1. Introduction
Electrodialysis (ED) is a unit operation for the separation or concentration of ions in solutions based on their selective electromigration through semi-permeable cationic (C) and anionic (A) membranes under the influence of an electric field [
1]. In addition to homopolar membranes (C and A membranes), electrodialysis stacks can also host bipolar membranes (B), which are membranes dissociating water molecules in protons (on the cationic side of the B) and hydroxyls (on the anionic side of the B) under the effect of an electric field. The use of ED is increasing in the agri-food industry thanks to the advantages associated with its low energy consumption, efficiency, ease of use and low thermal damage to the product. In the fruit juice industry ED can be applied for deacidification, desalting, and enzyme inhibition [
1]. Conventional juice deacidification methods involve the addition of additives such as sugar, CaCO
3 or the use of ion exchange resins. However, these methods can affect the sensory and functional properties of beverages and cause health problems [
2]. ED was studied in the deacidification of juices from several fruits such as cranberry [
2,
3,
4], mandarin orange [
5], passion fruit [
6,
7,
8,
9,
10], tropical fruits (passion fruit, naranjilla, araza, mulberry) [
7,
11,
12], and pineapple [
13]. Deacidification using ED was achieved by applying several process configurations with two or three compartments using a combination of cationic, anionic, bipolar and ultrafiltration membranes. The aim of the process is the removal of the acid (citric, malic, quinic, etc.) from the juice. This is accomplished by selectively removing the acid anion and the H
+ cation from the juice or by removing the anion and substituting it with OH
− either generated from a bipolar membrane or donated from a KOH or NaOH solution circulating in a compartment adjacent to the juice. Basically, four ED membrane stack configurations were studied for the deacidification of fruit juices: two-compartment conventional homopolar membrane electrodialysis using alternating cationic and anionic membranes (ED2AC) or just anionic membranes (ED2AA); three-compartment monopolar electrodialysis with repeating cells made up of two anionic membranes delimitated by two cationic ones (ED3CAAC); two-compartment homopolar/bipolar electrodialysis using alternating anionic and bipolar membranes (ED2AB). Substitution of the anionic membrane with an ultrafiltration membrane (U) in the different configurations was also studied.
A critical role in all the systems employing electrodialysis is represented by the anion exchange membrane, which facilitates the removal of organic anions present in fruit juices, such as citrate, malate, and tartrate anions.
