Production Methods, Biological Activity and Potential Application Prospects of Astaxanthin
Abstract
1. Introduction
2. AST Production Methods
2.1. Biosynthesis of AST
2.2. AST Extraction and Purification
3. Bioactivity of AST
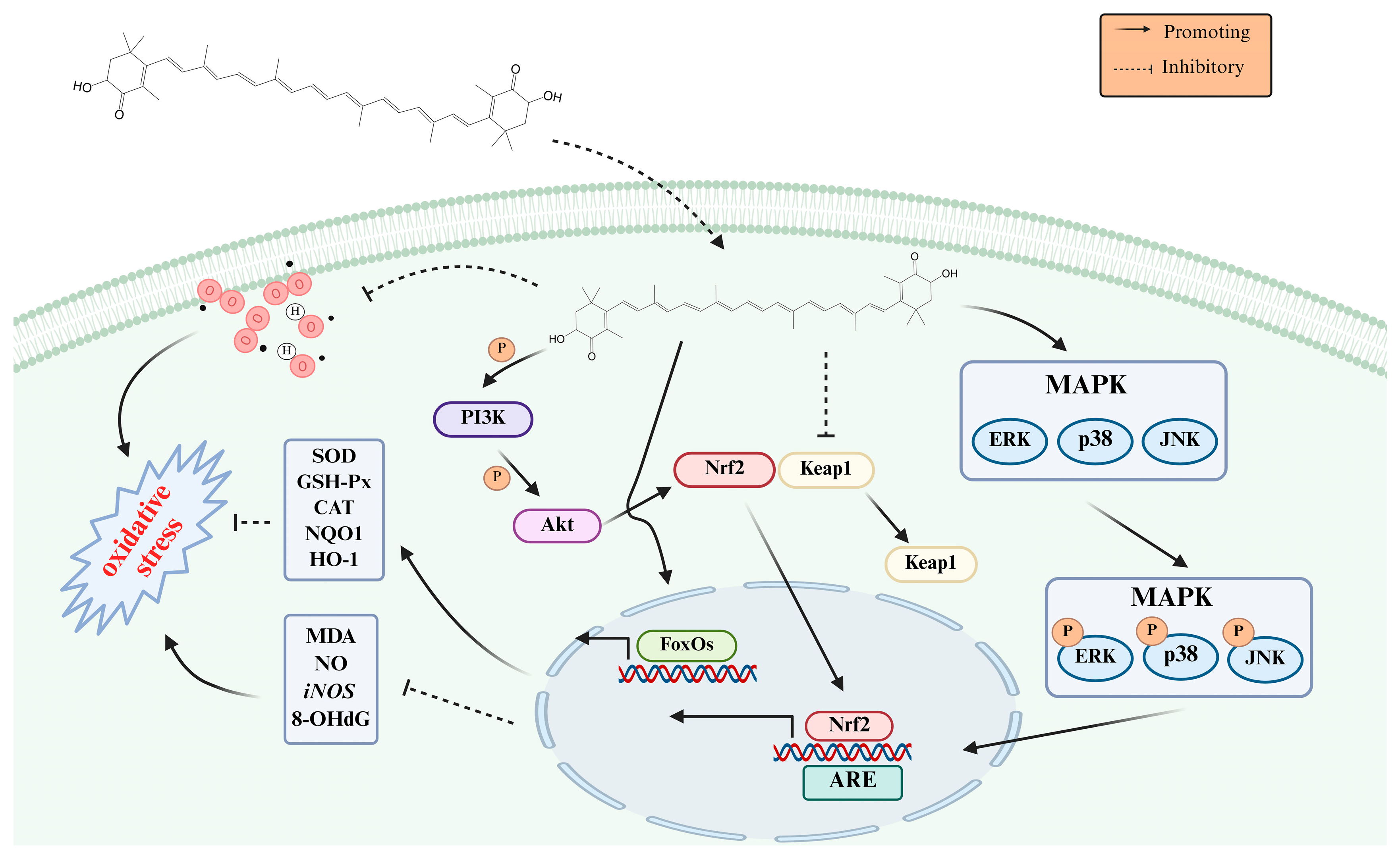
3.1. Antioxidation
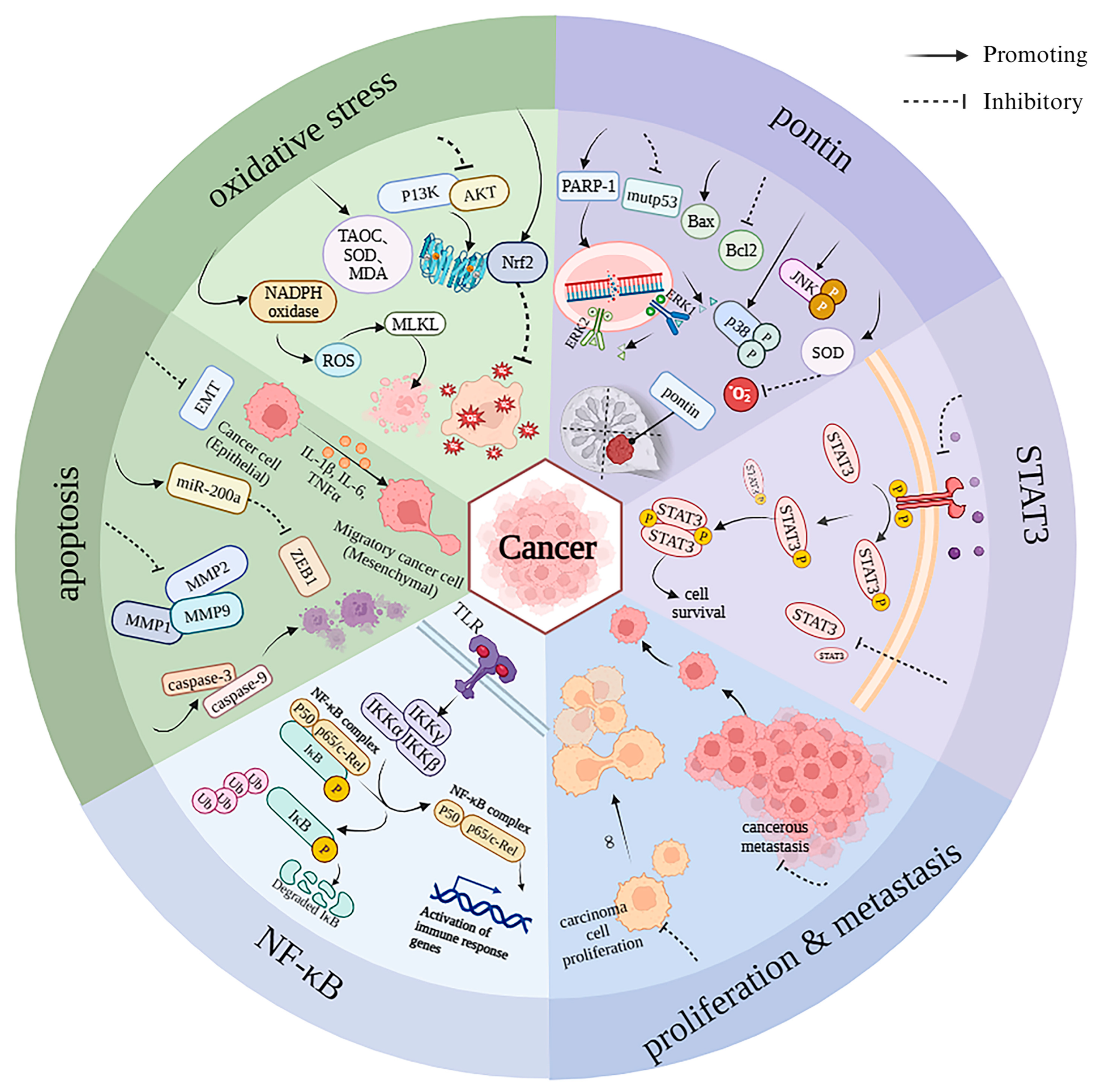
3.2. Anti-Cancer
3.3. Eye Protection
3.4. Anti-Inflammatory
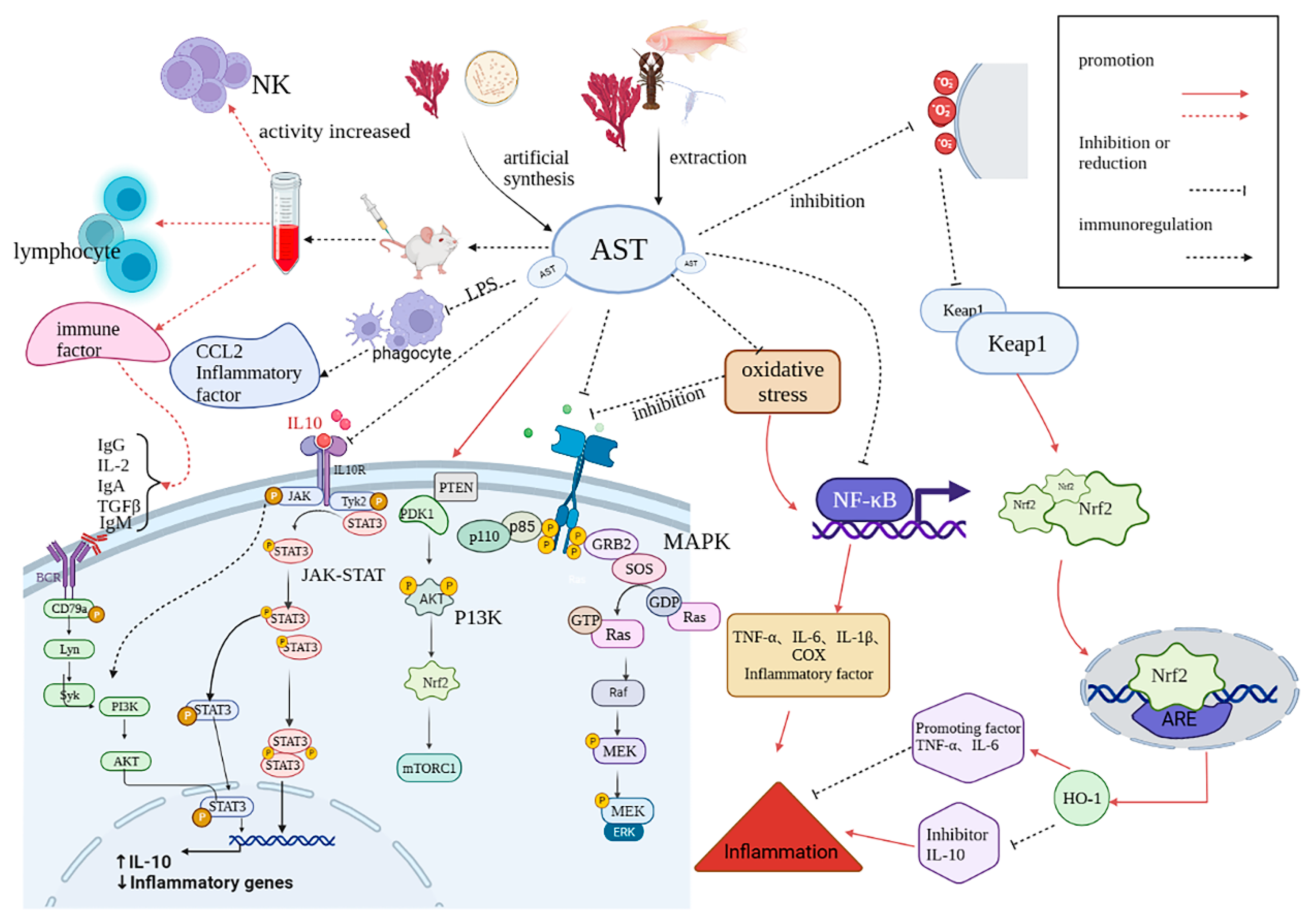
3.5. Immunoregulation
3.6. Skin Care
3.7. Antidiabetic
3.8. Neuroprotection
3.9. Others
4. Conclusions, Challenges, Opportunities and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ma, X.K.; Shao, X.; Xu, T.; Yan, H.; Teng, H.; Chen, Z.X.; Hu, M.X. New Horizons of Astaxanthin-loaded Akkermansia muciniphila as an Integrated Dietary Supplement: Physicochemical Structures and Gastrointestinal Fate. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef] [PubMed]
- Mohd Shafie, A.S.; Kamarudin, S.N.; Meor Mohd Affandi, M.M.R.; Siran, R. Exploring astaxanthin: A comprehensive review on its pharmacokinetics properties and neuroprotective potential. Nutr. Neurosci. 2025; Ahead-of-print. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Bharti, A.; Hooda, V.; Jain, U.; Chauhan, N. Astaxanthin: A nature’s versatile compound utilized for diverse applications and its therapeutic effects. 3 Biotech 2025, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhan, W.; Xie, S.; Peng, H.; Cao, H.; Tang, Z.; Tian, Y.; Zhu, T.; Sun, P.; Jin, M.; et al. Multi-omics analysis revealed the effects of different astaxanthin sources on the antioxidant properties of Scylla paramamosain. Food Chem. 2025, 478, 143470. [Google Scholar] [CrossRef] [PubMed]
- Basiony, M.; Ouyang, L.; Wang, D.; Yu, J.; Zhou, L.; Zhu, M.; Wang, X.; Feng, J.; Dai, J.; Shen, Y.; et al. Optimization of microbial cell factories for astaxanthin production: Biosynthesis and regulations, engineering strategies and fermentation optimization strategies. Synth. Syst. Biotechnol. 2022, 7, 689–704. [Google Scholar] [CrossRef]
- Czamara, K.; Adamczyk, A.; Stojak, M.; Radwan, B.; Baranska, M. Astaxanthin as a new Raman probe for biosensing of specific subcellular lipidic structures: Can we detect lipids in cells under resonance conditions? Cell. Mol. Life Sci. 2021, 78, 3477–3484. [Google Scholar] [CrossRef]
- Mosaad, Y.O.; Gobba, N.A.; Hussein, M.A. Astaxanthin; a Promising Protector Against Gentamicin-Induced Nephrotoxicity in Rats. Curr. Pharm. Biotechnol. 2016, 17, 1189–1197. [Google Scholar] [CrossRef]
- Huang, J.J.; Xie, Q.; Lin, S.; Xu, W.; Cheung, P.C.K. Microalgae-derived astaxanthin: Bioactivities, biotechnological approaches and industrial technologies for its production. Crit. Rev. Food Sci. Nutr. 2025; Ahead-of-print. [Google Scholar] [CrossRef]
- Wang, C.; Armstrong, D.W.; Chang, C.D. Rapid baseline separation of enantiomers and a mesoform of all-trans-astaxanthin, 13-cis-astaxanthin, adonirubin, and adonixanthin in standards and commercial supplements. J. Chromatogr. A 2008, 1194, 172–177. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Yuan, W.C.; Wu, T.Y.; Chu, P.Y.; Chang, F.R.; Wu, Y.C. High-Purity Bioactive Ingredient—3S,3′S-Astaxanthin: A New Preparation from Genetically Modified Kluyveromyces marxianus without Column Chromatography and Gel Filtration. Antioxidants 2023, 12, 875. [Google Scholar] [CrossRef]
- Giercuszkiewicz-Hecold, B.; Pajuelo, D.; Steczkiewicz, Z.; Cywinska, A.; Marycz, K. Astaxanthin supplementation in Arabian racing horses mitigates oxidative stress and inflammation in peripheral blood mononuclear cells through enhanced mitophagy. Sci. Rep. 2025, 15, 14633. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Agni, M.B.; Rai, P.; Sadananda, M.; Mirajkar, A.M.; Kumar, B.M.; Ranade, A.V.; Gowda, K.M.D. Unraveling the synergistic effects of Astaxanthin and DHA on perinatal undernutrition-induced oxidative stress and cognitive deficit. Learn. Behav. 2025. [Google Scholar] [CrossRef] [PubMed]
- Aslanbay Guler, B.; Saglam-Metiner, P.; Deniz, I.; Demirel, Z.; Yesil-Celiktas, O.; Imamoglu, E. Aligned with sustainable development goals: Microwave extraction of astaxanthin from wet algae and selective cytotoxic effect of the extract on lung cancer cells. Prep. Biochem. Biotechnol. 2023, 53, 565–571. [Google Scholar] [CrossRef]
- Jeon, Y.-N.; Ryu, S.-J.; Sathiyaseelan, A.; Baek, J.-S. Bioactive Molecules of Microalgae Haematococcus pluvialis–Mediated Synthesized Silver Nanoparticles: Antioxidant, Antimicrobial, Antibiofilm, Hemolysis Assay, and Anticancer. Bioinorg. Chem. Appl. 2025, 2025, 8876478. [Google Scholar] [CrossRef]
- Wang, P.; Yu, X.; Sun, P.; Pan, K.; Sun, J.; Guo, Y.; Liu, Z.; Jiao, M.; Deng, J.; Zhang, H. Astaxanthin Increases Tumor Suppressor Gene Expression and Affects Cellular Biological Behavior in Oral Dysplastic Keratinocytes by Regulating DNA Methylation. J. Oral Pathol. Med. 2025, 54, 39–48. [Google Scholar] [CrossRef]
- Kerlikowsky, F.; Bartsch, M.; Jonas, W.; Hahn, A.; Schuchardt, J.P. Calanus Oil and Lifestyle Interventions Improve Glucose Homeostasis in Obese Subjects with Insulin Resistance. Mar. Drugs 2025, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Phochantachinda, S.; Photcharatinnakorn, P.; Chatchaisak, D.; Sakcamduang, W.; Chansawhang, A.; Buranasinsup, S.; Suemanotham, N.; Chantong, B. Plasma-based proteomics analysis of molecular pathways in canine diabetes mellitus after astaxanthin supplementation. PLoS ONE 2025, 20, e0321509. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeon, S.H.; Ham, H.J.; Lee, H.P.; Song, M.J.; Hong, J.T. Improved Anti-Inflammatory Effects of Liposomal Astaxanthin on a Phthalic Anhydride-Induced Atopic Dermatitis Model. Front. Immunol. 2020, 11, 565285. [Google Scholar] [CrossRef]
