Abstract
The food logistics system is an essential sector for maintaining and monitoring the safety and quality of food products and becoming more crucial, especially during and after the pandemic of COVID-19. Kimchi is a popular traditional fermented food originally from Korea and easily changes because of the storage conditions. This study aims to evaluate the effects and the contributions of temperature to volatile compounds, quality indexes, and the shelf life of Halal-certified Kimchi, and to identify alcohol and find the correlation between the identified variables using an electronic nose and conventional method with the integration of multivariate analysis. Thirty-two volatile compounds (VOCs) were detected and correlated with pH, titratable acidity (TA), and lactic acid bacteria (LAB) counts during storage time. Ethanol was also found in the ripened Kimchi and possibly became the critical point of halal Kimchi products besides total acidity, pH, and LAB. Furthermore, the correlation between pH and benzaldehyde, titratable acidity and 3-methylbutanoic acid, and among lactic acid bacteria with ethanol, acetic acid, ethyl acetate, and 3-methylbutanoic acid properly can be used as a given set of variables in the prediction of food quality during storage and distribution.
1. Introduction
Kimchi is a traditional fermented food, originally from Korea, which can be produced from cabbage (Baechu), green onion, leaf mustard, and radish. It also has unique characteristics of flavor constructed by the combination of the main ingredients and the spices such as chili powder, ginger, green onion, garlic, and salted seafood in the process of making Kimchi that has been applied over a long time [1,2]. Fermentation generally is a biochemical change of organic substances induced by microorganisms or enzymes whether brought by anaerobic or partial anaerobic oxidation [3]. Lactic acid bacteria (LAB), Leuconostoc gelidum, Latilactobacillus sakei, and Weisella koreensis, possibly are major contributors to the quality, safety, and nutrition of the Kimchi during fermentation [4]. This slow decomposition process of organic materials continuously occurs and can highly affect the quality of the final products, whether advantageous or disadvantageous, during distribution and storage [5].
Previous researchers have demonstrated that starter cultures contributed to the production of Kimchi metabolites (organic acids and mannitol), the optimal ripening period, and sensory improvement, and they provide uniform quality for commercial production [6,7]. Other researchers have also investigated and attempted to halt over-acidification using a physicochemical approach by adding the antimicrobial agents and combining them with hurdle effects technology to extend the shelf life of Kimchi [8]. The initial density of microorganisms can be also reduced by 3.29 and 4.78 log cfu/g after implying 10 kGy of gamma irradiation [9]. The combination of high hydrostatic pressure (HHP) treatment and super-cooling storage conditions (−4.5 °C) effectively prolongs the ripening state of leaf mustard Kimchi [5]. Furthermore, the effects of various seasoning ingredients, food additives, and sodium chloride content in the Kimchi have been studied to identify not only the shelf life but also the health effects of the Kimchi diet [10,11,12]. The alteration of the initial temperature of storage to 4 °C for long-term storage (minimum of 31.3 days) with the comparison of one or two days of fermentation at room temperature storage was also conducted [13]. It is believed that temperature control in storage during distribution is the key factor and the easiest practice to control fermentation speed and the formation of early microbes such as Leuconostoc, Weissella, and Lactobacillus.
Aroma-active compounds, such as acetic acid, propionic acid, butanoic acid, 2-methyl propionic acid, hexanal, and ethanol, have been identified specifically as volatile metabolites produced by microorganisms during Kimchi fermentation. These volatile compounds have a positive correlation with lactic acid bacteria growth and can be determined by gas chromatography–mass spectrometry (GC/MS) with the combination of solid-phase micro-extraction (SPME) [14,15,16], vacuum simultaneous steam distillation–solvent extraction/gas chromatography/mass spectrometry (V-SDE/GC/MS) [17], or using GC-MS with an automated purge and trap sampler [18]. An electronic nose based on a fast gas chromatography-flame ionized detector (FGC-FID) system has also the potential to assess food quality (freshness) in the supply chain. The benefit of this method is not only fast measurement but also a small requirement of sample volume [19,20]. Rapid and non-destructive measurements such as Vis/NIR hyperspectral imaging, Fourier-transform infrared spectroscopy (FTIR), and hyperspectral imaging, nowadays, are also becoming more popular especially in the food sector, as they are not only fast in detection but also effective in determining the quality of food products [21,22,23,24]. Furthermore, an electronic nose has been proven and practically can be integrated with various non-destructive techniques for monitoring the food quality and halal status in food products [25,26].
This study aims to evaluate the effects and the contributions of storage conditions, especially storage at room temperature, volatile compounds, quality indexes, and the shelf life of halal-certified Kimchi over storage. Next, it aims to identify alcohol production qualitatively during fermentation in the storage room using an electronic nose and find its correlation with conventional methods of food quality analysis.
2. Materials and Methods
2.1. Preparation of Samples and Materials
Halal-certified Kimchi, 500 g weight each product, was obtained from a Kimchi manufacturer, and the experiment was conducted immediately after receiving samples at the Korea Food Research Institute (KFRI), Wanju-gun, Republic of Korea. The samples were stored in four storage rooms with different temperature conditions 0 °C, 5 °C, 10 °C, and 20 °C. The sampling procedure was managed variously based on temperature treatment, at 0 °C and 5 °C, and the interval of sampling was 3 days. On the other hand, sampling at 10 °C and 20 °C was performed every day from day 0 until day 6, with a 2-days interval of sampling from day 6 until day 14. The samples were determined by the value of quality indexes (pH, titratable acidity, and lactic acid bacteria count), temperature records, and volatile compounds.