In this work, we resorted to the theory of ampholyte transport through electrodialysis membranes to elucidate the behavior of anion exchange membranes in fruit juice deacidification. To this end, we considered the ED2AC configuration and used citric acid and pineapple juice as a model system. The new understanding of the behavior of the anionic membrane allowed us to explain why the ED2AC configuration leads to deacidification of fruit juices without pH increase.
2. Materials and Methods
2.1. Raw Materials
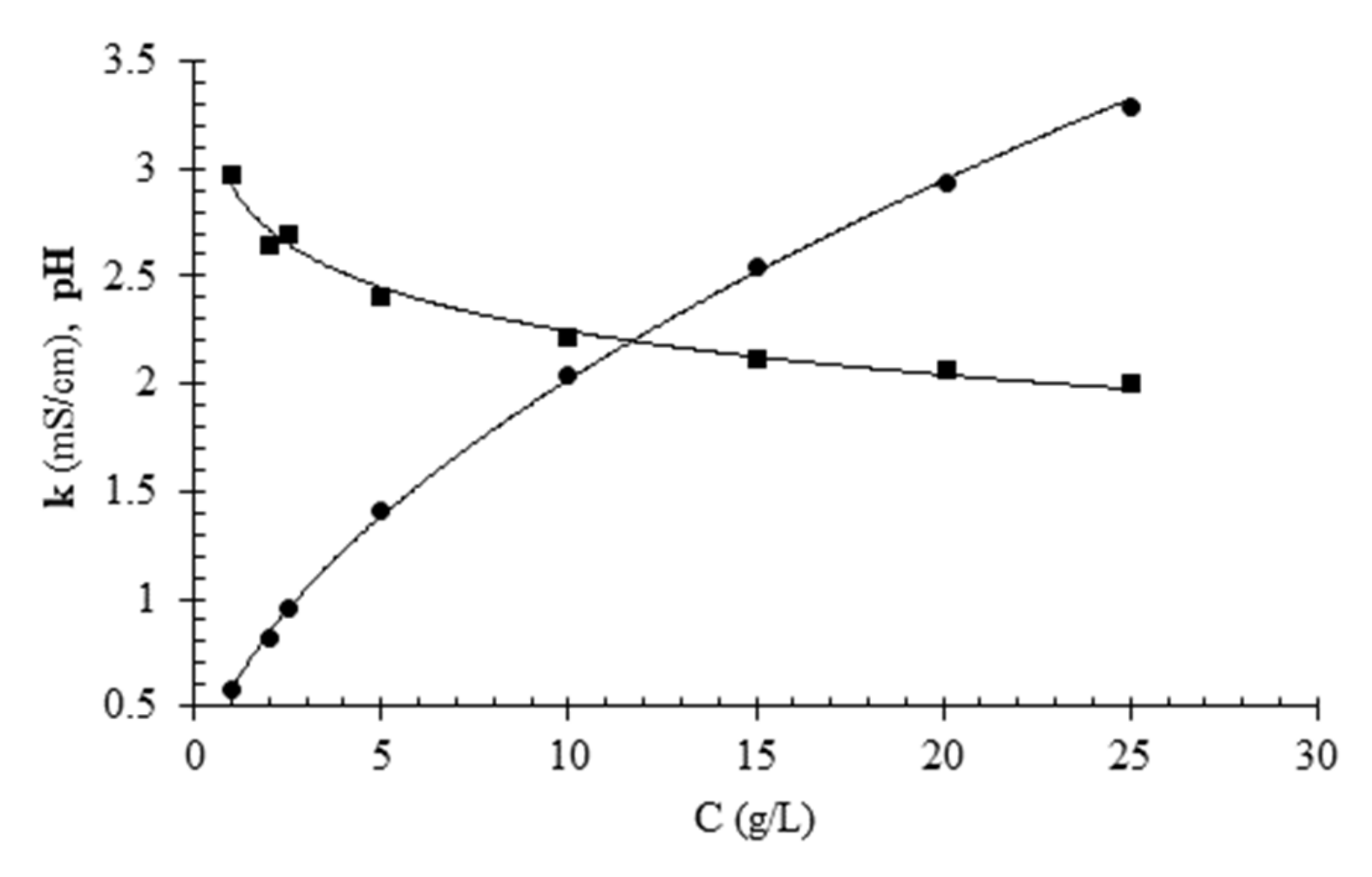
Aqueous solutions of citric acid were prepared by dissolving anhydrous citric acid in deionized water to obtain concentrations in the range of 325 g/L. These solutions were used to study the effect of citric acid concentration on pH and conductivity (k) and as feeds for the diluting, concentrating and electrode rinsing compartments in electrodialysis deacidification experiments.
For the electrodialysis tests on juice, a pulpy pineapple juice was used. The product contained only juice, not from concentrate, as the only ingredient and was purchased from a local supermarket (Conad, Bologna, Italy). The label stated the caloric and carbohydrate content as 50 kcal and 12 g of carbohydrate per 100 mL of product, respectively. Juices from the same production batch were selected.
2.2. Analytical Methods
Samples of model solutions prepared for calibration purposes or withdrawn during deacidification experiments were subjected to pH, conductivity, and titratable acidity measurements (TA). Samples of fruit juice withdrawn during deacidification experiments were additionally subjected to measurement of soluble solids content (SSC).
pH was measured using a multi-parameter instrument (model InoLab pH/Cond. 740, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany)) set at 20 °C. The instrument was connected to a pH probe with built-in temperature measurement (model SenTix81 PLUS, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany). Calibration for pH was performed using standard solutions of pH 2, pH 4, and pH 7.
Electrical conductivity was measured at 20 °C using a benchtop conductivity meter (InoLab Con Level 1, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany) to which a conductivity cell (model Tetracon 325, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany) was connected. The instrument was calibrated using standard solutions with conductivities of 12.88 mS/cm and 1413 µS/cm (HI 70030 and HI7030, Hanna Instruments Italia, Ronchi di Villafranca Padovana, Italy).
Total soluble solids of juice samples were determined using an electronic refractometer (model WM-7 Atago, Tokyo, Japan).
