1. Introduction
In the study of oxide materials with fluorescent properties, trivalent transition elements are often added as activators. Eu
2O
3, in particular, is a versatile and valuable doping material because it can exist in multiple oxidation states, allowing it to produce phosphors with various colors. When introduced into host materials, Eu
2O
3 can exist as either Eu
3+ or Eu
2+, depending on the synthesis conditions. Under normal conditions, Eu
2O
3 forms Eu
3+ ions, which emit strong red [
1] or near-infrared light [
2]. However, in a reducing atmosphere, Eu
3+ ions can be reduced to Eu
2+, which results in green [
3] or blue emissions [
4], useful for full-color displays and white light generation. The generation of blue-emitting phosphors can be achieved not only by using Eu
2⁺ ions as activators, but also through other activators [
5]. Additionally, Bi
3+ ions can be used as activators for different emission colors. Liu et al. synthesized a Bi
3+-doped La
3SnGa
5O
14 phosphor, which exhibited a narrow-band emission centered at 360 nm and a broadband emission centered at 540 nm under 290 nm excitation [
6]. Fu et al. developed a Bi
3+-doped CaYGaO
4 phosphor that showed a broadband emission centered at 435 nm when excited at 370 nm [
7]. Meng et al. prepared a Bi
3+-doped CaMgGeO
4 phosphor, which displayed a narrow-band emission centered at 346 nm under 316 nm excitation [
8]. Pi et al. reported a Bi
3+-doped Ba
3Ga
2O
6 phosphor with a broadband emission centered at 416 nm when excited at 340 nm [
9]. These previous studies demonstrate that Bi
3+-doped phosphors can emit across a broad spectral range, depending on the host matrix and excitation wavelength. However, most of these materials either exhibit emissions in the ultraviolet or blue region, or show relatively broad emission bands that may limit their color purity for specific applications.
In Liu et al.’s research, they conducted an in-depth investigation into the optical properties and compositional relationships of Ca
3Ga
4O
9 + 1 mol% Bi
2O
3 +
x mol% ZnO as a cyan-emitting phosphor [
10]. Through systematic adjustments of ZnO content, the research team observed several noteworthy phenomena. All Ca
3Ga
4O
9 + 1 mol% Bi
2O
3 +
x mol% ZnO phosphors exhibited only one primary peak in their photoluminescence excitation (PLE) spectra, located at approximately 340 nm. This characteristic peak demonstrated remarkable stability; even with variations in ZnO content, neither its wavelength position nor peak intensity showed significant shifts. This suggests that the excitation mechanism likely depends primarily on the intrinsic properties of the matrix material rather than the dopant concentration. Regarding emission characteristics, these materials similarly displayed a single emission peak located at approximately 490 nm, corresponding to visible cyan light. This emission peak’s wavelength position also showed low sensitivity to changes in ZnO content, remaining essentially constant. However, unlike the excitation spectra, the maximum photoluminescence emission intensity (PLmax) exhibited a pronounced trend with increasing ZnO concentration. Particularly noteworthy was the pattern of PLmax as ZnO dopant levels (
x values) increased, showing rapid initial growth followed by stabilization. When the
x value reached 3, the emission intensity approached its maximum, with further increases in ZnO content yielding minimal enhancement, demonstrating typical saturation behavior. This result suggests that ZnO at an appropriate concentration (
x = 3) likely achieves optimal lattice modification effects or activation center concentration, while further increases may lead to concentration quenching or other limiting factors. These findings hold significant implications for optimizing the performance of Ca
3Ga
4O
9-based phosphor materials, indicating the optimal doping level of ZnO as an additive, while also providing experimental evidence for understanding energy transfer mechanisms within this material system.
Yin and colleagues investigated the effect of ZnO doping on the fluorescent properties of Ca
3Ga
4O
9 phosphors doped with 3 mol% Bi
3+ as the active luminescent ion [
11]. By varying the ZnO concentration from 0 to 7 mol%, they studied changes in photoluminescent behavior. Under the same excitation conditions, the emission wavelength shifted from ~528 nm to 484 nm, indicating a blue shift. This suggests that ZnO doping can effectively tune the emission color, showing potential for creating tunable phosphors. However, the peak emission wavelength of PLmax changed only slightly with increased ZnO, indicating that the energy levels of Bi
3+ ions were only mildly affected. This is likely due to Zn
2+ ions replacing certain lattice sites, altering the crystal field around Bi
3+ without significantly changing their transition mechanisms. The PLmax showed a more noticeable and non-linear trend. It was lowest at 0 mol% ZnO and highest at 7 mol%, indicating improved emission efficiency at higher doping levels. However, the increase was not steady, suggesting that multiple factors influence the emission. PLE spectra revealed more complexity with increasing ZnO. At 0 mol%, the spectrum had a single peak, while at 1 mol%, it showed two peaks. With further ZnO addition, a triple-peak structure emerged, pointing to new energy levels or defects caused by Zn
2+-induced lattice distortions. In summary, ZnO doping not only adjusts the emission wavelength and intensity of Ca
3Ga
4O
9:Bi
3+ phosphors but also significantly alters their excitation energy structure.
