Statistical Method for Dental Clinics for Determining Presence and Stage of Periodontitis with aMMP-8 Mouth Rinse Point-of-Care Test and Digital Reader
Abstract
1. Introduction
2. Materials and Methods
2.1. Hypothesis and Modeling
2.2. aMMP-8 Point-of-Care Testing (POCT)
2.3. Other Oral Health Parameters
2.4. Periodontitis Classification
2.5. Age, WWI and Other Predictors
2.6. Sample Data
2.7. Mathematical Background
3. Results
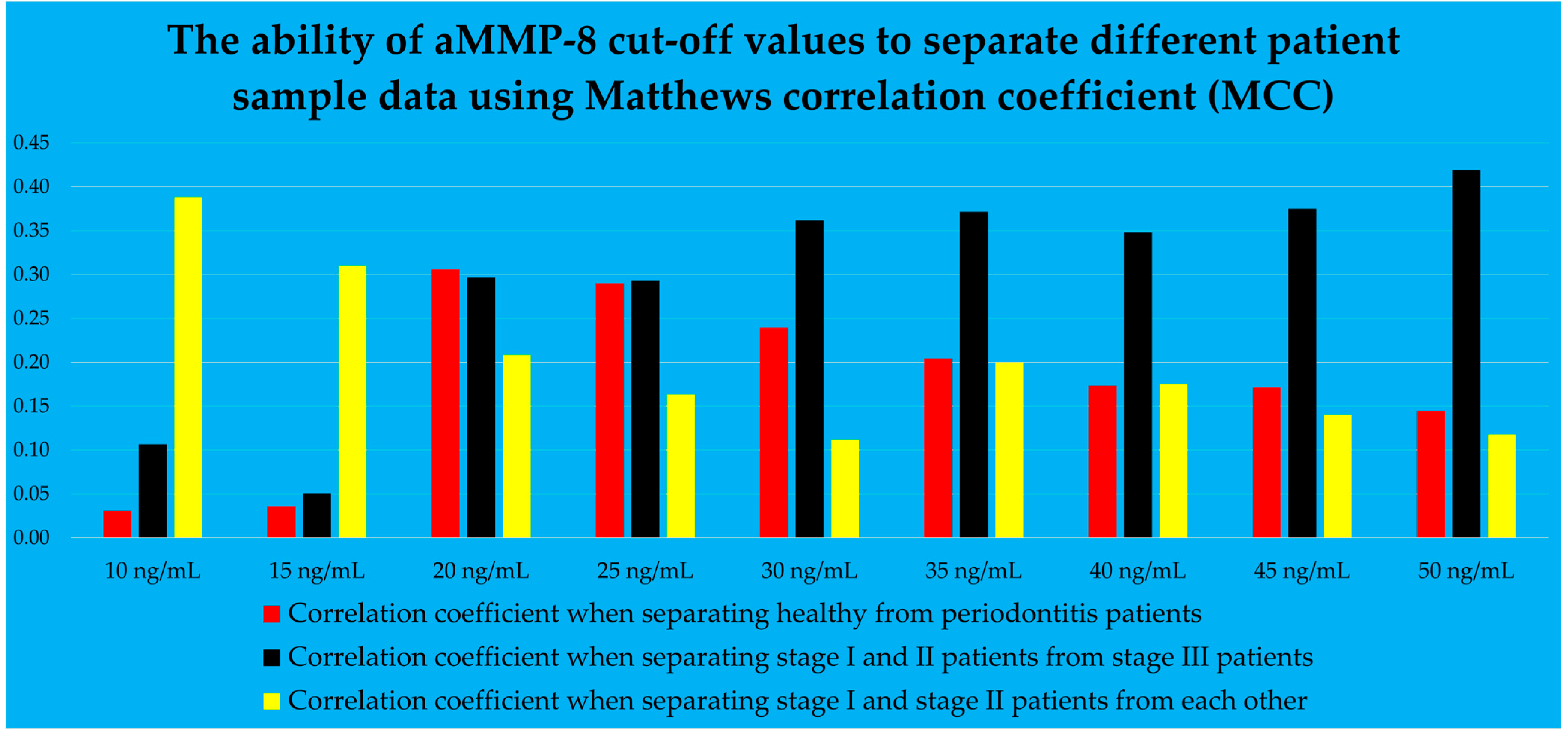
- aMMP-8 mouth rinse test result (cut-off value of 20 ng/mL)
- aMMP-8 mouth rinse test result (cut-off value of 50 ng/mL)
- aMMP-8 mouth rinse test result, examined as a continuous value at 5-point intervals in ng/mL
- Tobacco smoking status
- The visible plaque index (VPI) measured as a continuous value with a precision of two decimal places
- Visible plaque index VPI ≥ 72%
- Number of teeth present
- Number of teeth present: ≥5 teeth missing
- Number of teeth present: ≥8 teeth missing
- Age
- Waist-to-height ratio
- PERIORISK: sensitivity 111/118 = 0.94, specificity 23/31 = 0.74; chi-square goodness-of-fit test χ2 (1, N = 149) = 0.04, p = 0.838; phi coefficient φ = 0.69; AUC = 0.915 (95% CI = 0.865–0.964); Youden’s index = 0.683, cut-off 0.536; accuracy 134/149 = 0.90, F1 score 0.94.
