Analysis of Organic Sulphur Compounds in Coal Tar by Using Comprehensive Two-Dimensional Gas Chromatography-High Resolution Time-of-Flight Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples, Standard Compounds and Reagents
2.2. Instrumental Conditions
3. Results and Discussion
3.1. Instrumental Aspects and Performance
3.2. Target Analysis
3.3. Quantitative Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Phillips, J.B. Comprehensive two-dimensional gas chromatography using an on-column thermal modulator interface. J. Chromatogr. Sci. 1991, 29, 227–231. [Google Scholar] [CrossRef]
- Frysinger, G.S.; Gaines, R.B. Comprehensive two-dimensional gas chromatography with mass spectrometric detection (GC×GC/MS) applied to the analysis of petroleum. J. High Resol. Chromatogr. 1999, 22, 251–255. [Google Scholar] [CrossRef]
- Phillips, J.B.; Beens, J. Comprehensive two-dimensional gas chromatography: A hyphenated method with strong coupling between the two dimensions. J. Chromatogr. A 1999, 856, 331–347. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Brinkman, U.A.T. Recent developments in the application of comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2008, 1186, 67–108. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, P.Q.; Purcaro, G.; Dugo, P.; Mondello, L. Modulators for comprehensive two-dimensional gas chromatography. Trac-Trends Anal. Chem. 2011, 30, 1437–1446. [Google Scholar] [CrossRef]
- Seeley, J.V.; Seeley, S.K. Multidimensional gas chromatography: Fundamental advances and new applications. Anal. Chem. 2013, 85, 557–578. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, P.Q.; Aloisi, I.; Giocastro, B.; Mondello, L. Current state of comprehensive two-dimensional gas chromatography-mass spectrometry with focus on processes of ionization. Trac-Trends Anal. Chem. 2018, 105, 360–366. [Google Scholar] [CrossRef]
- Amaral, M.S.S.; Nolvachai, Y.; Marriott, P.J. Comprehensive two-dimensional gas chromatography advances in technology and applications—biennial update. Anal. Chem. 2020, 92, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Suzuki, K. Resources, properties and utilization of tar. Resour. Conserv. Recy. 2010, 54, 905–915. [Google Scholar] [CrossRef]
- Borah, D.; Baruah, M.K.; Haque, I. Oxidation of high sulphur coal. Part 2. Desulphurisation of organic sulphur by hydrogen peroxide in presence of metal ions. Fuel 2001, 80, 1475–1488. [Google Scholar] [CrossRef]
- Machado, M.E.; Caramão, E.B.; Zini, C.A. Investigation of sulphur compounds in coal tar using monodimensional and comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2011, 1218, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, M.; Tranchida, P.Q.; Mondello, L. On-line combination of high performance liquid chromatography with comprehensive two-dimensional gas chromatography-triple quadrupole mass spectrometry: A proof of principle study. Anal. Chem. 2015, 87, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Japes, A.; Penassa, M.; Andersson, J.T. Analysis of recalcitrant hexahydrodibenzothiophenes in petroleum products using a simple fractionation process. Energy Fuels 2009, 23, 2143–2148. [Google Scholar] [CrossRef]
- Nylén, U.; Delgado, J.F.; Järås, S.; Boutonnet, M. Characterization of alkylated aromatic sulphur compounds in light cycle oil from hydrotreated vacuum gas oil using GC-SCD. Fuel Process. Technol. 2004, 86, 223–234. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Vreuls, R.J.J.; Brinkman, U.A.T. Recent developments in comprehensive two-dimensional gas chromatography (GC×GC) III. Applications for petrochemicals and organohalogens. Trac-Trends Anal. Chem. 2006, 25, 726–741. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Salivo, S.; Franchina, F.A.; Mondello, L. Flow-modulated comprehensive two-dimensional gas chromatography combined with a high resolution time-of-flight mass spectrometry: A proof-of-principle study. Anal. Chem. 2015, 87, 2925–2930. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, M.M.; Beens, J.; Janssen, H.G.; Lecrercq, P.A.; Cramers, C.A. Evaluation of time-of-flight mass spectrometric detection for fast gas chromatography. J. Chromatogr. A 2000, 878, 205–213. [Google Scholar] [CrossRef]
- Byer, J.D.; Siek, K.; Jobst, K. Distinguishing the C3 vs CH4 mass split by comprehensive two-dimensional gas chromatography-high resolution time-of-flight mass spectrometry. Anal. Chem. 2016, 88, 6101–6104. [Google Scholar] [CrossRef] [PubMed]
- Dallüge, J.; Roose, P.; Brinkman, U.A.T. Evaluation of a high-resolution time-of-flight mass spectrometer for the gas chromatographic determination of selected environmental contaminants. J. Chromatogr. A 2002, 970, 213–223. [Google Scholar] [CrossRef]
- LECO Corporation. Mass Spectrometry Solution from LECO; LECO Corporation: St. Joseph, MI, USA, 2013; Available online: http://cires1.colorado.edu/jimenez/CHEM-5181/Lect/2013_GCxGC_Handouts.pdf (accessed on 10 April 2020).
