Abstract
Photocatalysis represents an efficient and environmentally friendly technology for wastewater treatment. In this study, a novel composite material, comprising AgBr nanospheres anchored on the surface of SrSnO3 nanorods, was synthesized via a co-precipitation method. Its photocatalytic activity was evaluated using Methyl Orange as the target pollutant. The results demonstrated that the composite photocatalyst achieved a degradation efficiency of 92% within 40 min, which is 7.16 times higher than AgBr. XPS analysis confirmed the successful construction of a built-in electric field between SrSnO3 and AgBr. Photoelectrochemical experiments further verified a significant enhancement in the charge carrier dynamics of the composite catalyst.
1. Introduction
The acceleration of global industrialization has been accompanied by a growing problem of water pollution [1]. In particular, the discharge of organic dye wastewater from textile and printing industries has become increasingly prominent [2]. Dyes such as Methyl Orange (MO), methylene blue (MB), and rhodamine B (RhB) pose significant hazards in natural water environments and are known to considerably increase the risk of cancer in humans [3,4]. Photocatalytic degradation represents a promising approach for treating organic wastewater using solar energy, offering advantages such as low energy consumption and no secondary pollution [5]. Therefore, it is crucial to develop efficient and environmentally friendly technologies for pollutant removal [6].
For a photocatalyst to be viable, it must exhibit a broad spectral response, achieve efficient separation of photogenerated charge carriers, and maintain structural stability [7]. AgBr emerges as an attractive candidate in this context. Being an inorganic halide with a relatively small band gap of approximately 2.55 eV, it possesses a strong capacity for visible light absorption, which is a fundamental prerequisite for harnessing solar energy effectively [8]. These optical and electrical properties make AgBr highly suitable for building heterostructures [9]. For example, forming a heterojunction with CuBi2O4 improves tetracycline degradation through a zigzag charge transfer mechanism [10]. Likewise, creating a ternary heterojunction with BiOBr and BiOCl greatly expands the visible-light absorption range, resulting in a RhB degradation rate that is significantly higher than that of any single-component photocatalyst [11]. As a typical perovskite oxide, SrSnO3 is valued in applications like superconductors and sensors due to its unique cubic lattice [12]. This structure provides numerous oxygen vacancies and metal active centers that naturally trap photogenerated electron–hole pairs, complemented by excellent chemical stability for harsh photocatalytic conditions [13]. While beneficial, SrSnO3 has a wide band gap (~4.0 eV) limits its light absorption to the UV region [14]. To address this and improve carrier separation, researchers have modified SrSnO3 with Ag to increase visible-light absorption [15]. Since AgBr itself suffers from instability and often performs best in heterostructures [16], combining it with SrSnO3 to create a novel composite offers a pathway to capitalize on their synergistic interactions.
An analysis of the photochemical properties of AgBr and SrSnO3 reveals a remarkable complementarity between them for photocatalysis. SrSnO3 offers high chemical stability and abundant active sites, effectively compensating for the poor photochemical stability of AgBr [17]. Conversely, the strong visible-light response of AgBr can significantly extend the absorption range of SrSnO3 into the visible spectrum. The establishment of an efficient heterojunction interface between the two materials is anticipated to significantly enhance the separation and migration of photogenerated charge carriers [18]. Consequently, this work presents a detailed study on the SrSnO3/AgBr heterojunction, systematically examining its crystal structure, optical characteristics, and performance in the photocatalytic degradation of Methyl Orange (MO) [19]. A thorough discussion of the fundamental reaction mechanism is also provided. The findings aim to provide a new material and theoretical foundation for the effective treatment of dye wastewater [20]. This approach seeks to enhance the photocatalyst’s activity, stability, and charge separation efficiency simultaneously, thereby overcoming the limitations of pure AgBr and offering a more effective solution for degrading organic pollutants.
2. Results and Discussion
2.1. Structure and Morphology
The crystal structure and phase composition of the photocatalysts were characterized using X-ray diffraction (XRD), with the corresponding patterns displayed in Figure 1. In the diffraction profile of SrSnO3, well-defined peaks are observed at 31.26°, 44.83°, and 55.69°, which can be indexed to the (200), (220), and (312) crystallographic planes, respectively [21]. These observed positions align well with the reference pattern PDF#04-010-2598, indicating the formation of a pure perovskite phase. Meanwhile, the AgBr component exhibits characteristic diffraction signals at 30.97°, 44.35°, and 55.07°, corresponding respectively to the (200), (220), and (222) planes [22]. This set of diffraction data matches the standard card PDF#04-005-4490, confirming the successful incorporation of AgBr into the composite. All materials exhibit sharp and high-intensity XRD peaks with no impurity signals, indicating high crystallinity. In the XRD patterns of the SrSnO3/AgBr composites with different molar ratios, characteristic peaks of both SrSnO3 and AgBr are present, while no impurity peaks are detected, suggesting that the composites consist solely of these two phases without the formation of any new impurities. Furthermore, the positions of the characteristic diffraction peaks of both phases remain unchanged after composite formation, indicating that the original crystal structures of the materials were preserved during the process.