- PERIOSTAGE I/II: sensitivity 67/81 = 0.83, specificity 8/14 = 0.57; chi-square goodness-of-fit test χ2 (1, N = 95) = 3.79, p = 0.052; phi coefficient φ = 0.33; AUC = 0.779 (95% CI = 0.663–0.894); Youden’s index = 0.399, cut-off 0.816 (The highest Youden’s index to determine the performance of the function, i.e., 0.610, yielded a cut-off value of 0.857, was not used in the model in this study because the selected Youden’s index of 0.399 (cut-off value 0.816) produced 75 correct stage predictions, while the aforementioned highest index resulted in two fewer, i.e., 73 correct stage predictions [76]); accuracy 75/95 = 0.79, F1 score 0.87.
- PERIOSTAGE III: sensitivity 13/23 = 0.57, specificity 90/95 = 0.95; chi-square goodness-of-fit test χ2 (1, N = 118) = 1.64, p = 0.201; phi coefficient φ = 0.56; AUC = 0.764 (95% CI = 0.633–0.895); Youden’s index = 0.513, cut-off 0.313; accuracy 103/118 = 0.87, F1 score 0.63.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US adults: National health and nutrition examination survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Bissett, S.M. Periodontitis and diabetes. Br. Dent. J. 2019, 227, 577–584. [Google Scholar] [CrossRef]
- Löe, H. Periodontal disease: The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef]
- Mattila, K.J.; Nieminen, M.S.; Valtonen, V.V.; Rasi, V.P.; Kesäniemi, Y.A.; Syrjälä, S.L.; Jungell, P.S.; Isoluoma, M.; Hietaniemi, K.; Jokinen, M.J. Association between dental health and acute myocardial infarction. Br. Med. J. 1989, 298, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases. Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, 1475. [Google Scholar] [CrossRef]
- Marini, L.; Tonetti, M.S.; Nibali, L.; Rojas, M.A.; Aimetti, M.; Cairo, F.; Cavalcanti, R.; Crea, A.; Ferrarotti, F.; Graziani, F.; et al. The staging and grading system in defining periodontitis cases: Consistency and accuracy amongst periodontal experts, general dentists and undergraduate students. J. Clin. Periodontol. 2021, 48, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Sanz, M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef]
- Chmielewski, M.; Pilloni, A.; Cuozzo, A.; D’Albis, G.; D’Elia, G.; Papi, P.; Marini, L. The 2018 Classification of Periodontitis: Challenges from Clinical Perspective. Dent. J. 2025, 13, 361. [Google Scholar] [CrossRef]
- Kornman, K.S.; Papapanou, P.N. Clinical application of the new classification of periodontal diseases: Ground rules, clarifications and “gray zones”. J. Periodontol. 2020, 91, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.V.O.; Fernandes, J.C.H. Revisiting and rethinking on staging (severity and complexity) periodontitis from the new classification system: A critical review with suggestions for adjustments and a proposal of a new flowchart. Dent. Med. Probl. 2025, 62, 371–391. [Google Scholar] [CrossRef]
- Sorsa, T.; Suomalainen, K.; Uitto, V.J. The role of gingival crevicular fluid and salivary interstitial collagenases in human periodontal diseases. Arch. Oral Biol. 1990, 35, 193S–196S. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Aitken, S.; Sodek, J.; McCulloch, C.A.G. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: Role of active enzyme in human periodontitis. J. Periodontal Res. 1995, 30, 23–33. [Google Scholar] [CrossRef]