- Masoudi, A.; Jorjani, M.; Alizadeh, M.; Mirzamohammadi, S.; Mohammadi, M. Anti-inflammatory and antioxidant effects of astaxanthin following spinal cord injury in a rat animal model. Brain Res. Bull. 2021, 177, 324–331. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, N.; Shi, Y.; Zhou, B.; Sun, D.; Ma, B.; Xu, Z.; Yang, J.; Li, C. Astaxanthin Protects Dendritic Cells from Lipopolysaccharide-Induced Immune Dysfunction. Mar. Drugs 2021, 19, 346. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Nagoya, C.; Ishikura, A.; Sugiyama, Y.; Takahashi, J.; Sugiyama, K. Effect of Astaxanthin on the Expression and Activity of Aquaporin-3 in Skin in an In-Vitro Study. Life 2020, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Lu, Y.; Li, W.; Tao, T.; Peng, L.; Wang, W.H.; Gao, S.; Liu, C.; Zhuang, Z.; Xia, D.Y.; et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br. J. Pharmacol. 2021, 178, 1114–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiu, Y.; Li, W.; Tang, A.; Huang, H.; Yao, W.; Li, H.; Zou, T. Astaxanthin Alleviates Foam Cell Formation and Promotes Cholesterol Efflux in Ox-LDL-Induced RAW264.7 Cells via CircTPP2/miR-3073b-5p/ABCA1 Pathway. Molecules 2023, 28, 1701. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Shuvo, A.A.; Bepari, A.K.; Hasan Apu, M.; Shill, M.C.; Hossain, M.; Uddin, M.; Islam, M.R.; Bakshi, M.K.; Hasan, J.; et al. Curcumin improves D-galactose and normal-aging associated memory impairment in mice: In vivo and in silico-based studies. PLoS ONE 2022, 17, e0270123. [Google Scholar] [CrossRef]
- Deng, M.; Tong, R.; Bian, Y.; Hou, G. Astaxanthin attenuates cigarette smoking-induced oxidative stress and inflammation in a sirtuin 1-dependent manner. Biomed. Pharmacother. 2023, 159, 114230. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway. Mar. Drugs 2019, 17, 123. [Google Scholar] [CrossRef]
- Gao, S.; Heng, N.; Liu, F.; Guo, Y.; Chen, Y.; Wang, L.; Ni, H.; Sheng, X.; Wang, X.; Xing, K.; et al. Natural astaxanthin enhanced antioxidant capacity and improved semen quality through the MAPK/Nrf2 pathway in aging layer breeder roosters. J. Anim. Sci. Biotechnol. 2021, 12, 112. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Zhang, Y.; Du, J.; Wang, Y.; Yu, H.; He, Y. Astaxanthin alleviates gestational diabetes mellitus in mice through suppression of oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2517–2527. [Google Scholar] [CrossRef]
- Kochi, T.; Shimizu, M.; Sumi, T.; Kubota, M.; Shirakami, Y.; Tanaka, T.; Moriwaki, H. Inhibitory effects of astaxanthin on azoxymethane-induced colonic preneoplastic lesions in C57/BL/KsJ-db/dbmice. BMC Gastroenterol. 2014, 14, 212. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lim, J.W.; Kim, H. Astaxanthin induces NADPH oxidase activation and receptor-interacting protein kinase 1-mediated necroptosis in gastric cancer AGS cells. Mol. Med. Rep. 2021, 24, 12477. [Google Scholar] [CrossRef]
- Cui, L.; Li, Z.; Xu, F.; Tian, Y.; Chen, T.; Li, J.; Guo, Y.; Lyu, Q. Antitumor Effects of Astaxanthin on Esophageal Squamous Cell Carcinoma by up-Regulation of PPARγ. Nutr. Cancer 2021, 74, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ahn, Y.T.; Lee, C.W.; Kim, H.; An, W.G. Astaxanthin Modulates Apoptotic Molecules to Induce Death of SKBR3 Breast Cancer Cells. Mar. Drugs 2020, 18, 266. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Zhao, Y.X.; Li, S.Y.; Qiang, J.W.; Ji, Y.Z. Anti-Tumor Effects of Astaxanthin by Inhibition of the Expression of STAT3 in Prostate Cancer. Mar. Drugs 2020, 18, 415. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, H. Astaxanthin Modulation of Signaling Pathways That Regulate Autophagy. Mar. Drugs 2019, 17, 546. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, Y.M.; Hong, S. Astaxanthin suppresses the metastasis of colon cancer by inhibiting the MYC-mediated downregulation of microRNA-29a-3p and microRNA-200a. Sci. Rep. 2019, 9, 9457. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kao, C.-J.; Huang, H.-Y.; Huang, S.-Y.; Chen, C.-Y.; Lin, Y.-S.; Wen, Z.-H.; Wang, H.-M.D. Astaxanthin reduces MMP expressions, suppresses cancer cell migrations, and triggers apoptotic caspases of In Vitro and In Vivo models in melanoma. J. Funct. Foods 2017, 31, 20–31. [Google Scholar] [CrossRef]
- Shin, J.; Saini, R.K.; Oh, J.W. Low Dose Astaxanthin Treatments Trigger the Hormesis of Human Astroglioma Cells by Up-Regulating the Cyclin-Dependent Kinase and Down-Regulated the Tumor Suppressor Protein P53. Biomedicines 2020, 8, 434. [Google Scholar] [CrossRef]
- Otsuka, T.; Shimazawa, M.; Inoue, Y.; Nakano, Y.; Ojino, K.; Izawa, H.; Tsuruma, K.; Ishibashi, T.; Hara, H. Astaxanthin Protects Against Retinal Damage: Evidence from In Vivo and In Vitro Retinal Ischemia and Reperfusion Models. Curr. Eye Res. 2016, 41, 1465–1472. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Y.; Zhao, T.; Wang, Z. Effect of astaxanthin on metabolic cataract in rats with type 1 diabetes mellitus. Exp. Mol. Pathol. 2020, 113, 104372. [Google Scholar] [CrossRef]
- Shimokawa, T.; Fukuta, T.; Inagi, T.; Kogure, K. Protective effect of high-affinity liposomes encapsulating astaxanthin against corneal disorder in the in vivo rat dry eye disease model. J. Clin. Biochem. Nutr. 2020, 66, 224–232. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Hou, C.; Li, J.; Peng, H.; Wang, Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp. Eye Res. 2020, 197, 108113. [Google Scholar] [CrossRef] [PubMed]
- Küçüködük, A.; Helvacioglu, F.; Haberal, N.; Dagdeviren, A.; Bacanli, D.; Yilmaz, G.; Akkoyun, I. Antiproliferative and anti-apoptotic effect of astaxanthin in an oxygen-induced retinopathy mouse model. Can. J. Ophthalmol. 2019, 54, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Yang, C.M.; Yang, C.H. Protective Effect of Astaxanthin on Blue Light Light-Emitting Diode-Induced Retinal Cell Damage via Free Radical Scavenging and Activation of PI3K/Akt/Nrf2 Pathway in 661W Cell Model. Mar. Drugs 2020, 18, 387. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhou, Y.; Li, X.F.; Wan, Q.J.; Yu, L.H. Preventive treatment of astaxanthin provides neuroprotection through suppression of reactive oxygen species and activation of antioxidant defense pathway after stroke in rats. Brain Res. Bull. 2017, 130, 211–220. [Google Scholar] [CrossRef]
