The Possible Use of Stable Carbon and Nitrogen Isotope Signal and Spectral Analysis to Identify Habitat Condition of Aquatic Plants
Abstract
:1. Introduction
2. Materials and Methods
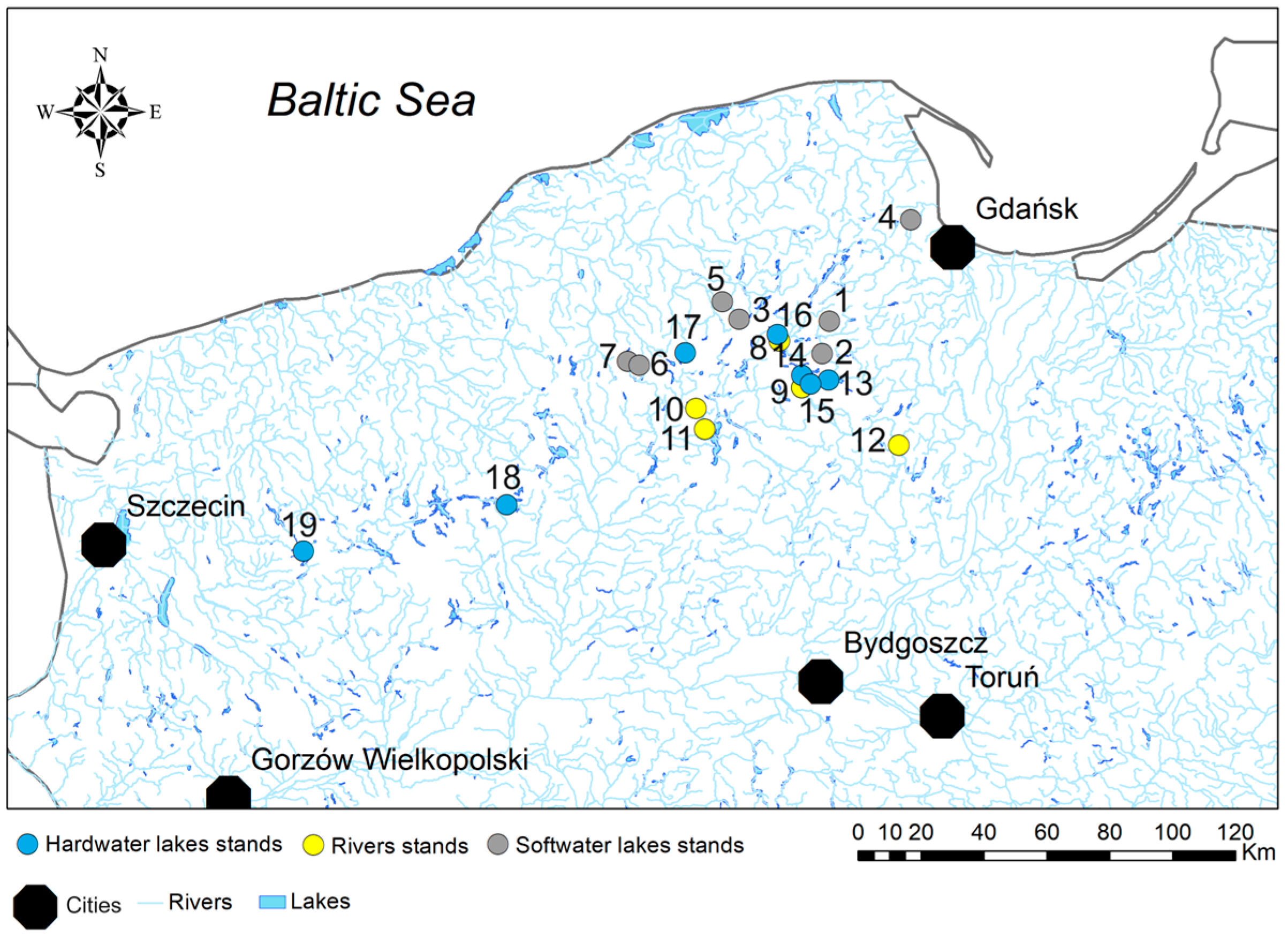
2.1. Study Area
2.2. Isotope Analyses of E. canadensis Plant Material
2.3. Physico-Chemical Analyses of Water from Research Sites
2.4. Statistical Analyses
3. Results
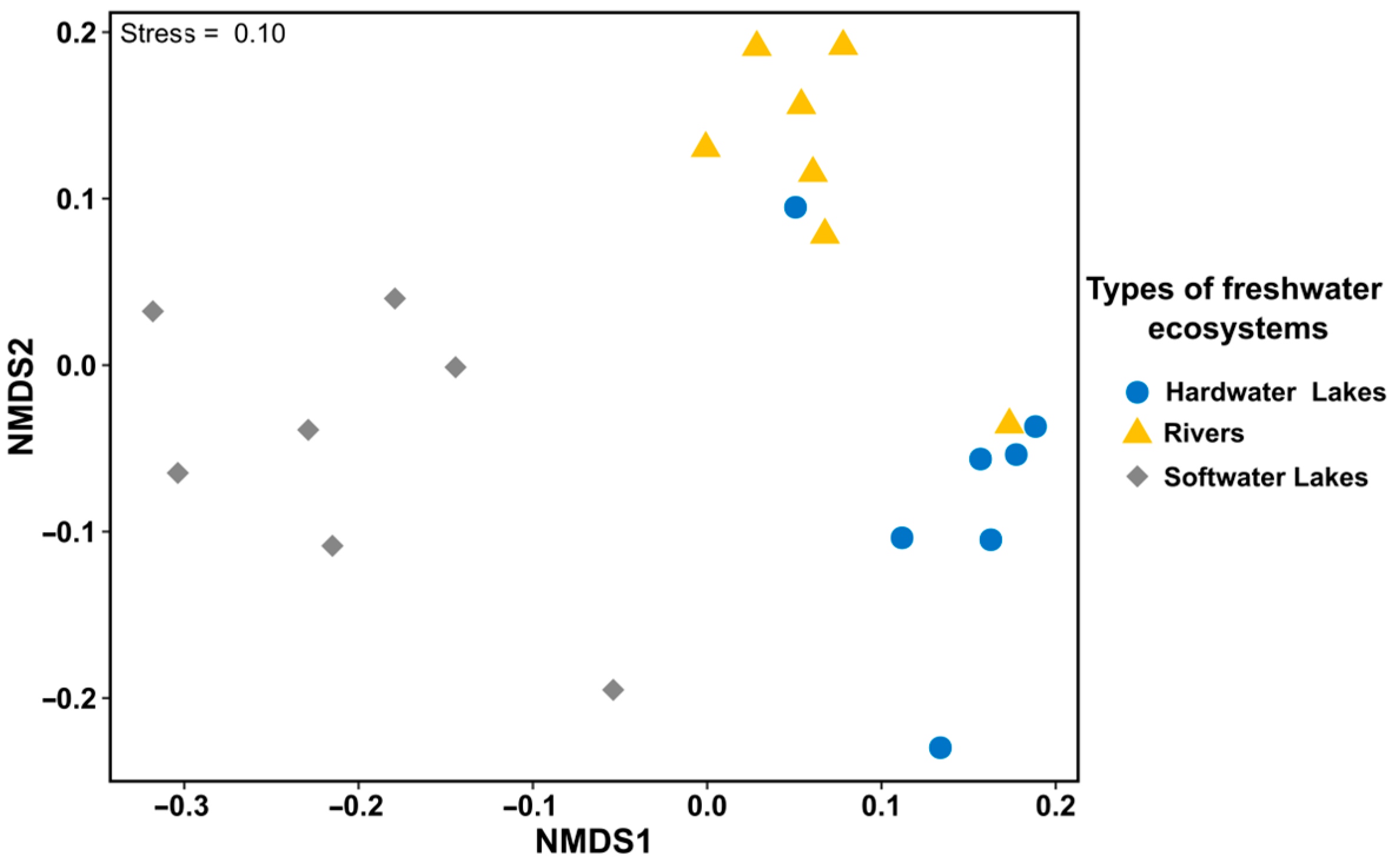
3.1. Diversity of Freshwater Ecosystems Based on the Physical and Chemical Characteristics of Water
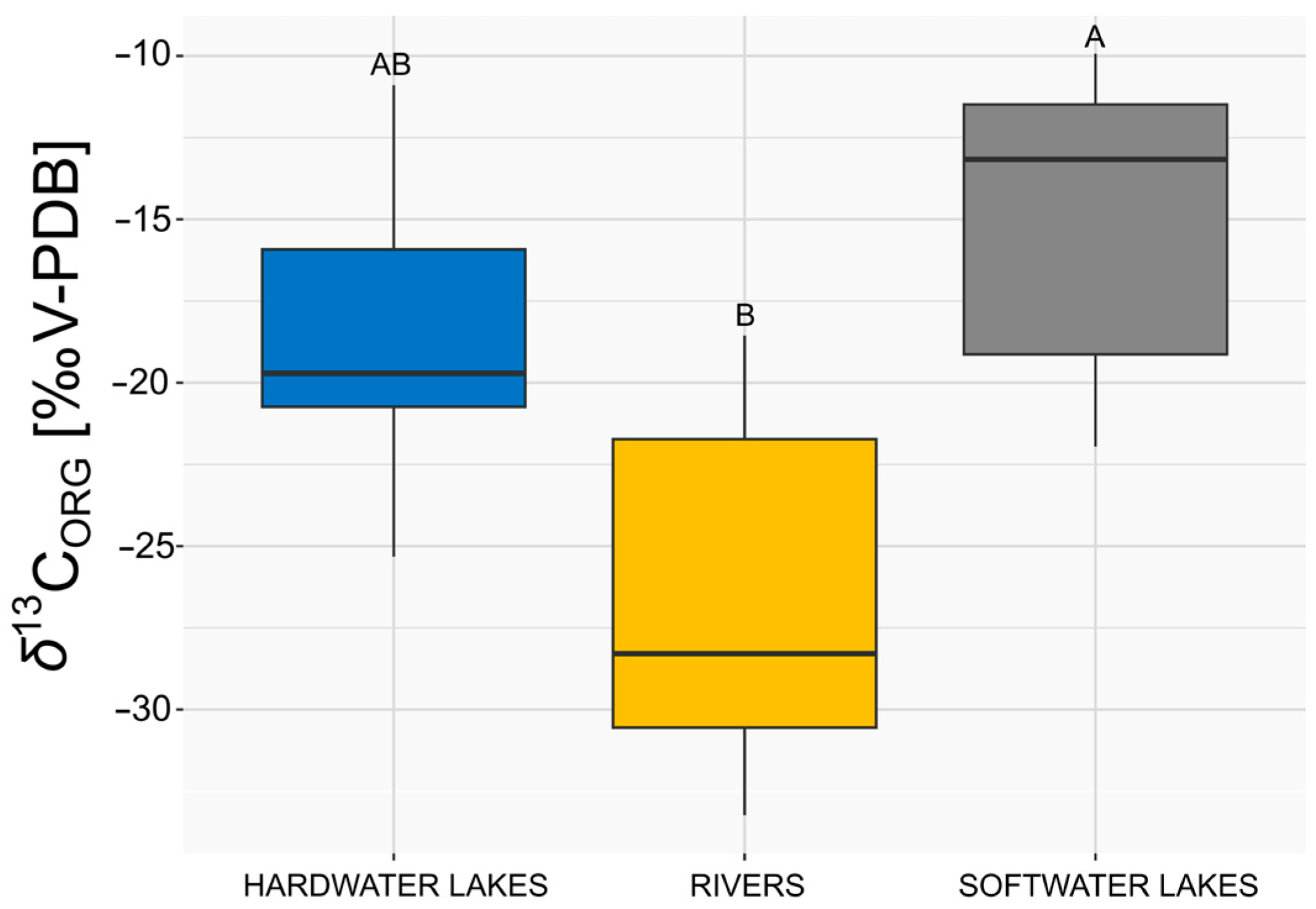
3.2. Comparison of δ13CORG of E. canadensis in Different Freshwater Ecosystems
3.3. Comparison of δ15NORG of E. canadensis in Different Freshwater Ecosystems
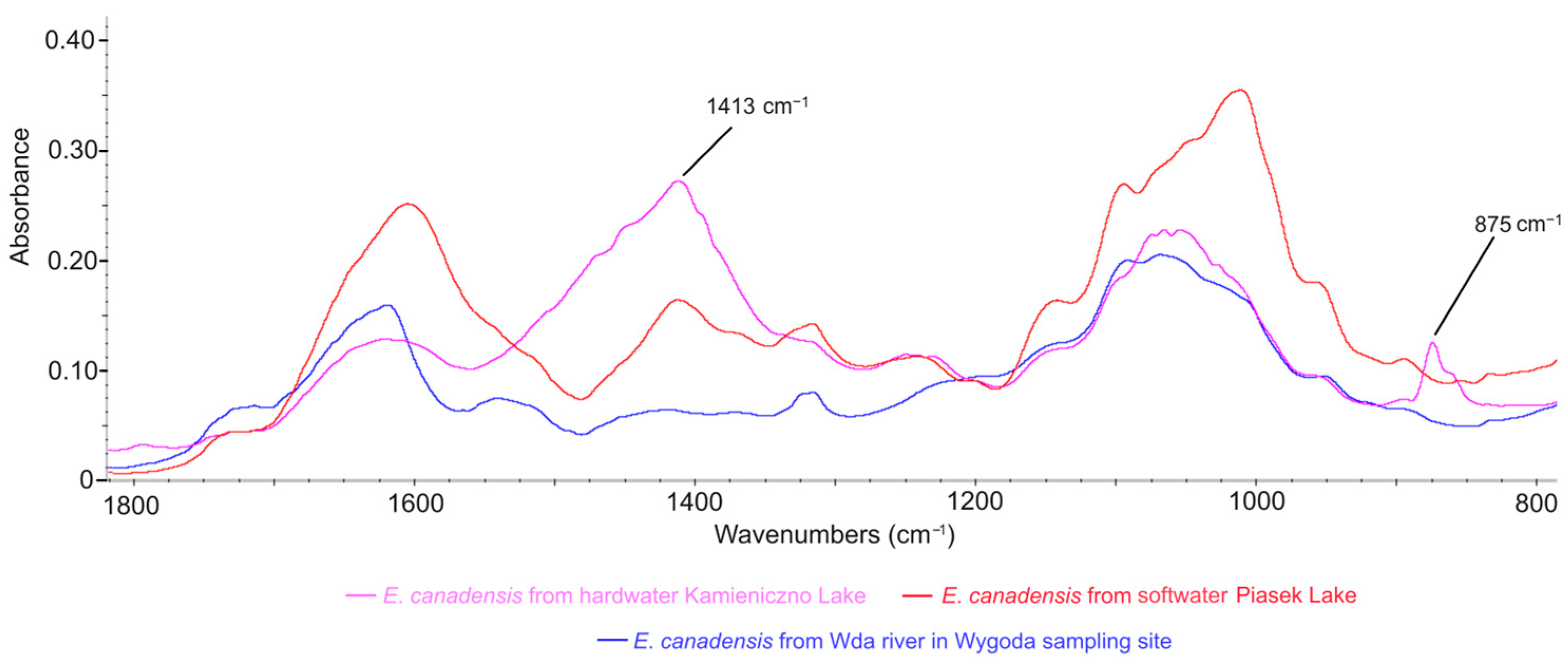
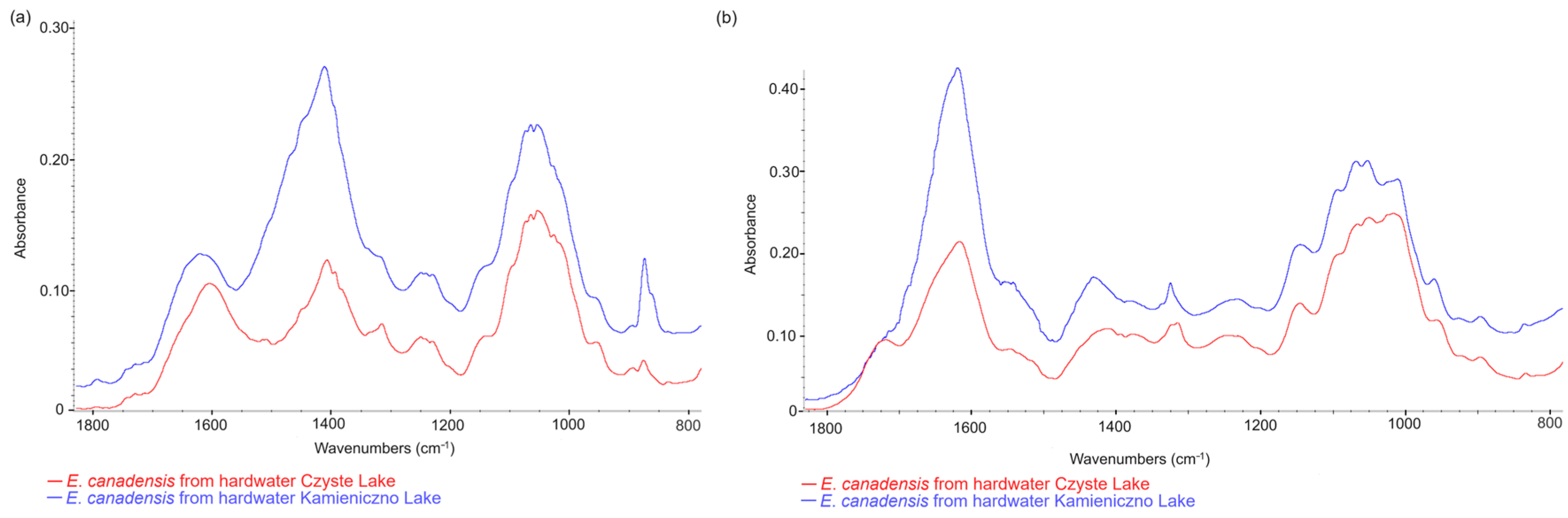
3.4. Comparison of E. canadensis FTIR Spectra in Different Freshwater Ecosystems
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the Curve of Global Freshwater Biodiversity Loss: An Emergency Recovery Plan. Bioscience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, M.; Alahuhta, J.; Heino, J.; Hjort, J.; Toivonen, H. Changes in the Functional Features of Macrophyte Communities and Driving Factors across a 70-Year Period. Hydrobiologia 2020, 847, 3811–3827. [Google Scholar] [CrossRef]