- Romanelli, R.; Mancini, S.; Laschinger, C.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Activation of neutrophil collagenase in periodontitis. Infect. Immun. 1999, 67, 2319–2326. [Google Scholar] [CrossRef]
- Mancini, S.; Romanelli, R.; Laschinger, C.A.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J. Periodontol. 1999, 70, 1292–1302. [Google Scholar] [CrossRef]
- Sorsa, T.; Alassiri, S.; Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Nwhator, S.O.; Gieselmann, D.-R.; Sakellari, D. Active MMP-8 (aMMP-8) as a Grading and Staging Biomarker in the Periodontitis Classification. Diagnostics 2020, 10, 61. [Google Scholar] [CrossRef]
- Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Tervahartiala, T.; Sorsa, T.; Sakellari, D. Prediabetes/diabetes screening strategy at the periodontal clinic. Clin. Exp. Dent. Res. 2021, 7, 85–92. [Google Scholar] [CrossRef]
- Penttala, M.; Sorsa, T.; Thomas, J.T.; Grigoriadis, A.; Sakellari, D.; Gupta, S.; Pärnänen, P.; Pätilä, T.; Räisänen, I.T. Periodontitis Home Screening with Mouth Rinse Cut-Off 20 Ng/mL aMMP-8 Test and Mobile Application. Diagnostics 2025, 15, 296. [Google Scholar] [CrossRef]
- Penttala, M.; Sorsa, T.; Thomas, J.T.; Grigoriadis, A.; Sakellari, D.; Sahni, V.; Gupta, S.; Pärnänen, P.; Pätilä, T.; Räisänen, I.T. Determination of the Stage of Periodontitis with 20 ng/mL Cut-Off aMMP-8 Mouth Rinse Test and Polynomial Functions in a Mobile Application. Diagnostics 2025, 15, 1411. [Google Scholar] [CrossRef]
- Penttala, M.; Sorsa, T.; Sakellari, D.; Räisänen, I.T. Using mouth rinse samples for screening periodontitis: Using mouth rinse samples for screening periodontitis. Br. Dent. J. 2025, 239, 367. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFPWorkshop Participants Methodological Consultants; Merete Aass, A.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Kinane, D.F.; Darby, I.B.; Said, S.; Luoto, H.; Sorsa, T.; Tikanoja, S.; Mäntylä, P. Changes in gingival crevicular fluid matrix metalloproteinase-8 levels during periodontal treatment and maintenance. J. Periodontal Res. 2003, 38, 400–404. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef]
- Aji, N.R.A.S.; Yucel-Lindberg, T.; Räisänen, I.T.; Kuula, H.; Nieminen, M.T.; Mc Crudden, M.T.C.; Listyarifah, D.; Lundmark, A.; Lundy, F.T.; Gupta, S.; et al. In Vivo Regulation of Active Matrix Metalloproteinase-8 (aMMP-8) in Periodontitis: From Transcriptomics to Real-Time Online Diagnostics and Treatment Monitoring. Diagnostics 2024, 14, 1011. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lo, E.C.M.; Kot, S.C.C.; Chan, K.C.W. Motivational interviewing in improving oral health: A systematic review of randomized controlled trials. J. Periodontol. 2014, 85, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Aji, N.R.A.S.; Sahni, V.; Penttala, M.T.; Sakellari, D.; Grigoriadis, A.; Pätilä, T.; Pärnänen, P.; Neefs, D.; Pfützner, A.; Gupta, S.; et al. Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe® aMMP-8 POCT, Lumoral® 2× PDT- and Lingora® Fermented Lingonberry Oral Rinse-Treatments. Dent. J. 2025, 13, 127. [Google Scholar] [CrossRef]
