Abstract
Colorectal cancer (CRC) is the third most common type of cancer and is caused by multiple factors. Chronic inflammation, known to cause inflammatory bowel disease (IBD), is closely associated with CRC. Cheonggukjang (CJ), a traditional Korean fermented soybean, is a functional food with anti-inflammatory effects in the intestines, but its anti-cancer effects have not yet been explored. In this study, we investigated the cancer-protective effects of cheonggukjang in an azoxymethane/DSS (AOM/DSS)-induced colitis-associated colorectal cancer (CAC) mouse model. The CJ alleviated AOM/DSS-induced pathological symptoms such as colonic shortening, increased spleen weight, tumor formation, and histological changes. It also modulated pro-inflammatory and anti-inflammatory cytokine levels via the suppression of NF-κB and inflammatory mediator signaling pathways. Furthermore, the CJ improved intestinal integrity by regulating mucin-associated and tight junction proteins. In addition, it suppressed tumor growth by regulating apoptosis and proliferation. These results highlight the anti-tumor effects of CJ in an AOM/DSS-induced CAC mouse model.
1. Introduction
Colorectal cancer (CRC) is the third most common type of cancer in both men and women worldwide [1]. In 2018, the overall CRC incidence rate in Korea was 11.4%, 12.9% in men and 9.8% in women, with a mortality rate of 10% in men and 12.6% in women [2]. Chronic inflammation leads to various organ-specific diseases, depending on where the inflammation occurs [3]. In particular, chronic inflammation in the intestine causes inflammatory bowel disease (IBD), which increases the risk of developing CRC, also known as colitis-associated colorectal cancer (CAC) [4].
IBD is associated with multiple genetic, microbial, environmental, and immune-mediated factors [5,6]. However, the accurate etiology of IBD is still unknown. Recent studies have indicated that pro-inflammatory cytokines and gut dysbiosis induce both intestinal inflammation and the disruption of normal mucosal immunity, resulting in IBD [7,8]. Consequently, the suppression of intestinal inflammation may help prevent inflammation-associated colon cancer [9].
Cheonggukjang (CJ) is a soybean paste made through the fermentation of boiled soybeans. It is rich in substances that exhibit various biological activities, such as poly γ-glutamic acid (γ-PGA), isoflavone, saponins, phenolic acids, and flavonoids [10]. In many studies, CJ has emerged as a functional food with anti-obesity, antioxidant, and anti-inflammatory effects in bowel disease [10,11,12]. We have previously shown that CJ contains beneficial probiotics and is effective in preventing inflammatory diseases by suppressing the inflammatory signaling pathway in dextran sodium sulfate (DSS)-induced colitis mice [13]. However, to the best of our knowledge, the preventive effect of CJ on inflammatory colorectal tumorigenesis has not been elucidated. In this study, we report the anti-tumor growth effects of CJ by inhibiting azoxymethane (AOM)/DSS-induced pathological symptoms in an AOM/DSS-induced CAC mouse model.
2. Materials and Methods
2.1. Preparation of the CJ
The CJ was obtained from the Microbial Institute for Fermentation Industry (Sunchang-gun, Jeollabuk-do, Republic of Korea) as described previously [13]. It was produced using the traditional method of Kangjin-gun (Jeollanam-do, Republic of Korea) and had a moisture content of 53.15%. The CJ was dissolved in distilled water at 500 mg/kg and then stored at −20 °C before being orally administered to mice.