- Cai, X.; Chen, Y.; Xie, X.; Yao, D.; Ding, C.; Chen, M. Erratum: Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-κB. Am. J. Transl. Res. 2021, 13, 7420–7421. [Google Scholar]
- Hwang, S.H.; Kim, J.M.; Kim, S.; Yoon, M.J.; Park, K.S. Chemical Transformation of Astaxanthin from Haematococcus pluvialis Improves Its Antioxidative and Anti-inflammatory Activities. ACS Omega 2020, 5, 19120–19130. [Google Scholar] [CrossRef]
- Mamun-Or-Rashid, A.N.M.; Lucy, T.T.; Yagi, M.; Yonei, Y. Inhibitory Effects of Astaxanthin on CML-HSA-Induced Inflammatory and RANKL-Induced Osteoclastogenic Gene Expression in RAW 264.7 Cells. Biomedicines 2021, 10, 54. [Google Scholar] [CrossRef]
- Yang, M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. Astaxanthin Prevents Diet-Induced NASH Progression by Shaping Intrahepatic Immunity. Int. J. Mol. Sci. 2021, 22, 11037. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, L.; Ge, J.; Wu, X.; Peng, Y.; Zhang, T.; Jiang, M. Astaxanthin supplementation enriches productive performance, physiological and immunological responses in laying hens. Anim. Biosci. 2021, 34, 443–448. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, W.; Gao, Y.; Yang, R.; Zhang, X.; Xu, J.; Tang, Q. Astaxanthin (ATX) enhances the intestinal mucosal functions in immunodeficient mice. Food Funct. 2020, 11, 3371–3381. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, J.; Liu, H.; Yao, K.; Hou, X.; Cao, Y.; Liu, X. Astaxanthin attenuates oxidative stress and immune impairment in D-galactose-induced aging in rats by activating the Nrf2/Keap1 pathway and suppressing the NF-κB pathway. Food Funct. 2020, 11, 8099–8111. [Google Scholar] [CrossRef]
- Ha, Y.; Lee, W.H.; Jeong, J.; Park, M.; Ko, J.Y.; Kwon, O.W.; Lee, J.; Kim, Y.J. Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-κB. Nutrients 2020, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Park, S.H.; Yun, S.Y.; Yu, D.S.; Lee, Y.B. Astaxanthin Protects Ultraviolet B-Induced Oxidative Stress and Apoptosis in Human Keratinocytes via Intrinsic Apoptotic Pathway. Ann. Dermatol. 2022, 34, 125–131. [Google Scholar] [CrossRef]
- Gürsoy, K.; Teymur, H.; Koca, G.; Işikçi, Ö.; Demircan, F.B.; Kankaya, Y.; Koçer, U. The Effect of Astaxanthin on Random Pattern Skin Flaps. Ann. Plast. Surg. 2020, 84, 208–215. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Zhang, Y.; Hibino, T.; Goto, M. Oral Supplementation with Z-Isomer-Rich Astaxanthin Inhibits Ultraviolet Light-Induced Skin Damage in Guinea Pigs. Mar. Drugs 2022, 20, 414. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-diabetic effects of astaxanthin on an STZ-induced diabetic model in rats. Endocr. J. 2021, 68, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Tian, D.C.; Wang, F.W.; Zhang, M.H.; Fan, C.D.; Chen, W.; Wang, M.H.; Fu, X.Y.; Ma, J.K. Astaxanthin inhibits homocysteine-induced endothelial cell dysfunction via the regulation of the reactive oxygen species-dependent VEGF-VEGFR2-FAK signaling pathway. Mol. Med. Rep. 2019, 19, 4753–4760. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhu, X.L.; Sun, M.H.; Dang, Y.K. Effects of astaxanthin onaxonal regeneration via cAMP/PKA signaling pathway in mice with focal cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 135–143. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zhang, X.; Zhou, M.L.; Zhou, X.M.; Li, N.; Li, W.; Cong, Z.X.; Sun, Q.; Zhuang, Z.; Wang, C.X.; et al. Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. J. Neurosurg. 2014, 121, 42–54. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, C.; Zhang, S.; Xu, Y. Neuroprotective effects of astaxanthin against oxygen and glucose deprivation damage via the PI3K/Akt/GSK3β/Nrf2 signalling pathway in vitro. J. Cell. Mol. Med. 2020, 24, 8977–8985. [Google Scholar] [CrossRef]
- Kim, R.E.; Shin, C.Y.; Han, S.H.; Kwon, K.J. Astaxanthin Suppresses PM2.5-Induced Neuroinflammation by Regulating Akt Phosphorylation in BV-2 Microglial Cells. Int. J. Mol. Sci. 2020, 21, 7227. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, S.E.; Abdel-Aziz, A.K.; Wahdan, S.; Esmat, A.; Azab, S.S. Astaxanthin Ameliorates Doxorubicin-Induced Cognitive Impairment (Chemobrain) in Experimental Rat Model: Impact on Oxidative, Inflammatory, and Apoptotic Machineries. Mol. Neurobiol. 2018, 55, 5727–5740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Too, H.-P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef]
- Wang, C.; Hong, Z.; Song, M.; Zheng, H.; Zhou, Q.; Yang, H.; Li, H.; Huang, D. Production of astaxanthin with high purity and activity based on engineering improvement strategies. J. Biotechnol. 2025, 405, 139–149. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Chen, D.; Wang, F.; Jiang, S.; Liu, P.; Zhu, H.; Li, X.; Wan, X. Research Progress on Chemical and Biological Synthesis of Astaxanthin. Sci. Technol. Food Ind. 2021, 42, 445–453. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.Y.; Kageyama, Y.; Tanzawa, N.; Hirono-Hara, Y.; Kikukawa, H.; Wakabayashi, K. Development of astaxanthin production from citrus peel extract using Xanthophyllomyces dendrorhous. Environ. Sci. Pollut. Res. 2021, 28, 12640–12647. [Google Scholar] [CrossRef]
- Šimat, V.; Rathod, N.B.; Čagalj, M.; Hamed, I.; Generalić Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- Vakarelova, M.; Zanoni, F.; Lardo, P.; Rossin, G.; Mainente, F.; Chignola, R.; Menin, A.; Rizzi, C.; Zoccatelli, G. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem. 2017, 221, 289–295. [Google Scholar] [CrossRef]
- De Aguiar Saldanha Pinheiro, A.C.; Martí-Quijal, F.J.; Barba, F.J.; Tappi, S.; Rocculi, P. Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Processing By-Products—An Opportunity for a Circular Economy Approach. Foods 2021, 10, 2030. [Google Scholar] [CrossRef] [PubMed]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Einafshar, S.; Ramaswamy, H.S. Optimization of ultrasonic-assisted extraction of astaxanthin from green tiger (Penaeus semisulcatus) shrimp shell. Ultrason. Sonochemistry 2021, 76, 105666. [Google Scholar] [CrossRef]