- Buduneli, N.; Bıyıkoğlu, B.; Kinane, D.F. Utility of gingival crevicular fluid components for periodontal diagnosis. Periodontology 2000 2024, 95, 156–175. [Google Scholar] [CrossRef]
- Aji, N.R.A.S.; Räisänen, I.T.; Rathnayake, N.; Lundy, F.T.; Mc Crudden MTC: Goyal, L.; Sorsa, T.; Gupta, S. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease. J. Clin. Periodontol. 2021, 48, 1051–1065. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Authors’ Response: “Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease”. J. Clin. Periodontol. 2021, 48, 1499–1500. [Google Scholar] [CrossRef]
- Deng, K.; Wei, S.; Xu, M.; Shi, J.; Lai, H.; Tonetti, M.S. Diagnostic accuracy of active matrix metalloproteinase-8 point-of-care test for the discrimination of periodontal health status: Comparison of saliva and oral rinse samples. J. Periodontal Res. 2022, 57, 768–779. [Google Scholar] [CrossRef]
- Wei, S.; Lin, T.; Sáenz-Ravello, G.; Gao, H.; Zhang, Y.; Tonetti, M.S.; Deng, K. Diagnostic accuracy of salivary active matrix metalloproteinase (aMMP)-8 point-of-care test for detecting periodontitis in adults: A systematic review and meta-analysis. J. Clin. Periodontol. 2024, 51, 1093–1108. [Google Scholar] [CrossRef]
- Guarnieri, R.; Reda, R.; Zanza, A.; Xhajanka, E.; Patil, S.; Di Nardo, D.; Testarelli, L. Relationship between gingival and peri-implant sulcular fluid active matrix metalloproteinase-8 concentration and clinical indices in healthy and diseased conditions. Explor. Med. 2024, 5, 243–256. [Google Scholar] [CrossRef]
- Tschesche, H.; Wenzel, H. Neutrophil Collagenase. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 725–734. [Google Scholar]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Bornes, R.; Montero, J.; Correia, A.; Marques, T.; Rosa, N. Peri-implant diseases diagnosis, prognosis and dental implant monitoring: A narrative review of novel strategies and clinical impact. BMC Oral Health 2023, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Reda, R.; Zanza, A.; Miccoli, G.; Nardo, D.D.; Testarelli, L. Can Peri-Implant Marginal Bone Loss Progression and a-MMP-8 Be Considered Indicators of the Subsequent Onset of Peri-Implantitis? A 5-Year Study. Diagnostics 2022, 12, 2599. [Google Scholar] [CrossRef] [PubMed]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef]
- Shochet, T.; Comstock, I.A.; Ngoc, N.T.N.; Westphal, L.M.; Sheldon, W.R.; Loc, L.T.; Blum, J.; Winikoff, B.; Blumenthal, P.D. Results of a pilot study in the US and Vietnam to assess the utility and acceptability of a multi-level pregnancy test (MLPT) for home monitoring of hCG trends after assisted reproduction. BMC Women’s Health 2017, 17, 67. [Google Scholar] [CrossRef]
- Prazuck, T.; Karon, S.; Gubavu, C.; Andre, J.; Legall, J.M.; Bouvet, E.; Kreplak, G.; Teglas, J.P.; Pialoux, G. A finger-stick whole-blood HIV self-test as an HIV screening tool adapted to the general public. PLoS ONE 2016, 11, e0146755. [Google Scholar] [CrossRef]