- Li, G.; Hu, S.; Hou, H.; Kimura, S. Heterophylly: Phenotypic Plasticity of Leaf Shape in Aquatic and Amphibious Plants. Plants 2019, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Cronin, G.; Lodge, D.M. Effects of Light and Nutrient Availability on the Growth, Allocation, Carbon/Nitrogen Balance, Phenolic Chemistry, and Resistance to Herbivory of Two Freshwater Macrophytes. Oecologia 2003, 137, 32–41. [Google Scholar] [CrossRef]
- Chmara, R.; Pronin, E.; Szmeja, J. Functional Macrophyte Trait Variation as a Response to the Source of Inorganic Carbon Acquisition. PeerJ 2021, 9, e12584. [Google Scholar] [CrossRef]
- Aiken, S.G. An Experiment Relating Vegetative Morphology of Myriophyllum alterniflorum DC. (Haloragaceae) to Growth Substrate. Aquat. Bot. 1981, 10, 383–388. [Google Scholar] [CrossRef]
- Kaplan, Z. Phenotypic Plasticity In Potamogeton (Potamogetonaceae). Folia Geobot. 2002, 37, 141–170. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2005; ISBN 8322614853. [Google Scholar]
- Kolada, A.; Kutyła, S. Elodea canadensis (Michx.) in Polish Lakes: A Non-Aggressive Addition to Native Flora. Biol. Invasions 2016, 18, 3251–3264. [Google Scholar] [CrossRef]
- Cegłowska, A.; Jusik, S.; Samecka-Cymerman, A.; Klink, A.; Szoszkiewicz, K. Habitat Requirements of Elodea canadensis Michx. in Polish Rivers. Oceanol. Hydrobiol. Stud. 2017, 46, 363–378. [Google Scholar] [CrossRef]