These results highlight the important role of composition in tuning luminescent properties and advancing the development of customizable phosphor materials. Zhou et al. investigated the optical properties of Ca
3Ga
4O
9:
xBi
3+ phosphors with
x = 0.005, 0.01, 0.03, and 0.07, and found that all samples exhibited a single emission peak [
12]. The wavelengths to reveal the maximum photoluminescence excitation (PLEmax) and PLmax peaks were observed at 345 nm and 490 nm, respectively. Yin et al. synthesized 1150 °C for 10 h, and the analysis results showed that for the excitation spectra of Ca
3Ga
4O
9: 3 mol% Bi
3+ with different co-doping of Zn
2+ ions, two distinct absorption bands were observed: a dominant band at approximately 340 nm and a weaker band around 295 nm, which correspond to emission peaks at 484 nm and 530 nm [
11]. While transitions from the
1S
0 ground state are theoretically restricted to the
1P
1 excited state within the 6s
1p
1 configuration, strong spin–orbit coupling enables interaction between the
3P
1 and 1P
1 energy levels, making the
1S
0 →
3P
1 transition feasible. Since the
1S
0 →
1P
1 transition typically occurs in the vacuum UV region, these two absorption bands are attributed to
1S
0 →
3P
1 transitions of Bi
3+ ions occupying different crystallographic sites.
In recent studies, the focus had predominantly been on the influence of ZnO content on the PLE and PL properties of phosphors. However, there has been a lack of detailed exploration regarding how varying the content of CaO affects these properties in the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors. In addition to the established findings from Yin et al., this study provides a deeper exploration into the specific role of CaO tuning in enhancing the luminescent properties of Ca3Ga4O9: Bi3+ phosphors. The unique ability of CaO to modulate the crystal structure and local environment of Bi3+ ions has not been fully investigated in previous works, especially when co-doped with Zn2+ ions. By varying the CaO content, we observe significant changes in both the absorption and emission profiles, demonstrating the unique influence of CaO on the excitation spectra and photoluminescence behavior. This approach not only enhances the understanding of the luminescence mechanism in Bi3+-doped phosphors but also opens new avenues for the design of high-performance phosphors with tunable optical properties. Compared to previous studies that primarily focused on Zn2+ doping or Bi3+ site occupancy, this work provides a more comprehensive analysis of how CaO contributes to fine-tuning the phosphor’s properties, offering valuable insights for future phosphor design strategies.
In this study, we proposed that the observed variations in the PLE and PL spectra of the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors are primarily attributed to the increasing CaO content in the Ca2+xGa4O8+x compositions. The main purpose of varying the CaO content in Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO is to modify the oxygen vacancies or metal ion coordination environment in the crystal structure. This adjustment can enhance the excitation process of Bi3+, thereby optimizing its absorption and emission characteristics at specific wavelengths. Additionally, altering the CaO content could modulate the charge balance within the crystal lattice, which would influence the electronic structure and transition probabilities of Bi3+, resulting in shifts in both the excitation and emission spectra. Therefore, the expected structural and electronic impact of varying CaO content lies in its ability to modify the defect dynamics and the local electronic environment of the luminescence centers, ultimately optimizing the photophysical properties of the phosphor material. To investigate this effect in detail, we synthesized a series of phosphors with the general formula Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO, where x ranges from 0 to 1. This compositional design enabled a systematic study of how varying CaO concentrations affect the structural and optical properties of the material. In addition, we examined the doping behavior of Bi3+ and Zn2+ ions within the Ca2+xGa4O8+x lattice to understand their incorporation mechanisms and interaction with the host matrix. We analyzed the influence of calcium content on the emission peak position, luminescence intensity, and color purity, aiming to clarify the underlying mechanisms driving these changes. Through this analysis, we identified the optimal x value for achieving a high-brightness, narrow-band blue-emitting phosphor with an emission wavelength centered around 484 nm and high color purity. Furthermore, we investigated the impact of varying calcium content on the thermal stability and quantum efficiency of the phosphor, providing critical insights for the development of high-performance luminescent materials.
2. Methodology
CaCO3, Ga2O3, ZnO, and Bi2O3 were used as the starting raw materials to synthesize ceramic compositions with the nominal formula Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO, where x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0. The raw materials were weighed precisely according to the target stoichiometry. To ensure homogeneity, the powders were thoroughly mixed by ball milling for 2 h using absolute ethanol as the milling medium. The mixed powders were then further ground in ethanol to produce a uniform slurry, which was dried at 80 °C and subsequently pulverized into a fine powder to ensure consistent particle size and reactivity. During the high-temperature synthesis, CaCO3 was expected to thermally decompose into CaO and CO2 gas. The resulting CaO would then react with Ga2O3, ZnO, and Bi2O3 to form the desired ceramic phase. Given that synthesis temperatures at or above 1250 °C could lead to partial melting or degradation of the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO compositions, the solid-state reaction was carried out at a slightly lower temperature of 1200 °C for 2 h. The selected temperature was chosen to promote the formation of the target phase while minimizing the risk of melting and maintaining phase stability. When the synthesis temperature exceeded 1200 °C, for instance at 1250 °C, some compositions melting of Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO occurred. On the other hand, when the temperature was below 1200 °C, a higher amount of the original phase, Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO compositions, remained intact. X-ray diffraction (XRD) analysis was performed using a Bruker D8 diffractometer operated at 40 kV and 40 mA. The diffraction patterns were recorded over a 2θ range of 20° to 80°, with a scanning rate of 1° per min to identify the phase composition and crystallographic structure of the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO samples.
