Nutritional and Chemical Composition of Sargassum zhangii and the Physical and Chemical Characterization, Binding Bile Acid, and Cholesterol-Lowering Activity in HepG2 Cells of Its Fucoidans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Extraction of Crude Fucoidans from Six Sargassum
2.3. Analysis of Chemical Constituents of Fucoidans
2.4. Bile Acid Binding Capacity Assay
2.5. Analysis of Basic Components of Sargassum zhangii
2.6. Fractionation of Crude Fucoidan from Sargassum zhangii
2.7. Monosaccharide Composition Analysis
2.8. Determination of Molecular Weight (Mw)
2.9. Fourier Transformed-Infrared (FTIR) Spectrum Analysis
2.10. Cell Culture and Cell Viability
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Statistical Analysis
3. Results
3.1. Chemical Composition Analysis of Six Crude Fucoidans
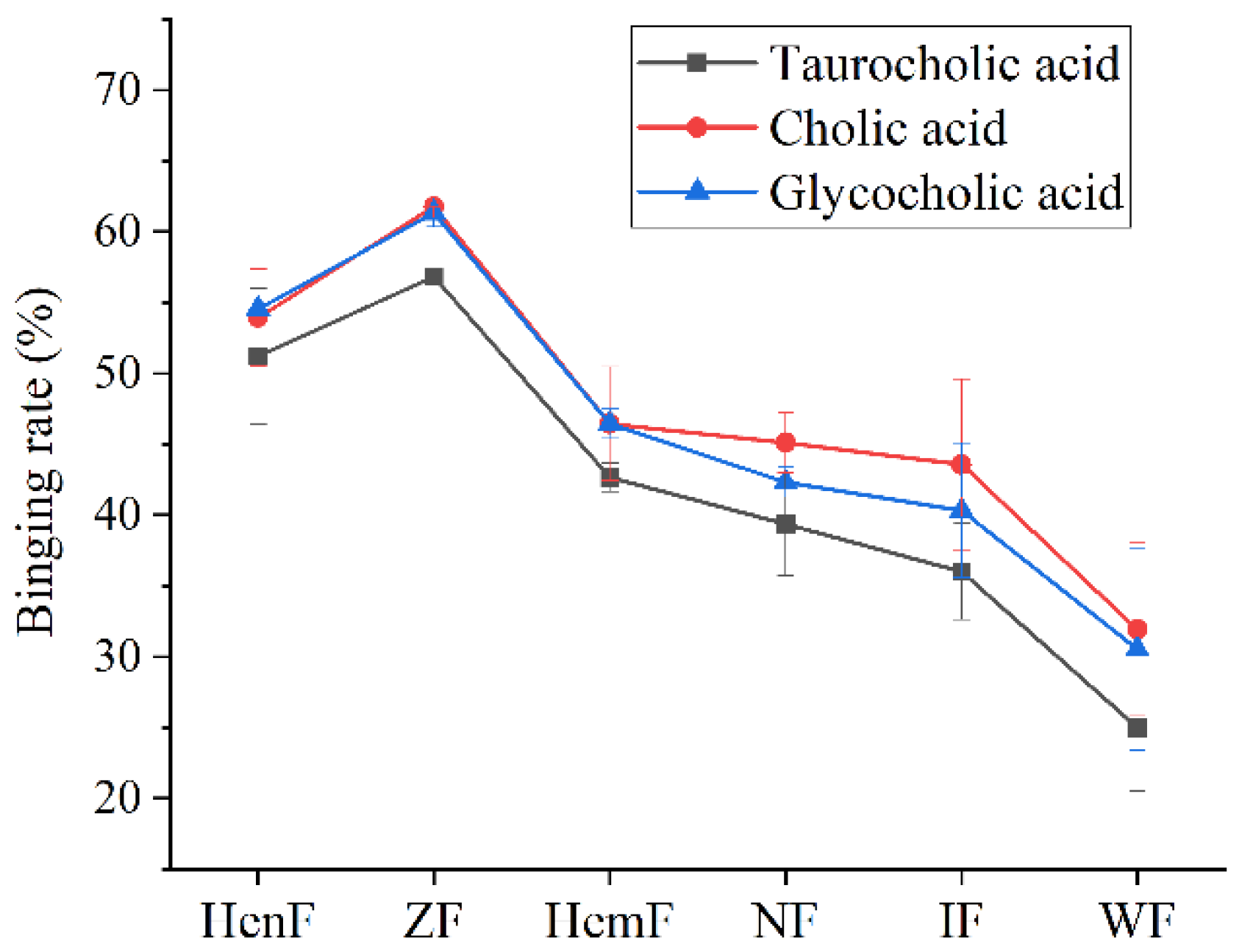
3.2. The Ability of Six Crude Fucoidans to Bind Bile Acids
3.3. Nutritional and Chemical Composition of Sargassum zhangii
3.4. Fractionation of ZF
3.5. Chemical Composition and Molecular Weight (Mw) of ZF1, ZF2, and ZF3
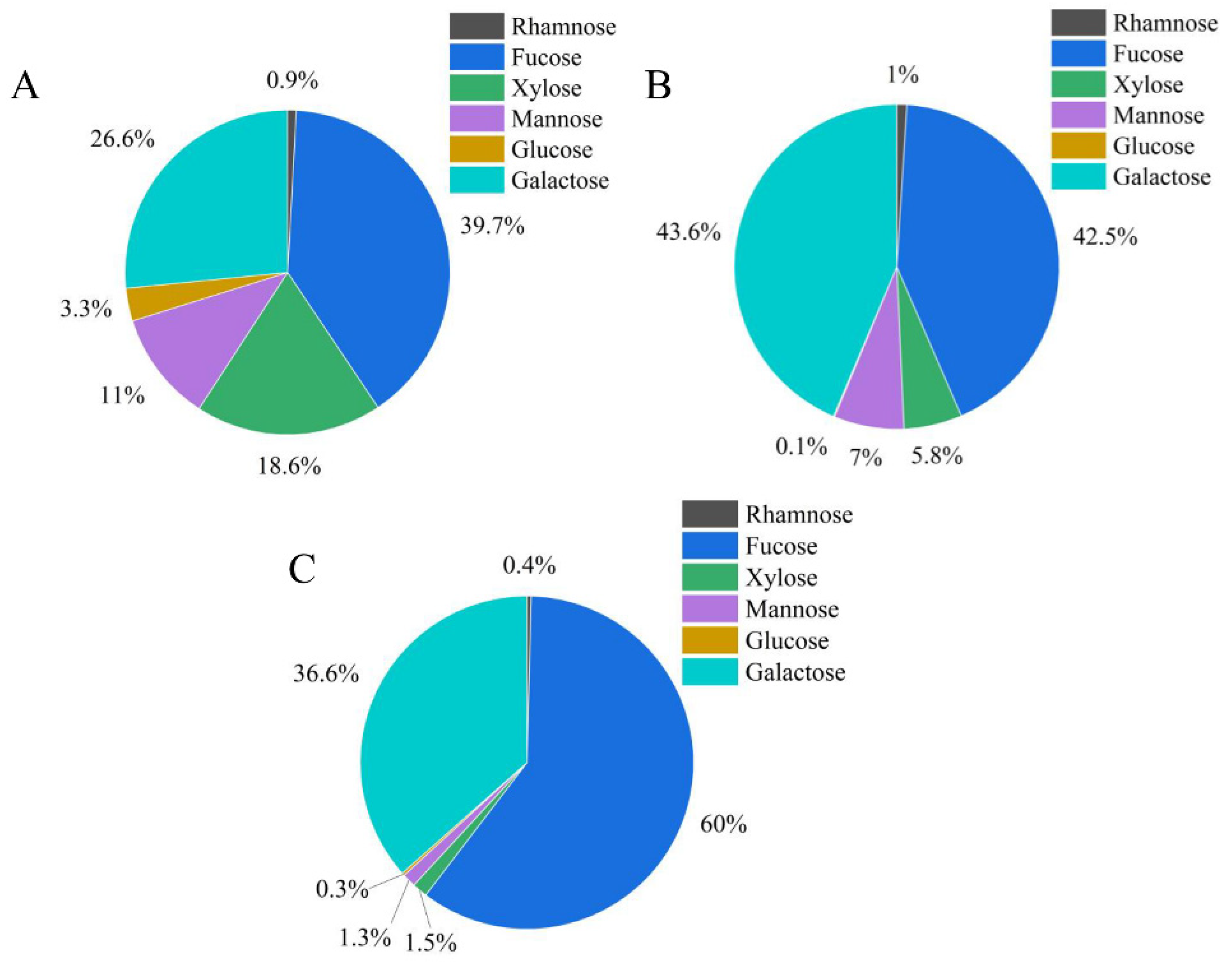
3.6. Analysis of Monosaccharide Composition of ZF1, ZF2, and ZF3
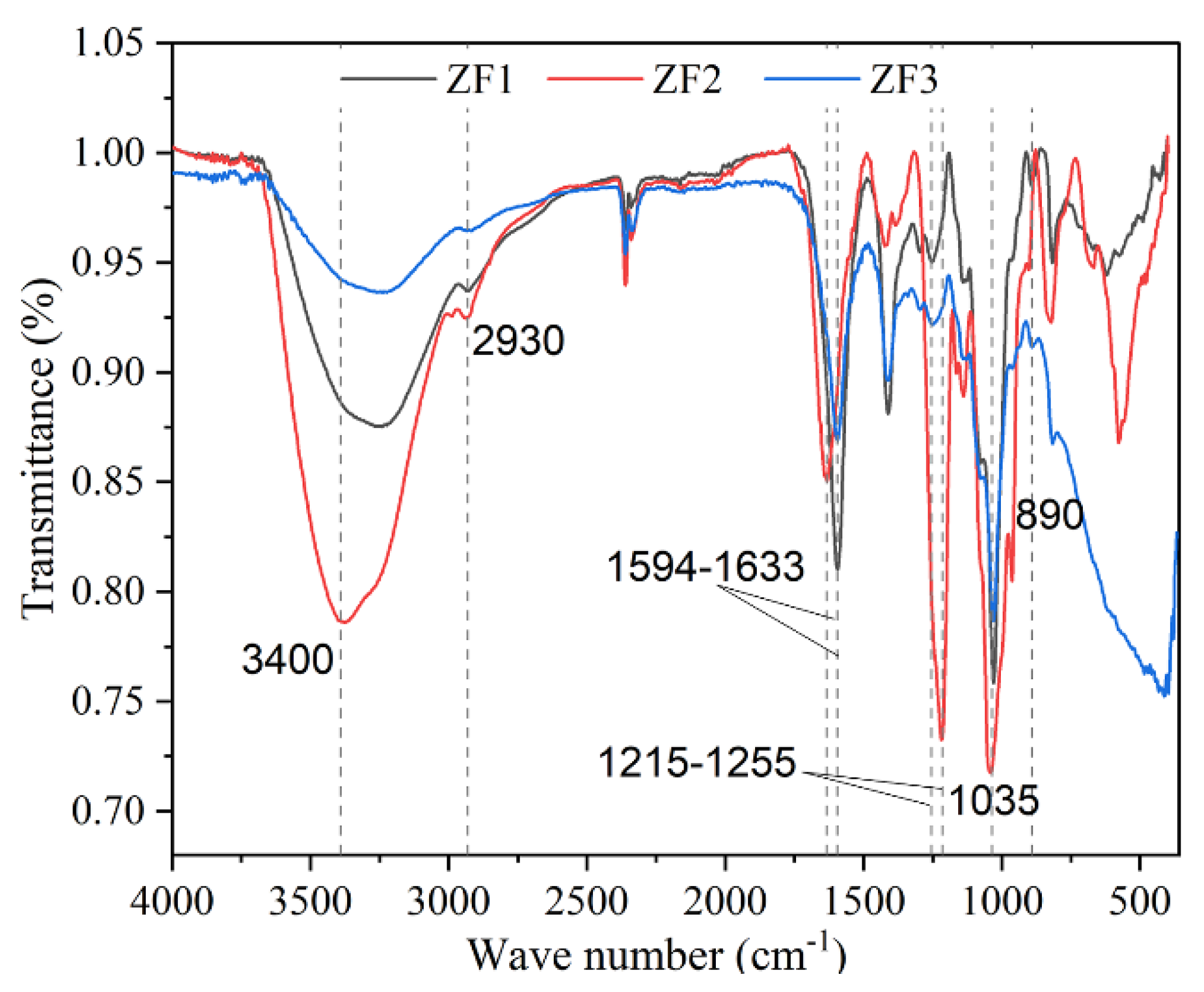
3.7. FTIR Spectrum of ZF1, ZF2, and ZF3
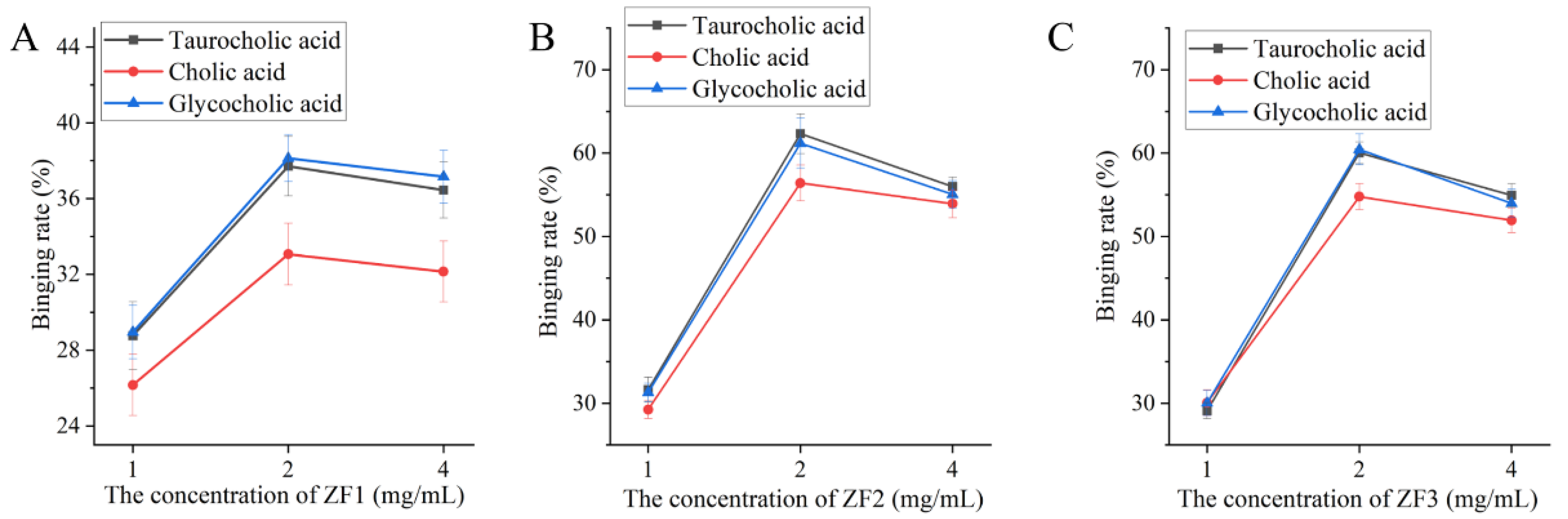
3.8. The Ability of ZF1, ZF2, and ZF3 to Bind Bile Acids
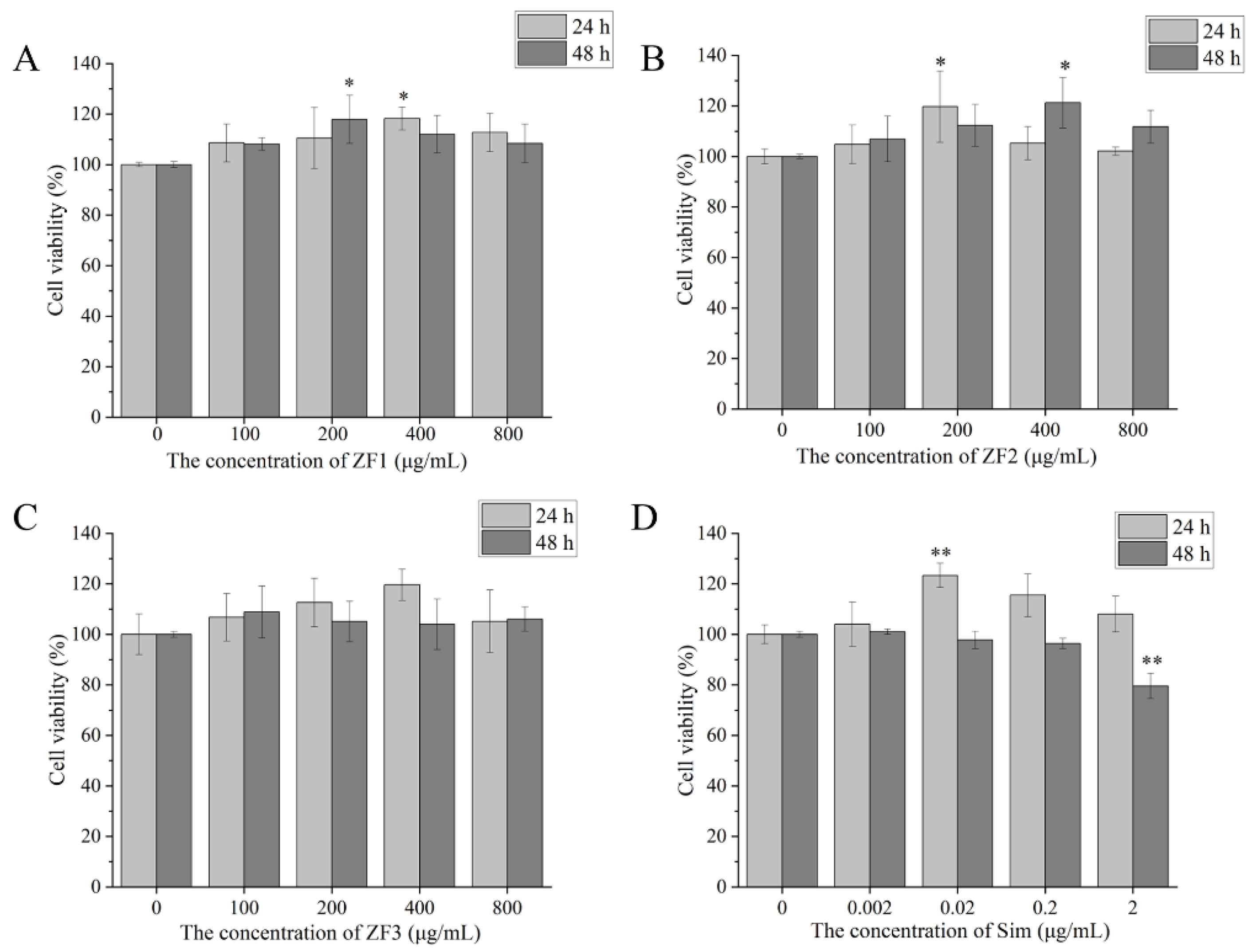
3.9. Cell Viability
3.10. Intracellular TC Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalita, P.; Ahmed, A.B.; Sen, S.; Chakraborty, R. A comprehensive review on polysaccharides with hypolipidemic activity: Occurrence, chemistry and molecular mechanism. Int. J. Biol. Macromol. 2022, 206, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Taddei, C.; Zhou, B.; Bixby, H.; Carrillo-Larco, R.M.; Danaei, G.; Jackson, R.T.; Farzadfar, F.; Sophiea, M.K.; Di Cesare, M.; Iurilli, M.L.C.; et al. Repositioning of the global epicentre of non-optimal cholesterol. Nature 2020, 582, 73. [Google Scholar]
