Abstract
This work comparably investigates the gas sensing potential of noble metal (Pd and Ru)-doped GeS2 monolayers upon three C4F7N decomposed species (FCN, CF3CN, and C2F4) using the first-principles theory, for operation status evaluation in C4F7N-insulated devices. The Pd- and Ru-doping effects on the pristine GeS2 monolayer are analyzed, followed by the adsorption mechanism and sensing performance of two doped monolayers. Our results demonstrate that while Ru doping induces stronger surface interactions with the GeS2 substrate and consequently exhibits superior adsorption strengths upon the three gases, the Pd-doped monolayer shows remarkable advantages in charge transfer capability that leads to exceptional room-temperature sensitivity responses of −99.6% (FCN), −95.0% (CF3CN), and −88.0% (C2F4), thus significantly outperforming the Ru-doped system. Combined with the instantaneous recovery for gas desorption, the Pd-GeS2 monolayer holds significance as an ideal room-temperature sensor to monitor the operation status of C4F7N-insulated devices in power systems. This research provides promising insights into the application of GeS2-based materials for gas sensing in power systems and emphasizes the importance of dopant selection in designing high-performance gas sensing materials, especially for developing advanced electrical equipment monitoring technologies.
1. Introduction
In the global shift toward carbon-neutral energy infrastructure, C4F7N-insulated equipment has emerged as a superior eco-friendly substitute for its SF6-based counterpart, offering comparable dielectric performance while boasting a significantly reduced environmental impact due to its minimal global warming potential [1]. Despite these advantages, the promising insulating medium (C4F7N) is susceptible to molecular degradation when subjected to electrical and thermal stresses, including partial discharge, arc discharges, and thermal decomposition. While C4F7N-decomposed species, including FCN, COF2, CF3CN, and C2F4 [2,3], are hazardous, research has identified that these gases can act as indicators of deteriorated insulation in equipment [4]. Crucially, the concentrations of these gaseous species exhibit a direct relationship with both the intensity and progression of insulation defects in C4F7N-based apparatus [5]. Consequently, the implementation of workable gas detection methodologies becomes imperative for real-time condition assessment, ensuring operational reliability and extending service life in power distribution systems [6].
Recent breakthroughs in two-dimensional (2D) nanomaterial engineering have revolutionized gas sensing capabilities, with graphene derivatives, transition metal dichalcogenides, and III-V compounds setting new standards for high-sensitivity, energy efficient, and compact detection platforms [7,8,9]. These advanced materials exhibit extraordinary physicochemical characteristics, including exceptional specific surface areas, modifiable electronic band structures, and remarkable sensitivity to ppb-level gases [10,11,12]. Apart from these innovative materials, IV-VI compounds, called group-IV monochalcogenides, (MX2, M=Ge, Sn; X=S, Se, Te) have attracted particular research interests recently, due to their unique structure and large specific surface area [13,14]. In particular, the GeS2 monolayer, as a new type of weak interlayer coupling 2D material, is widely investigated in terms of its geometric and electronic properties. It is found that GeS2 exhibits multiple crystalline phases, and the stable ones include the three-dimensional α-GeS2 (P3m1 space group) and the layered β-GeS2 structure (P4m2 space group) [13]. Previous reports show the successful synthesis of 2D GeS2 nanosheets with thicknesses of up to 1.2 nm, hence paving the way for further exploration into its potential role as a gas sensor [15].
Nevertheless, the practical application of pristine GeS2 in molecular detection faces inherent limitations due to its weak van der Waals interactions with the target gas species. To address this issue, researchers have developed various modification strategies, with noble metal doping emerging as a particularly effective approach, as proved from the theoretical and experimental investigations [16,17,18]. Recent studies have demonstrated theoretical improvements in gas sensing performance through atomic doping, as evidenced by the enhanced sensitivity and good reusability of a Pd-doped α-GeS2 monolayer for NO and NO2 detection [19]. This modification strategy combines the intrinsic advantages of GeS2 (notably its high charge carrier mobility) with the catalytic properties of metal dopants, positioning doped GeS2 as a highly promising material for next-generation gas sensing applications [20]. Despite these advances, research on the gas sensing properties of β-GeS2 monolayers remains largely unexplored. The P4m2 layer group in β-GeS2 exhibits a distinctive square-lattice arrangement of [GeS4] tetrahedra [21], creating highly symmetric in-plane atomic configurations and naturally occurring van der Waals gaps that are particularly conducive to gas sensing applications [22]. This unique structural characteristic suggests that metal-doped β-GeS2 could yield even more pronounced improvements in gas detection performance, making it a compelling subject for further investigation.
Building upon the established efficacy of metal-doped systems, this study employs theoretical methods to examine Pd- and Ru-doped β-GeS2 (short for Ru-GeS2 and Pd-GeS2) monolayers for detecting critical decomposed byproducts of C4F7N insulation gas (namely FCN, CF3CN, and C2F4). The selection of Pd and Ru as dopants is based on their well-documented catalytic properties and chemical reactivity from existing theoretical and experimental research, in order to enhance gas adsorption and sensing performances for various nanomaterials [17,23,24,25]. These target gases are chosen for their diagnostic value in insulation condition monitoring: FCN serves as an indicator of partial discharge, CF3CN signals sever arcing faults, and C2F4 emerges during thermal decomposition events [26]. The aims of the first-principles calculations in this work are not only to uncover the modification in the geometric and electronic properties of the GeS2 monolayer induced by Pd/Ru doping but also to illustrate the gas adsorption and sensing properties of the doped lattice from a theoretical aspect. These analyses can illustrate the importance of metal selection for surface modification, providing a theoretical screening tactic to predict the potential of doped GeS2 and thus guiding the experimental design of more effective gas sensors tailored for specific industrial needs. Such fundamental understanding is particularly relevant for developing detection systems with improved sensitivity and operational stability. The theoretical framework established in this study demonstrates the considerable potential of GeS2-based nanomaterials as key components in gas sensing technologies, positioning the MX2 monolayer as a viable solution for diagnostic applications in high-voltage electrical equipment.
2. Results and Discussion
2.1. Analysis of Pristine GeS2 Monolayer and Typical C4F7N Decompression
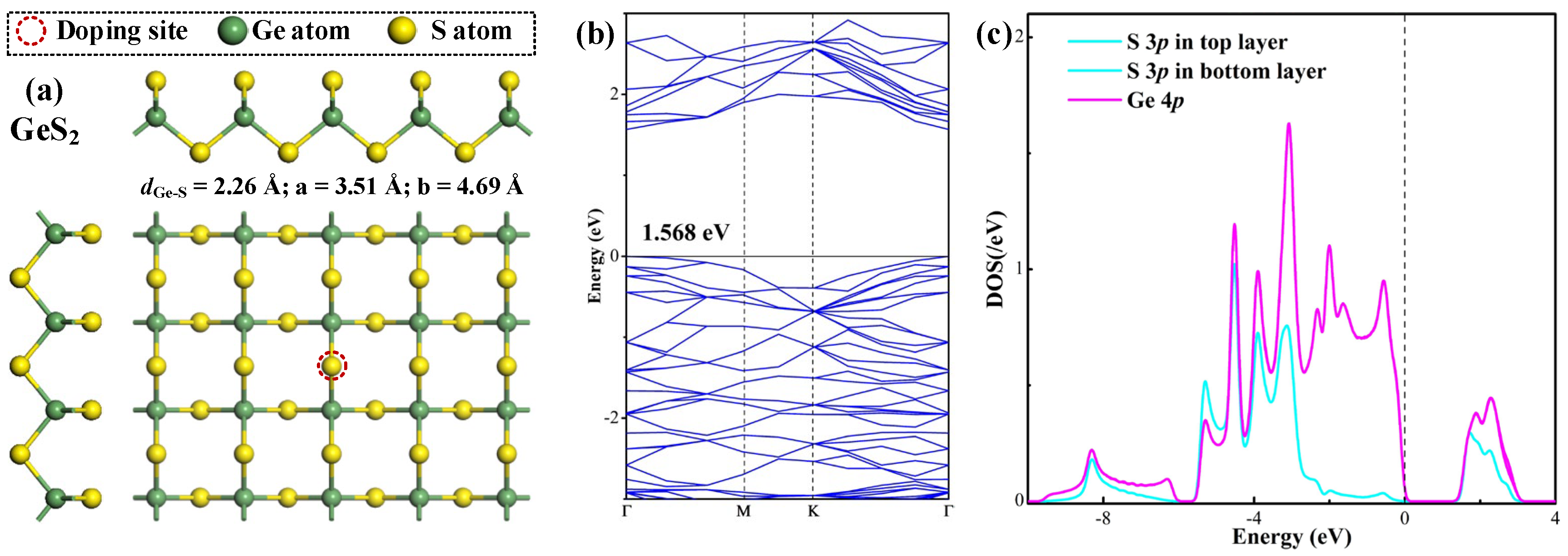
The optimized geometric configuration, accompanied by the corresponding band structure (BS) and density of states (DOS) for the pristine GeS2 monolayer are presented in Figure 1, to provide fundamental insights into its intrinsic properties. The relaxed structure depicted in Figure 1a exhibits the uniform Ge-S bond lengths of 2.26 Å throughout the lattice. The calculated lattice parameters (a = 3.51 Å, b = 4.69 Å) demonstrate agreement with previously reported values (a = 3.53 Å, b = 4.70 Å) [27], with the minor discrepancies (≤0.02 Å) well within the expected range of variation due to differences in basis set selections [28]. The observed consistency between our theoretical results and established findings is particularly significant as it confirms the reliability of our approach for subsequent investigations of doped GeS2 systems.
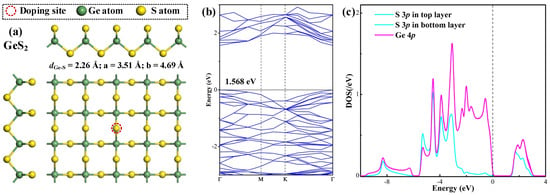
Figure 1.
Geometric configuration (a), BS (b), and DOS (c) of the pristine GeS2 monolayer. In DOS, the dashed line is the Fermi level.
The BS analysis in Figure 1b reveals that the GeS2 monolayer exhibits a direct bandgap of 1.568 eV, with both the conduction band minimum (CBM) and valence band maximum (VBM) located at the Γ point, in accordance with previous theoretical studies confirming its direct semiconductor characteristics [29]. As revealed by the DOS analysis in Figure 1c, the electronic structure shows perfect symmetry between the top and bottom S sub-lattices, as evidenced by the identical DOS profiles for S atoms in both atomic planes. The detailed orbital DOS demonstrates significant hybridization between S 3p and Ge 4p states at multiple energy levels (−4.5, −3.9, −3.1, −2.0, −0.6, 1.8, and 2.3 eV relative to the Fermi level), reflecting the strong covalent character of Ge-S bonds that contributes to the material’s structural stability. Moreover, Hirshfeld population analysis quantifies the charge distribution within the monolayer, revealing a net transfer of 0.244 electrons from Ge to each S atom (Ge: +0.244 e; S: −0.122 e). This charge redistribution is consistent with the relative electronegativities of the constituent elements (S: 2.58; Ge: 2.01) [30], and further confirms the ionic–covalent nature of the chemical bonding in the GeS2 monolayer.
In addition, a comprehensive evaluation of the structural integrity and thermodynamic stability of the pristine GeS2 monolayer is conducted through molecular dynamics (MD) simulations (Figure S1) and vibrational frequency analysis. MD simulations spanning 200~600 K confirm that although thermal vibrations induce atomic displacements, the full configuration of the GeS2 monolayer retains its structural integrity without significant distortion or bond dissociation. Vibrational spectrum analysis reveals exclusively real frequencies (6.71~455.68 cm−1), with the absence of imaginary modes validating dynamical stability [31]. Concurrently, the total energy fluctuated within 0.001 Ha (0.027 eV) throughout 2000 simulation steps, proving the outstanding resilience to thermal perturbations. These collective findings—structural persistence under thermal stress, dynamically stable phonon spectrum, and minimal energy variance—give rise to the compelling evidence of outstanding thermodynamic stability [32]. This robust behavior originates from the strong covalent bonding network that permeates the monolayer’s architecture.