PL and PLE measurements were initially conducted at room temperature using a Hitachi F-4500 fluorescence spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan) equipped with a xenon lamp as the excitation source. To optimize the optical performance of the synthesized phosphors, a three-dimensional (3D) scanning method was employed to systematically identify the most effective PLE wavelengths within the 300–400 nm range. Measurements were performed at 10 nm intervals across this excitation range, and the corresponding PL spectra were recorded within the 370–600 nm emission window. This composition-dependent analysis revealed significant variations in emission intensity and spectral profiles depending on the excitation wavelength, indicating the strong influence of composition on luminescence behavior. The 3D scanning approach thus proved effective in precisely determining the optimal excitation conditions for maximizing emission efficiency. Furthermore, due to the built-in heating system of the spectrophotometer, temperature-dependent PLE and PL spectra were directly acquired over a temperature range from room temperature (~30 °C) up to 210 °C. These measurements provided valuable insights into the thermal stability and temperature sensitivity of the optical properties of the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors.
3. Results and Discussion
3.1. XRD Analysis Results of the Synthesized Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO Powders
XRD patterns of the synthesized Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO powders were measured as a function of Ca content and are presented in
Figure 1. The analysis primarily referred to the following JCPDS standards: No. 82-1691 for CaO, which has a cubic phase with a main peak at (200) 37.469°, No. 87-1901 for Ga
2O
3 with an orthorhombic phase and a main peak at (421) 35.217°, No. 16-0593 for CaGa
2O
4 (also denoted as CaO·Ga
2O
3), which exhibits an orthorhombic phase with a main peak at (220) 30.273°, and No. 83-1477 for Ca
3Ga
4O
9 with an orthorhombic phase and a main peak at (421) 31.874°. In the synthesized Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors, four crystalline phases, CaGa
2O
4, CaO, Ga
2O
3, and Ca
3Ga
4O
9, were clearly identified in the sample with
x = 0. It was observed that synthesis temperatures above 1250 °C led to partial melting of the material, which adversely affected phase stability and homogeneity. Therefore, a synthesis temperature of 1200 °C was selected to avoid melting; however, this relatively lower temperature may have been insufficient to enable complete solid-state diffusion and reaction between CaO and Ga
2O
3, resulting in the retention of unreacted secondary phases. The presence of Ca
3Ga
4O
9 in the
x = 0 composition further indicates that the expected formation of a single-phase CaGa
2O
4 was disrupted. This likely interfered with the complete consumption of Ga
2O
3 and contributed to its residual presence.
As the x value increased, the diffraction intensity associated with the CaO phase progressively decreased and disappeared by x = 0.4, suggesting improved integration of excess Ca into the crystalline structure. In contrast, the diffraction intensity of Ga2O3 became more prominent with increasing x, suggesting that an increasing amount of Ga remained unincorporated in the host lattice, even under Ca-rich conditions. At x = 1.0, despite the highest Ca content, a distinct crystalline Ga2O3 phase was still observed. This phenomenon can be attributed to the complex interplay of several factors. One key factor is the incorporation of ZnO, which may partially substitute Ga3+ in the crystal lattice due to the comparable ionic radii of Zn2+ and Ga3+. This substitution introduces local charge imbalance and structural distortion, which can hinder complete Ga incorporation into the desired phases. As a result, excess Ga tends to segregate and crystallize as Ga2O3, especially when the host phases reach their solubility limit for gallium. Furthermore, the persistent coexistence of both CaGa2O4 and Ca3Ga4O9 phases throughout the compositional range, including at x = 1.0, implies that the system naturally favors the formation of multiple stable phases rather than a single-phase solid solution. This multiphase equilibrium constrains the incorporation of excess Ga, leading to the formation of separate Ga2O3 domains. The relatively moderate synthesis temperature of 1200 °C may also limit reaction kinetics and diffusion, preventing complete homogenization and allowing unreacted Ga2O3 to remain. The residual crystalline Ga2O3 observed at x = 1.0 is attributed to a combination of incomplete solid-state reactions at 1200 °C, partial substitution of Ga3+ by Zn2+, and the coexistence of multiple calcium gallate phases, which collectively limit the full incorporation of Ga into the host lattice.