Determinations of the titratable acidity of juice samples were carried out using an automatic titrator (model Flash Steroglass, Perugia, Italy) that had two probes, one for pH and one for temperature. A 0.25 M NaOH titrant solution was initially prepared. Following initialization of the instrument and calibration using pH standards (4 and 7), testing was started. For the analysis, samples were diluted 1 to 5 with water by adding deionized water to 10 mL of each sample up to a final volume of 50 mL. Titration was stopped when a pH value of 8.1 was reached. Titratable acidity was expressed in g/L of citric acid.
The accuracy and precision of all analytical methods was checked using aqueous solutions of citric acid, conductivity and pH calibration standards and reference literature values for citric acid physicochemical properties (electric conductivity).
2.3. Description of the Electrodialysis System
A laboratory-scale electrodialysis plant (model EUR2, Eurodia Industrie SA, Wissons, France) was used for the deacidification tests with model solutions and fruit juice.
The ED stack of the plant consisted of 7 desalination cells (Ncells) made up of 8 cationic (Neosepta CMX-Sb) and 7 anionic (Neosepta AMX-Sb) membranes (Tokugama Soda Co., Tokyo, Japan), separated by spacer gaskets (0.7 mm thick) and arranged in parallel between a platinum electrode (anode) and a titanium electrode (cathode). The ED stack was connected to a direct current generator (model N5767A, Agilent Technologies Inc., Santa Clara, CA, USA), which provided voltage (E) and current (I) in ranges of 0–60 V and 0–25 A, respectively. Two PVC tanks with a capacity of 1.6 L were used to hold the diluting and concentrating solution and a larger PVC tank with a capacity of 2 L was used to hold the electrode rinsing solution. The diluting and concentrating tanks were each equipped at their top with a 2 m high plexiglass tube with a metric scale to accurately measure the variation in the volume of liquid they contained. An ultrasonic distance sensor (model Tough Sonic 14, Senix Corporation, VT, USA) was inserted into the upper cavity of the concentrating tank tube to measure the liquid level in the reservoir, from which the corresponding liquid volume was estimated.
The plant was equipped with three polypropylene centrifugal pumps that allowed the recirculation of solutions inside the stack, with a nominal capacity of 0.25 m3/h and a total discharge head of 15 m of water.
The flow rates of solutions circulating in the diluting and concentrating tanks were adjusted using ball valves, in the range of 90–110 L/h, taking care to keep them almost similar between the two compartments.
For the electrode solution, the flow rate was set to 300 L/h.
To measure the conductivity and temperature of the solutions in the plant, three conductivity cells were used: two flow cells (model Tetracon DU/T, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany)) used for diluting and concentrating solution tanks, and one discontinuous cell (model Tetracon 325, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany ) for the electrode rinsing fluid.
Three instruments were used to measure conductivity and temperature data: two InoLab Con Level 1 devices (Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany) and the multi-parameter instrument InoLab pH/Cond. 740 (Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany).
The process temperature was regulated and controlled using a thermocryostat (model PL1, Lauda Master, Lauda-Königshofen, Germany) equipped with a thermocouple inserted into the electrode rinsing solution tank. The thermocryostat provided cooling water flowing inside coil heat exchangers inserted into the three tanks.
An application for the control of the ED unit (EDCP) developed in LabVIEW (National Instruments Italy S.R.L, Assago, Italy) facilitated control of the electric generator and acquisition and storage of real-time data using probes installed on the ED system and from manual operation. Parameters were acquired at an acquisition rate of 1 Hz (one acquisition per second).
2.4. Experiments with the ED Plant
Before using the system for the electrodialysis process, the stack was disassembled to observe the wear and condition of the membranes. Once reassembled, the membranes were washed with deionized water. Subsequently, washes were performed alternating deionized water with hydrochloric acid solution (HCl, 1 mol/L) and sodium hydroxide solution (NaOH, 3 g/L). Finally, the system was subjected to multiple washes with deionized water to ensure effective cleaning of residues from the HCl and NaOH solutions. The ED plant was used to perform limiting current intensity tests and deacidification experiments with model solutions of citric acid or fruit juice.