The temperature of the internal product was recorded using a Thermo recorder (TR-5i Series, T&D Corp., Matsumoto, Japan) with two repetitions in each storage room [27]. At the end of the experiment, all data were collected using optical communication port TR-50U2 and displayed through T&D Graph software for data management and visualization.
2.2. Analysis of Halal-Certified Kimchi Quality Indexes
2.2.1. pH and Titratable Acidity (TA)
Samples of Kimchi juice (20 mL), which was previously blended using a slow juicer (H-100-SBFA01, Hurom Corp., Seoul, Korea), were placed in a 50 mL beaker glass, and the pH was measured using a digital pH meter (TA-70, DKK-TOA Corp., Tokyo, Japan) [28,29].
The titratable acidity was titrated to 20 mL of Kimchi juice by adding 0.1 N NaOH until it reached pH 8.2. The consumed volume of NaOH was calculated and converted as lactic acid content in a percentage (%). The titratable acidity was calculated according to the next Equation (1) [30]:
TA (%) = [Volume NaOH (mL) × 90.08 (g) × 0.1 N × 100]/[20 mL × 1000]
2.2.2. Lactic Acid Bacteria Count (LAB)
Lactic acid bacteria were counted by the 3M Petrifilm lactic acid bacteria count plate method 6461/6492. The evaluation of Petrifilm is a reliable LAB enumeration method compared with the traditional methodology [31,32]. In sterile conditions, portions of Kimchi (10 g) were obtained from each sample and diluted 10-fold with 90 mL of 0.85% saline solution in a sterile filter bag. After homogenization for 1 min with four strokes/s using a stomacher (BagMixer 400 CC, Interscience Intl., Saint-Nom-la-Bretèche, France), 1 mL of solution was transferred and diluted stepwise with 9 mL of 0.85% saline solution. Afterward, the sample (1 mL) was plated in a 3MTM PetrifilmTM lactic acid bacteria count plate (PLAB) and incubated at 35 °C for 48 h. The counted colonies of the Kimchi sample were expressed as log cfu/g [28].
2.3. Analysis of Volatile Compounds
The volatile compound in the sample product was analyzed using GC-FID Heracles II (Alpha M.O.S., Toulouse, France). The instrument consisted of an auto sampling system (HS100 autosampler), two polarity columns, MXT-5 and MXT-1701, and two flame ionization detectors. Five milliliters of Kimchi juice were prepared in a vial and injected into the e-nose in six repeats of the sample at 0 °C, 5 °C, 10 °C, and 20 °C. Before analysis, a method was created with the following parameters: injection volume of 1000 μL with a speed of 125 μL/s at 200 °C of injector temperature, incubation at 40 °C for 20 min with agitation at 500 rpm, and 90 s flushing time between injections. This method was followed by Hydrogen carrier gas flow at 30 mL/min, trapping temperature at 40 °C, initial oven at 50 °C, the endpoint of oven temperature at 250 °C, the heating rate at 1–3 °C/s, an acquisition duration of 110 s, and an acquisition period of 0.01 s. For data calculation, Kovats indices were used to determine the retention time of C6–C16, and the processed-data acquisition was conducted through Alpha-Soft software. Alpha-Soft (V14.2, Alpha M.O.S, Toulouse, France) software is a data acquisition and processing system used for instrument control and raw data processing. The chromatographic results are treated and recorded as input data for chemometric analysis. Sensors (peak areas) with the highest performance power were selected using the function of the Alpha-Soft software. The Heracles e-nose was equipped additionally with the AroChembase (Alpha MOS, Toulouse, France) library which was used for confirming the chemical compound identification and allowed to pre-screen the compounds and sensory attributes from the chromatograms [20].
2.4. Bivariate and Multivariate Statistical Analysis
The analysis of variance (ANOVA) was conducted by SPSS statistics 24.0 software (SPSS Inc., Chicago, IL, USA) with Tukey-HSD as a post hoc test at the significance level of 0.05. Unsupervised multivariate chemometric analysis such as principal component (PC) was also performed using R programming version 4.2.0 and RStudio Desktop 2022.02.2 + 485 to calculate and analyze the contribution of temperature, storage period, and other variables of each sample. PCA, or principal component analysis, is a multivariate approach to find new variables that are linear functions of those in the original dataset that successively maximize variance, and that are uncorrelated with each other. PCA is applied to transform a high-dimensional dataset into a lower-dimensional dataset by using the first few principal components [33,34]. Besides, k-means clustering was performed to partition the observations with the nearest mean of volatile compounds and quality indexes of halal-certified Kimchi over storage time. K-means clustering is a distance-based clustering algorithm that aims to minimize the cluster performance index, square-error, error criterion, distances between the points, and their respective cluster centroid [35,36]. The packages used in R were as follows: FactoMineR 2.4 [37], factoextra 1.0.7 [38], ggplot2 3.3.5 [39], and ComplexHeatmap 2.10.0 [40].