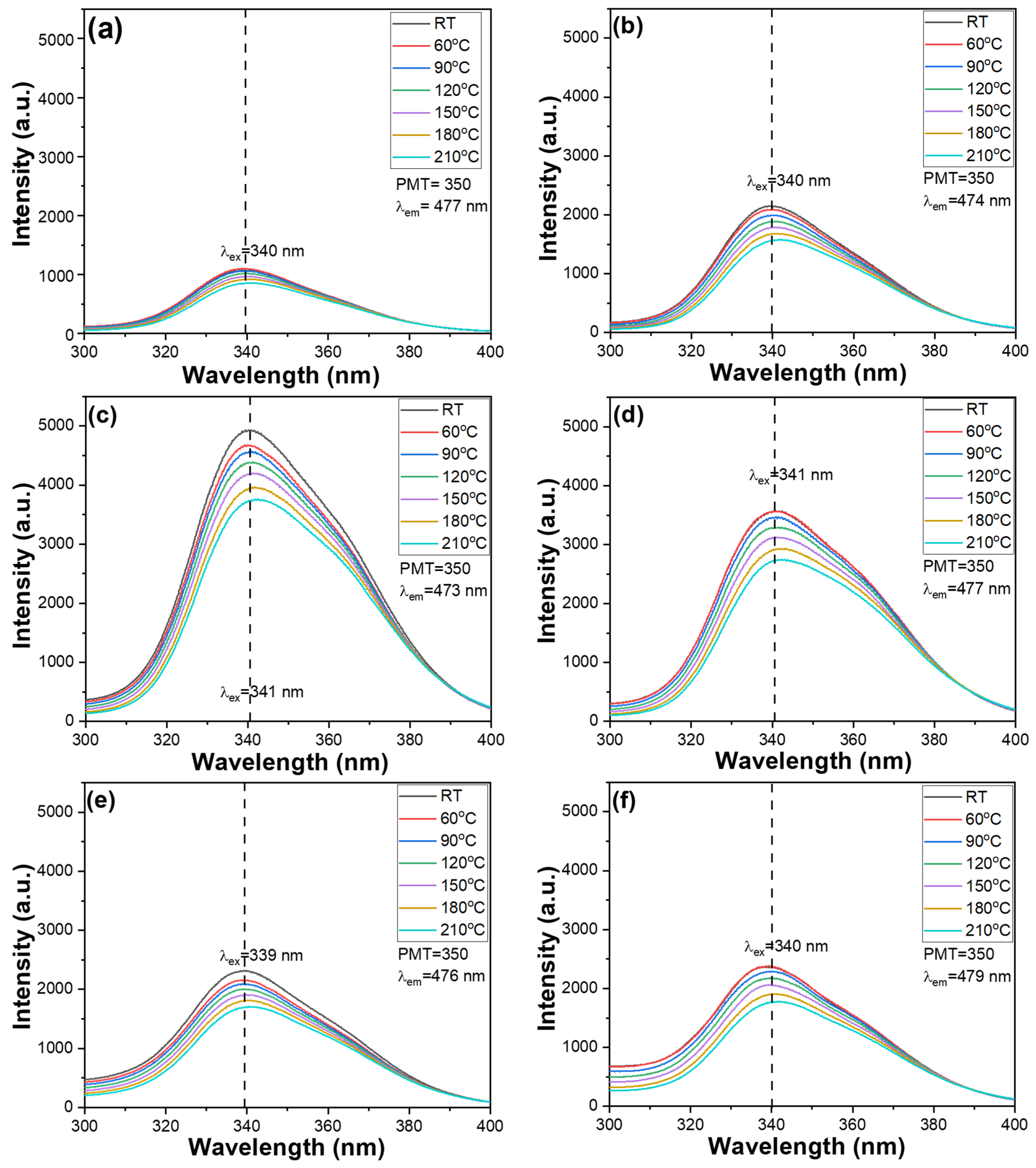
3.2. PLE and PL Properties of the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO Phosphors
Another important focus of this study is to investigate the effect of CaO content on the PLE and PL properties of the synthesized Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors. The PLE spectra of these phosphors were recorded under varying CaO concentrations, and the results are presented in
Figure 2. For samples with
x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0, the PLE spectra were measured using a spectrophotometer while monitoring emission wavelengths at 477, 474, 473, 477, 476, and 477 nm, respectively. The selection of different monitoring wavelengths is based on the variation in emission peak positions due to compositional changes and will be discussed in a later section. All measurements were conducted within the spectral range of 300–400 nm, and temperature conditions were varied from room temperature to 210 °C to assess thermal stability. For our Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors, all PLE spectra exhibited a single excitation peak. The PLEmax were observed at wavelengths of 340, 340, 341, 341, 339, and 341 nm, respectively, corresponding to varying
x values of 0, 0.2, 0.4, 0.6, 0.8, and 1.0. In contrast, a study by Yin et al. on Ca
3Ga
4O
9: 3 mol% Bi
3+ phosphors with varying Zn
2+ ion concentrations reported two distinct absorption bands, a strong one near 340 nm and a weaker one around 295 nm, leading to emission peaks at 484 nm and 530 nm, respectively [
11]. Notably, our phosphors share the strong excitation feature at approximately 340 nm, consistent with Yin et al.’s findings. However, unlike their results, we did not observe the secondary absorption band at 295 nm in any of our samples.
This indicates that our phosphor system supports a single dominant excitation pathway, which may be attributed to differences in crystal structure, dopant distribution, or energy transfer mechanisms. The reason for monitoring slightly different but similar emission wavelengths (e.g., 477, 474, 473, 477, 476, and 477 nm) in the PLE spectra is to confirm the consistency and robustness of the excitation profile across the emission band. These small variations in detection wavelength help ensure that the observed excitation behavior is not an artifact of a specific monitoring wavelength, but rather an intrinsic property of the luminescent center. The nearly identical PLE shapes at these wavelengths further support the presence of a single dominant emitting species and excitation mechanism, regardless of slight shifts in emission monitoring. The PLE spectra corresponding to
x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 are shown in
Figure 2a–f, respectively. As the CaO content increased from
x = 0 to
x = 0.4, both the overall emission intensities of the PLE spectra and the PLEmax exhibited a marked increase. This enhancement can be attributed to improved crystallinity and optimized local coordination environments around the luminescent centers, which facilitate more efficient energy absorption and transfer processes. However, when the CaO content was further increased from
x = 0.4 to
x = 0.8, a noticeable decline in both overall emission intensities of the PLE spectra and PLEmax was observed. The full width at half maximum (FWHM) values of the PLE of the synthesized Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors are 40.4, 42.8, 46.6, 47.8, 45.6, and 47.0 nm for
x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0, respectively. In the synthesized Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors, four crystalline phases, CaGa
2O
4, CaO, Ga
2O
3, and Ca
3Ga
4O
9, were clearly identified in the sample with
x = 0. It was observed that synthesis temperatures above 1250 °C led to partial melting of the material, which adversely affected phase stability and homogeneity. Therefore, a synthesis temperature of 1200 °C was selected to avoid melting; however, this relatively lower temperature may have been insufficient to enable complete solid-state diffusion and reaction between CaO and Ga
2O
3, resulting in the retention of unreacted secondary phases. The presence of Ca
3Ga
4O
9 phase in the
x = 0 composition further indicates that the expected formation of a single-phase CaGa
2O
4 was disrupted.