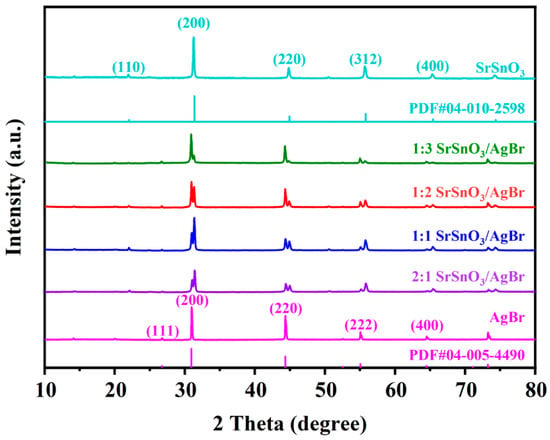
Figure 1.
XRD patterns of synthesized photocatalytics.
The surface morphology and microstructural characteristics of the synthesized samples were characterized using scanning electron microscopy (SEM). Pure SrSnO3 consists of densely packed, prism-like nanoparticles (Figure 2a) [23], while pure AgBr comprises relatively uniform and smooth microparticles (Figure 2b) [24]. The smooth surfaces of AgBr are unfavorable for forming grain boundaries, which is considered beneficial for the migration of photogenerated charge carriers. In the SrSnO3/AgBr composite, smaller AgBr nanospheres are successfully anchored onto the surfaces of larger SrSnO3 nanorods, forming intimate interfacial contact that facilitates charge transfer between the two phases (Figure 2c,d). To gain further insights into the microstructure, transmission electron microscopy (TEM) analysis was conducted. The observation that fine AgBr particles are attached to larger SrSnO3 particles indicates a tight combination between them. Figure 2h presents the high-resolution transmission electron microscopy (HRTEM) results, which allow for the clear resolution of two distinct lattice fringe patterns with interplanar spacings of 0.298 nm and 0.305 nm. These values are indexed to the (200) plane of orthorhombic SrSnO3 and the (200) plane of cubic AgBr, respectively [25]. The clear identification of these two phases in intimate contact offers direct verification of their coexistence at the interface, thereby substantiating the successful construction of a SrSnO3/AgBr heterojunction. Furthermore, the EDS elemental mapping of the composite (Figure 2i–n) shows a homogeneous distribution of all constituent elements, verifying the uniform dispersion of both components in the composite catalyst. In conclusion, the microscopic characterization confirms the successful synthesis of the SrSnO3/AgBr heterojunction photocatalyst. The intimate interfacial contact provides a structural foundation for effective charge separation and transfer during photocatalytic reactions.

Figure 2.
SEM images of (a) pure SrSnO3, (b) pure AgBr, (c,d) 1:2 SrSnO3/AgBr composite; TEM images of (e–g) 1:2 SrSnO3/AgBr nanocomposite, (h) HRTEM image; EDS mapping images of (i–n) Sr, Sn, O, Ag, and Br elements in the composite.
2.2. XPS Quantitative Surface Analysis
To probe the surface chemical composition and states of the catalysts, X-ray photoelectron spectroscopy (XPS) measurements were conducted. The Sr 3d core-level spectra (Figure 3b) reveal considerable modifications, not only in the spectral profile but also in the spin–orbit coupling energy, which shifts from ΔE = 1.6 eV in the pristine SrSnO3, ΔE = to 1.8 eV in SrSnO3/AgBr This energy value, being in close agreement with the work of Alammar et al. [26], serves as a clear indicator of an altered local electronic structure around Sr atoms, suggesting a potential electronic interaction at the heterojunction interface. The increase in spin orbital energy after heterostructure construction indicates disturbances in the local environment around Sr and reinforces the suggestion of interface composition in SrSnO3/AgBr [27]. The XPS spectra of Sn 3d in SrSnO3/AgBr samples show two peaks at 486.7 eV and at 495.1 eV with a peak separation of 8.4 eV, which are assigned to Sn 3d5/2 and Sn 3d3/2, respectively. Analysis of the Sn 3d region reveals an optimal spin–orbit splitting energy of 8.4 eV, which is consistent with values characteristic of Sn4+ in SnO2, as reported in prior studies [28,29,30]. Turning to the O 1s core-level spectrum (Figure 3d), deconvolution yields three distinct components. The two primary peaks, located at binding energies of 531.8 eV and 530.6 eV, correspond to structural lattice oxygen (Ostru) linked to Sn2+ and Sn4+ ions, respectively, on the catalyst surface. These observations collectively confirm the complex surface chemistry and the mixed valence states present in the SrSnO3/AgBr composite. Support for these assignments comes from prior research [31,32], where the peak at 530.6 eV has been connected to an unreduced lattice region, and the broader feature around 530–531 eV is attributed to an oxygen-deficient region. In a related context, Li et al. [33] assigned the third peak, observed at 532.3 eV, to surface hydroxyl groups. The presence of this protonated surface is plausibly a key factor, as it serves to stabilize the otherwise susceptible reduced Sn(II) cations, thereby maintaining the material’s specific surface chemistry. The chemical states of silver and bromine in the composite were determined by XPS. The diagnostic peaks for Ag 3d (367.4 eV and 373.4 eV) are indicative of Ag+ cations [34], and correspondingly, the Br 3d signals (68.0 eV and 69.0 eV, Figure 3f) confirm the presence of Br− anions [35]. A more pertinent observation pertains to the directional core-level shifts: elements originating from the SrSnO3 lattice (Sr, Sn, O) undergo a positive binding energy shift, while those from AgBr (Ag, Br) experience a negative shift. This reciprocal shifting pattern is a characteristic signature of charge redistribution, thereby confirming the establishment of a built-in electric field at the heterojunction interface [36].
