The Nallan–Nickel Effect: A Mechanistic Perspective on Burning Sensations and Lichenoid Reactions in Long-Serving Porcelain-Fused-to-Metal Restorations
Abstract
1. Background
2. Clinical Concern: Long-Term Wear and Symptom Development
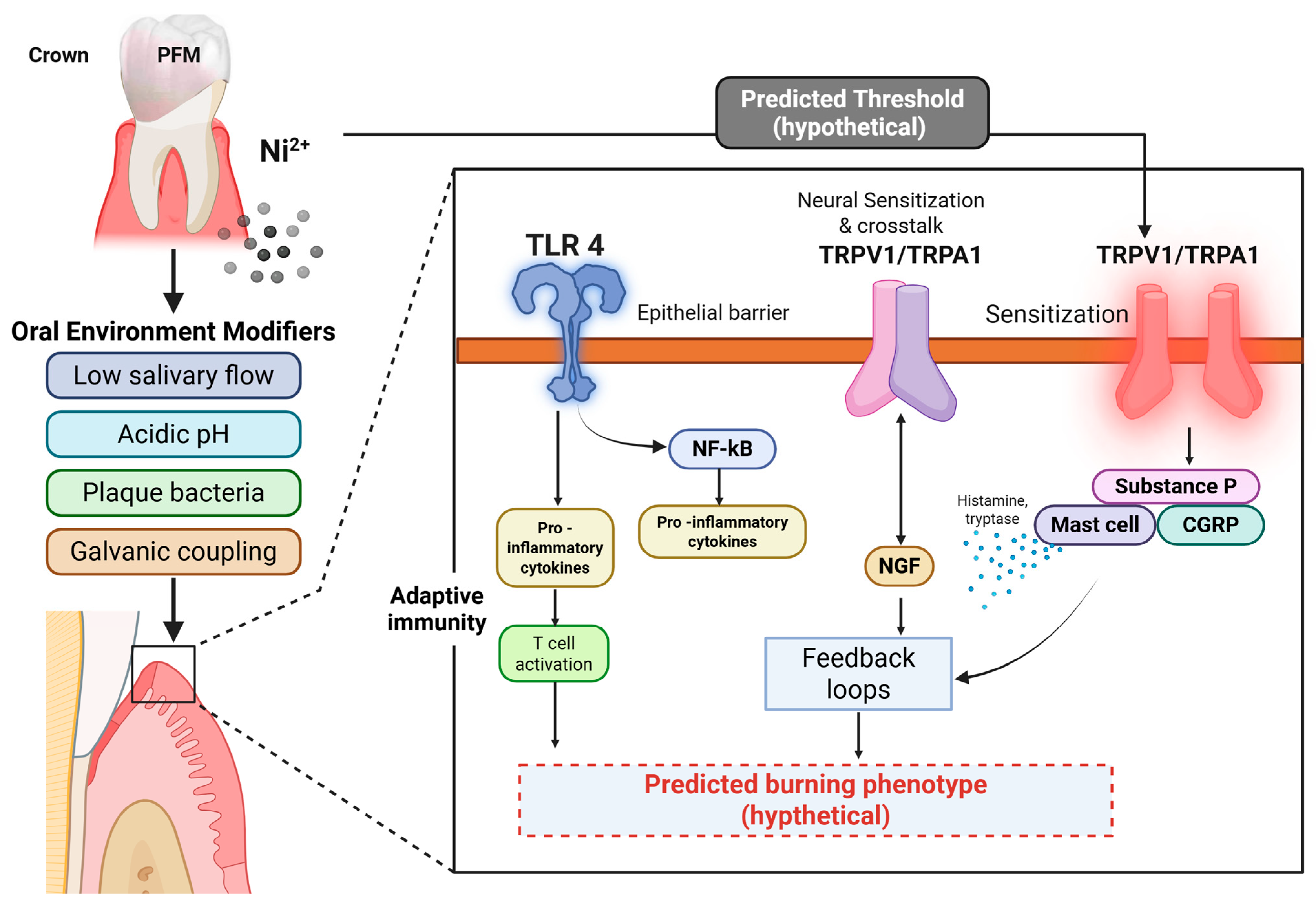
3. The Nallan–Nickel Effect
4. Timeframe for Development of Burning Sensations or Lichenoid Lesions
5. Individual Susceptibility
- Allergic Predisposition: Patients with a known sensitivity to nickel, palladium, or cobalt are at higher risk. Many cases of oral lichenoid contact reactions are essentially delayed hypersensitivity phenomena in predisposed individuals [91].
- Mucosal and Immune Status: Older individuals often show reduced mucosal barrier function and altered immune regulation, making them more vulnerable to irritation and hypersensitivity [92].