2.2. AOM/DSS-Induced Colorectal Cancer Model and CJ Treatment
A total of 32 male BALB/c mice (five-week-old) were purchased from Damool Science (Daejeon, Republic of Korea). The mice were maintained at 20–24 °C with a 12 h light/dark cycle and a relative humidity of 50–60%. After 1 week of acclimatization, the mice were divided into four groups (n = 8): NOR (normal group; only water), CON (control group; AOM (10 mg/kg)/DSS (2%)), PC (positive control group; AOM (10 mg/kg)/DSS (2%) and 5-aminosalicylic acid (75 mg/kg/day, 5-ASA, Sigma-Aldrich, St. Louis, MO, USA)), and CJ (CJ treated group; AOM (10 mg/kg)/DSS (2%) and CJ (100 mg/kg/day)). AOM/DSS-induced CAC was initiated by injecting the mice intraperitoneally with AOM (10 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) on day 0. After 1 week, the mice were administered DSS (2%, MP Biomedicals, Irvine, CA, USA) for another week, followed by water for 2 weeks for recovery. This treatment was repeated twice (days 7–14, 32–39). All experimental animal procedures were performed within the guidelines of and approved by the Jeonju AgroBio-Materials Institute’s Animal Care and Use Committee (JAMI IACUC 2022004).
2.3. ELISA Analysis
The serum levels of TNF-α (MTA00B), IFN-γ (MIF00), IL-6 (M6000B), IL-1β (MLB00C), IL-4 (M4000B), and IL-10 (M1000B) were analyzed using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s recommendations.
2.4. Quantitative Real-Time PCR (qRT-PCR) Analysis
Total RNA was extracted from colonic tissues using a Hybrid-RTM kit (GeneAll, Seoul, Republic of Korea). First, cDNA was synthesized with the BioFACTTM 2X RT Pre-Mix (BIOFACT, Daejeon, Republic of Korea). Next, qRT-PCR was undertaken using the BioFACTTM 2X Real-Time PCR Master Mix (Bio-Fact) and analyzed with a sequence detection system (CFX96, Bio-Rad, Hercules, CA, USA). The primer sequences used are listed in Table 1.

Table 1.
Primer sequences.
2.5. Western Blotting
Western blotting was performed as described previously [13]. Briefly, about 20 μg of cell lysate was separated using a 10% SDS-PAGE and transferred to a PVDF membrane using the Trans-Blot Turbo Transfer system (Bio-Rad). The membranes were probed with specific antibodies: anti-iNOS, anti-COX-2, anti-p-p65, anti-p65, anti-Bax, anti-p53, anti-Bcl-2, anti-Bcl-XL, and anti-β-actin (Cell Signaling Technology, Danvers, MA, USA). The protein–antibody binding was visualized using the enhanced chemiluminescence (ECL) detection system (Amersham Imager 600, GE Healthcare, Chicago, IL, USA).
2.6. Hematoxylin & Eosin (H&E) Staining and Immunohistochemistry
Colon tissue sections (4 μm thick) were stained with H&E. For immunolabeling, the sections were incubated with specific antibodies (anti-Muc2, anti-ZO-1, anti-occludin, anti-proliferating cell nuclear antigen (PCNA; Cell Signaling Technology, Danvers, MA, USA), and anti-Ki-67 (Abcam, Boston, MA, USA)) overnight at 4 °C in the dark. Next, the sections were incubated with either mouse or rabbit Envision plus polymer reagent (Dako, Glostrup Kommune, Denmark) for 30 min at 4 °C, and binding was detected by 3, 3’-diaminobenzidine (DAB) staining. Images were captured using a tissue slide scanner (Motic; Xiamen, China).
2.7. Statistical Analysis
A comparison of the statistical significance between groups was determined using a one-way ANOVA and a Tukey post-hoc test in GraphPad Prism (version 5.0; GraphPad Software, Inc., San Diego, CA, USA). The data are presented as mean ± standard deviation (SD). The statistical significance was set at p < 0.05.
