Abstract
Organic–inorganic hybrid metal halides (OIMHs) have attracted widespread attention due to their unique chemical properties, excellent electronic performance, and low-cost fabrication processes. These hybrid materials impose fewer size constraints on the organic components, providing an exciting platform for the molecular-level design of new materials and functionalities. In this review, we discuss the latest progress in OIMHs. Specifically, we summarize recent advances in their structures, synthetic strategies, and luminescence mechanisms, and highlight their applications in light-emitting diodes (LEDs), information encryption and anti-counterfeiting, sensors, and X-ray imaging. Finally, we discuss the challenges related to structural design, mechanistic understanding, and stability, along with perspectives on future opportunities for OIMHs.
1. Introduction
In recent years, organic–inorganic hybrid metal halides (OIMHs) have received extensive attention owing to their unique crystal structures and remarkable optoelectronic properties [1,2]. These materials can combine structural tunability, facile preparation, and cost advantages with a precisely adjustable optical bandgap, strong defect tolerance, and outstanding photophysical performance. As next-generation functional materials, OIMHs exhibit particularly excellent luminescence properties, including a high color rendering index (CRI), broadband tunable emission, wide color gamut coverage, and high photoluminescence quantum yields (PLQYs) [3]. Together, these advantages make OIMHs promising alternatives to conventional phosphors, with potential applications in LED lighting, X-ray imaging, and luminescent probes and sensors [4,5].
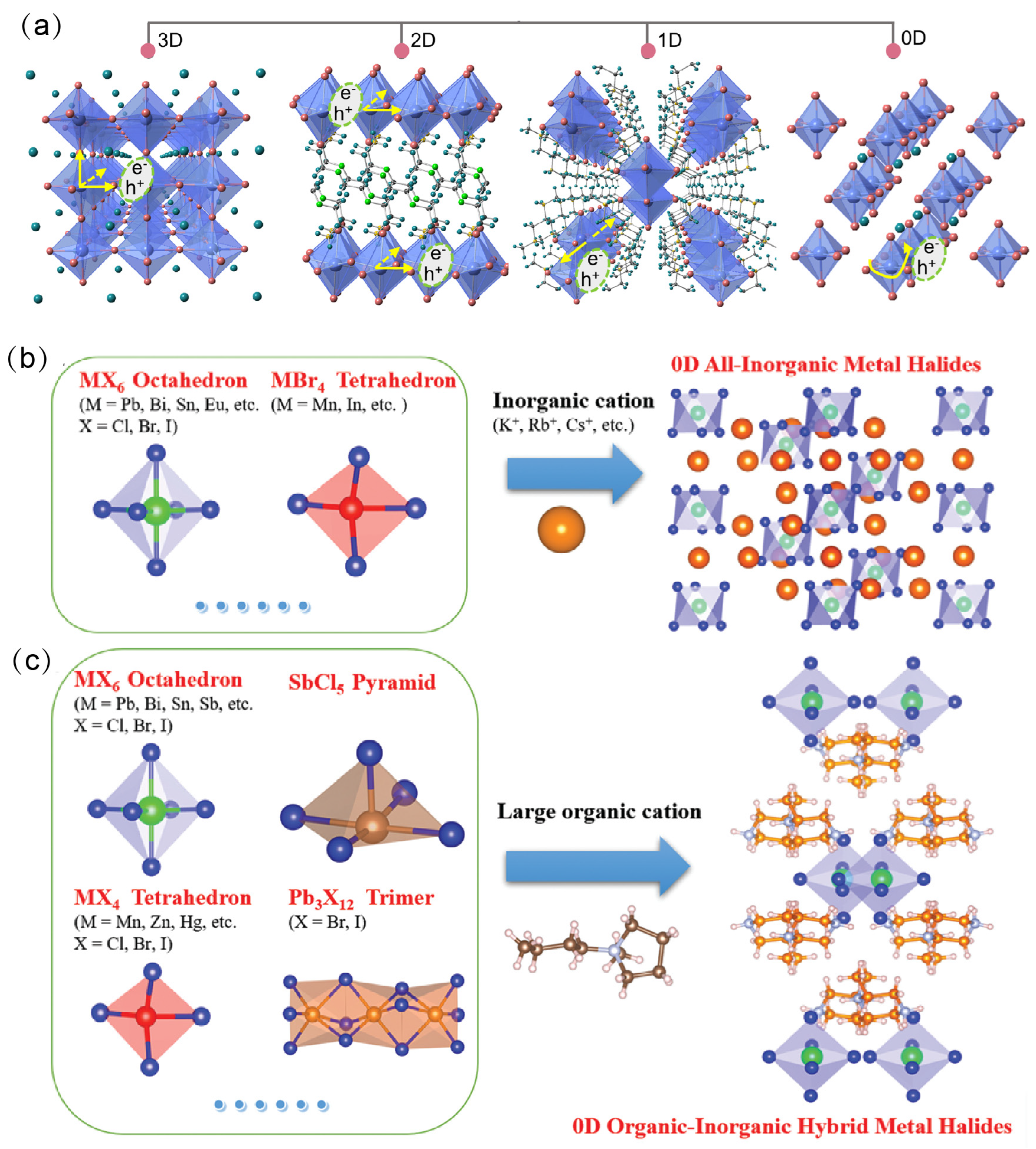
The dimensionality of OIMHs is defined by the arrangement and connectivity of inorganic polyhedra within the crystal lattice. Depending on the connectivity of metal–halide octahedra, OIMHs can adopt different dimensional structures. When the organic cations are relatively small, the inorganic octahedra extend continuously via metal–halide coordination bonds to form three-dimensional (3D) networks. In contrast, introducing bulky organic cations disrupts the octahedral network, reducing the structural dimensionality and yielding two-dimensional (2D) layered, one-dimensional (1D) chain-like, or zero-dimensional (0D) cluster-like OIMHs, collectively referred to as OIMHs (Figure 1a) [6]. Low-dimensional OIMHs exhibit higher exciton binding energies and therefore superior emissive properties and radiative recombination characteristics [7,8,9,10,11]. At the same time, low-dimensional OIMHs offer enhanced stability and reduced toxicity, making them attractive replacements for 3D Pb-based OIMHs; they display excellent optical performance and have achieved notable progress across optoelectronic applications. Consequently, the rational design and synthesis of functional OIMHs are of substantial research significance for enabling diverse practical uses [12,13,14,15]. Nevertheless, challenges persist in the structural design and mechanistic understanding of low-dimensional OIMHs. Issues such as limited stability and luminescence efficiency loss remain to be addressed. Against this backdrop of opportunities and challenges, a systematic review is essential for advancing the understanding and future development of OIMHs.
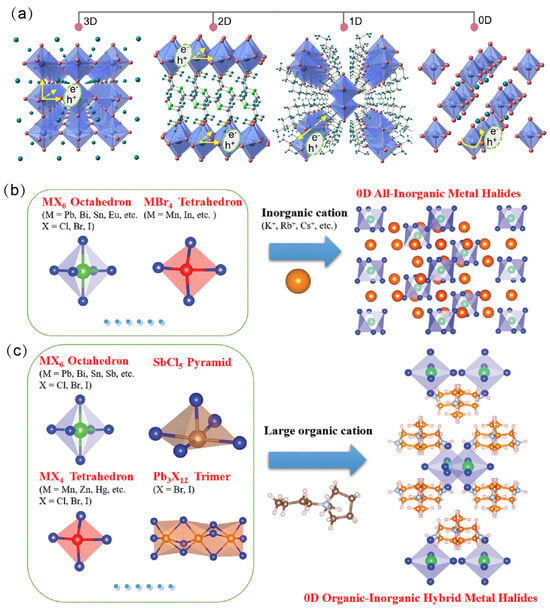
Figure 1.
(a) Typical structures of OIMHs [6]. Reprinted with permission from Ref. [6]. Copyright 2024, Wiley; (b,c) 0D OIMHs with different interlayer cations [2,16]. Reprinted with permission from Ref. [2]. Copyright 2020, Wiley. Reprinted with permission from Ref. [16]. Copyright 2021, Royal Society of Chemistry.
In this article, we have summarized recent advances in OIMHs, including their structures, synthetic methods, optical properties, luminescence mechanisms, and applications. We particularly emphasize the role of structural design in guiding the formation of OIMHs and analyze the relationships between structural composition and photophysical properties. Considering their excellent optical and multifunctional luminescent features, we highlight the potential applications of OIMHs in LEDs, information encryption and anti-counterfeiting, sensors, and X-ray imaging, and provide an outlook on future research directions.
2. Structural Characteristics and Stability of Organic–Inorganic Hybrid Metal Halides
2.1. Structural Characteristics
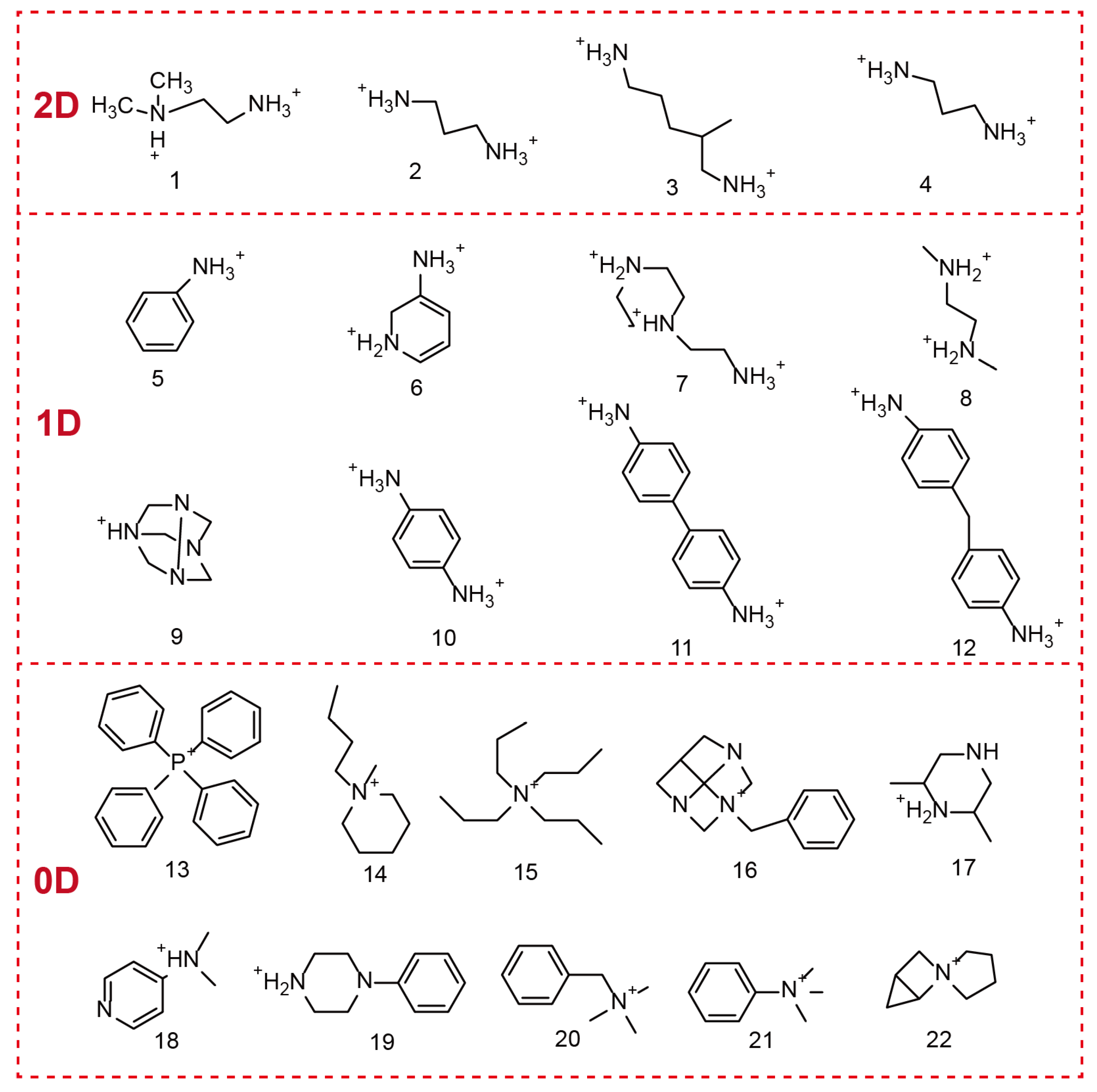
OIMHs are composed of metal–halide anions and organic cations. In terms of composition, the selection of the metal cation plays a pivotal role in shaping the properties of OHMHs. The chemistry of the ns2 ion (Pb2+, Bi3+, Sn2+, Sb3+, etc.) is closely related to the electronic properties and dielectric properties of ns2 cation-based OIMHs. Moreover, the organic unit in OIMHs is typically an ammonium species or a sulfur-/phosphorus-containing cation. The structural diversity of OIMHs is governed primarily by the types of organic cations present and by the spatial packing of the inorganic polyhedral framework units [12,13,14,17]. During formation, multiple intermolecular forces drive the orderly assembly of the organic and inorganic parts: organic cations attract each other through van der Waals interactions and hydrogen bonding, whereas the inorganic units connect via corner-, edge-, or face-sharing coordination. Strong hydrogen bonding between the organic cations and the inorganic polyhedra further ensures a highly ordered structure, endowing OIMHs with excellent optoelectronic properties [9,10,12,15]. Figure 2 displays some typical organic cations in the reported 2D, 1D, and 0D OIMHs [18].
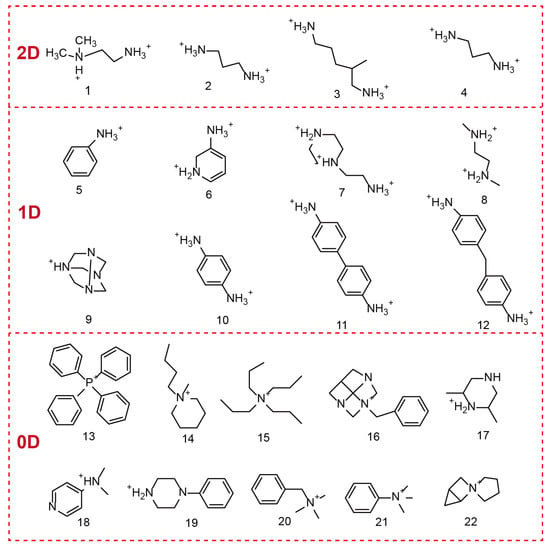
Figure 2.
Organic cations used in low-dimensional OIMHs.