- Genetic and Epigenetic Factors: Variability in pain receptor expression (e.g., TRPV1 polymorphisms) or immune response genes may explain why some individuals develop neurogenic burning sensations while others remain asymptomatic despite similar exposures [93].
- Oral Environment: Low salivary flow, acidic oral pH, and the presence of dissimilar metals (which induce galvanic currents) all increase corrosion and ion release, thereby raising the risk of symptoms [78].
- Duration of Exposure: The longer the crown remains in service after porcelain breakdown, the greater the cumulative release of ions and the higher the risk of symptomatic manifestation [94].
6. Management of Symptoms
- When patients present with burning sensations or lichenoid lesions associated with long-term PFM crowns, the first step is careful diagnosis. A thorough history and examination should be undertaken to establish a temporal link between the onset of symptoms and the restoration. Other systemic causes of burning mouth syndrome, such as endocrine, neurological, or psychogenic disorders, should also be excluded. Patch testing for nickel, cobalt, or palladium hypersensitivity can help confirm immunological involvement [95].
- Once a prosthesis-related cause is suspected, the definitive management involves the removal or replacement of the offending PFM crown. Metal-free restorations, particularly zirconia or lithium disilicate crowns, are the preferred substitutes because they eliminate the source of ion release while providing good esthetics and strength. In situations where financial constraints or clinical limitations necessitate continued use of PFMs, selecting high-noble alloys with a higher content of gold or platinum is advisable, as these materials exhibit significantly lower corrosion rates and better biocompatibility [96].
- For patients with active mucosal lesions or burning discomfort, symptomatic relief measures may be required until replacement is carried out. Topical corticosteroids or calcineurin inhibitors (e.g., tacrolimus) can reduce lichenoid inflammation, while topical anesthetics, clonazepam rinses, or capsaicin rinses may help attenuate burning sensations through desensitization of oral nociceptors. Strict oral hygiene maintenance, reduction in parafunctional habits, and avoidance of acidic foods or alcohol-containing mouth rinses further support mucosal healing. Importantly, patients with lichenoid lesions should be kept under regular surveillance, as these lesions carry a small but real risk of malignant transformation [97,98].
7. Other Potential Triggers and Confounders
8. Differentiation from Primary Burning Mouth Syndrome and Other Lichenoid Conditions
9. Limitations of the Hypothesis
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gallucci, G.O.; Grütter, L.; Nedir, R.; Bischof, M.; Belser, U.C. Esthetic outcomes with porcelain-fused-to-ceramic and all-ceramic single-implant crowns: A randomized clinical trial. Clin. Oral Implant. Res. 2011, 22, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R. A comparison of all-ceramic restorative systems: Part 2. Gen. Dent. 2000, 48, 35–38. [Google Scholar]
- Zarone, F.; Russo, S.; Sorrentino, R. From porcelain-fused-to-metal to zirconia: Clinical and experimental considerations. Dent. Mater. 2011, 27, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.G.; Cipra, D.L. Alternative crown systems: Is the metal-ceramic crown always the restoration of choice? Dent. Clin. N. Am. 1992, 36, 765–782. [Google Scholar] [CrossRef]
- Griffin, J.D., Jr. Combining monolithic zirconia crowns, digital impressioning, and regenerative cement for a predictable restorative alternative to PFM. Compend. Contin. Educ. Dent. 2013, 34, 212–222. [Google Scholar]
- Haugen, H.J.; Soltvedt, B.M.; Nguyen, P.N.; Ronold, H.J.; Johnsen, G.F. Discrepancy in alloy composition of imported and non-imported porcelain-fused-to-metal (PFM) crowns produced by Norwegian dental laboratories. Biomater. Investig. Dent. 2020, 7, 41–49. [Google Scholar] [CrossRef]
- Roach, M. Base metal alloys used for dental restorations and implants. Dent. Clin. N. Am. 2007, 51, 603–627. [Google Scholar] [CrossRef]