3. Results and Discussion
3.1. CJ Attenuates Pathological Symptoms in Mice with AOM/DSS-Induced CAC
Chronic inflammation, one of the major contributing factors in the development of IBD, is closely associated with CRC and can be prevented or delayed with anti-inflammatory agents [14,15]. We have previously studied the anti-inflammatory effects of CJ [13] and now aimed to determine the anti-cancer effects of CJ in CRC, which is closely related to chronic inflammation. Therefore, in this study, we investigated the anti-cancer effects of CJ using an AOM/DSS-induced CAC mouse model (Figure 1a). The administration of a 2% DSS reduced the body weights of AOM/DSS-treated mice. However, the lost body weight was regained when the DSS administration was discontinued (Figure 1b). The decrease in body weight was moderate in the PC (5-ASA treated) and CJ groups. Based on previous reports of reduced colon length in mice with AOM/DSS-induced CAC [16], we measured the colon lengths of the treated mice to evaluate the protective effects of CJ. Compared with the NOR group, the AOM/DSS-treated group (CON) had significantly shorter colons. However, the administration of 5-ASA and CJ restored the colon length (Figure 1c,d). Next, we measured the spleen weights, an indicator of the severity of tumor progression, of AOM/DSS-induced CAC mice [16]. The CON group showed an increase in spleen weight, which was reversed by the administration of 5-ASA and CJ (Figure 1e), supporting the anti-tumor effects of CJ. These findings indicate that CJ attenuates the pathological symptoms in mice with AOM/DSS-induced CAC.
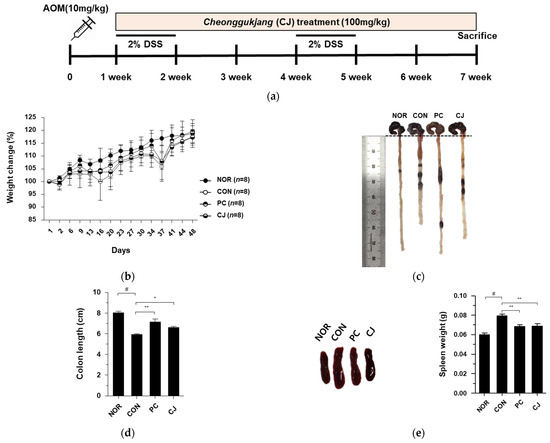
Figure 1.
Effects of CJ on pathological symptoms of AOM/DSS-induced colorectal cancer (CAC) mice. (a) Experimental design; (b) weight change (%); (c) representative pictures of colon in mice; (d) colon length in mice; (e) representative pictures of spleen and spleen weight in mice. Values are means ± standard deviation (n = 8); NOR, normal group; CON, control group; PC, positive control group; CJ, cheonggukjang treated group; #, p < 0.05 versus normal group; **, p < 0.005; *, p < 0.05 versus control group.
3.2. CJ Suppresses Tumorigenesis in AOM/DSS-Induced CAC Mouse Model
AOM/DSS-induced CAC in mice is characterized by severe colitis with weight loss and a reduction in colon length, followed by the development of multiple colon tumors [17]. Therefore, we investigated the effects of CJ on intestinal tumorigenesis in mice with AOM/DSS-induced CAC. The AOM/DSS-treated mice showed an increase in macroscopic tumors, which was reversed following the administration of 5-ASA and CJ (Figure 2a.). The increased number of tumors in the CON group was reduced following the administration of 5-ASA and CJ (Figure 2b). Moreover, a significant difference was seen in the mean tumor size between the CON group and the PC and CJ groups (Figure 2c). Tumor formation and the thickening of the mucous membrane have been reported to increase the ratio of colon weight to colon length [18]. Therefore, we calculated the ratio of colon weight to colon length to confirm the inhibitory effects of CJ on tumorigenesis. As shown in Figure 2d, the weight-to-length ratio was significantly higher in the CON group than in the NOR group. However, this increase was significantly attenuated by the administration of 5-ASA and CJ (Figure 2d). H&E staining is used for the histological diagnosis of various diseases, including IBD and cancer [19,20]. The pathological features of AOM/DSS-induced CAC in mice include inflammatory cell infiltration, crypt abscesses, and hyperchromatic nuclei [17,21]. The histopathological features related to CRC development were examined with H&E staining. Inflammatory cell infiltration and hyperchromatic nuclei were observed in the colon tissue of the CON group, and they were significantly ameliorated by the administration of PC and CJ (Figure 2e). These results indicate that CJ suppresses AOM/DSS-induced colitis-associated tumorigenesis in mice.
