Abstract
Molecular spectroscopic detection techniques, such as Fourier transform infrared spectroscopy (FTIR), provides additional specificity for isomers where often mass spectrometry (MS) fails, due to similar fragmentation patterns. A hyphenated system of gas chromatography (GC) with FTIR via a light-pipe interface is reported in this study to explore a number of GC–FTIR analytical capabilities. Various compound classes were analyzed—aromatics, essential oils and oximes. Variation in chromatographic peak parameters due to the light-pipe was observed via sequentially-located flame ionization detection data. Unique FTIR spectra were observed for separated mixtures of essential oil isomers having similar mass spectra. Presentation of GC×FTIR allows a ‘comprehensive’-style experiment to be developed. This was used to obtain spectroscopic/separation profiles for interconverting oxime species with their individual spectra in the overlap region being displayed on a color contour plot. Partial least square regression provides multivariate quantitative analysis of co-eluting cresol isomers derived from GC–FTIR data. The model resulted in an R2 of 0.99. Prediction was obtained with R2 prediction value of 0.88 and RMSEP of 0.57, confirming the method’s suitability. This study explores the potential of GC–FTIR hyphenation and re-iterates its value to derive unambiguous and detailed molecular information which is complementary to MS.
1. Introduction
The separation and precise identification of components in complex samples is a desirable goal of many analytical chemists. Capillary gas chromatography (GC) supports this goal through high separation efficiency and is capable of both quantitative and qualitative analysis of volatile and semivolatile compounds. Confirmation of identity of GC-separated components rely largely upon the use of suitable detection technologies, although retention time invariance compared with authentic standards and use of retention indices also support identification. Amongst various detection technologies used are a range of ionization-based detectors [1], of which the flame ionisation detector (FID) is the most popular (but does not, per se provide identification) and spectroscopic technique-based detectors. The latter includes infrared (IR), nuclear magnetic resonance (NMR; off-line), flame photometry, chemiluminescence and the recent commercially-developed vacuum ultraviolet (VUV) detector [2]. The online combination or hyphenation, of suitable detectors to GC has over the years brought new dimensions of identification to separation science [3].
The hyphenation of mass spectrometry (MS) to GC (GC–MS), has superseded many other detection techniques, due to its use of the highly ‘informing’ MS detector [4]. MS detection is based on the mass-to-charge ratio (m/z) of molecular and fragment ions of compounds, which can operate as a universal (in its total ion mode) or selective (in its single-ion mode) detector. This makes the MS a powerful tool with excellent selectivity and sensitivity, supported by the widespread availability of MS spectrum reference libraries [5]. These characteristics, however, conceal some erroneous measurements and interpretations arising from MS detection [6]. One main shortfall of MS is poor differentiation of molecules with similar fragmentation patterns [7], the reduced ability of MS to distinguish between, for example, diastereomers and positional isomers and information on functional groups and their position(s) within a molecule. This might be expressed as a need for caution of over-reliance on MS for molecular identification. These limitations suggest other detection techniques, such as spectroscopic tools, may play a support role for correct interpretation of compound identity. This has been re-confirmed recently by various studies conducted using GC–vacuum ultraviolet systems on a range of samples which have exemplified its capabilities to boost confidence in results [4], especially in instances where GC–MS is challenged by isomer differentiation. A recent review article amply demonstrates the complementary nature of molecular spectroscopic detection techniques to MS detection in GC to yield information-rich results [2].
Hyphenation of Fourier transform infrared (FTIR) spectroscopic detection to GC (GC–FTIR), provides unique spectra of eluted GC compounds based on the molecule’s various rotational and vibrational energy states [8]. These molecular absorbances have distinct IR frequencies, so GC–FTIR instruments impart information related to the functional groups and arrangement of atoms in molecules. Thus, FTIR detection boasts a molecular ‘fingerprinting’ ability, not available to MS, despite the relatively lower sensitivity of FTIR compared to MS. This places FTIR at an advantage in structural elucidation studies. FTIR detection is also non-destructive and universal, can be operated in series with other detectors and can be used for both selective and specific detection [9,10]. Its obedience to the Beer-Lambert law allows for quantitative analysis of data.
GC–FTIR is commonly implemented via one of three interface types. Two types – matrix-isolation and direct-deposition interfaces – use a trapping medium to concentrate and immobilise the GC effluent. The condensed phase GC–FTIR system has gained popularity in recent years for structural elucidation studies [11], with reportedly improved detection limits. The third, a light-pipe (LP) interface, offers on-line real-time detection. The characteristic IR absorption of GC eluted analytes is obtained by separation of sample components, with ideally sufficient resolution to obtain single compounds in the LP. Here, the GC column effluent passes into a heated gold-coated borosilicate glass flow cell into which the FTIR instrument’s light passes through KBr windows at either end of the cell, to provide complete vapour phase IR spectra.
IR spectroscopy can distinguish functional groups but may fail to adequately differentiate homologous series; MS may distinguish homologues and molecular masses, yet may be unable to adequately differentiate isomers. Use of MS with FTIR, with complementary detection capabilities, may be used simultaneously or sequentially for significantly improved identification of components in GC effluents. FTIR’s competence in hyphenation with other detectors in GC [3], allows integration of MS and FTIR for assignment of analyte structures and benefits complex mixture analysis [12]. These techniques can be applied by use of separate GC–MS and GC–FTIR instruments or as a hyphenated GC–FTIR/MS system [13,14,15,16]. However, GC–FTIR has not attained the same level of adoption as GC–MS due to performance criteria which include; (1) lower sensitivity compared to MS, (2) inferior dynamic detection range compared to MS, (3) relatively difficult quantification and (4) extensive MS databases available for component identification [17]. Limited access to libraries may also reduce GC–FTIR’s attraction. Yet the value of complementarity of GC–MS and GC–FTIR data must be emphasised; comprehensive identification applications include pharmaceuticals, petroleum hydrocarbons, fatty acids, essential oils, Li-ion battery degradation, polymers and plastics [2].
The value of GC–FTIR as a useful technique was further validated in recent studies that investigated elution profiles of individual isomers of oximes that undergo characteristic on-column interconversion in GC (dynamic GC). Unambiguous identification and elution order of E and Z isomers were obtained [18].
In this study, an integrated light-pipe GC–FTIR for real-time spectroscopic detection of compounds, together with GC–FID and GC–MS capability for comparative interpretation, was developed. A variety of analyses were conducted, in general to establish qualitative and quantitative relationships of retention, concentration and response using the FTIR detector. The ability of GC–FTIR to differentiate chemical structures for resolved compounds was contrasted with GC‒MS data and was also extended to unresolved compounds. The extra peak dispersion of the chromatographic peak arising from the LP interface was estimated by using the response of sequential FID detection. The system was tested for identification, quantification and peak deconvolution by using FTIR of different compounds and classes, such as aromatic essential oils and positional isomers, as detailed in the materials and methods section. Novel applicability of GC–FTIR for two-dimensional separation approaches presented as GC×FTIR retention/response data allowed clear display of the overlapping molecular interconversion isomers of oximes. Finally, multivariate analysis of overlapping m- and p-cresol isomer analysis permitted reliable identification and quantification of m-cresol.
2. Materials and Methods
2.1. Instrumentation
A PerkinElmer Clarus 680 GC with a flame ionisation detector (FID) hyphenated to a SQ 8T mass spectrometer (PerkinElmer Inc., Shelton, CT, USA) controlled by a PC (PC–1) was used for chromatographic analysis. For FTIR, a PIKE interface accessory (PIKE Technologies, Madison, WI, USA) consisting of a temperature (T) controllable gold coated 120 mm path-length × 1 mm I.D. heated gas cell (light-pipe; LP) and 13 mm diameter × 2 mm thick KBr windows at either end, with a narrow band mercury-cadmium-telluride (MCT) detector cooled to –196 °C by liquid N2, was used. The PIKE accessory was located a few cm from the right hand side panel of the Clarus 680 GC, which was customised by drilling of an opening into the side of the GC shroud and oven and installation of a short length of stainless-steel transfer sleeve (tubing) of 300 mm length × 1.59 mm I.D. through which the transfer column passes. The insulated transfer line (TL) is ca. 5.7 cm cross-section, with a controllable T heating element. The accuracy of T control was ±1 °C for the TL and LP. A PerkinElmer Frontier SP8000 Fourier transform IR (PerkinElmer Inc., Bucks, UK) with a mid-infrared source, connected to the LP interface, was used as the IR source. FTIR data acquisition was controlled by a separate PC (PC–2) which was connected to a trigger mechanism for timing purposes initiated by the GC injection.
