Correlation between Multilocus Sequence Typing and Antibiotic Resistance, Virulence Potential of Campylobacter jejuni Isolates from Poultry Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Genomic DNA Extraction
2.2. MLST Analysis
2.3. Adherence and Invasion to Caco-2 Cells
2.4. Antibiotic Susceptibility Testing
2.5. Statistical Analysis
3. Results
3.1. MLST Genotypes of C. jejuni
3.2. Antibiotic Susceptibility of C. jejuni Isolates
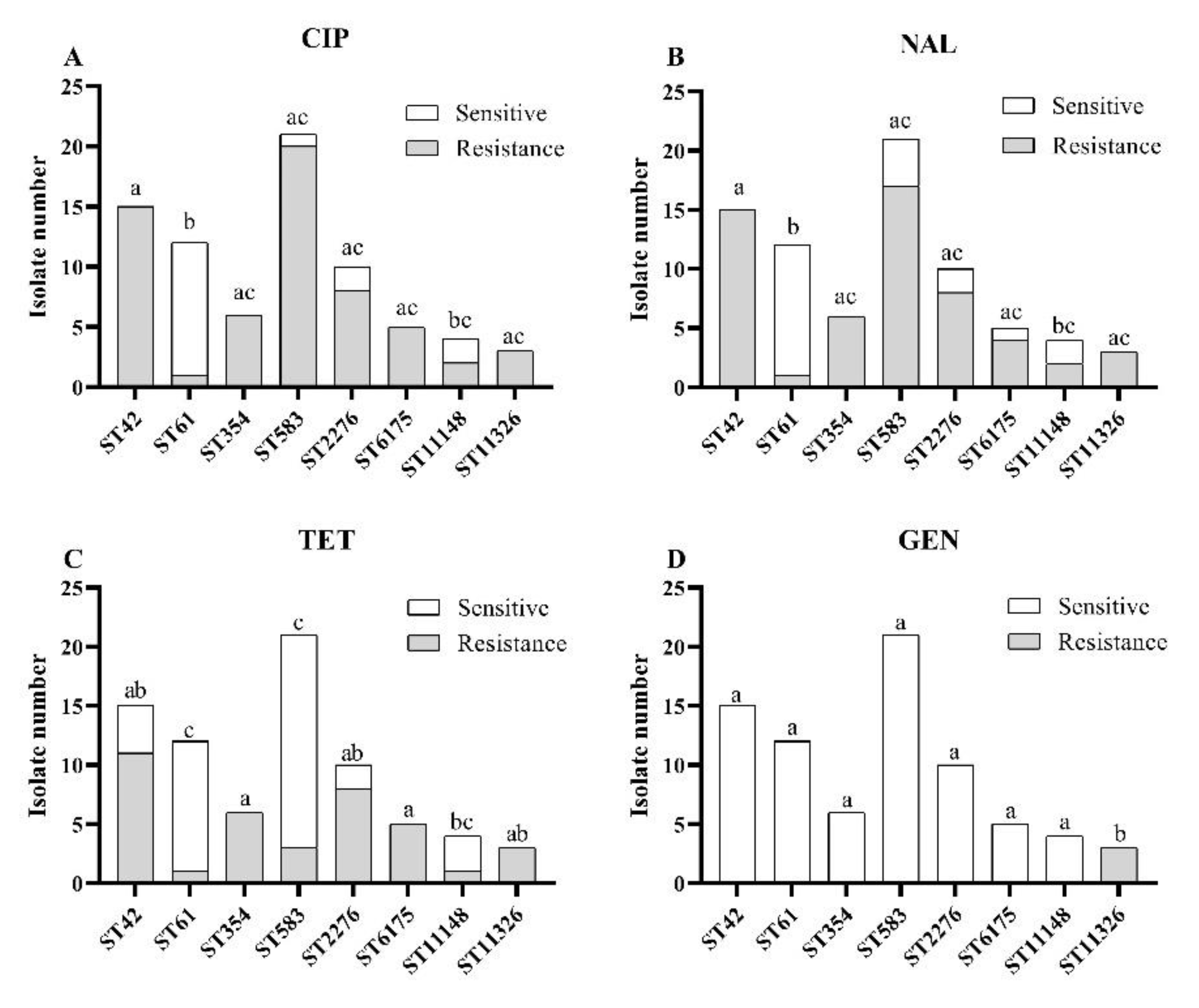
3.3. Associations between MLST Genotypes and Antibiotic Resistances
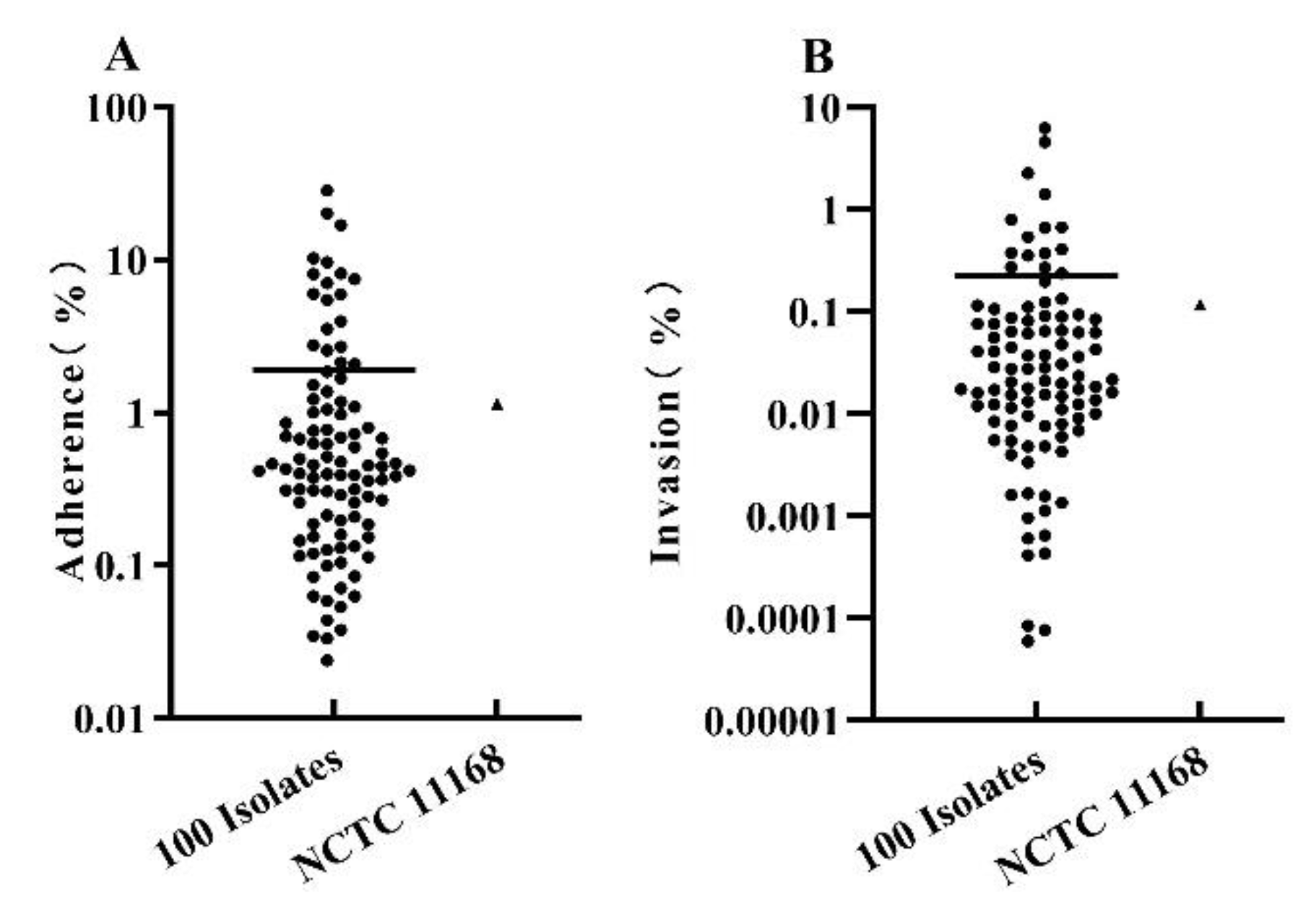
3.4. The Adherence and Invasion to Caco-2 Cells by C. jejuni Isolates
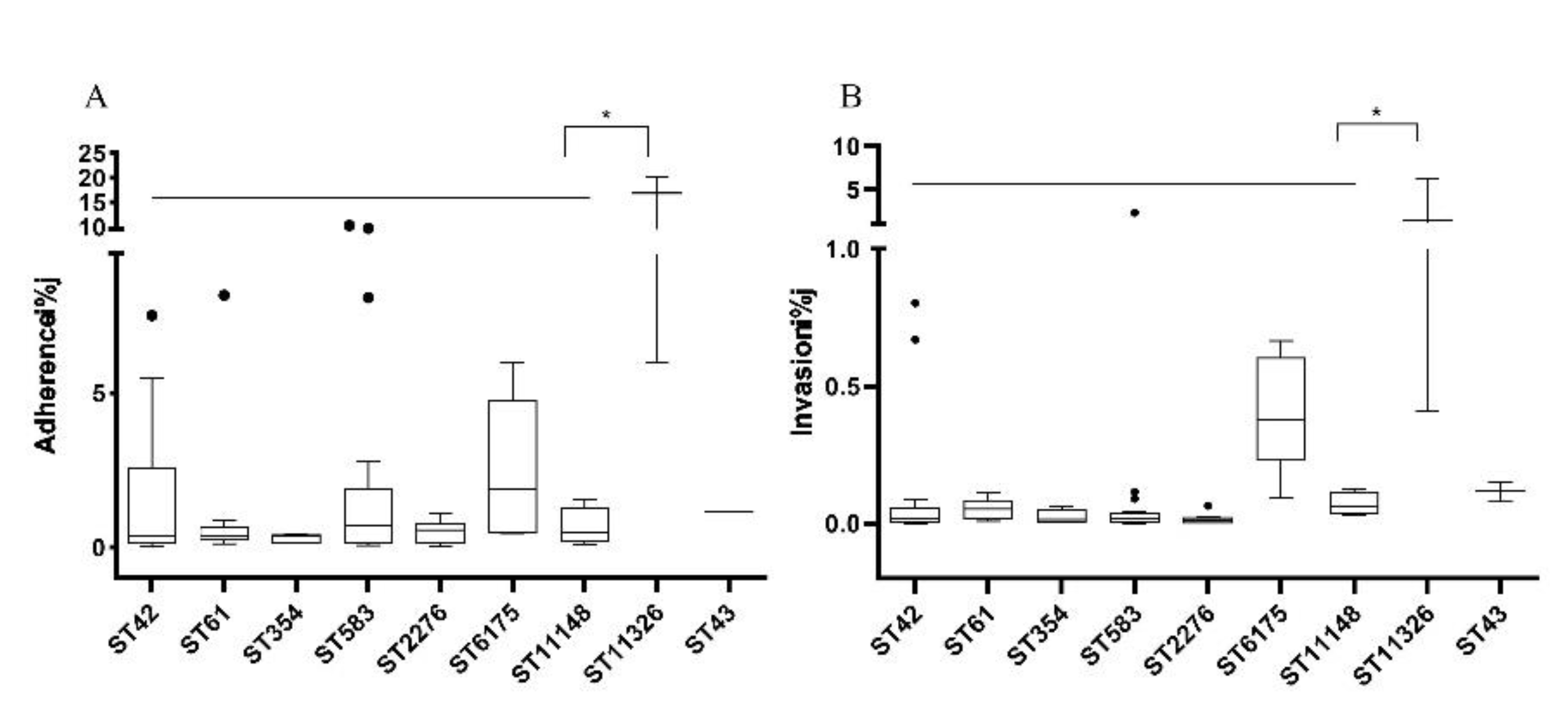
3.5. Correlation between MLST Genotypes and Pathogenic Ability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wysok, B.; Wojtacka, J.; Kivistö, R. Pathogenicity of Campylobacter strains of poultry and human origin from Poland. Int. J. Food Microbiol. 2020, 334, 108830. [Google Scholar] [CrossRef] [PubMed]
- Cróinín, T.Ó.; Backert, S. Host epithelial cell invasion by Campylobacter jejuni: Trigger or zipper mechanism? Front. Cell. Infect. Microbiol. 2012, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.N.; Campioni, F.; Vilela, F.P.; Duque, S.S.; Falcao, J.P. Campylobacter coli strains from Brazil can invade phagocytic and epithelial cells and induce IL-8 secretion. Braz. J. Microbiol. 2021, 52, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Hodges, L.M.; Carrillo, C.D.; Upham, J.P.; Borza, A.; Eisebraun, M.; Kenwell, R.; Mutschall, S.K.; Haldane, D.; Schleihauf, E.; Taboada, E.N. A strain comparison of Campylobacter isolated from retail poultry and human clinical cases in Atlantic Canada. PLoS ONE 2019, 14, e0215928. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, Q.; Chen, Y.; Li, T.; Wen, G.; Zhang, R.; Luo, L.; Lu, Q.; Ai, D.; Wang, H.; et al. Molecular epidemiology, virulence determinants and antimicrobial resistance of Campylobacter spreading in retail chicken meat in Central China. Gut Pathog. 2016, 8, 48. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, e07209. [Google Scholar] [CrossRef]
- Wysok, B.; Wojtacka, J. Detection of virulence genes determining the ability to adhere and invade in Campylobacter spp. from cattle and swine in Poland. Microb. Pathog. 2018, 115, 257–263. [Google Scholar] [CrossRef]
- Bang, D.D.; Borck, B.; Nielsen, E.M.; Scheutz, F.; Pedersen, K.; Madsen, M. PCR detection of seven virulence and toxin genes of Campylobacter jejuni and Campylobacter coli isolates from Danish pigs and cattle and cytolethal distending toxin production of the isolates. J. Appl. Microbiol. 2003, 94, 1003–1014. [Google Scholar] [CrossRef]
- Man, S.M. The clinical importance of emerging Campylobacter species. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 669–685. [Google Scholar] [CrossRef]
- Miller, W.G.; On, S.L.; Wang, G.; Fontanoz, S.; Lastovica, A.J.; Mandrell, R.E. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. J. Clin. Microbiol. 2005, 43, 2315–2329. [Google Scholar] [CrossRef]