From a crystallographic perspective, 3D OIMHs are built from metal–halide octahedra that are corner-linked into a 3D network, with small A-site cations uniformly occupying the interstitial cavities. In the archetypal 3D OIMH structure (ABX3), A denotes a monovalent cation such as methylammonium MA+, formamidinium FA+, or Cs+; M denotes a metal cation such as Pb2+, Sn2+, or Ge2+; and X denotes a halide anion (Cl−, Br−, I−). Owing to their high absorption coefficients, tunable optical bandgaps, low exciton binding energies, and long carrier-diffusion lengths, 3D OIMHs have shown broad application in solar photovoltaics [19]. Because 3D OIMHs generally possess small exciton binding energies (20–50 meV), their radiative recombination efficiencies and PLQYs tend to be low, which limits their performance in light-emitting devices.
Two-dimensional OIMHs comprise layered structures formed by corner-, edge-, or face-sharing polyhedra, with bulky organic cations residing between the layers to yield a characteristic “sandwich” architecture [20]. Two-dimensional OIMHs can be categorized structurally into ⟨100⟩-oriented, ⟨110⟩-oriented, and ⟨111⟩-oriented structures. Linear carbon chain cations are one of the first spacer types. For example, butylammonium cation is the extensively studied spacer, and other spacers such as propylammonium, pentylammonium, and hexylammonium cations have been demonstrated for the multilayer 2D structures [21]. Also, some cyclic monoammonium and aromatic cations have been widely studied. Organic amine cations with distinct intrinsic physical and chemical properties exert three main effects on 2D OIMHs: crystal structure, optical properties, and film properties [22].
Further reducing dimensionality to 1D systems produces chain-like inorganic backbones: metal–halide octahedra extend as single or double chains via point-, edge-, or face-sharing, while large organic cations spatially isolate neighboring chains [23]. In 1D OIMHs, organic cations are usually bulky diammonium or multiammonium species, sometimes cyclic or aromatic (ethylenediammonium derivatives, quinoline, naphthalene derivatives). Diammonium/multiamine spacers are used to enhance interchain hydrogen bonding and lattice robustness.
In 0D OIMHs, sufficiently large organic cations separate individual metal-centered tetrahedra, pentahedra, octahedra, or their clusters, embedding each unit within an insulating organic matrix and producing discrete “host–guest” assemblies (Figure 1b,c) [16,24]. The individual organic cations in 0D OIMHs usually encompass only one positive charge. Moreover, their unsaturated atoms (N/P/S) are surrounded in the center site of organic cations, such as tetraphenylphosphonium (TPP+) and triphenylsulfonium (Ph3S+) cations. The large-size organic cations can fully separate the inorganic anions, leading to highly localized excitons upon excitation. These localized excitons induce strong electron–phonon interactions, resulting in high PLQY. Moreover, the non-networked metal halide polyhedra or clusters in 0D OHMHs offer diverse coordination environments, providing opportunities to tune both structure and luminescence, and thus present significant potential for optoelectronic applications.
2.2. Stability
The stability of 3D, 2D, 1D, and 0D OHMHs is of paramount importance for their applications. While these materials have demonstrated exceptional optoelectronic properties—such as high PLQYs, tunable bandgaps, and strong excitonic effects—their practical application is often limited by sensitivity to environmental stressors, including moisture, oxygen, light, and heat [25]. Usually, different families of OHMHs display markedly different vulnerabilities to moisture, oxygen, light, heat, and charge stress.
Three-dimensional Pb-based OIMHs are highly sensitive to humidity and oxygen. Their dominant degradation routes involve hydration and precursor back-reaction (yielding PbI2, CH3NH2, HI), which can be accelerated by the synergistic action of illumination, trapped charges, and O2, promoting I− oxidation to I2/I3−, phase transitions, and vacancy growth. Sn-based OIMHs (e.g., FASnI3) are limited primarily by spontaneous Sn2+ → Sn4+ oxidation, accompanied by vacancy formation and deep defect states, leading to rapid degradation in air; even when short-term operation is feasible, synthesis and film deposition are typically conducted under low-O2/low-H2O conditions with antioxidant additives and/or reducing atmospheres [26]. Consequently, practical devices require encapsulation, and stability is typically improved via compositional engineering, defect passivation, and encapsulation strategies.
Two-dimensional OHMHs offer enhanced resistance due to their organic spacer barriers [27]. Two-dimensional/quasi-two-dimensional systems incorporate organic spacer layers that provide hydrophobic barriers and suppress ion migration, conferring substantially better tolerance to moisture and oxygen than their 3D counterparts. One-dimensional and zero-dimensional OHMHs, with their reduced dimensional connectivity, generally show greater robustness but may suffer from limited charge mobility and exciton confinement effects. A systematic understanding of these stability–property trade-offs is crucial, as it directly informs the design of devices ranging from solar cells and light-emitting diodes to scintillators and sensors [28]. 6s2 cation-based OHMHs and 0D/1D Cu-based OIMHs are comparatively more robust—particularly against moisture—yet they can still suffer photo- or thermally driven halide migration and surface-defect formation [16].
3. Common Techniques of Organic–Inorganic Hybrid Metal–Halide Crystals
Solution-based methods are widely employed for the synthesis of OIMH crystals owing to their low cost, mild reaction conditions, simple equipment requirements, and short synthesis cycles. They are particularly useful for exploratory studies of growing new OIMH crystals quickly.
3.1. Solution-Cooling Method
The solution-cooling method represents one of the most straightforward and widely employed strategies for growing OIMH crystals. In a typical process, the organic cation and the metal halide precursor are dissolved in a suitable solvent at a prescribed stoichiometric ratio and heated until the components fully react to form a homogeneous solution. As the solution is subsequently cooled, the solubility of OIMHs decreases with temperature, leading to supersaturation and the initiation of crystallization. The rate of cooling plays a decisive role: rapid cooling generally promotes the formation of numerous small crystallites, whereas controlled, slow cooling favors fewer nucleation sites and the growth of larger crystals with higher structural perfection. Consequently, by carefully adjusting the cooling gradient, OIMH crystals with improved crystallinity, reduced defect density, and enhanced optical quality can be obtained [29]. For example, by gradually cooling the reaction solution, the Ma group readily produced large quantities of 1D (C4N2H14)SnBr4 crystals [30].
3.2. Solvent Evaporation Method
The solvent evaporation method is another widely used route for growing OIMH crystals. Typically, the organic cation and metal–halide precursors are fully dissolved in a suitable solvent at a defined stoichiometric ratio; controlled, slow evaporation of the solvent then drives supersaturation and nucleation, followed by crystal growth. Because many OIMHs are moisture-sensitive, aprotic organic solvents are generally employed—most commonly DMF, DMSO, γ-butyrolactone (GBL), N-methyl-2-pyrrolidone (NMP), or acetonitrile—to mitigate hydrolysis and compositional drift. Compared with solution-cooling, solvent evaporation is often more time-consuming, as mass-transport is governed by the evaporation rate; however, it is experimentally straightforward and can yield high-quality single crystals when the evaporation flux is carefully regulated (via temperature, headspace, humidity, and exposed surface area) to minimize secondary nucleation and defects. Variants such as isothermal slow evaporation and vapor-mediated evaporation further refine nucleation–growth balance and are frequently adopted for OIMHs requiring narrow crystallization windows. For example, using solvent evaporation, Xiong’s group obtained 2D (C7NH10)2PbCl4 crystals measuring 5 × 10 × 2 mm3 within a few days [31].
3.3. Solvent Diffusion Method
In this method, the reactants are dissolved in two mutually miscible solvents that differ markedly in density and solvent quality. The lower-density solvent is gently layered over the higher-density phase to form a sharp interface; under gravity-assisted, Fickian interdiffusion, smooth concentration and solvent-quality gradients develop across the interface. These gradients drive controlled supersaturation, localizing nucleation near the moving diffusion front and enabling defect-suppressed crystal growth. Solvent diffusion is especially useful when a metal–halide/solvent system exhibits weak temperature dependence of solubility or when the good solvent struggles to evaporate: a poor solvent is allowed to diffuse into the precursor solution to trigger crystallization. For example, at room temperature, the Ma group diffused diethyl ether into DMF solution containing SbCl3 and Ph4PCl to afford 0D (Ph4P)2SbCl5 crystals [32].
3.4. Antisolvent Crystallization
Antisolvent crystallization lowers the solubility of dissolved precursors until the solution becomes supersaturated, thereby triggering nucleation and crystal growth [33]. In practice, crystal quality and grain size are governed by (i) the polarity/miscibility contrast between the good solvent and the antisolvent, (ii) crystallization temperature, and (iii) supersaturation kinetics dictated by the antisolvent addition/diffusion rate. After dissolving the metal–halide precursors in an appropriate coordinating solvent (e.g., DMF, DMSO, GBL), the controlled introduction of a poorly coordinating, volatile antisolvent (e.g., chlorobenzene, dichloromethane, ethanol, n-hexane, toluene, diethyl ether) reduces solubility and raises supersaturation according to the LaMer framework, initiating nucleation once the critical threshold is reached. To favor growth over secondary nucleation and to suppress defect-rich crystallites, the antisolvent flux must be carefully regulated—commonly by vapor-diffusion across a partially covered vessel or by metered liquid addition. The diffusion rate can be tuned by the aperture geometry and headspace path length, as well as by temperature (which simultaneously affects solvent vapor pressure, viscosity, and solubility). Although replenishing precursors is inconvenient with the antisolvent method, it enables crystallization at concentrations below the nominal solubility limit and is well suited for growing small-sized OIMH crystals [34].
3.5. Mechanochemical Method
Mechanochemical synthesis induces chemical transformation by imparting mechanical energy to solid reactants—typically under solvent-free or solvent-minimized conditions. Although the microscopic mechanism of solid-state grinding is not fully resolved, prevailing views attribute reactivity to repeated particle-size reduction, the continuous exposure of “fresh” reactive surfaces, and localized shear/pressure “hot spots,” all of which lower kinetic barriers and accelerate bond formation. For OIMHs, mechanochemistry has emerged as a fast, scalable, and solvent-lean route that offers (i) inherently lower toxicity by avoiding bulk organic solvents, (ii) precise stoichiometric control in closed systems, and (iii) straightforward implementation using a mortar and pestle or electrically powered ball mills. Variants such as liquid-assisted grinding further widen the crystallization window by introducing microliter-scale protic/aprotic additives to modulate ion mobility and intermediate adduct formation. In many cases, the approach affords high yields with few side products and is therefore attractive for upscaling OIMH production [35]. Practical considerations include brief post-annealing or vapor-phase treatments to enhance crystallinity and phase purity when required, as well as careful control of milling parameters (ball-to-powder ratio, frequency, time) to balance nucleation and growth while minimizing defect generation [36]. However, mechanochemical methods attractive for OIMHs typically yield polycrystalline powders or microcrystals suitable for PXRD, not the millimeter-scale single crystals required for full single-crystal XRD analysis or many device-physics studies. Mechanochemical methods are not the choice when device-grade or diffraction-quality OIMH single crystals are required.
4. Photophysical Mechanisms of Organic–Inorganic Hybrid Metal Halides
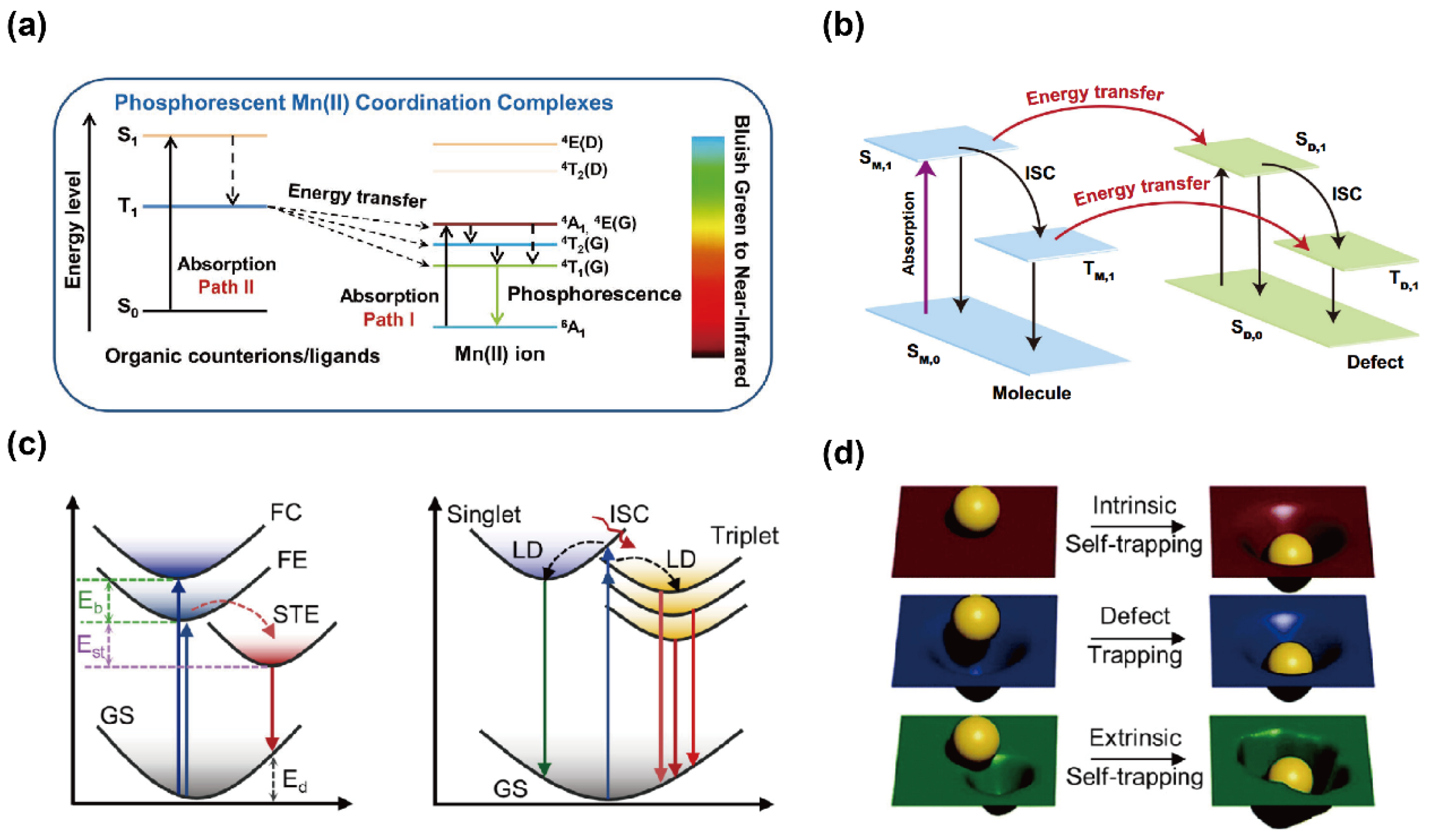
OIMHs feature rich chemistries and structural diversity. In particular, in low-dimensional OIMHs, photogenerated carriers are spatially confined within isolated metal–halide polyhedra, which effectively increases the probability of radiative recombination and thus enables high PLQY, broadband emission, large Stokes shifts, and self-absorption-free STE emission [37]. Despite rapid progress, key aspects of the emission mechanisms in OIMHs remain to be clarified—for example, dopant/ion-centered emission, defect-state emission, and emission behaviors regulated by external fields such as temperature and pressure. It is therefore important to probe the exciton dynamics and intrinsic physical properties of these systems. Current evidence indicates that the PL emission arises mainly from four pathways (Figure 3): (1) band-edge exciton dynamics; (2) ion-centered (activator) emission; (3) defect-induced emission; (4) STE emission [16,38].