3. Results and Discussion
3.1. Analysis of Halal-Certified Kimchi Quality Indexes
3.1.1. pH and Titratable Acidity (TA)
Changes in pH during the fermentation of Kimchi indicate the degradation of carbohydrates as the result of microbial activities in the formation of organic acids and other chemical compounds [41,42]. The changes in the pH value of halal-certified Kimchi at different temperatures in the storage room are illustrated in Figure 1a. From the results, the pH value of the halal Kimchi at 0 days or in the fresh condition was 5.96 ± 0.01. The Kimchi stored at 20 °C significantly reduced to a pH of 4.11 ± 0.10 after 2 days, and 6 days of the Kimchi stored at 10 °C reached the pH of 4.13 ± 0.02. On the other hand, the Kimchi stored at 5 °C was relatively stable until 6 days of storage from 5.96–5.55 and was followed by a rapid decrease to 4.50 ± 0.04 in the next 3 days. The Kimchi stored at 0 °C exhibited longer stability conditions of pH compared to the other treatments with a range of 5.96–5.88, with an extreme decline between 15 and 18 days of storage. In the following days, the pH values gradually decreased until 30 days and reached the final value of 4.3 ± 0.02 at 30 days. Another finding showed that the optimal flavor of Kimchi is in the range of 4.0–4.5 [43]. Thus, at 0 °C, the shelf life of the Kimchi was significantly longer (p < 0.05), and the ripening stage can be halted in the long-term storage period.
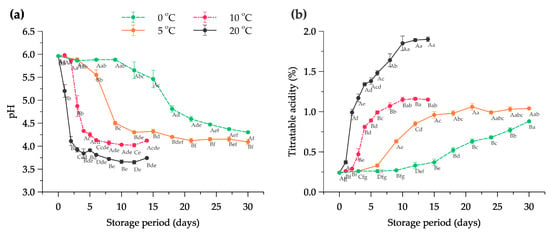
Figure 1.
Changes in (a) pH value and (b) titratable acidity as lactic acid of halal-certified Kimchi stored at different temperature conditions. Note: The data are expressed as mean ± SD with n = 3; Superscripts with different letters (A–D) considering on the same day and (a–g) considering at the same temperature condition represent significant differences at the p < 0.05.
Titratable acidity (TA) represents the total acidity of lactic acid generated from microorganisms in the Kimchi during fermentation. The percentages of TA in halal-certified Kimchi at different temperatures are presented in Figure 1b. The initial TA of fresh Kimchi (at day 0) showed 0.24 ± 0.01%. After 12 days, the TA of the Kimchi stored at 0 °C gradually increased and reached the maximum value of 0.88 ± 0.00% on day 30. The Kimchi acidity stored at 5 °C did not considerably change after 12 days of storage time with a range of 0.96–1.06%. In contrast, the rapid increase of TA occurred at the beginning of the Kimchi storage at 20 °C from day 1 to 2 with 0.99 ± 0.04%, whereas Kimchi stored at 10 °C increased sharply on day 3 from 0.47% to 0.81% of total acidity as lactic acid. The optimal fermentation in Kimchi occurs at a pH of 4.2 and 0.6% of total acidity [2]. Therefore, the ripening process of halal-certified Kimchi stored at 0 °C possibly can be delayed for a long time, and the results depicted significant differences compared to other treatments (p < 0.05). Kimchi at low temperatures (−1 °C) can be kept for 2 to 30 weeks and the acidity is maintained at 0.47–0.50% [44].
3.1.2. Lactic Acid Bacteria (LAB) and Temperature Records during Distribution
The lactic acid bacteria growth at four different temperatures and the history of the Kimchi’s internal temperature are presented in Figure 2. The initial LAB count in the Kimchi was 5.01 ± 0.24 log cfu/g; this result is relatively higher than the finding which exhibited 4.58 log cfu/g of LAB at the beginning of fermentation [45]. The gap number of the microbial count in the Kimchi can be caused by the initial temperature of halal-certified Kimchi in the distribution (8.46 ± 0.84 °C) shown in Figure 2a–d. Different temperatures and packaging conditions (open or closed) could alter the diversity of microorganisms in the Kimchi [46]. In addition, the Kimchi types and the composition of vegetables and seasoning possibly affected the microbial community in determining product qualities during the early phase of fermentation [47].
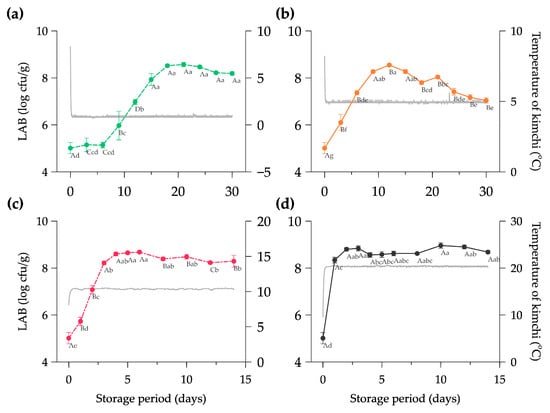
Figure 2.
Growth of lactic acid bacteria in halal-certified Kimchi at (a) 0 °C, (b) 5 °C, (c) 10 °C, and (d) 20 °C during storage period. Note: Data are expressed as mean ± SD from three repetitions; The mean values highlighted with different capital letters (A–D) considering the same storage time and different lowercase letters (a–g) considering the same temperature treatment are significantly different at the p < 0.05; the gray line indicates the evolution of the internal temperature of Kimchi during storage.