- Ramonaite, S.; Tamuleviciene, E.; Alter, T.; Kasnauskyte, N.; Malakauskas, M. MLST genotypes of Campylobacter jejuni isolated from broiler products, dairy cattle and human campylobacteriosis cases in Lithuania. BMC Infect. Dis. 2017, 17, 430. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.; Uyttendaele, M.; De Zutter, L. Survival of poultry-derived Campylobacter jejuni of multilocus sequence type clonal complexes 21 and 45 under freeze, chill, oxidative, acid and heat stresses. Food Microbiol. 2010, 27, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Zhang, X.; Xue, F.; Wang, Y.; Jiang, L.; Jiang, Y. Phenotypic Characters and Molecular Epidemiology of Campylobacter Jejuni in East China. J. Food Sci. 2016, 81, M106–M113. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.N.; Sheppard, S.K.; McCarthy, N.D.; Maiden, M.C.; Ingmer, H.; Krogfelt, K.A. MLST clustering of Campylobacter jejuni isolates from patients with gastroenteritis, reactive arthritis and Guillain-Barre syndrome. J. Appl. Microbiol. 2010, 108, 591–599. [Google Scholar] [CrossRef]
- Zang, X.; Huang, P.; Li, J.; Jiao, X.; Huang, J. Genomic relatedness, antibiotic resistance and virulence traits of Campylobacter jejuni HS19 isolates from cattle in china indicate pathogenic potential. Front. Microbiol. 2021, 12, 3676. [Google Scholar] [CrossRef]
- Peters, S.; Pascoe, B.; Wu, Z.; Bayliss, S.C.; Zeng, X.; Edwinson, A.; Veerabadhran-Gurunathan, S.; Jawahir, S.; Calland, J.K.; Mourkas, E.; et al. Campylobacter jejuni genotypes are associated with post-infection irritable bowel syndrome in humans. Commun. Biol. 2021, 4, 1015. [Google Scholar] [CrossRef]