2.2. Standards and Sample Preparation
Standards of ortho-, meta- and para-cresol (o-, m-, p-; >99.9% purity) and ethylbenzene (>99.9% purity) were purchased from Tokyo Chemicals Industry Co., Ltd., (Tokyo, Japan). Nonane, acetaldehyde oxime (acetaldoxime; 99%) and propionaldehyde oxime (≥96%) were purchased from Sigma-Aldrich Co., (St. Louis, MO, USA). Essential oil standards (~95%) of α-pinene, β-pinene, γ-terpinene, limonene, para-cymene (p-cymene), geraniol and eugenol were gratefully provided by Australian Botanical Products Pty. Ltd. (Hallam, Australia). Solvents used were dichloromethane (DCM) (≥99.8% purity), n-hexane (≥98.0% purity) and acetone (99.8% purity) purchased from Merck KGaA, Darmstadt, Germany and methanol (99.9% purity) purchased from Scharlab S.L., Spain. Stock solutions were prepared from the above standards and further diluted to obtain working solutions. n-hexane was used for ethylbenzene and essential oils and DCM was used for cresols, nonane and oximes. For oxime analysis a 20% (v/v) sample of acetaldehyde oxime and propionaldehyde oxime was prepared in acetone with 250 µL of 20% (v/v) butan-1-ol (99.5%, Merck, Kilsyth, Australia) as an internal standard.
2.3. Conditions
A PerkinElmer Elite-5MS column (30 m × 0.25 mm I.D. × 0.25 µm df; Shelton, CT, USA) connected injection port A to the MS. An SGE BPX5 column (30 m × 0.25 mm I.D. × 0.25 µm df; Ringwood, Australia) connected injection port B, with the column outlet joined to a deactivated fused silica (DFS) transfer line column (1 m × 0.25 mm I.D., Agilent Technologies, Mulgrave, Australia) using a universal Press-Tight connector (Restek, Bellefonte, PA, USA), which in turn was connected to the LP inlet. Another DFS transfer line column (1 m × 0.25 mm I.D., Agilent Technologies, Mulgrave, Australia) connected the LP outlet to the FID. Both inlet and outlet transfer line columns were housed within the insulated TL. A separate configuration connected the analytical column outlet to the FID using a DFS transfer line ensuring the total column length of both configurations remained the same. For oxime analysis a HP-INNOWax (30 m × 0.32 mm I.D. × 0.5 µm df: Agilent Technologies) column consisting of a polyethylene glycol (PEG) stationary phase was connected to injection port B, through the LP as above.
Experiments were carried out using the following operational conditions (unless stated otherwise). Injection volumes were 1 µL for essential oils and 1.5 µL for cresol isomer comparison, signal-to-noise ratio (SNR) observations and ethylbenzene calibration. Injections (2 µL) were made for GC×FTIR analysis and cresol calibration. Injections were made at 250 °C with split ratios of 2:1 for essential oil analysis, 20:1 for SNR observations and 10:1 for all other GC analyses. The carrier gas was He (99.999%) in constant flow mode with 2.0 mL min−1 column flow rate for essential oil analysis, 3.0 mL min−1 for oxime analysis and 1.0 mL min−1 for all other analyses. T programs for the oven had an initial T of 100 °C for essential oil analyses and 50 °C for all other analyses, with T ramps of 5 °C min−1 or 10 °C min−1 as specified in each section. Oximes were analysed at an isothermal temperature of 110 °C. MS parameters were electron ionisation (EI) mode with 70 eV ionisation energy, 200 °C source T and 20–500 u mass range.
The TL and LP Ts were thermostated between 100 °C and 300 °C (to prevent condensation of semi-volatile compounds within the LP flow cell interface) whilst ensuring these Ts were maintained at least 10 °C more than the maximum T of the GC oven program. Real time spectra were recorded in the wavelength range of 4000–700 cm−1 with weak apodization and 100 background scans. Optical resolutions of either 4 or 8 cm−1 were used. Scan speed was 1 cm s−1, which corresponds to data acquisition rates of 0.92 spectra s−1 (0.92 Hz) at 4 cm−1 and 1.68 spectra s−1 (1.68 Hz) at 8 cm−1.
2.4. Software
PerkinElmer TurboMass v6.1.0.1963 (PerkinElmer, Inc., Shelton, CT, USA) on PC–1 was used for GC and MS data. PerkinElmer Timebase v6.1.0.1963 (PerkinElmer, Inc., Bucks, UK) was used for FTIR data acquisition and PerkinElmer Spectrum v10.4.2.27 (PerkinElmer, Inc., Bucks, UK) for FTIR spectrum processing, both using PC–2. MS and FTIR library searching was conducted using National Institute of Standards and Technology (NIST) 2011, the Fluka IR library (library supplied by PerkinElmer Inc.) and the IR vapour phase library by Nicolet Corp. Matlab R2019a v9.6.0.1072779 (The Mathworks, Inc., Natick, MA, USA) and Partial Least Square Toolbox (Eigenvector Research Inc., Wenatchee, WA, USA) were used for chemometric data processing.
3. Results and discussion
3.1. Chromatographic Peak Variations
The instrument used in this study couples the GC and FTIR instruments via a light-pipe interface where IR radiation from the source is focussed onto the LP/carrier stream and the emerging beam is refocused onto the detector. Supplementary information Figure S1 shows a schematic of the GC/PIKE interface/FTIR source instrumental setup and an enlarged view of the effluent flow path and IR beam path within the LP interface. Being non-destructive, after acquisition of FTIR data, carrier flow can be directed to other detectors such as FID or MS. The reflective gold coating of the heated flow-through LP confers multiple internal reflection thereby increasing the practical cell length, which in turn maximises sensitivity (and also minimises reactivity). Use of a LP offers real-time analysis of GC effluent, simple design and least complexity of operation compared to other interfaces [19] as well as spectrum matching to vapour-phase IR libraries. Minimal solute–solute interference (i.e., by ensuring adequate resolution) in the LP results in vapour phase IR spectra with sharp spectral features [20].
Resolution of components achieved on the GC column can be degraded due to the re-combination of the FTIR response of closely separated components, within the mixing volume of the LP and connecting tube(s) [21]. The flow cell volume of 120 mm length with 1 mm I.D. may be comparable to the average peak volume of capillary GC columns [9]. These dimensions are critical so as not to excessively reduce chromatographic resolution without compromising chromatographic peak distortion and maximising IR sensitivity [10]. A key consideration to designing the system was to use a short TL to minimise dead volume.