This multiphase structure likely contributes to the observed variations in luminescence intensity with CaO content. The reduction in emission intensity may be attributed to the formation of lattice distortions or structural defects, such as oxygen vacancies, which act as non-radiative recombination centers and quench luminescence. Increasing the CaO content introduces excess Ca
2+ ions into the host matrix, potentially leading to an imbalance in charge compensation and lattice strain due to ionic radius mismatch between Ca
2+ and other constituent ions. This local lattice distortion can increase the defect density, thereby facilitating non-radiative relaxation pathways. Moreover, the behavior of Bi
3+ ions in different local environments also plays a significant role. As the crystal structure becomes increasingly distorted with higher CaO content, the crystal field symmetry surrounding Bi
3+ ions is altered. These changes can affect the 6s
2 → 6s6p transitions of Bi
3+ by modifying the energy level splitting and radiative transition probabilities. In highly disordered or strained environments, Bi
3+ ions may occupy energetically less favorable sites or experience strong perturbations, thereby reducing the emission efficiency. At
x = 1.0, the overall emission intensities of the PLE spectra and PLEmax continued to decline slightly, as shown in
Figure 3e,f, possibly indicating the saturation of structural modification and further accumulation of defects. These findings clearly demonstrate that CaO content plays a critical role in modulating the optical properties of Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors by influencing their phase composition, lattice structure, defect density, and the local coordination environment of Bi
3+ ions, which collectively govern the excitation efficiency and emission behavior.
For the Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors with varying compositions (
x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0), photoluminescence (PL) spectra were recorded using excitation wavelengths of 340, 340, 341, 341, 339, and 341 nm, respectively. The emission spectra were collected in the 370–600 nm wavelength range while systematically varying the CaO content. Additional measurements were conducted over a temperature range from room temperature to 210 °C to assess thermal stability. The PL spectra corresponding to different CaO concentrations are presented in
Figure 3a–f, revealing the wavelengths at which the PL intensity reaches its maximum (PLmax). Specifically, the PLmax wavelengths for
x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 were observed at 477, 474, 473, 477, 476, and 477 nm, respectively. The FWHM values of the PL of the synthesized Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors are 112, 110, 114, 116, 122, and 122 nm for
x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0, respectively. These relatively broad FWHM values indicate strong spectral coverage, which is advantageous for applications such as white light emission and broadband optical devices, where a wide emission range is desirable for improved color rendering and performance. These results indicate that there is no significant shift in PLmax wavelength with increasing
x, suggesting that the emission band remains essentially stable across the compositional range. Furthermore, the PLmax intensity initially increases as
x increases, peaking at
x = 0.4, then decreases as
x continues to increase, eventually plateauing around
x = 0.8. This trend suggests that moderate substitution of Ca
2+ may enhance luminescence efficiency, possibly due to the optimal local environment and reduced defect density around the luminescent centers.
3.3. Emission Mechanisms of the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO Phosphors
However, excess CaO content beyond x = 0.4 may introduce defects or structural distortions that act as non-radiative recombination centers, thus quenching the emission intensity. Most notably, both PLmax and PLEmax wavelengths remain nearly unchanged with increasing x, indicating that the fundamental electronic transitions responsible for the luminescence are not significantly affected by CaO content. The luminescence of Bi3+ ions originates from the electronic transition of their 6s2 configuration; specifically, the spin–orbit allowed transition from the excited state 3P1 to the ground state 1S0. This type of transition typically results in broadband emission, with the peak position strongly influenced by the local crystal field environment surrounding the Bi3+ ions. In the Ca3Ga4O6 host lattice, Bi3+ ions tend to substitute for Ca2+ sites (denoted Ca1 to Ca4). These calcium sites exhibit different coordination geometries, either six-fold or eight-fold, leading to variations in the local crystal field strength and thus altering the emission characteristics of Bi3+. When Bi3+ occupies six-fold coordinated sites such as Ca1 or Ca3, the emission peak appears at approximately 484 nm, corresponding to blue–green light. Conversely, when Bi3+ substitutes into the eight-fold coordinated Ca2 or Ca4 sites, the emission peak redshifts to around 528 nm, corresponding to yellow–green light. Photoluminescence measurements reveal two distinct emission bands.