2.4.1. Limiting Current Intensity Tests
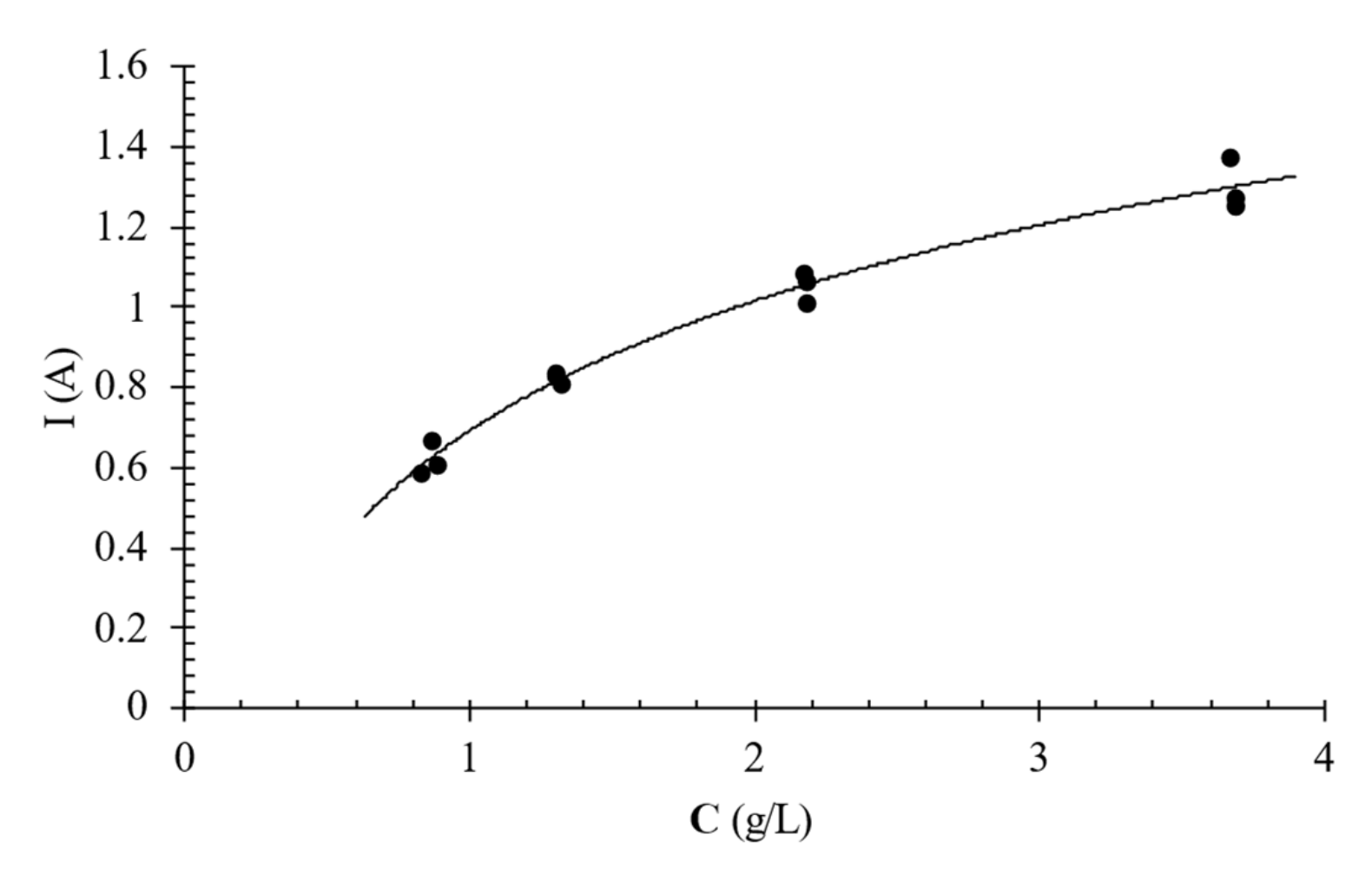
Limiting current intensity tests were carried out to establish the maximum current intensity to which the membranes could be subjected without being damaged, the so-called limiting current intensity (Ilim). This parameter made it possible to identify the current at which to perform the deacidification tests, which had to be lower than Ilim. In order to keep the concentration of the solutions flowing in the C and D compartments approximately the same, the tubes of D and C connected to the stack were reversed. A solution of citric acid with a concentration of 25 g/L was used as an electrode rinsing solution while in the concentrating and diluting tanks a solution of 25 g/L was initially used for the first experiments and then its concentration was reduced by removing an aliquot of solution and replacing it with deionized water to vary its electric conductivity from approximately 3.15 mS/cm to 437 µS/cm.