- Gul, S.S.; Abdulkareem, A.A.; Sha, A.M.; Rawlinson, A. Diagnostic Accuracy of Oral Fluids Biomarker Profile to Determine the Current and Future Status of Periodontal and Peri-Implant Diseases. Diagnostics 2020, 10, 838. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The nexus between periodontal inflammation and dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 2018, 45, 44–67. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing an indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721. [Google Scholar] [PubMed]
- Belikov, A.V. Age-related diseases as vicious cycles. Ageing Res. Rev. 2019, 49, 11–26. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Shaju, J.P.; Zade, R.M.; Das, M. Prevalence of periodontitis in the Indian population: A literature review. J. Indian Soc. Periodontol. 2011, 15, 29–34. [Google Scholar] [CrossRef]
- Hewlett, S.A.; Anto, F.; Blankson, P.K.; Tormeti, D.; Ayettey-Adamafio, M.; Bayitse, P.; Danso-Appiah, T.; Amoah, A.G. Periodontitis prevalence and severity in an African population: A cross-sectional study in the Greater Accra Region of Ghana. J. Periodontol. 2022, 93, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Dawson, D.A., 3rd; Emecen Huja, P.; Pandruvada, S.; Basu, A.; Nguyen, L.; Zhang, Y.; Gonzalez, O.A. Age and Periodontal Health—Immunological View. Curr. Oral Health Rep. 2018, 5, 229–241. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef]
- Baelum, V.; López, R. Periodontal disease epidemiology—Learned and unlearned? Periodontology 2000 2013, 62, 37–58. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Alves-Costa, S.; Romandini, M. Burden of severe periodontitis and edentulism in 2021, with projections up to 2050: The Global Burden of Disease 2021 study. J. Periodontal Res. 2024, 59, 823–867. [Google Scholar] [CrossRef]
- Kangas, S.; Timonen, P.; Knuuttila, M.; Jula, A.; Ylöstalo, P.; Syrjälä, A.M.H. Waist circumference and waist-to-height ratio are associated with periodontal pocketing—Results of the Health 2000 Survey. BMC Oral Health 2017, 17, 48. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhao, C.; Wang, J.; Zhou, H. Association between weight-adjusted waist index and periodontitis: A population-based study. PLoS ONE 2024, 19, e0305010. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L.; Oates, T.W. Diabetes mellitus and periodontal diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Ding, Z.; Yang, Y. The impact of diabetes on periodontal diseases. Periodontology 2000 2020, 82, 214–224. [Google Scholar] [CrossRef]
- Tsai, C.; Hayes, C.; Taylor, G.W. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent. Oral Epidemiol. 2002, 30, 182–192. [Google Scholar] [CrossRef]
- Bergström, J. Tobacco smoking and chronic destructive periodontal disease. Odontology 2004, 92, 1–8. [Google Scholar] [CrossRef]
- Johnson, G.K.; Slach, N.A. Impact of tobacco use on periodontal status. J. Dent. Educ. 2001, 65, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Nociti Jr, F.H.; Casati, M.Z.; Duarte, P.M. Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontology 2000 2015, 67, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Allison, P. Logistic Regression Using SAS: Theory and Application; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- LaValley, M.P. Logistic regression. Circulation 2008, 117, 2395–2399. [Google Scholar] [CrossRef]
