Abstract
The EU’s regulatory framework for genetically modified organisms (GMOs) was developed for “classical” transgenic GMOs, yet advancements in so-called “new genomic techniques (NGTs)” have led to implementation challenges regarding detection and identification. As traceability can complement detection and identification strategies, improvements to the existing traceability strategy for GMOs are investigated in this study. Our results are based on a comprehensive analysis of existing traceability systems for globally traded agricultural products, with a focus on soy. Alternative traceability strategies in other sectors were also analysed. One focus was on traceability strategies for products with characteristics for which there are no analytical verification methods. Examples include imports of “conflict minerals” into the EU. The so-called EU Conflict Minerals Regulation requires importers of certain raw materials to carry out due diligence in the supply chain. Due diligence regulations, such as the EU’s Conflict Minerals Regulation, can legally oblige companies to take responsibility for certain risks in their supply chains. They can also require the importer to prove the regional origin of imported goods. The insights from those alternative traceability systems are transferred to products that might contain GMOs. When applied to the issue of GMOs, we propose reversing the burden of proof: All companies importing agricultural commodities must endeavour to identify risks of unauthorised GMOs (including NGTs) in their supply chain and, where appropriate, take measures to minimise the risk to raw material imports. The publication concludes that traceability is a means to an end and serves as a prerequisite for due diligence in order to minimise the risk of GMO contamination in supply chains. The exemplary transfer of due diligence to a company in the food industry illustrates the potential benefits of mandatory due diligence, particularly for stakeholders actively managing non-GMO supply chains.
1. Introduction
Genetically modified organisms (GMO) may only be placed on the EU market following an authorisation procedure, and products derived from GMOs, including GM food and feed products, have to be labelled accordingly [1,2,3] (see [4] for a comprehensive overview). Compliance with these regulatory requirements is ensured by inspections (including documentation checks and laboratory tests). Importers of products that might contain GMOs have to make sure that their charges are free of unauthorised GMOs; the EU has in principle zero tolerance for unauthorised GMOs. To support compliance through the detection of authorised GMOs, a quantitative, event-specific analytical detection method is provided by the developer of the GMO in the framework of the application for market authorisation [5]. These detection methods are available for authorised GMOs as well as for those with ongoing approval procedures and allow the unique identification of the GM event.
The current regulatory framework for GMOs in the EU was developed when “classical” GMOs produced by transgenesis were state-of-the-art. However, since the entry into force of EU Directive 2001/18/EC [1], techniques were developed to produce GMOs allowing various modifications in the genome. In the EU, these methods are summarised under the term “new genomic techniques” (NGTs) and include targeted mutagenesis using CRISPR/Cas [6]. In 2018, the European Court of Justice clarified that products developed by targeted mutagenesis are considered GMOs and are subject to the same regulatory requirements as “classical” transgenic GMOs [7].
Since then, the implementation of the detection and traceability requirements for NGT products [4,8,9,10] and regulatory changes have been discussed, with the latest development being a proposal for a regulation on plants obtained using certain NGTs published by the European Commission in July 2023 [11].
Control regarding authorised GMOs and GM products covers compliance with labelling requirements, considering the thresholds for the adventitious and technically unavoidable presence of minimal amounts of GMOs in other products [2]. Control measures, including analytical control, are also important to ensure that no GMOs are present in products that are marketed as being non-GMO, i.e., conventional non-GM products and products of organic production, the latter prohibiting the use of GMOs according to Regulation (EU) 2018/848 [12]. Inspections are also essential to ensure that no unauthorised GMOs enter the EU market. Analytical methods for unauthorised GMOs, however, have to be developed by control laboratories based on publicly available information. The development of such detection methods focusses on GMOs whose presence in supply chains is suspected or known. GMOs are usually detected in the routine application of screening methods. However, the range of newly developed transgenic GMOs that are not detectable with the Matrix approach used in the EU has significantly increased in the last years [4].
In order to develop event-specific analytical detection methods for NGTs, some key information is needed. On the one hand, information on products on the market worldwide is needed, and on the other hand, product-specific information that is crucial for developing detection methods is required, e.g., (sequence) information regarding the genetic modifications present in these products. However, such information may not be readily available for different reasons: Firstly, GMOs are regulated differently worldwide, and many non-EU countries do not require GMOs to be labelled. Secondly, NGT products are not considered GMOs in several countries [13]. Thus, necessary information regarding these products is not available from regulatory authorities. The placing on the market of NGT products will increase the existing challenges regarding the detection of non-authorised GMOs, which are currently not detectable with the available screening methods but only with event-specific detection methods. Although NGT products are detectable if respective sequence information is available, the specific challenge associated with NGT products might be their unequivocal identification. It might be challenging to link the respective modification in the genome to the new genomic technique applied [9,10]. In order to solve this challenge, sufficient information on the NGT plant needs to be available [4,8,13].
Currently, no internationally consistent approach for the development and publication of detection methods exists [8]. The Biosafety Clearing House (BCH) [14] established under the Cartagena Protocol on Biosafety provides information for organisms that are subject to a national authorisation procedure. In addition, only Parties to the Cartagena Protocol participate in the BCH information exchange together with some non-Parties like the US or Canada. Against this background, an attempt to provide an overview of NGT products was made by Wageningen Food Safety Research (WFSR) and the German Federal Office of Consumer Protection and Food Safety (BVL). They have jointly developed a database that collects information relevant to GMO products (including NGT products) and their detection and identification, called “The European GMO Database” (EUginius Database) [15]. Information in the database is provided by WFST and BVL, in collaboration with several other project partners, the Austrian Agency for Health and Food Safety Ltd. (AGES), the Polish Plant Breeding and Acclimatization Institute (IHAR) and the Italian Experimental Zooprophylactic Institute of Lazio and Tuscany (IZSLT).
Globally only a few NGT plant products are already being marketed. However, several are in the pre-commercial stage, complemented by many applications in the advanced research and development stage [16]. Since an increasing number of NGT products is expected to be developed in the future for the agri-food sector, the issue of detection and traceability is urgent in order to ensure compliance with GMO regulation in the EU (for unauthorised NGTs) and maintain non-GMO production chains (for authorised NGTs in the future). In the absence of detection methods, authorities have to explore traceability strategies that are not based on analytical testing.
The traceability of food products has become increasingly important as the food and feed trade has become more global. Historically, the need for traceability arises from concerns about health and safety risks within food systems [17]. In recent years, the traceability of global food and feed supply chains has become increasingly important, also in the context of growing sustainability awareness [18].