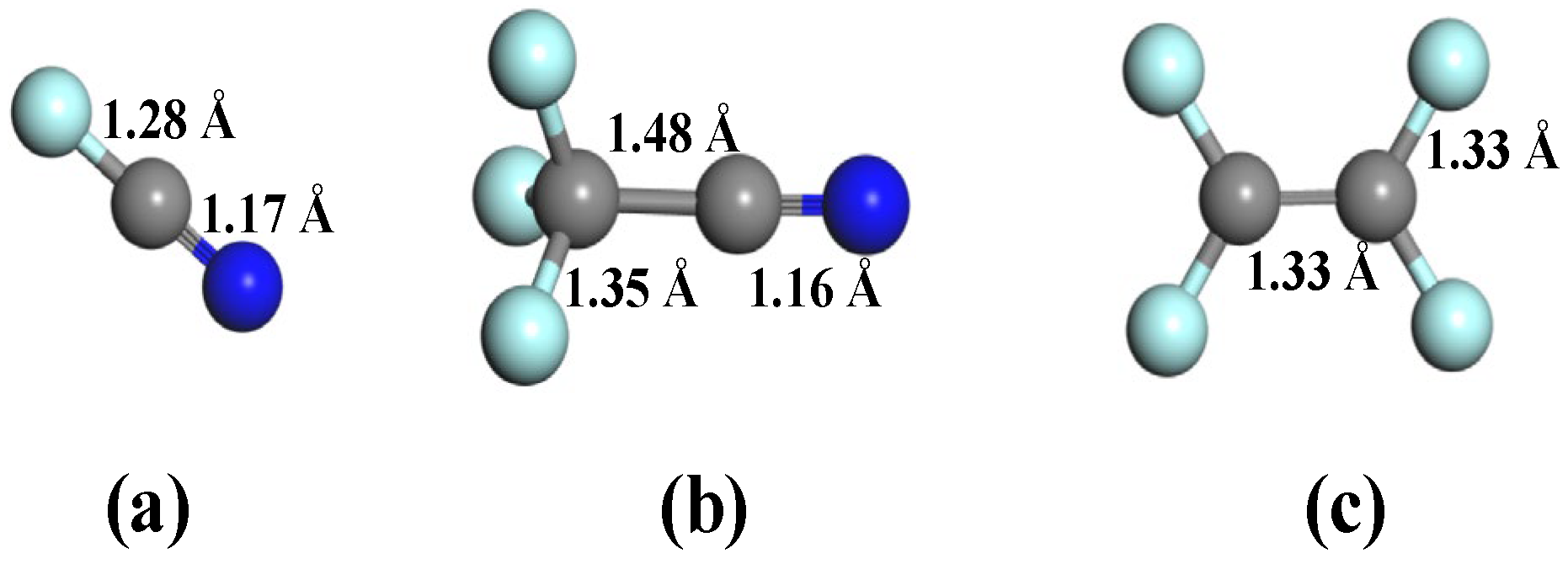
The optimized configurations of three decomposed byproducts of C4F7N (FCN, CF3CN, and C2F4) are displayed in Figure 2, in which each gas species contains distinct functional moieties critical for surface interactions. These molecules feature two principal reactive centers: (i) carbon–carbon double bonds (C=C, 1.33 Å) and (ii) cyano groups (C≡N, 1.16 or 1.17 Å), with bond lengths conforming to established values [33,34]. The electron-rich nature of these functional groups, particularly the lone pair electrons on unsaturated C and N atoms, renders them highly reactive on nanomaterial surfaces, serving as primary adsorption sites in our interfacial studies. Furthermore, a comparative analysis of C-F bond lengths reveals significant structural variations (1.28–1.35 Å) across different molecular species, reflecting the electronic impact of adjacent functional groups [35]. This bond length modulation suggests varying degrees of bond polarization and differential chemical reactivity among the decomposed products, which are expected to profoundly influence their adsorption thermodynamics and kinetics on the doped GeS2 surface.
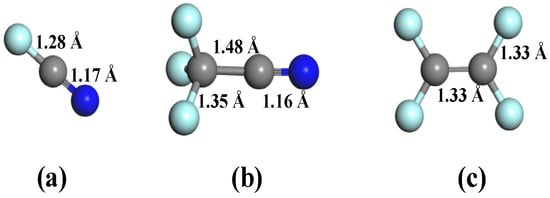
Figure 2.
Molecular structures of C4F7N decomposed species. (a) FCN, (b) CF3CN, and (c) C2F4.
2.2. Pd- and Ru-Doping Properties Within the GeS2 Monolayer
To systematically investigate the Pd- and Ru-doping effects, we construct the doped GeS2 monolayer through atomic substitution, namely by replacing an S atom with a Pd or Ru atom. Such configurations make the metal atom covalently bonded within the 2D lattice, representing a metastable configuration with a significantly higher diffusion barrier. This “anchoring” effect effectively suppresses metal atom migration and aggregation, thereby guaranteeing the long-term structural integrity and consistent performance of the sensor material [36]. The dynamic stability of these doped configurations are evaluated using formation energy (Eform) calculations, defined as [37]
where and denote the total energies of doped and pristine systems, respectively, while µM and µS correspond to the chemical potentials of the dopant atoms and the replaced S atoms in their stable bulk phases. For comparison, we also analyze the doping configuration via the dopant adsorption manner, in which a single Pd or Ru atom as well as Pd2 or Ru2 dimer are considered, with the optimized configurations shown in Figure S2. Specifically, single Pd or Ru atoms adsorbed on the pristine GeS2 surface, exhibiting high mobility, and leading to a strong propensity for aggregation and the formation of metallic clusters. This migration compromises the uniformity and stability of the active sites. Furthermore, when a Pd2 or Ru2 dimer is adsorbed, the configurations undergo significant geometric distortion. This structural instability not only alters the intended electronic properties of the doping sites but also increases the system’s energy, making it susceptible to reconstruction or decomposition under operational conditions. All these findings highlight the significance of substitutional doping strategy in this work.
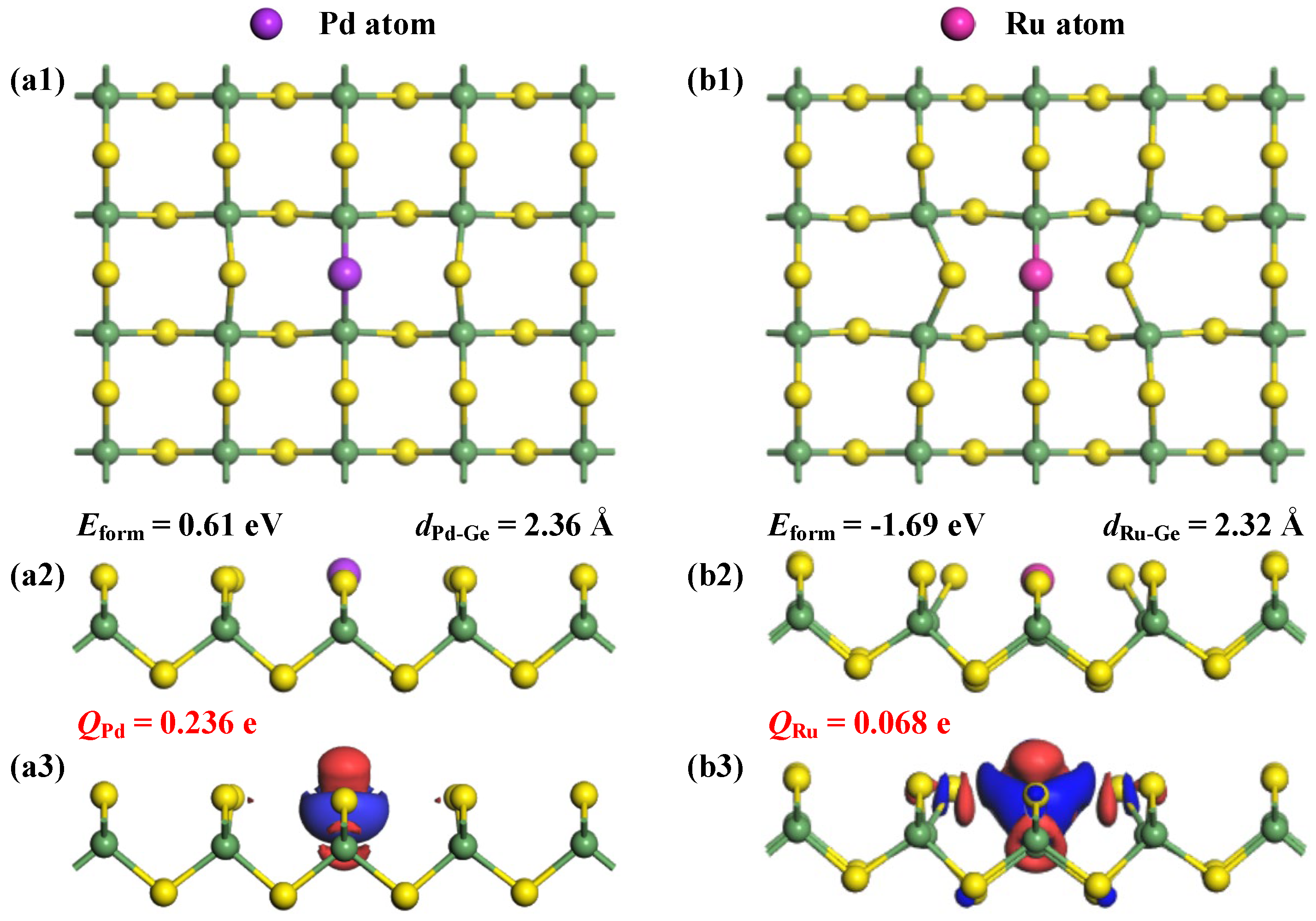
The analysis of Eform can uncover critical insights into the doping characteristics of Pd and Ru within the GeS2 monolayer, with the optimized structures and the related charge density difference (CDD) of these doped systems presented in Figure 3. The calculated Eform values manifest dramatically different doping behaviors upon the two noble metal atoms. Specifically, Pd doping exhibits endothermic properties with a positive value of 0.61 eV, indicating the requirement of substantial energy input in this process [38]; while Ru doping shows strongly exothermic behavior with a negative energy value of −1.69 eV, suggesting spontaneous incorporation of the Ru atom into the GeS2 lattice under appropriate synthesis conditions [39]. Such a remarkable difference primarily stems from the enhanced surface interactions between the Ru atom and the GeS2 substrate compared to Pd atom, as quantitatively reflected in their respective Eform values. Further structural analysis reveals additional evidence supporting this conclusion. The optimized Pd-Ge and Ru-Ge bond lengths measure 2.36 Å and 2.32 Å, respectively. Notably, despite Pd having a slightly smaller covalent radius than Ru (1.39 Å vs. 1.42 Å) [40], the observed longer bond length for Pd-Ge compared to Ru-Ge clearly indicates weaker bonding interactions in the Pd-doped system. This structural observation provides direct confirmation of the more stable integration of Ru atoms into the GeS2 monolayer [41]. Moreover, the CDD distributions reveal significant electron depletion around Pd and Ru dopants, clearly demonstrating their electron-donating properties within the doped GeS2 lattice. This observation is quantitatively corroborated by the Hirshfeld charge analysis, which shows net positive charges of +0.236 e and +0.068 e for Pd and Ru dopants, respectively. The higher charge value associated with the Pd dopant indicates its stronger electron-donating capability compared to Ru, suggesting more favorable electron redistribution and pronounced modifications in the electronic properties of the Pd-doped system.
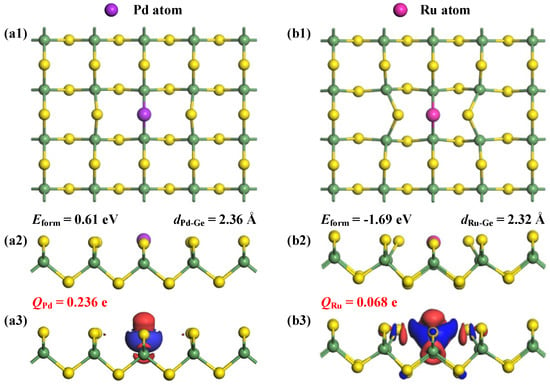
Figure 3.