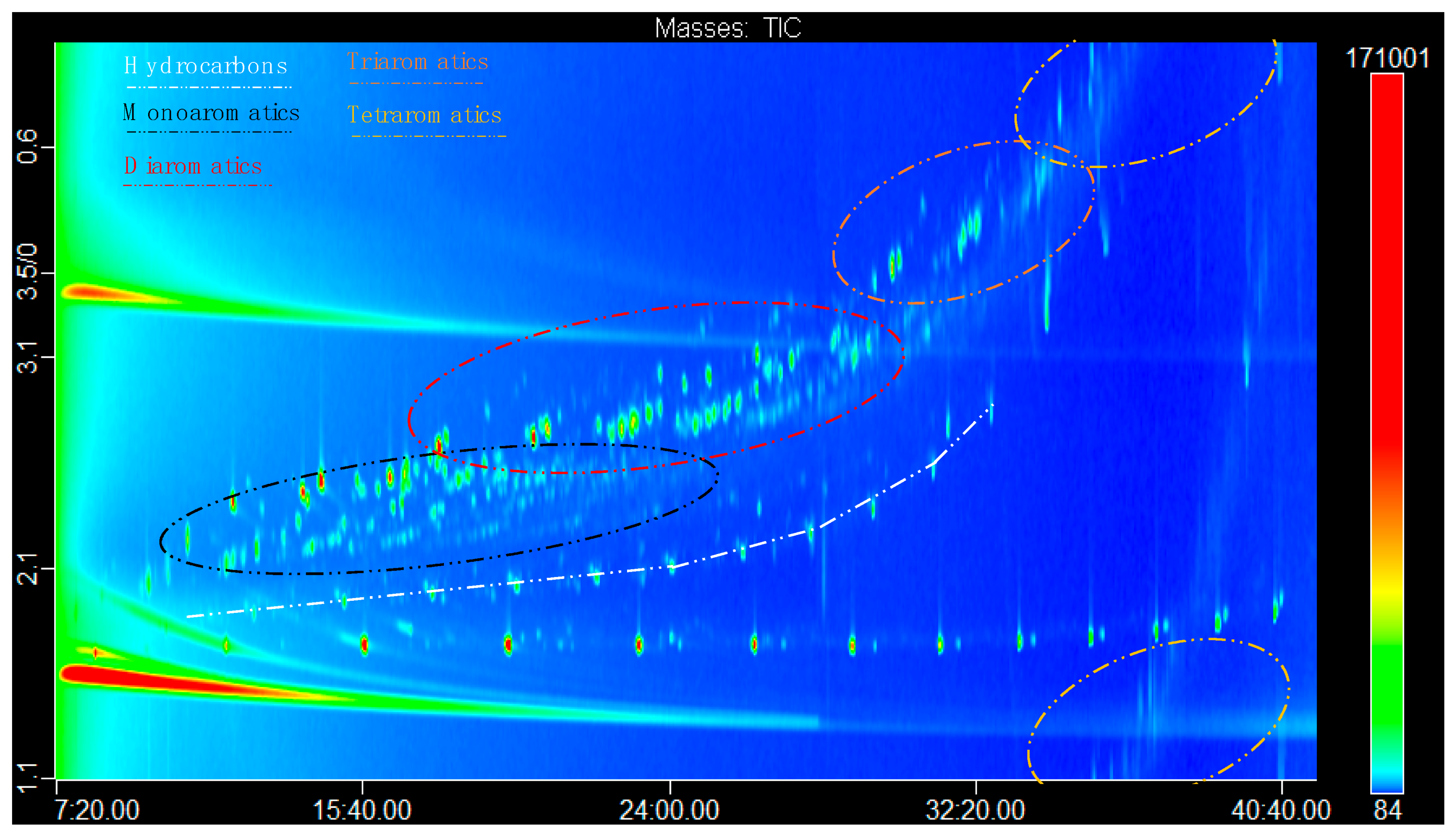
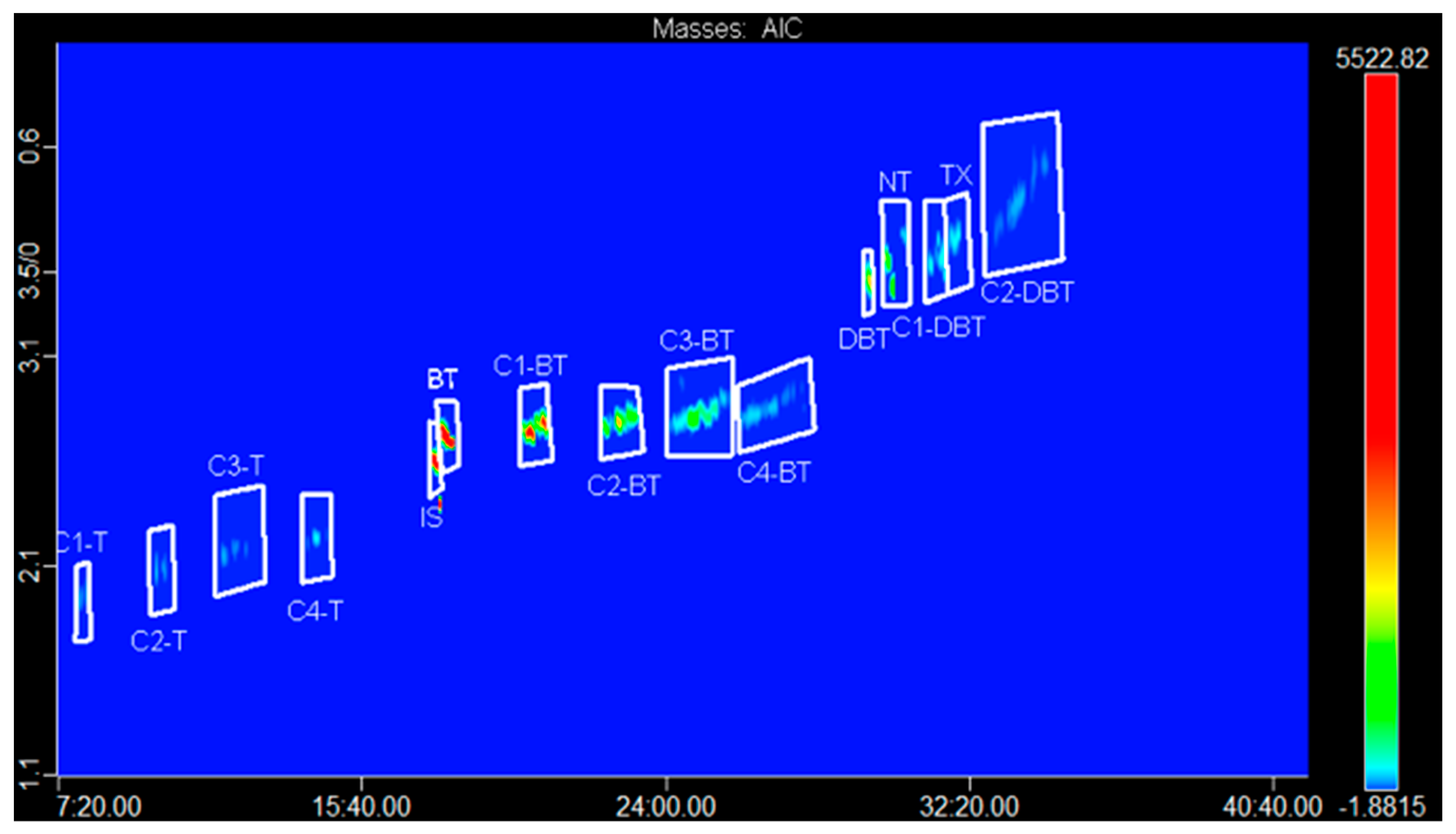

| Compound | 1D tR (min) | 2D tR (sec) | Formula | Theor. [M]+• | Average Error (ppm) | Match |
|---|---|---|---|---|---|---|
| 3-Methylthiophene | 8.0 | 2.0 | C5H6S | 98.018473 | 1.2 | 954 |
| 2,5-Dimethylthiophene | 9.7 | 2.1 | C6H8S | 112.034123 | 0.4 | 901 |
| 2-Propylthiophene | 11.5 | 2.1 | C7H10S | 126.049773 | 0.5 | 906 |
| 3-Butylthiophene | 14.4 | 2.3 | C8H12S | 140.065423 | 0.6 | 935 |
| 1-Fluoronaphthalene (IS) | 17.4 | 2.6 | C10H7F | 146.052630 | 1.0 | 942 |
| Benzo[b]thiophene | 17.6 | 2.7 | C8H6S | 134.018473 | 0.5 | 937 |
| 2-Methylbenzo[b]thiophene | 20.2 | 2.7 | C9H8S | 148.034123 | 2.7 | 928 |
| Dibenzothiophene | 29.3 | 3.5 | C12H8S | 184.034123 | 3.7 | 917 |
| 4,6-Dimethyldibenzothiophene | 32.5 | 3.7 | C14H12S | 212.065423 | 4.6 | 954 |
| Class | 1D tR (min) | 2D tR (sec) | TAF Ion | Coeluting Class and Base Peak |
|---|---|---|---|---|
| C1-Thiophene | 8.0 | 2.0 | 97.010648 | |
| C1-T | 8.1 | 2.0 | ||
| C2-Thiophene | 10.0 | 2.1 | 111.026298 | |
| C2-T | 10.3 | 2.1 | ||
| C3-Thiophene | 11.8 | 2.2 | 97.010648 | 105.069877 (C3-B) |
| C3-T | 11.9 | 2.1 | ||
| C3-T * | 12.2 | 2.2 | ||
| C3-T | 12.5 | 2.2 | ||
| C4-Thiophene | 14.2 | 2.2 | 97.010648 | 105.069877 (C4-B) |