- Rábago-Panduro, L.M.; Morales-de la Peña, M.; Romero-Fabregat, M.P.; Martín-Belloso, O.; Welti-Chanes, J. Effect of Pulsed Electric Fields (PEF) on Extraction Yield and Stability of Oil Obtained from Dry Pecan Nuts (Carya illinoinensis (Wangenh. K. Koch)). Foods 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Irna, C.; Jaswir, I.; Othman, R.; Jimat, D.N. Comparison Between High-Pressure Processing and Chemical Extraction: Astaxanthin Yield from Six Species of Shrimp Carapace. J. Diet. Suppl. 2018, 15, 805–813. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, Y.; He, C.; Wang, X.; Chen, S.; Huang, Z.; Chen, F. Enhancement of linoleic acid content stimulates astaxanthin esterification in Coelastrum sp. Bioresour. Technol. 2020, 300, 122649. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, X.L.; Liu, J.Z. Adaptive laboratory evolution and shuffling of Escherichia coli to enhance its tolerance and production of astaxanthin. Biotechnol. Biofuels Bioprod. 2022, 15, 17. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Gao, L.; Yang, F. Advances and trends for astaxanthin synthesis in Phaffia rhodozyma. Microb. Cell Factories 2025, 24, 100. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, X.; Yu, X.; Li, S.; Wang, K.; Wei, L.; Li, R.; Qin, S. Advancements of astaxanthin production in Haematococcus pluvialis: Update insight and way forward. Biotechnol. Adv. 2025, 79, 108519. [Google Scholar] [CrossRef]
- Han, X.; Wang, X.; Chen, Y.; Yang, Y.; Du, X.; Li, Z.; Jiang, Z.; Ni, H.; Li, Q. Optimized separation of astaxanthin stereoisomers from microbial sources using chiral HPLC. Anal. Methods 2025, 17, 504–513. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Liu, J.; Li, L.; Qin, S.; Huang, Q. Transcriptomic and metabolic analysis of an astaxanthin-hyperproducing Haematococcus pluvialis mutant obtained by low-temperature plasma (LTP) mutagenesis under high light irradiation. Algal Res. 2020, 45, 101746. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Han, B.; Zhao, Y.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Simultaneous improvement of astaxanthin and lipid production of Haematococcus pluvialis by using walnut shell extracts. Algal Res. 2021, 54, 102171. [Google Scholar] [CrossRef]
- Vadalà, R.; Di Salvo, E.; De Maria, L.; Vecchio, G.L.; Bartolomeo, G.; De Pasquale, R.; Genovese, C.; Cicero, N.; Costa, R. Leftover Food as a Sustainable Source of Astaxanthin Through Fermentation Using Phaffia rhodozyma. Foods 2025, 14, 1232. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wang, H.; Tang, J.; Bi, C.; Li, Q.; Zhang, X. Coordinated Expression of Astaxanthin Biosynthesis Genes for Improved Astaxanthin Production in Escherichia coli. J. Agric. Food Chem. 2020, 68, 14917–14927. [Google Scholar] [CrossRef]
- Debnath, T.; Bandyopadhyay, T.K.; Vanitha, K.; Bobby, M.N.; Tiwari, O.N.; Bhunia, B.; Muthuraj, M. Astaxanthin from microalgae: A review on structure, biosynthesis, production strategies and application. Food Res. Int. 2024, 176, 113841. [Google Scholar] [CrossRef]
- Cabanillas-Bojórquez, L.A.; Gutiérrez-Grijalva, E.P.; González-Aguilar, G.A.; López-Martinez, L.X.; Castillo-López, R.I.; Bastidas-Bastidas, P.d.J.; Heredia, J.B. Valorization of Fermented Shrimp Waste with Supercritical CO2 Conditions: Extraction of Astaxanthin and Effect of Simulated Gastrointestinal Digestion on Its Antioxidant Capacity. Molecules 2021, 26, 4465. [Google Scholar] [CrossRef] [PubMed]
- Radzali, S.A.; Baharin, B.S.; Othman, R.; Markom, M.; Rahman, R.A. Co-solvent selection for supercritical fluid extraction of astaxanthin and other carotenoids from Penaeus monodon waste. J. Oleo Sci. 2014, 63, 769–777. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.; Na, X.; Chen, Y.; Tan, M. High internal phase Pickering emulsions stabilized by a cod protein–chitosan nanocomplex for astaxanthin delivery. Food Funct. 2021, 12, 11872–11882. [Google Scholar] [CrossRef] [PubMed]
- Tintchev, F.; Wackerbarth, H.; Kuhlmann, U.; Toepfl, S.; Knorr, D.; Hildebrandt, P.; Heinz, V. Molecular effects of high-pressure processing on food studied by resonance Raman. Ann. N. Y. Acad. Sci. 2010, 1189, 34–42. [Google Scholar] [CrossRef]
- Wan, X.; Zhou, X.R.; Moncalian, G.; Su, L.; Chen, W.C.; Zhu, H.Z.; Chen, D.; Gong, Y.M.; Huang, F.H.; Deng, Q.C. Reprogramming microorganisms for the biosynthesis of astaxanthin via metabolic engineering. Prog. Lipid Res. 2021, 81, 101083. [Google Scholar] [CrossRef]
- Pitacco, W.; Samorì, C.; Pezzolesi, L.; Gori, V.; Grillo, A.; Tiecco, M.; Vagnoni, M.; Galletti, P. Extraction of astaxanthin from Haematococcus pluvialis with hydrophobic deep eutectic solvents based on oleic acid. Food Chem. 2022, 379, 132156. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Su, D.; Zhao, L.; Leng, K.; Miao, J.; Yu, Y. Effective astaxanthin production from flocculated Haematococcus pluvialis via biofilm cultivation in a tri-layer tray bioreactor. J. Biotechnol. 2025, 405, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Tambat, V.S.; Chen, C.-W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.-Y.; Dong, C.-D.; Singhania, R.R. Recent advancements in astaxanthin production from microalgae: A review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Munir, M.; Qureshi, R.; Hanif, U.; Gulzar, N.; Sheikh, A.A. Advanced methods of algal pigments extraction: A review. Crit. Rev. Food Sci. Nutr. 2023, 64, 9771–9788. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.M.; Manuguerra, S.; Arena, R.; Renda, G.; Ficano, G.; Randazzo, M.; Fricano, S.; Sadok, S.; Santulli, A. In Vitro Bioactivity of Astaxanthin and Peptides from Hydrolisates of Shrimp (Parapenaeus longirostris) By-Products: From the Extraction Process to Biological Effect Evaluation, as Pilot Actions for the Strategy “From Waste to Profit”. Mar. Drugs 2021, 19, 216. [Google Scholar] [CrossRef]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernández, N.; Bronze, M.R.; Matias, A.A. Astaxanthin Extraction from Marine Crustacean Waste Streams: An Integrate Approach between Microwaves and Supercritical Fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Panagiotakopoulos, I.; Karantonis, H.C.; Kartelias, I.G.; Nasopoulou, C. Ultrasonic-Assisted Extraction of Astaxanthin from Shrimp By-Products Using Vegetable Oils. Mar. Drugs 2023, 21, 467. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Impact of pulsed electric field pretreatment on yield and quality of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasound-assisted process. Int. J. Food Sci. Technol. 2020, 55, 619–630. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Effect of pre-treatments on yield and properties of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasonic assisted process. LWT 2019, 100, 106–113. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Hamdi, M.; Nasri, R.; Dridi, N.; Li, S.; Nasri, M. Development of novel high-selective extraction approach of carotenoproteins from blue crab (Portunus segnis) shells, contribution to the qualitative analysis of bioactive compounds by HR-ESI-MS. Food Chem. 2020, 302, 125334. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Muskhazli, M.; Azwady, A.A.N.; Ahmad, S.A.; Adli, A.A. Three dimensional optimisation for the enhancement of astaxanthin recovery from shrimp shell wastes by Aeromonas hydrophila. Biocatal. Agric. Biotechnol. 2020, 27, 101649. [Google Scholar] [CrossRef]
- Ahmed, F.; Li, Y.; Fanning, K.; Netzel, M.; Schenk, P.M. Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res. Int. 2015, 74, 231–236. [Google Scholar] [CrossRef]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar]
- Sun, L.; Li, Y.; Yang, A.; Xie, M.; Xiong, R.; Huang, C. Astaxanthin: A comprehensive review of synthesis, biological activities and applications. Food Chem. 2025, 488, 144847. [Google Scholar] [CrossRef]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta (BBA)-Biomembr. 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- Savikj, M.; Kostovski, E.; Lundell, L.S.; Iversen, P.O.; Massart, J.; Widegren, U. Altered oxidative stress and antioxidant defence in skeletal muscle during the first year following spinal cord injury. Physiol. Rep. 2019, 7, e14218. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sun, H.; Wei, H.; Dong, M.; Zhang, Y.; Xu, W.; Fang, Y.; Zhao, J. Astaxanthin alleviates spinal cord ischemia-reperfusion injury via activation of PI3K/Akt/GSK-3β pathway in rats. J. Orthop. Surg. Res. 2020, 15, 275. [Google Scholar] [CrossRef]
- Song, E.-K.; Park, H.; Kim, H.-S. Additive effect of walnut and chokeberry on regulation of antioxidant enzyme gene expression and attenuation of lipid peroxidation in d-galactose-induced aging-mouse model. Nutr. Res. 2019, 70, 60–69. [Google Scholar] [CrossRef]
- Ma, J.; Wang, H.; Liu, B.; Shan, Y.; Zhou, H.; Qi, X.; Wu, W.; Jia, L. Combination of chick embryo and nutrient mixture prevent D-galactose-induced cognitive deficits, immune impairment and oxidative stress in aging rat model. Sci. Rep. 2019, 9, 4092. [Google Scholar] [CrossRef]
- Han, J.; Guo, X.; Koyama, T.; Kawai, D.; Zhang, J.; Yamaguchi, R.; Zhou, X.; Motoo, Y.; Satoh, T.; Yamada, S. Zonarol Protected Liver from Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients 2021, 13, 3455. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Xiao, J.; Song, M.; Cao, Y.; Xiao, H.; Liu, X. Astaxanthin attenuates d-galactose-induced brain aging in rats by ameliorating oxidative stress, mitochondrial dysfunction, and regulating metabolic markers. Food Funct. 2020, 11, 4103–4113. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.-K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective Effects of Haematococcus Astaxanthin on Oxidative Stress in Healthy Smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, S.M.; Aljuaydi, S.H.; AbuBakr, H.O.; Tahoun, E.A.; Di Cerbo, A.; Alagawany, M.; Khalil, S.R.; Farag, M.R. Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats. Mar. Drugs 2021, 19, 525. [Google Scholar] [CrossRef]
- Cui, G.; Li, L.; Xu, W.; Wang, M.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Yang, S.; Long, M.; et al. Astaxanthin Protects Ochratoxin A-Induced Oxidative Stress and Apoptosis in the Heart via the Nrf2 Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 7639109. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Jiao, D.; Yang, S.; Li, L.; Li, P. Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules 2020, 25, 1386. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Guo, X.S.; Yue, Y.Y.; Wang, Y.; Jin, X.L. Astaxanthin Promotes the Survival of Adipose-Derived Stem Cells by Alleviating Oxidative Stress via Activating the Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 3850. [Google Scholar] [CrossRef]
- Gao, F.; Wu, X.; Mao, X.; Niu, F.; Zhang, B.; Dong, J.; Liu, B. Astaxanthin provides neuroprotection in an experimental model of traumatic brain injury via the Nrf2/HO-1 pathway. Am. J. Transl. Res. 2021, 13, 1483–1493. [Google Scholar]
- Zarneshan, S.N.; Fakhri, S.; Farzaei, M.H.; Khan, H.; Saso, L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem. Toxicol. 2020, 145, 111714. [Google Scholar] [CrossRef]
- Yuan, L.; Qu, Y.; Li, Q.; An, T.; Chen, Z.; Chen, Y.; Deng, X.; Bai, D. Protective effect of astaxanthin against La2O3 nanoparticles induced neurotoxicity by activating PI3K/AKT/Nrf-2 signaling in mice. Food Chem. Toxicol. 2020, 144, 111582. [Google Scholar] [CrossRef]
- Mohammadi, S.; Barzegari, A.; Dehnad, A.; Barar, J.; Omidi, Y. Astaxanthin protects mesenchymal stem cells from oxidative stress by direct scavenging of free radicals and modulation of cell signaling. Chem. Interact. 2021, 333, 109324. [Google Scholar] [CrossRef]