The LAB count of Kimchi stored at 20 °C drastically increased to 8.34 ± 0.12 log cfu/g in less than 24 h (Figure 2d) and was significantly different compared to other storage rooms (p < 0.05) on the first day. The Kimchi stored at 10 °C reached the maximum number of LAB of 8.68 ± 0.05 log cfu/g on day 6, and at 5 °C it was 8.55 log cfu/g on day 12 (Figure 2b,c). In contrast, the Kimchi at 0 °C showed a relatively stable number of LAB (5.01–5.15 log cfu/g) until 6 days of storage and continuously grew to 8.57 ± 0.09 log cfu/g on day 18. The external temperature could likely affect the maximum number of LAB counts in the Spring, Autumn, and Winter seasons with the range of 8.30–8.85 log cfu/g [48].
3.2. Analysis of Volatile Compounds
All the data of volatile compounds (VOCs) produced during fermentation and their odor descriptors individually are summarized in Table 1. Different from conventional methods, the response should be pre-treated using chemometric analysis with the additional module software from the instrument in order to find the qualitative and quantitative data output and reveal the useful information such as chemical compound, peak area, functional group, and odor descriptor [49]. The changes in the e-nose sensor (chromatogram peak) of the Kimchi stored at 20 °C are provided in Figure 3. It can be seen that during fermentation, the peak of the volatile compound changed gradually from day 0 until day 14. Six major compounds, ethyl isobutyrate, 2,3-dimethyl pyrazine, nonan-2-one, ethanol, ethyl 3-(methylthio)propanoate, and acetaldehyde, were detected in the early fermentation at all temperatures. Each compound represented the sweet, nutty, fresh, alcoholic, sulfury, and pungent nuance of Kimchi, respectively. Changes in the VOCs of the Kimchi varied depending on the functional group of chemical compounds as shown in Figure 4. There were nine groups of VOCs including acids, alcohols, aldehydes, alkenes, benzenes, esters, ketones, pyrazines, and sulfides. Alcohol, esters, and acids in the Kimchi stored at 20 °C rapidly increased compared to the Kimchi at 0 °C, 5 °C, and 10 °C with esters as predominant compounds and followed by alcohols. However, extreme decreases occurred in aldehydes, alkenes, ketones, pyrazines, and sulfides at 20 °C on the first day of storage. Five groups of compounds could be identified in the Kimchi such as alcohol, aldehyde, ketone, ester, and nitrile, of which alcohols were the predominant compounds in the optimum conditions of the fermented Kimchi [50]. Furthermore, thirty-two types of volatile compounds were identified in the fresh and fermented Kimchi during distribution (Table 2). Significant changes, that frankly could be found in Kimchi, were the production of ethyl acetate and butanoic acid at 20 °C (p < 0.05). The latter compounds are defined as ethereal, fruity, sweet, acetic, and buttery odors after 14 days of fermentation. Other compounds were also identified at 20 °C in high intensity such as ethanol, butanal, acetic acid, 3-methylbutanoic acid, and 2-heptanone which could give a stronger alcoholic, pungent, sour, stinky, and spicy aroma than the Kimchi at 0 °C, 5 °C, and 10 °C.

Table 1.
Volatile compound composition and odor descriptor of fresh Kimchi.
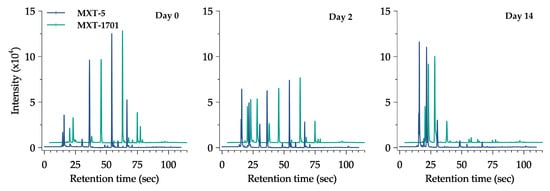
Figure 3.
Response change of e-nose chromatogram of halal-Certified Kimchi stored at 20 °C and performed on three different days.
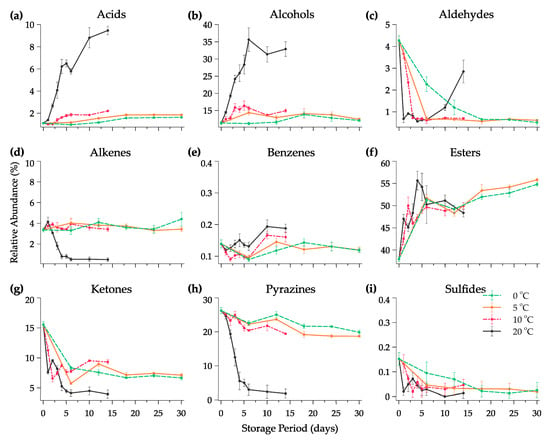
Figure 4.
Changes in the relative abundance of volatile compounds (a–i) as functional groups in halal Kimchi under different temperature conditions over a storage period. Note: Data are expressed as mean ± standard deviations; n = 6.

Table 2.
Volatile compound composition and odor descriptor of fresh Kimchi.
3.3. Multivariate Analysis
3.3.1. Relationship of Volatile Compounds and Quality Indexes by Cluster Analysis
Clustering is an unsupervised algorithm which calculates the similarity or proximity based on the distance of measurement [51]. Cluster analysis was used to normalize the concentration of volatile compounds by squared Euclidean and applied the standard k-means to a dataset of identified VOCs in the Kimchi. Data mining and clustering related to chemometric databases are common in chemical processing data [52,53]. In the same way, hierarchical cluster analysis can be used to find the correlation between volatile compounds and sensory attributes of traditional sweets [54]. Figure 5 depicts the matrix of the normalized enrichment distance score of 32-volatile compounds and three quality indexes as variables in the function of the correlation between temperature (°C) and storage period (day) by k-means clustering. The variables, measured by e-nose and conventional methods, were divided into two clusters which have different patterns of shifting between increasing and decreasing distances during the fermentation of Kimchi at different temperatures. In general, the Parameter of pH was in the group of negative responses (red dendrogram) during storage together with benzaldehyde, acetaldehyde, and dimethyl trisulfide. By contrast, the titratable acidity and lactic acid bacteria counts were in the positive response group (blue dendrogram) together with 3-methylbutanoic acid. Interestingly, the ethanol content also had a close relationship with the increase in acetic acid, ethyl acetate, 3-methylbutanoic acid, and total acidity and has a negative relationship with acetaldehyde. The increase in ethanol was caused by the conversion of acetaldehyde metabolized by microorganisms [55]. Furthermore, based on the dendrogram tree, the relationship between pH and benzaldehyde, titratable acidity and 3-methylbutanoic acid, and among lactic acid bacteria with ethanol, acetic acid, ethyl acetate, and 3-methylbutanoic acid can be used in the future as a given set of variables to predict a target variable in determining the food quality during storage and distribution using supervised multivariate analysis.