- Culley, T.M. Why Vouchers Matter in Botanical Research. Appl. Plant Sci. 2013, 1, 1300076. [Google Scholar] [CrossRef]
- Guiry, E. Complexities of Stable Carbon and Nitrogen Isotope Biogeochemistry in Ancient Freshwater Ecosystems: Implications for the Study of Past Subsistence and Environmental Change. Front. Ecol. Evol. 2019, 7, 313. [Google Scholar] [CrossRef]
- Pronin, E.; Panettieri, M.; Torn, K.; Rumpel, C. Stable Carbon Isotopic Composition of Dissolved Inorganic Carbon (DIC) as a Driving Factor of Aquatic Plants Organic Matter Build-up Related to Salinity. Ecol. Indic. 2019, 99, 230–239. [Google Scholar] [CrossRef]
- Pronin, E.; Banaś, K.; Chmara, R.; Ronowski, R.; Merdalski, M. Do Stable Carbon and Nitrogen Isotope Values of Nitella flexilis Differ between Softwater and Hardwater Lakes? Aquat. Sci. 2023, 85, 79. [Google Scholar] [CrossRef]
- Apolinarska, K.; Pełechaty, M.; Pronin, E. Discrepancies between the Stable Isotope Compositions of Water, Macrophyte Carbonates and Organics, and Mollusc Shells in the Littoral Zone of a Charophyte-Dominated Lake (Lake Lednica, Poland). Hydrobiologia 2016, 768, 1–17. [Google Scholar] [CrossRef]
- Pronin, E.; Pełechaty, M.; Apolinarska, K.; Pukacz, A.; Frankowski, M. Sharp Differences in the Δ13C Values of Organic Matter and Carbonate Encrustations but Not in Ambient Water DIC between Two Morphologically Distinct Charophytes. Hydrobiologia 2016, 773, 177–191. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Hu, J.; Cao, Y.; Xiao, S.; Liu, W. Systematical δ 13C Investigations of TOC in Aquatic Plants, DIC and Dissolved CO2 in Lake Water from Three Tibetan Plateau Lakes. Ecol. Indic. 2022, 140, 109060. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, L.; Liu, Q.; Yao, S.; Wang, X.; Liu, Y.; Zhang, Y.; Xue, B. Sedimentary Macrophyte δ13Ccellulose Record of Environmental Evolution over the Past Century in East Taihu Lake, China. Ecol. Indic. 2023, 154, 110716. [Google Scholar] [CrossRef]
- Chappuis, E.; Seriñá, V.; Martí, E.; Ballesteros, E.; Gacia, E. Decrypting Stable-Isotope (δ13C and δ15N) Variability in Aquatic Plants. Freshw. Biol. 2017, 62, 1807–1818. [Google Scholar] [CrossRef]
- Campbell, L.M.; Hecky, R.E.; Wandera, S.B. Stable Isotope Analyses of Food Web Structure and Fish Diet in Napoleon and Winam Gulfs, Lake Victoria, East Africa. J. Great Lakes Res. 2003, 29, 243–257. [Google Scholar] [CrossRef]
- Morkūnė, R.; Bučas, M.; Kataržytė, M.; Politi, T.; Vaičiūtė, D.; Vizzini, S.; Martin, G. Food Sources for Benthic Grazers in Trophic Networks of Macrophyte Habitats in a Transitional Baltic Ecosystem. Water 2022, 14, 1565. [Google Scholar] [CrossRef]