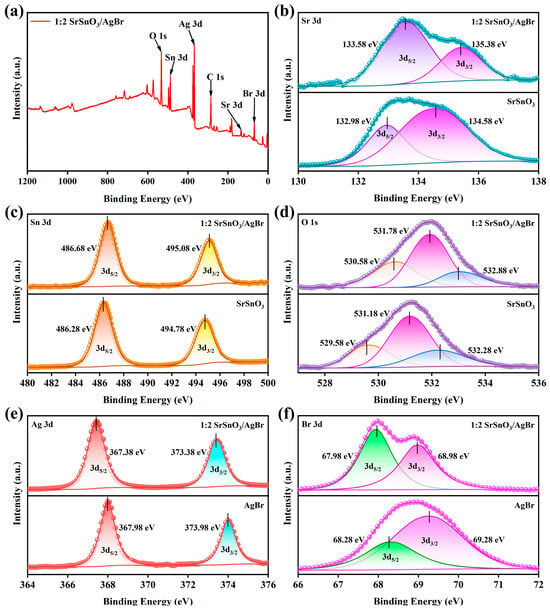
Figure 3.
(a) Full spectrum of the 1:2 SrSnO3/AgBr nanocomposite, High-resolution XPS spectra of (b) Sr 3d, (c) Sn 3d, (d) O 1s, (e) Ag 3d, (f) Br 3d.
2.3. Evaluation of the Catalytic Activity
Using MO as the target pollutant to evaluate the photocatalytic activity, 0.05 g of different types of photocatalysts were added to an MO solution and stirred in the dark for 30 min until the mixture reached equilibrium. As shown in Figure 4a–f, the degradation of MO by SrSnO3 was almost negligible under visible light irradiation after 40 min, with its characteristic absorption peak around 460 nm remaining nearly unchanged over time [37]. Pure AgBr exhibited a low degradation efficiency of approximately 40% [38]. In contrast to these single-phase materials, the SrSnO3/AgBr composites with different molar ratios showed significantly enhanced photocatalytic activity [39]. The characteristic absorption peak of MO decreased markedly within 10 min for the 1:2 SrSnO3/AgBr composite, continued to diminish over time, and nearly disappeared after 40 min [40,41,42]. The 1:2 SrSnO3/AgBr composite exhibited the highest degradation efficiency (Figure 5a), which was 7.16 times higher than that of pure AgBr. This result indicates a strong synergistic interaction between SrSnO3 and AgBr [43,44,45]. To identify the dominant active species in the photocatalytic reaction, different scavengers were introduced into the pollutant solution: isopropanol (IPA) and disodium ethylenediaminetetraacetate (EDTA-2Na) to scavenge hydroxyl radicals (·OH) and holes (h+), respectively. To avoid interference from the chemical reaction between p-benzoquinone (BQ) and the photocatalytic solution, electron paramagnetic resonance (EPR) was employed to monitor the superoxide radical (·O2−). As presented in Figure 5c, the photocatalytic efficiency was only slightly reduced by the addition of IPA compared to the blank experiment. In contrast, EDTA-2Na markedly suppressed the efficiency to 51.2%. According to the EPR results, superoxide radicals played almost no role in the reaction (Supplementary Material S2). Additionally, the 1:2 SrSnO3/AgBr composite demonstrated good stability, maintaining 68.9% of its efficiency after three successive cycles.
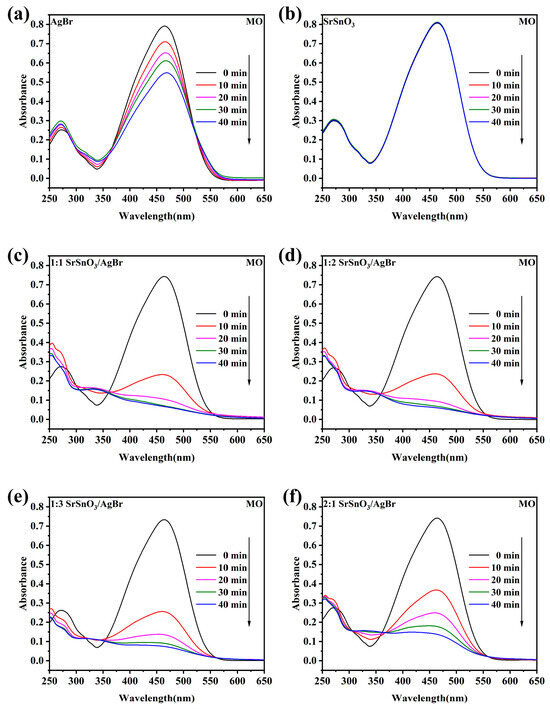
Figure 4.