- Lai, T.T.; Yang, C.M.; Yang, C.H. Astaxanthin Protects Retinal Photoreceptor Cells against High Glucose-Induced Oxidative Stress by Induction of Antioxidant Enzymes via the PI3K/Akt/Nrf2 Pathway. Antioxidants 2020, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef]
- Wang, H.Q.; Sun, X.B.; Xu, Y.X.; Zhao, H.; Zhu, Q.Y.; Zhu, C.Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010, 1360, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhang, Z.; Hu, W.; Lou, M.; Zhai, B.; Mei, S.; Hu, Z.; Zhang, L.; Liu, D.; Liu, Z.; et al. Attenuation of the Na/K-ATPase/Src/ROS amplification signaling pathway by astaxanthin ameliorates myocardial cell oxidative stress injury. Mol. Med. Rep. 2020, 22, 5125–5134. [Google Scholar] [CrossRef]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.T.; Kim, M.S.; Kim, Y.S.; An, W.G. Astaxanthin Reduces Stemness Markers in BT20 and T47D Breast Cancer Stem Cells by Inhibiting Expression of Pontin and Mutant p53. Mar. Drugs 2020, 18, 577. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef]
- Atalay, P.B.; Kuku, G.; Tuna, B.G. Effects of carbendazim and astaxanthin co-treatment on the proliferation of MCF-7 breast cancer cells. Vitr. Cell. Dev. Biol.-Anim. 2018, 55, 113–119. [Google Scholar] [CrossRef]
- Ko, J.C.; Chen, J.C.; Wang, T.J.; Zheng, H.Y.; Chen, W.C.; Chang, P.Y.; Lin, Y.W. Astaxanthin down-regulates Rad51 expression via inactivation of AKT kinase to enhance mitomycin C-induced cytotoxicity in human non-small cell lung cancer cells. Biochem. Pharmacol. 2016, 105, 91–100. [Google Scholar] [CrossRef]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef]
- Yamashita, E. Extensive Bioactivity of Astaxanthin from Haematococcus pluvialis in Human. Adv. Exp. Med. Biol. 2021, 1261, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Q.; Chu, C.; Liu, S. Astaxanthin protects retinal ganglion cells from acute glaucoma via the Nrf2/HO-1 pathway. J. Chem. Neuroanat. 2020, 110, 101876. [Google Scholar] [CrossRef] [PubMed]
- Fındık, H.; Tumkaya, L.; Yılmaz, A.; Aslan, M.G.; Okutucu, M.; Akyildiz, K.; Mercantepe, T. The protective effects of astaxanthin against cisplatin-induced retinal toxicity. Cutan. Ocul. Toxicol. 2019, 38, 59–65. [Google Scholar] [CrossRef]
- Janani, R.; Anitha, R.E.; Perumal, M.K.; Divya, P.; Baskaran, V. Astaxanthin mediated regulation of VEGF through HIF1α and XBP1 signaling pathway: An insight from ARPE-19 cell and streptozotocin mediated diabetic rat model. Exp. Eye Res. 2021, 206, 108555. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, T.; Yoshida, M.; Fukuta, T.; Tanaka, T.; Inagi, T.; Kogure, K. Efficacy of high-affinity liposomal astaxanthin on up-regulation of age-related markers induced by oxidative stress in human corneal epithelial cells. J. Clin. Biochem. Nutr. 2019, 64, 27–35. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2010, 55, 150–165. [Google Scholar] [CrossRef]
- Hafez, M.H.; El-Far, A.H.; Elblehi, S.S. Astaxanthin alleviates fipronil-induced neuronal damages in male rats through modulating oxidative stress, apoptosis, and inflammatory markers. Sci. Rep. 2025, 15, 4299. [Google Scholar] [CrossRef]
- Rastinpour, Z.; Fakhri, S.; Abbaszadeh, F.; Ranjbari, M.; Kiani, A.; Namiq Amin, M.; Echeverría, J. Neuroprotective effects of astaxanthin in a scopolamine-induced rat model of Alzheimer’s disease through antioxidant/anti-inflammatory pathways and opioid/benzodiazepine receptors: Attenuation of Nrf2, NF-κB, and interconnected pathways. Front. Pharmacol. 2025, 16, 1589751. [Google Scholar] [CrossRef]
- Chae, S.Y.; Park, R.; Hong, S.W. Surface-mediated high antioxidant and anti-inflammatory effects of astaxanthin-loaded ultrathin graphene oxide film that inhibits the overproduction of intracellular reactive oxygen species. Biomater. Res. 2022, 26, 30. [Google Scholar] [CrossRef]
- Wang, L.; Song, D.; Wei, C.; Chen, C.; Yang, Y.; Deng, X.; Gu, J. Telocytes inhibited inflammatory factor expression and enhanced cell migration in LPS-induced skin wound healing models in vitro and in vivo. J. Transl. Med. 2020, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.R.; Zhao, Y.L.; Liu, W.W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ogura, T.; Onodera, S.; et al. Type I collagen or gelatin stimulates mouse peritoneal macrophages to aggregate and produce pro-inflammatory molecules through upregulated ROS levels. Int. Immunopharmacol. 2019, 76, 105845. [Google Scholar] [CrossRef] [PubMed]
- Lauver, D.A.; Lockwood, S.F.; Lucchesi, B.R. Disodium Disuccinate Astaxanthin (Cardax) Attenuates Complement Activation and Reduces Myocardial Injury following Ischemia/Reperfusion. J. Pharmacol. Exp. Ther. 2005, 314, 686–692. [Google Scholar] [CrossRef]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin stimulates cell-mediated and humoral immune responses in cats. Veter- Immunol. Immunopathol. 2011, 144, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Grimmig, B.; Kim, S.-H.; Nash, K.; Bickford, P.C.; Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience 2017, 39, 19–32. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014, 5, 158–166. [Google Scholar] [CrossRef]
- Fan, Q.; Chen, Z.; Wu, Y.; Zhu, J.; Yu, Z. Study on the Enhancement of Immune Function of Astaxanthin from Haematococcus pluvialis. Foods 2021, 10, 1847. [Google Scholar] [CrossRef]
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol. 2020, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kuo, C.-C.; Chou, J.; Delvolve, A.; Jackson, S.N.; Post, J.; Woods, A.S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009, 23, 1958–1968. [Google Scholar] [CrossRef]
- Lee, J.; Lim, J.W.; Kim, H. Astaxanthin Inhibits Matrix Metalloproteinase Expression by Suppressing PI3K/AKT/mTOR Activation in Helicobacter pylori-Infected Gastric Epithelial Cells. Nutrients 2022, 14, 3427. [Google Scholar] [CrossRef] [PubMed]