- Gaillard, D.; Masson, D.; Garo, E.; Souidi, M.; Pais de Barros, J.-P.; Schoonjans, K.; Grober, J.; Besnard, P.; Thomas, C. Muricholic acids promote resistance to hypercholesterolemia in cholesterol-fed mice. Int. J. Mol. Sci. 2021, 22, 7163. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, F.; Zalzala, M.; Xu, J.; Gonzalez, F.J.; Adorini, L.; Lee, Y.-K.; Yin, L.; Zhang, Y. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 2016, 64, 1072–1085. [Google Scholar] [CrossRef] [Green Version]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.-T.; Jeon, Y.-J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef]
- Rao, Y.; Wen, Q.; Liu, R.; He, M.; Jiang, Z.; Qian, K.; Zhou, C.; Li, J.; Du, H.; Ouyang, H.; et al. PL-S2, a homogeneous polysaccharide from Radix Puerariae lobatae, attenuates hyperlipidemia via farnesoid X receptor (FXR) pathway-modulated bile acid metabolism. Int. J. Biol. Macromol. 2020, 165, 1694–1705. [Google Scholar] [CrossRef]
- Ren, D.; Wang, Q.; Yang, Y.; Hu, Y.; Song, Y.; He, Y.; Liu, S.; Wu, L. Hypolipidemic effects of fucoidan fractions from Saccharina sculpera (Laminariales, Phaeophyceae). Int. J. Biol. Macromol. 2019, 140, 188–195. [Google Scholar] [CrossRef]
- Raghavendran, H.R.B.; Sathivel, A.; Devaki, T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol. Cell. Biochem. 2005, 276, 89–96. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jeon, J.; Lee, J.-S. Fucoidan Prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, G.; Wang, Y.; Yin, J.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hou, P.; Hu, S.; et al. Fucoidan A2 from the brown seaweed Ascophyllum nodosum lowers lipid by improving reverse cholesterol transport in C57BL/6J mice fed a high-fat diet. J. Agric. Food Chem. 2019, 67, 5782–5791. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Y.R.; Lee, H.-G.; Fu, X.; Wang, K.; Xu, J.; Gao, X.; Jeon, Y.