Absorption spectra of different materials for MO degradation under sunlight irradiation: (a) pure SrSnO3; (b) pure AgBr; (c–f) x-SrSnO3/AgBr nanocomposites.
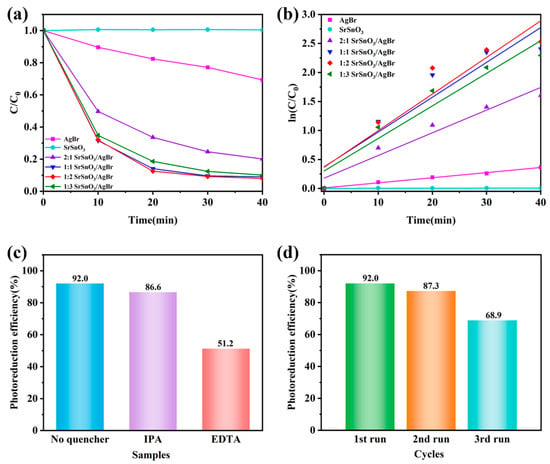
Figure 5.
(a) Changes in pure SrSnO3, pure AgBr, and x-SrSnO3/AgBr nanocomposites in MO solution upon light irradiation. (b) Corresponding kinetics of MO degradation. (c) Effect of scavengers on MO degradation. (d) Photocyclic performance of the 1:2 SrSnO3/AgBr composite.
2.4. I-T and EIS
The dynamics of photogenerated electron–hole pairs were investigated using transient photocurrent response (I-T) and electrochemical impedance spectroscopy (EIS). As shown in Figure 6a, the photocurrent density of pure SrSnO3 was almost negligible, indicating an extremely weak photoresponse and low efficiency of charge carrier generation, which resulted in a negligible number of detectable carriers participating in the electrode reaction. Although AgBr exhibited a certain photocurrent response, its density was still significantly lower than that of the 1:2 SrSnO3/AgBr composite. The composite demonstrated the strongest photocurrent response, suggesting the most efficient separation of photogenerated charge carriers. Furthermore, the EIS Nyquist plot showed that the arc diameter followed the order of 1:2 SrSnO3/AgBr < AgBr < SrSnO3, indicating the best charge carrier dynamics in the composite.
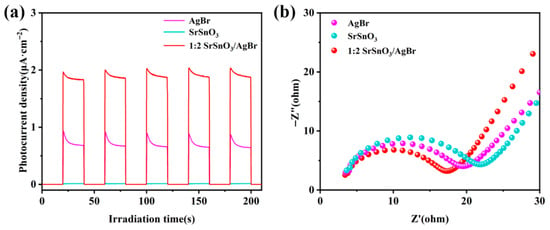
Figure 6.
(a) Transient photocurrent response spectrum. (b) Electrochemical impedance spectrum.
2.5. Mechanism of Photocatalytic Reactions
To investigate the light absorption properties and band structures of the composite materials, UV-Vis diffuse reflectance spectroscopy (DRS) was performed on the as-prepared samples. As shown in Figure 7a, the absorption edges of AgBr, SrSnO3, and the 1:2 SrSnO3/AgBr composite were observed at 312 nm, 522 nm, and 554 nm, respectively. A noticeable red shift in the absorption edge of the composite indicates a broadened visible-light absorption range and a significant enhancement of light-harvesting capability. Based on the UV-Vis absorption data, the band gap energies (Eg) of the materials were calculated (Supplementary Material S3). The Eg values for AgBr, SrSnO3, and 1:2 SrSnO3/AgBr were determined to be 2.63 eV, 4.18 eV and 3.00 eV, respectively. Based on the series of analyses above, a catalytic mechanism for the SrSnO3/AgBr composite photocatalyst is proposed (Figure 8). Under visible light irradiation, electron–hole pairs are generated in the valence band (VB) and conduction band (CB) of the SrSnO3/AgBr composite. The photogenerated carriers generated in wide-bandgap SrSnO3 rapidly migrate to the valence band (VB) and conduction band (CB) of narrow-bandgap AgBr. The reaction potential of H2O/·OH (2.27 eV) is located between the VB (2.615 eV) and CB (−0.015 eV) of AgBr, leading to the production of ·OH radicals on AgBr [46]. The potential necessary for oxidizing MO is 0.955 eV. Because the EVB of AgBr (2.615 eV) is greater than 0.955 eV [47], the holes in the VB of AgBr also exhibit strong oxidation capability and can directly oxidize MO. The large number of highly oxidative h+ generated by the catalyst preferentially degrade MO instead of transforming into ·OH during the photocatalytic process. In this type-I heterojunction system, holes are further transferred from the VB of SrSnO3 to AgBr, which enhances the oxidative degradation of MO in the AgBr domain [48].