- Douglas, P.M.J.; Stratigopoulos, E.; Park, S.; Keenan, B. Spatial Differentiation of Sediment Organic Matter Isotopic Composition and Inferred Sources in a Temperate Forest Lake Catchment. Chem. Geol. 2022, 603, 120887. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H.; Yu, W.; Yin, C.; He, Y.; Zhang, Z.; Xu, D.; Li, Q. Effect of Ecosystem Degradation on the Source of Particulate Organic Matter in a Karst Lake: A Case Study of the Caohai. Water 2022, 14, 1867. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, C.; Wei, R.; Zhu, G.; Cui, M.; Okolic, C.P. Qualitative and Quantitative Analysis of Source for Organic Carbon and Nitrogen in Sediments of Rivers and Lakes Based on Stable Isotopes. Ecotoxicol. Environ. Saf. 2020, 195, 110436. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, H.; Chang, F.; Li, D.; Liu, Q.; Zhang, X.; Liu, F.; Zhang, Y. Isotopic Constraints on Sources of Organic Matter in Surface Sediments from Two North–South Oriented Lakes of the Yunnan Plateau, Southwest China. J. Soils Sediments 2022, 22, 1597–1608. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and Quantification of Valuable Plant Substances by IR and Raman Spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Jebsen, C.; Norici, A.; Wagner, H.; Palmucci, M.; Giordano, M.; Wilhelm, C. FTIR Spectra of Algal Species Can Be Used as Physiological Fingerprints to Assess Their Actual Growth Potential. Physiol. Plant. 