- Aribisala, J.O.; Nkosi, S.; Idowu, K.; Nurain, I.O.; Makolomakwa, G.M.; Shode, F.O.; Sabiu, S. Astaxanthin-Mediated Bacterial Lethality: Evidence from Oxidative Stress Contribution and Molecular Dynamics Simulation. Oxidative Med. Cell. Longev. 2021, 2021, 7159652. [Google Scholar] [CrossRef]
- Donà, G.; Andrisani, A.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Ambrosini, G.; Ragazzi, E.; Bordin, L. Astaxanthin Prevents Human Papillomavirus L1 Protein Binding in Human Sperm Membranes. Mar. Drugs 2018, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, M.; Song, H.; Peng, J.; Zhao, W.; Zhang, W. Astaxanthin Inhibits STING Carbonylation and Enhances Antiviral Responses. J. Immunol. 2024, 212, 1188–1195. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, Q.; Liu, F.; Jing, C.; Ding, L.; Zhang, L.; Pang, C. Chronic trans-astaxanthin treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with chronic pain. Neurosci. Lett. 2018, 662, 36–43. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachex-Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef]
- Liu, C.; Dong, X.; Jia, J.; Ha, M. Effects of Astaxanthin Supplementation on Fatigue, Motor Function and Cognition: A Meta-Analysis of Randomized Controlled Trials. Biol. Res. Nurs. 2024, 26, 469–480. [Google Scholar] [CrossRef]
- Cai, X.; Hua, S.; Deng, J.; Du, Z.; Zhang, D.; Liu, Z.; Khan, N.U.; Zhou, M.; Chen, Z. Astaxanthin Activated the Nrf2/HO-1 Pathway to Enhance Autophagy and Inhibit Ferroptosis, Ameliorating Acetaminophen-Induced Liver Injury. ACS Appl. Mater. Interfaces 2022, 14, 42887–42903. [Google Scholar] [CrossRef]
- Le Goff, M.; Le Ferrec, E.; Mayer, C.; Mimouni, V.; Lagadic-Gossmann, D.; Schoefs, B.; Ulmann, L. Microalgal carotenoids and phytosterols regulate biochemical mechanisms involved in human health and disease prevention. Biochimie 2019, 167, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Eren, B.; Tuncay Tanrıverdi, S.; Aydın Köse, F.; Özer, Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2018, 18, 242–250. [Google Scholar] [CrossRef]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar]
- Martínez-Delgado, A.A.; Khandual, S.; Villanueva–Rodríguez, S.J. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Kneip, J.S.; Kniepkamp, N.; Jang, J.; Mortaro, M.G.; Jin, E.; Kruse, O.; Baier, T. CRISPR/Cas9-Mediated Knockout of the Lycopene ε-Cyclase for Efficient Astaxanthin Production in the Green Microalga Chlamydomonas reinhardtii. Plants 2024, 13, 1393. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef]
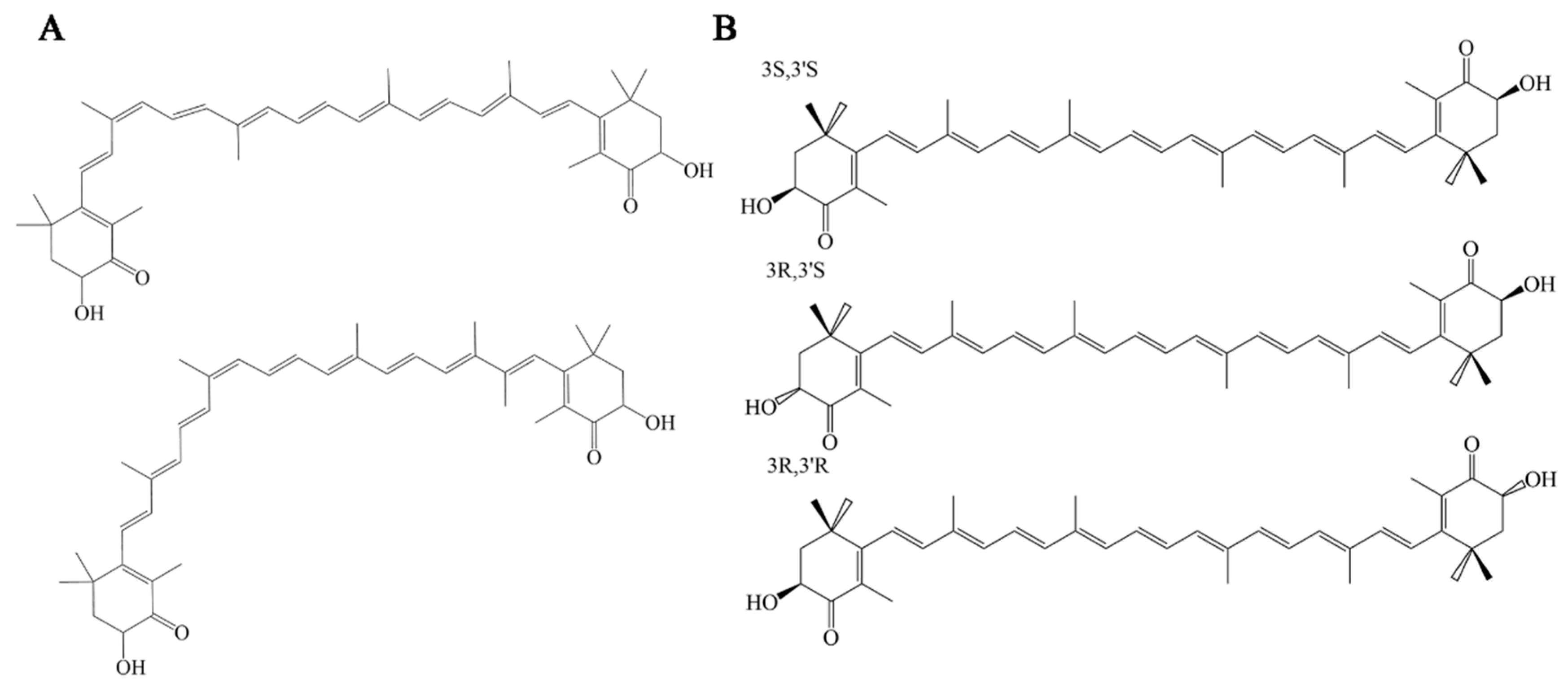

| Bioactivity | Models | Mechanisms | References |
|---|---|---|---|
| Antioxidation | Mice aging model | ↓ MDA, NO, AOPP ↑ SOD, GSH, CAT | [26] |
| Mouse model of chronic obstructive pulmonary disease | ↓ MDA ↑ SOD, GSH, HO-1, T-AOC, Nrf2 | [27] | |
| Memory loss mouse model | ↓ TNF-α, IL-1β, IL-6, ROS, NO | [28] | |
| Aging cock model | ↓ MDA, -OH, O2− ↑ SOD, GSH-Px, CAT, T-AOC, HO-1, ERK, p38, JNK1/2/3, Nrf2 | [29] | |
| Genetic gestational diabetes mellitus model | ↓ Glucose intolerance ↑ SOD, GSH-Px, CAT, Nrf2, HO-1 | [30] | |
| Anti-cancer | Obese mouse colon cancer model | ↓ Cell proliferation, oxidative stress, inflammatory response, NF-κB | [31] |
| Human gastric cancer cell line AGS cells | ↑ NQO1, ROS, p-RIP1 | [32] | |
| Rat model of esophageal cancer | ↓ MDA ↑ T-AOC, SOD, PPARγ, Bax/Bcl-2, Caspase3 | [33] | |
| Breast cancer cell line SKBR3 cells | ↓ mutp53, Cleavage of PARP-1 fragment, Bcl2, ROS ↑ G0/G1 cycle arrest, Bax, ERK1/2, p-JNK, p-p38, SOD Regulating bridge protein | [34] | |
| Prostate cancer du145 cell | ↓ Cell migration and proliferation, STAT3 ↑ Apoptosis | [35] | |
| ↓ p-Akt/Akt, NF-κB, STAT3, Wnt | [36] | ||
| Colorectal cancer cells CT26 and HCT116 | ↓ EMT, MMP2, ZEB1 ↑ miR-29a-3p, miR-200a | [37] | |
| Melanoma cell A375 and A2058 models | ↓ MMP1/2/9 ↑ caspase3, caspase7 | [38] | |
| Astrocytic glioma cell line U251-MG | ↓ p53 ↑ Cdk2, p-Cdk2/3 | [39] | |
| Eye-protection | Retinal ischemia/reperfusion model | ↓ ERG, ROS | [40] |
| Type I diabetic rat model | ↓ AGE, MDA, Lipid hydrogen peroxide ↑ CAT, SOD, GSH | [41] | |
| DED rat model | ↓ SPK lesions | [42] | |
| DED mouse model and HCECs cells | ↓ HMGB1, TNF-α, IL-1β ↑ p-Akt | [43] | |
| ARPE-19 cells and HIR mouse model | ↓ HIF1α, XBP1, VEGF, Cell permeability Protect ZO-1 | [44] | |
| 661w cells | ↓ ROS, Nitrotyrosine, 8-OHdG, Acrolein, Mitochondrial damage ↑ Bcl-2/Bax, PI3K/Akt, HO-1, NQO1 | [45] | |
| Anti-inflammatory | MCAO rats’ model | ↑ SOD, HO-1, NQO1 ↓ O2−, MDA | [46] |
| Mouse primary peritoneal macrophage model | ↓ IκB-α, ERK1/2, P38, JNK | [47] | |
| Mouse macrophage Raw 264.7 cell model | ↓ TNF-α, IL-1β, IL-6 | [48] | |
| Mouse macrophage Raw 264.7 model | ↓ NF-κB, NFATC1 | [49] | |
| Immunoregulation | NASH mouse model | ↓ Proinflammatory cytokine, CCL2 | [50] |
| Roman Brown laying hens | ↓ MDA ↑ GSH-Px, SOD, IgG | [51] | |
| C57BL/6 mice | ↑ IgA ↓ Reg-3γ, Lysozyme | [52] | |
| Male Sprague Dawley rats | ↓ IL-2, IgM, IgG, IL-1β, IL-6, IκBα, p65 ↑ Nrf2, Keap1 | [53] | |
| Skin care | HaCaT | ↓ IκB-α, ERK, JNK, p38, p65 | [54] |
| NHEKs | ↓ Bax, PARP, ROS, Caspase3 ↑ Bcl-2 | [55] | |
| Episkin 3D human skin model | ↑ AQP3 | [23] | |
| Sprague Dawley rats | ↑ Flap survival rate, Angiogenesis | [56] | |
| UV light-induced guinea pig model | ↓ Pigmentation, decreased elasticity, transcutaneous water loss | [57] | |
| Antidiabetic | Genetic gestational diabetes mellitus model | ↓ Glucose intolerance ↑ SOD, GSH-Px, CAT, Nrf2, HO-1 | [30] |
| Streptozotocin diabetic rat model | ↑ Adiponecti, AdipoR1, AdipoR2, PPARγ ↓ TC | [58] | |
| HuVecs | ↓ VEGF-VEGFR2-FAK signaling pathway | [59] | |
| Neuroprotection | Mouse model of traumatic brain injury | ↓ p-ASK1, p-p38 ↑ SIRT1, Nrf2, Prxs | [24] |
| MCAO rats’ model | ↑ cAMP, CREB, PKA | [60] | |
| db/db mice | ↓ MDA ↑ GSH, SOD | [61] | |
| Human neuroblastoma SH-SY5Y cells | ↓ HO-1, Nrf2, p-Akt/Akt, p-GSK3β/GSK3β | [62] | |
| BV-2 microglial cells | ↓ iNOS, NF-κB, ↑ Nrf2, HO-1 | [63] | |