Under excitation at 338 nm (which matches the peak of the PLE spectra shown in
Figure 2), the emission peak is centered around 484 nm, while excitation at a shorter wavelength of 295 nm shifts the emission peak to 530 nm. These two emission bands are both attributed to the
3P
1 →
1S
0 transition of Bi
3+ ions situated at different lattice sites. Specifically, the 484 nm emission is associated with Bi
3+ substituting at six-fold coordinated Ca I sites, whereas the 530 nm emission arises from Bi
3+ located at eight-fold coordinated Ca II sites [
13,
14]. Therefore, if Bi
3+ ions predominantly occupy six-fold coordinated sites (such as Ca1 or Ca3), the emission peak is expected to be centered around 484 nm, which is consistent with the experimentally observed peak near 477 nm. The results confirm the structural robustness of the host matrix in maintaining consistent luminescent properties despite compositional variation, making this material system promising for stable optical applications. The study of Bi
3+-doped CaGa
2O
4 phosphors remains relatively limited in the current literature. Among the few relevant works, Wang et al. synthesized a series of Ca
1-xGa
2O
4:
xBi
3+ (
x = 0–0.0125) phosphors and investigated their crystalline structures and photoluminescent properties [
15]. The PLE spectra, monitored at an emission wavelength of 584 nm, exhibited a prominent excitation peak at 337 nm, corresponding to the
1S
0 →
3P
1 transition of Bi
3+ ions, along with a broad excitation band centered at 280 nm, attributed to the Ga
3+–O
2− charge transfer transition from the CaGa
2O
4 host lattice. Under 337 nm excitation, an asymmetric emission band centered at 584 nm was observed, which is characteristic of the
3P
1→
1S
0 transition of Bi
3+ ions. However, the peak position of the PL spectrum reported in their study significantly differs from those observed in our measurements.
Fu et al. investigated the luminescent properties of CaY
1−xGaO
4:
xBi
3+ (
x = 0.002–0.020) [
7]. In their study, the phosphors exhibited an excitation wavelength of 370 nm and an emission peak at 435 nm, which corresponds to the characteristic absorption and radiative transition of Bi
3+ ions. However, it is noteworthy that the PLmax wavelength reported in their study shows a slight deviation from the results observed in our investigation. According to Fu et al., the luminescence behavior of CaYGaO
4:Bi
3+ is predominantly governed by the
1S
0→
3P
1 electronic transition of Bi
3+, which is highly sensitive to the local coordination environment of the dopant within the host lattice. In our synthesis of samples with compositions Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO at
x = 0 and
x = 0.2, the primary crystalline phase identified was CaGa
2O
4, which is structurally analogous to Ca
3Ga
4O
9. Given that the emission wavelength of Bi
3+ ions is largely influenced by its local coordination geometry, particularly the coordination number and crystal field strength, a similar coordination environment in different host lattices (e.g., six-fold coordination in both CaGa
2O
4 and Ca
3Ga
4O
9) could result in comparable luminescence wavelengths. While Fu et al. reported an emission peak at 435 nm for CaGa
2O
4:Bi
3+, it is plausible that in host matrices such as CaGa
2O
4 and Ca
3Ga
4O
9, where the Bi
3+ ions experience a comparable local environment, the emission characteristics may shift slightly, resulting in an observed peak near 474 nm. The
1S
0→
3P
1 transition of Bi
3+ is subject to modulation by crystal field splitting and electron–phonon coupling. Therefore, even subtle differences in lattice parameters or bond lengths across similar host structures can lead to a discernible shift in the emission wavelength. This implies that the red-shifted emission observed in our study may be attributed to such variations in the local structural environment of Bi
3+ within the CaGa
2O
4 and Ca
3Ga
4O
9 lattices, in contrast to that in CaYGaO
4.
Notably, all the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors exhibited similarly narrow PLE spectra, each featuring a prominent excitation peak centered around 340 nm. The presence of this single excitation peak across all compositions further supports a stable emission mechanism that remains unaffected by changes in CaO content. This behavior can be attributed to the structural and electronic characteristics of the phosphor system. Specifically, the excitation at ~340 nm (∼3.65 eV) corresponds to electronic transitions of Bi3+ ions incorporated into the host lattice. According to Liu et al., these Bi3+ ions preferentially substitute Ca2+ at the six coordinated Ca1 and Ca3 sites, located within the four-membered ring channels of the Ca3Ga4O9 structure, which is built on a GaO4 tetrahedral framework. This site-specific substitution produces a uniform 6s→6p (6S→6P) transition environment for Bi3+, with the excitation energy falling within the energy range permitted by the host’s bandgap (~4.67 eV). The consistency of the PLE spectra across varying CaO contents further supports the stability and robustness of this excitation mechanism. It is evident that the wavelengths corresponding to the PLEmax and PLmax of the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors remain unchanged regardless of the CaO content; no noticeable blue shift or red shift occurs.