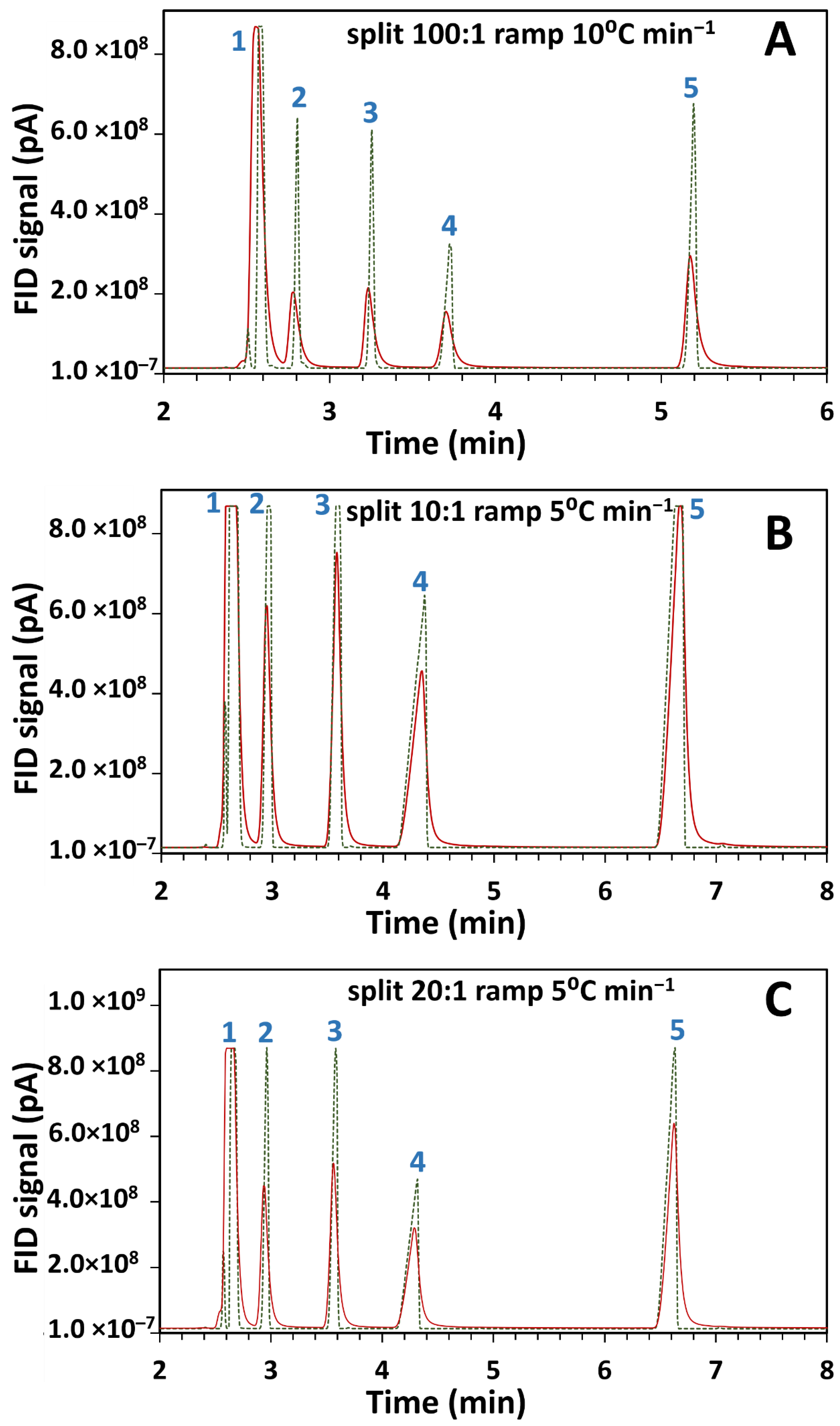
The current in-house designed system which allows post-light-pipe FID analysis was used to determine the influence of the LP on peak shape and width. A standard test mixture of 2-butanol, 2-pentanone, 1-nitropropane and m-xylene in DCM solvent was injected into the GC with two configurations and were chosen for their good chromatographic peak shape. Individual compound injection confirmed their elution order, further checked by library matching (Section 2.4) of the corresponding GC–FTIR spectra at each peak’s maxima, as reported in Supplementary information Table S1. Consistent correlation of the library match with the given injected isomer was observed, with only one dissenting match, being for the third match for 1-nitropropane erroneously matched to nitroethane; alkyl chain homologue matching may be less specific for FTIR, although for this small dataset matching appears good. Comparisons were done on two setups as outlined in Supplementary information Figure S1, where (a) the terminal end of the analytical column was connected directly to the FID (GC–FID) and (b) the LP outlet was connected to the FID (GC–FTIR–FID). The total column length remained the same for both configurations. The overlaid chromatograms for the same test mixture separately injected on the two configurations at 50 °C and at varying split ratios and temperature ramps is given in Figure 1.
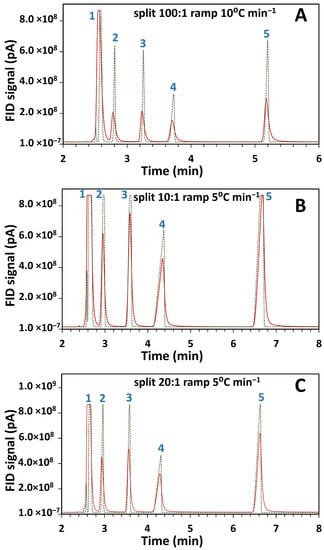
Figure 1.
Comparison of flame ionisation detector (FID) results for overlaid chromatograms of gas chromatography-Fourier transform infrared-FID (GC–FTIR–FID) (continuous line) and GC–FID (dotted line) using a standard component mix injected at 50 °C and analyzed with different split ratios and temperature ramps as (A) 100:1, 10 °C min−1, (B) 10:1, 5 °C min−1, and (C) 20:1, 5 °C min−1. Components corresponding to the peaks are (1) dichloromethane (DCM) (solvent peak), (2) 2-butanol, (3) 2-pentanone, (4) 1-nitropropane and (5) m-xylene.
Retention times and full width half maximum (FWHM) values for the chromatograms of Figure 1 are presented in Table 1. These values were not calculated for the solvent peak (peak 1) due to its high concentration.

Table 1.
Comparison of GC chromatographic FID peaks between direct FID and post-light-pipe FID results at various split ratio and temperature ramp combinations. Retention times and full width half maximum (FWHM) values are presented for peaks 2 to 5 depicted in Figure 1. Peaks 2, 3, 4 and 5 correspond to the compounds 2-butanol, 2-pentanone, 1-nitropropane and m-xylene respectively.
Comparison of peak widths between the two setups revealed an observed reduction in peak height and concomitant peak broadening in the GC–FTIR–FID configuration. In light-pipe GC–FTIR this could be ascribed to the dispersion of the chromatographic peak within the volume of the LP. Excessive band broadening can reduce separation efficiency resulting in poorer resolution in chromatographic systems. However, FTIR spectra in the gas phase display reduced spectral band broadening compared to those in condensed- or liquid-phases. Although gas-phase spectra are less common in literature than liquid-phase spectra, they are less confusing to interpret since the latter may contain multiple narrow bands within a broad band. From Figure 1A, the light-pipe contributes about a two-fold increase in peak width.
Effect of Varying Parameters on Signal-to-Noise (SNR) Ratio
The importance of having good signal-to-noise-ratio (SNR) in optical systems is not to be underestimated. The SNR determines the sensitivity of an instrument and relates to the measurement precision [22]. Attempts to improve SNR in LP GC–FTIR systems include optimising volume/length ratios to minimise chromatographic peak broadening [23], variations in the optical configurations [24], introduction of rapid scanning interferometers [25] and the use of suitable low-noise detectors [26]. The photoconductive liquid-nitrogen-cooled MCT detector used here, is well suited for LP measurements because of its sensitivity being almost two orders of magnitude greater than its pyroelectric predecessors [27]. The LP (and TL) needs to be maintained at a T sufficiently high to prevent condensation of GC solutes. High T has disadvantages by contributing to the LP acting as an IR source and increasing the intensity of the unmodulated radiation [28], which in turn could negatively affect the linear response of the MCT detector and the SNR [29].
This system’s ability to maintain measurement with a good SNR was validated where LP (and TL) T were raised in 40 °C increments (120, 160, 200, 240 and 280 °C) to monitor its effect on SNR. The resulting root mean square (RMS) intensity of absorbance as a function of time is illustrated in Supplementary information Figure S2 for each LP T setting and is seen to increase with T. SNR was calculated by taking the ratio of the peak height of the 2936 cm−1 C–H stretch (signal) against the peak-to-peak noise of the 2200–2000 cm−1 region. This wavelength range was chosen as it displays the greatest single beam intensity [22]. A plot of this SNR against increasing LP T is illustrated in Supplementary information Figure S3A. The negative trend with T is consistent with prior studies on LP T effect on SNR, with the SNR in some GC–FTIR systems was seen to decrease by a factor of up to 10 with an increase of LP T up to 300 °C [28]. Thus, the LP should be maintained at as low a T as possible, consistent with ensuring no condensation in the LP.