| Male albino rats | ↓ AChE, TNF-α, PGE2, COX-2, Cytochrome c | [64] |
| Production Methods | Classification | Advantages and Disadvantages | References |
|---|---|---|---|
| Biological accumulation | Direct extraction of waste from crustaceans | Low output and high costs. | [65] |
| Traditional extraction | Solvent extraction | The overall processing conditions are very strict, resulting in poor quality, yield, and stability of AST; high energy consumption and multiple separation steps. | [71,72] |
| Oil extraction | |||
| Chemical synthesis | Total synthesis method | The cost is relatively low, with a market application rate of 90%. It is mainly used in aquaculture and can produce a mixture of AST stereoisomers (L: racemic: R in a ratio of 1: 2: 1); there are unknown components and potential risks. | [70] |
| Semi-synthesis | |||
| Modern green technology | SFE-CO2 | Low viscosity, high diffusivity, and high density, enhancing the penetration of the biomass structure and the dissolution of target compounds; reducing extraction time and solvent usage. | [73] |
| UAE | The oxidation of lipids was triggered and intensified, resulting in significant increases in PV and TBARS. Among them, the presence of tannic acid (0.1%) resulted in the highest yield. | [74] | |
| PEF | Increases the extraction rate of lipid and carotenoid components in the shrimp’s head and thorax; has an inhibitory effect on enzymes; increases the content of bioactive substances in the oil. | [75] | |
| HPP | High quality; green and pollution-free; short time consumption. | [76] | |
| Biosynthesis | H. pluvialis | Maintains high photosynthetic activity and promotes the biosynthesis of AST; microalgae grow slowly and the production process is relatively long, making them prone to contamination during the production stage. | [67] |
| Coelastrum sp. HA-1 | Under the original conditions, the esterification rate of AST molecules was low, and the accumulation of AST was lower than that of H. pluvialis. When LA and ethanol were added, the content of AST esters and TA increased exponentially. | [77] | |
| X. dendrorhous | Providing yeast and AST with abundant carbon sources, nitrogen sources, minerals, and arabinose can significantly promote and increase the production and yield of AST. | [70] | |
| E. coli CAR026 | It cannot accumulate AST by itself, but it can enhance the tolerance and production of AST. | [78] |
| Production Mechanisms | Methods | Sources or Strains | Yield | References |
|---|---|---|---|---|
| Active synthesis | Microbial Biosynthesis | Mutant strain named as M3 of H. pluvialis | The accumulation of fatty acids and AST was higher than that of wild strains. | [82] |
| H. pluvialis | The contents of AST and lipids were increased by 77.57% and 23.39%, respectively. | [83] | ||
| Coelastrum sp. HA-1 | The contents of AST esters and TA were 3.82 times and 2.18 times (treated with LA) or 2.42 times and 1.61 times (treated with ethanol) those of the control group, respectively. | [77] | ||
| X. dendrorhous | Ponkan peel extract used alone (40 g/L): 0.92 mg/L; Ponkan peel extract was added to Synthetic Dropout medium: 1.22 mg/L; Ponkan peel extract was added to YM medium: 2.05 mg/L. | [70] | ||
| E. coli CAR026 (Coordinate the expression of CrtW and CrtZ, increase the copy number of crtY, and regulate groES-groEL) | 1.18 g/L | [85] | ||
| Passive accumulation | SFE-CO2 (300 bar, 60 °C and 6 mL/min) | Shrimp residue lactic acid fermentation broth | 0.6353 μg/g | [87] |
| SPD (TFA obtained from crude viscera oil as solvent) (160 °C, 0.002 mbar) | By-products of Parapenaeus longirostris | 114.80 ± 1.23 µg/mL | [96] | |
| Combination of MW pretreatment and SFE (0–30 min, 200–500 bar, 40–60 °C), ethanol content (8–13 wt%) | Brown crab (Cancer pagurus) shell waste | 1023 μg/g | [97] | |
| UAE (ultrasonic amplitude: 23.6%, 13.9 min, 26.3 °C) | Penaeus semisulcatus shell | It accounted for 51.5% of the extract. | [74] | |
| UAE (Preheat and add 0.1% tannic acid) (ultrasound amplitude: 80%, 25 min) | Pacific white shrimp (Litopenaeus vannamei) | Lipid: 133–141 mg/g sample | [100] | |
| UAE (ultrasound amplitude: 80%, 25 min) plus PEF pretreatment | Pacific white shrimp (Litopenaeus vannamei) | Lipid: 303.4 mg/g solids | [99] | |
| HPP (acetone and methanol (7: 3, v/v), 210 MPa, 10 min) | Penaeus monodon | 59.9744 µg/gdw total carotenoid: 68.26 µg/ml | [76] | |
| Enzyme-assisted extraction (20 units of P. segnis digestive alkaline proteases/g of blue crab shells for 60 min at 50 °C and pH 8.0.) combined with impregnation (MAC by using the binary organic system HxIPA (50/50, v/v), 120 h, solvent/raw material ratio of 4/1 (v/w)) | Blue crab (Portunus segnis) | 5045 µg/g | [102] | |
| microbiological degradation (the culture media-conditions is pH 7.0, monosodium glutamate 3% (w/v), glucose (1% w/v) and 30 °C) | SSW | 2.16 U/mL | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, F.; Rao, C.; Xiang, Q.; Wen, J.; Dai, Q.; Li, H.; Liang, J.; Chen, Y.; Peng, C. Production Methods, Biological Activity and Potential Application Prospects of Astaxanthin. Foods 2025, 14, 2103. https://doi.org/10.3390/foods14122103
Ren F, Rao C, Xiang Q, Wen J, Dai Q, Li H, Liang J, Chen Y, Peng C. Production Methods, Biological Activity and Potential Application Prospects of Astaxanthin. Foods. 2025; 14(12):2103. https://doi.org/10.3390/foods14122103
Chicago/Turabian StyleRen, Fajian, Chaolong Rao, Qiwen Xiang, Jiayu Wen, Qiuju Dai, He Li, Jiayu Liang, Yan Chen, and Cheng Peng. 2025. "Production Methods, Biological Activity and Potential Application Prospects of Astaxanthin" Foods 14, no. 12: 2103. https://doi.org/10.3390/foods14122103
APA StyleRen, F., Rao, C., Xiang, Q., Wen, J., Dai, Q., Li, H., Liang, J., Chen, Y., & Peng, C. (2025). Production Methods, Biological Activity and Potential Application Prospects of Astaxanthin. Foods, 14(12), 2103. https://doi.org/10.3390/foods14122103