Optimized structures and CDD of (a1–a3) Pd-GeS2 monolayer and (b1–b3) Ru-GeS2 monolayer. In CDD, the electron accumulations are in blue while the electron depletions are in red, with the isosurface at 0.05 e/Å3.
The dynamic stability and structural integrity of both metastable Pd- and Ru-GeS2 monolayers are investigated through molecular dynamic (MD) simulations and vibrational frequency analysis, as exhibited in Figures S3 and S4. The MD trajectories reveal structural preservation throughout the 2000 simulation steps, with only minimal atomic displacements observed around the dopant centers (Pd and Ru) and no significant distortion of the overall monolayer framework. Complementary vibrational analysis identifies characteristic frequency ranges of 19.90~454.25 and 12.11~467.69 cm−1 for the Pd- and Ru-doped systems, respectively, with all modes being real and positive and thus acting as a definitive indicator of dynamical stability [42]. Furthermore, the total energy fluctuations remain constrained within 0.001 Ha (0.027 eV), demonstrating thermal stability that is comparable to that of pristine GeS2. These analyses confirm the favorable dynamic stabilities of both metastable configurations, strongly suggesting that these doped monolayers can maintain their fundamental electronic and structural properties when subjected to thermal and mechanical stresses. However, the high sulfophilicity of palladium must be considered, as it presents a significant experimental challenge, namely the potential formation of palladium sulfide (PdxSy) phases under sulfur-rich conditions. Therefore, the experimental realization of the proposed Pd-GeS2 monolayer necessitates precise control over the chemical potential during synthesis. The use of a sulfur-poor atmosphere, for instance, would be essential to suppress the nucleation of bulk sulfides and promote the successful incorporation of isolated Pd atoms into the lattice.
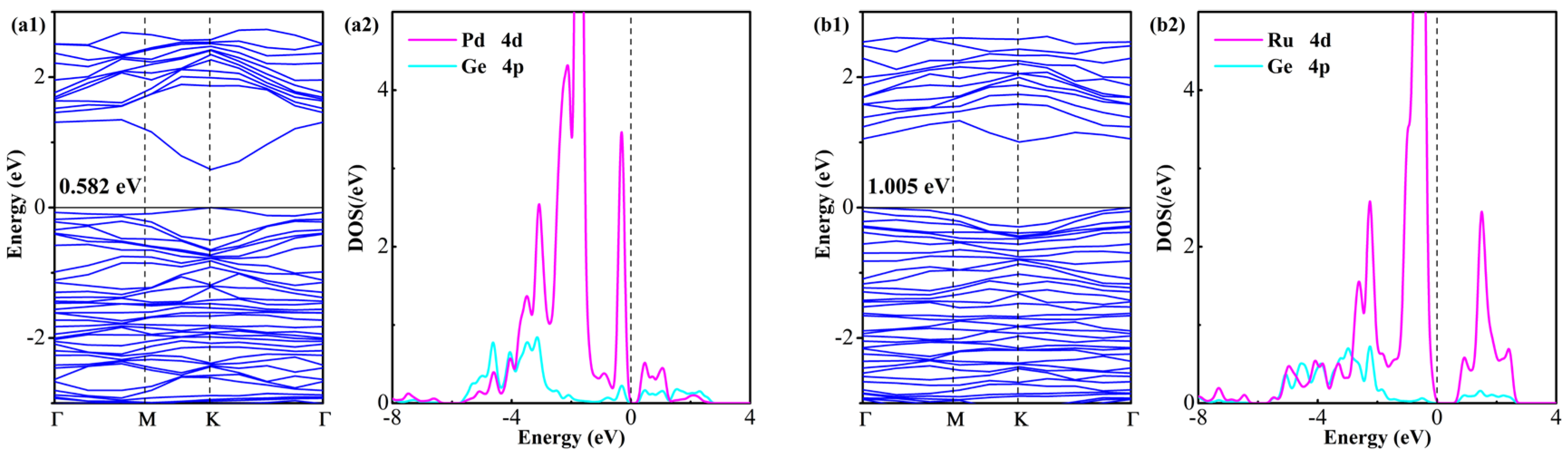
Following the above assumptions, electronic structure analyses through the examination of BS and DOS of Ru- and Pd-GeS2 monolayers are carried out to provide fundamental insights into the doping-induced electronic modifications, as plotted in Figure 4. Notably, both dopants induce significant bandgap reduction relative to pristine GeS2, wherein the Pd-doped system exhibits a more substantial bandgap narrowing to 0.582 eV (62.9% reduction), compared to the Ru-doped system’s bandgap of 1.005 eV (27.9% reduction). In addition, both doped systems retain the direct semiconductor character of pristine GeS2, with the VBM and CBM maintaining their coincident positions in the Brillouin zone (K point for Pd-doped systems and Γ point for Ru-doped systems). However, the largely narrowed bandgap in the Pd-doped system suggests significantly enhanced electrical conductivity compared with the Ru-doped counterpart, as evidenced by the reduced energy barrier for charge carrier excitation [43]. Specifically, the stronger bandgap reduction and enhanced charge transfer characteristics of Pd-doping may be particularly advantageous for gas sensing applications, where improved electrical conductivity can enhance sensitivity [44].
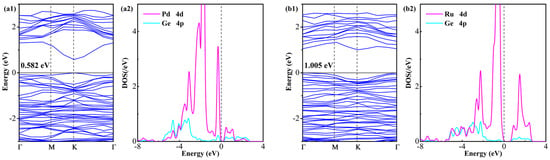
Figure 4.
BS and DOS of (a1,a2) Pd-GeS2 monolayer and (b1,b2) Ru-GeS2 monolayer. The Fermi level in DOS is set to 0 eV.
An examination of the DOS data provides critical insights into the electronic modifications induced by Pd and Ru doping in the GeS2 monolayer. The analysis reveals significant hybridization between the noble metal d-orbitals and Ge p-states, with differences in the energy ranges and intensities of these interactions. Specifically, the Pd 4d-Ge 4p orbital coupling occurs predominantly in two energy windows (−4.4 to −0.1 eV and 0.5 to 2.6 eV), while the Ru 4d-Ge 4p interactions span different ranges (−4.9 to −0.2 eV and 0.8 to 2.5 eV). These hybridization features demonstrate substantial electronic coupling between dopant and host atoms, with the Ru-doped system exhibiting stronger interactions near the Fermi level as supported by broader energy distributions and stronger orbital overlaps [45]. This finding aligns perfectly with the thermodynamic preference for Ru incorporation, as evidenced by its more negative Eform. These newly hybrid states serve as active centers for molecular adsorption while maintaining favorable charge transport properties in doped GeS2 systems, enabling high-performance surface reactivity and sensing applications [46].
2.3. Gas Adsorptions on Pd- and Ru-GeS2 Monolayers
This section evaluates the adsorption characteristics of Pd- and Ru-GeS2 monolayers toward three decomposed gases of C4F7N. The adsorption configurations are constructed by positioning each target gas molecule approximately 2.5 Å above the dopant center (Pd or Ru) within the GeS2 lattice, followed by geometric optimization to determine the most stable configurations (MSCs). The thermodynamic stability of each adsorption complex is quantified through adsorption energy (Ead) calculations, defined as [47]
where Emonolayer/gas, Emonolayer and Egas represent the total energies of the adsorption complex, the isolated doped monolayer, and free gas molecule, respectively. Such parameters can serve two purposes, including identifying the MSC for each gas–surface system through the most negative Ead value, and enabling quantitative comparison of adsorption strengths across various gas species. Complementary to the energetic analysis, CDD calculations are also performed for each MSC in the Pd- and Ru-doped systems to elucidate the fundamental nature of gas–surface interactions, including charge transfer pathways and chemical bonding mechanisms at the adsorption interface.
For reference, the adsorption behavior of C4F7N and its decomposition products on the pristine GeS2 monolayer are also investigated, with configurations shown in Figure S5. Calculation via Equation (2) reveals consistently weak interactions for all species, with Ead values of −0.2 eV for C4F7N and −0.12 to −0.21 eV for the decomposition products. These findings demonstrate the inherently low adsorption capability of the pristine GeS2 monolayer toward the target gases, underscoring its limited potential for direct sensing applications and highlighting the critical need for surface modification to achieve usable sensitivity.
2.3.1. Analysis of Pd-GeS2/Gas Systems
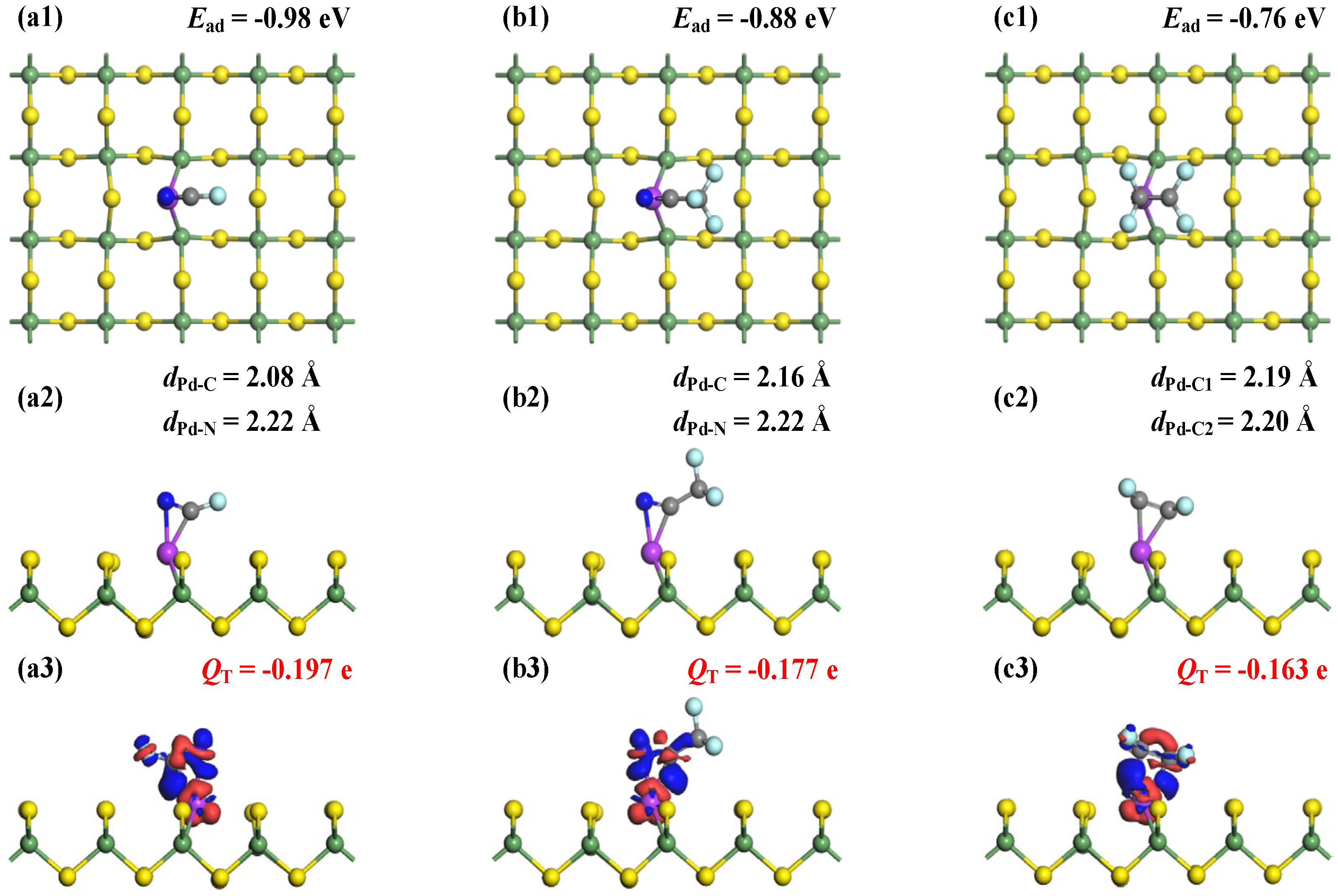
Figure 5 illustrates the MSC and corresponding CDD for FCN, CF3CN, and C2F4 adsorption on Pd-GeS2 monolayers. For FCN and CF3CN systems, simultaneous bond formations occur between the Pd dopant and both C and N atoms, with Pd-C bond lengths of 2.08 Å (FCN) and 2.16 Å (CF3CN), and Pd-N distances of 2.22 Å in both systems. For the adsorbed C2F4 molecule, two C atoms are captured by the Pd dopant forming Pd-C bonds measuring 2.19 Å and 2.20 Å. These structural parameters reveal strong interfacial interactions in all three systems [48], corroborated by the calculated Ead of −0.98 eV (FCN), −0.88 eV (CF3CN), and −0.76 eV (C2F4). These binding preferences can be attributed to the Pd dopant’s pronounced affinity for π-electron systems, particularly the C≡N and C=C functional groups, through strong π-backdonation effects [49].