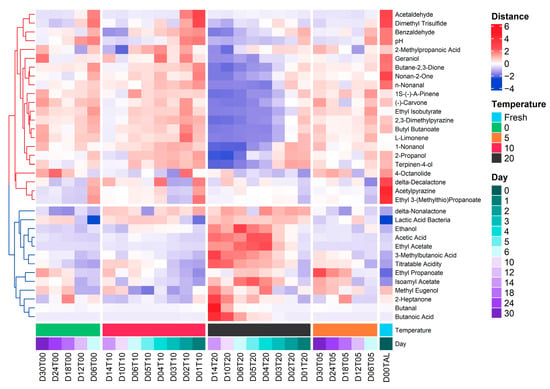
Figure 5.
Heatmap representing the volatile compounds (VOCs) composition and quality indexes (pH, TA, and LAB) among different storage conditions (temperature (°C) and time (day)) of halal-certified Kimchi products.
3.3.2. Correlation between Volatiles Compounds and Storage Conditions
Principal component analysis (PCA) is an unsupervised multivariate analysis approach to visualize the correlation between variables in order to find the differences and similarities among data points in the graph [56]. Loading plots and Principal Components (PC)-Biplots of VOCs with PC1 50.8% and PC2 12.7%, and volatile compounds as functional groups with PC1 52.4% and PC2 18.5% are presented in Figure 6. It can be seen in Figure 6A that 2-heptanone, butanal, delta-decalactone, delta-nonalactone, methyl eugenol, geraniol, 2-methylpropanoic acid, and 4-octanolide had a low contribution to the ripening stage of halal Kimchi. On the other hand, the rest of the twenty-four compounds had moderate to high contribution to the fermentation. In addition, Figure 6B shows the relationship of storage temperatures to the identified VOCs during storage in which the fresh Kimchi had a strong concentration in Ethyl 3-(methylthio)propanoate, acetylpyrazine, dimethyl trisulfides, and acetaldehyde (or Aldehyleds and Sulfides in Figure 6D). The halal-certified Kimchi stored at 20 °C produced VOCs separately compared to the Kimchi stored at 0 °C, 5 °C, and 10 °C, which gave a strong ethereal, sour, acetic, sweet, alcohol aroma as represented by ethyl acetate, acetic acid, butanoic acid, 3-methylbutanoic acid, isoamyl acetate, and ethanol (Acids and Alcohols in Figure 6C). In contrast, in the last observation, the Kimchi stored at 0 °C, 5 °C, and 10 °C identically had similar aromas such as sweet, butter, aldehydic, pepper, green, nutty, herbal, peppery, pine, ethereal, floral, slightly bitter, and musty odors produced by nonan-2-one, butane-2,3-dione, n-nonanal, terpinen-4-ol, butyl butanoate, 2,3-dimethylpyrazine, (-)-carvone, L-limonene, 1S-(-)-a-pinen, ethyl isobutyrate, 1-nonanol, benzaldehyde, and 2-propanol, respectively. Allyl mercaptan, methyl allyl sulfide, diallyl sulfide, and ethanol increased in line with the increase in temperature during the ripening process [57]. Thus, the determination of the volatile compound (ethanol) is the important key in halal logistics and distribution, especially in the storage stage, not only to control the quality of Kimchi but also to maintain the validity of halal status in the registered product.
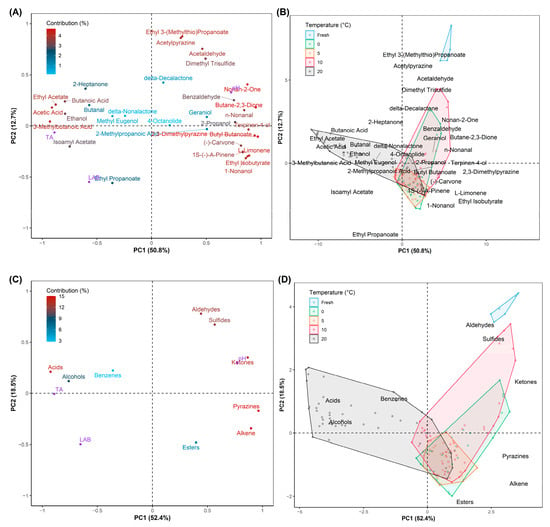
Figure 6.
Relevant loadings of (A) volatile compounds and (C) functional groups of VOCs with additional information of quality indexes as predicted contributors (purple color) on principal component analysis (PCA); PCA-biplot of (B) volatile compounds and (D) functional groups of VOCs of halal-certified Kimchi over storage time.