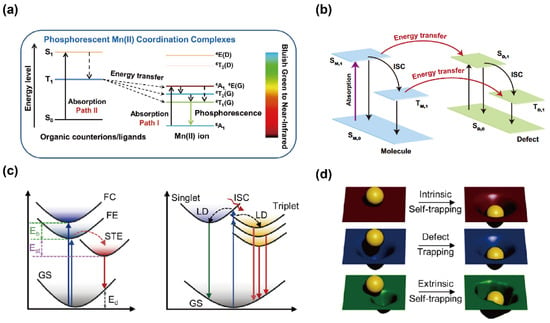
Figure 3.
(a) Emission mechanism of Mn-based OIMHs [39]. Reprinted with permission from Ref. [39]. Copyright 2020, Wiley. (b) Schematic of energy transfer between singlet and triplet defect states [40]. Reprinted with permission from Ref. [40]. Copyright 2024, Springer Nature. (c) Typical STE formation process, where Eβ is the exciton binding energy, Est is the self-trapping energy, and Ed is the lattice deformation energy; schematic STE formation pathways in 0D OIMHs, where LD denotes lattice distortion; (d) intrinsic vs. extrinsic STE formation in 0D OIMHs [6]. Reprinted with permission from Ref. [6]. Copyright 2024, Wiley.
4.1. Band-Edge Exciton Dynamics
Band-edge exciton dynamics refer to radiative recombination of tightly bound excitons formed at the conduction- and valence-band edges. In low-dimensional OIMHs, reduced dielectric screening together with enhanced quantum confinement markedly increases the exciton binding energy, enabling stable, room-temperature excitonic emission. As a result, band-edge processes typically produce narrowband, high-purity photoluminescence with short lifetimes, a combination that is highly attractive for optoelectronic applications. Excitons are strongly governed by structural dimensionality: in 3D frameworks, photogenerated excitons experience negligible spatial confinement and behave as free excitons (FEs) with low binding energies, which readily dissociate into free carriers at room temperature. While such dissociation suppresses radiative recombination, it benefits carrier transport and thus device operation in, for example, photodetectors and solar cells [41]. By contrast, 2D and lower-dimensional lattices confine carriers within metal–halide layers or clusters, stabilizing band-edge excitons and favoring radiative decay. Representative cases include 2D (PEA)2PbI4, which exhibits sharp excitonic emission around ~520 nm at room temperature due to strong confinement within Pb–I layers [42], and 3D CsPbBr3 nanocrystals, which show intense green band-edge emission (~515 nm) with PLQY approaching unity [43].
4.2. Ion Emission
Incorporation of transition-metal and rare-earth ions in OIMHs can bring emissive centers. Mn2+ ions with distinctive 3d5 configuration exhibit coordination-environment-dependent optics: it emits green light in four-coordinate, tetrahedral (weak-field) sites and red light in six-coordinate, octahedral (strong-field) sites; under certain conditions, even dynamic interconversion between these two coordination environments can be realized [44,45,46]. The PL emission of Mn-based OIMHs arises primarily from spin-forbidden d–d transitions of Mn2+. As illustrated in Figure 3a, two archetypal excitation pathways are distinguished by complex type. For neutral Mn complexes (Path II), the ligand first absorbs a photon and is promoted to a singlet excited state, and then it undergoes intersystem crossing (ISC) and transfers energy to triplet levels of Mn2+, culminating in the radiative transition 4T1 → 6A1 to yield efficient, long-lived phosphorescence. For ionic Mn-based OIMHs (Path I), UV excitation promotes the Mn2+ center directly from 6A1 to higher-energy 4T1, 4T2, or 4A1 states, followed by the radiative 4T1→ 6A1 emission [39,47].
4.3. Defect Emission
Defect luminescence can be divided into intrinsic and extrinsic types. “Intrinsic defects” arise when atoms in the lattice deviate from their ideal crystallographic sites; such defects are highly localized and, under external stimuli (e.g., illumination, temperature), can enable nonradiative recombination. Whenever electrons or holes reside on intrinsic defect levels, the resulting emission should be classified as intrinsic defect-induced luminescence, whereas “extrinsic defects” are introduced by impurity ions during doping [48].
Although defects are often deemed detrimental to emission, in some cases, the introduction of sub-bandgap states with suitable energies produces distinctive luminescence, turning defects into new radiative recombination centers [49]. The presence of defects can generate additional localized electronic levels in any material, where a fraction of electrons/holes become trapped within bandgap defect states. These carriers may lose energy through lattice thermal vibrations or, via exciton trapping and radiative transitions, give rise to defect-induced luminescence. As shown in Figure 3b, upon photoexcitation, carriers are promoted from the ground state (S0) to the excited singlet state (S1); a portion of the excited electrons then transfers to triplet states (Tn) through ISC. Because defect-related triplet states can also store energy, bidirectional energy transfer between the singlet and triplet manifolds can occur via mixed energy transfer pathways, producing defect-state emission and, consequently, afterglow.
4.4. Self-Trapped Exciton Emission
Exciton emission in OIMHs exhibits a pronounced dependence on structural dimensionality. In 3D OIMHs, emission is typically dominated by FEs, whereas in 2D and 1D systems, both FE and self-trapped exciton (STE) emission can coexist due to partial confinement. The reduced dimensionality enhances quantum confinement effects, restricting exciton migration and simultaneously promoting self-trapping through strong electron–phonon coupling, which manifests as distinctive broadband luminescence [50,51]. When the dimensionality is reduced to 0D, extreme exciton localization suppresses FE features, and only broadband STE emission is observed at room temperature [52].
Two principal models have been proposed to explain the large-Stokes-shift broadband emission from STEs in OIMHs [6]. In conventional low-dimensional OIMHs (Figure 3c), photogenerated carriers form FEs that relax into STEs via lattice distortions induced by electron–phonon coupling. The resulting large Stokes shift reflects the combined contributions of exciton binding energy, self-trapping energy, and lattice reorganization. However, 0D OIMHs display a distinct STE formation route (Figure 1c), and excitons directly drive excited-state lattice distortions within isolated metal–halide polyhedra, producing multiple STE states without an FE intermediate. The absence of an energy barrier between FE and STE states in 0D systems creates ideal conditions for efficient self-trapping.
Experimental studies further confirm that the extent of lattice distortion in isolated polyhedra correlates directly with emission bandwidth. Strong electron–phonon coupling, described by the Huang–Rhys factor (S), broadens the spectrum according to the following [53]:
where ℏ, ω, kB, and T are the reduced Planck constant, the phonon frequency, the Boltzmann constant, and temperature, respectively. S is the Huang–Rhys factor, reflecting the strength of electron–phonon coupling. In general, larger S favors STE formation; however, excessively strong coupling enhances nonradiative recombination and leads to quenching. Thus, moderating the coupling strength is essential for efficient broadband STE emission [54].
Because STE emission originates from excited-state lattice distortions, it is sometimes described as a defect of the excited state, as shown in Figure 3d. Unlike defect-induced luminescence, which depends on intrinsic defect density, STE emission arises from a perfect ground-state lattice with only transient excited-state perturbations. Moreover, extrinsic STEs can form when dopant ions introduce local inhomogeneity. In both intrinsic and extrinsic cases, distributions of self-trapping depths promote broadband emission [8,50]. While STE-based luminescence offers unique advantages for OIMHs, limitations such as restricted spectral tunability and thermal quenching motivate exploration of alternative or hybrid emission pathways [55].
5. Applications of Organic–Inorganic Hybrid Metal Halides
OIMHs, owing to their excellent optoelectronic properties, band-structure tunability, near-unity PLQYs, and compatibility with low-cost solution processing, have found broad applications in optoelectronic devices such as LEDs [56], information encryption and anti-counterfeiting [57], luminescent probes and sensors [58], and X-ray scintillation imaging [59]. Among these, Pb-based OIMHs remain the mainstream materials because of their nearly 100% PLQY and wide bandgap tunability. Representative compounds include 3D CsPbBr3 and 2D (R-MBA)2PbI4, which have been extensively employed in LEDs, encryption, anti-counterfeiting, and scintillation imaging. Beyond lead-based systems, Mn-based OIMHs have demonstrated significant potential in information security, anti-counterfeiting, and sensing applications due to their long-lived orange–red emission arising from Mn2+ d–d transitions. In parallel, Bi- and Sb-based OIMHs, benefiting from the high atomic numbers of Bi3+ and Sb3+, exhibit strong X-ray absorption, making them attractive candidates for scintillation imaging. Their unique energy-level structures also provide opportunities for use in luminescent probes and sensors. Notably, Sb3+-based 0D OIMHs, which share the 5s2 lone-pair electronic configuration with Sn2+ but avoid the oxidation instability of Sn, have emerged as particularly promising long-wavelength broadband emitters [60,61].
5.1. Light-Emitting Diodes
White LEDs have become leading replacements for traditional incandescent sources because of their high brightness, energy efficiency, and long lifetime. At present, two main technologies are used to fabricate white LEDs: (i) a UV chip that simultaneously excites three primary-color emitters to produce white light; and (ii) a down-conversion approach in which a blue chip and a color-conversion layer (typically a yellow phosphor) are co-packaged in a polymer matrix, yielding white emission by mixing the chip’s blue light with the converted yellow light. However, white LEDs produced by these routes still suffer from complex processing, limited tunability, insufficient luminous efficiency, and a relatively low CRI [62]. OIMHs have emerged as promising alternatives for next-generation WLEDs. These materials offer the combined advantages of low-cost processing, high PLQYs, and fully tunable emission spectra, which collectively address many of the limitations of rare-earth phosphors [63,64].
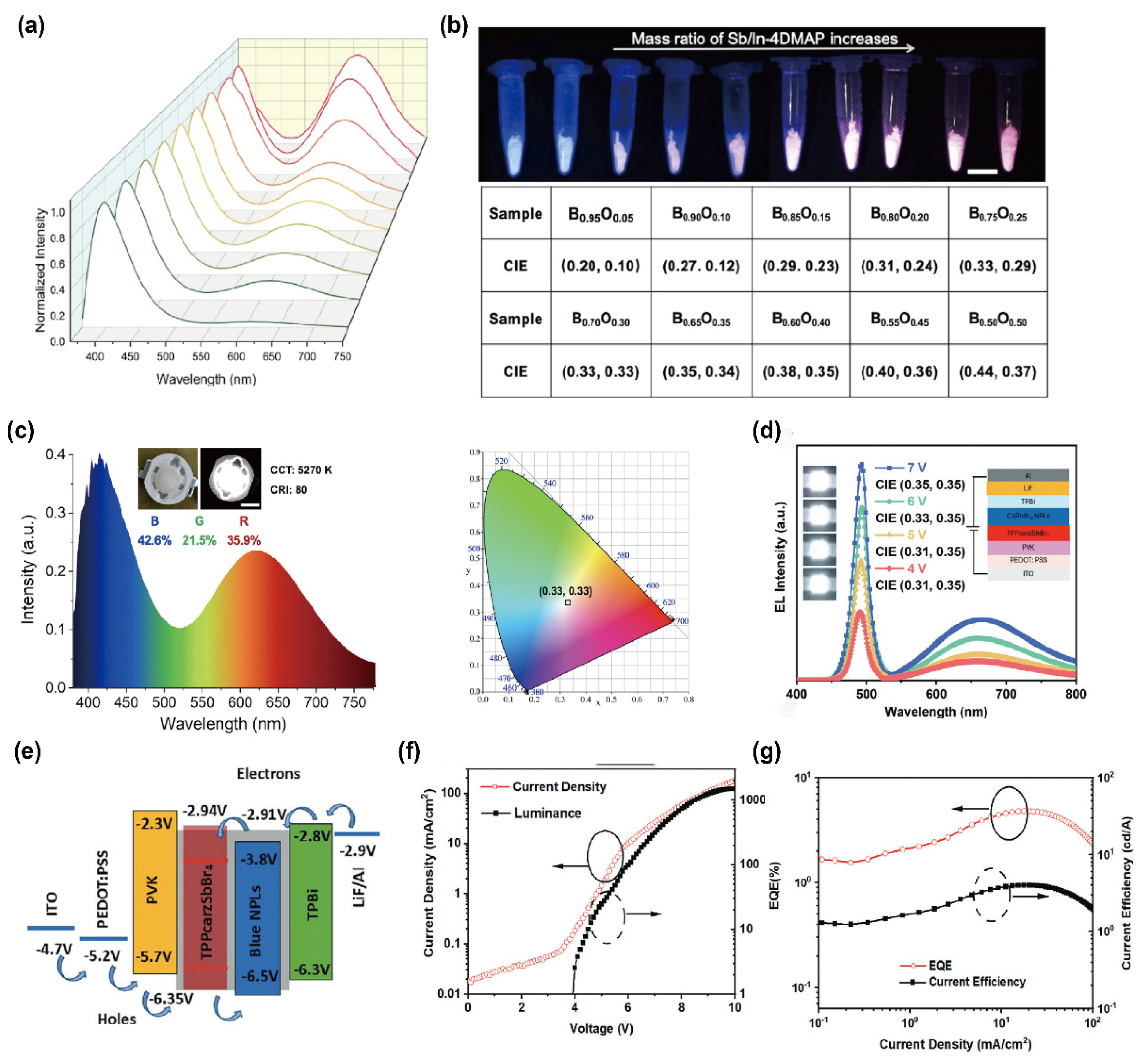
Recent advances in Mn2+-based 0D OIMHs highlight their potential for high-efficiency display and lighting applications. For example, Xia et al. discovered high-efficiency 0D OIMHs, (C10H16N)2MnBr4, through structural design engineering, and its excellent thermal stability is also highly competitive in practical LCD applications. The as-fabricated WLED device exhibited a high luminous efficiency of 120 lm W−1, together with CIE chromaticity coordinates of (0.3054, 0.3088), which fall within the white-light region [65]. In parallel, Yan’s group prepared a series of 0D Sb3+-based OIMHs. Driven by noncovalent interactions between inorganic cores and organic shells, the monomer Sb-2DMAP forms well-defined 1D microrods, while the dimer Sb/In-4DMAP yields 2D microplates. Leveraging the complementarity between deep-blue thermally activated delayed fluorescence (TADF) and orange room-temperature phosphorescence (RTP), they proposed that tuning the ratio of deep-blue Sb-2DMAP to orange-emitting Sb/In-4DMAP would enable white emission. As shown in Figure 4a, increasing the Sb/In-4DMAP mass fraction enhances the orange band at 627 nm, and the emission color can be continuously tuned from blue (CIE: 0.20, 0.10) to orange (CIE: 0.44, 0.37). At a 30% Sb/In-4DMAP mass ratio, deep-blue and orange emissions reach an optimal balance to produce standard white light (CIE: 0.33, 0.33), as shown in Figure 4b. This 30% mixture was then integrated with a commercial 365 nm UV chip to construct a WLED (Figure 4c). The device exhibits a well-balanced distribution of the three primaries (blue 42.6%, green 21.5%, red 35.9%), achieving standard white emission, along with a low correlated color temperature (CCT = 5270 K) and a high color rendering index (CRI = 80), fully meeting the requirements for high-quality white-light illumination.