| C4-T | 14.4 | 2.2 | ||
| C4-T | 14.7 | 2.3 | ||
| Benzo[b]thiophene | 17.9 | 2.7 | 134.018473 | |
| C1-Benzothiophene | 20.1 | 2.7 | 147.026298 | 142.077702 (C1-N) |
| C1-BT * | 20.3 | 2.7 | ||
| C1-BT | 20.5 | 2.8 | ||
| C1-BT * | 20.6 | 2.8 | ||
| C2-Benzothiophene | 22.3 | 2.8 | 161.051948 | 156.093352 (C2-N) |
| C2-BT * | 22.4 | 2.8 | ||
| C2-BT * | 22.7 | 2.8 | ||
| C2-BT * | 22.8 | 2.8 | ||
| C2-BT * | 23.0 | 2.8 | ||
| C3-Benzothiophene | 24.1 | 2.8 | 161.051948 | 155.085527 (C3-N) |
| C3-BT | 24.3 | 2.8 | ||
| C3-BT | 24.4 | 3.0 | ||
| C3-BT | 24.5 | 2.8 | ||
| C3-BT * | 24.7 | 2.8 | ||
| C3-BT | 24.8 | 2.8 | ||
| C3-BT * | 25.0 | 2.8 | ||
| C3-BT | 25.1 | 2.8 | ||
| C3-BT * | 25.2 | 2.9 | ||
| C3-BT | 25.3 | 2.9 | ||
| C3-BT | 25.5 | 2.8 | ||
| C3-BT * | 25.6 | 2.9 | ||
| C3-BT | 25.7 | 2.9 | ||
| C4-Benzothiophene | 26.1 | 2.8 | 175.057598 | |
| C4-BT | 26.2 | 2.8 | ||
| C4-BT | 26.5 | 2.8 | ||
| C4-BT | 26.6 | 2.8 | ||
| C4-BT | 26.7 | 2.8 | ||
| C4-BT | 27.0 | 2.9 | ||
| C4-BT | 27.3 | 2.9 | ||
| C4-BT | 27.5 | 2.9 | ||
| C4-BT | 27.7 | 3.0 | ||
| Dibenzothiophene | 29.6 | 3.5 | 184.034123 | |
| Naphthothiophene * | 30.1 | 3.6 | 184.034123 | 178.077702 (P) |
| NT | 30.6 | 3.7 | ||
| C1-Dibenzothiophene | 31.3 | 3.5 | 198.049773 | |