3.4. Temperature Stability and Emission Efficiency of the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO Phosphors
These phosphors were all synthesized at the same temperature, and the variation in CaO content leads to the formation of the different Ca
2+xGa
4O
8+x compounds, including CaGa
2O
4 and Ca
3Ga
4O
9. However, the constant doping levels of Bi
2O
3 and ZnO result in identical PLEmax and PLmax wavelengths across all samples. This strongly suggests that the emission mechanism of these phosphors is governed primarily by the incorporation of Bi
2O
3 and ZnO. To evaluate the influence of temperature on the optical properties of the Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors, the characteristic peak wavelengths, those at which PLEmax and PLmax occur, were selected for monitoring the evolution of PLE and PL spectra. These wavelengths served as reference points to assess how emission intensities varied with temperature. Upon heating the samples from 30 °C to 210 °C, only slight reductions in emission intensity were observed in both the PLE (
Figure 2) and PL (
Figure 3 and
Figure 4) spectra. Repeated measurements confirmed that the PLmax intensity of the Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors at 210 °C decreased to approximately 77.0% ± 1.9%, 72.5% ± 1.8%, 72.4% ± 2.0%, 73.8% ± 2.0%, 72.8% ± 2.0%, and 72.2% ± 1.7% for
x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0, respectively, relative to their initial intensities at room temperature. Since the PLEmax exhibited a similar trend of variation to the PLmax, detailed presentation was omitted for brevity.
To further investigate the luminescent behavior of the synthesized Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors, 3D scanning PL spectrum mapping was performed under various excitation wavelengths. The results revealed that, for all x values, the maximum PL intensity was consistently observed at an excitation wavelength of 340 nm, indicating that this is the optimal excitation condition for these materials. The emission stability of the phosphors was also evaluated under ambient laboratory conditions (approximately 25 °C and 50% relative humidity). The phosphors exhibited relatively stable emission behavior, with a decrease of approximately 20% in luminescence intensity after 30 min of continuous exposure. This degradation is mainly attributed to the hygroscopic nature of the material system. In particular, the presence of CaO, which is known to be sensitive to moisture, leads to the absorption of water molecules from the air. The incorporated moisture may promote the formation of surface hydroxyl groups or induce microstructural changes that create non-radiative recombination centers, thereby reducing the photoluminescence efficiency. These observations suggest that while the phosphors show promising emission characteristics, proper surface treatment or encapsulation strategies may be necessary to enhance their stability for practical applications under ambient conditions.
The observed decrease in luminescence efficiency with increasing temperature, often referred to as thermal quenching, has significant implications for the practical application of this material in optoelectronic devices. In real-world conditions, especially in high-power lighting or display technologies, devices can experience elevated operating temperatures. A strong thermal quenching effect may lead to reduced brightness and performance degradation over time. Therefore, understanding the thermal stability of the luminescent material is critical. If the material exhibits considerable efficiency loss at moderately elevated temperatures, it may not be suitable for high-temperature environments without additional thermal management. Conversely, if the efficiency drop is minimal or occurs only at temperatures higher than typical operating conditions, the material could still be viable for practical applications. The results of the PLmax variation with temperature indicate that the Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors possess higher thermal stability compared to phosphors of other compositions [
16].
The absolute photoluminescence quantum yield (PLQY) was employed as a quantitative metric to evaluate the emission efficiency of the synthesized Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors, as referenced in [
17]. To ensure the reliability and reproducibility of the measurements, PLQY values were averaged over eight independent measurements for each
x value, and the associated errors were calculated based on the corresponding standard deviations. The measured PLQY values for
x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 were 0.228 ± 0.020, 0.432 ± 0.025, 0.834 ± 0.030, 0.627 ± 0.028, 0.495 ± 0.027, and 0.547 ± 0.028, respectively. As the Ca content (
x) increased from 0 to 0.4, a significant enhancement in PLQY was observed, peaking at
x = 0.4, where the emission efficiency more than tripled compared to
x = 0. This improvement is likely due to more efficient incorporation of Bi
3+ and Zn
2+ ions into the host lattice, facilitated by optimal local environments and minimized non-radiative defects. Beyond
x = 0.4, however, the PLQY values began to decline, suggesting the onset of concentration quenching or increased formation of non-radiative centers, possibly due to structural distortions or excess Ga
2O
3 phase formation. Interestingly, at
x = 1.0, a slight recovery in PLQY was noted compared to
x = 0.8, which may be attributed to subtle phase evolution or improved crystallinity under Ca-rich conditions, though it still remained below the maximum value observed at
x = 0.4. These results indicate that emission performance is highly sensitive to compositional tuning, with
x = 0.4 representing an optimal balance between structural integration, dopant activation, and suppression of non-radiative pathways.