Similarly, the effect of variations in FTIR resolution and carrier gas flow on SNR was validated. GC requires rapid data acquisition and adequate interferometer scan rates (i.e. number of sampling data points) to avoid chromatographic resolution degradation whilst providing sufficient GC peak characterisation ability. Supplementary information Figure S3B depicts the SNR trend for spectra collected under increasing optical resolution settings of 0.5, 1, 2, 4, 8, 32 and 64 cm−1. The band broadening evident for lower resolution scans such as 64 cm−1 (and the high scan speed required by higher resolution) depicted in Supplementary information Figure S3D confirms the suitability of using spectral resolutions of 4 cm−1 or 8 cm−1 in routine use. The carrier gas flow conditions have an effect on migration of zones along the GC column [30] and variations in this affect the retention time of chromatographic peaks and peak widths. SNR was plotted as a function of carrier gas flows of 1.0, 1.5, 2.0 and 2.5 mL min−1. As expected, increases in carrier gas flow contributed to progressively shorter analysis times and reduced retention times of the compound as evident in Supplementary information Figure S3E. This however was accompanied by a progressive reduction in SNR as seen in Supplementary information Figure S3C. Further, due to the sequential arrangement of FTIR and FID detection, the retention time of the same peak in GC–FTIR and GC–FID differed slightly as would be expected and is depicted in Supplementary information Figure S4.
LP-based spectrum bands are quite broad and most vapour-phase spectra of molecules have a bandwidth of at least 10 cm−1. This minimises the necessity for measuring spectra at high resolutions, with GC–IR spectral measurements at 4 or 8 cm−1 resolution sufficing [31]. This can result when FTIR spectrometers measure 5–20 interferograms when operated in their highest scan speed.
3.2. Co-Eluting Isomer Analysis
The popularity of GC–MS for the analysis of complex mixtures has been unsurpassed due to its ease of use, applicability to multiple analytes, superior analyte detectability and identification capability based on mass spectrum libraries [32,33]. Its inability to differentiate isomers, which have almost identical mass spectra, has been amply discussed in literature [17,34]. MS data for such isomers can be ambiguous, resulting in similar fragmentation patterns from the molecular ion. In contrast, discrete vibrational and rotational absorption bands [35] in localised positions in FTIR spectra give rise to unique spectra, rendering a “fingerprinting” role, which can correlate to structural characteristics of the GC effluent molecules. Preliminary studies using o-, m- and p-cresol isomers validated the ability of this system to demonstrate the complementary nature of GC–MS and GC–FTIR as shown in Supplementary information Figure S5.
Essential oils consist of compounds that belong to diverse classes of compounds with various functional groups and structures [36], some of which may also have different isomeric forms. They include compounds including those with unsaturated bonds, branched and cyclic compounds, as well as oxygenated analogues. GC–FTIR spectra of selected essential oil standard compounds were obtained to validate the system’s applicability in identifying isomeric compounds that have similar mass spectra. Resulting GC–FTIR spectra of the essential oils geraniol, eugenol, limonene, α-pinene, β-pinene and γ-terpinene are depicted in Supplementary information Figure S6(A–F respectively) showing characteristic absorption bands in both functional and fingerprint regions. Ambiguities arising from the similarities in mass spectra of isomeric compounds, here α-pinene, β-pinene and γ-terpinene [Figure S6(G–I)], yield mismatches in library searches rendering a failure in MS identification. This can be resolved using the distinct FTIR spectra of these essential oils [Figure S6(D–F) respectively]. This complementary identification of FTIR and MS is a valuable tool in the characterisation of isomeric essential oil compounds in both one-dimensional and multidimensional GC.
Many components in essential oils overlap in GC analysis due to the similarity in their retention indices. It is common to require essential oils to be analysed on two complementary stationary phases in order to calculate retention indices on each phase to aid in identification. Thus, the analysis of essential oils depends on both successful separation as well as identification of their components. The strategy of using a combination of detection methods benefits the identification and evaluation of such similar compounds [37]. Although the separation of essential oils can be improved by the use of multidimensional GC [38], separation of most co-eluting essential oils on one-dimensional GC remains a challenge.
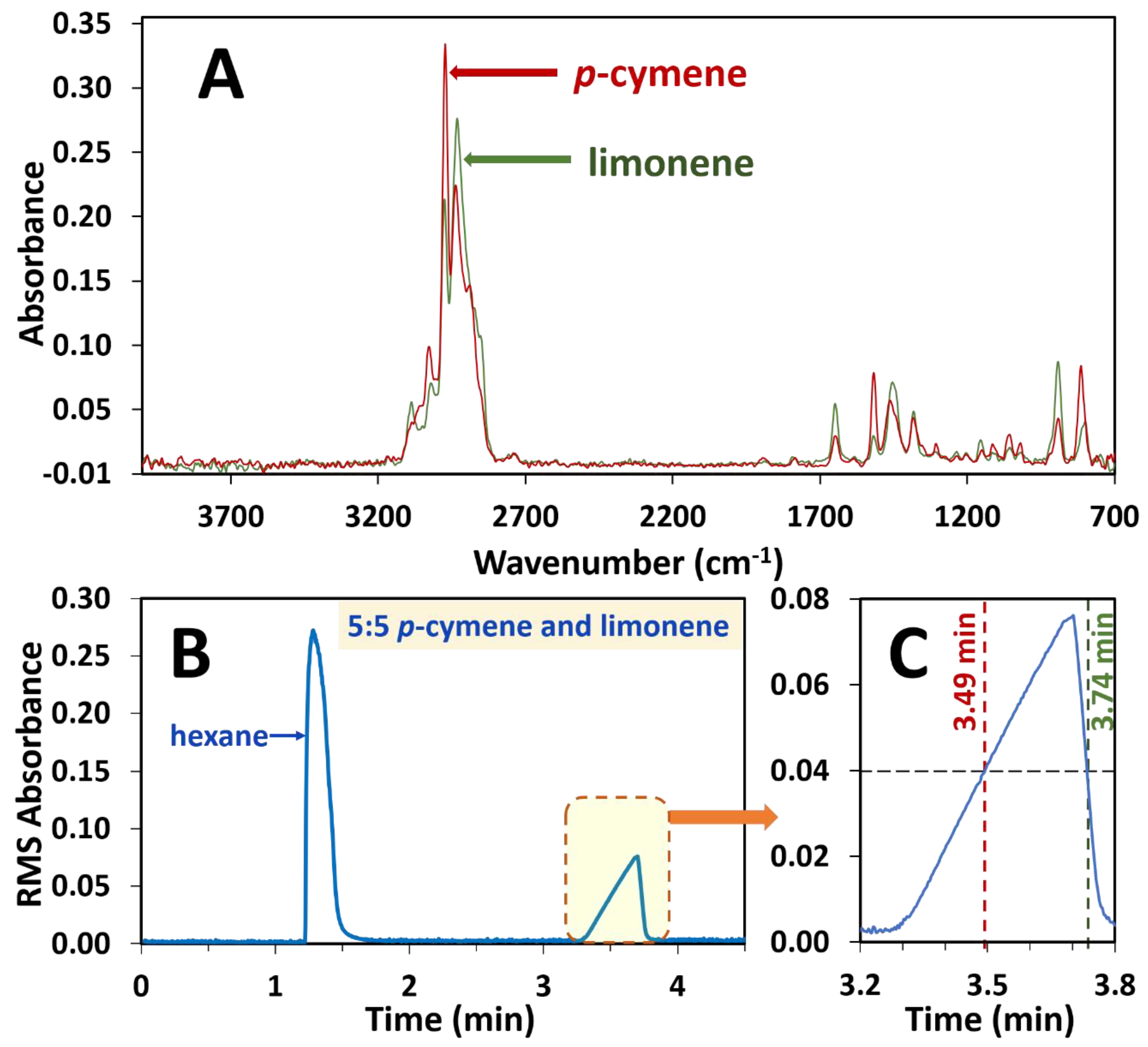
The ability to use this system to separately observe the spectra of the coeluting essential oils p-cymene and limonene on a single dimension was confirmed. p-cymene and limonene were mixed in the ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2 and 9:1 in hexane. Solutions of only p-cymene and limonene were also prepared in hexane and analysed. A single unresolved chromatographic peak was observed for both components as a result of deliberate coelution (Figure 2B). The spectrum on each side of the peak apex (Figure 2A) corresponding to a 0.04 absorbance (i.e., at 3.49 and 3.74 min, Figure 2C) was extracted and a library search conducted on each spectrum. In each instance of the different ratio mixtures, p-cymene was observed to be the early eluting isomer and limonene eluting as the later isomer. For example, in the sample of 5:5 limonene to p-cymene, the spectrum at 3.49 mins gave a search score of 0.87 for p-cymene and the spectrum at 3.74 mins gave a result of limonene with a search score of 0.95.