- Sperandei, S. Understanding logistic regression analysis. Biochem. Medica 2014, 24, 12–18. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common pitfalls in statistical analysis: Logistic regression. Perspect. Clin. Res. 2017, 8, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.J.; Lee, K.L.; Ingersoll, G.M. An introduction to logistic regression analysis and reporting. J. Educ. Res. 2002, 96, 3–14. [Google Scholar] [CrossRef]
- Harris, J.K. Primer on binary logistic regression. Fam. Med. Community Health 2021, 9, e001290. [Google Scholar] [CrossRef]
- Menard, S. Applied Logistic Regression Analysis; SAGE Publications: Thousand Oaks, CA, USA, 2001. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Long, S.J. Regression models for categorical and limited dependent variables. In Advanced Quantitative Techniques in the Social Sciences; SAGE Publications: Thousand Oaks, CA, USA, 1997. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Wallis, S. Binomial confidence intervals and contingency tests: Mathematical fundamentals and the evaluation of alternative methods. J. Quant. Linguist. 2013, 20, 178–208. [Google Scholar] [CrossRef]
- Eke, P.I.; Wei, L.; Borgnakke, W.S.; Thornton-Evans, G.; Zhang, X.; Lu, H.; McGuire, L.C.; Genco, R.J. Periodontitis prevalence in adults≥ 65 years of age, in the USA. Periodontology 2000 2016, 72, 76–95. [Google Scholar] [CrossRef]
- Huang, Q.; Dong, X. Prevalence of periodontal disease in middle-aged and elderly patients and its influencing factors. Am. J. Transl. Res. 2022, 14, 5677. [Google Scholar]
- Al-Majid, A.; Alassiri, S.; Rathnayake, N.; Tervahartiala, T.; Gieselmann, D.R.; Sorsa, T. Matrix metalloproteinase-8 as an inflammatory and prevention biomarker in periodontal and peri-implant diseases. Int. J. Dent. 2018, 2018, 7891323. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yan, H.; Huang, L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: A PRISMA-compliant systematic review and meta-analysis. Medicine 2018, 97, e9642. [Google Scholar] [CrossRef]
- Ismail, A.I.; Morrison, E.C.; Burt, B.A.; Caffesse, R.G.; Kavanagh, M.T. Natural history of periodontal disease in adults: Findings from the Tecumseh Periodontal Disease Study, 1959–1987. J. Dent. Res. 1990, 69, 430–435. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.; Gul, S.S.; Sha, A.; Chapple, I.L. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Ding, Y.L.; Ingman, T.; Salo, T.; Westerlund, U.; Haapasalo, M.; Tschesche, H.; Konttinen, Y.T. Cellular source, activation and inhibition of dental plaque collagenase. J. Clin. Periodontol. 1995, 22, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Lertpimonchai, A.; Rattanasiri, S.; Vallibhakara, S.A.O.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef]
- Eke, P.I.; Wei, L.; Thornton-Evans, G.O.; Borrell, L.N.; Borgnakke, W.S.; Dye, B.; Genco, R.J. Risk indicators for periodontitis in US adults: NHANES 2009 to 2012. J. Periodontol. 2016, 87, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Munoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2020, 116, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.; Ryan, M.E. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol. Res. 2011, 63, 114–120. [Google Scholar] [CrossRef]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef]