- Tramontana, M.; Bianchi, L.; Hansel, K.; Agostinelli, D.; Stingeni, L. Nickel allergy: Epidemiology, pathomechanism, clinical patterns, treatment and prevention programs. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 992–1002. [Google Scholar] [CrossRef]
- Moslehifard, E.; Ghaffari, T.; Mohammadian-Navid, S.; Ghafari-Nia, M.; Farmani, A.; Nasirpouri, F. Effect of chemical passivation on corrosion behavior and ion release of a commercial chromium-cobalt alloy. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 171. [Google Scholar] [CrossRef]
- Rokni, A.; Zakeralhosseini, R.; Moayed, M.H. An investigation on the effect of molybdenum alloy element and molybdate inhibitor on the stable and metastable pits and its correlation with the pit morphology. J. Taiwan Inst. Chem. Eng. 2023, 153, 105203. [Google Scholar] [CrossRef]
- Bajraktarova-Valjakova, E.; Korunoska-Stevkovska, V.; Kapusevska, B.; Gigovski, N.; Bajraktarova-Misevska, C.; Grozdanov, A. Contemporary dental ceramic materials: A review of chemical composition, physical and mechanical properties, and indications for use. Open Access Maced. J. Med. Sci. 2018, 6, 1742. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C. Biocompatibility of dental casting alloys: A review. J. Prosthet. Dent. 2000, 83, 223–234. [Google Scholar] [CrossRef]
- Cai, Z.; Vermilyea, S.G.; Brantley, W.A. In vitro corrosion resistance of high-palladium dental casting alloys. Dent. Mater. 1999, 15, 202–210. [Google Scholar] [CrossRef]
- Vaillant-Corroy, A.; Corne, P.; De March, P.; Fleutot, S.; Cleymand, F. Influence of recasting on the quality of dental alloys: A systematic review. J. Prosthet. Dent. 2015, 114, 205–211.e3. [Google Scholar] [CrossRef]
- Ansarifard, E.; Farzin, M.; Zohour Parlack, A.; Taghva, M.; Zare, R. Comparing castability of nickel-chromium, cobalt-chromium, and non-precious gold color alloys using two different casting techniques. J. Dent. 2022, 23, 7–12. [Google Scholar]
- Brukl, C.E.; Ocampo, R.R. Compressive strengths of a new foil and porcelain-fused-to-metal crowns. J. Prosthet. Dent. 1987, 57, 404–410. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Hagmann, E.; Weiger, R. What is the prevalence of various types of prosthetic dental restorations in Europe? Clin. Oral Implants Res. 2007, 18 (Suppl. 3), 20–33. [Google Scholar] [CrossRef]
- Eliasson, A.; Arnelund, C.F.; Johansson, A. A clinical evaluation of cobalt-chromium metal-ceramic fixed partial dentures and crowns: A three- to seven-year retrospective study. J. Prosthet. Dent. 2007, 98, 6–16. [Google Scholar] [CrossRef]
- Alnazzawi, A. Effect of fixed metallic oral appliances on oral health. J. Int. Soc. Prev. Community Dent. 2018, 8, 93. [Google Scholar] [CrossRef]
- Marino, R.; Capaccio, P.; Pignataro, L.; Spadari, F. Burning mouth syndrome: The role of contact hypersensitivity. Oral Dis. 2009, 15, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Näpänkangas, R.; Haikola, B.; Oikarinen, K.; Söderholm, A.L.; Remes-Lyly, T.; Sipilä, K. Prevalence of single crowns and fixed partial dentures in elderly citizens in Finland. J. Oral Rehabil. 2011, 38, 328–332. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.C.; Wu, B.; Yu, L.Y.; Wang, X.P.; Liu, Y. A comparative analysis of metal allergens associated with dental alloy prostheses and the expression of HLA-DR in gingival tissue. Mol. Med. Rep. 2016, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, M.; Kim, H.; Kim, Y.; Kim, C. Evaluation of marginal and internal gaps of Ni–Cr and Co–Cr alloy copings manufactured by microstereolithography. J. Adv. Prosthodont. 2017, 9, 176. [Google Scholar] [CrossRef]
- Grosgogeat, B.; Vaicelyte, A.; Gauthier, R.; Janssen, C.; Le Borgne, M. Toxicological risks of cobalt–chromium alloys in dentistry: A systematic review. Materials 2022, 15, 5801. [Google Scholar] [CrossRef] [PubMed]
- Majerič, P.; Lazić, M.M.; Mitić, D.; Lazić, M.; Lazić, E.K.; Vastag, G.; Anžel, I.; Lazić, V.; Rudolf, R. The thermomechanical, functional and biocompatibility properties of a Au–Pt–Ge alloy for PFM dental restorations. Materials 2023, 17, 5491. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Rylska, D.; Januszewicz, B.; Konieczny, B.; Cichomski, M.; Matinlinna, J.P.; Radwanski, M.; Sokolowski, J.; Lukomska-Szymanska, M. Corrosion resistance of titanium dental implant abutments: Comparative analysis and surface characterization. Materials 2023, 16, 6624. [Google Scholar] [CrossRef]