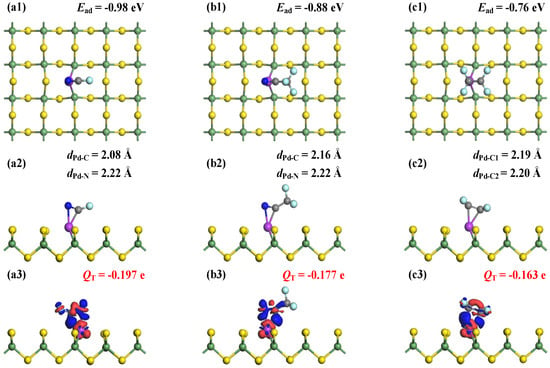
Figure 5.
MSCs and CDDs of gas-adsorbed Pd-GeS2 systems. (a1–a3) FCN, (b1–b3) CF3CN, and (c1–c3) C2F4. in CDD; the sets are same as Figure 3.
Hirshfeld charge analysis quantifies significant electron transfer from the doped monolayer to all adsorbed species, with net charges of −0.197 e (FCN), −0.177 e (CF3CN), and −0.163 e (C2F4). The consistent electron-accepting behavior of the gas molecules highlights the Pd’s exceptional electron-donating capability, facilitating substantial charge redistribution in adsorption. Notably, both Ead and QT follow the same trend across the three systems: FCN > CF3CN > C2F4. Moreover, the CDD plots provide spatial confirmation of these electronic interactions, showing pronounced electron accumulation at all Pd-C and Pd-N interfacial regions. This electron density redistribution, combined with the measured bond lengths and charge transfer values, unequivocally demonstrates the formation of covalent bonds between the Pd dopant and adsorbed molecules [50]. These comprehensive analyses collectively establish the chemisorption nature of all three gas–surface systems, with covalent bonding as the dominant interaction mechanism.
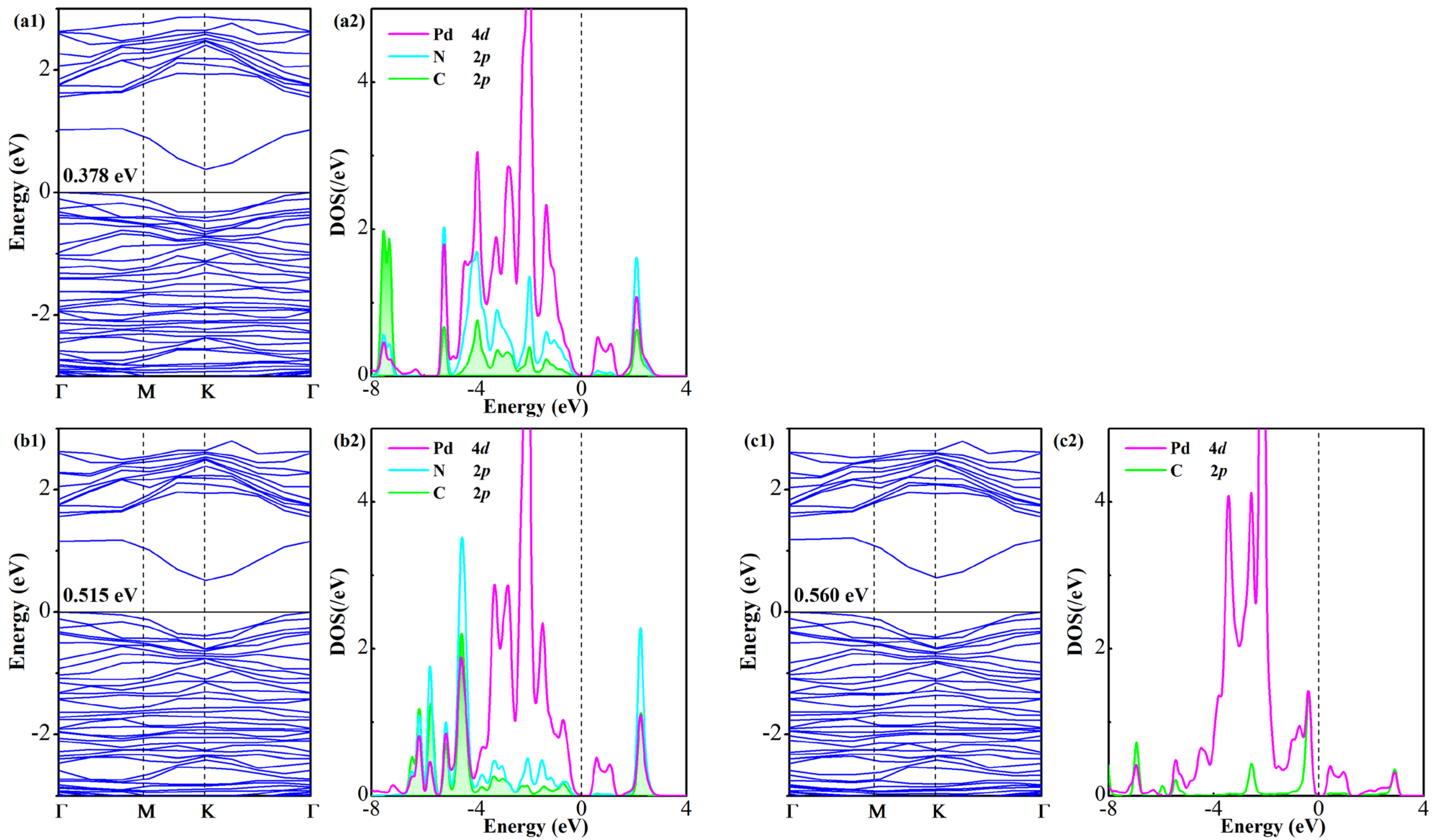
An investigation on BS and DOS of the gas-adsorbed system is conducted to elucidate the electronic modifications in the Pd-GeS2 monolayer following adsorption of three gas species, as illustrated in Figure 6. The calculations reveal non-magnetic ground states for all configurations, confirmed by the absence of spin polarization in their respective DOS profiles. Notably, gas adsorption induces substantial bandgap reduction relative to the isolated Pd-GeS2 monolayer (0.669 eV), with the modified bandgaps measuring 0.378 eV (FCN), 0.515 eV (CF3CN), and 0.560 eV (C2F4), representing reductions of 43.5%, 23.0%, and 16.3%, respectively. Such remarkable bandgap modulation exhibits a clear trend (FCN > CF3CN > C2F4) that directly correlates with the adsorption strength established in previous energetic analyses. It should be noted that the observed electronic restructuring stems from two mechanisms: (i) charge transfer between the adsorbates and the monolayer that shifts the Fermi level position, and (ii) interfacial orbital hybridization that reconstructs the band edges. Thus, further orbital DOS analysis is conducted to reveal the basic interactions driving these modifications, implying significant hybridization between Pd 4d-orbitals and C 2p/N 2p states of the adsorbed gases. The DOS decomposition shows particularly strong orbital mixing in all three systems, with C 2p and N 2p states forming new electronic features near the Fermi level through coupling with Pd 4d-orbitals. This interfacial interaction is most pronounced in the FCN system, consistent with its strongest adsorption energy and greatest bandgap reduction. These electronic structure analyses provide fundamental insights into the adsorption-induced property modifications of the Pd-GeS2 monolayer at the atomic level, establishing its potential as a gas-sensitive 2D candidate [51].
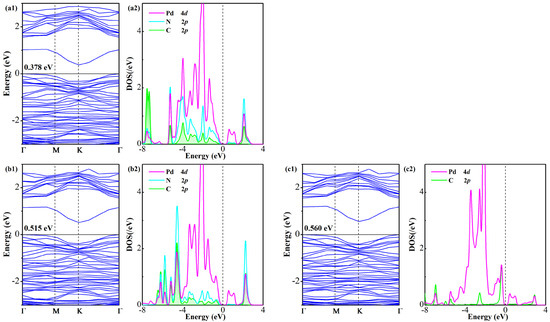
Figure 6.
BS and DOS of Pd-GeS2/gas systems (a1,a2) FCN, (b1,b2) CF3CN, and (c1,c2) C2F4.
2.3.2. Analysis of Ru-GeS2/Gas Systems
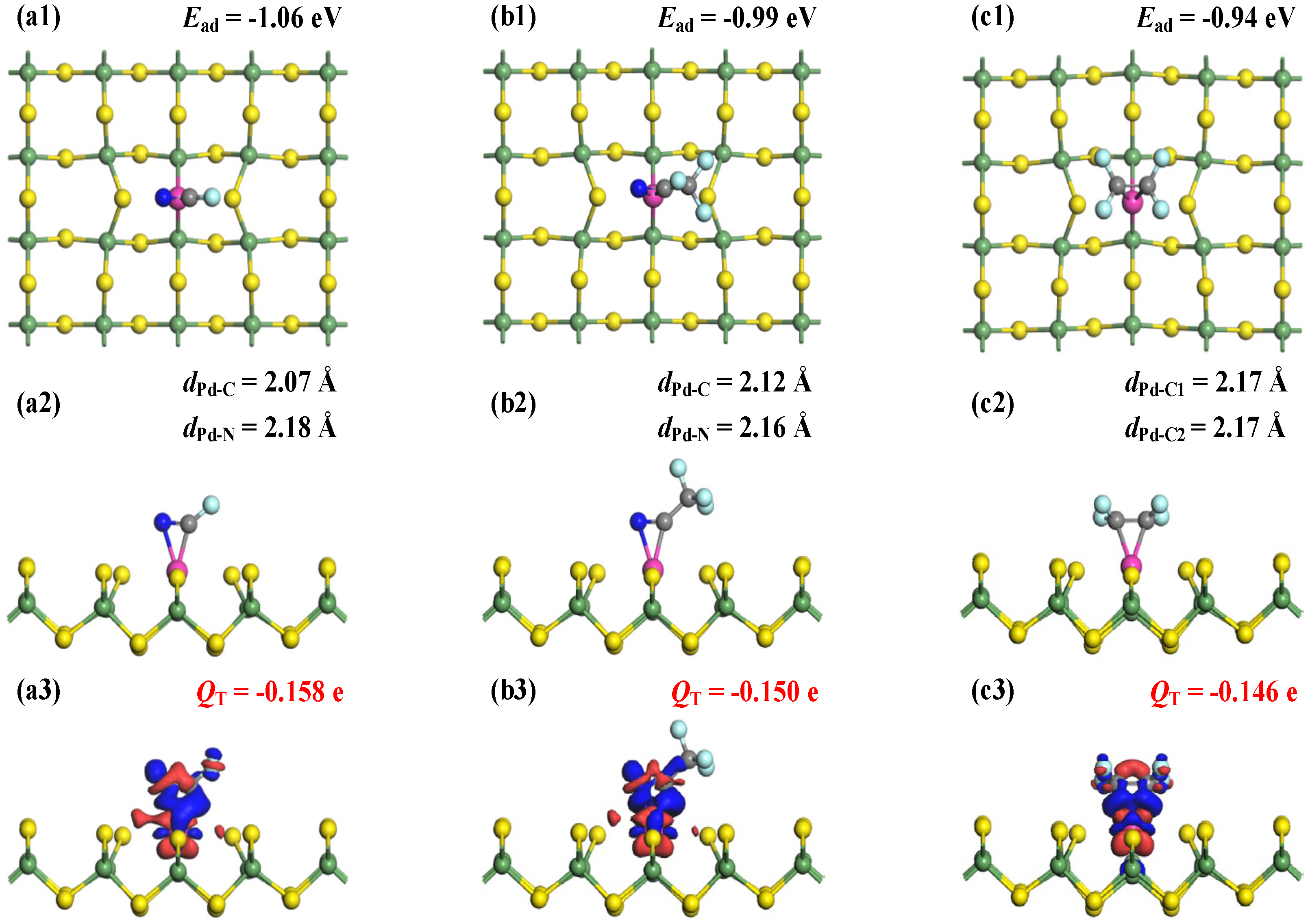
The MSC and CDD plots for FCN, CF3CN, and C2F4 adsorption on the Ru-GeS2 monolayer are displayed in Figure 7, which reveals interaction mechanisms analogous to those in Pd-doped systems. Structural analysis shows equivalent coordination in FCN and CF3CN systems, with Ru-C bond lengths of 2.07 Å and 2.12 Å, and Ru-N distances of 2.18 Å and 2.16 Å, respectively. In addition, the C2F4 molecule forms two equivalent Ru-C bonds measuring 2.17 Å. Notably, these interfacial bond lengths are all shorter than the Pd-doped counterparts, suggesting the marginally stronger adsorption interactions in Ru-GeS2 systems upon three C4F7N decomposed gases. The enhanced adsorption strength is quantitatively confirmed by calculated Ead of −1.06 eV (FCN), −0.99 eV (CF3CN), and −0.94 eV (C2F4), all indicative of chemisorption [52].