Beyond down-conversion WLEDs, direct electroluminescence from OIMHs represents an exciting frontier. In such devices, holes injected from the p-type hole-transport layer (HTL) and electrons from the n-type electron-transport layer (ETL) recombine within the emissive interlayer to form excitons, which subsequently radiatively decay [66,67,68]. While significant progress has been made in monochromatic LEDs spanning the blue, green, red, and near-infrared regions, achieving stable and efficient white EL within a single device remains challenging. A notable strategy was proposed by Ma and co-workers [69], who combined narrowband sky-blue emission from 2D CsPbBr3 nanoplatelets (NPLs) with broadband orange/red emission from 0D TPPcarzSbBr3. The bilayer structure (40 nm/70 nm) was solution-processed, yielding stable white emission with a CCT of 6000 K. Importantly, charge-carrier mobility studies confirmed well-matched transport between the 0D and 2D components, ensuring balanced exciton recombination. The optimized device achieved CIE coordinates of (0.32, 0.35), a peak external quantum efficiency (EQE) of 4.8%, and a luminance of 1507 cd m−2. Although the efficiency remains below theoretical limits, these results underscore the promise of 0D/2D heterostructure designs for realizing high-performance, solution-processable white EL devices (Figure 4e–g).
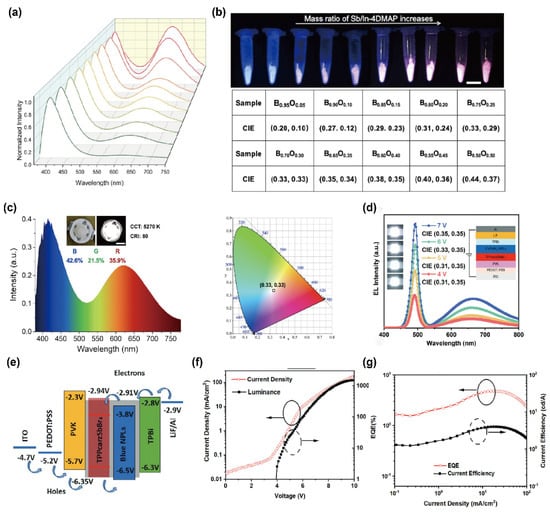
Figure 4.
(a) PL spectra of Sb/In-4DMAP at mass ratios of 5–50%; (b) photographs under 365 nm excitation and corresponding CIE coordinates (“B”: Sb-2DMAP; “O”: Sb/In-4DMAP; scale bar 1 cm); (c) photographs (inset), EL spectra, and CIE coordinates of the WLED before/after operation [64]. Reprinted with permission from Ref. [64]. Copyright 20123, Wiley. (d) EL spectra and CIE coordinates of the 0D TPPcarzSbBr4/2D CsPbBr3 NPLs white LED at different drive voltages (inset: device photo and schematic); (e) working principle; (f) current density–voltage–luminance curves; (g) EQE–current density–current efficiency plots [69]. Reprinted with permission from Ref. [69]. Copyright 2024, Wiley.
Figure 4.
(a) PL spectra of Sb/In-4DMAP at mass ratios of 5–50%; (b) photographs under 365 nm excitation and corresponding CIE coordinates (“B”: Sb-2DMAP; “O”: Sb/In-4DMAP; scale bar 1 cm); (c) photographs (inset), EL spectra, and CIE coordinates of the WLED before/after operation [64]. Reprinted with permission from Ref. [64]. Copyright 20123, Wiley. (d) EL spectra and CIE coordinates of the 0D TPPcarzSbBr4/2D CsPbBr3 NPLs white LED at different drive voltages (inset: device photo and schematic); (e) working principle; (f) current density–voltage–luminance curves; (g) EQE–current density–current efficiency plots [69]. Reprinted with permission from Ref. [69]. Copyright 2024, Wiley.
5.2. Information Encryption and Anti-Counterfeiting
Information recording, storage, and security technologies represent a cornerstone of modern information systems, with vital applications spanning national defense, financial protection, and daily life [70]. Among these, information encryption ensures confidentiality, integrity, and authenticity, thereby preventing tampering, forgery, and unauthorized access. Optical-materials-based encryption has gained considerable interest owing to its unique advantages: by modulating optical responses, materials can achieve efficient information encoding, covert storage, and selective decryption under specific excitation conditions. Mainstream approaches currently include laser holography, photoluminescence (PL) encoding, magnetic tagging, and pattern-based anti-counterfeiting. Notably, PL-based fluorescent encryption has emerged as particularly attractive, due to its high visibility, operational convenience, and excellent concealability, making it suitable for a wide range of application scenarios [71].
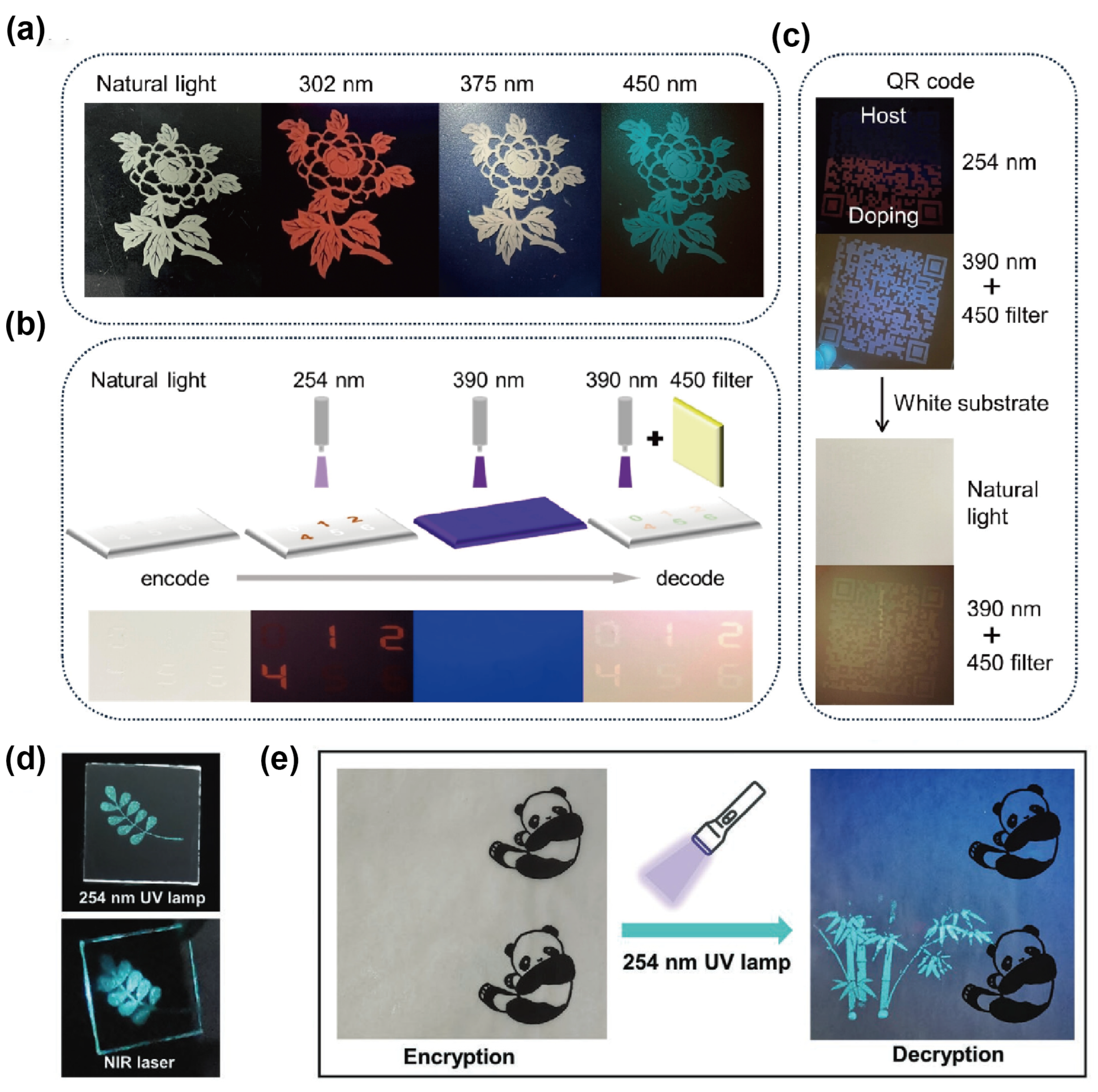
To overcome the poor stability of Sn2+-based OIMHs, Xia’s group developed the Sn4+-based 0D compound ATPP2SnCl6 (ATPP = acetyltriphenylphosphine) and its Sb3+-doped derivatives, which exhibit excitation-wavelength-dependent luminescence. Owing to the rigid Sn4+ lattice and Sb3+ dopants as emission centers, the materials display tunable emission across red, white, and green. Specifically, orange–red STE emission is observed under 302 nm excitation, intrinsic blue–green emission emerges at 450 nm, and simultaneous activation under 375 nm yields white light. For practical validation, flexible ATPP2SnCl6:Sb3+@PDMS composites were fabricated and applied in anti-counterfeiting demonstrations. Screen-printed floral and numeric patterns exhibited dynamic, wavelength-dependent color switching, enabling selective decryption under UV or filtered excitation. Furthermore, QR-code encryption systems combining doped and undoped composites achieved secure information storage: full decoding was only possible under specific excitation (390 nm) with an optical filter, while the code remained invisible under other conditions. These results highlight the stability, multicolor tunability, and excitation-dependent encryption capability of Sn4+-based OIMHs, underscoring their promise for advanced luminescent security applications (Figure 5a–c).
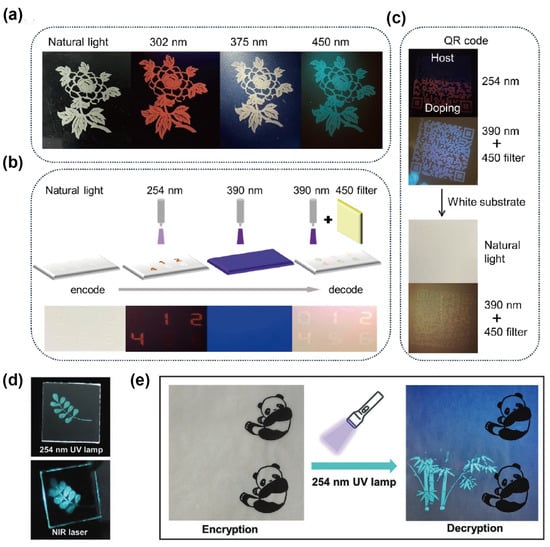
Figure 5.
(a) Photographs of ATPP2SnCl6:Sb3+@PDMS floral patterns on glass under ambient and different excitation wavelengths; (b) schematic of digit encoding/decoding on a white substrate; (c) QR codes screen-printed on glass/white substrates demonstrating anti-counterfeiting [57]. Reprinted with permission from Ref. [57]. Copyright 2024, Wiley. (d) Images of (AEP)2Cu2I6·2I·2H2O patterns under UV and NIR; (e) encrypted (visible) vs. decrypted (UV) bamboo patterns screen-printed on real documents using (AEP) 2Cu2I6·2I·2H2O@PDMS [72]. Reprinted with permission from Ref. [72]. Copyright 2022, Wiley.
Copper-based OIMHs offer complementary advantages, including low cost, low toxicity, and natural abundance. Kuang et al. demonstrated that the 0D (AEP)2Cu2I6·2I·2H2O (AEP = N-aminoethylpiperazinium) is a promising information-storage and anti-counterfeiting luminophore. By formulating (AEP)2Cu2I6·2I·2H2O@PDMS as a printable gel ink, well-defined patterns were produced via screen printing. The printed “branch” motif is invisible under ambient light yet readily revealed under UV excitation and NIR laser, enabling dual-channel authentication. As a proof of concept for document security, a monochrome “panda” image was first laser-printed on sulfuric paper; a bamboo-shaped overprint using the (AEP)2Cu2I6·2I·2H2O@PDMS ink allowed selective verification under UV illumination. These results highlight the material’s stimulus-responsive, covert-to-overt contrast and compatibility with scalable printing, underscoring its potential for covert tagging, multi-modal encryption, and practical anti-counterfeiting of paper-based records (Figure 5d,e) [72].