| C1-DBT | 31.4 | 3.6 | ||
| C1-DBT | 31.6 | 3.6 | ||
| C1-DBT | 31.8 | 3.6 | ||
| Thioxanthene * | 31.9 | 3.7 | 198.049773 | 192.093352 (C1-A) |
| C2-Dibenzothiophene | 32.8 | 3.7 | 212.065423 | |
| C2-DBT | 33.1 | 3.7 | ||
| C2-DBT | 33.5 | 3.8 | ||
| C2-DBT | 33.7 | 3.9 | ||
| C2-DBT | 34.1 | 3.9 | ||
| C2-DBT | 34.1 | 4.0 | ||
| C2-DBT | 34.2 | 4.0 | ||
| C2-DBT | 34.3 | 4.0 | ||
| C2-DBT | 34.4 | 4.0 |
| Compound | Quantifier | Average Error (ppm) | Range (ppb) | R2 | Coal Tar (mg L−1) | LoD (mg L−1) | LoQ (mg L−1) | RSD (%) |
|---|---|---|---|---|---|---|---|---|
| 3-Methylthiophene | 97.010648 | 2.5 | 5.0–500 | 0.98941 | >LoD | 30.0 | 110.0 | 2 |
| 2,5-Dimethylthiophene | 111.026298 | 2.5 | 5.8–580 | 0.98908 | 87.6 | 5.0 | 17.0 | 4 |
| 2-Propylthiophene | 97.010648 | 3.4 | 5.2–520 | 0.98819 | >LoD | 7.0 | 25.0 | 2 |
| 3-Butylthiophene | 97.010648 | 2.8 | 5.2–525 | 0.98876 | >LoD | 7.0 | 23.0 | 2 |
| 1-Fluoronaphthalene (IS) | 146.052630 | 1.0 | 2 | |||||
| Benzo[b]thiophene | 134.018473 | 2.0 | 5.5–555 | 0.97414 | 1516.8 | 6.0 | 20.0 | 1 |
| 2-Methylbenzo[b]thiophene | 147.026298 | 1.7 | 5.0–500 | 0.98723 | 767.4 | 10.0 | 35.0 | 1 |
| Dibenzothiophene | 184.034123 | 2.6 | 5.1–510 | 0.98630 | 599.7 | 9.0 | 30.0 | 2 |