-J. Fucoidan isolated from fermented Sargassum fusiforme suppresses oxidative stress through stimulating the expression of superoxidase dismutase and catalase by regulating Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 209, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.; Cai, H.; Li, R.; Jia, X.; Liu, X.; Ji, H.; Zhong, S. Physicochemical properties and immunomodulatory effects of fucoidan from different brown algae. J. Guangdong Ocean Univ. 2022, 42, 62–71. [Google Scholar]
- Diaz-Resendiz, K.J.G.; Covantes-Rosales, C.E.; Benitez-Trinidad, A.B.; Navidad-Murrieta, M.S.; Razura-Carmona, F.F.; Carrillo-Cruz, C.D.; Frias-Delgadillo, E.J.; Perez-Diaz, D.A.; Diaz-Benavides, M.V.; Zambrano-Soria, M.; et al. Effect of fucoidan on the mitochondrial membrane potential (ΔΨm) of leukocytes from patients with active COVID-19 and subjects that recovered from SARS-CoV-2 infection. Mar. Drugs 2022, 20, 99. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, N.; Zuo, Z.; Li, S.; Zheng, W.; Shi, X.; Liu, Q.; Ma, T.; Yin, R.; Li, X.; et al. Five distinct fucan sulfates from sea cucumber Pattalus mollis: Purification, structural characterization and anticoagulant activities. Int. J. Biol. Macromol. 2021, 186, 535–543. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Wang, J.; Yao, J.; Xie, E.; Liu, D.; Duan, D.; Zhang, Q. A study of neuroprotective and antioxidant activities of heteropolysaccharides from six Sargassum species. Int. J. Biol. Macromol. 2014, 67, 336–342. [Google Scholar] [CrossRef]
- Li, W.; Ding, Z. Common brown algae in Guangdong and Hainan Island. J. Guangdong Ocean Univ. 1990, 2, 23–31. [Google Scholar]
- Lin, P.; Chen, S.; Liao, M.; Wang, W. Physicochemical characterization of fucoidans from Sargassum henslowianum C.Agardh and their antithrombotic activity in vitro. Mar. Drugs 2022, 20, 300. [Google Scholar] [CrossRef]
- Roe, J.H.; Dailey, R.E. Determination of glycogen with the anthrone reagent. Anal. Biochem. 1966, 15, 245–250. [Google Scholar] [CrossRef]