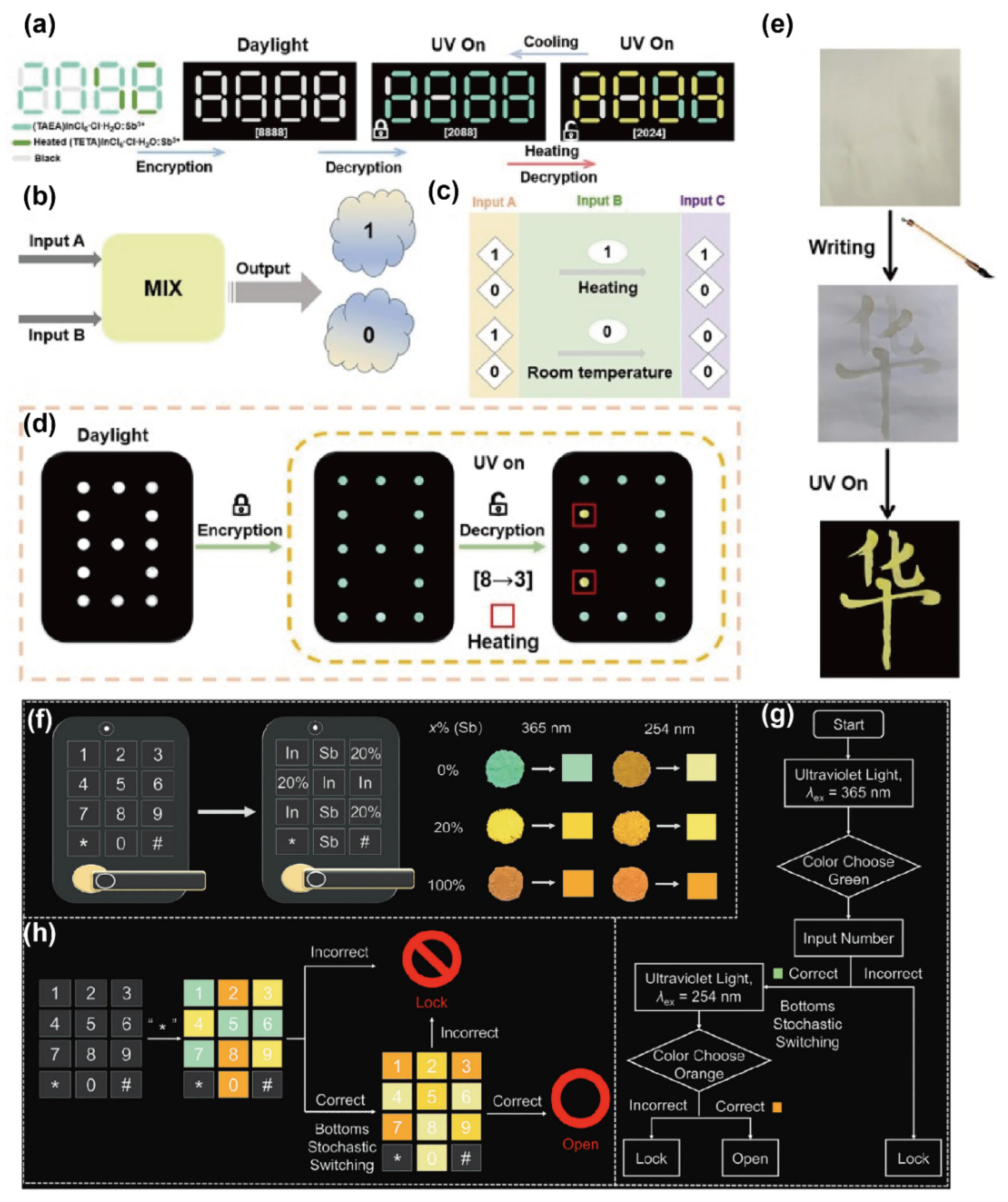
Stimuli-responsive smart luminescent materials are drawing intense interest for optical data recording and storage devices. In particular, such materials—capable of reversible luminescent responses to external stimuli such as light, electricity, magnetism, heat, and mechanical force—are becoming a research focus in optical information storage. They enable multi-mode information encoding, including fluorescence-intensity modulation, emission-wavelength shifts, and lifetime control, thereby showing great potential for high-density optical data storage, dynamic anti-counterfeiting labels, and rewritable display devices [73,74,75]. Li’s group successfully synthesized two 0D OIMHs: a green broadband emitter (TAEA)InCl6·Cl·4H2O:xSb3+ (denoted as 1:xSb3+) and a yellow emitter (TETA)InCl6·Cl·H2O:xSb3+ (denoted as 2:xSb3+). Notably, 1:0.5% Sb3+ exhibits a thermally driven, reversible green→orange color change with a fast response time of 20 s and a low transition temperature of 333 K. By contrast, heat-treated 2:1% Sb3+ does not display cold–hot reversible luminescence over short timescales. Combining the two materials enables a heating-triggered digital encryption–decryption system. As shown in Figure 6a, a numeric template “8888” with spatially segregated regions (2:1% Sb3+ and 1:0.5% Sb3+) was designed: under sunlight, it displays the decoy “8888”; after the first decryption with 365 nm UV, it shows the false message “2088”; following a second thermal step, regions containing 1:0.5% Sb3+ switch to orange emission, revealing the true message “2044.” Upon cooling to room temperature, the orange emission reverts to green, re-masking the true information and achieving cyclic encryption. Figure 6b–d further demonstrate a two-input logic gate constructed from the temperature-responsive PL-switch pair (1:0.5% Sb3+ and heat-treated 2:1% Sb3+) [76]. Define input A as the material identity (1:0.5% Sb3+ = “1”; heat-treated 2:1% Sb3+ = “0”) and input B as the ambient-temperature signal (heated = 1; room temperature = 0); the emission color serves as the output (orange = 1; green = 0). The output becomes “1” (gate ON) only when both inputs A and B are 1. In addition, 2:1% Sb3+ was formulated as a writable fluorescent ink: as shown in Figure 6e, patterns written with this ink exhibit bright yellow emission under UV illumination, highlighting its practical promise for anti-counterfeiting labels.
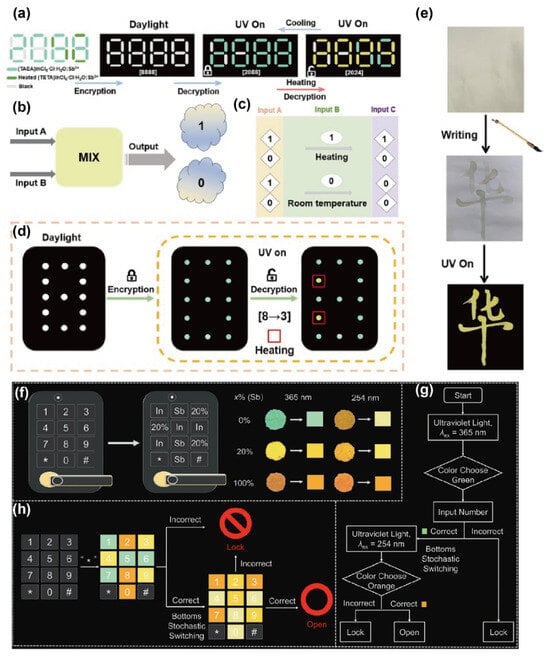
Figure 6.
(a) Schematic of dual encryption–decryption using the “8888” template; (b,c) optical logic-OR gate based on 1:0.5% Sb3+ and 2:1% Sb3+; (d) experimental design of the light-controlled logic gate; (e) fluorescent ink application of 1:0.5% Sb3+. (f–h) Dynamic color-code keypad using B-site Sb3+/In3+ alloying [77]. Reprinted with permission from Ref. [77]. Copyright 2024, Wiley. * stands for the encryption–decryption process.
Zhang’s group found that 0D (Me2NH2)4MCl6·Cl (M = Sb, In) exhibit multicolor emission under 254 nm and 365 nm excitation, enabling an anti-counterfeiting workflow for access-control applications. As shown in Figure 6f, the color distribution under 365 nm and 254 nm excitation varies with the Sb3+ doping level. Figure 6g,h depict the unlocking protocol: after the first press of the “” key, excitation with 365 nm UV light reveals the green-emitting digits that constitute the correct password; an incorrect input locks the keypad. Following the first decryption, the emitters are randomly reconfigured. Pressing “*” again and switching to 254 nm excitation reveals the orange-emitting digits that form the correct password. Only by entering the correct code twice in succession will the system unlock; otherwise, it remains locked. Featuring randomness and exclusivity, this encryption scheme markedly enhances security and shows great promise for access-control anti-counterfeiting systems [77]. Compared with established security inks and tags, OIMHs uniquely combine multi-channel optical keys with reconfigurable chemistry and low-temperature patternability, enabling high-density, hard-to-replicate encryption. While environmental stability and lead content remain concerns, 2D/0D architectures and Pb-free chemistries provide practical pathways. These attributes explain the growing interest in OIMHs for next-generation information encryption and anti-counterfeiting.
5.3. Luminescent Probes and Sensors
Owing to their distinctive optical properties, luminescent probes and sensors can transduce specific environmental inputs (e.g., temperature, pH, metal ions, gases, or solution composition) into sensitive optical signals, enabling rapid detection. Key performance metrics for such applications include response speed, selectivity, and reversibility, and certain low-dimensional OIMHs with special optical functionalities have already shown outstanding performance in this arena [58,78].
Zhang’s group found that (Me2NH2)4MCl6·Cl(M = Sb, In) exhibits composition- and temperature-dependent multicolor emission, arising from the competition between triplet STE emission (Tₙ→S0) in the inorganic [MCl6]3− units and singlet emission (S1→S0) from the organic Me2NH2+ cations. Under UV excitation, the crystals show distinct temperature-responsive color switching (cyan → yellow → orange, 80–305 K), with dual-band cooperative emission observed for the In-based variant. Notably, the Sb3+ compound enables visual temperature indication in the ultralow-temperature range (5–80 K) and was demonstrated in temperature-indicator strips integrated into a dial for extreme-environment sensing. This work highlights the potential of OIMH as multifunctional luminescent materials for spacecraft and cryogenic temperature monitoring (Figure 7a) [77].
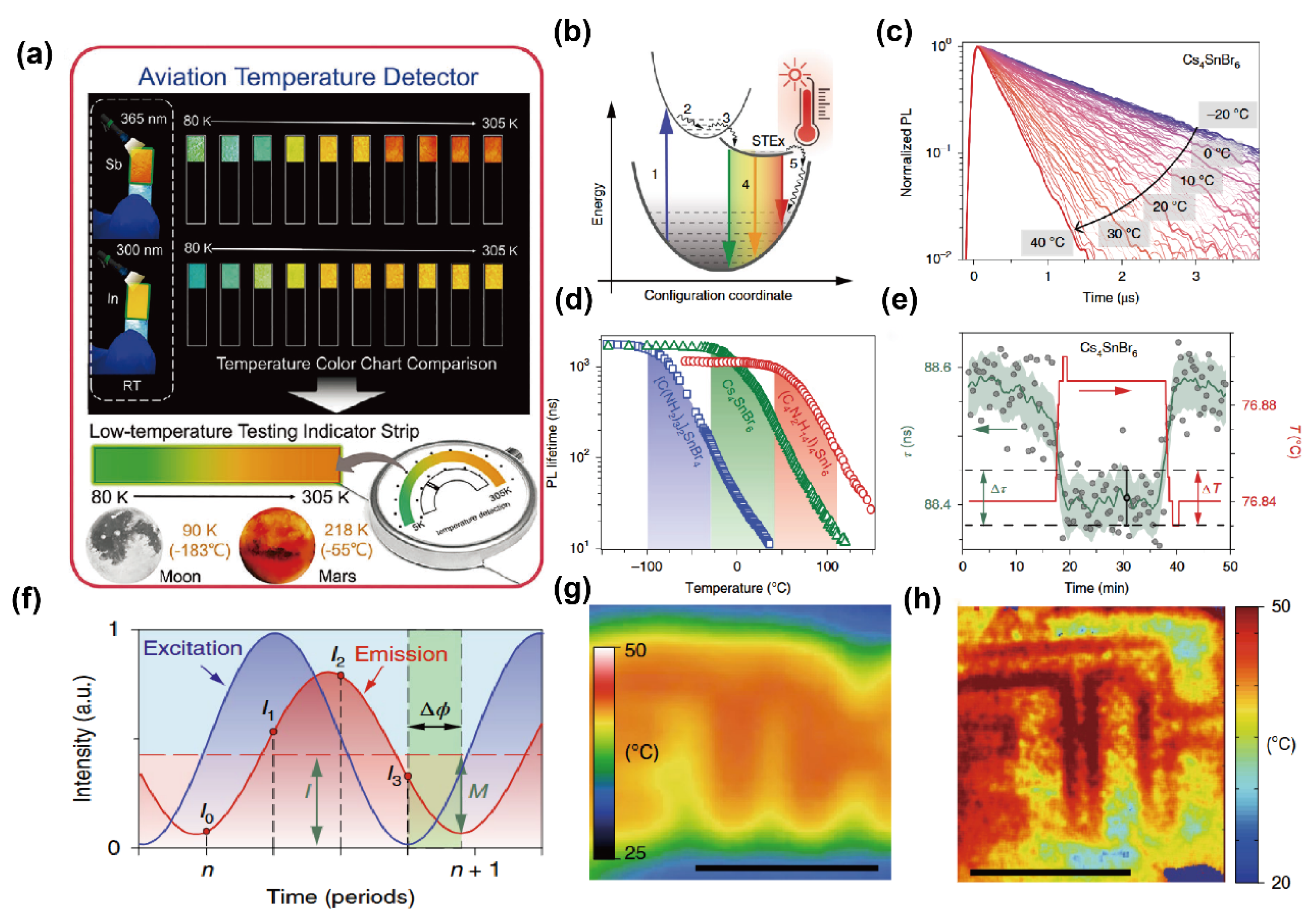
Remote thermometry and thermographic detection based on temperature-sensitive luminophores represent an emerging sensing application of OIMHs with strong value in medicine and biology. Leveraging the pronounced temperature dependence of PL lifetimes in low-dimensional OIMHs, particularly Sn2+-based 0D and 1D halides, Kovalenko’s group demonstrated precise lifetime-based thermography. The mechanism relies on STEs: photoexcited carriers relax into STE states, and thermally assisted detrapping accelerates nonradiative pathways, shortening PL lifetimes with increasing temperature. Representative compounds provide distinct sensing windows from −100 to 110 °C, with steep lifetime responses (up to 20 ns·°C−1) and excellent reproducibility. To overcome the limitations of single-channel time-resolved detection, these OIMHs were further integrated with time-of-flight fluorescence-lifetime imaging (ToF-FLI), enabling phase-resolved lifetime mapping. Compared with conventional infrared thermography, ToF-FLI based on Sn-halide OIMHs delivers markedly improved lateral resolution, high precision, and fast response, highlighting the potential of low-dimensional OIMHs for low-cost, high-performance thermographic imaging (Figure 7) [79].