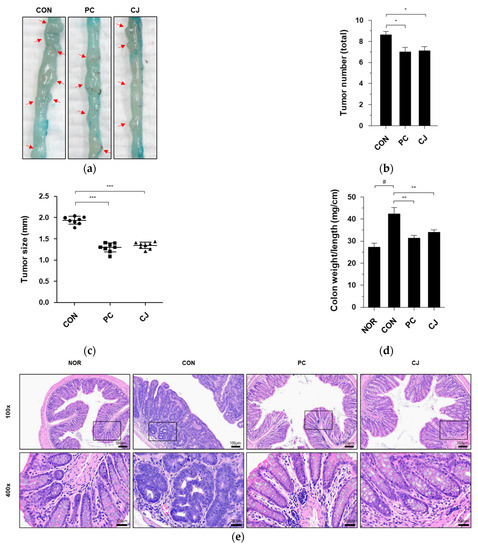
Figure 2.
Effects of CJ on tumorigenesis in mice with AOM/DSS-induced CAC. (a) Representative pictures of colon tissue in the different groups of mice. Arrow indicates a tumor. Shown are the (b) total number of tumors, (c) tumor size, and (d) ratio of colon weight-to-colon length in the different groups of mice. All values are mean ± standard deviation (n = 8). (e) Representative histology for H&E staining of colon tissue. Magnification, 100× and 400×; Scale bar; 100 μm and 300 μm; NOR, normal group; CON, control group; PC, positive control group; CJ, cheonggukjang treated group. #, p < 0.05 versus normal group; ***, p < 0.001; **, p < 0.005; *, p < 0.05 versus control group.
3.3. CJ Modulates the Expression of Inflammatory Cytokines in AOM/DSS-Induced CAC Mice
AOM/DSS-induced CAC is associated with the expression of pro-inflammatory and anti-inflammatory cytokines, such as TNF-α, IFN-γ, IL-1β, IL-6, IL-4, and IL-10 [22,23]. As shown in Figure 3a-f, mRNA levels of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, and IL-6) were increased in the CON group, whereas those of anti-inflammatory cytokines (IL-4 and IL-10) were decreased. However, these changes in mRNA levels were reversed by the administration of 5-ASA and CJ (Figure 3a–f). To confirm these effects, we evaluated the levels of TNF-α, IFN-γ, IL-1β, IL-6, IL-4, and IL-10 in a serum by ELISA. Consistent with the mRNA results, the CON group showed increased TNF-α, IFN-γ, IL-1β, and IL-6 levels and decreased IL-4 and IL-10 levels (Figure 3g–l). The increase/decrease in cytokine levels was reversed in the PC and CJ groups, indicating that CJ modulates the expression of pro-inflammatory and anti-inflammatory cytokines in mice with AOM/DSS-induced CAC.
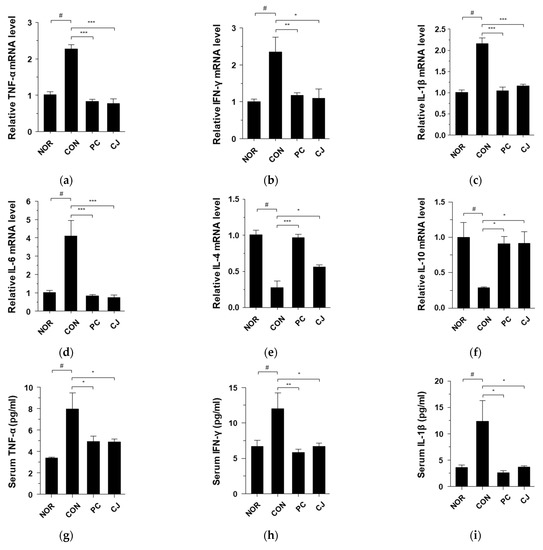

Figure 3.