- Saetan, U.; Nontasak, P.; Palasin, K.; Saelim, H.; Wonglapsuwan, M.; Mayakun, J.; Pongparadon, S.; Chotigeat, W. Potential health benefits of fucoidan from the brown seaweeds Sargassum plagiophyllum and Sargassum polycystum. J. Appl. Phycol. 2021, 33, 3357–3364. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Bio. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, H.; Gu, X.; Zhou, N.; Zhu, Z.; Wang, C.; Liu, X.; Zhao, M. Physicochemical characterization and bile acid-binding capacity of water-extract polysaccharides fractionated by stepwise ethanol precipitation from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 150, 654–661. [Google Scholar] [CrossRef]
- Deng, W.; Yang, X.; Zhu, Y.; Yu, J.; Xu, X. Structural characterization and hypolipidemic activities of purified stigma maydis polysaccharides. Food Sci. Nutr. 2019, 7, 2674–2683. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Dou, W.; Alaxi, S.; Niu, Y.; Yu, L. Modified soluble dietary fiber from black bean coats with its rheological and bile acid binding properties. Food Hydrocolloid. 2017, 62, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Maehre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein determination method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, H.; Wang, D.; He, P. Nutrient components analysis of eight kinds of seaweeds in Zhanjiang sea area. J. Guangdong Ocean Univ. 2008, 28, 5. [Google Scholar]
- Qu, Y.; Zhou, S.; Zhong, S.; Chen, S.; Su, W.; Wu, X. Extraction and antioxidant activity of mucopolysaccharide of from swim bladder of Aristichthys nobilis. J. Guangdong Ocean Univ. 2018, 38, 47–53. [Google Scholar]
- Cui, Y.; Zhu, L.; Li, Y.; Jiang, S.; Sun, Q.; Xie, E.; Chen, H.; Zhao, Z.; Qiao, W.; Xu, J.; et al. Structure of a laminarin-type beta-(1 -> 3)-glucan from brown algae Sargassum henslowianum and its potential on regulating gut microbiota. Carbohydr. Polym. 2021, 255, 117389. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.; He, Y.; Ren, D.; Kow, F.; Qiao, Z.; Liu, S.; Yu, X. Structural characterisation of algae Costaria costata fucoidan and its effects on CCl4-induced liver injury. Carbohydr. Polym. 2014, 107, 247–254. [Google Scholar] [CrossRef]