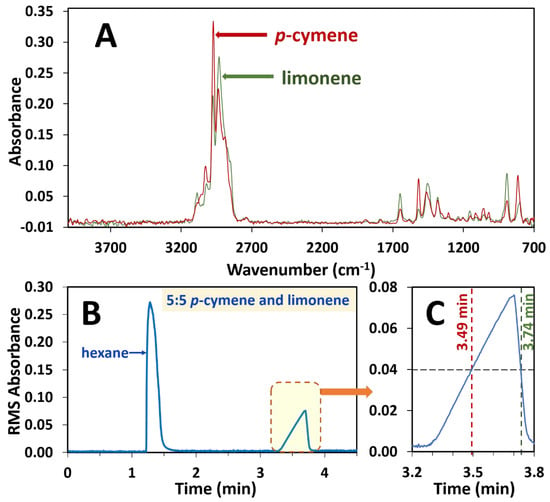
Figure 2.
Depiction of extraction of spectra at 0.04 RMS absorbance intensity of the single unresolved chromatographic peak due to co-eluting components in a 5:5 ratio mixture of p-cymene and limonene essential oil standards. p-cymene and limonene often overlap on GC columns and are deliberately overloaded here to illustrate the power of FTIR to deconvolute overlapping peaks. (A) Spectrum of p-cymene at 3.49 min and spectrum of limonene at 3.74 min, both at 0.04 RMS absorbance. (B) RMS absorbance chromatogram with the overloaded peak, which is enlarged (C) to illustrate the extraction of each spectrum at 0.04 RMS absorbance.
3.3. GC×FTIR Analysis of Oximes
The reversible molecular interconversion, in which chemical species show transformation on the time scale of chromatographic separation, is referred to as dynamic GC (DGC). This invariably involves isomers, which might be rotamers or involve other transformations and the efforts to better differentiate these isomers over the years have included both one-dimensional and multidimensional GC methods [39]. Here, on-column isomerisation (A⇌B) results in incompletely resolved peaks which have a characteristic broadening or plateau region between the terminal peaks known as the ‘interconversion region’ (Figure 3B). This has been observed in oximes, imines with the general formula RR’C=NOH, where the configuration of substituents around the C=N double bond produces E and Z isomers. Due to the isomers having similar (equivalent) mass spectra, conventional one-dimensional GC hyphenated to MS fails to separately identify or deconvolute the peak envelope or render information on the distribution of the individual isomers. In contrast the FTIR spectra of these isomers are distinct (Figure 3C). The suitability of light-pipe GC–FTIR for dynamic GC studies of oxime isomers was amply demonstrated in our recent studies [18], where the isomer profiles were traced using absorption bands stronger in each isomer, as well as by chemometric methods.
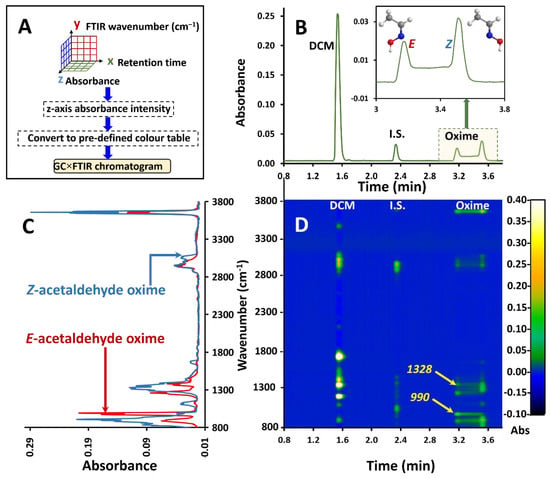
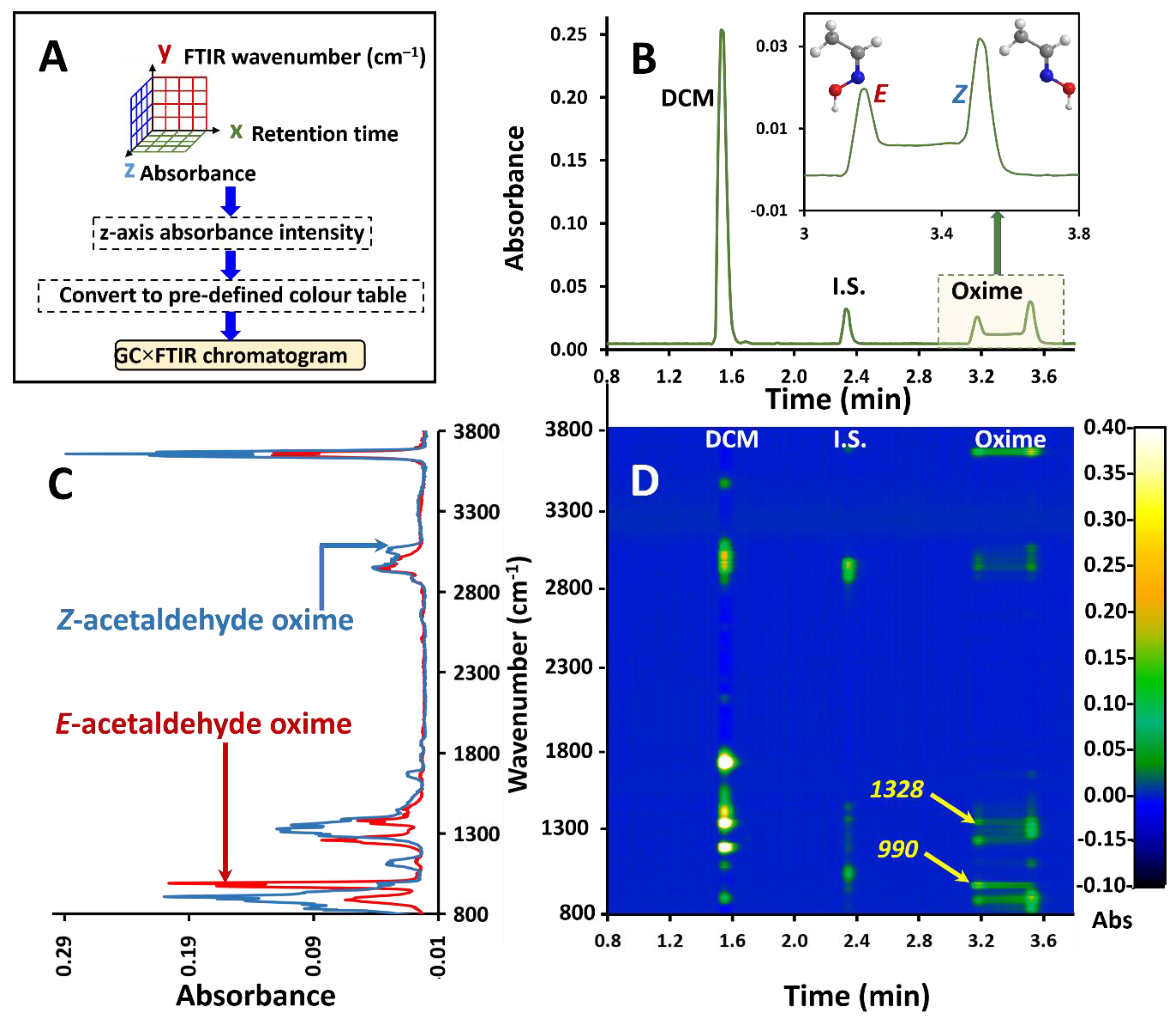
Figure 3.
(A) Flow chart of the approach to GC×FTIR. (B) GC–FTIR RMS response profile of interconversion of E and Z acetaldehyde oximes on the GC PEG phase column. (C) Overlay of corresponding FTIR spectra for E and Z acetaldehyde oxime acquired from the GC–FTIR experiment. (D) GC×FTIR 2D colour contour profile illustrating absorbance responses for each compound in the time/wavenumber space. Arrows 990 and 1328 refer to the bands at 990 cm−1 and 1328 cm−1 which are stronger and more selective for the E and Z isomer respectively.