4. Conclusions
Thirty-two volatile compounds, acids and alcohols as the predominant groups, contributed to the qualities and odor of the Kimchi. The changes in temperature conditions also had enormous impacts on the Kimchi’s qualities. The Kimchi stored at 20 °C fast fermented in two days and produced alcohols qualitatively higher than the Kimchi under other storage conditions. Furthermore, a fast and reliable instrument, an electronic nose, could be applied and integrated with conventional methods and multivariate analysis to evaluate the influences of storage conditions and control the qualities (ethanol) of halal-certified Kimchi during distribution. Besides, the relationship between the variables (conventional method and e-nose responses) such as pH with benzaldehyde, titratable acidity with 3-methylbutanoic acid, and among lactic acid bacteria with ethanol, acetic acid, ethyl acetate, and 3-methylbutanoic acid appropriately can be used as a known set of variables to predict a target variable in determining the food quality during storage and distribution.
Author Contributions
Conceptualization, B.-S.K. and J.-Y.K.; methodology, A.J.L. and J.-Y.K.; software, A.J.L.; validation, A.J.L., J.-Y.K. and B.-S.K.; formal analysis, A.J.L.; investigation, Y.-M.C.; writing—original draft preparation, A.J.L.; writing—review and editing, J.-Y.K.; visualization, A.J.L.; supervision, B.-S.K. and J.-Y.K.; project administration, B.-S.K. and J.-Y.K.; funding acquisition, B.-S.K. and J.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The Development of the Halal logistics system based on the IoT project (Project No. 20009244, Korea Evaluation Institute of Industrial Technology) and The Main Research Program (Project No. E0210902-02) of Korea Food Research Institute funded by the Ministry of Science and ICT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented within the article are available at request from corresponding author.
Conflicts of Interest
The corresponding author declares that all authors have no conflicts of interest in this article.
References
- Song, H.S.; Lee, S.H.; Ahn, S.W.; Kim, J.Y.; Rhee, J.-K.; Roh, S.W. Effects of the Main Ingredients of the Fermented Food, Kimchi, on Bacterial Composition and Metabolite Profile. Food Res. Int. 2021, 149, 110668. [Google Scholar] [CrossRef] [PubMed]
- You, S.-Y.; Yang, J.-S.; Kim, S.H.; Hwang, I.M. Changes in the Physicochemical Quality Characteristics of Cabbage Kimchi with Respect to Storage Conditions. J. Food Qual. 2017, 2017, 9562981. [Google Scholar] [CrossRef]
- Battcock, M.; Azam-Ali, S. Fermented Fruits and Vegetables: A Global Perspective; FAO Agricultural Services Bulletin; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Jung, M.-J.; Kim, J.; Lee, S.H.; Whon, T.W.; Sung, H.; Bae, J.-W.; Choi, Y.-E.; Roh, S.W. Role of Combinated Lactic Acid Bacteria in Bacterial, Viral, and Metabolite Dynamics during Fermentation of Vegetable Food, Kimchi. Food Res. Int. 2022, 157, 111261. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, E.J.; Chang, J.Y.; Song, K.B.; Chun, H.H. Effect of High Hydrostatic Pressure (HHP) and Supercooling Storage in Leaf Mustard (Brassica Juncea L.) Kimchi: Modelling of Microbial Activity and Preservation of Physicochemical Properties. LWT 2021, 145, 111325. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.-Y.; Park, W.-S.; Jeon, C.O. Effects of Leuconostoc Mesenteroides Starter Cultures on Microbial Communities and Metabolites during Kimchi Fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-E.; Jang, J.-Y.; Lee, J.-H.; Park, H.-W.; Choi, H.-J.; Kim, T.-W. Starter Cultures for Kimchi Fermentation. J. Microbiol. Biotechnol. 2015, 25, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bang, J.; Beuchat, L.R.; Kim, H.; Ryu, J.-H. Controlled Fermentation of Kimchi Using Naturally Occurring Antimicrobial Agents. Food Microbiol. 2012, 32, 20–31. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Yang, J.E.; Park, J.H.; Ko, S.H.; Choi, I.S.; Kim, H.M.; Chun, H.H.; Kwon, M.-J.; Park, H.W. Gamma Irradiation Improves the Microbiological Safety and Shelf-Life of Kimchi Seasoning Mixture. LWT 2020, 134, 110144. [Google Scholar] [CrossRef]
- Choi, S.J.; Yang, S.Y.; Yoon, K.S. Lactic Acid Bacteria Starter in Combination with Sodium Chloride Controls Pathogenic Escherichia coli (EPEC, ETEC, and EHEC) in Kimchi. Food Microbiol. 2021, 100, 103868. [Google Scholar] [CrossRef]
- Jin, Y.H.; Lee, J.; Pawluk, A.M.; Mah, J.-H. Inhibitory Effects of Nicotinic Acid, Glycine, and Other Food Additives on Biogenic Amine Formation in Baechu Kimchi Fermentation. LWT 2022, 155, 112921. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Dang, Y.-M.; Ha, J.-H. Effect of Various Seasoning Ingredients on the Accumulation of Biogenic Amines in Kimchi during Fermentation. Food Chem. 2022, 380, 132214. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Seo, S.-H.; Park, S.-E.; Lim, Y.-W.; Roh, S.W.; Son, H.-S. Initial Storage of Kimchi at Room Temperature Alters Its Microbial and Metabolite Profiles. LWT 2020, 134, 110160. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.-R.; Park, S.-H.; Lee, M.-A. Changes in Volatile and Non-Volatile Compounds of Model Kimchi through Fermentation by Lactic Acid Bacteria. LWT 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Shim, S.