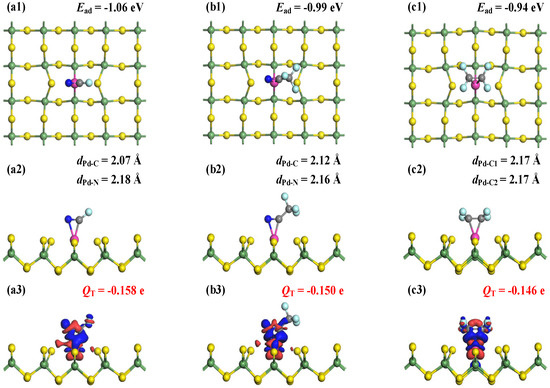
Figure 7.
MSCs and CDDs of gas-adsorbed Ru-GeS2 systems. (a1–a3) FCN, (b1–b3) CF3CN, and (c1–c3) C2F4. The sets for CDD are the same as those in Figure 3.
Hirshfeld charge analysis reveals consistent electron transfer from the monolayer to the adsorbates, with net charges of −0.158 e (FCN), −0.150 e (CF3CN), and −0.146 e (C2F4). While maintaining the same charge transfer direction as those in Pd-doped systems, these values indicate slightly weaker electron-accepting behavior by gas molecules on Ru-GeS2 surfaces. CDD analysis demonstrates pronounced electron accumulation at Ru-C and Ru-N regions, verifying the formation of covalent bond formation [53]. Importantly, both Ead and QT magnitude follow identical hierarchical trends (FCN > CF3CN > C2F4) across Ru- and Pd-doped systems, which may suggest a representative gas affinity ordering of noble metal-doped GeS2 monolayers. These analyses demonstrate comparable but subtly enhanced adsorption performance in Ru-GeS2 systems, providing valuable insights for dopant selection in designing 2D sensing materials [54].
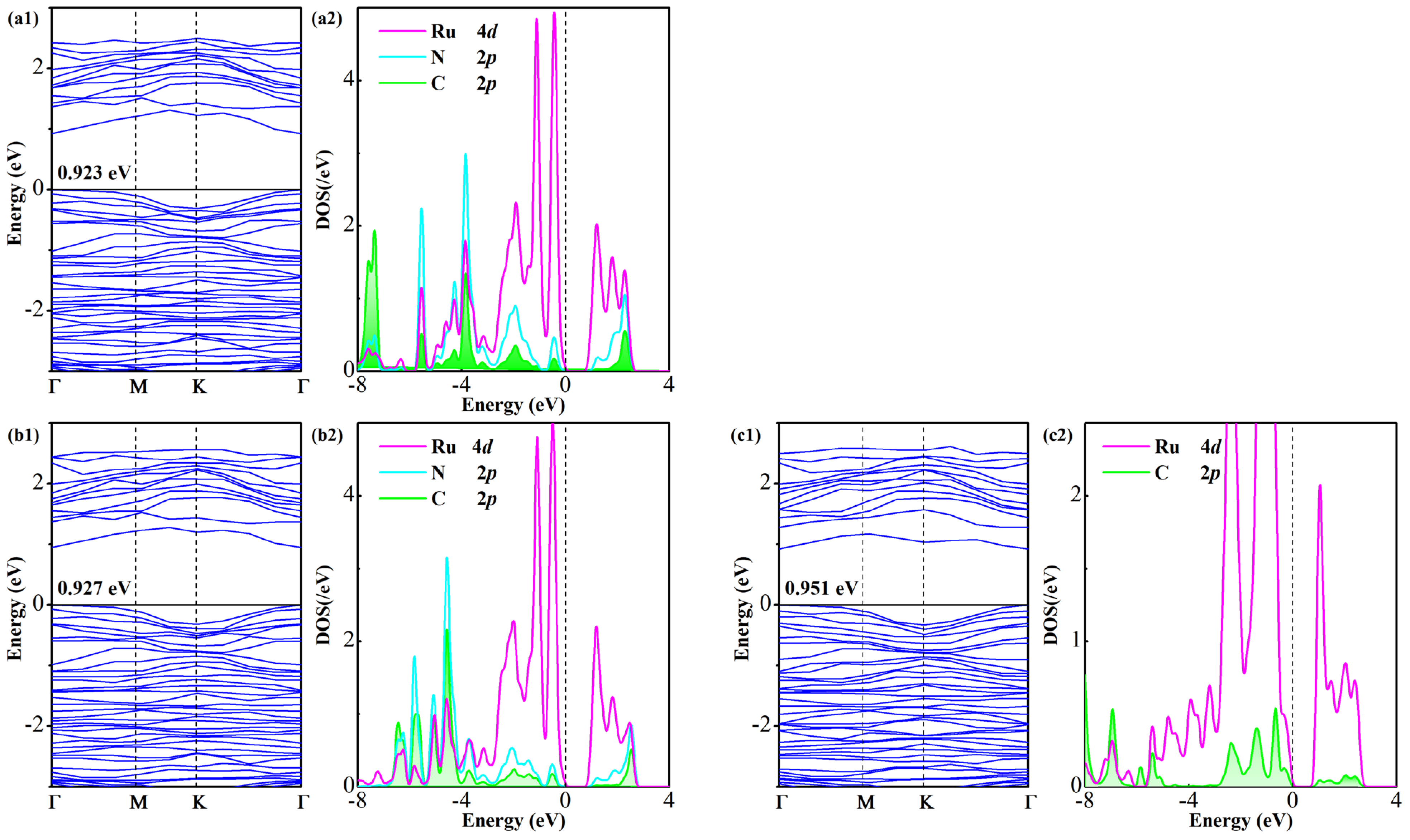
Figure 8 illustrates a comparative analysis of BS and DOS for gas-adsorbed Ru-GeS2 systems. Similarly to their Pd-doped counterparts, all three systems maintain non-magnetic ground states, as evidenced by spin-unpolarized electronic calculations. The bandgap evolution reveals a distinct pattern: the original 1.005 eV bandgap of the pristine Ru-GeS2 monolayer undergoes relatively modest reductions to 0.923 eV (FCN), 0.927 eV (CF3CN), and 0.951 eV (C2F4), representing decreases of only 9.2%, 7.8%, and 5.4%, respectively. This attenuated bandgap modulation follows the same hierarchy (FCN > CF3CN > C2F4) as Ead and QT, mirroring the trend in Pd-doped systems but with a significantly smaller magnitude. These findings indicate the higher sensitivity of the Pd-GeS2 monolayer for sensing C4F7N decomposed gases.
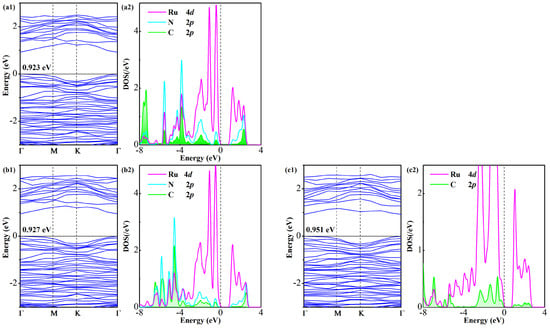
Figure 8.
BS and DOS of Ru-GeS2/gas systems (a1,a2) FCN, (b1,b2) CF3CN, and (c1,c2) C2F4.
Detailed DOS analysis uncovers substantial electronic reorganization in the Ru-GeS2 monolayer upon gas adsorption, manifested through pronounced state hybridization near the Fermi level. A closer examination of orbital interactions identifies: (i) distinct coupling peaks at −3.8, −3.2, −1.9, −0.4, and 2.3 eV (N/C 2p-Ru 4d) in the FCN system, (ii) hybridization features at −3.7, −3.2, −2.0, −1.4, −0.5, and 2.5 eV (N/C 2p-Ru 4d) in the CF3CN system and (iii) interaction peaks at −2.3, −1.3, −0.7, 0.3, 1.0, 2.0, and 2.4 eV (C 2p-Ru 4d) in the C2F4 system. These high-intensity hybridizations feature electronic-level evidence for the distinct chemisorption characteristics of the Ru-GeS2 monolayer [48]. The relatively minor bandgap perturbations in the Ru-GeS2/gas systems, coupled with localized hybridization peaks, underscore the critical role of dopant selection in determining both the strength and nature of gas–surface interactions in doped monolayers that tailor their sensing properties.
Apart from the above anslyses, a practical gas sensor must exhibit a significant ability to selectively detect trace-level decomposition products in the presence of a high background concentration of the parent gas. To evaluate this critical aspect of selectivity, we systematically investigated the adsorption behavior of the C4F7N molecule on both Ru- and Pd-GeS2 monolayers. Our computational results, summarized in Figure S6, explicitly demonstrate that the interaction between the doped surfaces and the parent C4F7N is notably weak, with Ead values of only −0.36 eV and −0.39 eV for the Pd- and Ru-doped systems, respectively. These Ead values are indctive of weak physisorption, which typically results in negligible sensor responses [55]. In contrast, the adsorption of key decomposition products, such as FCN, CF3CN, and C2F4, on the same doped surfaces is substantially stronger. This marked disparity in binding strength provides a clear theoretical foundation for high selectivity. The weakly adsorbed parent C4F7N molecules are unlikely to persistently block or “poison” the active sites. Instead, these sites remain available for the strong and preferential adsorption of the target decomposition analytes. Consequently, the proposed Ru- and Pd-doped GeS2 sensors are theoretically capable of distinguishing and detecting critical fault gases within a atmosphere dominated by the parent C4F7N.