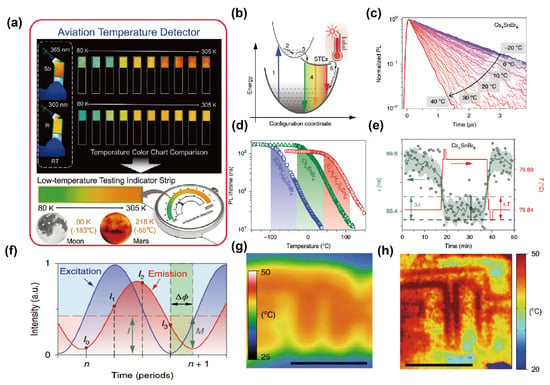
Figure 7.
(a) Multicolor emission of (Me2NH2)4MCl6·Cl (M = Sb, In) under 365/300 nm excitation and aerospace thermometry concept [77]; reprinted with permission from Ref. [77]. Copyright 2024, Wiley. (b) STE-related processes; (c) temperature evolution of TRPL traces for Cs4SnBr6 (355 nm excitation); (d) temperature-dependent PL lifetimes for [C(NH2)3]2SnBr4, Cs4SnBr6, and (C4N2H14I)4SnI6; (e) response to 0.05 °C steps (lifetime SD 0.04 ns; temperature SD 0.013 °C); (f) phase-domain reconstruction; (g) LWIR thermograph; (h) ToF-FLI thermograph under identical heating [79]. Reprinted with permission from Ref. [79]. Copyright 2021, Wiley.
Figure 7.
(a) Multicolor emission of (Me2NH2)4MCl6·Cl (M = Sb, In) under 365/300 nm excitation and aerospace thermometry concept [77]; reprinted with permission from Ref. [77]. Copyright 2024, Wiley. (b) STE-related processes; (c) temperature evolution of TRPL traces for Cs4SnBr6 (355 nm excitation); (d) temperature-dependent PL lifetimes for [C(NH2)3]2SnBr4, Cs4SnBr6, and (C4N2H14I)4SnI6; (e) response to 0.05 °C steps (lifetime SD 0.04 ns; temperature SD 0.013 °C); (f) phase-domain reconstruction; (g) LWIR thermograph; (h) ToF-FLI thermograph under identical heating [79]. Reprinted with permission from Ref. [79]. Copyright 2021, Wiley.
5.4. X-Ray Scintillation Imaging
Compared with direct X-ray detectors that convert X-ray photons directly into electrical signals, scintillator-based indirect detectors first convert high-energy X-ray photons into lower-energy photons and then transduce them into electrical signals. Owing to their fast response and low cost, indirect detectors are attracting considerable attention in medical diagnostics and security screening [80,81,82]. Scintillators play a pivotal role in multiple fields such as medical imaging, radioactive contaminant detection, non-destructive testing, high-energy physics, and homeland security. To obtain high-quality scintillators with high light yield, fast decay, large absorption coefficients, and high energy resolution, extensive research has been conducted.
In recent years, OIMH scintillators have become a focal point. Relative to conventional scintillators (which often require high-temperature synthesis, have inferior mechanical properties, and offer limited tunability in the visible range), OIMHs are easier to prepare and feature tunable emission wavelengths. In particular, low-dimensional OIMHs—by virtue of strongly localized emissive centers, large Stokes shifts (enabling efficient optical coupling), and strong X-ray absorption from heavy elements—are regarded as ideal candidates for achieving high scintillation efficiency [4,59]. Wang’s group introduced two kinds of 1D chiral OIMHs, namely (R/S-3AP)PbBr3Cl·H2O, which exhibit circularly polarized RL and a light yield of about 19,000 photons/MeV. Furthermore, they demonstrated that circularly polarized RL offers a novel strategy to mitigate optical crosstalk in X-ray imaging [83]. In 2021, Zhao’s group presented the Mn-doped STA2PbBr4 film as a fast neutron scintillator with a high hydrogen density of 9.51 × 1028 m−3 and a PLQY of 58.58%. This film served as a scintillator screen for fast neutron radiography, demonstrating a spatial resolution of 0.5 lp mm−1 [84].
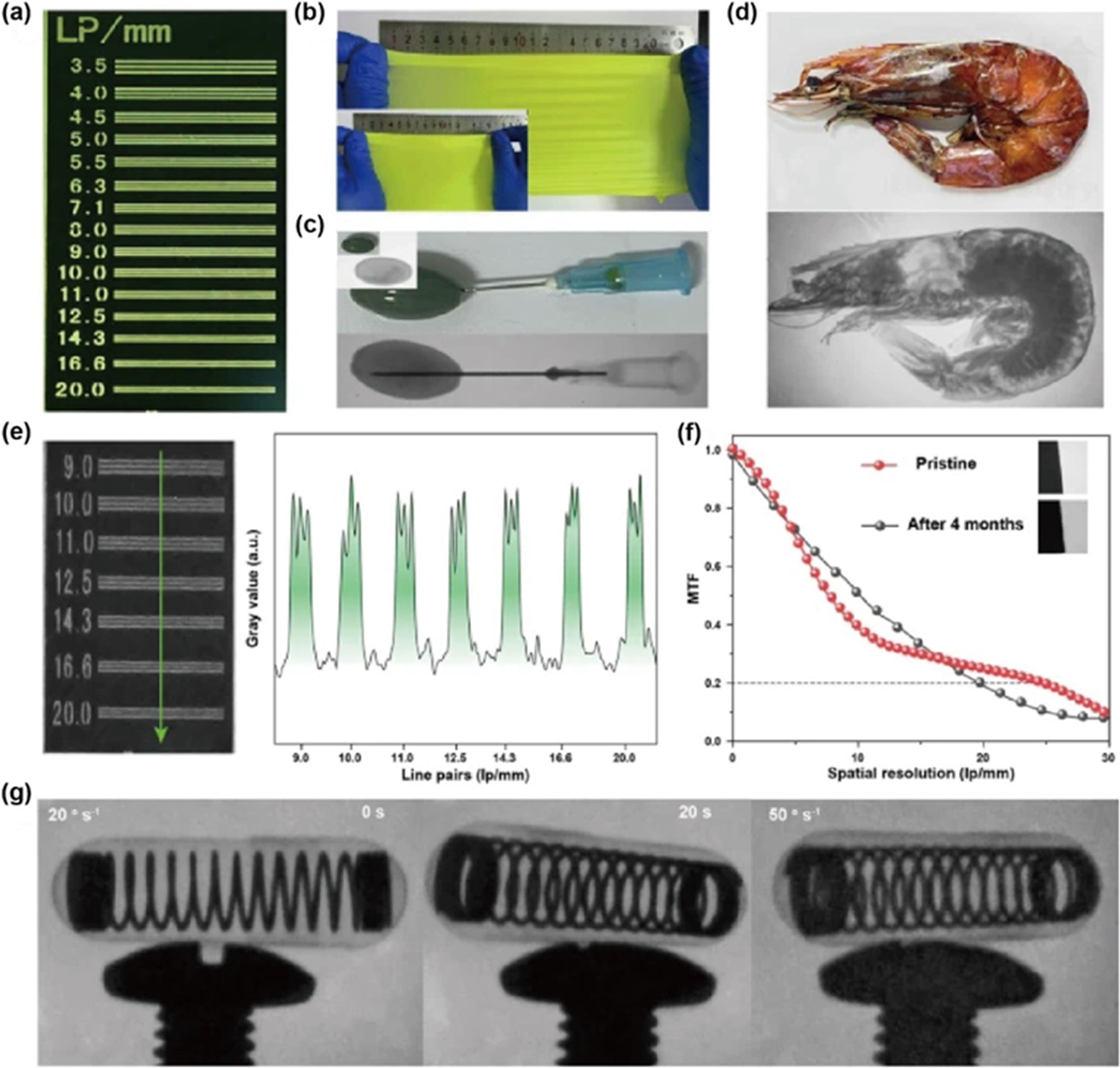
Other Pb-free OIMHs are also reported to show great potential in X/γ-ray detection. In 2020, Ma’s group demonstrated high-performance, eco-friendly X-ray scintillators utilizing a 0D (C38H34P2)MnBr4. Remarkably, high-quality (C38H34P2)MnBr4 SCs with sizes exceeding 1 inch exhibited exceptional thermal stability and emitted bright green light peaking at 517 nm. The scintillator showcased a high PLQY of ≈95%, a high light yield of 80,000 photons/MeV, and a low detection limit of 72.8 nGy s−1. The scintillator powder can be combined with polymer for flexible X-ray imaging. However, there is still incompatibility between the powder and the polymer material for the scintillation screen, impacting spatial resolution. To create large-area screens, in 2023, Jin’s group presented a scalable fabrication method for transparent films of HTP2MnBr4 through low-temperature melt-quenching. Impressively, the largest film achieved dimensions of 30 × 30 cm, making it well suited for flat panel detector applications [85]. Huang’s group reported that (C12H24O6)2Na2(H2O)3Cu4I6 (CDSCI) flexible scintillator films exhibit low optical scattering and high transparency, enabling clear imaging of line-pair charts. They also show excellent mechanical compliance, maintaining optical performance under elongations up to 125%. In X-ray imaging, the films convert transmitted intensity into visible emission with high fidelity, resolving fine internal structures such as a capsule with oil and a shrimp with heterogeneous tissue density. At a dose rate of 2.9 mGy s−1, the films achieve a spatial resolution of 20 lp mm−1, approaching the theoretical limit of 24.8 lp mm−1 determined by MTF analysis. Moreover, their ultrashort emission lifetime supports dynamic real-time imaging, capturing rapid capsule motion without motion blur even at 50° s−1. These results highlight the potential of CDSCI flexible scintillators to combine processability, high spatial resolution, and dynamic imaging capability, offering a promising pathway for next-generation medical diagnostics and industrial X-ray inspection (Figure 8) [70].
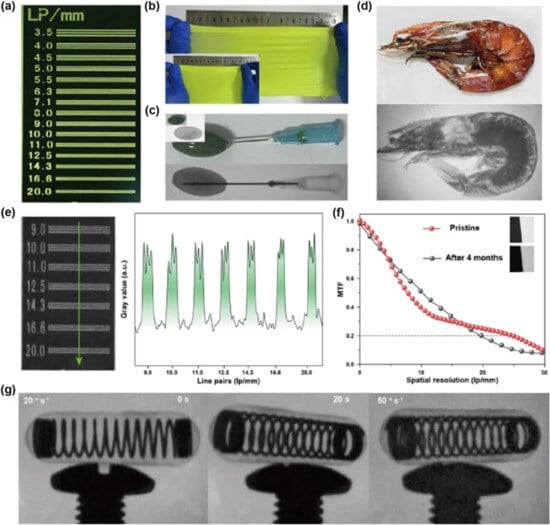
Figure 8.
Flexible CDSCI scintillator films for X-ray imaging: (a) line-pair chart; (b) photographs at different stretch ratios; (c,d) X-ray images and photographs of an oil-filled capsule (top) and shrimp (bottom); (e) X-ray image and grayscale profile of the line-pair chart; (f) MTF via slanted-edge; (g) real-time dynamic imaging of a rotating spring capsule [86]. Reprinted with permission from Ref. [86]. Copyright 2024, Springer Nature.
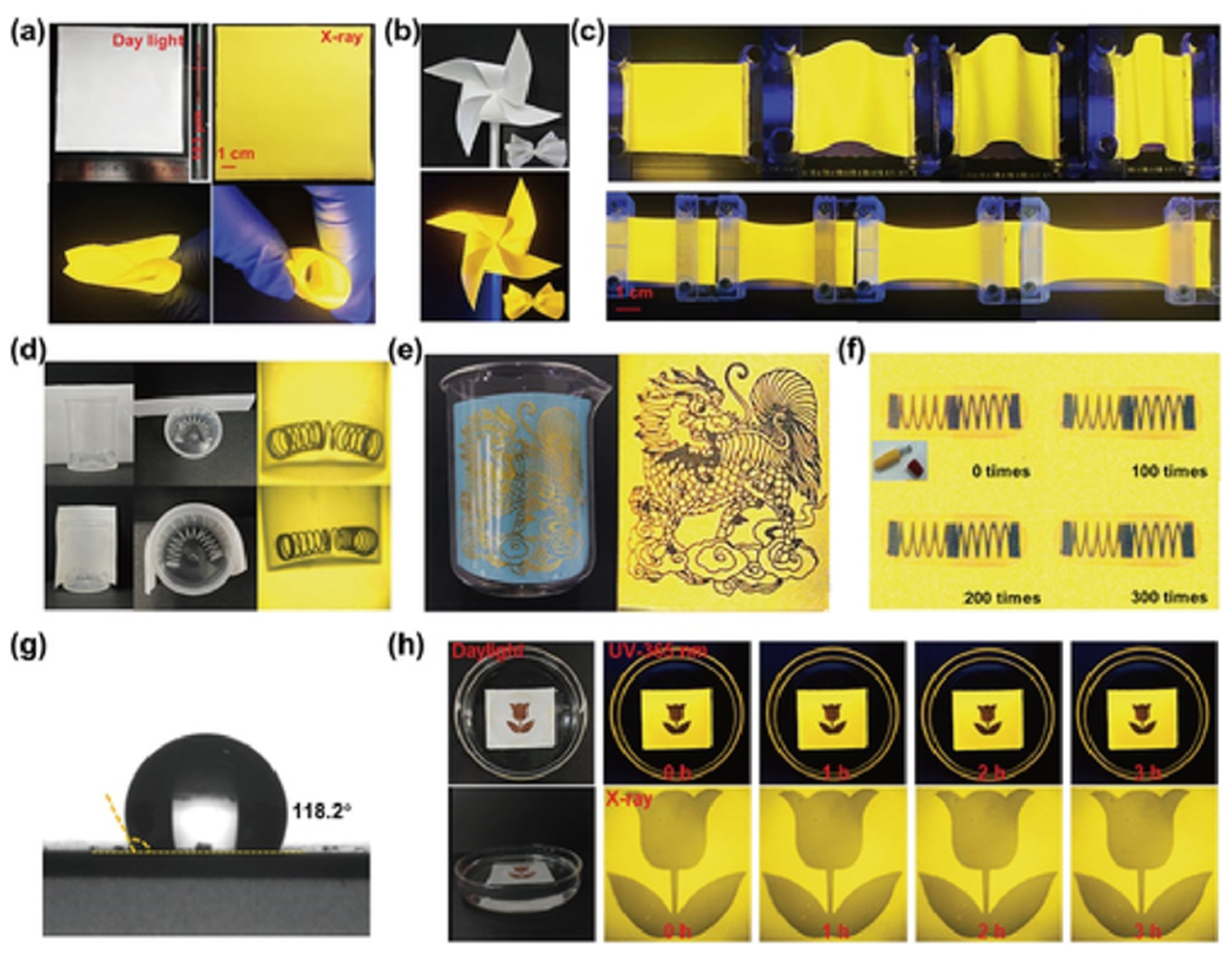
Shen and colleagues developed a 0D C38H36P2Sb2Cl8, which exhibits a wide bandgap, large Stokes shift, high PLQY, and strong X-ray absorption. They fabricated large-area, ultraflexible scintillator films with excellent mechanical compliance, capable of being stretched, rolled, or folded into complex shapes while maintaining bright yellow fluorescence and stable emission under 365 nm illumination. When applied to X-ray imaging, these films outperform conventional planar scintillators by conformally attaching to curved surfaces, thereby eliminating blur and data loss and enabling clear visualization of fine structural details. Durability studies showed that even after repeated stretching cycles at 150% strain, the films retained high-resolution imaging capability. In addition, their hydrophobic nature (contact angle 118.2°) ensures water resistance and stability; after prolonged immersion, imaging resolution was essentially unchanged. Together, these results highlight the unique advantages of C38H36P2Sb2Cl8 flexible scintillator films for nonplanar and underwater X-ray imaging, pointing to strong potential in advanced non-destructive testing applications (Figure 9) [87].