The EDCP program was used to perform the tests. Eight tests consisting of three replicates each were performed. The voltage (E)-current (I) data were automatically saved on the computer and used to calculate I
lim. The limiting current intensity was determined from the E-I response of the ED stack using the Cowan and Brown plot [
14], a graph in which the E/I is reported as a function of 1/I. The current value corresponding to the minimum on the Cowan and Brown diagram was chosen as the limiting current value (I
lim).
2.4.2. Deacidification Experiments with Citric Acid Solutions
The tests were conducted in batch mode at a temperature of 20 °C, continuously recirculating the diluting and concentrating solutions. All deacidification experiments were carried out at an electric current intensity lower than the limiting one (Ilim).
Before each test, the system was emptied of the liquid left over from the previous test and several washes with deionized water were performed until a conductivity between 200 and 600 µS/cm was reached in the tanks. The tanks were filled with citric acid solutions, using 1.9 L, 1.8 L and 2 L for the diluting, concentrating and electrode rinsing solutions, respectively. The concentrations of the solutions used for the concentrating and electrode rinsing solution tanks were kept constant for all tests, 5 g/L and 25 g/L, respectively.
The tests were conducted following a 2 × 2 factorial design added with a center point replicated three times. The two factors of the experiments were the initial concentration of the solution present in the diluting tank (C
D) and the intensity of the electric current (I). Their low, middle and high levels are reported in
Table 1.
The level of the initial concentrations in the diluting tank in the various tests carried out differed from that indicated in
Table 1 due to the residual volumes of solution inside the plant which modified the starting concentrations. The experimental conditions adopted are shown in
Table 2.
After starting the pumps for recirculation of the solutions, a few minutes’ wait allowed the solutions just inserted into the tanks to mix completely with the residual liquid and the temperature, controlled with the cryothermostat, to reach the desired value of 20 °C. The test was then started by turning on the current generator using the EDCP program. Several data were acquired by the program automatically or read on the instrument displays and entered manually through the graphical interface every two minutes. A sample of solution (20 mL) was taken every 10 min from the diluting stream and used to measure conductivity and pH.
The duration of each test was a function of the initial concentration of citric acid and varied according to the current intensity applied. In each test, deacidification was carried out until the final conductivity of the diluent solution reached 1.4 mS/cm, except in one case.
The efficiency of the deacidification process using ED was characterized in terms of Faraday efficiency (Ω). It relates the mass of electrolyte that is transported across membranes from diluting to concentrating compartments during the deacidification test, expressed in moles (∆n
B; ∆n
BD or ∆n
BC for the diluting or concentrating solution, respectively), to the amount of charge provided by the generator, expressed in moles of elemental charge (n
F):
where n
F can be calculated based on the following expression:
and N
cell is the number of ED cells used, I is the electric current intensity, F is the Faraday constant (=96,485.3 C/mol) and t is the process time.
2.4.3. Deacidification Experiments with Fruit Juice
Before using the juice in the ED plant, it was clarified by filtration to remove the pulp and limit fouling problems. In fact, for pulpy juices it is suggested that deacidification using ED is carried out on the filtered or centrifuged juice [
10]. Filtration was carried out using a vacuum filtration device employing a pump (model UN840 KNF Neuberger Laboport, Germany) and a filter (model WHA-10370319, Whatman, UK). The filtered juice was introduced into a two liter flask and its conductivity and pH were measured.
Two deacidification tests were performed under the same flow rates and temperature as the tests conducted with the citric acid solutions. For the first test the filtered fruit juice and an electric current intensity of 0.75 A were used. For the second test, the filtered juice was enriched with citric acid (40 g of citric acid were brought to a final volume of 2 L using the fruit juice) and the applied electric current was 1 A.
For the concentrating or electrode rinsing solutions a volume of 1.8 L or 2 L and a citric acid solution of 5 g/L or 25 g/L were used, respectively.
Once the pumps were turned on, the generator turned on, and the EDCP program set, the deacidification test was started. Data were recorded using the EDCP program both automatically from the sensors or manually every 2 min.
Samples of 20 mL were withdrawn from the diluting tank every 5 min (first trial) or at varying times (second trial) using a Falcon tube and placed in a refrigerator for subsequent analysis.