-M.; Kim, J.Y.; Lee, S.M.; Park, J.-B.; Oh, S.-K.; Kim, Y.-S. Profiling of Fermentative Metabolites in Kimchi: Volatile and Non-Volatile Organic Acids. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 463–469. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Lee, J.J.; Lee, H.J.; Choi, Y.-J.; Lee, J.-H.; Park, S.J.; Park, S.H.; Seo, H.-Y.; Min, S.G. Comparison of Quality Characteristics of Commercial Kimchi Manufactured in Korea, China, and the United States. Foods 2021, 10, 2488. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, H.; Cadwallader, K.R. Aroma-Active Compounds in Kimchi during Fermentation. J. Agric. Food Chem. 1998, 46, 1944–1953. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, M.-A.; Lee, K.-G. Determination of Compositional Quality and Volatile Flavor Characteristics of Radish-Based Kimchi Suitable for Chinese Consumers and Its Correlation to Consumer Acceptability. Food Sci. Biotechnol. 2018, 27, 1265–1273. [Google Scholar] [CrossRef]
- Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Guzek, D.; Sun, D.-W.; Wierzbicka, A. Differentiation of Chill-Stored and Frozen Pork Necks Using Electronic Nose with Ultra-Fast Gas Chromatography. J. Food Process Eng. 2017, 40, e12540. [Google Scholar] [CrossRef]
- Yimenu, S.M.; Kim, J.Y.; Kim, B.S. Prediction of Egg Freshness during Storage Using Electronic Nose. Poult. Sci. 2017, 96, 3733–3746. [Google Scholar] [CrossRef]
- Chu, X.; Li, R.; Wei, H.; Liu, H.; Mu, Y.; Jiang, H.; Ma, Z. Determination of Total Flavonoid and Polysaccharide Content in Anoectochilus formosanus in Response to Different Light Qualities Using Hyperspectral Imaging. Infrared Phys. Technol. 2022, 122, 104098. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, X.; Ru, Y.; Chen, Q.; Li, X.; Xu, L.; Zhou, H.; Shi, M. Rapid and Non-Destructive Detection of Natural Mildew Degree of Postharvest Camellia oleifera Fruit Based on Hyperspectral Imaging. Infrared Phys. Technol. 2022, 123, 104169. [Google Scholar] [CrossRef]
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and Quality Assessment of Meat Products by Fourier-Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2021, 13, 66–91. [Google Scholar] [CrossRef]
- El-Mesery, H.; Mao, H.; Abomohra, A. Applications of Non-Destructive Technologies for Agricultural and Food Products Quality Inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, J.; Chen, Q.; Zhang, Y. Nondestructive Measurement of Total Volatile Basic Nitrogen (TVB-N) in Pork Meat by Integrating near Infrared Spectroscopy, Computer Vision and Electronic Nose Techniques. Food Chem. 2014, 145, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Nurjuliana, M.; Che Man, Y.B.; Mat Hashim, D.; Mohamed, A.K.S. Rapid Identification of Pork for Halal Authentication Using the Electronic Nose and Gas Chromatography Mass Spectrometer with Headspace Analyzer. Meat Sci. 2011, 88, 638–644. [Google Scholar] [CrossRef]
- Kim, B.-S.; Lee, M.; Kim, J.-Y.; Jung, J.-Y.; Koo, J. Development of a Freshness-Assessment Model for a Real-Time Online Monitoring System of Packaged Commercial Milk in Distribution. LWT-Food Sci. Technol. 2016, 68, 532–540. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, B.-S.; Kim, J.-H.; Oh, S.-I.; Koo, J. Development of Dynamic Model for Real-Time Monitoring of Ripening Changes of Kimchi during Distribution. Foods 2020, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Yimenu, S.M.; Kim, J.Y.; Koo, J.; Kim, B.S. Predictive Modeling for Monitoring Egg Freshness during Variable Temperature Storage Conditions. Poult. Sci. 2017, 96, 2811–2819. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Lee, M.-K.; Choi, K.-S.; Koo, Y.-J.; Park, W.-S. Changes of Fermentation Characteristics and Sensory Evaluation of Kimchi on Different Storage Temperature. Korean J. Food Sci. Technol. 1998, 30, 644–649. [Google Scholar]
- Nero, L.A.; de FREITAS, C.F.; Flores Carvalho, L.M.V.; Constantino, C. 3M Petrifilm Lactic Acid Bacteria Count Plate Is a Reliable Tool for Enumerating Lactic Acid Bacteria in Bacon. J. Food Prot. 2020, 83, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.M.J.; Costa, A.C.; Nero, L.A.; Couto, E.P.; Ferreira, M.d.A. Evaluation of Petrifilm™ System Compared with Traditional Methodology in Count of Indicators of Sanitary-Hygienic Quality and Pathogenic Microorganisms in Sheep Milk. Food Sci. Technol. Camp. 2015, 35, 375–379. [Google Scholar] [CrossRef][Green Version]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Mahmoudi, M.R.; Heydari, M.H.; Qasem, S.N.; Mosavi, A.; Band, S.S. Principal Component Analysis to Study the Relations between the Spread Rates of COVID-19 in High Risks Countries. Alex. Eng. J. 2021, 60, 457–464. [Google Scholar] [CrossRef]
- Ahmed, M.; Seraj, R.; Islam, S.M.S. The K-Means Algorithm: A Comprehensive Survey and Performance Evaluation. Electronics 2020, 9, 1295. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H. A Clustering Method Based on K-Means Algorithm. Phys. Procedia 2012, 25, 1104–1109. [Google Scholar] [CrossRef]
- FactoMineR: An R Package for Multivariate Analysis, Journal of Statistical Software. Available online: https://www.jstatsoft.org/article/view/v025i01 (accessed on 13 June 2022).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 13 June 2022).