2.4. Gas Sensor Exploration
The above sections provide a comprehensive investigation on the geometric and electronic properties of gas-adsorbed Pd- and Ru-doped GeS2 monolayers, which reveals critical structure–property relationships. Specifically, it is demonstrated that while both doped systems exhibit identical hierarchical adsorption strengths for C4F7N-decomposed products (FCN > CF3CN > C2F4) that correspondingly lead to similar sequences of bandgap modulation, the Pd-doped configurations display more pronounced electronic modifications. This enhanced responsiveness in Pd-GeS2/gas systems directly correlates with their greater charge transfer magnitudes, as quantified by Hirshfeld population analysis. These observations establish a clear structure–property paradigm where Ead determines the relative ordering of bandgap modulation and QT magnitude primarily controls the absolute scale of electronic modifications. Also, the bandgap variations have direct consequences for electrical conductivity under gas exposure, as illustrated by the semiconductor transport equation [56]:
wherein σ represents conductivity, A is charge carrier concentration, Bg represents the bandgap, T denotes the operating temperature, and k is the Boltzmann constant (8.617 × 10−5 eV/K) [57]. This relationship demonstrates that the adsorption-induced bandgap reductions we observed would lead to exponential increases in conductivity, providing a robust transduction mechanism of engineered 2D materials for sensitive gas detection. As a resistive gas sensor, sensing response (SR) related to electrical conductivity can be quantitatively described by the relationship:
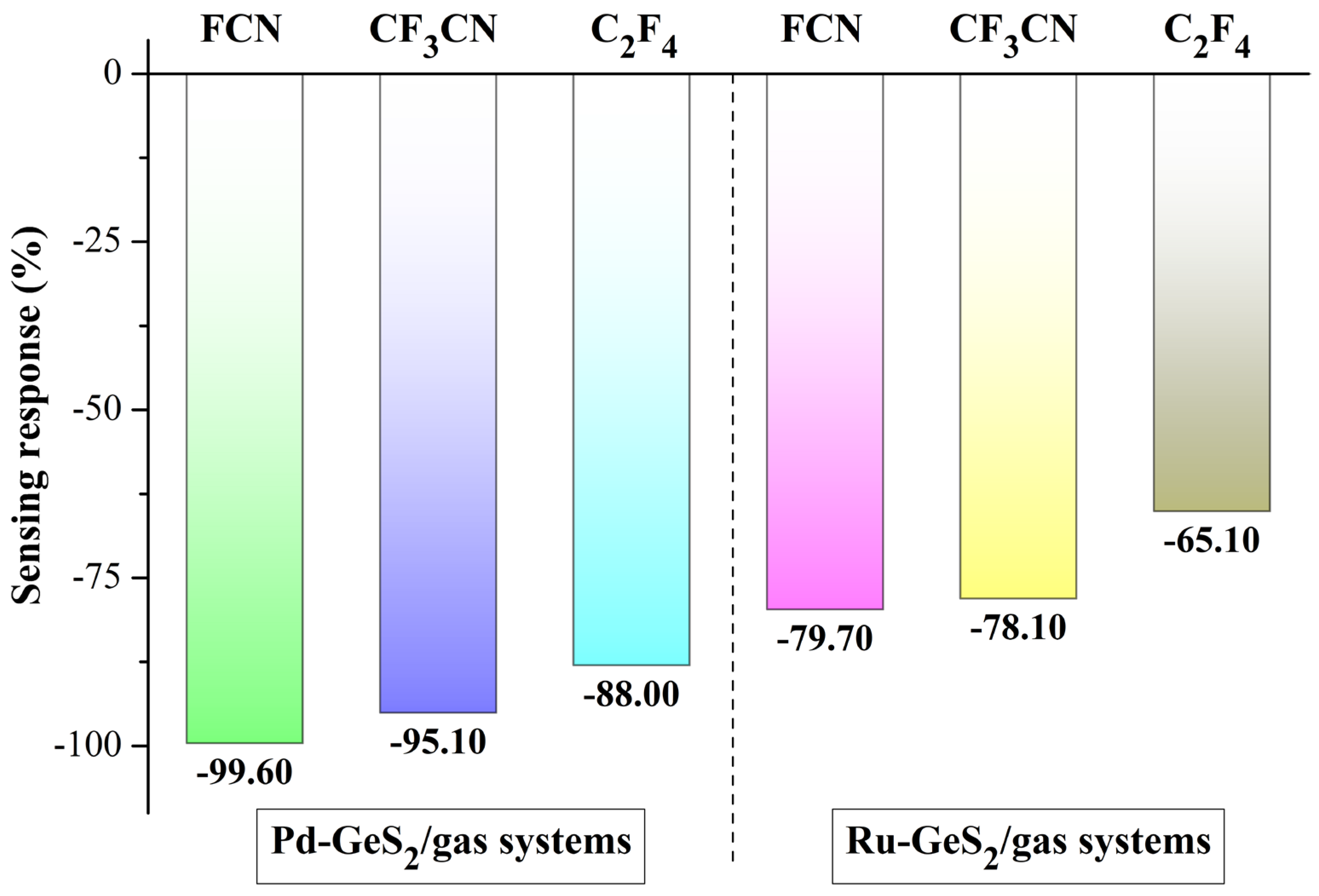
with ΔBg representing the adsorption-induced bandgap shift relative to the isolated monolayer. According to Equation (4), the SR values of two purposed monolayers could be calculated, as depicted in Figure 9.
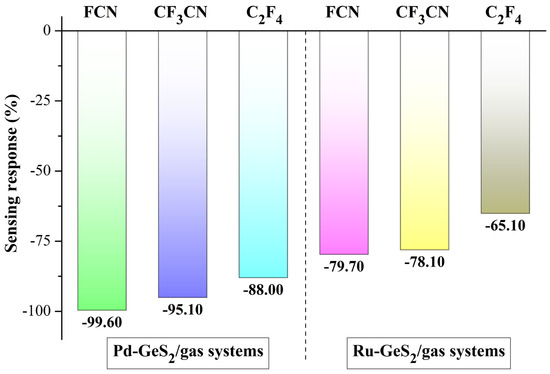
Figure 9.
SRs of Pd- and Ru-GeS2 monolayers upon three C4F7N decomposed species.
As illustrated in Figure 9, both Pd- and Ru-GeS2 monolayers exhibit negative SR values to the three C4F7N decomposed gases, though with different sensitivity characteristics. Specifically, the Pd-doped system shows more favorable response magnitudes, achieving SR values of −99.60% for FCN, −95.10% for CF3CN, and −88.00% for C2F4. In comparison, the Ru-doped monolayer demonstrates more moderate sensitivities, with corresponding responses of −79.70%, −78.10%, and −65.10%, respectively. This pronounced performance difference can be attributed to the more substantial bandgap reduction and enhanced charge transfer capability in Pd-doped systems, as evidenced by our previous electronic structure analyses. It should be noted that although both monolayers show limited selectivity upon different decomposed products, it does not diminish their practical utility for insulation-monitoring purposes. This is because in electrical equipment diagnostics, the primary aim is early detection of any decomposition gases as indicators of insulation degradation, rather than the precise identification of specific gas species [58].
All these findings confirm the stronger potential of the Pd-GeS2 monolayer for monitoring C4F7N decomposed species. Apart from that, the dopant selection principle in designing sensing materials can also be established, where the sensitivity of the material could be modulated through choice of noble metal dopant (e.g., Pd versus Ru).
2.5. Recovery Property
Given the outstanding sensing performance of the Pd-GeS2 monolayer, a thorough comparison of its recovery property with its Ru-doped counterpart becomes imperative to assess its practical viability for repeated detection cycles of C4F7N decomposed gases. The material’s recovery property governs its suitability for continuous monitoring applications in electrical equipment, where rapid recovery ensures both sensor reusability and the timely detection of subsequent insulation degradation events. The recovery time (τ) is calculated by employing the van’t Hoff–Arrhenius formalism [59], expressed as
where k is the Boltzmann constant (8.617 × 10−5 eV/K), A represents the attempt frequency (1016 s−1 [60]), and T is the operating temperature (K). The analysis provides essential insights into the material’s potential for long-term deployment in real-world electrical monitoring applications, where a lower Ead and higher operating T can largely promote the recovery process.
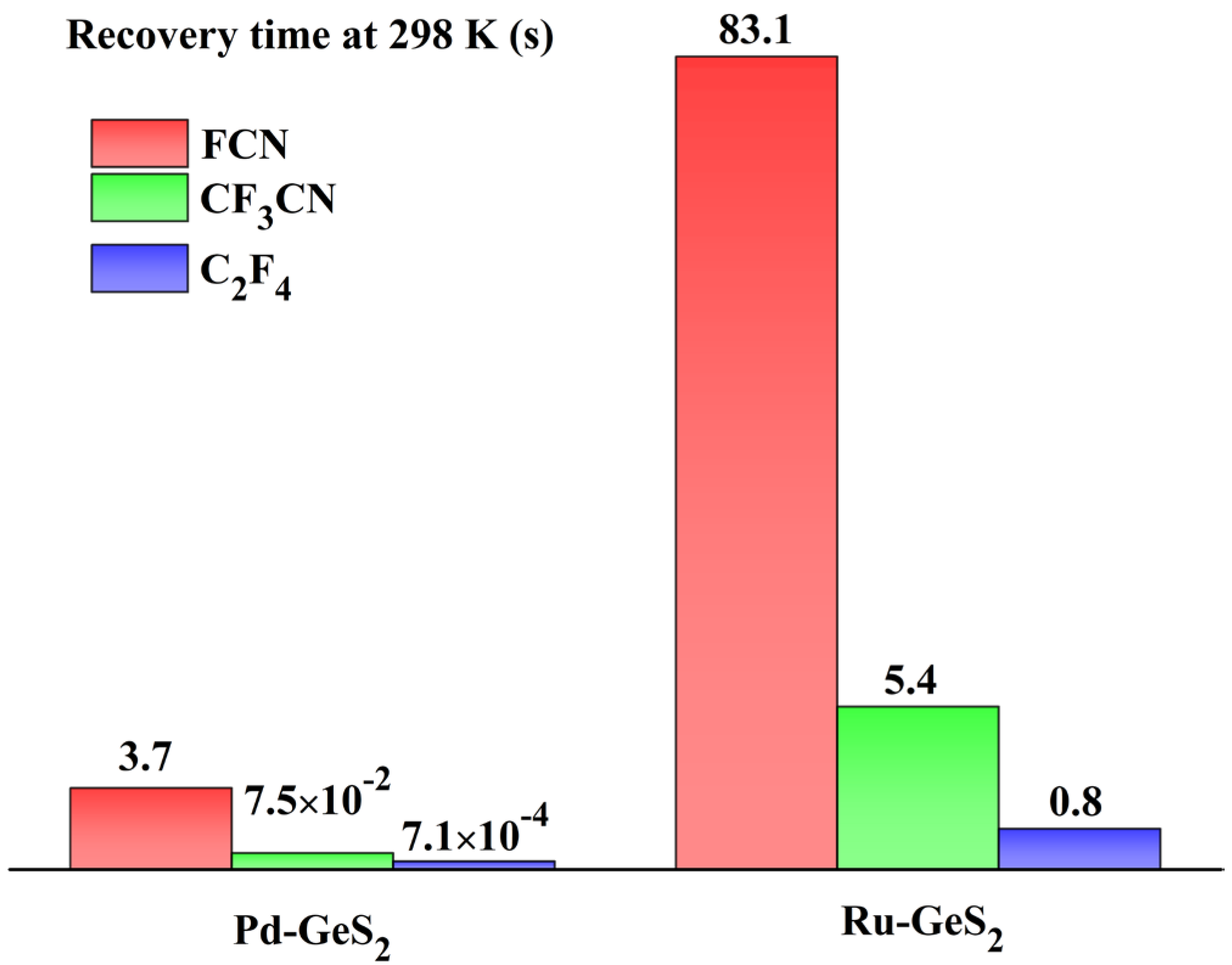
The computational recovery time of Pd- and Ru-GeS2 monolayers are exhibited in Figure 10, which uncovers their practical applicability for gas sensing in electrical insulation monitoring. As illustrated in this figure, the Pd-GeS2 system demonstrates outstanding desorption kinetics at ambient temperature (298 K), exhibiting recovery times of 3.7 s for FCN, 75 ms for CF3CN, and a remarkably fast 0.71 ms for C2F4. These time scales are particularly noteworthy as they fall well within the operational requirements for continuous monitoring systems in power equipment applications. While the Ru-GeS2 monolayer also shows acceptable recovery characteristics (83.1 s for FCN, 5.4 s for CF3CN, and 0.8 s for C2F4), its performance is notably slower compared to its Pd-GeS2 counterpart. In other words, the Pd-GeS2 monolayer is more suitable for gas sensor exploration in comparison with the Rh-doped counterpart.