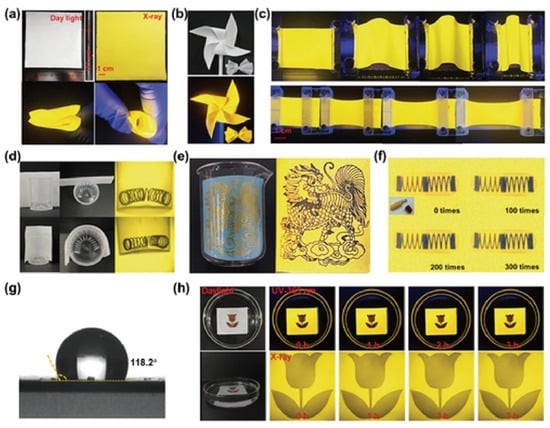
Figure 9.
Curved-surface and underwater X-ray imaging using large-area ultraflexible C38H36P2Sb2Cl8 scintillator films [87]. Reprinted with permission from Ref. [87]. Copyright 2024, Wiley. (a) Scintillator screen under daylight, X-ray, folding, and curling. (b) Scintillator film patterned as a windmill and bow, under daylight and UV light. (c) Scintillator screen under UV light during bending and stretching. (d) X-ray images of a curved spring via planar vs. curved scintillator screens. (e) Angled X-ray imaging of a curved object. (f) Scintillator screen X-ray images after 0, 100, 200, and 300 stretching cycles at 150% strain. (g) Scintillator screen water contact angle. (h) Scintillator screen in water over time, under UV and X-ray..
6. Conclusions
In this work, we summarize the common synthetic approaches of organic–inorganic hybrid metal halides, along with the recent advances in structures, PL mechanisms, and applications. In particular, their emission mechanisms are governed by multiple factors, including dimensionality, organic–inorganic hybrid nature, and local structural distortions. Although progress has been made, several challenges remain: the absence of a unified model, the coexistence of multiple emission pathways that are difficult to disentangle, and—on the design/synthesis side—a lack of in-depth mechanistic studies and systematic, reliable theoretical guidance. Unlike the many design rules established for assembling 3D and 2D structures by tuning the organic/inorganic components and synthesis conditions, rational strategies for constructing low-dimensional hybrids are not yet well developed.
Despite the excellent performance reported to date—for example, PLQYs approaching unity and promising prospects in X-ray detection, white-light LED lighting, and information anti-counterfeiting/encryption—expanding application scenarios are imposing stricter requirements, particularly for X-ray instrumentation in specialized contexts such as precision non-destructive testing under extreme conditions, thermally driven pathological diagnosis, and device thermal-failure analysis. These use cases often demand stable operation at elevated temperatures, posing a severe challenge to the radioluminescence stability of scintillators. Moreover, the intrinsic chemical fragility of low-dimensional hybrids—e.g., thermolysis of organic constituents and the high volatility of halide ions—renders them susceptible to oxidative degradation in humid and oxygen-containing environments, severely limiting long-term deployment.
Funding
This research was funded by Shandong Provincial Natural Science Foundation, grant number ZR2024QF165.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xuan, T.; Xie, R.-J. Recent Processes on Light-Emitting Lead-Free Metal Halide Perovskites. Chem. Eng. J. 2020, 393, 124757. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, L.J.; Lee, S.; Lin, H.; Ma, B. Recent Advances in Luminescent Zero-Dimensional Organic Metal Halide Hybrids. Adv. Opt. Mater. 2020, 9, 2001766. [Google Scholar] [CrossRef]
- Yao, J.-S.; Wang, J.-J.; Yang, J.-N.; Yao, H.-B. Modulation of Metal Halide Structural Units for Light Emission. Acc. Chem. Res. 2021, 54, 441–451. [Google Scholar] [CrossRef]
- Han, Y.; Yue, S.; Cui, B.B. Low-Dimensional Metal Halide Perovskite Crystal Materials: Structure Strategies and Luminescence Applications. Adv. Sci. 2021, 8, 2004805. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, C.; Tian, Y.; Siegrist, T.; Ma, B. Low-Dimensional Organometal Halide Perovskites. ACS Energy Lett. 2017, 3, 54–62. [Google Scholar] [CrossRef]
- Liu, D.; Dang, P.; Zhang, G.; Lian, H.; Li, G.; Lin, J. Near-Infrared Emitting Metal Halide Materials: Luminescence Design and Applications. InfoMat 2024, 6, e12542. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, H.; Tong, J.; Berry, J.J.; Beard, M.C.; Zhu, K. Advances in Two-Dimensional Organic–Inorganic Hybrid Perovskites. Energy Environ. Sci. 2020, 13, 1154–1186. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, H.; Sheng, M.; Shao, B.; He, Y.; Liu, Z.; Zhou, G. Advances of Low-Dimensional Organic-Inorganic Hybrid Metal Halide Luminescent Materials: A Review. Crystals 2025, 15, 364. [Google Scholar] [CrossRef]
- Lu, A.; Wu, Y.; Ji, Q.; Wang, J.; Ju, M.-G. Toward Tunable Low-Dimensional Metal Halide Perovskites with Functional Pseudohalides. Sci. Chin. Mater. 2024, 67, 2335–2344. [Google Scholar] [CrossRef]
- Mao, L.; Stoumpos, C.C.; Kanatzidis, M.G. Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. J. Am. Chem. Soc. 2018, 141, 1171–1190. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, S.; Zhou, Q.; Ju, M.G.; Zeng, X.C.; Wang, J. Two-Dimensional Perovskites with Tunable Room-Temperature Phosphorescence. Adv. Funct. Mater. 2022, 32, 2204579. [Google Scholar] [CrossRef]
- Hossain, A.; Bandyopadhyay, P.; Karmakar, A.; Ullah, A.K.M.A.; Manavalan, R.K.; Sakthipandi, K.; Alhokbany, N.; Alshehri, S.M.; Ahmed, J. The Hybrid Halide Perovskite: Synthesis Strategies, Fbrications, and Modern Applications. Ceram. Int. 2022, 48, 7325–7343. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Deng, H.; Qiao, K.; Farooq, U.; Ishaq, M.; Yi, F.; Liu, H.; Tang, J.; Song, H. Low-Dimensional Halide Perovskites and Their Advanced Optoelectronic Applications. Nano-Micro Lett. 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zou, G.; Wu, Y.; Tang, B.; Rogach, A.L.; Yip, H.L. Metal Halide Perovskite LEDs for Visible Light Communication and Lasing Applications. Adv. Mater. 2024, 1, 2414745. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qiu, L.; Liu, J.; Guan, M.; Dai, Z.; Li, G. Narrow Bandgap Metal Halide Perovskites: Synthesis, Characterization, and Optoelectronic Applications. Adv. Opt. Mater. 2022, 10, 2102661. [Google Scholar] [CrossRef]
- Li, M.; Xia, Z. Recent progress of zero-dimensional luminescent metal halides. Chem. Soc. Rev. 2021, 50, 2626–2662. [Google Scholar] [CrossRef]
- Yan, J.; Li, H.; Aldamasy, M.H.; Frasca, C.; Abate, A.; Zhao, K.; Hu, Y. Advances in the Synthesis of Halide Perovskite Single Crystals for Optoelectronic Applications. Chem. Mater. 2023, 35, 2683–2712. [Google Scholar] [CrossRef]
- Han, K.; Jin, J.; Su, B.; Xia, Z. Molecular dimensionality and photoluminescence of hybrid metal halides. Trends Chem. 2022, 4, 1034–1044. [Google Scholar] [CrossRef]
- Yang, G.; Deng, C.; Li, C.; Zhu, T.; Liu, D.; Bai, Y.; Chen, Q.; Huang, J.; Li, G. Towards efficient, scalable and stable perovskite/silicon tandem solar cells. Nat. Photonics 2025, 19, 913–924. [Google Scholar] [CrossRef]
- Guo, K.; Lv, W.; Wang, H.; Li, M.; Xu, L.; Chen, R.; Xing, G.; Wu, G. Rational Engineering of Phase-Pure 2D Perovskite Solar Cells. Adv. Funct. Mater. 2025, 35, 2412482. [Google Scholar] [CrossRef]
- Sun, J.; Wang, K.; Ma, K.; Park, J.Y.; Lin, Z.Y.; Savoie, B.M.; Dou, L. Emerging Two-Dimensional Organic Semiconductor-Incorporated Perovskites horizontal line A Fascinating Family of Hybrid Electronic Materials. J. Am. Chem. Soc. 2023, 145, 20694–20715. [Google Scholar] [CrossRef]
- Cao, Q.; Li, P.; Chen, W.; Zang, S.; Han, L.; Zhang, Y.; Song, Y. Two-dimensional perovskites: Impacts of species, components, and properties of organic spacers on solar cells. Nano Today 2022, 43, 101394. [Google Scholar] [CrossRef]
- Yang, Y.; Du, K.; Zhou, C.; Xu, M.; Wang, J.; Xu, Y.; Dong, X.; Yuan, N.; Li, B.; Zhang, X.; et al. Buried Interlayer Induced 1D Perovskite Seeds Enable Over 31%-Efficiency Perovskite/TOPCon Tandem Solar Cells. Small 2025, 21, 2504346. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Ge, C.; Rahaman, M.Z.; Lin, C.-H.; Shi, Y.; Lin, H.; Hu, H.; Wu, T. Recent Progress with One-Dimensional Metal Halide Perovskites: From Rational Synthesis to Optoelectronic Applications. NPG Asia Mater. 2023, 15, 8. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Anoop, K.M.; Devadiga, D.; Sunitha, M.S.; Ahipa, T.N. Advancements in Preventing Sn2+ Oxidation in Tin-Based Perovskite Solar Cells: A Review. phys. status solidi 2025, 222, 2400569. [Google Scholar]
- Leung, T.L.; Ahmad, I.; Syed, A.A.; Ng, A.M.C.; Popović, J.; Djurišić, A.B. Stability of 2D and quasi-2D perovskite materials and devices. Commun. Mater. 2022, 3, 63. [Google Scholar] [CrossRef]
- Sun, S.; Lu, M.; Gao, X.; Shi, Z.; Bai, X.; Yu, W.W.; Zhang, Y. 0D Perovskites: Unique Properties, Synthesis, and Their Applications. Adv. Sci. 2021, 8, e2102689. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Yin, J.; Zhou, H.; Chai, N.; Chen, B.; Zhang, Y.; Qu, K.; Shen, G.; Ma, H.; Li, Y.; et al. Layered and Pb-Free Organic–Inorganic Perovskite Materials for Ultraviolet Photoresponse: (010)-Oriented (CH3NH3)2MnCl4 Thin Film. ACS Appl. Mater. Interfaces 2016, 8, 28187–28193. [Google Scholar] [CrossRef]
- Zhou, C.; Tian, Y.; Wang, M.; Rose, A.; Besara, T.; Doyle, N.K.; Yuan, Z.; Wang, J.C.; Clark, R.; Hu, Y.; et al. Low-Dimensional Organic Tin Bromide Perovskites and Their Photoinduced Structural Transformation. Angew. Chem. Int. Ed. 2017, 56, 9018–9022. [Google Scholar] [CrossRef]
- Wang, Z.X.; Li, P.F.; Liao, W.Q.; Tang, Y.; Ye, H.Y.; Zhang, Y. Structure-Triggered High Quantum Yield Luminescence and Switchable Dielectric Properties in Manganese(II) Based Hybrid Compounds. Chem. Asian. J. 2016, 11, 981–985. [Google Scholar] [CrossRef]
- Zhou, C.; Worku, M.; Neu, J.; Lin, H.; Tian, Y.; Lee, S.; Zhou, Y.; Han, D.; Chen, S.; Hao, A.; et al. Facile Preparation of Light Emitting Organic Metal Halide Crystals with Near-Unity Quantum Efficiency. Chem. Mater. 2018, 30, 2374–2378. [Google Scholar] [CrossRef]
- Kim, B.W.; Im, S.H. Supersaturated Antisolvent-Assisted Crystallization for Highly Efficient Inorganic Perovskite Light-Emitting Diodes. ACS Nano 2024, 18, 28691–28699. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Yadav, P.; Prochowicz, D.; Sponseller, M.; Osherov, A.; Bulović, V.; Kong, J. Controllable Perovskite Crystallization via Antisolvent Technique Using Chloride Additives for Highly Efficient Planar Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803587. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Yépez, A.; Luque, R.; Camacho, L.; de Miguel, G. Benign-by-Design Solventless Mechanochemical Synthesis of Three-, Two-, and One-Dimensional Hybrid Perovskites. Angew. Chem. Int. Ed. 2016, 55, 14972–14977. [Google Scholar]
- Palazon, F.; El Ajjouri, Y.; Bolink, H.J. Making by Grinding: Mechanochemistry Boosts the Development of Halide Perovskites and Other Multinary Metal Halides. Adv. Energy Mater. 2020, 10, 1902499. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Sun, D.; Gao, B.; Zhang, B.; Yang, D.; Wang, Y. Low-Dimensional Organic–Inorganic Hybrid Metal Halide with Large Optical Anisotropy. Adv. Opt. Mater. 2025, 13, e01501. [Google Scholar] [CrossRef]
- Goetz, K.P.; Taylor, A.D.; Paulus, F.; Vaynzof, Y. Shining Light on the Photoluminescence Properties of Metal Halide Perovskites. Adv. Funct. Mater. 2020, 30, 1910004. [Google Scholar] [CrossRef]
- Tao, P.; Liu, S.-J.; Wong, W.-Y. Phosphorescent Manganese(II) Complexes and Their Emerging Applications. Adv. Opt. Mater. 2020, 8, 2000985. [Google Scholar] [CrossRef]