Although current definitions of traceability differ in focus, the main principles of transparency regarding identification of origin, quantities and movements along different stages of the value chain remain at the core of the concept. Traceability, as defined by the International Organization for Standardization (ISO) and the Regulation (EC) No 178/2002 [19], involves the ability to monitor the movement of food and feed through specified stages of production, processing and distribution.
Traceability enables the effective management of the flows of goods (commodities and products) in two ways: tracing (upstream) and tracking (downstream) [17]. From the perspective of food safety, it serves as a tool for effective management, allowing for the swift removal of potentially harmful products from the market in the event of incidents.
In the context of global value chains, the emphasis of this paper is set on the tracing aspect of imported agricultural products that might contain (unauthorised) GMOs (including NGTs). The aim of this article is to review the state of the art of traceability systems and to explore strategies to ensure that only authorised GMOs (including NGTs) enter the EU market and non-GMO production chains remain GMO (and NGT)-free.
2. Methodological Approach
The methodological approach followed in the present study consisted of an iterative process in which findings from literature and desk research were complemented by expert inputs from selected stakeholders via interviews and workshops (Table 1).

Table 1.
Type of stakeholders and number of interviews conducted.
Initial data collection was conducted through a literature review and desktop research covering scientific literature, as well as trade data and grey literature from governmental agencies. This research and its evaluation were not limited to GMOs but also covered traceability approaches in other global value chains, taking into account approaches not based on analytical evidence.
For the review of the status quo (Section 3), in addition to the literature review, a comparative analysis of traceability systems currently applied in different global supply chains was carried out. This analysis compared the traceability-specific requirements of different sustainability standards for global agricultural commodities. Representative standards were selected and compared using the ITC platform standardsmap.org [20]. The result of this comparison is compiled in Table 2.

Table 2.
Comparative analysis of traceability systems in selected voluntary sustainability standards for globally traded agro-food products.
On 22 December 2022, potential interviewees were contacted by email, some of them several times. Some individuals were also contacted by telephone. A total of 10 semi-structured online interviews were conducted in January and February 2023 with different types of stakeholders as listed in Table 1 (The Interview Questionnaire is available in Appendix A). The objective of the interviews was to understand current strategies applied in selected agricultural supply chains that support non-GMO labelling claims as well as to understand how conventional, non-GMO-labelled agricultural products are imported into Europe and how current monitoring of compliance with EU legislation is carried out. Interviews with experts were intended to supplement the literature and desktop research by gathering additional details on identified key topics of addressing data gaps through questions targeted at specific stakeholder groups.
Moreover, the regulatory perspective of traceability in global value chains was assessed in a literature review. For this purpose, the general concept of due diligence and specific due-diligence-based regulatory instruments applicable to agricultural and non-agricultural global supply chains such as soy, conflict minerals and timber were examined.
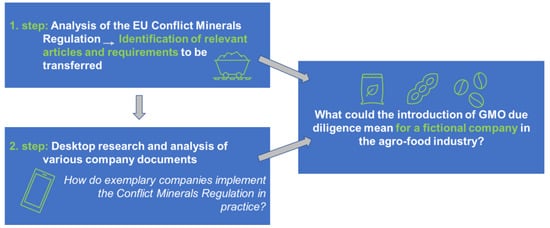
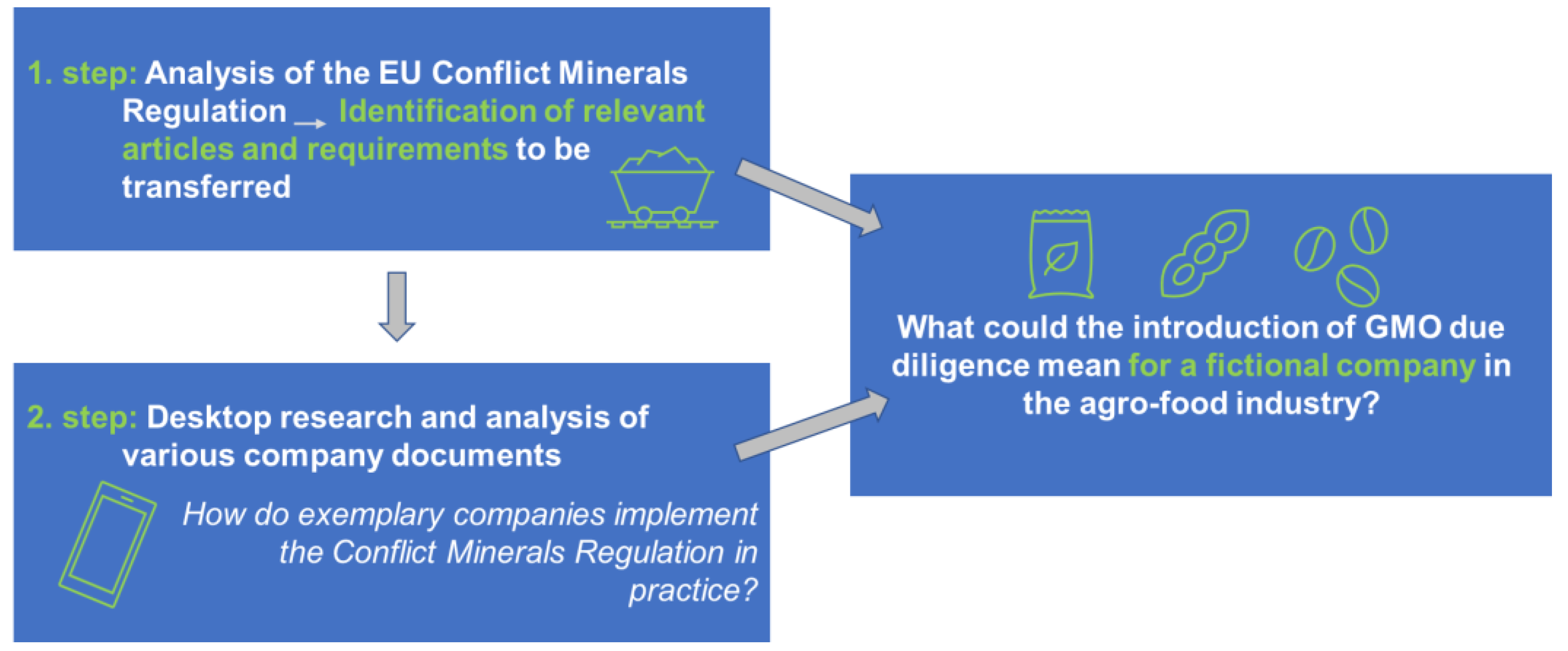
In exploring possible alternatives for the traceability of unauthorised GMOs in Europe, the elements of due diligence were examined using a specific example. The EU Conflict Minerals Regulation [21] was analysed in detail, and requirements relevant to a theoretical transfer to the field of GMOs were identified. The practical implementation was showcased by the transfer of the conflict mineral due diligence regulation to a specific hypothetical company from the food sector.