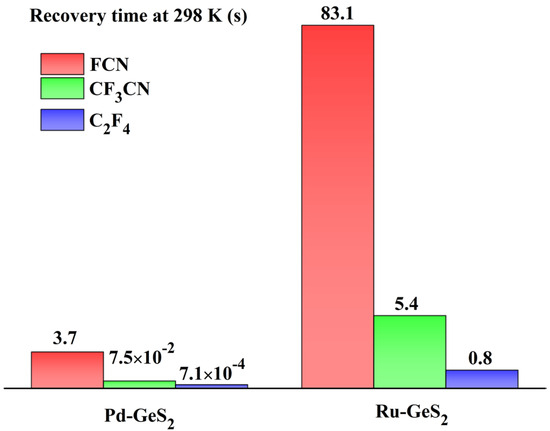
Figure 10.
Recovery time of Pd- and Ru-GeS2 monolayers upon three gases at room temperature.
Consequently, these findings, combining excellent sensitivity with instantaneous recovery, hold significance for intended applications of Pd-GeS2 as an ideal room-temperature sensor to monitor the operational statuses of C4F7N insulated devices. This unique combination of properties enables two critical operational advantages. First, the sub-second recovery times for CF3CN and C2F4 allow quasi real-time tracking of developing fault conditions through rapid sequential measurements [61]. Second, the uniform recovery behavior across different gas species ensures reliable operation regardless of the specific decomposition chemistry. Overall, this work on GeS2-based material not only paves the way for diagnosing insulation degradation in eco-friendly insulating media, but also emphasizes the importance of dopant selection in designing high-performance gas sensing materials. Future work can particularly focus on bridging the gap between theoretical predictions and practical implementation under continuous operation conditions, to accelerate technological adoption.
3. Computational Details
The first-principles calculations in this study were performed using the DMol3 module [62], employing the generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional [63]. To accurately model the weakly interacting systems under investigation, we incorporated empirical dispersion corrections through the DFT-D3 method, which effectively describes van der Waals interactions and long-range electron correlations [64]. The electronic structure calculations utilized a double numerical basis set with polarization functions (DNP), while the treatment of relativistic effects—important for transition metal dopants—was achieved through the density functional semi-core pseudopotential (DSSP) approach [65]. For Brillouin zone integration, a Monkhorst–Pack k-point mesh of 10 × 10 × 1 was employed to ensure convergence in both geometric optimization and electronic structure calculations [66]. Some other parameters were carefully optimized, including an energy convergence threshold of 1 × 10−6 Ha for self-consistent field iterations, a global orbital cutoff radius of 5.0 Å, and an electronic smearing value of 0.005 Ha [67]. These carefully designed computational protocols ensure the reliable prediction of electronic and adsorption properties for the doped GeS2 systems. For vibrational analysis, we employed the finite displacement method within the DMol3 framework, constructing the Hessian matrix through numerical differentiation of atomic forces using Cartesian displacements of ±0.015 Å [68]. Additionally, molecular dynamics simulations were performed at 298 K for 2 ps, with a time step of 1 fs to examine the thermal stability of the monolayers [69].
The initial framework was established by constructing an optimized 3 × 4 supercell of the pristine GeS2 monolayer, comprising 12 Ge and 24 S atoms as its fundamental structural unit. To accurately simulate the 2D characteristics of the system while mitigating artificial periodicity effects, a substantial vacuum spacing of 18 Å was introduced normal to the monolayer plane [70]. For comprehensive electronic structure characterization, we implemented the Hirshfeld population analysis method to quantitatively assess (i) the effective atomic charges of the dopants (denoted as QPd or QRu for Pd and Ru dopants, respectively) within the modified GeS2 lattice and (ii) the charge transfer (QT) in the gas adsorption processes. This approach provides fundamental insights into electronic redistribution, where positive charge values correspond to electron-donating properties of either the metal dopants or adsorbed gas molecules. The resulting charge distribution data offers a crucial mechanistic understanding of the underlying electronic interactions governing the sensing behavior at the atomic scale.
4. Conclusions
Through systematic first-principles investigations, this study presents a comparative evaluation of Pd- and Ru-doped GeSe2 monolayers for detecting critical C4F7N decomposed gases (FCN, CF3CN, and C2F4) in electrical insulation monitoring applications. Our calculations reveal the fundamental differences in metal doping that significantly influence gas sensing performance. While Ru doping demonstrates stronger surface interactions with the GeS2 substrate (as evidenced by Ef analysis) and consequently exhibits greater adsorption strengths (Ead = −0.94 to −1.06 eV) compared to Pd-doped systems (Ead = −0.76 to −0.98 eV), the Pd-doped monolayer surprisingly outperforms in practical sensing metrics. This apparent paradox is resolved through detailed charge transfer analysis, which shows that Pd doping facilitates more substantial electron donation during gas adsorption processes. The enhanced charge redistribution in Pd-GeS2 translates to exceptional room-temperature SRs of −99.6% (FCN), −95.0% (CF3CN), and −88.0% (C2F4), which largely surpasses the Ru-doped system’s responses of 79.7%, 78.1%, and 65.1%, respectively. Furthermore, the Pd-doped configuration demonstrates superior practical applicability through its instantaneous recovery properties (3.7 s for FCN, 75 ms for CF3CN, and 0.71 ms for C2F4 desorption at 298 K), making it an ideal candidate for real-time monitoring of C4F7N-insulated power equipment. These findings not only establish GeS2-based materials as promising materials for eco-friendly insulation monitoring but also highlight the critical importance of dopant selection in 2D material design, where optimal performance requires the balancing of multiple factors including adsorption strength, charge transfer capability, and recovery kinetics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/inorganics13110348/s1: Figure S1: MS simulation for pristine GeS2 monolayer. (a) configuration after MD simulation, and (b) fluctuations of temperature and energy during the simulation; Figure S2: Adsorption configurations of (a) Pd atom, (b) Ru atom, (c) Pd2 dimer and (d) Ru2 dimer on the pristine GeS2 monolayer; Figure S3: MS simulation for Pd-GeS2 monolayer. (a) configuration after MD simulation, and (b) fluctuations of temperature and energy during the simulation; Figure S4: MS simulation for Ru-GeS2 monolayer. (a) configuration after MD simulation, and (b) fluctuations of temperature and energy during the simulation; Figure S5: Adsorption configurations for (a) C4F7N, (b) FCN, (c) CF3CN and (d) C2F4 onto the pristine GeS2 monolayer. Figure S6: Adsorption configurations for C4F7N onto the (a) Pd- and (b) Ru-GeS2 monolayers.
Author Contributions
Methodology, X.G.; Formal analysis, S.M.; Investigation, S.M.; Writing—original draft, X.G.; Writing—review & editing, Y.L. and H.C.; Supervision, H.C.; Funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52207175).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Dr. Xinyu Guo and Dr. Shuoxiao Ma were employed by CHN Energy Qinghai Electric Power Co., Ltd. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Li, Y.; Zhang, X.; Zhang, J.; Xiao, S.; Xie, B.; Chen, D.; Gao, Y.; Tang, J. Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas. J. Hazard. Mater. 2019, 368, 653–660. [Google Scholar] [CrossRef]
- Xiao, S.; Gao, B.; Pang, X.; Zhang, X.; Li, Y.; Tian, S.; Tang, J.; Luo, Y. The sensitivity of C4F7N to electric field and its influence to environment-friendly insulating gas mixture C4F7N/CO2. J. Phys. D Appl. Phys. 2020, 54, 055501. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Xiong, J.; Li, X.; Murphy, A.B. Decomposition mechanism and kinetics of iso-C4 perfluoronitrile (C4F7N) plasmas. J. Appl. Phys. 2019, 126, 163303. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, A.; Wang, X.; Rong, M. Theoretical study of the decomposition mechanism of C4F7N. J. Phys. D Appl. Phys. 2019, 52, 245203. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Zhang, J.; Li, Y.; Xiao, S.; Zhuo, R.; Tang, J. Experimental study on power frequency breakdown characteristics of C4F7N/CO2 gas mixture under quasi-homogeneous electric field. IEEE Access 2019, 7, 19100–19108. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Jiang, X.; Zhang, Z.; Li, T.; Ma, L.; Niu, S.; Chen, Z.; Xiao, S.; Dan, M.; et al. Ir-doped MoSe2: A promising candidate for C4F7N decomposed species detection and scavenging. Surf. Interfaces 2024, 51, 104634. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, X.; Wu, Z. Fabrication of tin disulfide/graphene oxide nanoflower on flexible substrate for ultrasensitive humidity sensing with ultralow hysteresis and good reversibility. Sens. Actuators B Chem. 2019, 287, 398–407. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Li, Y.; Chen, D.; Zhang, Y. First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 859–866. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, W.; Duan, X.; Yu, T.; Cui, W.; Quan, W.; Chen, Y.; Xu, D. The optimization of noble metal-modified MoSe2 nanoflowers gas sensor for hazardous NO2 detection. Microchem. J. 2025, 209, 112892. [Google Scholar] [CrossRef]
- Cui, H.; Hu, J.; Jiang, X.; Zhang, X. A first-principles study of SOF2 and SO2F2 adsorption onto PdSe2-based monolayers: Favorable sensitivity and selectivity by doping single Cu or Rh atom. Environ. Res. 2025, 269, 120843. [Google Scholar] [CrossRef]
- Li, Z.; Liao, Y.; Liu, Y.; Zeng, W.; Zhou, Q. Room temperature detection of nitrogen dioxide gas sensor based on Pt-modified MoSe2 nanoflowers: Experimental and theoretical analysis. Appl. Surf. Sci. 2023, 610, 155527. [Google Scholar] [CrossRef]
- Hou, C.; Li, J.; Huo, D.; Luo, X.; Dong, J.; Yang, M.; Shi, X. A portable embedded toxic gas detection device based on a cross-responsive sensor array. Sens. Actuators B Chem. 2012, 161, 244–250. [Google Scholar] [CrossRef]
- Yan, H.-J.; Li, Z.; Liu, S.-C.; Wang, X.; Zhang, X.; Xue, D.-J.; Hu, J.-S. Investigation of weak interlayer coupling in 2D layered GeS2 from theory to experiment. Nano Res. 2022, 15, 1013–1019. [Google Scholar] [CrossRef]
- He, X.; Cen, W.; Zou, P. The Electronic Structure and Optical Properties of GeSe2: A First-Principles Study. Phys. Status Solidi 2025, 2500383. [Google Scholar] [CrossRef]
- Li, C.C.; Wang, B.; Chen, D.; Gan, L.-Y.; Feng, Y.; Zhang, Y.; Yang, Y.; Geng, H.; Rui, X.; Yu, Y. Topotactic Transformation Synthesis of 2D Ultrathin GeS2 Nanosheets toward High-Rate and High-Energy-Density Sodium-Ion Half/Full Batteries. ACS Nano 2020, 14, 531–540. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, W.; Hou, W.; Zeng, W.; Zhou, Q. Experimental and density functional theory study of the gas sensing property of Pt and Au doped WS2 to partial discharge gas CO in air switchgear. Sens. Actuators A Phys. 2024, 379, 115905. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, D.; Li, Q. Room Temperature Acetone-Sensing Properties of Ru-Doped MoSe2 Nanoflowers: Experimental and Density Functional Theory Study. IEEE Electron Device Lett. 2021, 42, 739–742. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 612. [Google Scholar] [CrossRef]