- Li, Y.; Kung, J.C.K.; Shi, J.; Wu, X.; Lam, S.L.T.; Deng, K.; Zhang, X.; Lai, H.; Pelekos, G.; Jin, L.; et al. Diagnostic Accuracy of a Point-Of-Care aMMP-8 Test for Discriminating Periodontal Health Status in Adults: Validation Trials and Updated Meta-Analysis. J. Clin. Periodontol. 2025, 52, 510–515. [Google Scholar] [CrossRef]
- Teles, R.; Teles, F.; Frias-Lopez, J.; Paster, B.; Haffajee, A. Lessons learned and unlearned in periodontal microbiology. Periodontology 2000 2013, 62, 95–162. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Holde, G.E.; Oscarson, N.; Trovik, T.A.; Tillberg, A.; Jönsson, B. Periodontitis prevalence and severity in adults: A cross-sectional study in Norwegian circumpolar communities. J. Periodontol. 2017, 88, 1012–1022. [Google Scholar] [CrossRef]
- Wilimitis, D.; Walsh, C.G. Practical considerations and applied examples of cross-validation for model development and evaluation in health care: Tutorial. JMIR AI 2023, 2, e49023. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Harrell Jr, F.E.; Borsboom, G.J.; Eijkemans, M.J.C.; Vergouwe, Y.; Habbema, J.D.F. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Imamura, T.; Fukuo, A.; Fukui, T.; Koi, T.; Ueno, Y.; Onoda, H.; Tanaka, S.; Ushijima, R.; Sobajima, M.; et al. Validation of inter-rater and intra-rater reliability of remote dielectric sensing measurement. Int. Heart J. 2022, 63, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Bernardelli, F.; Giulianelli, E.; Toselli, I.; Moscatelli, M.; Pagliaro, U.; Nieri, M. Inter-rater agreement in the diagnosis of mucositis and peri-implantitis. J. Clin. Periodontol. 2014, 41, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.B.; Obermeyer, Z.; Navathe, A.S. Regulation of predictive analytics in medicine. Science 2019, 363, 810–812. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]


| Patient’s Data | Stage of Periodontitis Status | |||
|---|---|---|---|---|
| No Evidence of Periodontitis (n = 31) | Stage I (n = 14) | Stage II (n = 81) | Stage III (n = 23) | |
| Gender | ||||
| Female | 11 | 13 | 39 | 12 |
| Male | 20 | 1 | 42 | 11 |
| Smoking status | ||||
| Tobacco smoker | 3 | 7 | 24 | 10 |
| Non-smoker | 28 | 7 | 57 | 13 |
| Diabetic status | ||||
| Diabetic | 0 | 0 | 3 | 4 |
| Non-diabetic | 31 | 14 | 78 | 19 |
| Age (years) | 43 ± 11 | 62 ± 8 | 55 ± 10 | 56 ± 10 |
| Body mass index (kg/m2) | 30.6 ± 4.5 | 28.6 ± 4.4 | 30.5 ± 4.7 | 29.3 ± 5.9 |
| Weight (kg) | 93 ± 17 | 78 ± 11 | 89 ± 17 | 85 ± 23 |
| Height (cm) | 174 ± 10 | 165 ± 6 | 171 ± 9 | 169 ± 10 |
| Waist circumference (cm) | 100 ± 17 | 98 ± 12 | 103 ± 14 | 105 ± 21 |
| Waist-to-height ratio (cm/cm) | 0.57 ± 0.08 | 0.60 ± 0.09 | 0.60 ± 0.08 | 0.62 ± 0.11 |
| WWI (cm/√kg) | 10.36 ± 1.02 | 11.16 ± 1.08 | 10.93 ± 1.01 | 11.49 ± 1.23 |
| aMMP-8 levels | ||||
| aMMP-8 ≥ 20 ng/mL | 2 | 2 | 31 | 17 |
| aMMP-8 < 20 ng/mL | 29 | 12 | 50 | 6 |
| aMMP-8 ≥ 50 ng/mL | 0 | 0 | 3 | 8 |
| aMMP-8 < 50 ng/mL | 31 | 14 | 78 | 15 |