From those results, approaches for improving the traceability of products that might contain GM plants (including NGTs) were deduced. Two expert workshops with interview partners and other relevant stakeholders were conducted in April and September 2023 to validate and prioritise the identified alternative approaches to GMO traceability. The first expert workshop was held online on 26 April 2023; the second expert workshop took place on 29 September 2023 at the Federal Agency for Nature Conservation in Bonn. Hybrid participation was made possible. Thirty-four international experts from different stakeholder groups (non-governmental organisations, standard organisations, industry associations, feed trade, food producers, research, authorities, ministries) were invited to the first workshop. A total of 9 experts participated, including 6 experts from Germany and one expert each from Brazil, Ukraine and Belgium.
Thirty-six experts from all relevant stakeholder groups were also invited to the second workshop. A total of 23 experts participated, including 19 experts from Germany, 1 expert from the Netherlands, 2 from Belgium and 1 from Ukraine. The inputs from the workshop facilitated the discussion and optimisation of the alternative traceability strategies for unauthorised GMOs in Europe as presented in Section 4.
3. Traceability Strategies and Their Implementation in Global Supply Chains
In order to understand the status quo of traceability practices, this section explores different existing systems and instruments. The selected instruments include voluntary sustainability standards and due-diligence-based regulations applicable to goods imported into the EU. In this section, the authors also present the case study of soy, selected due to its relevance in terms of import volumes for the European market, in further detail. The results of this analysis should contribute to understanding which traceability strategies are used in the non-GM soy supply chain imported from Brazil and how compliance with EU regulation is ensured. The instruments examined in this section provide elements for an alternative traceability strategy for GMOs not authorised in Europe as presented in Section 4.
3.1. Existing Traceability Systems within Voluntary Sustainability Standards for Globally Traded Agro-Food Products
Voluntary sustainability standards (VSSs) play a pivotal role in ensuring transparency, accountability and ethical practices within global value chains. When integrated with traceability requirements, these standards can significantly enhance the management of different value chains—from primary production to market—and address increasing consumer demands regarding sustainability concerns inherent to global value chains [22].
The central concept underlying all traceability systems commonly used in voluntary sustainability standards is that the origin of a product is shown transparently and—depending on the traceability requirements—without gaps along the entire supply chain. This means that the key function of a traceability system is to collect and compile data on specific product characteristics and to trace them back along the entire value chain. This requires the establishment of a so-called chain-of-custody (CoC) system.
The CoC system and the underlying control system are key components of most sustainability standards. Both elements serve to verify the claims made through the labelling of a product, process, company or service with a corresponding sustainability standard [23]. To achieve this goal, sustainability standards must define a set of requirements, measures and means of proof that must be met along the entire supply chain. The control system, also defined and established by the standard owner, serves to verify that the stated requirements are adhered to.
Systems used to ensure traceability vary widely and are designed to be fit for purpose (e.g., could be paper-based or only go to a limited level of detail) [23]. Due to the differences in globally traded raw materials, intermediate products or finished products, each industry and standard applies different approaches to traceability.
In supply chain management, there are four main CoC models [23]:
- “Identity Preserved” ensures the physical separation of the certified raw material from its extraction or agricultural production to the final consumer good, allowing traceability.
- “Segregation” keeps certified and non-certified materials separate, allowing the mixing of compliant materials from different producers.
- “Mass Balance” tracks certified raw material by weight, allowing mixing before separation for accounting.
- “Book & Claim” involves purchasing sustainability certificates based on raw material quantity, without guaranteeing physical traceability. This model helps manufacturers meet procurement goals when direct sourcing is not possible.
Only the two CoC models “Identity Preserved” and “Segregation” can be considered for a traceability strategy for GMOs (including NGTs). The Mass Balance and Book & Claim models only provide administrative traceability. There is no physical link between the raw material used and the production supported by the certificates. These models allow producers to meet their sourcing targets if they are unable to source the required quantities of the certified product directly.
In order to understand the status quo of different approaches to traceability, the authors conducted a comparative analysis of selected recognised voluntary standards established for the certification of different agro-food products and commodities. The aim of this analysis was to identify current practices as well as initial insights for deriving alternative traceability strategies for unauthorised GMOs in Europe. Table 2 provides an overview of the aspects considered in the analysis. All of the selected VSSs are third-party-certified, requiring respective independent audits to ensure compliance with the standard criteria.
The current landscape of traceability systems within voluntary sustainability standards adhering to the “Identity Preserved” and “Segregation” models in agricultural product supply chains is characterised by the meticulous documentation and archiving of pertinent information throughout the entire continuum. In certain instances, standards incorporate online platforms or digital tools to facilitate the documentation of all actors along the supply chain. The use of blockchain-based systems for documentation is also on the horizon. Appropriately developed applications based on blockchain technology—as a new form of data storage—raise a number of expectations. It is usually pointed out that appropriately developed blockchain applications can facilitate the work of documentation required for traceability and minimise the risk of fraud in documentation. However, a research team led by Commandré was unable to confirm the fulfilment of these expectations of blockchain technology based on their study results [24].
The operational framework for documentation in the standards analysed includes the following key aspects:
- At the outset, the farmer is obliged to meticulously maintain purchase receipts for seeds or relevant inputs, alongside comprehensive records of yields and resale transactions. In most cases, products are endowed with a unique identification number right at the farm level.
- Subsequently, the initial processor or intermediary entity is tasked with documenting vital details, such as the quantity and origin of certified raw materials received. Moreover, in cases of resale, pertinent information including the buyer’s name and address, loading or dispatch/delivery dates, document issuance dates, certificate number, product description and delivered quantity has to be recorded, along with all associated transport documents.
- The verification process is conducted by external auditors hailing from accredited certification bodies who conduct on-site assessments. These audits encompass a comprehensive review of documents in conjunction with physical site visits including plausibility checks, e.g., to ensure that the seed input is consistent with the crop yield. The frequency of the audits and their intervals are specified in the respective standards. Often, standards employ a risk-based approach, wherein regions with an elevated risk of misdeclaration necessitate a higher frequency of audits, as opposed to regions deemed to have a lower risk of misdeclaration.