- Lin, P.; Lu, Z.; Zhang, Y.; Liao, X.; He, L.; Guo, Y.; Zhou, C.; Qian, Z.-J.; Hong, P.; Liang, Y.-Q.; et al. Do polystyrene nanoplastics aggravate the toxicity of single contaminants (okadaic acid)? Using AGS cells as a biological model. Environ. Sci. Nano 2021, 8, 3186–3201. [Google Scholar] [CrossRef]
- Somasundaram, S.N.; Shanmugam, S.; Subramanian, B.; Jaganathan, R. Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line HCT-15. Int. J. Biol. Macromol. 2016, 91, 1215–1223. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Song, Y.; He, Y.; Ren, D.; Cong, H.; Wu, L. Studies on the hepatoprotective effect of fucoidans from brown algae Kjellmaniella crassifolia. Carbohydr. Polym. 2018, 193, 298–306. [Google Scholar] [CrossRef]
- Qin, L.; Xu, H.; He, Y.; Liang, C.; Wang, K.; Cao, J.; Qu, C.; Miao, J. Purification, chemical characterization and immunomodulatory activity of a sulfated polysaccharide from marine brown algae Durvillaea antarctica. Mar. Drugs 2022, 20, 223. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Nishino, T.; Nagumo, T. Anticoagulant and antithrombin activities of over sulfated fucans. Carbohydr. Res. 1992, 229, 355–362. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Chen, X.; Xie, E. Extraction technology of mannitol in Sargassum zhangii. Food Res. Dev. 2021, 42, 4. [Google Scholar]
- Peng, Y.; Xie, E.; Zheng, K.; Fredimoses, M.; Yang, X.; Zhou, X.; Wang, Y.; Yang, B.; Lin, X.; Liu, J.; et al. Nutritional and chemical composition and antiviral activity of cultivated seaweed Sargassum naozhouense. Mar. Drugs 2013, 11, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Xiong, H.; Huang, T.; Ji, H.; Shao, H. Analysis on basic nutrition constituents of Codium cylindricum Holme and extraction of its polysaccharides. J. Guangdong Ocean Univ. 2007, 27, 82–85. [Google Scholar]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zhao, S. Chemical constituents analysis of three edible chloraphytas in south china sea and their nutrition evaluation. J. Guangdong Ocean Univ. 2005, 25, 19–23. [Google Scholar]