- Chen, X.; Che, M.; Xu, W.; Wu, Z.; Suh, Y.D.; Wu, S.; Liu, X.; Huang, W. Matrix-induced defects and molecular doping in the afterglow of SiO2 microparticles. Nat. Commun. 2024, 15, 8111. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zou, B. Effects of Electron−Phonon Coupling and Spin−Spin Coupling on the Photoluminescence of Low-Dimensional Metal Halides. J. Phys. Chem. Lett. 2022, 13, 1752–1764. [Google Scholar] [CrossRef]
- Sheikh, M.A.K.; Kowal, D.; Mahyuddin, M.H.; Cala’, R.; Auffray, E.; Witkowski, M.E.; Makowski, M.; Drozdowski, W.; Wang, H.; Dujardin, C.; et al. A2Bn–1PbnI3n+1 (A = BA, PEA.; B = MA; n = 1, 2): Engineering Quantum-Well Crystals for High Mass Density and Fast Scintillators. J. Phys. Chem. C. 2023, 127, 10737–10747. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Wu, Y.; Cai, W.; Wang, Y.; Zhang, S.; Zeng, H.; Li, X. High-Temperature, Reversible, and Robust Thermochromic Fluorescence Based on Rb2MnBr4(H2O)2 for Anti-Counterfeiting. Adv. Mater. 2023, 35, 2301914. [Google Scholar] [CrossRef]
- Xue, S.H.; Shi, C.M.; Xu, L.J.; Chen, Z.N. Thermal and Vapor Induced Triple-Mode Luminescent Switch of Manganese (II) Halides Hybrid. Adv. Opt. Mater. 2024, 12, 2302854. [Google Scholar] [CrossRef]
- Lun, M.M.; Ni, H.F.; Zhang, Z.X.; Li, J.Y.; Jia, Q.Q.; Zhang, Y.; Zhang, Y.; Fu, D.W. Unusual Thermal Quenching of Photoluminescence from an Organic–Inorganic Hybrid [MnBr4]2−-based Halide Mediated by Crystalline–Crystalline Phase Transition. Angew. Chem. Int. Ed. 2023, 63, e202313590. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, H.; Cai, W.; Lu, S.; Zhao, S.; Zang, Z.; Xie, L. Mn2+-Based Luminescent Metal Halides: Syntheses, Properties, and Applications. Adv. Opt. Mater. 2023, 11, 202202997. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Li, B.; Liu, Z.; Li, Y.; Shi, C.; Xu, Y. Emission Regulation in 0D Hybrid Copper Halides via Structural Transformation: From Defect to Non-Defect States for Information Encryption and Storage. Chin. Chem. Lett. 2025, 36, 110858. [Google Scholar] [CrossRef]
- Levine, I.; Menzel, D.; Musiienko, A.; MacQueen, R.; Romano, N.; Vasquez-Montoya, M.; Unger, E.; Mora Perez, C.; Forde, A.; Neukirch, A.J.; et al. Revisiting Sub-Band Gap Emission Mechanism in 2D Halide Perovskites: The Role of Defect States. J. Am. Chem. Soc. 2024, 146, 23437–23448. [Google Scholar] [CrossRef]
- Zhou, G.; Su, B.; Huang, J.; Zhang, Q.; Xia, Z. Broad-Band Emission in Metal Halide Perovskites: Mechanism, Materials, and Applications. Mater. Sci. Eng. R Rep. 2020, 141, 100548. [Google Scholar] [CrossRef]
- Gao, X.; Guo, F.; Chen, R.; Lin, F.; Li, Q.; Xu, D. Solvent Effects in Structural Engineering for Photoluminescent Low-Dimensional Metal Halides. Laser Photonics Rev. 2024, 18, 2400440. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, X.; Song, B.; Luo, J.; Tang, J. Light Emission of Self-Trapped Excitons in Inorganic Metal Halides for Optoelectronic Applications. Adv. Mater. 2022, 34, 2201008. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, Z.; Lu, M.; Gao, Y.; Li, X.; Bai, X.; Ji, Y.; Zhang, Y. Broadband Emission Origin in Metal Halide Perovskites: Are Self-Trapped Excitons or Ions? Adv. Mater. 2023, 35, 2211088. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.F.; Kuang, D.B. An Overview for Zero-Dimensional Broadband Emissive Metal-Halide Single Crystals. Adv. Opt. Mater. 2021, 9, 2100544. [Google Scholar] [CrossRef]
- Ran, Q.; Zhang, Y.; Yang, J.; He, R.; Zhou, L.; Hu, S. White-Light Defect Emission and Enhanced Photoluminescence Efficiency in a 0D Indium-Based Metal Halide. J. Mater. Chem. C. 2022, 10, 1999–2007. [Google Scholar] [CrossRef]
- Yan, S.; Tian, W.; Chen, H.; Tang, K.; Lin, T.; Zhong, G.; Qiu, L.; Pan, X.; Wang, W. Synthesis of 0D Manganese-Based Organic–Inorganic Hybrid Perovskite and Its Application in Lead-Free Red Light-Emitting Diode. Adv. Funct. Mater. 2021, 31, 2100855. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Han, K.; Xia, Z. Rigid Phase Formation and Sb3+ Doping of Tin (IV) Halide Hybrids toward Photoluminescence Enhancement and Tuning for Anti-Counterfeiting and Information Encryption. Angew. Chem. Int. Ed. 2024, 63, e202408653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liao, J.F.; Huang, Z.G.; Wei, J.H.; Wang, X.D.; Li, W.G.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. AHighly Red-EmissiveLead-Free Indium-Based Perovskite Single Crystal for SensitiveWater Detection. Angew. Chem. Int. Ed. 2019, 58, 5277–5281. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, W.; Xiao, J.; Li, A.; Han, X. Low-Dimensional Metal Halide for High Performance Scintillators. Adv. Funct. Mater. 2024, 34, 2402902. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.S.; Lee, T.W. Strategies to Improve Luminescence Efficiency of Metal-Halide Perovskites and Light-Emitting Diodes. Adv. Mater. 2018, 31, 1804595. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Jin, X.; Fu, H. Tunable Halide Perovskites for Miniaturized Solid-State Laser Applications. Adv. Opt. Mater. 2019, 7, 1900099. [Google Scholar] [CrossRef]
- Yao, J.; Xu, L.; Wang, S.; Song, J. Metal Halide Perovskites-Based White Light-Emitting Diodes. J. Phys. Photonics 2022, 4, 042001. [Google Scholar] [CrossRef]
- Wu, L.-K.; Li, R.-F.; Wen, W.-Y.; Zou, Q.-H.; Ye, H.-Y.; Li, J.-R. Lead-Free Hybrid Indium Perovskites with Near-Unity PLQY and White-Light Emission by Sb3⁺ Doping Strategy. Inorg. Chem. Front. 2023, 10, 3297–3306. [Google Scholar] [CrossRef]
- Zhou, B.; Qi, Z.; Dai, M.; Xing, C.; Yan, D. Ultralow-loss Optical Waveguides through Balancing Deep-Blue TADF and Orange Room Temperature Phosphorescence in Hybrid Antimony Halide Microstructures. Angew. Chem. Int. Ed. 2023, 62, e202309913. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Z.; Huang, J.; Molokeev, M.S.; Xiao, Z.; Ma, C.; Xia, Z. Unraveling the Near-Unity Narrow-Band Green Emission in Zero-Dimensional Mn2+-Based Metal Halides: A Case Study of (C10H16N)2Zn1–xMnxBr4 Solid Solutions. J. Phys. Chem. Lett. 2020, 11, 5956–5962. [Google Scholar] [CrossRef]
- Xue, S.-H.; Yao, J.-Y.; Xu, L.-J.; Chen, Z.-N. Advances in Electrically Driven Light-Emitting Diodes Based on Lead-Free Metal Halides. Chem. Commun. 2023, 59, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Bi, C.; Guo, R.; Zhang, M.; Qu, X.; Tian, J. Electroluminescence Principle and Performance Improvement of Metal Halide Perovskite Light-Emitting Diodes. Adv. Opt. Mater. 2021, 9, 2002167. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Jie, J.; Tian, C.; Jia, R.; Wu, X.; Zhang, X.; Zhang, X. Metal Halide Perovskite Single Crystals toward Electroluminescent Applications. Adv. Funct. Mater. 2024, 34, 2401189. [Google Scholar] [CrossRef]
- Liu, H.; Shonde, T.B.; Olasupo, O.J.; Manny, T.F.; Islam, M.S.; Viera, J.; Khizr, M.; Moslemi, S.; Lin, X.; Winfred, J.S.R.V.; et al. Solution Processed Bilayer Metal Halide White Light Emitting Diodes. Adv. Mater. 2024, 36, 2412239. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Y.; Zhou, L.; Wu, J.; Pan, Z.; Sun, F.; Gu, H.; Liu, R.; Ye, T.; Hou, J.; et al. Flexible Luminescent Fibers Based on Novel Antimony Halides Toward Advanced Anti-Counterfeiting and Information Encryption. Adv. Opt. Mater. 2025, 13, 2402641. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Li, S.; Zhao, L.; Hu, W.; Cai, A.; Zeng, Y.; Wang, Q.; Wu, M.; Li, G.; et al. A General Strategy for Developing Ultrasensitive “Transistor-Like” Thermochromic Fluorescent Materials for Multilevel Information Encryption. Adv. Mater. 2023, 35, 2305472. [Google Scholar] [CrossRef]
- Wei, J.H.; Yu, Y.W.; Luo, J.B.; Zhang, Z.Z.; Kuang, D.B. Bright Cyan-Emissive Copper(I)-Halide Single Crystals for Multi-Functional Applications. Adv. Opt. Mater. 2022, 10, 2200724. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X. Luminescent Organic-Inorganic Hybrid Metal Halides: An Emerging Class of Stimuli-Responsive Materials. Chem. Eur. J. 2022, 28, 202200609. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Li, H.Y.; Han, R.P.; Liu, H.L.; Zang, S.Q. Multiple Stimuli-Responsive Luminescent Chiral Hybrid Antimony Chlorides for Anti-Counterfeiting and Encryption Applications. Angew. Chem. Int. Ed. 2023, 62, e202307875. [Google Scholar] [CrossRef]
- Liu, K.; Liu, K.; Hao, S.; Hou, A.; Cao, J.; Quan, M.; Wang, Y.; Wolverton, C.; Zhao, J.; Liu, Q. Stimuli-Responsive Emission from Hybrid Metal Halides. Adv. Funct. Mater. 2024, 34, 2309296. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, L.; Sun, H.; Yin, H.; Zou, Q.; Deng, J.; Li, R.; Ye, H.; Li, J. Antimony Doping towards Fast Digital Encryption and Decryption and High-efficiency WLED in Zero-Dimensional Hybrid Indium Chlorides. Chem. Eng. J. 2024, 494, 153060. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Mao, Y.; Guo, C.; Zhang, J.; Molokeev, M.S.; Xia, Z.; Zhang, X.-M. Temperature/Component-Dependent Luminescence in Lead-Free Hybrid Metal Halides for Temperature Sensor and Anti-Counterfeiting. Adv. Funct. Mater. 2024, 34, 2401860. [Google Scholar] [CrossRef]
- Hong, D.; Xu, X.; Song, X.; Zhang, L.; Sun, L.; Yuan, C.; Jiang, R. Emerging Metal Halide Perovskite for Photoluminescence Sensing: Transitioning Photophysics into Practical Applications. Chem. Eng. J. 2025, 505, 159092. [Google Scholar] [CrossRef]
- Yakunin, S.; Benin, B.M.; Shynkarenko, Y.; Nazarenko, O.; Bodnarchuk, M.I.; Dirin, D.N.; Hofer, C.; Cattaneo, S.; Kovalenko, M.V. High-resolution remote thermometry and thermography using luminescent low-dimensional tin-halide perovskites. Nat. Mater. 2019, 18, 846–852. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, M.; Liu, Y.; Yu, X.; Pi, C.; Yang, Z.; Zhang, H.; Liu, Z.; Wang, T.; Qiu, J.; et al. All-Inorganic Perovskite Polymer–Ceramics for Flexible and Refreshable X-Ray Imaging. Adv. Funct. Mater. 2021, 32, 2107424. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Deng, Y.; Jiang, T.; Yu, A.; Chen, H.; Liu, S.; Zhao, Q. Lead-Free Organic–Inorganic Hybrid Scintillators for X-ray Detection. Aggregate 2023, 5, e454. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Bakr, O.M.; Mohammed, O.F. Metal Halide Perovskites for X-ray Imaging Scintillators and Detectors. ACS Energy Lett. 2021, 6, 739–768. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Yang, L.; Chai, Z.; Wang, Y.; Wang, S. Circularly Polarized Radioluminescence from Chiral Perovskite Scintillators for Improved X-ray Imaging. Angew. Chem. Int. Ed. 2022, 61, e202208440. [Google Scholar] [CrossRef]
- Zheng, J.; Zeng, Y.; Wang, J.; Sun, C.; Tang, B.; Wu, Y.; Zhang, Y.; Yi, Y.; Wang, N.; Zhao, Y.; et al. Hydrogen-Rich 2D Halide Perovskite Scintillators for Fast Neutron Radiography. J. Am. Chem. Soc. 2021, 143, 21302–21311. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Peng, G.; Qiu, F.; Li, Z.; Lei, Y.; Deng, Y.; Wang, H.; Liu, Z.; Jin, Z. Organic Cation Design of Manganese Halide Hybrids Glass toward Low-Temperature Integrated Efficient, Scaling, and Reproducible X-Ray Detector. Adv. Opt. Mater. 2023, 11, 2300216. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Li, R.; Liu, X.; Huang, W. Flash synthesis of high-performance and color-tunable copper(I)-based cluster scintillators for efficient dynamic X-ray imaging. npj Flex. Electron. 2024, 8, 77. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Zhang, F.; Niu, S.; Zhu, M.; Shi, Z.; Shen, G. Stable Organic-Inorganic Hybrid Sb(III) Halide Scintillator for Nonplanar Ultra-Flexible X-Ray Imaging. Adv. Funct. Mater. 2024, 35, 2412597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).