3.2. Status Quo of Labelling and Traceability of GM Crop Imports to Europe
In the complex web of global trade for agro-food products, the traceability of GMOs has emerged as a critical concern. Ensuring the accurate identification and tracking of GMOs within international supply chains is not only pivotal for regulatory compliance but also fundamental for meeting consumer demands for transparency. This section dives into the current landscape of labelling and traceability for the import of GM crops (and derived products) to Europe.
Current traceability practices for agricultural products containing GMOs rely on the data shared by actors from the first stages of the supply chain. When commodities produced through genetic modification techniques move along the value chain, specialised business-to-business (B2B) markers are essential. These markers, also known as unique identifiers, serve to track the specific GMO lineage but are not communicated to the end consumer. Instead, the obligation to furnish this information extends solely to the subsequent actor in the supply chain. At the same time, whether data indicating the presence of GMOs are shared with the next tier in the supply chain is determined both by the regulation in the country of origin of these commodities and the regulation in the importing country. If the national regulation of the country of origin does not require GMO labelling, the obligation to report data about the corresponding B2B marker will be determined only by the importing country.
In this context, importers into the EU are required to rely on the information received from their suppliers while ensuring compliance with EU GMO labelling requirements. Conventionally, these data are transmitted through the delivery note, without necessitating documentation of the goods’ origin, cultivation details or primary producer information.
It is crucial to note that the challenge of traceability transcends GMOs alone. It is, e.g., also of paramount importance in substantiating compliance with various criteria of sustainability standards (see Section 3.1). This encompasses an array of requisites, aimed at ensuring compliance with tangible but also intangible attributes. Common intangible attributes for which standards must apply traceability are claims about fair trade or production without child labour. Other common intangible areas of application for traceability in standards include verifying that agro-food commodities originate from supply chains free of deforestation or are sourced from organic and fair cultivation systems. Additionally, traceability serves as a cornerstone for certifying non-GMO agricultural supply chains, further underscoring its broad-reaching significance in contemporary agricultural trade practices.
3.3. Traceability of Non-GMO-Labelled Globally Traded Commodities: The Case of Soy from Brazil
The top four GM crops in the world are soybean, corn, cotton and canola [25]. The case of soy, which is used in a wide range of food and feed products, has become a key issue in the international trade in agricultural raw materials. Soybeans account for 50% of the world’s GMO cultivated area [25]. A substantial portion of the traded volumes originate from regions with a significant use of GMOs in agricultural practices. According to a 2021 report from the Global Agricultural Information Network (GAIN), the share of GMO varieties currently used for soy cultivation in selected countries ranges from 50–65% for Ukraine to 100% for Argentina. For Brazil, the share of GMO varieties used in soy production is 96% [26]. These data indicate that in the main soy-growing countries, almost exclusively GM varieties are cultivated. By cross-checking these findings with available data from international trade databases [27], it becomes evident that imports of soy and its byproducts (oil, meal, oil cake) account for the largest share of agricultural commodity imports to the EU in terms of volume. According to the UN’s COMTRADE database, imports of soybeans and their byproducts into the EU totalled around 31 million tonnes in 2021. Most of the soy imported into Europe is genetically modified and used as animal feed. Along with rapeseed meal, soy meal is one of the most important protein feeds. A closer look at the country of origin shows that the largest share is imported from Brazil [25]. Despite the high proportion of GM soy grown (96%), Brazil is still one of the few countries that exports large quantities of non-GM soy. Against this background, the import of non-GM soy from Brazil to Europe was selected as a case study to analyse the traceability strategies implemented for these import flows.
The case study provides an overview of the detailed practices involved in the traceability of non-GM soy produced in Brazil and its chain of custody to the point of entry into the Europe to ensure compliance with current regulations. Insights into the traceability practices described in the case study are based on interviews with a Norwegian company that exclusively imports non-GM soy and whose entire supply chain is also certified to the ProTerra standard [28] (see Table 2).
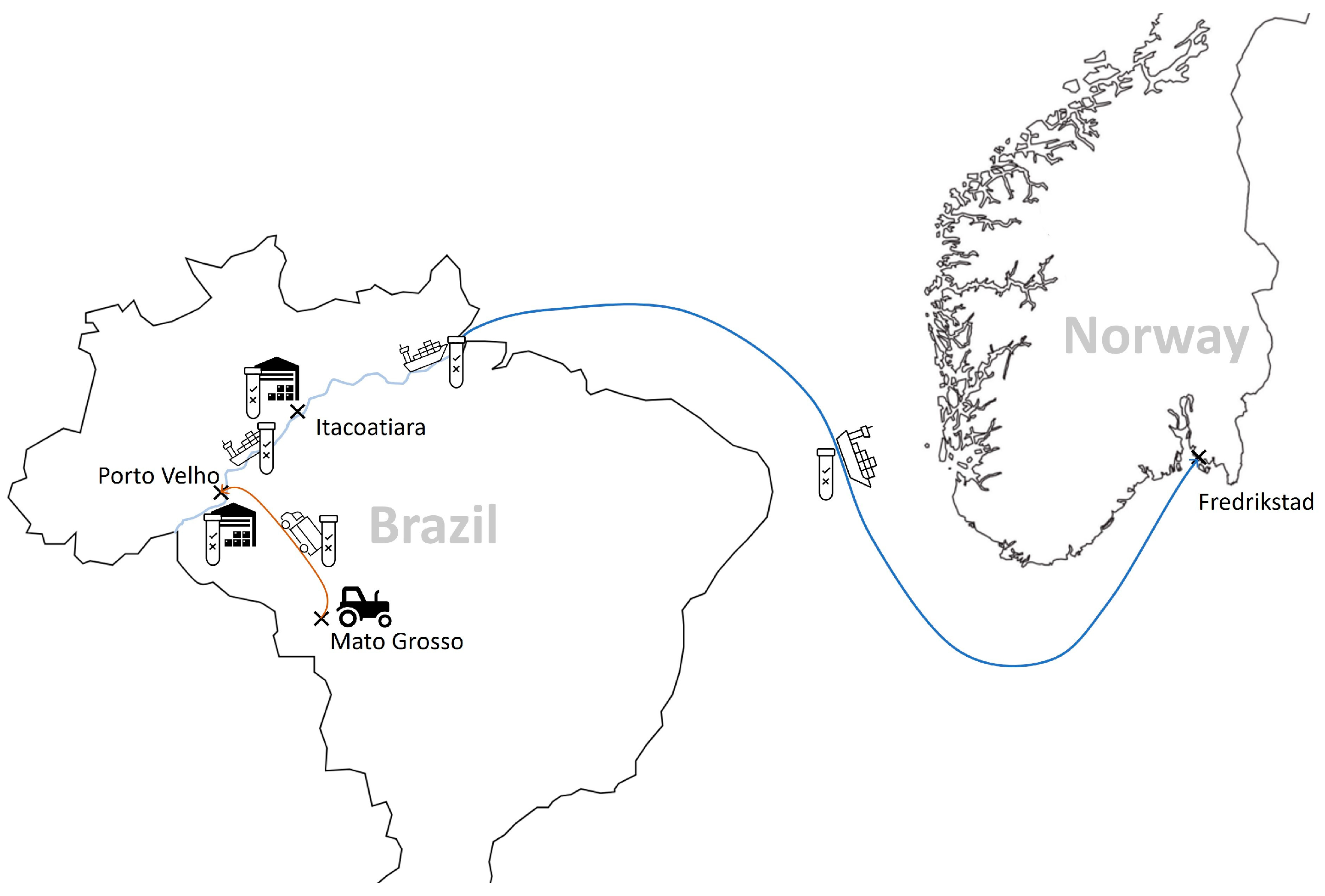
The company’s main supplier is based in Brazil. The soybeans are grown in the Mato Grosso region, a key location for soy production in Brazil. All actors in the supply chain follow a strict process to ensure the segregation of non-GM soybeans at all stages of production, storage and transport. Non-GM soy is only harvested using machinery specifically designed for this purpose or machinery that has been thoroughly cleaned to avoid contamination with GM soy. Once harvested, non-GM soybeans are stored separately in special silos. These silos are then transported to Porto Velho for storage before being shipped down the Madeira River to the port of Itacoatiara, where they are stored in warehouses dedicated to non-GM soybeans. From there, the soybeans are shipped by sea (see Figure 1).