- Succaria, F.; Morgano, S.M. Prescribing a dental ceramic material: Zirconia vs lithium-disilicate. Saudi Dent. J. 2011, 23, 165. [Google Scholar] [CrossRef]
- Imirzalioglu, P.; Alaaddinoglu, E.; Yilmaz, Z.; Oduncuoglu, B.; Yilmaz, B.; Rosenstiel, S. Influence of recasting different types of dental alloys on gingival fibroblast cytotoxicity. J. Prosthet. Dent. 2012, 107, 24–33. [Google Scholar] [CrossRef]
- Abu-Hassan, M.I.; Abu-Hammad, O.A.; Harrison, A. Stress distribution associated with loaded ceramic onlay restorations with different designs of marginal preparation: An FEA study. J. Oral Rehabil. 2000, 27, 294–298. [Google Scholar] [CrossRef]
- Lu, C.; Zheng, Y.; Zhong, Q. Corrosion of dental alloys in artificial saliva with Streptococcus mutans. PLoS ONE 2017, 12, e017444. [Google Scholar] [CrossRef]
- Schmalz, G.; Garhammer, P. Biological interactions of dental cast alloys with oral tissues. Dent. Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, M.; Huang, M.; Wang, C.; Hung, C.; Chen, C. Survival assessment of fractured porcelain-fused-to-metal crowns surface roughened by sandblasting and repaired by composite resin after in vitro thermal fatigue. J. Dent. Sci. 2023, 18, 1706. [Google Scholar] [CrossRef] [PubMed]
- Bilhan, H.; Bilgin, T.; Cakir, A.F.; Yuksel, B.; Von Fraunhofer, J. The effect of mucine, IgA, urea, and lysozyme on the corrosion behavior of various non-precious dental alloys and pure titanium in artificial saliva. J. Biomater. Appl. 2007, 22, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Poljak-Guberina, R.; Knezović-Zlatarić, D.; Katunarić, M. Dental alloys and corrosion resistance. Acta Stomatol. Croat. 2002, 36, 447–450. [Google Scholar]
- Piñeda-Zayas, A.; Menendez Lopez-Mateos, L.; Palma-Fernández, J.C.; Iglesias-Linares, A. Assessment of metal ion accumulation in oral mucosa cells of patients with fixed orthodontic treatment and cellular DNA damage: A systematic review. Crit. Rev. Toxicol. 2021, 51, 622–633. [Google Scholar] [CrossRef]
- Trombetta, D.; Mondello, M.R.; Cimino, F.; Cristani, M.; Pergolizzi, S.; Saija, A. Toxic effect of nickel in an in vitro model of human oral epithelium. Toxicol. Lett. 2005, 159, 219–225. [Google Scholar] [CrossRef]
- Waldman, S.D. Burning Mouth Syndrome. In Atlas of Uncommon Pain Syndromes, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2011; pp. 45–52. [Google Scholar]
- Ditrichova, D.; Kapralova, S.; Tichy, M.; Ticha, V.; Dobesova, J.; Justova, E.; Eber, M.; Pirek, P. Oral lichenoid lesions and allergy to dental materials. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2007, 151, 333–339. [Google Scholar] [CrossRef]
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2503. [Google Scholar] [CrossRef]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of neural transient receptor potential channels in peripheral inflammation. Front. Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef]
- Gao, N.; Li, M.; Wang, W.; Liu, Z.; Guo, Y. The dual role of TRPV1 in peripheral neuropathic pain: Pain switches caused by its sensitization or desensitization. Front. Mol. Neurosci. 2024, 17, 1400118. [Google Scholar] [CrossRef]
- Muris, J.; Scheper, R.J.; Kleverlaan, C.J.; Rustemeyer, T.; Feilzer, A.J. Palladium-based dental alloys are associated with oral disease and palladium-induced immune responses. Contact Dermat. 2014, 71, 82–91. [Google Scholar] [CrossRef]
- Sadrolvaezin, A.; Pezhman, A.; Zare, I.; Nasab, S.Z.; Chamani, S.; Naghizadeh, A.; Mostafavi, E. Systemic allergic contact dermatitis to palladium, platinum, and titanium: Mechanisms, clinical manifestations, prevalence, and therapeutic approaches. MedComm 2023, 4, e386. [Google Scholar] [CrossRef] [PubMed]
- Vrbova, R.; Podzimek, S.; Himmlova, L.; Roubickova, A.; Janovska, M.; Janatova, T.; Bartos, M.; Vinsu, A. Titanium and other metal hypersensitivity diagnosed by MELISA® test: Follow-up study. BioMed Res. Int. 2021, 2021, 5512091. [Google Scholar]