| aMMP-8 (ng/mL) | 15 ± 5 | 15 ± 10 | 20 ± 10 | 40 ± 25 |
| Stage of periodontitis status | ||||
| No evidence of periodontitis | 31 | 0 | 0 | 0 |
| Stage I | 0 | 14 | 0 | 0 |
| Stage Il | 0 | 0 | 81 | 0 |
| Stage III | 0 | 0 | 0 | 23 |
| Grade of periodontitis status | ||||
| No evidence of progression | 31 | 0 | 0 | 0 |
| Grade A | 0 | 7 | 7 | 0 |
| Grade B | 0 | 7 | 70 | 13 |
| Grade C | 0 | 0 | 4 | 10 |
| CAL (mm) | 2.4 ± 0.5 | 2.3 ± 0.5 | 3.4 ± 0.8 | 4.8 ± 1.2 |
| PPD (mm) | 2.2 ± 0.3 | 2.2 ± 0.4 | 3.0 ± 0.7 | 3.9 ± 0.9 |
| Number of teeth present (No.) | 27 ± 2 | 25 ± 2 | 24 ± 3 | 22 ± 4 |
| VPI (%) | 43 ± 22 | 29 ± 20 | 48 ± 27 | 63 ± 28 |
| BOP (%) | 42 ± 25 | 33 ± 17 | 56 ± 23 | 63 ± 22 |
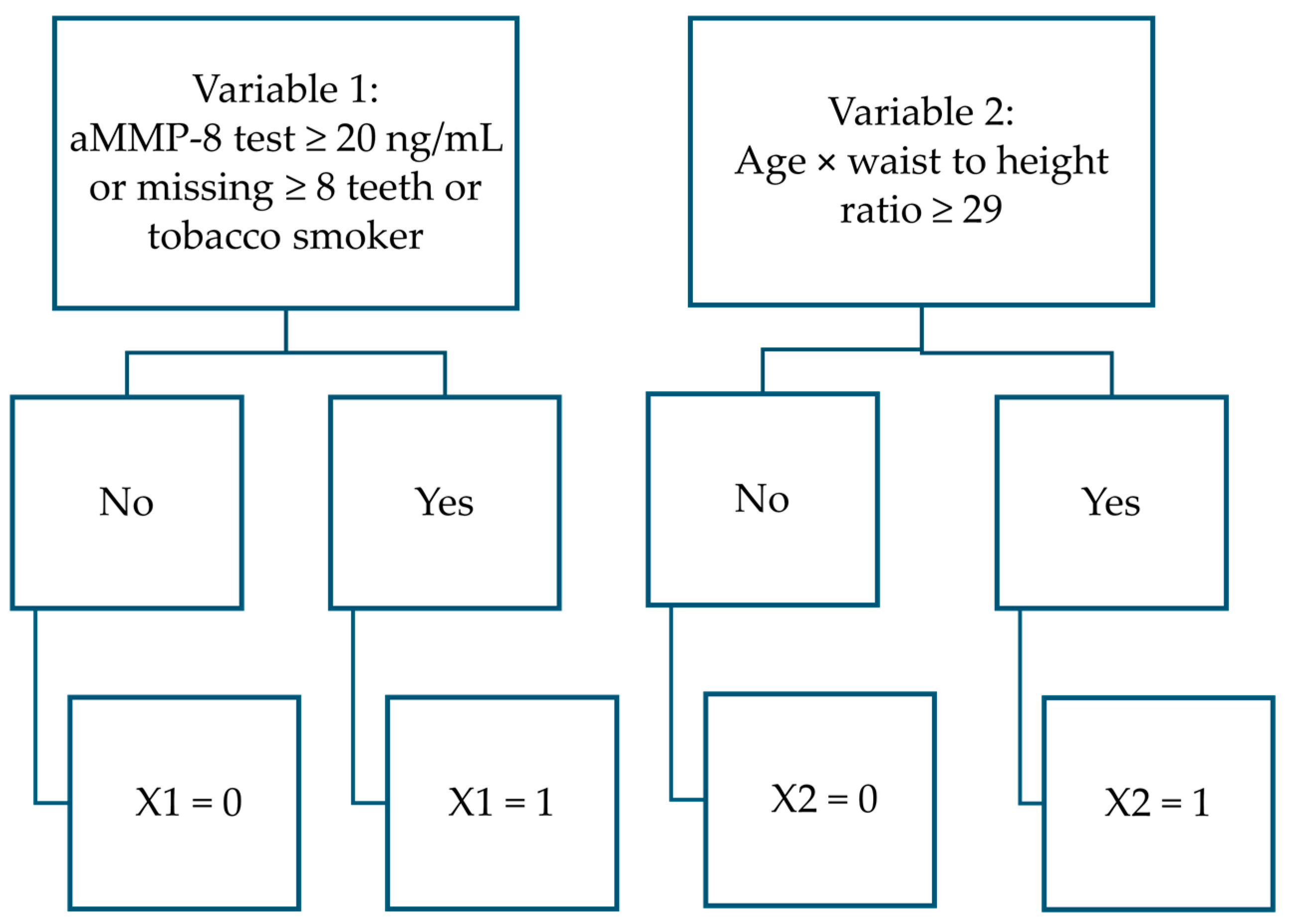
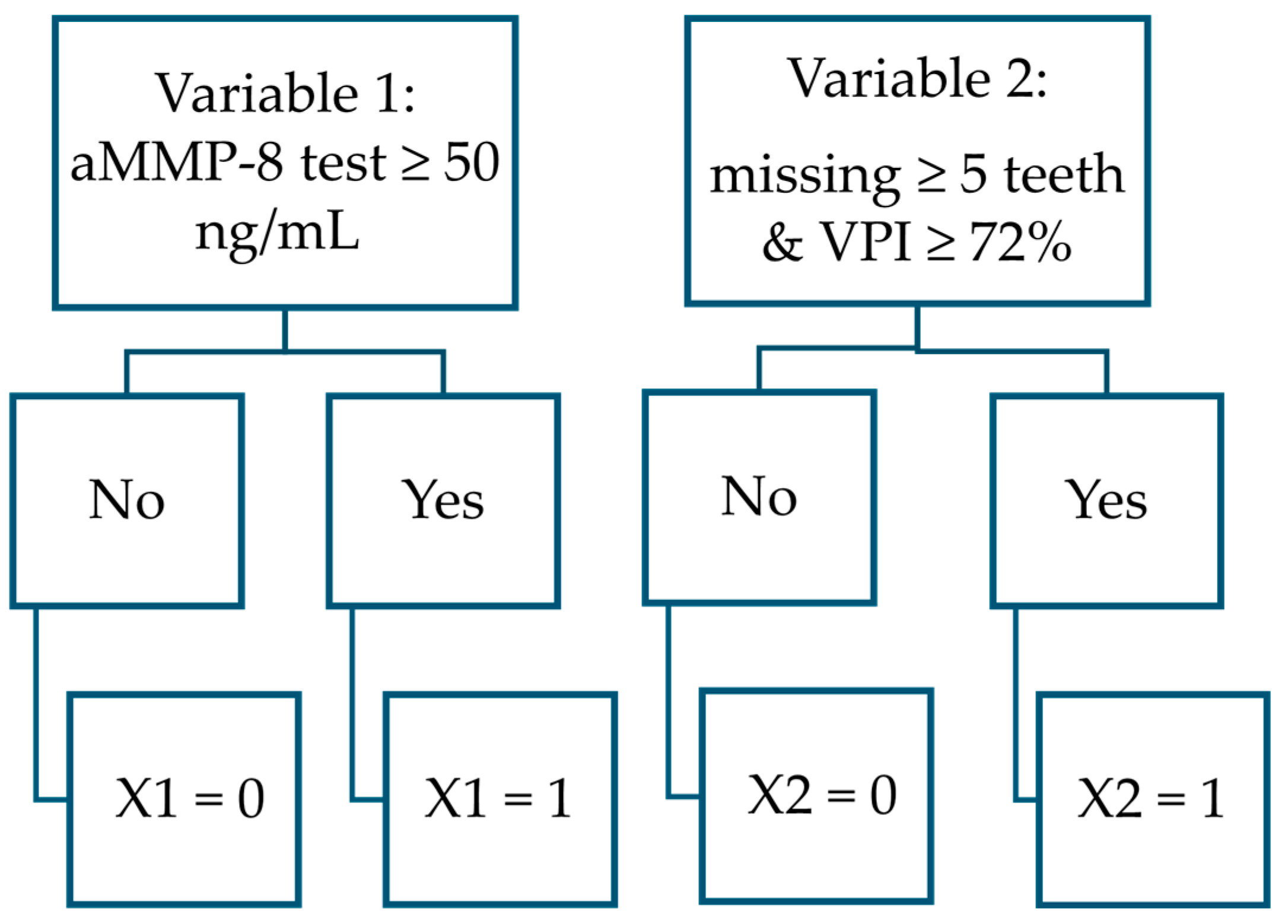
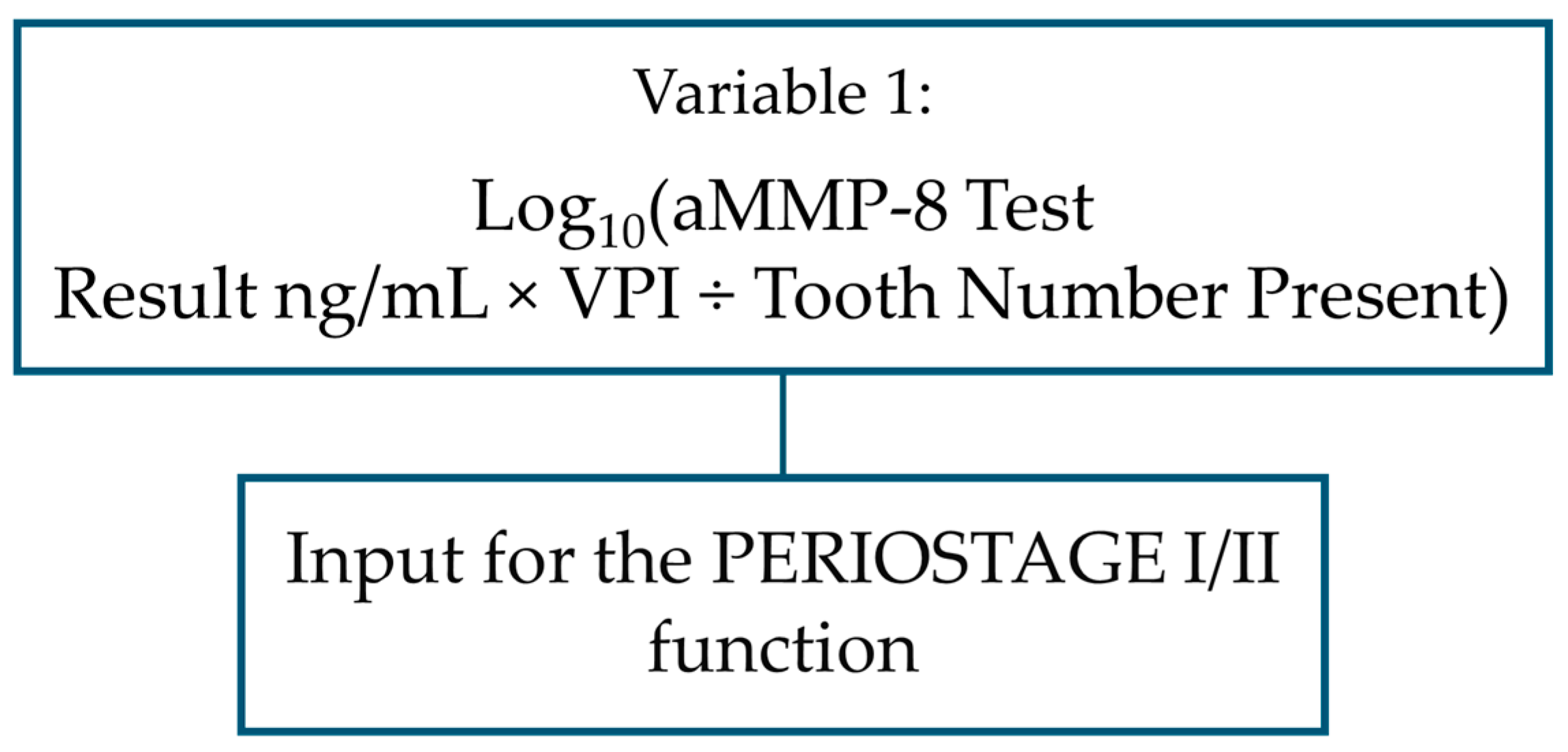
| Functions and Variables | B | S.E. | Wald | df | Sig | Exp(B) |
|---|---|---|---|---|---|---|
| PERIORISK: function separating healthy patients from stage I, II and III patients | ||||||
| aMMP-8 test ≥ 20 ng/mL or tobacco smoker or missing ≥8 teeth | 3.399 | 0.716 | 22.554 | 1 | <0.001 | 29.937 |
| Age × waist-to-height ratio ≥ 29 yrs. × cm/cm | 2.998 | 0.626 | 22.941 | 1 | <0.001 | 20.054 |
| Constant | −1.259 | 0.428 | 8.658 | 1 | 0.003 | 0.284 |
| PERIOSTAGE III: function separating stage III patients from stage I and II patients | ||||||
| aMMP-8 test ≥ 50 ng/mL | 2.328 | 0.838 | 7.714 | 1 | 0.005 | 10.253 |
| Missing ≥ 5 teeth and VPI ≥ 72% | 3.252 | 0.871 | 13.960 | 1 | <0.001 | 25.853 |
| Constant | −2.216 | 0.333 | 44.182 | 1 | <0.001 | 0.109 |
| PERIOSTAGE I/II: function separating stage I and stage II patients from each other | ||||||
| log10 (aMMP-8 concentration in mouth rinse in ng/mL × VPI ÷ number of teeth present) | 1.408 | 0.564 | 6.238 | 1 | 0.013 | 4.089 |
| Constant | −0.035 | 0.728 | 0.002 | 1 | 0.962 | 0.966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penttala, M.; Räisänen, I.T.; Sakellari, D.; Grigoriadis, A.; Sorsa, T. Statistical Method for Dental Clinics for Determining Presence and Stage of Periodontitis with aMMP-8 Mouth Rinse Point-of-Care Test and Digital Reader. Dent. J. 2025, 13, 508. https://doi.org/10.3390/dj13110508
Penttala M, Räisänen IT, Sakellari D, Grigoriadis A, Sorsa T. Statistical Method for Dental Clinics for Determining Presence and Stage of Periodontitis with aMMP-8 Mouth Rinse Point-of-Care Test and Digital Reader. Dentistry Journal. 2025; 13(11):508. https://doi.org/10.3390/dj13110508
Chicago/Turabian StylePenttala, Miika, Ismo T. Räisänen, Dimitra Sakellari, Andreas Grigoriadis, and Timo Sorsa. 2025. "Statistical Method for Dental Clinics for Determining Presence and Stage of Periodontitis with aMMP-8 Mouth Rinse Point-of-Care Test and Digital Reader" Dentistry Journal 13, no. 11: 508. https://doi.org/10.3390/dj13110508
APA StylePenttala, M., Räisänen, I. T., Sakellari, D., Grigoriadis, A., & Sorsa, T. (2025). Statistical Method for Dental Clinics for Determining Presence and Stage of Periodontitis with aMMP-8 Mouth Rinse Point-of-Care Test and Digital Reader. Dentistry Journal, 13(11), 508. https://doi.org/10.3390/dj13110508