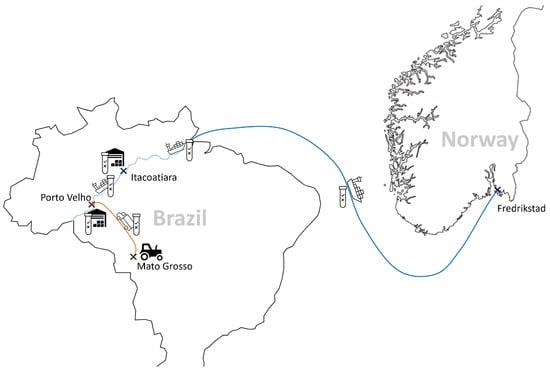
Figure 1.
The chain of custody of certified non-GM soy from Brazil imported into Europe (source: © Öko-Institut e.V. 2023).
All relevant stages in the value chain are documented. This means that the farmer has to document the purchase of non-GMO seed (e.g., with the relevant purchase receipts). It is double-checked whether the receipts for non-GM seeds match up with the actual harvest. The certified crop is given a batch number, which is passed down the supply chain. Silos are clearly marked and sealed for extra security. The quantities and origins of the certified raw materials received and their processing must then be documented throughout the supply chain (e.g., as follows: name and address of the buyer, name and address of the seller, date of loading or dispatch/delivery, date of issue of documents, certificate number, quantity of product delivered, relevant transport documents).
Furthermore, samples are taken for GMO testing using both rapid tests and PCR tests right from the early sowing stage through to each storage phase. This means seeds are also tested for the presence of GMOs. Accredited and independent laboratories handle these tests. Only shipments with negative test results move on to the next stage, with all cargoes held in quarantine until they receive the green light based on the test results. All the test results (including date and time) are carefully logged in a database run by the independent laboratory, giving a detailed record of responsibilities and shipments. The company also keeps a parallel paper record of the entire supply chain.
The interviewed Norwegian company stated that every batch of soy arriving in its port goes through about 3800 GMO tests, including quick tests. The company also regularly trains and inspects its suppliers to stress the importance of following the rules. When a ship with soybeans docks, the company promptly informs the relevant food authorities. This means that when the soybeans are unloaded, the authorities take samples right there at the port and test them for GMO content.
It is crucial to highlight that in order to be sure about the GMO status of a product, one needs to have separate supply chains for non-GM and GM products. Only machines, like harvesters and tractors, exclusively used for non-GM products can be used for the harvest and transport to the storage silos. In countries like Brazil, this is mostly doable on large industrial farms. The basic idea of a non-GM soy supply chain is the same for all traceability systems of agricultural products that follow the “Identity Preserved” and the “Segregation” model, even if there are small differences in the specifics. Using one of these two models is necessary for non-GM goods when attempting to comply with EU regulations.
The efforts described above for the segregation of non-GMO and GMO-containing raw materials and intermediate products result in additional costs. This point was raised in the interviews conducted. Many respondents were unable to quantify the costs of ensuring non-GM supply chains. This was generally due to the fact that most respondents did not market both GMO and non-GM products. The standard organisations interviewed claimed that the cost of certification is minimal compared to the cost of segregation. However, certification gives the legitimacy to claim a premium in the European market. One German food retailer stated that the prices for globally traded certified non-GM soybean meal are about 17% higher than those for conventional soybean meal. Another interviewee from a food producers’ association explained that, in general, there are no major price differences between non-GM soy from European value chains and globally traded non-GM soy. Prices are very volatile and depend on various factors, such as weather-related fluctuations in crop yields.
The additional costs of non-GM supply chains were also included in the literature review, and two different studies were found and analysed. In Brazil, the cost of non-GM soy includes several variables, including the availability of non-GM seeds; the need to establish a 20-metre buffer zone to prevent cross-pollination; increased maintenance costs to control weed growth; and the aforementioned efforts to avoid contamination during harvest, storage and transport [29].
In a study published in 2014, the additional costs of production and supply chain segregation in Brazil and the premium surcharge for non-GM soy were calculated and estimated to be around 21%. Just over half of the additional costs (around 51%) are borne by the farmer. Just under half (about 49%) of the additional costs are due to separate storage and transport [30].
3.4. Due-Diligence-Based Regulatory Instruments Contributing to Traceability of Imported Goods
The concept of supply chain due diligence and its relevance for the traceability of imported goods has its roots in the UN Guiding Principles on Business and Human Rights which were published in 2011 at the end of the so-called “Ruggie process” [31,32]. Those principles assigned companies a central role in respecting human rights worldwide. Until then, the question of the human rights responsibility of private companies was at the discretion of nation-states, which can pose challenges in their enforcement [31]. The UN Guiding Principles thus for the first time assigned their respective roles to both states and globally operating businesses. In the following years, the due diligence concept, developed in the context of human rights, has been increasingly expanded to include, depending on the standard or legislation, impacts on the environment as well as society in a broader sense [33,34].