Effects of CJ on the expression of inflammatory cytokines in mice with AOM/DSS-induced CAC. Shown are the mRNA levels of (a) TNF-α, (b) IFN-γ, (c) IL-1β, (d) IL-6, (e) IL-4, and (f) IL-10 in the colon and protein levels of (g) TNF-α, (h) IFN-γ, (i), IL-1β, (j) IL-6, (k) IL-4, and (l) IL-10 in the serum. Values are mean ± standard deviation (n = 8); NOR, normal group; CON, control group; PC, positive control group; CJ, cheonggukjang treated group. #, p < 0.05 versus normal group; ***, p < 0.001; **, p < 0.005; *, p < 0.05 versus control group.
3.4. CJ Suppresses Activation of NF-κB Signaling in AOM/DSS-Induced CAC Mice
Nuclear factor-κB (NF-κB) is involved in inflammatory responses, proliferation, differentiation, and apoptosis. Moreover, the activation of NF-κB contributes to IBD and tumor development [24,25]. In a previous study, we showed that the NF-κB signaling pathway is activated by DSS and suppressed by CJ in DSS-treated mice [13]. Therefore, we investigated whether CJ suppresses NF-κB activation in mice with AOM/DSS-induced CAC. The CON group showed a significant increase in phospho-p65 NF-κB expression, which was decreased in the PC and CJ groups (Figure 4a). NF-κB activation induced several inflammatory enzymes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2) [26]. Therefore, western blotting and quantitative real-time PCR (qRT-PCR) were performed to evaluate the iNOS and COX-2 levels in the treated mice. The protein levels of iNOS and COX-2 were increased in the CON group (Figure 4a). However, 5-ASA and CJ administration inhibited iNOS and COX-2 expression (Figure 4a). These findings were confirmed by the mRNA levels of iNOS and COX-2 measured by qRT-PCR (Figure 4b,c). These findings indicate that CJ suppresses the phospho-NF-κB, iNOS, and COX-2 expression in mice with AOM/DSS-induced CAC.
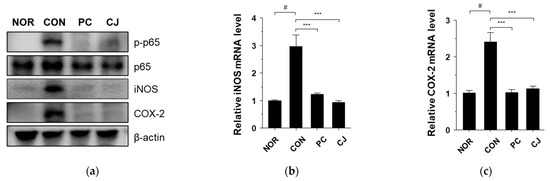
Figure 4.
Effects of CJ on AOM/DSS-induced CAC-associated signaling pathways: (a) protein levels of phosphorylated p65, p65, iNOS, and COX2; and mRNA levels of (b) iNOS and (c) COX-2. Values are means ± standard deviation (n = 8); NOR, normal group; CON, control group; PC, positive control group; CJ, cheonggukjang treated group. #, p < 0.05 versus normal group; ***, p < 0.001 versus control group.
3.5. CJ Improves Intestinal Integrity in Mice with AOM/DSS-Induced CAC
The colonic mucus layer and tight junctions act as a layered defensive barrier in the colon and play a vital role in regulating mucosal permeability to ions, nutrients, and water [27,28]. The mucus layer, protected by the secreted mucin protein MUC2, is in contact with the epithelial cells lining the intestine and is joined via tight junctions [29]. Tight junctions consist of transmembrane proteins such as occludin, claudin, and junctional adhesion molecules [30]. Occludin directly interacts with zonula occludens-1 (ZO-1) and regulates paracellular permeability [31]. The loss of the colonic mucus layer is responsible for various intestinal diseases, including IBD and CRC [32,33].