- Wickham, H. Ggplot2; Use R! Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Kim, J.; Moon, B. Effect of Main Vegetable Ingredient on the Glucosinolate, Carotenoids, Capsaicinoids, Chlorophylls, and Ascorbic Acid Content of Kimchis. J. Food Compos. Anal. 2022, 110, 104523. [Google Scholar] [CrossRef]
- Maoloni, A.; Ferrocino, I.; Milanović, V.; Cocolin, L.; Corvaglia, M.R.; Ottaviani, D.; Bartolini, C.; Talevi, G.; Belleggia, L.; Cardinali, F.; et al. The Microbial Diversity of Non-Korean Kimchi as Revealed by Viable Counting and Metataxonomic Sequencing. Foods 2020, 9, 1568. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Lee, J.-E.; Lee, C.-H. Importance of Lactic Acid Bacteria in Asian Fermented Foods. Microb. Cell Factories 2011, 10, S5. [Google Scholar] [CrossRef]
- Yoo, M.-J.; Kim, H.-R.; Chung, H.-J. Changes in Physicochemical and Microbiological Properties in Low-Temperature and Long-Term Fermented Kimchi during Fermentation. J. Korean Soc. Food Cult. 2001, 16, 431–441. [Google Scholar]
- Lee, J.-J.; Choi, Y.-J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.-R.; Min, S.G.; Seo, H.-Y.; Park, S.-H.; Lee, M.-A. Effects of Combining Two Lactic Acid Bacteria as a Starter Culture on Model Kimchi Fermentation. Food Res. Int. 2020, 136, 109591. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Lee, H.-W.; Kim, J.Y.; Kang, S.E.; Roh, S.W.; Hong, S.W.; Yoo, S.R.; Kim, T.-W. Impact of Fermentation Conditions on the Diversity of White Colony-Forming Yeast and Analysis of Metabolite Changes by White Colony-Forming Yeast in Kimchi. Food Res. Int. 2020, 136, 109315. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Song, J.H.; Park, J.M.; Chang, J.Y. Bacterial Diversity in Korean Temple Kimchi Fermentation. Food Res. Int. 2019, 126, 108592. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Song, J.H.; Lee, S.H.; Jung, M.Y.; Chang, J.Y. Effect of Seasonal Production on Bacterial Communities in Korean Industrial Kimchi Fermentation. Food Control 2018, 91, 381–389. [Google Scholar] [CrossRef]
- Singh, I.; Juneja, P.; Kaur, B.; Kumar, P. Pharmaceutical Applications of Chemometric Techniques. ISRN Anal. Chem. 2013, 2013, 795178. [Google Scholar] [CrossRef]
- Hong, S.P.; Lee, E.J.; Kim, Y.H.; Ahn, D.U. Effect of Fermentation Temperature on the Volatile Composition of Kimchi: Volatiles of Kimchi. J. Food Sci. 2016, 81, C2623–C2629. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.G.; Kang, M.S.; Heo, J. Clustering Performance Comparison Using K -Means and Expectation Maximization Algorithms. Biotechnol. Biotechnol. Equip. 2014, 28, S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Noviandy, T.R.; Maulana, A.; Sasmita, N.R.; Suhendra, R.; Muslem; Idroes, G.M.; Paristiowati, M.; Helwani, Z.; Yandri, E.; Rahimah, S.; et al. The Implementation of K-Means Clustering in Kovats Retention Index on Gas Chromatography. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1087, 012051. [Google Scholar] [CrossRef]
- Thomas, M.C.; Zhu, W.; Romagnoli, J.A. Data Mining and Clustering in Chemical Process Databases for Monitoring and Knowledge Discovery. J. Process Control 2018, 67, 160–175. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Zhan, P.; Liu, P.; Tian, H.; Fan, J. Correlation of Volatile Compounds and Sensory Attributes of Chinese Traditional Sweet Fermented Flour Pastes Using Hierarchical Cluster Analysis and Partial Least Squares-Discriminant Analysis. J. Chem. 2017, 2017, 3213492. [Google Scholar] [CrossRef]
- Ryu, J.-Y.; Lee, H.-S.; Rhee, H.-S. Changes of Organic Acids and Volatile Flavor Compounds in Kimchis Fermented with Different Ingredients. Korean J. Food Sci. Technol. 1984, 16, 169–174. [Google Scholar]
- Yoon, S.-R.; Kim, S.H.; Lee, H.-W.; Ha, J.-H. A Novel Method to Rapidly Distinguish the Geographical Origin of Traditional Fermented-Salted Vegetables by Mass Fingerprinting. PLoS ONE 2017, 12, e0188217. [Google Scholar] [CrossRef]
- Ko, Y.-T.; Kang, J.-H. Changes of Volatile Odor Components in Kimchi by Freeze-drying. Korean J. Food Sci. Technol. 2002, 34, 559–564. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).