Here we use a GC×FTIR, ‘two-dimensional’ approach, proposed by Wang and Edwards [40] for ‘spectrometric resolution’ of close-eluting C2-naphthalene isomeric mixtures, to independently trace the two oxime isomers. Whilst multidimensional separations focus on multiple column configurations, the multidimensional analysis system here can also be applied to instances where a detector is used as a separate second dimension to the first dimension GC separation, as proposed for MS where the molecular ion mass corresponds to a mass-domain separation dimension [41]. In a similar manner, Wang [42] recently presented a GC×VUV study of a diesel sample as a 2D analysis approach with VUV data collected over the range 125−430 nm, contrasting the result with GC×GC–FID. Whilst it was concluded that “All structural features are readily visualized in a manner complementary to two-dimensional gas chromatography, GC×GC,” it must be acknowledged that most features will correspond to incompletely resolved components and so represent overlapping VUV spectra.
Thus, GC×FTIR renders a two-dimensional presentation of GC retention time against the FTIR response for chemical species, with its inherent value in isomer mixture resolution. Here, in addition to its ability to detect and identify compounds, the FTIR enables spectrum display in the wavenumber dimension, together with separation in the time dimension by GC. The FTIR result is based on its ability to discriminate/separate functional groups or different component mixtures, based on differences of their absorption band attributes, rather than a physical separation. In conventional GC–FTIR presentation, the root mean square (RMS) of the total absorbance is plotted against retention time. In GC×FTIR, the whole spectrum for each retention data point is plotted and aligned vertically along the retention time axis, with their respective intensities in the third dimension (perpendicular to the 2D plane). This results in a 3D chromatogram with retention time on the x-axis, FTIR wavenumber on the y-axis and absorbance on the z-axis (or as a 2D contour plot of spectrum intensity) as depicted in the flow chart in Figure 3A. Converting the z-axis to a predefined colour scale renders a 2D plane presentation. The GC×FTIR result is shown in Figure 3D, with the RMS absorbance chromatogram given in Figure 3B and overlaid FTIR spectra given in Figure 3C. The polyethylene glycol (PEG) stationary phase was chosen due to its polar nature which is known to promote interconversion. FTIR spectra were recorded at 4 cm−1 resolution. This technique can plot multiple spectra in 3D space as demonstrated using a simple GC application.
Such 2D/3D plots allow for a comprehensive overview of the spectroscopic/separation information achievable by FTIR detection in GC. For instance, it might be of use in determining the purity of compound elution. For example, the band at 990 cm−1 is stronger for the E isomer (Figure 3D, arrowed, 990) which is confirmed by the contour plot at an elution time of 3.2 min and wavenumber of 990 cm−1. This is accompanied by a decay of the distribution of contour intensity at this wavelength which implies the gradual decrease of the E isomer with progressing elution time. Likewise, on Figure 3D, arrow 1328 (more selective for the Z isomer) shows an increase in contour intensity of the 1328 cm−1 band through to 3.255 min which is the elution time of original injected Z isomer. Previous attempts to trace the individual profiles of oximes undergoing dynamic interconversion have used contour plots in multidimensional and comprehensive 2D GC experiments [43,44].
The above discussion corresponds to the acetaldehyde oxime isomer elution. A chromatogram of a mixture of acetaldehyde oxime and propionaldehyde oximes is also depicted in the Supplementary information Figure S7A, displaying the expanded region of oximes to highlight the different bands corresponding to the interconversion regions of the E and Z isomers together with the corresponding GC×FTIR colour contour plot over the same retention time scale (Figure S7B) and an expanded region (Figure S7C) displaying IR bands corresponding to terminal (a, 4.4 min) and (c, 4.9 min) and an intermediate elution (b, 4.7 min) position of P.O. which shows absorption bands characteristic of both isomers. To the best of our knowledge this is however the first time multidimensional separations and contour plots in the form of a GC×FTIR experiment have been used with one-dimensional GC and FTIR spectroscopy to study isomer distribution of interconverting species. This is of value in recognising co-eluting isomers and suggests where deconvolution of spectra may be used in a simpler operator-friendly manner with a linear GC–FTIR presentation.
3.4. Multivariate Analysis of Co-Eluting Isomers
The capability of the GC–FTIR instrument in terms of rapid scan speeds is significant for both qualitative and quantitative analysis. Direct quantification of GC–FTIR data is possible as IR absorption obeys the Beer-Lambert law, where the absorbance (A) can be related to concentration of an absorber (c) and path length (l) as A=ɛcl, where ɛ is molar absorptivity. Despite the best FTIR spectrometers having relatively poor sensitivity, the quantification ability of FTIR can be demonstrated [12] and is also of use in compound characterisation [45]. We validated the ability of this system to carry out univariate quantitative analyses—analysis of a single variable—with the IR detector’s linear range response using several compounds with varying functional groups. Calibration curves for FTIR data can be constructed using peak area or peak absorption maxima as demonstrated in Supplementary information Figures S8 and S9. The R2 values of o-cresol (Figure S8A), ethylbenzene (Figure S8B), α-pinene (Figure S9A) and γ-terpinene (Figure S9B) were 0.997, 0.996, 0.979 and 0.9662 respectively. Conditions of analysis are tabulated in Supplementary information Table S2.
As opposed to univariate data analysis, multivariate data analysis, which assesses multiple variables, is a growing trend in predicting the response or change in a chemical system using the development of models. As such, the development of multivariate models that relate to multi-wavelength spectrum responses to variations in analyte concentrations has been proved to be valuable in the quantitative analysis of FTIR spectra [46,47] with the advantages of being fast, non-destructive and often cheap with the ability to be used in complex mixtures as well. The efficiency of these models to predict the concentration of new samples outweighs that of calibration curves based on a single wavelength of the FTIR spectrum. One such multivariate calibration approach, partial least squares regression (PLSR) was used to determine its applicability to GC–FTIR analysis of isomeric species, which is a useful test of co-eluting positional isomers. PLSR uses the whole FTIR spectrum region rather than a specific absorption band for analysing the component of interest and establishes a linear relationship between a known variable set (X) and predicted variables (Y). Here X constitutes the known concentration and Y the predicted values based on the spectra acquired. A set of latent variables (LVs) explain the source of variation in the Y block correlated to the X vector are computed to yield the latent variables which are used to calculate the regression vector b as set out in Equation (1), where e is the error vector (i.e., residuals).
In this study, the co-eluting isomers m- and p-cresol were chosen due to their FTIR spectra having marked differences, whilst having close similarity of mass spectra which precludes their individual quantification (Supplementary information Figure S2). The PLSR model was developed using a calibration data set X (M × N) prepared using m- and p-cresol isomers in the ratios 0:10, 1:9, 2:8, 4:6, 6:4, 8:2, 9:1 and 10:1. The validation set had these isomers in ratios of 3:7, 5:5 and 7:3. Spectra were obtained in *.spc format with a resolution of 4 cm−1 with an initial GC oven T of 90 °C increased to 135 °C at the rate of 5 °C min−1.