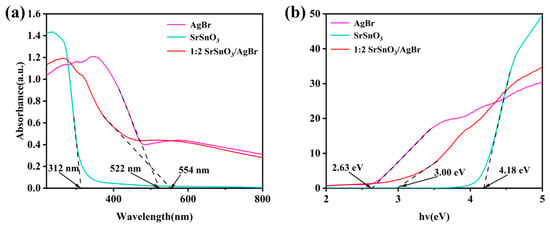
Figure 7.
(a) UV-visible diffuse reflectance spectra. (b) Bandgap energy diagrams for pure SrSnO3, pure AgBr, and a 1:2 SrSnO3/AgBr mixture.
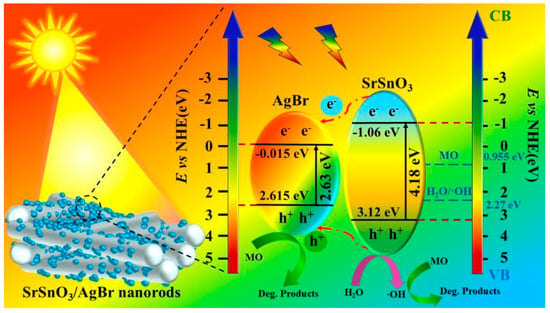
Figure 8.
Schematic illustration of MO degradation on a 1:2 SrSnO3/AgBr composite under simulated sunlight irradiation.
3. Conclusions
In summary, this study successfully designed an efficient SrSnO3/AgBr composite photocatalyst through a heterojunction engineering strategy. Under simulated sunlight, the composite demonstrated exceptional performance in degrading Methyl Orange (MO), achieving a removal rate of 92% within 40 min. Notably, the degradation efficiency of the composite was measured to be twice that of pure AgBr and twenty times that of pure SrSnO3. Furthermore, electron microscopy confirmed its microstructure, revealing that AgBr nanoparticles were homogeneously deposited on the SrSnO3 nanorod surfaces, creating a well-defined and intimate interfacial contact. XPS analysis further revealed significant binding energy shifts for the constituent elements, providing strong evidence for the formation of a built-in electric field at the interface. This field, as supported by photoelectrochemical measurements, greatly promoted the separation and migration of photogenerated charges. Meanwhile, the clearance test found that holes (h+) were the main active substance. This composite material also demonstrates excellent stability and maintains high activity over three consecutive cycles. These findings provide a theoretical basis for the rational design of high-performance heterojunction photocatalysts and open new avenues for photocatalytic degradation and other related applications.
4. Experimental
4.1. Materials
Strontium nitrate (Sr(NO3)2, AR), sodium stannate trihydrate (Na2SnO3·3H2O, AR), sodium bromide (NaBr, AR), silver nitrate (AgNO3, AR), and Methyl Orange (MO) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. All experiments used analytical-grade deionized water.
4.2. Preparation of SrSnO3
Pure SrSnO3 was synthesized according to the previous method.
4.3. Preparation of SrSnO3/AgBr
As illustrated in Figure 9, the SrSnO3/AgBr nanocomposite was synthesized via a co-precipitation method. First, 0.2572 g of NaBr and 0.4247 g of AgNO3 were separately dissolved in 50 mL of deionized water under continuous stirring until complete dissolution. A measured amount of SrSnO3 was then added to the NaBr solution under stirring. Finally, the AgNO3 solution was slowly introduced into the mixed solution. After continuous stirring for 30 min, the resulting mixture was washed 3–4 times with deionized water and absolute ethanol. The product was dried in a forced-air oven at 60 °C for 24 h. After grinding, x-SrSnO3/AgBr nanocomposites (where x = 2:1, 1:2, 1:3 and 1:1 represents the molar ratio of SrSnO3 to AgBr) were obtained.
4.4. Characterizations and Activity Testing of Photocatalysts
Characterization information is detailed in Supplementary Material S1.
4.5. Electrochemical Testing Experiment
Electrochemical testing information is detailed in Supplementary Material S1.
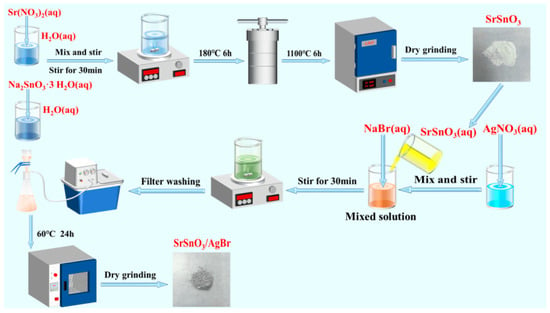
Figure 9.
Schematic illustration of the preparation of x-SrSnO3/AgBr nanocomposites.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics13120406/s1.