- Dingle, K.E.; Van Den Braak, N.; Colles, F.M.; Price, L.J.; Woodward, D.L.; Rodgers, F.G.; Endtz, H.P.; Van Belkum, A.; Maiden, M.C. Sequence typing confirms that Campylobacter jejuni strains associated with Guillain-Barre and Miller-Fisher syndromes are of diverse genetic lineage, serotype, and flagella type. J. Clin. Microbiol. 2001, 39, 3346–3349. [Google Scholar] [CrossRef][Green Version]
- Hsu, C.H.; Harrison, L.; Mukherjee, S.; Strain, E.; McDermott, P.; Zhang, Q.; Zhao, S. Core genome multilocus sequence typing for food animal source attribution of human Campylobacter jejuni infections. Pathogens 2020, 9, 532. [Google Scholar] [CrossRef]
- Costard, S.; Espejo, L.; Groenendaal, H.; Zagmutt, F.J. Outbreak-related disease burden associated with consumption of unpasteurized cow’s milk and cheese, United States, 2009–2014. Emerg. Infect. Dis. 2017, 23, 957–964. [Google Scholar] [CrossRef]
- Karenlampi, R.; Rautelin, H.; Schonberg-Norio, D.; Paulin, L.; Hanninen, M.L. Longitudinal study of Finnish Campylobacter jejuni and C. coli isolates from humans, using multilocus sequence typing, including comparison with epidemiological data and isolates from poultry and cattle. Appl. Environ. Microbiol. 2007, 73, 148–155. [Google Scholar] [CrossRef]
- Collado, L.; Munoz, N.; Porte, L.; Ochoa, S.; Varela, C.; Munoz, I. Genetic diversity and clonal characteristics of ciprofloxacin-resistant Campylobacter jejuni isolated from Chilean patients with gastroenteritis. Infect. Genet. Evol. 2018, 58, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Maesaar, M.; Meremae, K.; Ivanova, M.; Roasto, M. Antimicrobial resistance and multilocus sequence types of Campylobacter jejuni isolated from Baltic broiler chicken meat and Estonian human patients. Poult. Sci. 2018, 97, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Denis, E.; Lachtara, B.; Osek, J. Distribution of Campylobacter jejuni multilocus sequence types isolated from chickens in Poland. Poult. Sci. 2017, 96, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Redondo, N.; Carroll, A.; McNamara, E. Molecular characterization of Campylobacter causing human clinical infection using whole-genome sequencing: Virulence, antimicrobial resistance and phylogeny in Ireland. PLoS ONE 2019, 14, e0219088. [Google Scholar] [CrossRef]
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef]
- Ganan, M.; Campos, G.; Munoz, R.; Carrascosa, A.V.; de Pascual-Teresa, S.; Martinez-Rodriguez, A.J. Effect of growth phase on the adherence to and invasion of Caco-2 epithelial cells by Campylobacter. Int. J. Food Microbiol. 2010, 140, 14–18. [Google Scholar] [CrossRef]
- Russell, R.G.; Blake, D.C., Jr. Cell association and invasion of Caco-2 cells by Campylobacter jejuni. Infect. Immun. 1994, 62, 3773–3779. [Google Scholar] [CrossRef]
- Birk, T.; Wik, M.T.; Lametsch, R.; Knøchel, S. Acid stress response and protein induction in Campylobacter jejuni isolates with different acid tolerance. BMC Microbiol. 2012, 12, 174. [Google Scholar] [CrossRef]
- Zeitouni, S.; Guyard-Nicodeme, M.; Kempf, I. Comparison of adhesion, invasion, motility, and toxin production of Campylobacter strains and their resistant mutants. Microb. Drug Resist. 2013, 19, 130–137. [Google Scholar] [CrossRef]
- EUCAST (The European Committee on Antimicrobial Susceptibility Testing). Tables Interpret. MICs Zo. Diameters. 2013. Available online: http://www.eucast.org (accessed on 24 January 2022).