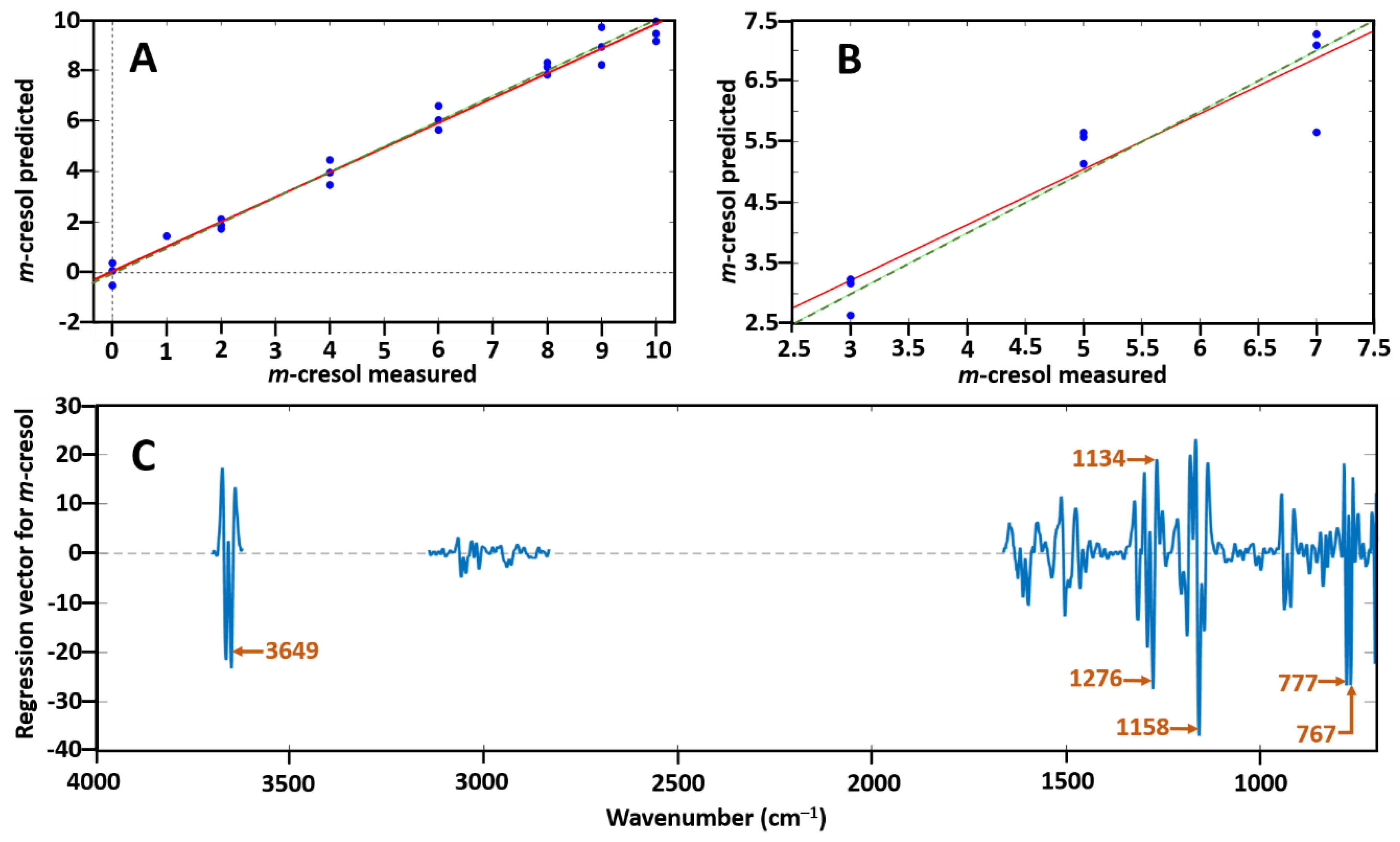
Spectrum pre-processing was Savitzky–Golay second-derivative polynomial order 3 and 15 points of smoothing. Data were excluded from the regions 1699–2841, 3106–3159, 3715–3733 and 3735–4000 cm−1 to reduce the effect of noise. Two data points were removed as outliers due to high Q residuals and Hotelling T2 plots to enable better prediction. The coefficient of determination (R2) for the calibration and validation sets were measured as well as the root mean square error of calibration (RMSEC) and root mean square error of prediction (RMSEP). The PLSR model was seen to follow a linear regression of near perfect linearity with a R2 value of 0.99 and thus was confirmed to be suitable to use as a prediction model as demonstrated in Figure 4A. The RMSEC was 0.38 which reflects the internal prediction accuracy. Figure 4C shows the loading plots of the regression vector for m-cresol corresponding to the PLSR model together with the labelling of the most significant bands [767 cm−1, 777 cm−1 (C–H bending), 1134 cm−1, 1158 cm−1, 1158 cm−1 (C–O stretching), 3649 cm−1 and 3662 cm−1 (O–H stretching)]. When tested using the validation set a R2 prediction value of 0.88 and an RMSEP of 0.57 was obtained by the prediction set presented in Figure 4B, which represents the external predictive ability. The variability here is due to the decreasing sensitivity and IR response of the MCT detector below 700 cm−1. In both Figure 4A,B the green dotted line represents the expected proportional 1:1 fit of the samples and the red continuous line represents the fit obtained using the model and test data.
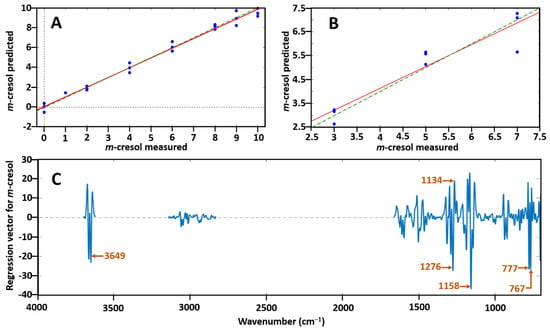
Figure 4.
(A) Predicted m-cresol concentration ratio vs. measured m-cresol concentration ratio with the use of the partial least squares (PLS) regression model; (B) prediction set of m-cresol; and (C) loadings plot corresponding to the PLS model with labelling of the most significant bands which allow prediction of m-cresol from p-cresol. In both (A) and (B) the green dotted line represents the expected 1:1 proportional fit whilst the red continuous line shows the fit calculated by the model on the test data.
The low value of RMSEP and high value of R2 indicate the accuracy of this approach. This confirms the suitability of combining GC–FTIR data with the multivariate calibration method of PLS regression for quantifying coeluting isomer mixtures, especially for isomeric species with identical mass spectra.
4. Conclusions
One of GC–FTIR’s greatest strengths is its ability to differentiate functional groups or chemical structures for resolved compounds. This is largely achieved by the distinct combination of peaks in the absorption spectrum, which creates a unique pattern for individual compounds. In contrast, MS library searches rely largely on defined fragmentation patterns (mass; intensity) of the molecule and these usually comprise less informative structural details. Identification is commonly achieved by the closest match of an acquired spectrum to the ‘hit-list’ of compound spectra submitted to reference libraries [32]. As aptly outlined by Ausloos et. al. [48], the structure identification ability of MS library searches depends mainly on the abundance and diversity of that structure in the library database of reference compounds—and it is possible to retrieve a ‘proposed MS library match’ even if the compound is not present in the library. Due to the complexity of ions which can be generated by dissociation, electron-ionisation mass spectra cannot be reliably predicted. This is mainly because these ions can undergo multiple pathways to achieve a given ion, the dependence of the mass spectrum on experimental conditions and the possibility of ions rearranging before dissociation [49]. Contrasting the progress in MS detection for GC in recent decades, there is a corresponding scarcity of GC–FTIR literature. However, a revival of interest in condensed phase GC–FTIR and the development of other spectroscopic detectors such as the VUV has reinvigorated the value of spectroscopic detectors and complementary identification approaches in chemical structure deduction. The relatively lower sensitivity characteristic of light-pipe GC–FTIR systems contrasts with its favourable identification power. The success of GC–FTIR optimisation rests upon a compromise of chromatography and spectroscopy and when used in combination with the spectrometric data of GC–MS can result in greater confidence in component identification rendering dual-qualitative information. Ongoing research is directed at setting up multidimensional configurations and combining alternative GC techniques. We believe applications to a range of samples to further demonstrate the value of using multiple detection methodologies will yield results with reliable and absolute identification of compounds, adding new dimensions to analytical capabilities of FTIR and similar spectroscopic detection techniques with GC.
Supplementary Materials
The following are available online at https://www.mdpi.com/2297-8739/7/2/27/s1, The detailed captions for these Figures and Tables are included in the supplementary file. Figure S1: Instrumental setup schematic for the light-pipe GC–FTIR–FID system., Figure S2: Effect of increasing light-pipe (and transfer line) temperatures on the root mean square intensity absorbance of GC–FTIR chromatograms of nonane., Figure S3: Effect of increasing LP and TL T, varying FTIR resolution and varying flow on signal-to-noise ratio calculations., Figure S4: GC–FTIR and GC–FTIR–FID chromatograms of 5% o-cresol in MeOH at varying injection volumes of (a) 1.5 µL, (b) 2.0 µL and (c) 3.0 µL., Figure S5: Virtually identical GC–MS spectra for o-, m- and p-cresol isomers and their corresponding GC–FTIR spectra., Figure S6: GC–FTIR spectra of α-pinene, β-pinene, geraniol, γ-terpinene, limonene and eugenol essential oil standard compounds., Figure S7: GC×FTIR colour contour plot of acetaldehyde oxime (A.O.) and propionaldehyde oxime (P.O.) elution with FTIR spectra corresponding to P.O. isomers at different positions over the dynamic P.O. peak., Figure S8: Calibration curves for peak area of o-cresol and peak height of ethylbenzene and GC–FTIR spectra for o-cresol and ethylbenzene together., Figure S9: Calibration curves for α-pinene and γ-terpinene at their absorption maxima and maximum absorption peak responses of α-pinene and γ-terpinene., Table S1: Library match results for elution of compounds used for GC–FID and GC–FTIR–FID peak comparison., Table S2: Chromatography conditions for univariate calibration of select compounds depicted in Figure S8 and Figure S9.