- Jeong, M.-H.; Lim, C.-W.; Shim, K.B.; Je, C.Y. Mineral analysis and nutritional evaluation according to production area of Laver Porphyra tenera, Japanese kelp Saccharina japonicus, Sea mustard Undaria pinnatifida and hijiki Sargassum fusiforme in Korea. J. Fish. Mar. Sci. Educ. 2017, 29, 1624–1632. [Google Scholar]
- Palanisamy, S.; Vinosha, M.; Manikandakrishnan, M.; Anjali, R.; Rajasekar, P.; Marudhupandi, T.; Manikandan, R.; Vaseeharan, B.; Prabhu, N.M. Investigation of antioxidant and anticancer potential of fucoidan from Sargassum polycystum. Int. J. Biol. Macromol. 2018, 116, 151–161. [Google Scholar] [CrossRef]
- Je, J.-G.; Lee, H.-G.; Fernando, K.H.N.; Jeon, Y.-J.; Ryu, B. Purification and structural characterization of sulfated polysaccharides derived from brown algae, Sargassum binderi: Inhibitory mechanism of iNOS and COX-2 pathway interaction. Antioxidants 2021, 10, 822. [Google Scholar] [CrossRef]
- Fauziee, N.A.M.; Chang, L.S.; Mustapha, W.A.W.; Nor, A.R.M.; Lim, S.J. Functional polysaccharides of fucoidan, laminaran and alginate from Malaysian brown seaweeds (Sargassum polycystum, Turbinaria ornata and Padina boryana). Int. J. Biol. Macromol. 2021, 167, 1135–1145. [Google Scholar] [CrossRef]
- Wang, S.-H.; Huang, C.-Y.; Chen, C.-Y.; Chang, C.-C.; Huang, C.-Y.; Dong, C.-D.; Chang, J.-S. Structure and biological activity analysis of fucoidan isolated from Sargassum siliquosum. ACS Omega 2020, 5, 32447–32455. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yim, E.K.F. Fucoidan for cardiovascular application and the factors mediating its activities. Carbohydr. Polym. 2021, 270, 118347. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; White, P.J. In vitro bile-acid binding and fermentation of high, medium, and low molecular weight beta-glucan. J. Agric. Food Chem. 2010, 58, 628–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, X.; Yue, L.; Jiang, Q.; Xia, W. Synthesis, characterization and bioactivities of N,O-carbonylated chitosan. Int. J. Biol. Macromol. 2016, 91, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. Comparison study on polysaccharide fractions from Laminaria japonica: Structural characterization and bile acid binding capacity. J. Agric. Food Chem. 2017, 65, 9790–9798. [Google Scholar] [CrossRef]
- Niu, Y.; Xie, Z.; Zhang, H.; Sheng, Y.; Yu, L. Effects of structural modifications on physicochemical and bile acid-binding properties of Psyllium. J. Agric. Food Chem. 2013, 61, 596–601. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; Lin, L. Effects of extraction methods on structural characteristics and bile acid-binding capacities of Moringa oleifera leaf polysaccharide fractions. Int. J. Food Sci. Tech. 2020, 55, 1539–1546. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; McCord, J.M. Effects of the phytochemical combination PB123 on Nrf2 Activation, gene expression, and the cholesterol pathway in HepG2 cells. OBM Integr. Complement. Med. 2022, 7, 2. [Google Scholar] [CrossRef]
- Huang, Y.; Tocmo, R.; Nauman, M.C.; Haughan, M.A.; Johnson, J.J. Defining the cholesterol lowering mechanism of bergamot (Citrus bergamia) extract in HepG2 and Caco-2 cells. Nutrients 2021, 13, 3156. [Google Scholar] [CrossRef]


| Crude Fucoidans | Total Sugar (%) | Fucose (%) | Sulfate (%) |
|---|---|---|---|
| HenF | 31.78 | 9.14 | 12.44 |
| ZF | 33.37 | 7.91 | 13.63 |
| HemF | 28.64 | 8.21 | 10.56 |
| NF | 30.01 | 7.20 | 9.82 |
| IF | 25.42 | 6.40 | 8.59 |
| WF | 22.56 | 5.73 | 5.94 |
| Protein | Lipid | Carbohydrate | Dietary Fiber | Ash |
|---|---|---|---|---|
| 13.97 | 1.24 | 70.25 | 13.09 | 14.54 |
| Amino Acid | Content | Amino Acid | Content |
|---|---|---|---|
| Methionine | 0.32 | Glutamate | 1.66 |