| 4,6-Dimethyldibenzothiophene | 212.065423 | 3.4 | 5.1–515 | 0.98882 | >LoD | 8.0 | 28.0 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloisi, I.; Zoccali, M.; Tranchida, P.Q.; Mondello, L. Analysis of Organic Sulphur Compounds in Coal Tar by Using Comprehensive Two-Dimensional Gas Chromatography-High Resolution Time-of-Flight Mass Spectrometry. Separations 2020, 7, 26. https://doi.org/10.3390/separations7020026
Aloisi I, Zoccali M, Tranchida PQ, Mondello L. Analysis of Organic Sulphur Compounds in Coal Tar by Using Comprehensive Two-Dimensional Gas Chromatography-High Resolution Time-of-Flight Mass Spectrometry. Separations. 2020; 7(2):26. https://doi.org/10.3390/separations7020026
Chicago/Turabian StyleAloisi, Ivan, Mariosimone Zoccali, Peter Q. Tranchida, and Luigi Mondello. 2020. "Analysis of Organic Sulphur Compounds in Coal Tar by Using Comprehensive Two-Dimensional Gas Chromatography-High Resolution Time-of-Flight Mass Spectrometry" Separations 7, no. 2: 26. https://doi.org/10.3390/separations7020026
APA StyleAloisi, I., Zoccali, M., Tranchida, P. Q., & Mondello, L. (2020). Analysis of Organic Sulphur Compounds in Coal Tar by Using Comprehensive Two-Dimensional Gas Chromatography-High Resolution Time-of-Flight Mass Spectrometry. Separations, 7(2), 26. https://doi.org/10.3390/separations7020026