2012, 146, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Straková, P.; Larmola, T.; Andrés, J.; Ilola, N.; Launiainen, P.; Edwards, K.; Minkkinen, K.; Laiho, R. Quantification of Plant Root Species Composition in Peatlands Using FTIR Spectroscopy. Front. Plant Sci. 2020, 11, 597. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Kaiser, M. Stability and Composition of Different Soluble Soil Organic Matter Fractions—Evidence from Δ13C and FTIR Signatures. Geoderma 2005, 128, 28–37. [Google Scholar] [CrossRef]
- Castro, K.; Pérez, M.; Rodriguez-Laso, M.D.; Madariaga, J.M. FTIR Spectra Database of Inorganic Art Materials. Anal. Chem. 2003, 75, 214A–221A. [Google Scholar] [CrossRef]
- Kiefer, J.; Strk, A.; Kiefer, A.L.; Glade, H. Infrared Spectroscopic Analysis of the Inorganic Deposits from Water in Domestic and Technical Heat Exchangers. Energies 2018, 11, 798. [Google Scholar] [CrossRef]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Murphy, K.J. Plant Communities and Plant Diversity in Softwater Lakes of Northern Europe. Aquat. Bot. 2002, 73, 287–324. [Google Scholar] [CrossRef]
- Körner, C.; Leuzinger, S.; Riedl, S.; Siegwolf, R.T.; Streule, L. Carbon and Nitrogen Stable Isotope Signals for an Entire Alpine Flora, Based on Herbarium Samples. Alp. Bot. 2016, 126, 153–166. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; de la Barrera, E. Drying Protocol Does Not Alter Plant δ13C and δ15N: A Baseline Survey for Ecological Studies. Isot. Environ. Health Stud. 2019, 55, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Stock, W.D.; Chuba, D.K.; Verboom, G.A. Distribution of South African C3 and C4 Species of Cyperaceae in Relation to Climate and Phylogeny. Austral Ecol. 2004, 29, 313–319. [Google Scholar] [CrossRef]
- Cai, G.-B.; Chen, S.-F.; Liu, L.; Jiang, J.; Yao, H.-B.; Xu, A.-W.; Yu, S.-H. 1,3-Diamino-2-Hydroxypropane-N,N,N′,N′-Tetraacetic Acid Stabilized Amorphous Calcium Carbonate: Nucleation, Transformation and Crystal Growth. CrystEngComm 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Merdalski, M.; Banaś, K.; Ronowski, R. Environmental Factors Affecting Pondweeds in Water Bodies of Northwest Poland. Biodivers. Res. Conserv. 2019, 56, 13–28. [Google Scholar] [CrossRef]
- R Core Team R Core Team. R: A Language and Environment for Statistical Computing; The R Project for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J. Vegan: An Introduction to Ordination. 2017. Available online: https://cran.r-hub.io/web/packages/vegan/vignettes/intro-vegan.pdf (accessed on 20 September 2023).
- Wickham, H. Ggplot2; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Dinno, A. Package ‘Dunn.Test’. CRAN Repos. 2017, pp. 1–7. Available online: https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf (accessed on 20 September 2023).