- Muris, J.; Goossens, A.; Gonçalo, M.; Bircher, A.J.; Giménez-Arnau, A.; Foti, C.; Rustemeyer, T.; Feilzer, A.J.; Kleverlaan, C.J. Sensitization to palladium and nickel in Europe and the relationship with oral disease and dental alloys. Contact Dermat. 2015, 72, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Chipinda, I.; Hettick, J.M.; Siegel, P.D. Haptenation: Chemical reactivity and protein binding. J. Allergy 2011, 2011, 839682. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Kumagai, K.; Matsubara, R.; Nasu, K.; Kitaura, K.; Suzuki, M.; Hamada, Y.; Suzuki, R. Characterization of metal-specific T-cells in inflamed oral mucosa in a novel murine model of chromium-induced allergic contact dermatitis. Int. J. Mol. Sci. 2022, 24, 2807. [Google Scholar] [CrossRef]
- Guo, J.; Tian, B.; Wei, R.; Wang, W.; Zhang, H.; Wu, X.; He, L.; Zhang, S. Investigation of the time-dependent wear behavior of veneering ceramic in porcelain-fused-to-metal crowns during chewing simulations. J. Mech. Behav. Biomed. Mater. 2014, 40, 23–32. [Google Scholar] [CrossRef]
- Alghazzawi, T.F. Clinical survival rate and laboratory failure of dental veneers: A narrative literature review. J. Funct. Biomater. 2024, 15, 131. [Google Scholar] [CrossRef]
- Procházková, J.; Podzimek, S.; Tomka, M.; Kucerová, H.; Mihaljevic, M.; Hána, K.; Miksovský, M.; Sterzl, I.; Vinsová, J. Metal alloys in the oral cavity as a cause of oral discomfort in sensitive patients. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 1), 53–58, Erratum in Neuro Endocrinol. Lett. 2007, 28, iii. [Google Scholar]
- Quadras, D.D.; Krishna Nayak, U.S.; Kumari, N.S.; Priyadarshini, H.R.; Gowda, S.; Fernandes, B. In vivo study on the release of nickel, chromium, and zinc in saliva and serum from patients treated with fixed orthodontic appliances. Dent. Res. J. 2019, 16, 209. [Google Scholar] [CrossRef]
- Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular mechanisms of nickel allergy. Int. J. Mol. Sci. 2016, 17, 202. [Google Scholar] [CrossRef]
- Carroll, S.; Wood, E.J. Exposure of human keratinocytes and fibroblasts in vitro to nickel sulphate ions induces synthesis of stress proteins Hsp72 and Hsp90. Acta Derm. Venereol. 2000, 80, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Shuba, Y.M. Beyond neuronal heat sensing: Diversity of TRPV1 heat-capsaicin receptor-channel functions. Front. Cell. Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef]
- Luebbert, M.; Radtke, D.; Wodarski, R.; Damann, N.; Hatt, H.; Wetzel, C.H. Direct activation of transient receptor potential V1 by nickel ions. Pflügers Arch. 2010, 459, 737–750. [Google Scholar] [CrossRef]
- Willis, W.D., Jr. The role of TRPV1 receptors in pain evoked by noxious thermal and chemical stimuli. Exp. Brain Res. 2009, 196, 5–11. [Google Scholar] [CrossRef]
- Tsushima, F.; Sakurai, J.; Shimizu, R.; Kayamori, K.; Harada, H. Oral lichenoid contact lesions related to dental metal allergy may resolve after allergen removal. J. Dent. Sci. 2021, 17, 1300. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Schultze, J.; Lohrengel, M. Stability, reactivity and breakdown of passive films: Problems of recent and future research. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Mlinaric, M.R.; Kanizaj, L.; Zuljevic, D.; Katic, V.; Spalj, S.; Curkovic, H.O. Effect of oral antiseptics on the corrosion stability of nickel–titanium orthodontic alloys. Mater. Corros. 2018, 69, 510–518. [Google Scholar] [CrossRef]
- Kurowska, E.; Bonna, A.; Goch, G.; Bal, W. Salivary histatin-5, a physiologically relevant ligand for Ni(II) ions. J. Inorg. Biochem. 2011, 105, 1220–1225. [Google Scholar] [CrossRef]
- Bowman, C.; Benet, L. An examination of protein binding and protein-facilitated uptake relating to in vitro–in vivo extrapolation. Eur. J. Pharm. Sci. 2018, 123, 502–509. [Google Scholar] [CrossRef]
- Tanaka, F.; Uda, M.; Hirose, Y.; Hirai, Y. Restoration of calcium-induced differentiation potential and tight junction formation in HaCaT keratinocytes by functional attenuation of overexpressed high mobility group box-1 protein. Cytotechnology 2020, 72, 165–173. [Google Scholar] [CrossRef]