Author Contributions
Investigation, H.L.; Data curation, B.-S.Z.; Writing—original draft, S.-H.T.-W. and S.-M.L.; Writing—review & editing, Y.Z., Z.-Y.X., T.S. and Q.Z.; Funding acquisition, Y.Z., C.-H.H. and D.-H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Project of Guangxi (Grant No. AA24263076), the Guangxi Key Laboratory of Information Materials & Guangxi Collaborative Innovation Center of Structure and Property for New Energy Materials (Grant No. 231067-Z), the College Students’ innovation and Entrepreneurship Competition (Grant No. 202310595054), the Guangxi Natural Science Foundation (Grant No. 2025GXNSFAA069270), the National Natural Science Foundation of China (Grant No. 52061006), the Guangxi Key Laboratory of Information Materials (Grant No. 231007-Z).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- do Rosário, L.O.; Silva, U.C.; Tranquilin, R.L.; Teodoro, M.D.; Melo, M.C.N.; Motta, F.V.; Bomio, M.R.D. Development and application of a novel CaTiO3/NiO/AgBr ternary heterostructure for the treatment of highly concentrated dyes and its performance as an antimicrobial agent. Ceram. Int. 2025, 51, 43156–43177. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Chen, P.; Liang, Y.; Xu, Y.; Zhao, Y.; Song, S. Synchronous photosensitized degradation of methyl orange and methylene blue in water by visible-light irradiation. J. Mol. Liq. 2021, 334, 116159. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Zenebe, A.; Dikamu, M.; Ezez, D. Synthesis of Chitosan-Natrolite modified magnetite nanocomposite (Chio/Fe3O4@NAT) for photocatalytic degradation of methyl orange using response surface model (RSM). Sci. Afr. 2025, 30, e03004. [Google Scholar] [CrossRef]
- Faisal, M.; Rashed, M.A.; Ahmed, J.; Alsaiari, M.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A. Ag nanoparticle-decorated chitosan/SrSnO3 nanocomposite for ultrafast elimination of antibiotic linezolid and methylene blue. Environ. Sci. Pollut. Res. 2022, 29, 52900–52914. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; Xu, Y.; Song, Y.; Li, H.; Xia, J.; Huang, C.; Wan, H. Novel visible-light-driven AgX/graphite-like C3N4 (X = Br, I) hybrid materials with synergistic photocatalytic activity. Appl. Catal. B Environ. 2013, 129, 182–193. [Google Scholar] [CrossRef]
- Guo, H.; Niu, C.-G.; Wen, X.-J.; Zhang, L.; Liang, C.; Zhang, X.-G.; Guan, D.-L.; Tang, N.; Zeng, G.-M. Construction of highly efficient and stable ternary AgBr/Ag/PbBiO2Br Z-scheme photocatalyst under visible light irradiation: Performance and mechanism insight. J. Colloid Interface Sci. 2018, 513, 852–865. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Wang, Y.; Fan, C. Novel visible-light AgBr/Ag3PO4 hybrids photocatalysts with surface plasma resonance effects. J. Solid State Chem. 2013, 202, 51–56. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Guan, W.; Huang, H.; Liu, Y.; Kang, Z. Study on highly enhanced photocatalytic tetracycline degradation of type II AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater. 2018, 349, 111–118. [Google Scholar] [CrossRef]
- Zheng, X.-h.; Chen, W.; Xu, M.-n.; Cai, C.-b.; Yang, F.-e. Photocatalytic properties of BiOBr/BiOCl/AgBr ternary photocatalysts for degradation of RhB dye. J. Nanoparticle Res. 2023, 25, 96. [Google Scholar] [CrossRef]
- Baba, E.; Kan, D.; Yamada, Y.; Haruta, M.; Kurata, H.; Kanemitsu, Y.; Shimakawa, Y. Optical and transport properties of transparent conducting La-doped SrSnO3 thin films. J. Phys. D Appl. Phys. 2015, 48, 455106. [Google Scholar] [CrossRef]
- Zhang, W.F.; Tang, J.; Ye, J. Photoluminescence and photocatalytic properties of SrSnO3 perovskite. Chem. Phys. Lett. 2006, 418, 174–178. [Google Scholar] [CrossRef]
- Teixeira, A.R.F.A.; de Meireles Neris, A.; Longo, E.; de Carvalho Filho, J.R.; Hakki, A.; Macphee, D.; dos Santos, I.M.G. SrSnO3 perovskite obtained by the modified Pechini method—Insights about its photocatalytic activity. J. Photochem. Photobiol. A Chem. 2019, 369, 181–188. [Google Scholar] [CrossRef]
