled, Multi-Arm, Double-Blinded Clinical Trial. Gynecol. Endocrinol. 2021, 37, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Kensler, T.W. The Challenges of Designing and Implementing Clinical Trials With Broccoli Sprouts… and Turning Evidence Into Public Health Action. Front. Nutr. 2021, 8, 648788. [Google Scholar] [CrossRef] [PubMed]
| Glucosinolate (GSL) | Molecular Structure | Isothiocyanate (ITC) | Molecular Structure | Cruciferous Vegetable |
|---|---|---|---|---|
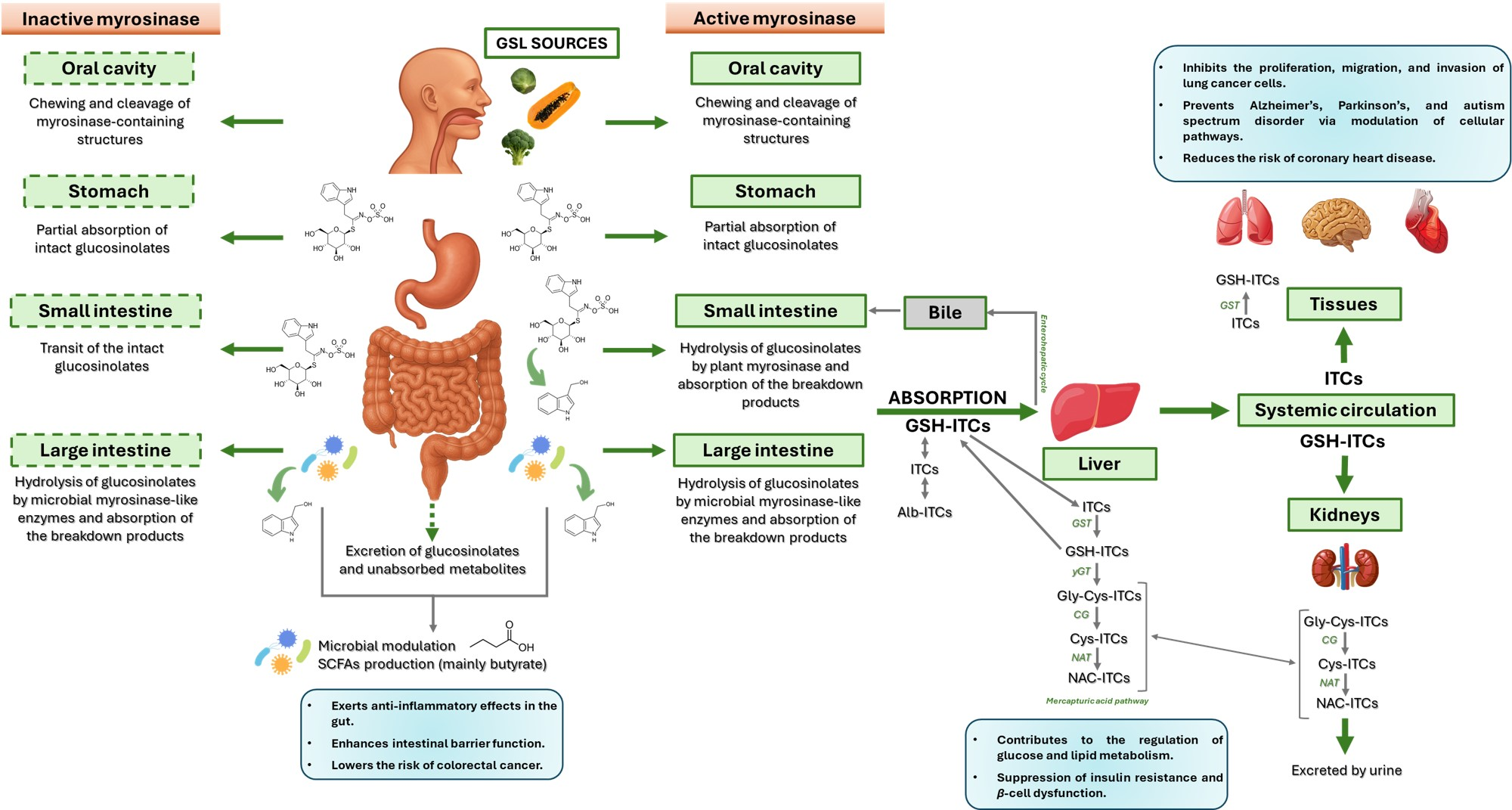
| Glucobrassicin, Neoglucobrassicin | 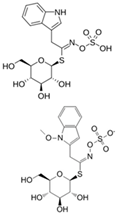 | Indole-3-carbinol (I3C) 3,3-diindolylmethane (DIM) | 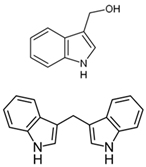 | All crucifers |
| Sinigrin | 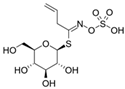 | Allyl isothiocyanate (AITC) |  | Mustard Brussels sprouts Cauliflower |
| Glucoraphanin |  | Sulforaphane (SFN) |  | Broccoli Arugula |
| Gluconasturtiin | 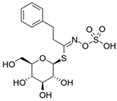 | Phenyl isothiocyanate (PEITC) | 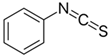 | Cabbage Chinese cabbage Radish Watercress |
| Glucotropaeolin | 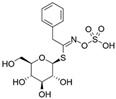 | Benzyl isothiocyanate (BITC) |  | Garden cress Horseradish White mustards |
| Sinalbin, Glucosinalbin | 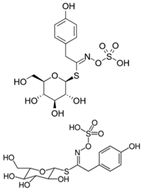 | 4-hydroxybenzyl isothiocyanate | 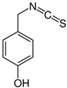 | White mustards |
| Progoitrin Epiprogoitrin | 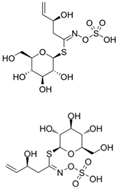 | 5-vinyloxazolidine-2-thione/Goitrin |  | Brussels sprouts Salad rocket Sea kale Turnips |
| Glucoerucin |  | Erucin | 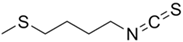 | Salad rocket Radish Chinese cabbage Wild rocket |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narra, F.; Galgani, G.; Harris, C.B.; Moreno, D.A.; Núñez-Gómez, V. Bioavailability, Human Metabolism, and Dietary Interventions of Glucosinolates and Isothiocyanates: Critical Insights and Future Perspectives. Foods 2025, 14, 2876. https://doi.org/10.3390/foods14162876
Narra F, Galgani G, Harris CB, Moreno DA, Núñez-Gómez V. Bioavailability, Human Metabolism, and Dietary Interventions of Glucosinolates and Isothiocyanates: Critical Insights and Future Perspectives. Foods. 2025; 14(16):2876. https://doi.org/10.3390/foods14162876
Chicago/Turabian StyleNarra, Federica, Giulia Galgani, Cassidy Bo Harris, Diego A. Moreno, and Vanesa Núñez-Gómez. 2025. "Bioavailability, Human Metabolism, and Dietary Interventions of Glucosinolates and Isothiocyanates: Critical Insights and Future Perspectives" Foods 14, no. 16: 2876. https://doi.org/10.3390/foods14162876
APA StyleNarra, F., Galgani, G., Harris, C. B., Moreno, D. A., & Núñez-Gómez, V. (2025). Bioavailability, Human Metabolism, and Dietary Interventions of Glucosinolates and Isothiocyanates: Critical Insights and Future Perspectives. Foods, 14(16), 2876. https://doi.org/10.3390/foods14162876