- Wu, H.; Fang, J.; Yuan, S.; Liu, Y.; Zeng, J.; Jiang, T. Adsorption and gas sensing properties of Cun and Pdn (n = 1–3) clusters modified MoSe2 for lithium battery thermal runaway gases. Appl. Surf. Sci. 2024, 648, 158963. [Google Scholar] [CrossRef]
- Chen, J.; Jia, L.; Cui, X.; Zeng, W.; Zhou, Q. Adsorption and gas-sensing properties of SF6 decomposition components (SO2, SOF2 and SO2F2) on Co or Cr modified GeSe monolayer: A DFT study. Mater. Today Chem. 2023, 28, 101382. [Google Scholar] [CrossRef]
- Tverjanovich, A.S.; Tsiok, O.B.; Brazhkin, V.V.; Bokova, M.; Cuisset, A.; Bychkov, E. Remarkably Stable Glassy GeS2 Densified at 8.3 GPa: Hidden Polyamorphism, Contrasting Optical Properties, Raman and DFT Studies, and Advanced Applications. J. Phys. Chem. B 2023, 127, 9850–9860. [Google Scholar] [CrossRef]
- Tse, G. The structural, electronic, optical, elastic, and vibrational properties of GeS2 using HSE03: A first-principle investigation. J. Comput. Electron. 2024, 23, 968–976. [Google Scholar] [CrossRef]
- Xiao, L.; Shu, S.; Liu, S. A facile synthesis of Pd-doped SnO2 hollow microcubes with enhanced sensing performance. Sens. Actuators B Chem. 2015, 221, 120–126. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.; Peng, X. Adsorption of SO2 and NO2 molecule on intrinsic and Pd-doped HfSe2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 513, 145863. [Google Scholar] [CrossRef]
- Peng, R.; Zeng, W.; Zhou, Q. Adsorption and gas sensing of dissolved gases in transformer oil onto Ru3-modified SnS2: A DFT study. Appl. Surf. Sci. 2023, 615, 156445. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Tang, J.; Chen, D.; Wang, D. Decomposition properties of C4F7N/N2 gas mixture: An environmentally friendly gas to replace SF6. Ind. Eng. Chem. Res. 2018, 57, 5173–5182. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.C.; Wang, X.; Li, Z.; Zhang, Y.; Zhang, G.; Xue, D.J.; Hu, J.S. Polarization-sensitive ultraviolet photodetection of anisotropic 2D GeS2. Adv. Funct. Mater. 2019, 29, 1900411. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Xiong, H.; Deng, G.; Gan, L. Theoretical investigations of adsorption and sensing properties of M2Pc (M = Cr, Mo) monolayers towards volatile organic compounds. Colloids Surf. A Physicochem. Eng. Asp. 2025, 717, 136750. [Google Scholar] [CrossRef]
- Celino, M.; Le Roux, S.; Ori, G.; Coasne, B.; Bouzid, A.; Boero, M.; Massobrio, C. First-principles molecular dynamics study of glassy GeS 2: Atomic structure and bonding properties. Phys. Rev. B—Condens. Matter Mater. Phys. 2013, 88, 174201. [Google Scholar] [CrossRef]
- Li, K.; Xue, D. Estimation of electronegativity values of elements in different valence states. J. Phys. Chem. A 2006, 110, 11332–11337. [Google Scholar] [CrossRef]
- Ma, Z.; Fu, M.; Gao, C.; Fan, S.; Chi, H.; Li, W.; Hou, D.; Cao, Y. Trenched microwave resonator integrated with porous PDMS for detection and classification of VOCs with enhanced performance. J. Hazard. Mater. 2024, 472, 134553. [Google Scholar] [CrossRef]
- Zhai, S.; Jiang, X.; Wu, D.; Chen, L.; Su, Y.; Cui, H.; Wu, F. Single Rh atom decorated pristine and S-defected PdS2 monolayer for sensing thermal runaway gases in a lithium-ion battery: A first-principles study. Surf. Interfaces 2023, 37, 102735. [Google Scholar] [CrossRef]
- He, W.; Xu, Y.; Wu, Z.; Hou, Z.; Qin, W.; Liu, X.; Shi, J.; Long, Y. Adsorption of Nitrogen-Containing Toxic Gases on Transition Metal (Pt, Ag, Au)-Modified Janus ZrSSe Monolayers for Sensing Applications: A DFT Study. ACS Appl. Nano Mater. 2025, 8, 3163–3174. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, D.; Xiao, S.; Tang, J. Adsorption behavior of COF2 and CF4 gas on the MoS2 monolayer doped with Ni: A first-principles study. Appl. Surf. Sci. 2018, 443, 274–279. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, B.; Wang, H.; Muhammad, I.; Liu, R.; Zhou, Y. Monolayer B3C2P3: A promising candidate as N-containing gases sensor and/or capturer with high Sensitivity, selectivity and reversibility. Appl. Surf. Sci. 2024, 655, 159531. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Zhao, B.; Li, C.Y.; Liu, L.L.; Zhou, B.; Zhang, Q.K.; Chen, Z.Q.; Tang, Z. Adsorption of gas molecules on Cu impurities embedded monolayer MoS2: A first-principles study. Appl. Surf. Sci. 2016, 382, 280–287. [Google Scholar] [CrossRef]
- Gu, M.; Tao, L.; Dastan, D.; Dang, J.; Fang, T.; An, B. Metal-Modified C3N1 Monolayer Sensors for Battery Instability Monitoring. J. Mater. Chem. A 2024, 12, 15254–15264. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goel, N. A DFT study of superior adsorbate–surface bonding at Pt-WSe2 vertically aligned heterostructures upon NO2, SO2, CO2, and H2 interactions. Sci. Rep. 2024, 14, 15708. [Google Scholar] [CrossRef]
- Pyykkö, P.; Atsumi, M. Molecular single-bond covalent radii for elements 1–118. Chemistry 2009, 15, 186–197. [Google Scholar] [CrossRef]
- Wu, D.; He, B.; Wang, Y.; Lv, P.; Ma, D.; Jia, Y. Double-atom catalysts for energy-related electrocatalysis applications: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 203001. [Google Scholar] [CrossRef]
- Cui, H.; Ran, M.; Peng, X.; Zhang, G. First-principles design of noble metal (Rh and Pd) dispersed Janus WSTe monolayer for toxic gas sensing applications. J. Environ. Chem. Eng. 2024, 12, 112047. [Google Scholar] [CrossRef]
- Zheng, D.; Peng, W.; Wu, R.; Quan, S.; Qian, L.; Cui, H. Sensing property of Pt-doped Janus SnSSe monolayer upon thermal runaway gases in the lithium-ion battery with effects of biaxial strains and electric fields. J. Environ. Chem. Eng. 2023, 11, 109711. [Google Scholar] [CrossRef]
- Yang, W.; Chen, T.; Zhou, G. Unveiling the tunable electronic, optoelectronic, and strain-sensitive gas sensing properties of Janus ZrBrCl: Insights from DFT study. Appl. Surf. Sci. 2025, 680, 161283. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Wu, W.; Liu, Y.; Zhou, Z. Transition metal (Pd, Pt, Ag, Au) decorated InN monolayer and their adsorption properties towards NO2: Density functional theory study. Appl. Surf. Sci. 2018, 455, 106–114. [Google Scholar] [CrossRef]
- Li, Z.; Rao, Y.; Wang, Z.; Zhang, T.; Wu, G.; Sun, L.; Ren, Y.; Tao, L. Universal Synthesis of Core–Shell-Structured Ordered Mesoporous Transition Metal Dichalcogenides/Metal Oxides Heterostructures with Active Edge Sites. Small Struct. 2025, 6, 2400376. [Google Scholar] [CrossRef]
- Xue, R.; Guo, Y.; He, H.; Zhang, Y.; Yang, N.; Xie, G.; Nie, Z. Bimetallic Phthalocyanine Monolayers as Promising Materials for Toxic H2S, SO2, and SOF2 Gas Detection: Insights from DFT Calculations. Langmuir 2025, 41, 4059–4075. [Google Scholar] [CrossRef]
- Wu, H.; Shan, Z.; Yuejing, B.; Xiaoping, J.; Cui, H. Ni-decorated WS2-WSe2 heterostructure as a novel sensing candidate upon C2H2 and C2H4 in oil-filled transformers: A first-principles investigation. Mol. Phys. 2025, e2492391. [Google Scholar] [CrossRef]
- Tantardini, C.; Oganov, A.R. Thermochemical electronegativities of the elements. Nat. Commun. 2021, 12, 2087. [Google Scholar] [CrossRef]
- Allian, A.D.; Kazuhiro, T.; Fujdala, K.L.; Xianghong, H.; Truex, T.J.; Juan, C.; Corneliu, B.; Matthew, N.; Enrique, I. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498. [Google Scholar] [CrossRef]
- Wu, J.; Wu, D.; Li, H.; Song, Y.; Lv, W.; Yu, X.; Ma, D. Tailoring the coordination environment of double-atom catalyst to boost electrocatalytic nitrogen reduction: A first-principles study. Nanoscale 2023, 15, 16056–16067. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.V.; Pham, K.D. The theoretical prediction of the structural characteristics and SO2 adsorption-sensing properties of pristine HfS2 and TM-doped HfS2 monolayers (TM = Ni, Pd, or Pt). New J. Chem. 2023, 47, 17252–17260. [Google Scholar] [CrossRef]
- Luo, C.; Yang, N.; Dong, X.; Qin, D.; Zhou, G.; Chen, T. Susceptible Detection of Organic Molecules Based on C3B/Graphene and C3N/Graphene van der Waals Heterojunction Gas Sensors. ACS Sens. 2024, 9, 4822–4832. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, M.; Poh, H.L.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Šaněk, F.; Khezri, B.; Webster, R.D.; Pumera, M. Noble metal (Pd, Ru, Rh, Pt, Au, Ag) doped graphene hybrids for electrocatalysis. Nanoscale 2012, 4, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, Y.; Guo, M.; Niu, C.; Lu, J.; Huang, B. Electronic and magnetic properties of perfect, vacancy-doped, and nonmetal adsorbed MoSe2, MoTe2 and WS2 monolayers. Phys. Chem. Chem. Phys. PCCP 2011, 13, 15546. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, Y.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X. Adsorption of SF6 decomposition components on Pt3-TiO2 (1 0 1) surface: A DFT study. Appl. Surf. Sci. 2018, 459, 242–248. [Google Scholar] [CrossRef]
- Cui, H.; Wu, H.; He, D.; Ma, S. Noble metal (Pd, Pt)-functionalized WSe2 monolayer for adsorbing and sensing thermal runaway gases in LIBs: A first-principles investigation. Environ. Res. 2025, 269, 120847. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, D.; Chen, Q.; Wang, Z.; Wang, D.; Yang, Z.; Xu, W.; Wang, L.; Zhu, L.; An, F. Heterostructure construction of SnS2 Debye nanowires modified with ZnO nanorods for chemiresistive H2S detection in sulfur hexafluoride decomposition products. Sens. Actuators B Chem. 2023, 390, 133952. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.B.; Zhou, K.G.; Liu, C.H.; Zeng, J.; Zhang, H.L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Kushwaha, A.; Kumar, R.; Goel, N. Chemiresistive gas sensors beyond metal oxides: Using ultrathin two-dimensional nanomaterials. FlatChem 2024, 43, 100584. [Google Scholar] [CrossRef]
- Yong, Y.; Gao, R.; Yuan, X.; Zhao, Z.; Hu, S.; Kuang, Y. Gas sensing and capturing based on the C7N6 monolayer with and without metal decoration: A first-principles investigation. Appl. Surf. Sci. 2022, 591, 153129. [Google Scholar] [CrossRef]
- Cui, H.; Liu, T.; Zhang, Y.; Zhang, X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sens. J. 2019, 19, 5249–5255. [Google Scholar] [CrossRef]
- Tkatchenko, A.; DiStasio, R.A.; Head-Gordon, M.; Scheffler, M. Dispersion-corrected Møller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 171. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Z.; Jia, P. Pd-doped C3N monolayer: A promising low-temperature and high-activity single-atom catalyst for CO oxidation. Appl. Surf. Sci. 2021, 537, 147881. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Ding, G.; Gao, G.Y.; Huang, Z.; Zhang, W.; Yao, K. Thermoelectric properties of monolayer MSe2 (M = Zr, Hf): Low lattice thermal conductivity and a promising figure of merit. Nanotechnology 2016, 27, 375703. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gong, X.; Xiao, F.; Liu, Y.; Ming, X. First-Principles Study on the Selective Detection of Toxic Gases by 2D Monolayer PdS2: Insight into Charge Transfer Dynamics and Alignment of Frontier Molecular Orbitals. ACS Appl. Nano Mater. 2023, 6, 12470–12478. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Q.; Li, P.; Pang, M.; Luo, Y. Fabrication of Pd-decorated MoSe2 nanoflowers and density functional theory simulation toward ammonia sensing. IEEE Electron Device Lett. 2019, 40, 616–619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).