In recent years, a number of binding legal provisions based on the concept of human rights due diligence or incorporating some of its elements have been enacted. Those regulations include comprehensive regulatory approaches like the German Supply Chain Act (Lieferkettensorgfaltspflichtengesetz—LkSG) [35] as well as sector-specific solutions such as the EU Conflict Minerals Regulation [21] and the EU Regulation on deforestation-free products [36]. Finally, the European Commission has published a legislative proposal for a comprehensive due diligence regulation (Corporate Sustainability Due Diligence Directive (CSDDD)) [37]. Looking at the above-mentioned regulations, valuable insights were drawn regarding their transferability to alternative traceability strategies to prevent the import of unauthorised GMOs in the EU.
Due diligence defines companies´ responsibility for human rights violations and adverse environmental impacts even when they are not directly committed by the company but by business partners [38]. This means that the respective responsibilities apply to the entire value chain of a company and its products. So, if legislation is in place, the due diligence requirements apply to companies that are headquartered in or import into the regulated area (here, the EU), but they also indirectly affect companies in the supply chain that may be located outside the regulated area.
Critics often argue that it is impossible for businesses to meet such a comprehensive responsibility, especially if, for example, it is a distant supplier that has caused the negative impacts [39,40]. This argument, however, is based on a misconception. Firstly, due diligence differentiates with regard to the degree of responsibility according to the level of actual “involvement”. For example, companies that directly cause a human rights violation should stop doing so, avoid doing so in the future and also provide remediation. If companies are merely “linked” to the human rights violation, they are only required to use their influence on the company that has caused the damage [38,41,42]. Secondly, due diligence is a risk-based approach. It does not establish an “obligation of result” but an “obligation of means” or “reasonable care”, meaning that companies have to first identify their risks along the value chain. Subsequently, they must take measures to prevent, avoid and mitigate those risks and, in case harm has already been caused, provide remedy. However, companies do not have to guarantee that no negative impacts occur [38,42]. The concrete solutions resulting from the regulations under consideration are explained in more detail in the following section.
5. Discussion
Focussing on GMOs, it is important to note that traceability requirements exist in the EU for authorised GMOs. Additionally, the non-GM sector currently employs traceability, supported by analytical controls to ensure non-GMO status. Analytical controls in the GMO sector, including NGTs, would be the optimal solution to guarantee non-GMO status and ensure that only authorised GMOs enter the EU market. The challenge is that the required detection methods or the appropriate information for developing such detection methods might not be readily available. Here, the existing international GMO databases could play a central role in providing access to information concerning detection and identification. Consideration should be given to supplementing these databases with additional information on the type of GMO and data to help assess the risk that a particular supply chain may contain unauthorised GMOs. Another option would be to create a completely new database that hosts information to facilitate the detection and identification of GMOs including NGT products. Ideally, such databases should provide access to DNA sequence information regarding the respective genetic modification(s) present in a particular GMO and should be retrievable in automated ways. As the analytical detection and identification of NGTs will likely be challenging for some products even with an improved information base, an alternative traceability strategy needs to be developed in addition.
Today, there are many products on the European food market that have claims that are not discernible by visible product characteristics (e.g., grown without the use of various pesticides, grown on land that does not contribute to further deforestation, grown and harvested without child labour, European certificate of origin, non-GMO, etc.). Not all existing claims can be substantiated by analytical methods. Our analysis has shown that there are traceability strategies that ensure the postulated properties, even if they cannot be proven analytically. The central concept underlying all traceability systems is that the ingredients of a product can be traced back through the whole supply chain up to the agricultural production or even to the origin of the seed material used to grow the respective crops.
Given the current GMO legislation in Europe, it is theoretically possible to implement full traceability of agricultural products that comply with existing EU legislation. In practice, there are now a large number of traceability systems for globally traded agricultural products that are certified to meet certain sustainability requirements. However, these systems are voluntary. The information required for traceability is provided voluntarily by actors throughout the supply chain. Typically, operators benefit by obtaining a premium price for their product on the European market. However, operators marketing conventionally produced products or NGT products may have no interest and no added value in providing the relevant batch traceability information. For those operators that would have to provide the necessary data for traceability, there is no reason or motivation to do so. However, this means that importers into the EU have to be legally obliged to prove that they only import approved GMOs. In the event of false declarations, importers would face severe sanctions.
The traceability systems currently in use in different sustainability standards are voluntary and do not in themselves provide a solution for the traceability of GMO products under current European GMO legislation, because the implementation of traceability is not defined. However, the transfer into an appropriate legal regulation could be an important step for an effective traceability strategy.
It must be emphasised that traceability itself is not the regulatory objective. It is merely a means to an end, a prerequisite for fulfilling the described due diligence obligations in place or a hypothetical due diligence regulation for GMOs. Only when a supply chain is known can risks be assessed and, if necessary, addressed.
The risk-based perspective of due diligence would have additional implications. Above all, it means that the tracing system should take into account different risk levels. This means, for example, that depending on the product or the country of origin, the extent of the controls would differ. Accordingly, the regulatory bodies or supervisory authorities should develop respective risk categories. On the one hand, these categories would be for their own use so they can, e.g., focus document checks or laboratory tests on high-risk cases; on the other hand, this information would be made available to other companies (e.g., in an international database) so that risk categorisations can be harmonised.
The exemplary transfer of due diligence to a company in the food industry shows that many important due diligence requirements are already met by selling certified non-GM products. The main elements that can be assumed to have already (partly) been introduced in the context of non-GMO certification comprise (1) a policy on how to avoid GMOs in supply chains, (2) an overview of agricultural supply chains and actors, (3) a traceability system based on the respective information about supply chains and involved actors, (4) the use of third-party audits to ensure compliance and minimise risks and (5) a management position with responsibility for the tasks described before.
Selling certified non-GM products is very likely to even go beyond compliance with a due diligence regulation, as it excludes all GMOs, including those authorised in the EU.
In this context, it should be considered that the existing concerns of stakeholders currently implementing non-GMO supply chains may be unfounded. On the contrary, a mandatory introduction of due diligence might give certain companies advantages over players who have not yet been actively investigating and managing their supply chains with regard to GMOs so far.
6. Conclusions
Undoubtedly, for some NGT products, challenges exist regarding detection and especially unequivocal identification. For this reason, an adaptation of the current GMO traceability system might be necessary. However, the argument that European GM legislation needs to be amended because of the existing challenges regarding analytical control methods is not entirely valid. The existing challenges might be compensated by other means since there are a number of regulations that prohibit the import of certain products without analytical control methods available to prove that the imported products comply with the regulation. Notable examples are the EU Conflict Minerals Regulation or the new Regulation on deforestation-free products. Consequently, there is no need to reduce GMO regulatory standards for NGTs just because detection of them poses a challenge.