- Pronin, E.; Wrosz, Z.; Banaś, K.; Merdalski, M. Izotopy Stabilne Azotu i Węgla Zanurzonych Roślin Wodnych Rzek Jako Potencjalny Wskaźnik Dopływu Zanieczyszczeń. In Funkcjonowanie I Ochrona Wód Płynących; Czerniawski, R., Bilski, P., Eds.; University of Szczecin Institute of Biology: Szczecin, Poland, 2023; pp. 153–166. [Google Scholar]
- Schulte, P.; van Geldern, R.; Freitag, H.; Karim, A.; Négrel, P.; Petelet-Giraud, E.; Probst, A.; Probst, J.L.; Telmer, K.; Veizer, J.; et al. Applications of Stable Water and Carbon Isotopes in Watershed Research: Weathering, Carbon Cycling, and Water Balances. Earth-Sci. Rev. 2011, 109, 20–31. [Google Scholar] [CrossRef]
- King, L.; Maberly, S.C.; De Ville, M.M.; Kitschke, M.; Gibson, C.E.; Jones, R.I. Nitrogen Stable Isotope Ratios of Lake Macrophytes in Relation to Growth Form and Nutrient-Limitation. Fundam. Appl. Limnol. 2009, 175, 307–315. [Google Scholar] [CrossRef]
- Osmond, C.B.; Valaane, N.; Haslam, S.M.; Uotila, P.; Roksandic, Z. Comparisons of Δ13C Values in Leaves of Aquatic Macrophytes from Different Habitats in Britain and Finland; Some Implications for Photosynthetic Processes in Aquatic Plants. Oecologia 1981, 50, 117–124. [Google Scholar] [CrossRef]
- Guo, H.R.; Wu, Y.; Hu, C.C.; Liu, X.Y. Elevated Nitrate Preference Over Ammonium in Aquatic Plants by Nitrogen Loadings in a City River. J. Geophys. Res. Biogeosci. 2022, 127, e2021JG006614. [Google Scholar] [CrossRef]
- Raven, J.A. Nutritional Strategies of Submerged Benthic Plants: The Acquisition of C, N and P By Rhizophytes and Haptophytes. New Phytol. 1981, 88, 1–30. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lucassen, E.C.H.E.T.; Roelofs, J.G.M. The Isoetid Environment: Biogeochemistry and Threats. Aquat. Bot. 2002, 73, 325–350. [Google Scholar] [CrossRef]


| Physicochemical Parameters | Kruskal–Wallis Test Results |
|---|---|
| pH | H = 0.013, p = 0.99 |
| conductivity (µS cm−1) | H = 14.200, p = 0.001 |
| DIC (mg C/L) | H = 14.466, p = 0.001 |
| CO2 (mg C/L) | H = 6.2252, p = 0.040 |
| CO2 (CO3−2) (mg C/L) | H = 14.764, p = 0.001 |
| CO2 (HCO3−) (mg C/L) | H = 12.625, p = 0.001 |
| DOC (mg C/L) | H = 6.633, p = 0.040 |
| Ca2+ (mg Ca/L) | H = 14.716, p = 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pronin, E. The Possible Use of Stable Carbon and Nitrogen Isotope Signal and Spectral Analysis to Identify Habitat Condition of Aquatic Plants. Limnol. Rev. 2024, 24, 17-29. https://doi.org/10.3390/limnolrev24010002
Pronin E. The Possible Use of Stable Carbon and Nitrogen Isotope Signal and Spectral Analysis to Identify Habitat Condition of Aquatic Plants. Limnological Review. 2024; 24(1):17-29. https://doi.org/10.3390/limnolrev24010002
Chicago/Turabian StylePronin, Eugeniusz. 2024. "The Possible Use of Stable Carbon and Nitrogen Isotope Signal and Spectral Analysis to Identify Habitat Condition of Aquatic Plants" Limnological Review 24, no. 1: 17-29. https://doi.org/10.3390/limnolrev24010002
APA StylePronin, E. (2024). The Possible Use of Stable Carbon and Nitrogen Isotope Signal and Spectral Analysis to Identify Habitat Condition of Aquatic Plants. Limnological Review, 24(1), 17-29. https://doi.org/10.3390/limnolrev24010002