During deacidification, in addition to the liquid level and conductivity measurements from online sensors, the pH was measured automatically thanks to the multi-parameter instrument InoLab pH/Cond. 740 (Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany) that allowed connection with a pH probe with built-in temperature measurement (model SenTix81 PLUS, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany). A juice recirculation system was constructed consisting of a tube (internal diameter of 3 mm and length of 1.50 m) that used a peristaltic pump (model 7518-10, Masterflex easy load, Cole-Parmer North America, Vernon Hills, IL, USA) to move the juice from the diluting tank and, before reintroducing it into the tank itself, sent it through a glass flow cell (model D01/T, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany) that housed the pH probe (model SenTix81 PLUS, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany).
2.5. Statistical Methods
Experimental design and univariate and multivariate regression analysis were carried out using JMP statistical software package v. 16.1.0 (SAS Institute, Cary, NC, USA).
4. Discussion
The experiments carried out in this work facilitate for the first time a better understanding of the deacidification mechanism of citric acid solutions using conventional ED in light of recent research findings. The statistically designed deacidification experiments showed that the Faraday efficiency was independent of the electric current intensity and acid concentration used and equal to approximately 1/3, indicating that the triple-charged anion Cit
3− migrated through the anionic membrane. Citric acid is an anpholyte, i.e., a substance with a chemical structure and electrical charge that depend on the pH of the medium due to its participation in protonation-deprotonation reactions. In the pH range of the citric acid solutions used in ED experiments, approximately 2.0–2.5, the undissociated form of citric acid accounts for approximately 95 to 80%, the remaining being H
2Cit
−, as can be roughly estimated from the citric acid dissociation diagram [
23]. It was recently demonstrated with reference to the ED of NaH
2PO
4 solutions, that transformation of one form into another takes place not only in the solution, which is located in the intermembrane space, but also inside ion-exchange membranes [
26,
27,
28]. The transformation of an ampholyte from one form to another is thus possible when it enters or leaves the membrane. This is because the pH of the internal solution present in the membrane is different than that of the outside solution. In anion exchange membranes, the pH of the internal solutions is reported to be one to three units higher than that of the external solution because H
+ ions are pushed out from the membrane as coions. Thus, the transport of ampholytes in systems with ion-exchange membranes is coupled with the chemical reactions of protonation-deprotonation of species entering/leaving the membrane. These considerations can be applied to citric acid migrations through anion exchange membranes. Since the feed contained only H
3Cit and H
2Cit
− species, we can assume that deprotonation reactions occurred at the anion exchange membrane/solution interface leading to the release of the triple-charged citrate ion that migrated through the anionic membrane and to the H
+ ions that migrated through the solution in the opposite direction. Thanks to the migration of H
+ ions through the cation exchange membranes, the overall process results in a decrease in citric acid concentration from the diluting stream leading to a corresponding increase in pH. When submitting the pineapple juice to ED, several species are present in solutions with citric acid forms and potassium as the main acid and cation respectively. In this case, what may happen is that the H
+ ions compete with the K
+ cations at the cation exchange membrane; thus, the H
+ ions generated at the anion exchange membrane in the diluting compartment are not balanced by their migration through the cation exchange membrane resulting in an increase in their concentration, and a corresponding decrease in pH. Once most of the K
+ ions are removed from the diluting compartment, the H
+ ion migrations through the cationic membranes increase, and thus the pH starts to increase, as observed in the deacidification of pineapple juice carried out at I = 1 A.
5. Conclusions
In this paper, the deacidification of juices derived from fruits presenting high concentrations of organic acids, mainly citric, using monopolar membrane electrodialysis in the classical two-compartment configuration, was analyzed. Through a design of experiments approach, it was shown that ED enables a decrease in the acid concentration and a simultaneous increase in the pH of citric acid solutions. ED treatment of pineapple juice resulted in a decrease in titratable acidity without pH increase, as previously reported in a few articles. This led some researchers to consider conventional ED as ineffective in the deacidification of fruit juices. However, sourness is correlated to both titratable acidity and pH; thus, the reduction in titratable acidity, even if not accompanied by an increase in pH, can lead to fruit juices with a less perceived sourness, as already shown. The mechanism of pH decrease is explained by the theory of ampholyte transport through electrodialysis membranes. Thus, this work contributes to a better understanding of citric acid anion transport through anion exchange membranes, a process that is involved in several ED configurations proposed for fruit juice deacidification. Further research to validate the proposed transport mechanism with other organic acids/fruit juices may lead to improvements of fruit juice deacidification processes based on electrodialysis.