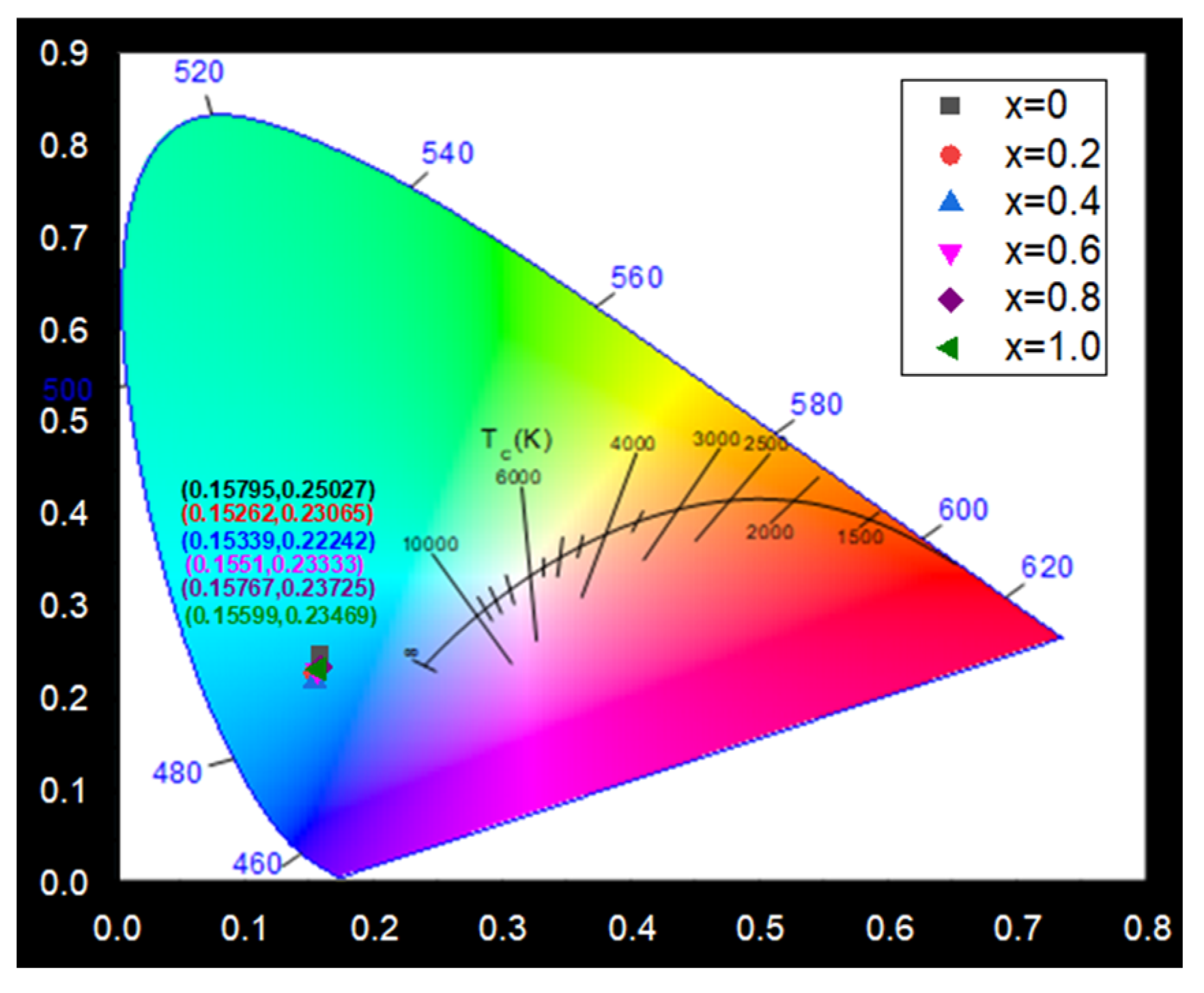
The chromaticity coordinate (CIE) analysis provides valuable insight into the color characteristics of the synthesized phosphors. As illustrated in
Figure 5, the CIE diagram displays the PL spectra of the Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors with varying CaO concentrations. The measured CIE coordinates are listed in
Table 1. Notably, as the CaO content increases from
x = 0 to
x = 1.0, the peak emission (PLmax) wavelength remains nearly unchanged. Despite this stability, all CIE coordinates consistently fall within the blue region of the chromaticity diagram, confirming that the Ca
2+xGa
4O
8+x + 0.01 Bi
2O
3 + 0.07 ZnO phosphors exhibit blue light emission. This stable blue emission is particularly advantageous for applications in display technologies, solid-state lighting, and other optical devices requiring precise color control.
4. Conclusions
In the synthesized Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors, four crystalline phases, CaGa2O4, CaO, Ga2O3, and Ca3Ga4O9, were identified at x = 0. This lower temperature of 1200 °C may not have allowed complete solid-state reactions between CaO and Ga2O3, leading to leftover secondary phases. The presence of Ca3Ga4O9 suggests that the intended single-phase CaGa2O4 did not fully form, leaving some Ga2O3 unreacted. As x increased, the CaO diffraction signal weakened and disappeared by x = 0.4, indicating better incorporation of extra Ca into the structure. The Ga2O3 signal grew stronger, suggesting that more Ga remained outside the lattice even with excess Ca. At x = 1.0, a clear Ga2O3 phase was still present, likely due to complex interactions during synthesis. For our Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors, all PLE and PL spectra exhibited a single excitation peak, and the PLEmax were located at 340, 340, 341, 341, 339, and 341 nm, and the PLmax were located at 477, 474, 473, 477, 476, and 477 nm, respectively, corresponding to varying x values of 0, 0.2, 0.4, 0.6, 0.8, and 1.0. The measured PLQY values for x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0 were 0.228 ± 0.020, 0.432 ± 0.025, 0.834 ± 0.030, 0.627 ± 0.028, 0.495 ± 0.027, and 0.547 ± 0.028, and all of the CIE coordinates consistently confirmed that the Ca2+xGa4O8+x + 0.01 Bi2O3 + 0.07 ZnO phosphors exhibit blue light emission. These results suggest that precise control of the Ca:Ga ratio significantly affects the phase composition, defect formation, and energy transfer efficiency of Bi3+-activated phosphors. The observed non-linear relationship between Ca content and quantum yield highlights the importance of optimizing the host lattice composition for maximum luminescence. This study provides a useful guideline for tuning host structures to enhance the performance of Bi3+-based phosphors, which can be extended to other oxide-based systems in the design of efficient blue-emitting materials for solid-state lighting or display applications.