| Leucine | 1.13 | Glycine | 0.72 |
| Valine | 0.82 | Alanine | 0.87 |
| Lysine | 0.67 | Cystine | 0.15 |
| Isoleucine | 0.68 | Tyrosine | 0.33 |
| Phenylalanine | 0.74 | Arginine | 0.59 |
| Tryptophan | 0.20 | Proline | 0.56 |
| Threonine | 0.60 | Total amino acid (TAA) | 12.15 |
| Histidine | 0.24 | Essential amino acid (EAA) | 5.40 |
| Aspartic acid | 1.30 | EAA/TAA | 0.44 |
| Serine | 0.57 | EAA/NEAA | 0.80 |
| K | Ca | Mg | Fe | Zn | Mn |
|---|---|---|---|---|---|
| 1000 | 1980 | 1080 | 85 | 22 | 16.4 |
| Fucoidan | Total Sugar (%) | Fucose (%) | Sulfate (%) | Mw (Da) |
|---|---|---|---|---|
| ZF1 | 64.77 | 4.94 | 3.29 | 4.026 × 105 |
| ZF2 | 68.75 | 17.81 | 19.39 | 2.893 × 105 |
| ZF3 | 73.48 | 26.01 | 18.89 | 3.368 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.; Chen, S.; Zhong, S. Nutritional and Chemical Composition of Sargassum zhangii and the Physical and Chemical Characterization, Binding Bile Acid, and Cholesterol-Lowering Activity in HepG2 Cells of Its Fucoidans. Foods 2022, 11, 1771. https://doi.org/10.3390/foods11121771
Lin P, Chen S, Zhong S. Nutritional and Chemical Composition of Sargassum zhangii and the Physical and Chemical Characterization, Binding Bile Acid, and Cholesterol-Lowering Activity in HepG2 Cells of Its Fucoidans. Foods. 2022; 11(12):1771. https://doi.org/10.3390/foods11121771
Chicago/Turabian StyleLin, Peichun, Suhua Chen, and Siyan Zhong. 2022. "Nutritional and Chemical Composition of Sargassum zhangii and the Physical and Chemical Characterization, Binding Bile Acid, and Cholesterol-Lowering Activity in HepG2 Cells of Its Fucoidans" Foods 11, no. 12: 1771. https://doi.org/10.3390/foods11121771
APA StyleLin, P., Chen, S., & Zhong, S. (2022). Nutritional and Chemical Composition of Sargassum zhangii and the Physical and Chemical Characterization, Binding Bile Acid, and Cholesterol-Lowering Activity in HepG2 Cells of Its Fucoidans. Foods, 11(12), 1771. https://doi.org/10.3390/foods11121771