- Anyachor, C.P.; Dooka, D.B.; Orish, C.N.; Amadi, C.N.; Bocca, B.; Ruggieri, F.; Senofonte, M.; Frazzoli, C.; Orisakwe, O.E. Mechanistic considerations and biomarker levels in nickel-induced neurodegenerative diseases: An updated systematic review. IBRO Neurosci. Rep. 2022, 13, 136–146. [Google Scholar] [CrossRef]
- Germande, O.; Ducret, T.; Quignard, F.; Deweirdt, J.; Freund-Michel, V.; Errera, H.; Cardouat, G.; Vacher, P.; Muller, B.; Berger, P.; et al. NiONP-induced oxidative stress and mitochondrial impairment in an in vitro pulmonary vascular cell model mimicking endothelial dysfunction. Antioxidants 2022, 11, 847. [Google Scholar] [CrossRef]
- Peana, M.; Zdyb, K.; Medici, S.; Pelucelli, A.; Simula, G.; Gumienna-Kontecka, E.; Zoroddu, M.A. Ni(II) interaction with a peptide model of the human TLR4 ectodomain. J. Trace Elem. Med. Biol. 2017, 44, 151–160. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Z.; Qiu, Y.; Gao, Y.; Fan, Y.; Wang, Q.; Zhou, Q. NLRP3 inflammasome activation in response to metals. Front. Immunol. 2023, 14, 1055788. [Google Scholar] [CrossRef]
- Moretti, J.; Blander, J.M. Increasing complexity of NLRP3 inflammasome regulation. J. Leukoc. Biol. 2020, 109, 561–575. [Google Scholar] [CrossRef]
- Tapia, V.S.; Daniels, M.J.D.; Palazón-Riquelme, P.; Dewhurst, M.; Luheshi, N.M.; Rivers-Auty, J.; Green, J.; Redondo-Castro, E.; Kaldis, P.; Lopez-Castejon, G.; et al. The three cytokines IL-1β, IL-18, and IL-1α share related but distinct secretory routes. J. Biol. Chem. 2019, 294, 8325–8335. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, K.; Bruze, M.; Ahlborg, N. T-helper 22 cell type responses to nickel in contact allergic subjects are associated with T-helper 1, T-helper 2, and T-helper 17 cell cytokine profile responses and patch test reactivity. Int. Arch. Allergy Immunol. 2023, 184, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; Lebonvallet, N.; Gall-Ianotto, C.L.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, L.; Lefeuvre, L.; Misery, L.; Le Garrec, R. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and sensitization. Protein Cell 2017, 8, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, Z.; Sun, Y.; Kingery, W.S.; Clark, J.D. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology 2012, 116, 882–895. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Zhang, L.; Shi, X.; Liao, T.; Jie, L.; Yu, L.; Wang, P. NGF signaling exacerbates KOA peripheral hyperalgesia via increased TRPV1-labeled synovial sensory innervation in KOA rats. Pain Res. Manag. 2024, 2024, 1552594. [Google Scholar] [CrossRef]
- Jose, C.C.; Wang, Z.; Tanwar, V.S.; Zhang, X.; Zang, C.; Cuddapah, S. Nickel-induced transcriptional changes persist post exposure through epigenetic reprogramming. Epigenetics Chromatin 2019, 12, 75. [Google Scholar] [CrossRef]
- Morou-Bermudez, E.; Elias-Boneta, A.; Billings, R.; Burne, R.; Garcia-Rivas, V.; Brignoni-Nazario, V.; Suarez-Perez, E. Urease activity in dental plaque and saliva of children during a three-year study period and its relationship with other caries risk factors. Arch. Oral Biol. 2011, 56, 1282–1289. [Google Scholar] [CrossRef]
- Guo, C.; Shi, S.; Dai, H.; Yu, J.; Chen, X. Corrosion mechanisms of nickel-based alloys in chloride-containing hydrofluoric acid solution. Eng. Fail. Anal. 2022, 140, 106580. [Google Scholar] [CrossRef]
- Chepelova, N.; Antoshin, A.; Voloshin, S.; Usanova, A.; Efremov, Y.; Makeeva, M.; Evlashin, S.; Stepanov, M.; Turkina, A.; Timashev, P. Oral galvanism side effects: Comparing alloy ions and galvanic current effects on the mucosa-like model. J. Funct. Biomater. 2023, 14, 564. [Google Scholar] [CrossRef]
- Tomova, Z.; Vlahova, A.; Zlatev, S.; Stoeva, I.; Tomov, D.; Davcheva, D.; Hadzhigaev, V. Clinical evaluation of corrosion resistance, ion release, and biocompatibility of CoCr alloy for metal-ceramic restorations produced by CAD/CAM technologies. Dent. J. 2023, 11, 166. [Google Scholar] [CrossRef]
- Selvaraj, M.; Mohaideen, K.; Sennimalai, K.; Gothankar, G.S.; Arora, G. Effect of oral environment on contemporary orthodontic materials and its clinical implications. J. Orthod. Sci. 2023, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Kamath, V.V.; Setlur, K.; Yerlagudda, K. Oral lichenoid lesions—A review and update. Indian J. Dermatol. 2015, 60, 102. [Google Scholar] [CrossRef] [PubMed]