- Junploy, P.; Thongtem, T.; Thongtem, S.; Phuruangrat, A.; Liu, T. Decolorization of Methylene Blue by Ag/SrSnO3 Composites under Ultraviolet Radiation. J. Nanomater. 2014, 2014, 261395. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, M.; Wang, Q.; Sun, X.; Lan, J. One-step reduction synthesis of Ag/Ag3PO4/AgBr sub-micron composites for enhanced visible photocatalytic degradation of methyl orange. Mater. Res. Bull. 2018, 104, 149–154. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, P.; Liang, Y.; Zhao, M.; Jiang, Y.; Wang, G.; Zou, P.; Zeng, J.; Zhang, Y.; Wang, Y. Fabrication of a three-dimensional porous Z-scheme silver/silver bromide/graphitic carbon nitride@nitrogen-doped graphene aerogel with enhanced visible-light photocatalytic and antibacterial activities. J. Colloid Interface Sci. 2019, 536, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Ma, B.; Liu, K.; Pu, F.; Yang, Z.; Yang, S. Templated-synthesis of hierarchical Ag-AgBr hollow cubes with enhanced visible-light-responsive photocatalytic activity. Appl. Surf. Sci. 2018, 443, 492–496. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Zhou, D.; Dong, S. Fabrication of Ag/CDots/BiOBr ternary photocatalyst with enhanced visible-light driven photocatalytic activity for 4-chlorophenol degradation. J. Mol. Liq. 2018, 262, 194–203. [Google Scholar] [CrossRef]
- Gu, R.; Huang, Z.; Lv, L.; Zhang, J.; Feng, S.; Xu, Y.; Xie, M. Functionalized fabric with Ag/AgBr/Fe2O3 for optimized outdoor applications. J. Mater. Sci. Technol. 2025, 227, 32–40. [Google Scholar] [CrossRef]
- Kaynar, Ü.H.; Coban, M.B.; Madkhli, A.Y.; Ayvacikli, M.; Can, N. Phase transition and luminescence characteristics of dysprosium doped strontium stannate phosphor synthesized using hydrothermal method. Ceram. Int. 2023, 49, 11641–11646. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, M.; Yu, M.; Zhang, T.; Wang, J.; Bi, J.; Dong, F. Efficient photo-degradation of antibiotics by waste eggshells derived AgBr-CaCO3 heterostructure under visible light. Sep. Purif. Technol. 2023, 314, 123573. [Google Scholar] [CrossRef]
- Ghubish, Z.; Kamal, R.; Mahmoud, H.R.; Saif, M.; Hafez, H.; El-Kemary, M. Photocatalytic activation of Ag-doped SrSnO3 nanorods under visible light for reduction of p-nitrophenol and methylene blue mineralization. J. Mater. Sci. Mater. Electron. 2022, 33, 24322–24339. [Google Scholar] [CrossRef]
- Huang, S.; Yuan, H.; Dong, W.; Wu, X.; Wang, G. Visible-light-driven removal of AO7 by peroxymonosulfate-assisted Z-scheme AgBr/LaFeO3 heterojunction vis accelerated electronic transfer. Mater. Sci. Semicond. Process. 2023, 160, 107414. [Google Scholar] [CrossRef]
- Yasin, U.A.A.; Ahmed, M.M.; Zhang, J.; Jia, Z.; Guo, T.; Zhao, R.; Shi, J.; Du, J. Engineering the band structure of CuO via decoration with AgBr to enhance its photocatalytic degradation performance. J. Mater. Sci. 2023, 58, 7333–7346. [Google Scholar] [CrossRef]
- Alammar, T.; Hamm, I.; Grasmik, V.; Wark, M.; Mudring, A.-V. Microwave-Assisted Synthesis of Perovskite SrSnO3 Nanocrystals in Ionic Liquids for Photocatalytic Applications. Inorg. Chem. 2017, 56, 6920–6932. [Google Scholar] [CrossRef]
- Honorio, L.M.C.; Oliveira, A.L.M.d.; Silva Filho, E.C.d.; Osajima, J.A.; Hakki, A.; Macphee, D.E.; Santos, I.M.G.d. Supporting the photocatalysts on ZrO2: An effective way to enhance the photocatalytic activity of SrSnO3. Appl. Surf. Sci. 2020, 528, 146991. [Google Scholar] [CrossRef]
- Stranick, M.A.; Moskwa, A. SnO2 by XPS. Surf. Sci. Spectra 1993, 2, 50–54. [Google Scholar] [CrossRef]
- Barreca, D.; Garon, S.; Tondello, E.; Zanella, P. SnO2 Nanocrystalline Thin Films by XPS. Surf. Sci. Spectra 2000, 7, 81–85. [Google Scholar] [CrossRef]
- Alammar, T.; Slowing, I.I.; Anderegg, J.; Mudring, A.V. Ionic-Liquid-Assisted Microwave Synthesis of Solid Solutions of Sr1−xBaxSnO3 Perovskite for Photocatalytic Applications. ChemSusChem 2017, 10, 3387–3401. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Kim, D.W.; Cho, I.S.; Park, S.; Shin, S.S.; Seo, S.W.; Hong, K.S. Simple synthesis and characterization of SrSnO3 nanoparticles with enhanced photocatalytic activity. Int. J. Hydrogen Energy 2012, 37, 10557–10563. [Google Scholar] [CrossRef]