Regarding improvements in traceability within the non-GMO sector, the concept of the “reversal of the burden of proof” is relevant. It necessitates the declaration of not only authorised GMOs (as per current requirements) but also the absence of GMOs. This declaration would have to apply to everyone, not just the non-GM sector. This could be ensured through a specific due diligence regulation that requires that importers introduce a traceability system for their supply chains and that importers identify and assess the risk of GMOs in their supply chain. This risk assessment in turn would require a range of information on GMOs, such as a regularly updated overview of the main countries exporting plant products to the EU for which GM varieties are available worldwide and an overview of the main points of entry into the EU (e.g., ports). Preferably, these data should be made available in a robust international database that also contains easily retrievable DNA sequence information on GMOs. This information would enable importers of agricultural products to conduct the required non-analytical assessment of risks regarding GMO contamination.
Overall, there is an urgent need for more transparency in our global agricultural supply chains, not only with regard to GMOs. Against the backdrop of the global climate crisis and the dramatic loss of biodiversity, the European Parliament and the Council of the European Union adopted the EU Regulation on deforestation-free products (EU) 2023/1115 in May 2023.
This regulation now needs to be implemented by European importers. It obliges them to disclose their supply chains and assess them for risks related to deforestation. As soy is one of the agricultural products with a high risk of deforestation, there are synergies with the issue of GMOs. In this context, better traceability alternatives applied to globally traded agricultural products could also contribute to ensuring that only authorised GMOs are imported to the European Union.
Author Contributions
Conceptualisation, S.S.; methodology, J.T., N.K. and A.G.; investigation, V.L.H., I.H., A.G. and J.T.; writing—original draft preparation, V.L.H., J.T., A.G. and N.K.; writing—review and editing, S.S. and M.E.; visualisation, V.L.H. and J.T.; supervision, J.T.; validation, F.N. and A.H.; project administration, J.T.; funding acquisition, J.T., N.K. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV) with grant number 3521842200. The project was commissioned by the Federal Agency for Nature Conservation (BfN) of Germany.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The interview data are not publicly available due to privacy restrictions of the interviewees.
Acknowledgments
The authors would like to acknowledge the contribution of all experts who agreed to be interviewed and who participated in the expert workshops for the validation of the results.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. S.S. is employed by the institution that commissioned the study.
Appendix A. Interview Questionnaire
| Questions | Relevant Stakeholders |
| All |
| Standard organisations; associations; logistics; businesses; importers |
| Standards; importers, businesses; control laboratories and other stakeholders responsible for spot checks |
| All |
| Non-GMO standards or companies working with isotope analysis; logistics; control laboratories |
| Control laboratories |
| All |
| All |
| All |
References
- European Commission. Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the Deliberate Release into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC; European Parliament: Strasbourg, France, 2001. [Google Scholar]
- European Commission. Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on Genetically Modified Food and Feed; European Parliament: Strasbourg, France, 2003. [Google Scholar]
- European Commission. Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 Concerning the Traceability and Labelling of Genetically Modified Organisms and the Traceability of Food and Feed Products Produced from Genetically Modified Organisms and Amending Directive 2001/18/EC; European Parliament: Strasbourg, France, 2003. [Google Scholar]
- Ribarits, A.; Narendja, F.; Stepanek, W.; Hochegger, R. Detection Methods Fit-for-Purpose in Enforcement Control of Genetically Modified Plants Produced with Novel Genomic Techniques (NGTs). Agronomy 2021, 11, 61. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) No 503/2013 of 3 April 2013 on Applications for Authorisation of Genetically Modified Food and Feed in Accordance with REGULATION (EC) No 1829/2003 of the European Parliament and of the Council and Amending Commission Regulations (EC) No 641/2004 and (EC) No 1981/200; European Parliament: Strasbourg, France, 2013. [Google Scholar]
- Broothaerts, W.; Jacchia, S.; Angers, A.; Petrillo, M.; Querci, M.; Savini, C.; van den Eede, G.; Emons, H. New Genomic Techniques: State-of-the-Art Review; Publications Office of the European Union: Luxembourg, 2021; ISBN 9789276246961. [Google Scholar]
- Court of Justice of the European Union. Judgement of the Court (Grand Chamber) of 25 July 2018 in Case C-528/16 Concerning the Request for a Preliminary Ruling under Article 267 TFEU from the Conseil d’État (Council of State, France); European Parliament: Strasbourg, France, 2018. [Google Scholar]
- Ribarits, A.; Eckerstorfer, M.; Simon, S.; Stepanek, W. Genome-Edited Plants: Opportunities and Challenges for an Anticipatory Detection and Identification Framework. Foods 2021, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- European Network of GMO Laboratories. Detection of Food and Feed Plant Products Obtained by New Mutagenesis Techniques; Publications Office of the European Union: Luxembourg, 2019; JRC116289. [Google Scholar]
- European Network of GMO Laboratories. Detection of Food and Feed Plant Products Obtained by Targeted Mutagenesis and Cisgenesis; Publications Office of the European Union: Luxembourg, 2023; ISBN 9789268039342. [Google Scholar]
- European Commission. Proposal for a Regulation of the European Parliament and of the Council on Plants Obtained by Certain New Genomic Techniques and Their Food and Feed, and Amending Regulation (EU) 2017/625 COM(2023) 411 Final; European Parliament: Strasbourg, France, 2023. [Google Scholar]
- European Commission. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007; European Parliament: Strasbourg, France, 2018. [Google Scholar]
- Eckerstorfer, M.F.; Engelhard, M.; Heissenberger, A.; Simon, S.; Teichmann, H. Plants Developed by New Genetic Modification Techniques-Comparison of Existing Regulatory Frameworks in the EU and Non-EU Countries. Front. Bioeng. Biotechnol. 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Secretariat of the Convention on Biological Diversity. BCH—Biosafety Clearing House. Available online: https://bch.cbd.int/en/ (accessed on 16 January 2024).
- EUginius. The European GMO Database. Available online: https://euginius.eu/euginius/pages/home.jsf (accessed on 16 January 2024).