We investigated the levels of mucin-associated protein (MUC2) and tight junction structural proteins (occludin and ZO-1) in the treated mice to assess the effects of AOM/DSS treatment on the colonic mucus layer. The mRNA levels of MUC2, occludin, and ZO-1 were significantly decreased in the CON group (Figure 5a–c). However, the administration of 5-ASA and CJ reversed the effects of AOM/DSS and significantly increased the mRNA levels of these markers (Figure 5a–c). Immunohistochemical staining confirmed the reduced expression of MUC2, Occludin, and ZO-1 in the CON group (Figure 5d). However, the administration of 5-ASA and CJ reversed these effects and increased the levels of MUC2, occludin, and ZO-1. These findings indicate that CJ may improve intestinal integrity by inducing the expression of mucin-associated and tight junction proteins in mice with AOM/DSS-induced CAC.

Figure 5.
Effects of CJ on the expression of mucin-associated protein and tight junction proteins in mice with AOM/DSS-induced CAC. Shown are the mRNA levels of (a) Muc2, (b) occludin, and (c) ZO-1 in the different groups of mice. (d) Representative images of the colonic tissue immunohistochemically stained with MUC2, occludin, and ZO-1. Magnification, 200 X; scale bar, 60 μm. Values are means ± standard deviation (n = 8); NOR, normal group; CON, control group; PC, positive control group; CJ, cheonggukjang treated group. #, p < 0.05 versus normal group; ***, p < 0.001; **, p < 0.005; *, p < 0.05 versus control group.
3.6. CJ Suppresses Tumor Growth by Regulating Apoptosis and Proliferation
The occurrence of CRC is closely associated with abnormal cell growth via the rapid proliferation and evasion of apoptosis [34]. The NF-κB signaling pathway regulates apoptosis and cell proliferation factors such as p53, Bax, Bcl-2, Bcl-XL, PCNA, and Ki67 [24,35]. In Figure 4a, we observed that the NF-κB signaling pathway was activated in the CON group. Thus, we evaluated the apoptosis and cell proliferation factors following treatment with AOM/DSS in this group. As shown in Figure 6a–d, the mRNA levels of pro-apoptotic markers, p53 and Bax, were significantly lower, and the mRNA levels of anti-apoptotic markers, Bcl-2 and Bcl-XL, were higher in the AOM/DSS-treated CON group compared with the NOR group. Treatment with 5-ASA and CJ reversed these effects (Figure 6a–d). Western blotting confirmed these mRNA results at the protein level (Figure 6e). Next, to evaluate the regulation of cell proliferation by AOM/DSS, cell proliferation markers were studied with immunohistochemical staining. PCNA and Ki-67 expression were higher in the AOM/DSS-treated CON group compared with the NOR group, while 5-ASA and CJ reversed this effect (Figure 6f). These findings indicate that CJ inhibits tumor growth by inducing apoptosis and suppressing the proliferation of tumor cells in mice with AOM/DSS-induced CAC.
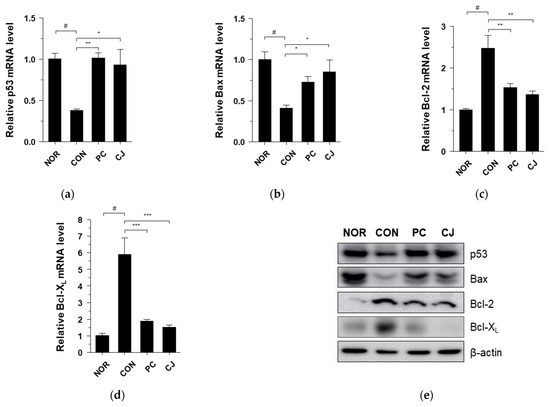
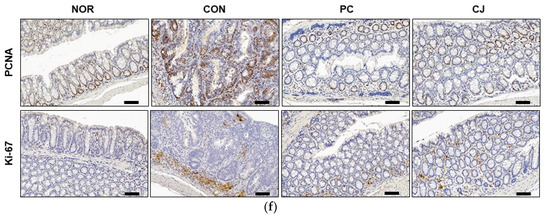
Figure 6.