- Mohan, V.; Stevenson, M.; Marshall, J.; Fearnhead, P.; Holland, B.R.; Hotter, G.; French, N.P. Campylobacter jejuni colonization and population structure in urban populations of ducks and starlings in New Zealand. MicrobiologyOpen 2013, 2, 659–673. [Google Scholar] [CrossRef]
- Habib, I.; Miller, W.G.; Uyttendaele, M.; Houf, K.; De Zutter, L. Clonal population structure and antimicrobial resistance of Campylobacter jejuni in chicken meat from Belgium. Appl. Environ. Microbiol. 2009, 75, 4264–4272. [Google Scholar] [CrossRef]
- French, N.P.; Midwinter, A.; Holland, B.; Collins-Emerson, J.; Pattison, R.; Colles, F.; Carter, P. Molecular epidemiology of Campylobacter jejuni isolates from wild-bird fecal material in children’s playgrounds. Appl. Environ. Microbiol. 2009, 75, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Sakata, J.; Nakamura, H.; Yamamoto, S.; Murakami, S. Phylogenetic diversity and antimicrobial resistance of Campylobacter coli from humans and animals in Japan. Microbes Environ. 2019, 34, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ladely, S.R.; Berrang, M.E.; Meinersmann, R.J.; Cox, N.A. Campylobacter multi-locus sequence types and antimicrobial susceptibility of broiler cecal isolates: A two year study of 143 commercial flocks. J. Food Saf. 2017, 37, e12366. [Google Scholar] [CrossRef]
- Kovanen, S.M.; Kivisto, R.I.; Rossi, M.; Hanninen, M.L. A combination of MLST and CRISPR typing reveals dominant Campylobacter jejuni types in organically farmed laying hens. J. Appl. Microbiol. 2014, 117, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Guyard-Nicodeme, M.; Rivoal, K.; Houard, E.; Rose, V.; Quesne, S.; Mourand, G.; Rouxel, S.; Kempf, I.; Guillier, L.; Gauchard, F.; et al. Prevalence and characterization of Campylobacter jejuni from chicken meat sold in French retail outlets. Int. J. Food Microbiol. 2015, 203, 8–14. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Dallas, J.F.; MacRae, M.; McCarthy, N.D.; Sproston, E.L.; Gormley, F.J.; Strachan, N.J.; Ogden, I.D.; Maiden, M.C.; Forbes, K.J. Campylobacter genotypes from food animals, environmental sources and clinical disease in Scotland 2005/6. Int. J. Food Microbiol. 2009, 134, 96–103. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Kim, J.; Kim, J.H.; Jung, J.I.; Cho, S.; Ryu, S.; Jeon, B. Comparative analysis of aerotolerance, antibiotic resistance, and virulence gene prevalence in Campylobacter jejuni isolates from retail raw chicken and duck meat in south korea. Microorganisms 2019, 7, 433. [Google Scholar] [CrossRef]
- Bolinger, H.; Kathariou, S. The current state of macrolide resistance in Campylobacter spp.: Trends and impacts of resistance mechanisms. Appl. Environ. Microbiol. 2017, 83, e00416-17. [Google Scholar] [CrossRef]
- Awad, E.A.; Aliyu, A.B.; Bitrus, A.A.; Puan, C.L.; Khairani-Bejo, S.; Jalila, A.; Abdul-Aziz, S.; Mohamed-Yousif, I.M. Occurrence of antibiotic resistant Campylobacter in wild birds and poultry. Malays. J. Microbiol. 2019, 15, 143–151. [Google Scholar] [CrossRef]
- Jain, D.; Sinha, S.; Prasad, K.N.; Pandey, C.M. Campylobacter species and drug resistance in a north Indian rural community. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Marotta, F.; Di Marcantonio, L.; Janowicz, A.; Pedonese, F.; Di Donato, G.; Ardelean, A.; Nuvoloni, R.; Di Giannatale, E.; Garofolo, G. Genotyping and antibiotic resistance traits in Campylobacter jejuni and coli from pigs and wild boars in Italy. Front. Cell. Infect. Microbiol. 2020, 10, 592512. [Google Scholar] [CrossRef] [PubMed]