- Aragón, F.H.; Gonzalez, I.; Coaquira, J.A.H.; Hidalgo, P.; Brito, H.F.; Ardisson, J.D.; Macedo, W.A.A.; Morais, P.C. Structural and Surface Study of Praseodymium-Doped SnO2 Nanoparticles Prepared by the Polymeric Precursor Method. J. Phys. Chem. C 2015, 119, 8711–8717. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Gao, D.; Wang, Y. Effect of oxide defect on photocatalytic properties of MSnO3 (M = Ca, Sr, and Ba) photocatalysts. Appl. Catal. B Environ. 2019, 243, 428–437. [Google Scholar] [CrossRef]
- Chen, Y.; Pu, S.; Wang, D.; Zhang, Y.; Wan, G.; Zhao, Q.; Sun, Y. Facile synthesis of AgBr@ZIF-8 hybrid photocatalysts for degradation of Rhodamine B. J. Solid State Chem. 2023, 321, 123857. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, J.; Shi, H. Double-vacancy-induced polarization and intensified built-in electric field in S-Scheme heterojunction for removal of antibiotics and Cr (VI). Chin. J. Catal. 2025, 76, 50–64. [Google Scholar] [CrossRef]
- Zhang, L.; Xuan, Y.; Wang, Q.; Zhao, D.; Liu, X. Synergistic Effect of Photochromism and Vacancy Engineering within Z-Scheme Doped BiOBr-BaTiO3 Heterostructures Promoting CO2 Photocatalysis. Adv. Funct. Mater. 2025, e14966. [Google Scholar] [CrossRef]
- de Sousa Filho, I.A.; Arana, L.R.; Doungmo, G.; Grisolia, C.K.; Terrashke, H.; Weber, I.T. SrSnO3/g-C3N4 and sunlight: Photocatalytic activity and toxicity of degradation byproducts. J. Environ. Chem. Eng. 2020, 8, 103633. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Lin, H.; Xu, B.; Chen, S. Ag/AgBr/g-C3N4: A highly efficient and stable composite photocatalyst for degradation of organic contaminants under visible light. Mater. Res. Bull. 2013, 48, 3873–3880. [Google Scholar] [CrossRef]
- Gómez-Solís, C.; Oliva, J.; Diaz-Torres, L.A.; Bernal-Alvarado, J.; Reyes-Zamudio, V.; Abidov, A.; Torres-Martinez, L.M. Efficient photocatalytic activity of MSnO3 (M: Ca, Ba, Sr) stannates for photoreduction of 4-nitrophenol and hydrogen production under UV light irradiation. J. Photochem. Photobiol. A Chem. 2019, 371, 365–373. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Li, J.; Yin, J. Photocatalytic degradation of methyl orange by TiO2-coated activated carbon and kinetic study. Water Res. 2006, 40, 1119–1126. [Google Scholar] [CrossRef]
- Lin, H.; Cao, J.; Luo, B.; Xu, B.; Chen, S. Visible-light photocatalytic activity and mechanism of novel AgBr/BiOBr prepared by deposition-precipitation. Chin. Sci. Bull. 2012, 57, 2901–2907. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and Characterization of Co–ZnO and Evaluation of Its Photocatalytic Activity for Photodegradation of Methyl Orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Wang, D.; Duan, Y.; Luo, Q.; Li, X.; Bao, L. Visible light photocatalytic activities of plasmonic Ag/AgBr particles synthesized by a double jet method. Desalination 2011, 270, 174–180. [Google Scholar] [CrossRef]
- Wang, X.J.; Yang, W.Y.; Li, F.T.; Zhao, J.; Liu, R.H.; Liu, S.J.; Li, B. Construction of amorphous TiO2/BiOBr heterojunctions via facets coupling for enhanced photocatalytic activity. J. Hazard. Mater. 2015, 292, 126–136. [Google Scholar] [CrossRef]
- Tun, P.; Wang, K.; Naing, H.; Wang, J.; Zhang, G. Facile preparation of visible-light-responsive kaolin-supported Ag@AgBr composites and their enhanced photocatalytic properties. Appl. Clay Sci. 2019, 175, 76–85. [Google Scholar] [CrossRef]
- Lin, C.; Wang, H.; Liu, S.; Li, C.; Chu, B.; Yan, Q. Preparation of magnetic Co0.5Zn0.5Fe2O4/AgBr hybrids for the visible-light-driven degradation of methyl orange. Mater. Sci. Semicond. Process. 2018, 73, 67–71. [Google Scholar] [CrossRef]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. A visible light driven AgBr/g-C3N4 photocatalyst composite in methyl orange photodegradation: Focus on photoluminescence, mole ratio, synthesis method of g-C3N4 and scavengers. Compos. Part B Eng. 2020, 183, 107712. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.H.; Monsef, R.; Salavati-Niasari, M.; Majdi, H.S.; Al-Azzawi, W.K.; Hashim, F.S. Schiff-base ligand assisted synthesis of DyVO4/AgBr nanocomposites, characterization, and investigation of photocatalytic activity over organic dye contaminants. Arab. J. Chem. 2023, 16, 105020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).