- Riedel, F.; Curato, C.; Thierse, H.; Siewert, K.; Luch, A. Immunological mechanisms of metal allergies and the nickel-specific TCR–pMHC interface. Int. J. Environ. Res. Public Health 2020, 18, 10867. [Google Scholar] [CrossRef]
- Özcan, M. Fracture reasons in ceramic-fused-to-metal restorations. J. Oral Rehabil. 2003, 30, 265–269. [Google Scholar] [CrossRef]
- Arakelyan, M.; Spagnuolo, G.; Iaculli, F.; Dikopova, N.; Antoshin, A.; Timashev, P.; Turkina, A. Minimization of adverse effects associated with dental alloys. Materials 2022, 15, 7476. [Google Scholar] [CrossRef] [PubMed]
- Kinsel, R.P.; Lin, D. Retrospective analysis of porcelain failures of metal–ceramic crowns and fixed partial dentures supported by 729 implants in 152 patients: Patient-specific and implant-specific predictors of ceramic failure. J. Prosthet. Dent. 2009, 101, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Farga-Niñoles, I.; Agustín-Panadero, R.; Román-Rodríguez, J.L.; Solá-Ruíz, M.F.; Granell-Ruíz, M.; Fons-Font, A. Fractographic study of the behavior of different ceramic veneers on full coverage crowns in relation to supporting core materials. J. Clin. Exp. Dent. 2013, 5, e260. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Mozaffari, H.R.; Ramezani, M.; Sadeghi, M. Effect of fixed orthodontic treatment on salivary nickel and chromium levels: A systematic review and meta-analysis of observational studies. Dent. J. 2019, 7, 21. [Google Scholar] [CrossRef]
- Dwivedi, A.; Tikku, T.; Khanna, R.; Maurya, R.P.; Verma, G.; Murthy, R.C. Release of nickel and chromium ions in the saliva of patients with fixed orthodontic appliance: An in-vivo study. Natl. J. Maxillofac. Surg. 2015, 6, 62. [Google Scholar] [CrossRef]
- Behr, M.; Zeman, F.; Baitinger, T.; Galler, J.; Koller, M.; Handel, G.; Rosentritt, M. The clinical performance of porcelain-fused-to-metal precious alloy single crowns: Chipping, recurrent caries, periodontitis, and loss of retention. Int. J. Prosthodont. 2014, 27, 153–160. [Google Scholar] [CrossRef]
- Lipsky, M.S.; Singh, T.; Zakeri, G.; Hung, M. Oral health and older adults: A narrative review. Dent. J. 2024, 12, 30. [Google Scholar] [CrossRef]
- Shah, K.M.; Agrawal, M.R.; Chougule, S.A.; Mistry, J.D. Oral lichenoid reaction due to nickel alloy contact hypersensitivity. BMJ Case Rep. 2013, 2013, bcr2013009754. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Zhu, Z.; Lu, C.; Zhang, C.; Zeng, L.; Xie, F.; Zhang, L.; Zhou, F. Mucosal immune response in biology, disease prevention and treatment. Signal Transduct. Target. Ther. 2025, 10, 7. [Google Scholar] [CrossRef]
- Okamoto, N.; Okumura, M.; Tadokoro, O.; Sogawa, N.; Tomida, M.; Kondo, E. Effect of single-nucleotide polymorphisms in TRPV1 on burning pain and capsaicin sensitivity in Japanese adults. Mol. Pain 2018, 14, 1744806918804439. [Google Scholar] [CrossRef] [PubMed]
- Hawthan, M.; Chrcanovic, B.R.; Larsson, C. Retrospective clinical study of tooth-supported single crowns: A multifactor analysis. Eur. J. Oral Sci. 2022, 130, e12871. [Google Scholar] [CrossRef]
- Alberdi-Navarro, J.; Aguirre-Urizar, J.M.; Ginestal-Gómez, E. Clinical presentation of burning mouth syndrome in patients with oral lichenoid disease. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e805. [Google Scholar] [CrossRef]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef]
- Chancellor, M.B. Rationale for the use of topical calcineurin inhibitors in the management of oral lichen planus and mucosal inflammatory diseases. Cureus 2024, 16, e74570. [Google Scholar] [CrossRef] [PubMed]
- Utz, S.; Suter, V.; Cazzaniga, S.; Borradori, L.; Feldmeyer, L. Outcome and long-term treatment protocol for topical tacrolimus in oral lichen planus. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2459. [Google Scholar] [CrossRef] [PubMed]
- Wylie, C.M.; Shelton, R.M.; Fleming, G.J.; Davenport, A.J. Corrosion of nickel-based dental casting alloys. Dent. Mater. 2007, 23, 714–723. [Google Scholar] [CrossRef]
- Ouédraogo, Y.; Sakira, A.K.; Ouédraogo, M.; Tapsoba, I.; Konsem, T.; Beugré, J.B. Influence of pH on the release of nickel ions from fixed orthodontic appliances in artificial saliva. J. Orthod. Sci. 2024, 13, 49. [Google Scholar] [CrossRef]