- Kittl, S.; Heckel, G.; Korczak, B.M.; Kuhnert, P. Source attribution of human Campylobacter isolates by MLST and fla-typing and association of genotypes with quinolone resistance. PLoS ONE 2013, 8, e81796. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.; Cadez, N.; Stessl, B.; Stingl, K.; Gruntar, I.; Ocepek, M.; Trkov, M.; Wagner, M.; Smole Mozina, S. High genetic similarity of ciprofloxacin-resistant Campylobacter jejuni in central Europe. Front. Microbiol. 2015, 6, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Meng, J.; Zhao, S.; Singh, R.; Song, W. Adherence to and invasion of human intestinal epithelial cells by Campylobacter jejuni and Campylobacter coli isolates from retail meat products. J. Food Prot. 2006, 69, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ge, Y.; Xu, H.; Zhang, J.; Kuang, D.; Yang, X.; Su, X.; Huang, Z.; Shi, X.; Xu, X.; et al. Molecular characterization, antimicrobial resistance and Caco-2 cell invasion potential of Campylobacter jejuni/coli from young children with diarrhea. Pediatr. Infect. Dis. J. 2016, 35, 330–334. [Google Scholar] [CrossRef]
| CCs | STs (No. of Isolates) | Total Number |
|---|---|---|
| CC45 | ST583 (21) | 21 |
| CC42 | ST42 (15) | 15 |
| CC61 | ST61 (12) | 12 |
| CC21 | STST760 (2), ST1811 (1), ST6175 (5), ST6500 (1) | 9 |
| CC354 | ST354 (6) | 6 |
| CC460 | ST113 (2) | 2 |
| CC464 | ST464 (1), ST11325 1(1) | 2 |
| CC574 | ST2031 (1), ST8149 (1) | 2 |
| CC828 | ST1586 (2) | 2 |
| CC22 | ST22 (1) | 1 |
| CC353 | ST11322 1 (1) | 1 |
| CC403 | ST8003 (1) | 1 |
| CC443 | ST51 (1) | 1 |
| NA 2 | ST1962 (1), ST2276 (10), ST4258 (1), ST8881 (1), ST8887 (1), ST11148 1 (4), ST11149 1 (1), ST11150 1 (1), ST11321 1 (1), ST11326 1 (3), ST11327 1 (1) | 25 |
| Sequence Type | Clonal Complex | No. of Isolates | MLST Allelic Profile | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aspA | glnA | gltA | glyA | pgm | tkt | uncA | |||
| ST11148 | NA 1 | 4 | 1 | 172 | 34 | 4 | 3 | 9 | 6 |
| ST11149 | NA | 1 | 9 | 17 | 5 | 10 | 350 | 164 | 3 |
| ST11150 | NA | 1 | 37 | 61 | 292 | 545 | 127 | 24 | 35 |
| ST11321 | NA | 1 | 8 | 364 | 4 | 470 | 127 | 29 | 35 |
| ST11322 | CC353 | 1 | 7 | 114 | 16 | 2 | 2 | 3 | 6 |
| ST11325 | CC464 | 1 | 8 | 2 | 2 | 2 | 13 | 3 | 147 |
| ST11326 | NA | 3 | 37 | 23 | 263 | 496 | 127 | 1 | 6 |
| ST11327 | NA | 1 | 37 | 367 | 292 | 26 | 127 | 57 | 541 |
| Antibiotic Resistance Profiles | NO. of Isolates |
|---|---|
| Sensitive | 12 |
| AZI | 2 |
| CIP | 4 |
| TET | 3 |
| AZI-CLI | 1 |
| CIP-TET | 1 |
| CIP-NAL | 17 |
| CIP-TET-NAL | 38 |
| CIP-NAL-CLI | 4 |
| AZI-TET-NAL | 1 |
| AZI-CIP-TET-NAL | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhuo, Q.; Hong, Y.; Wu, Y.; Gu, Q.; Yuan, D.; Dong, Q.; Shao, J. Correlation between Multilocus Sequence Typing and Antibiotic Resistance, Virulence Potential of Campylobacter jejuni Isolates from Poultry Meat. Foods 2022, 11, 1768. https://doi.org/10.3390/foods11121768
Wang X, Zhuo Q, Hong Y, Wu Y, Gu Q, Yuan D, Dong Q, Shao J. Correlation between Multilocus Sequence Typing and Antibiotic Resistance, Virulence Potential of Campylobacter jejuni Isolates from Poultry Meat. Foods. 2022; 11(12):1768. https://doi.org/10.3390/foods11121768
Chicago/Turabian StyleWang, Xiang, Qiyun Zhuo, Yi Hong, Yufan Wu, Qiang Gu, Dawei Yuan, Qingli Dong, and Jingdong Shao. 2022. "Correlation between Multilocus Sequence Typing and Antibiotic Resistance, Virulence Potential of Campylobacter jejuni Isolates from Poultry Meat" Foods 11, no. 12: 1768. https://doi.org/10.3390/foods11121768
APA StyleWang, X., Zhuo, Q., Hong, Y., Wu, Y., Gu, Q., Yuan, D., Dong, Q., & Shao, J. (2022). Correlation between Multilocus Sequence Typing and Antibiotic Resistance, Virulence Potential of Campylobacter jejuni Isolates from Poultry Meat. Foods, 11(12), 1768. https://doi.org/10.3390/foods11121768