- Parisi, C.; Rodríguez-Cerezo, E. Current and Future Market Applications of New Genomic Techniques; Publications Office of the European Union: Luxembourg, 2021; ISBN 9789276302063. [Google Scholar]
- Montet, D.; Dey, G. History of Food Traceability. In Food Traceability and Authenticity: Analytical Techniques; Montet, D., Ray, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–30. ISBN 9781351228435. [Google Scholar]
- Sun, S.; Wang, X.; Zhang, Y. Sustainable Traceability in the Food Supply Chain: The Impact of Consumer Willingness to Pay. Sustainability 2017, 9, 999. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety: (EC) No 178/2002; European Parliament: Strasbourg, France, 2002. [Google Scholar]
- International Trade Centre. Standards Map: The World’s Largest Database for Sustainability Standards. Available online: https://www.standardsmap.org/en/home (accessed on 27 June 2023).
- European Commission. Regulation (EU) 2017/821 of the European Parliament and of the Council of 17 May 2017 Laying down Supply Chain Due Diligence Obligations for Union Importers of Tin, Tantalum and Tungsten, Their Ores, and Gold Originating from Conflict-Affected and High-Risk Areas: (EU) 2017/821; European Parliament: Strasbourg, France, 2017. [Google Scholar]
- Fernandes Martins, K.; Teixeira, D.; de Oliveira Corrêa, R. Gains in sustainability using Voluntary Sustainability Standards: A systematic review. Clean. Logist. Supply Chain. 2022, 5, 100084. [Google Scholar] [CrossRef]
- ISEAL Alliance. Chain of Custody Models and Definitions: A Reference Document for Sustainability Standards Systems, and to Complement ISEAL’s Sustainability Claims Good Practice Guide; Cersion 1.0; ISEAL Alliance: London, UK, 2016. [Google Scholar]
- Commandré, Y.; Macombe, C.; Mignon, S. Implications for Agricultural Producers of Using Blockchain for Food Transparency, Study of 4 Food Chains by Cumulative Approach. Sustainability 2021, 13, 9843. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef] [PubMed]
- GAIN. Biotechnology and Other New Production Technologies Annual: European Union E42021-0088; European Union: Brussels, Belgium, 2021. [Google Scholar]
- United Nation’s Statistics Division. UN Comtrade Database. Available online: https://comtradeplus.un.org/ (accessed on 16 January 2024).
- ProTerra Foundation. ProTerra Standard: Social Responsibility and Environmental Sustainability. Version 4.1. Available online: https://www.proterrafoundation.org/wp-content/uploads/2020/10/ProTerra-Standard-V4.1_EN-2.pdf (accessed on 25 October 2023).
- Thadani, R.; Rocha, A. Soja não geneticamente modificada no Brasil: A diferenciação interessa ao Produtor? In Proceedings of the XI CASI—Congresso de Administração, Sociedade e Inovação, ECEME, Rio de Janeiro, Brazil, 6–7 December 2018; Even3: Recife, Brazil, 2019. [Google Scholar]
- Leitão, F.O. Análise Sistêmica da Segregação na Cadeia Logística da Soja Após o Advento e a Difusão dos Transgênicos; Universidade de Brasília: Brasilia, Brazil, 2014. [Google Scholar]
- Ruggie, J.G. Business and human rights: The evolving international agenda. Am. J. Int. Law 2007, 101, 819–840. [Google Scholar] [CrossRef]
- Ruggie, J.G. Just Business: Multinational Corporations and Human Rights; W. W. Norton & Company: New York, NY, USA; London, UK,, 2013. [Google Scholar]
- Scherf, C.-S.; Gailhofer, P.; Kampffmeyer, N.; Hilbert, I.; Schleicher, T. Umweltbezogene und Menschenrechtliche Sorgfaltspflichten als Ansatz zur Stärkung Einer Nachhaltigen Unternehmensführung: Zwischenbericht Arbeitspaket 1—Analyse der Genese und des Status quo; TEXTE 102/2019; Umweltbundesamt: Dessau, Germany, 2019. [Google Scholar]
- Kampffmeyer, N.; Gailhofer, P.; Scherf, C.-S.; Schleicher, T.; Westphal, I. Umweltschutz wahrt Menschenrechte! Unternehmen und Politik in der Verantwortung; Öko-Institut Working Paper 3/2018; Öko-Institut: Berlin, Germany, 2018. [Google Scholar]
- Altenschmidt, S.; Helling, D. Gesetz Über die Unternehmerischen Sorgfaltspflichten zur Vermeidung von Menschenrechtsverletzungen in Lieferketten: Lieferkettensorgfaltspflichtengesetz—LkSG; Bundesministerium für Bildung und Forschung: Bonn, Germany, 2021. [Google Scholar]
- European Commission. Regulation (EU) 2023/1115 of the European Parliament and of the Council of 31 May 2023 on the Making Available on the Union Market and the Export from the Union of Certain Commodities and Products Associated with Deforestation and Forest Degradation and Repealing Regulation (EU) No 995/2010: (EU) 2023/1115; European Parliament: Strasbourg, France, 2023. [Google Scholar]
- European Commission. Proposal for a Directive of the European Parliament and of the Council on Corporate Sustainability Due Diligence and amending Directive (EU) 2019/1937: CSDDD; European Parliament: Strasbourg, France, 2022. [Google Scholar]
- United Nations. Guiding Principles on Business and Human Rights: Implementing the United Nations Implementing the United Nations “Protect, Respect and Remedy” Framework. Available online: https://www.ohchr.org/documents/publications/GuidingprinciplesBusinesshr_eN.pdf (accessed on 22 March 2019).
- BDI. EU-Lieferkettengesetz: Entwurf Droht Unternehmen zu Überfordern. Available online: https://bdi.eu/artikel/news/entwurf-droht-unternehmen-zu-ueberfordern (accessed on 1 November 2023).
- DIHK. EU-Lieferkettengesetz Belastet Unternehmen Unverhältnismäßig: DIHK Sieht Auch den Aufbau Resilienterer Wertschöpfungsketten in Gefahr. Available online: https://www.dihk.de/de/aktuelles-und-presse/aktuelle-informationen/eu-lieferkettengesetz-belastet-unternehmen-unverhaeltnismaessig-96298 (accessed on 1 November 2023).
- Debevoise & Plimpton; Enodo Rights. Practical Definitions of Cause, Contribute, and Directly Linked to Inform Business Respect for Human Rights: Discussion Draft. 2017. Available online: https://www.business-humanrights.org/sites/default/files/documents/Debevoise-Enodo-Practical-Meaning-of-Involvement-Draft-2017-02-09.pdf (accessed on 7 May 2018).
- Klinger, R.; Krajewski, M.; Krebs, D.; Hartmann, C. Verankerung Menschenrechtlicher Sorgfaltspflichten von Unternehmen im Deutschen Recht; Germanwatch: Bonn, Germany, 2016; ISBN 3943704459. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).