- Bonefeld, C.M.; Nielsen, M.M.; Vennegaard, M.T.; Johansen, J.D.; Geisler, C.; Thyssen, J.P. Nickel acts as an adjuvant during cobalt sensitization. Exp. Dermatol. 2015, 24, 229–231. [Google Scholar] [CrossRef]
- Derr, R.; Moelijker, N.; Hendriks, G.; Brandsma, I. A tiered approach to investigate the inhalation toxicity of cobalt substances. Tier 2b: Reactive cobalt substances induce oxidative stress in ToxTracker and activate hypoxia target genes. Regul. Toxicol. Pharmacol. 2022, 129, 105120. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Váchová, L.; Palková, Z. Hostile environments: Modifying surfaces to block microbial adhesion and biofilm formation. Biomolecules 2025, 15, 754. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Kannoorpatti, K.; Padovan, A.; Thennadil, S. Effect of pH regulation by sulfate-reducing bacteria on corrosion behaviour of duplex stainless steel 2205 in acidic artificial seawater. R. Soc. Open Sci. 2021, 8, 200639. [Google Scholar] [CrossRef]
- Rahmadhini, E.N. Burning tongue and taste alteration in xerostomic undiagnosed diabetic patients with vitamin D deficiency. Diabetes Metab. Syndr. Obes. 2024, 17, 4585. [Google Scholar] [CrossRef]
- Renton, T. Burning mouth syndrome. Rev. Pain 2011, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Binnie, R.; Dobson, M.L.; Chrystal, A.; Hijazi, K. Oral lichen planus and lichenoid lesions—Challenges and pitfalls for the general dental practitioner. Br. Dent. J. 2024, 236, 285. [Google Scholar] [CrossRef] [PubMed]
| Alloy Family | Contains Nickel? | Typical Use with PFM | Corrosion/Galvanic Considerations | Practical Notes | Ref. |
|---|---|---|---|---|---|
| Ni-Cr base-metal | Yes | Common legacy PFM frameworks | Susceptible if porcelain thins; galvanic risk with dissimilar metals | Avoid mixing with Co-Cr or amalgam; polish exposed areas; consider replacement | [23] |
| Co-Cr base-metal | No (nickel-free) | PFM frameworks, RPDs | Generally passive oxide, but galvanic vs. other metals | Prefer over Ni-Cr in sensitized patients; avoid mixed-metal couples | [24] |
| High noble (Au-Pt-Pd) | Trace/none (formulation dependent) | Premium PFM | Good corrosion resistance; lower galvanic risk | Safer replacement option for nickel-sensitive patients; all-ceramic also viable | [25] |
| Titanium (cp Ti) | No | Implant abutments/frames | Excellent corrosion resistance; possible galvanic with other metals | Insulate from base-metals; avoid acidulated fluoride agents | [26] |
| All-ceramic (zirconia, lithium disilicate) | No metal | Full-coverage crowns/veneers | No corrosion | Preferred definitive option when Nallan–Nickel Effect is suspected | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaitanya, N.C.S.K.; Hashim, N.T.; Padmanabhan, V.; Islam, M.S.; Babiker, R.; Mohammed, R.; Rahman, M.M. The Nallan–Nickel Effect: A Mechanistic Perspective on Burning Sensations and Lichenoid Reactions in Long-Serving Porcelain-Fused-to-Metal Restorations. Dent. J. 2025, 13, 507. https://doi.org/10.3390/dj13110507
Chaitanya NCSK, Hashim NT, Padmanabhan V, Islam MS, Babiker R, Mohammed R, Rahman MM. The Nallan–Nickel Effect: A Mechanistic Perspective on Burning Sensations and Lichenoid Reactions in Long-Serving Porcelain-Fused-to-Metal Restorations. Dentistry Journal. 2025; 13(11):507. https://doi.org/10.3390/dj13110507
Chicago/Turabian StyleChaitanya, Nallan C. S. K., Nada Tawfig Hashim, Vivek Padmanabhan, Md Sofiqul Islam, Rasha Babiker, Riham Mohammed, and Muhammed Mustahsen Rahman. 2025. "The Nallan–Nickel Effect: A Mechanistic Perspective on Burning Sensations and Lichenoid Reactions in Long-Serving Porcelain-Fused-to-Metal Restorations" Dentistry Journal 13, no. 11: 507. https://doi.org/10.3390/dj13110507
APA StyleChaitanya, N. C. S. K., Hashim, N. T., Padmanabhan, V., Islam, M. S., Babiker, R., Mohammed, R., & Rahman, M. M. (2025). The Nallan–Nickel Effect: A Mechanistic Perspective on Burning Sensations and Lichenoid Reactions in Long-Serving Porcelain-Fused-to-Metal Restorations. Dentistry Journal, 13(11), 507. https://doi.org/10.3390/dj13110507