Effects of CJ on the expression of apoptosis and proliferation-related proteins in mice with DSS-induced colitis. mRNA levels of (a) p53, (b) Bax, (c) Bcl-2, and (d) Bcl-XL in the different groups of mice were measured by qRT-PCR. (e) Protein levels of p53, Bax, Bcl-2, and Bcl-XL were determined by western blotting. (f) Representative images of the colonic tissue immunohistochemically stained with PCNA and Ki67. Magnification, 200×; Scale bar, 60 μm. Values are means ± standard deviation (n = 8); NOR, normal group; CON, control group; PC, positive control group; CJ, cheonggukjang treated group. #, p < 0.05 versus normal group; ***, p < 0.001; **, p < 0.005; *, p < 0.05 versus control group.
4. Conclusions
This study demonstrates that CJ, made from the fermentation of boiled soybeans, significantly ameliorates AOM/DSS-induced pathological symptoms in mice including colonic shortening, spleen hypertrophy, tumor formation, and histopathological colonic changes, similar to the positive control (5-ASA). We also show that CJ and 5-ASA suppress the expression of pro-inflammatory cytokines and inflammatory mediators while increasing the expression of anti-inflammatory cytokines through the activation of NF-κB signaling pathways. Furthermore, CJ and 5-ASA attenuate tumorigenesis by inhibiting tumor growth through the induction of apoptosis and the inhibition of cell proliferation. These results suggest that CJ, which shows anti-cancer effects similar to 5-ASA, can be used as a functional food to prevent chronic inflammatory CRC.
Author Contributions
Conceptualization, S.-Y.K. and C.-H.J.; investigation, H.-J.L., I.-S.P., S.-J.J., G.-S.H. and H.-J.Y.; writing—original draft preparation, C.-H.J.; writing—review and editing, S.-Y.K. and C.-H.J.; funding acquisition, D.-Y.J. and S.-Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Functional research of fermented soybean food (safety monitoring)” under the Ministry of Agriculture, Food, and Rural Affairs, and partly Korea Agro-Fisheries and Food Trade Corporation in 2022.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Committee of Jeonju AgroBio-Materials Institute (JAMI IACUC 2022004, Jeonju, Republic of Korea).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| CRC | Colorectal Cancer |
| IBD | Inflammatory Bowel Disease |
| CAC | Colitis-Associated Colorectal Cancer |
| CJ | Cheonggukjang |
| γ-PGA | poly γ-glutamic acid |
| DSS | Dextran Sulfate Sodium |
| AOM | Azoxymethane |
| NOR | Normal |
| CON | Control |
| PC | Positive Control |
| 5-ASA | 5-Aminosalicylic acid |
| qRT-PCR | Quantitative Real-Time PCR |
| H&E | Hematoxylin & Eosin |
| NF-κB | Nuclear Factor-κB |
| iNOS | Inducible Nitric Oxide Synthase |
| COX-2 | Cyclooxygenase |
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kong, H.J.; Im, J.S.; Seo, H.G. Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2018. Cancer Res. Treat. 2021, 53, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Schetter, A.J.; Heegaard, N.H.; Harris, C.C. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Teng, S.; Hao, J.; Bi, H.; Li, C.; Zhang, Y.; Zhang, Y.; Han, W.; Wang, D. The Protection of Crocin Against Ulcerative Colitis and Colorectal Cancer via Suppression of NF-κB-Mediated Inflammation. Front. Pharmacol. 2021, 12, 639458. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Hwang, C.W.; Yang, W.S.; Kim, C.H. Current Perspectives on the Physiological Activities of Fermented Soybean-Derived Cheonggukjang. Int. J. Mol. Sci. 2021, 22, 5746. [Google Scholar] [CrossRef]
- Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases. Foods 2021, 10, 636. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Ryu, M.S.; Yang, H.J.; Park, S. γ-PGA-Rich Chungkookjang, Short-Term Fermented Soybeans: Prevents Memory Impairment by Modulating Brain Insulin Sensitivity, Neuro-Inflammation, and the Gut-Microbiome-Brain Axis. Foods 2021, 10, 221. [Google Scholar] [CrossRef]
- Lim, H.J.; Kim, H.R.; Jeong, S.J.; Yang, H.J.; Ryu, M.S.; Jeong, D.Y.; Kim, S.Y.; Jung, C.H. Protective Effects of Fermented Soybeans (Cheonggukjang) on Dextran Sodium Sulfate (DSS)-Induced Colitis in a Mouse Model. Foods 2022, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Lucafò, M.; Curci, D.; Franzin, M.; Decorti, G.; Stocco, G. Inflammatory Bowel Disease and Risk of Colorectal Cancer: An Overview from Pathophysiology to Pharmacological Prevention. Front. Pharmacol. 2021, 12, 772101. [Google Scholar] [CrossRef] [PubMed]
- van Staa, T.P.; Card, T.; Logan, R.F.; Leufkens, H.G. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: A large epidemiological study. Gut 2005, 54, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, Y.; Zhang, S.; Kong, G.; Li, Z. Association between cellular immune response and spleen weight in mice with hepatocellular carcinoma. Oncol. Lett. 2021, 22, 625. [Google Scholar] [CrossRef]
- Parang, B.; Barrett, C.W.; Williams, C.S. AOM/DSS Model of Colitis-Associated Cancer. Methods Mol. Biol. 2016, 1422, 297–307. [Google Scholar]
- Chen, L.H.; Song, J.L.; Qian, Y.; Zhao, X.; Suo, H.Y.; Li, J. Increased preventive effect on colon carcinogenesis by use of resistant starch (RS3) as the carrier for polysaccharide of Larimichthys crocea swimming bladder. Int. J. Mol. Sci. 2014, 15, 817–829. [Google Scholar] [CrossRef]
- Cornaggia, M.; Leutner, M.; Mescoli, C.; Sturniolo, G.C.; Gullotta, R. Chronic idiopathic inflammatory bowel diseases: The histology report. Dig. Liver Dis. 2011, 43, S293–S303. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Yu, X.; Huang, K.; Zheng, T.; Cheng, X.; Zeng, S.; Liu, X. Hematoxylin and eosin staining of intact tissues via delipidation and ultrasound. Sci. Rep. 2018, 8, 12259. [Google Scholar] [CrossRef]
- Wu, S.; Luo, W.; Wu, X.; Shen, Z.; Wang, X. Functional Phenotypes of Peritoneal Macrophages Upon AMD3100 Treatment during Colitis-Associated Tumorigenesis. Front. Med. 2022, 9, 840704. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, J.Y.; Kim, Y.T.; Kim, S.H.; Lee, J.H. Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes. 2020, 12, 1785803. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Brantley, D.M.; Chen, C.L.; Muraoka, R.S.; Bushdid, P.B.; Bradberry, J.L.; Kittrell, F.; Medina, D.; Matrisian, L.M.; Kerr, L.D.; Yull, F.E. Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol Biol Cell. 2001, 12, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.; Samowitz, W.S.; Wolff, R.K.; Stevens, J.R.; Herrick, J.S. The NF-κB signalling pathway in colorectal cancer: Associations between dysregulated gene and miRNA expression. J. Cancer Res. Clin. Oncol. 2018, 144, 269–283. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning from Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front. Cell Infect. Microbiol. 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Choi, W.; Buckley, A.; Shashikanth, N.; Joseph, N.E.; Wang, Y.; Warren, M.H.; Buschmann, M.M.; Pavlyuk, R.; Hildebrand, J.; et al. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J. Cell Sci. 2017, 130, 243–259. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Clay, S.L.; Fonseca-Pereira, D.; Garrett, W.S. Colorectal cancer: The facts in the case of the microbiota. J. Clin. Investig. 2022, 132, e155101. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. Colorectal cancer: Genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med. Sci. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shen, D.; Kong, C.; Zhang, Z.; Zeng, Y.; Lin, X.; Liu, X. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci. Rep. 2017, 7, 40723. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).