Author Contributions
Conceptualization, P.J.M. and J.S.Z.; methodology, J.S.Z., S.B., J.S.P.S., H.D.W. and Y.N.; software, J.S.Z.; formal analysis, J.S.Z. and H.D.W.; writing—original draft preparation, J.S.Z.; writing—review and editing, Y.N., P.J.M. and J.S.Z.; supervision, P.J.M. and B.R.W.; funding acquisition, P.J.M. All authors have read and agreed to the published version of the manuscript
Funding
This work was supported by the Australian Research Council, Linkage Grant scheme, with partner PerkinElmer (Grant LP150100465). Funding from PerkinElmer to the Monash University GRIP program is also acknowledged. Monash University support to J.S.Z. and H.D.W. for Deans’ Research Scholarships is acknowledged.
Acknowledgments
Assistance given by Chadin Kulsing to J.S.S.P and J.S.Z. and assistance in PLSR to J.S.Z. by David Perez-Guaita, Zach Richardson and Miguela Martin is greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poole, C.F. Ionization-based detectors for gas chromatography. J. Chromatogr. A 2015, 1421, 137–153. [Google Scholar] [CrossRef]
- Zavahir, J.S.; Nolvachai, Y.; Marriott, P.J. Molecular spectroscopy—Information rich detection for gas chromatography. TrAC Trends Anal. Chem. 2018, 99, 47–65. [Google Scholar] [CrossRef]
- Wilson, I.D.; Brinkman, U.A.T. Hyphenation and hypernation: The practice and prospects of multiple hyphenation. J. Chromatogr. A 2003, 1000, 325–356. [Google Scholar] [CrossRef]
- Santos, I.C.; Schug, K.A. Recent advances and applications of gas chromatography vacuum ultraviolet spectroscopy. J. Sep. Sci. 2017, 40, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Nolvachai, Y.; Kulsing, C.; Marriott, P.J. Pesticides analysis: Advantages of increased dimensionality in as chromatography and mass spectrometry. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2135–2173. [Google Scholar] [CrossRef]
- Andersson, J.T. Detectors. In Practical Gas Chromatography: A Comprehensive Reference; Dettmer-Wilde, K., Engewald, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 205–248. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Jimenez-Gordon, I.; Eshetu, G.G.; Boyanov, S.; Lecocq, A.; Marlair, G.; Pilard, S.; Laruelle, S. Gas chromatography/Fourier transform infrared/mass spectrometry coupling: A tool for Li-ion battery safety field investigation. Anal. Methods 2014, 6, 6120–6124. [Google Scholar] [CrossRef]
- Skoog, D.A. Principles of Instrumental Analysis, 6th ed.; Thomson, Brooks/Cole: Belmont, CA, USA, 2007. [Google Scholar]
- Visser, T. FT-IR detection in gas chromatography. TrAC Trends Anal. Chem. 2002, 21, 627–636. [Google Scholar] [CrossRef]
- Visser, T. Gas Chromatography/Fourier Transform Infrared Spectroscopy. In Handbook of Vibrational Spectroscopy; Griffiths, P.R., Chalmers, J.M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1605–1626. [Google Scholar] [CrossRef]
- Sciarrone, D.; Schepis, A.; De Grazia, G.; Rotondo, A.; Alibrando, F.; Cipriano, R.R.; Bizzo, H.; Deschamps, C.; Sidisky, L.M.; Mondello, L. Collection and identification of an unknown component from: Eugenia uniflora essential oil exploiting a multidimensional preparative three-GC system employing apolar, mid-polar and ionic liquid stationary phases. Faraday Discuss. 2019, 218, 101–114. [Google Scholar] [CrossRef]
- Demirgian, J.C. Gas chromatography—Fourier transform infrared spectroscopy—Mass spectrometry. A powerful tool for component identification in complex organic mixtures. TrAC Trends Anal. Chem. 1987, 6, 58–64. [Google Scholar] [CrossRef]
- Wilkins, C.L. Directly-linked gas chromatography–infrared–mass spectrometry (GC/IR/MS). In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1627–1633. [Google Scholar] [CrossRef]
- Krock, K.A.; Ragunathan, N.; Wilkins, C.L. Multidimensional gas chromatography coupled with infrared and mass spectrometry for analysis of Eucalyptus essential oils. Anal. Chem. 1994, 66, 425–430. [Google Scholar] [CrossRef]
- Cooper, J.R.; Wilkins, C.L. Utilization of spectrometric information in linked gas chromatography-Fourier transform infrared spectroscopy-mass spectrometry. Anal. Chem. 1989, 61, 1571–1577. [Google Scholar] [CrossRef]
- Cai, J.; Lin, P.; Zhu, X.L.; Su, Q. Comparative analysis of clary sage (S. sclarea L.) oil volatiles by GC-FTIR and GC-MS. Food Chem. 2006, 99, 401–407. [Google Scholar] [CrossRef]
- Ragunathan, N.; Krock, K.A.; Klawun, C.; Sasaki, T.A.; Wilkins, C.L. Gas chromatography with spectroscopic detectors. J. Chromatogr. A 1999, 856, 349–397. [Google Scholar] [CrossRef]
- Zavahir, J.S.; Nolvachai, Y.; Wood, B.R.; Marriott, P.J. Gas chromatography-Fourier transform infrared spectroscopy reveals dynamic molecular interconversion of oximes. Analyst 2019, 144, 4803–4812. [Google Scholar] [CrossRef]
- Poole, C.F. Spectroscopic Detectors for Identification and Quantification. In The Essence of Chromatography; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 719–792. [Google Scholar] [CrossRef]
- White, R.L. Chromatography-IR, methods and instrumentation. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 251–255. [Google Scholar] [CrossRef]
- Mark, H.; Workman, J., Jr. Experimental designs: Part 8—β, the power of a test. In Chemometrics in Spectroscopy; Academic Press: Amsterdam, The Netherlands, 2007; pp. 101–102. [Google Scholar] [CrossRef]
- Blitz, J.P.; Klarup, D.G. Signal-to-noise ratio, signal processing, and spectral information in the instrumental analysis laboratory. J. Chem. Educ. 2002, 79, 1358–1360. [Google Scholar] [CrossRef]
- Giss, G.N.; Wilkins, C.L. Effects of lightpipe dimensions on gas chromatography/Fourier transform infrared sensitivity. Appl. Spectrosc. 1984, 38, 17–20. [Google Scholar] [CrossRef]
- Henry, D.E.; Giorgetti, A.; Haefner, A.M.; Griffiths, P.R.; Gurka, D.F. Optimizing the optical configuration for light-pipe gas chromatography/Fourier transform infrared spectrometry interfaces. Anal. Chem. 1987, 59, 2356–2361. [Google Scholar] [CrossRef]
- Brissey, G.M.; Henry, D.E.; Giss, G.N.; Yang, P.W.; Griffiths, P.R.; Wilkins, C.L. Comparison of gas chromatography/Fourier transform infrared spectrometric Gram-Schmidt reconstructions from different interferometers. Anal. Chem. 1984, 56, 2002–2006. [Google Scholar] [CrossRef]
- Theocharous, E.; Birch, J.R. Detectors for mid- and far-infrared spectrometry: Selection and use. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 349–367. [Google Scholar] [CrossRef]
- Griffiths, P.R.; Heaps, D.A.; Brejna, P.R. The gas chromatography/infrared interface: Past, present, and future. Appl. Spectrosc. 2008, 62, 259A–270A. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.R.; de Haseth, J.A. Coupled Techniques. In Fourier Transform Infrared Spectrometry; John Wiley & Sons, Inc.: Chichester, UK, 2006; pp. 481–507. [Google Scholar] [CrossRef]
- Brown, R.S.; Cooper, J.R.; Wilkins, C.L. Lightpipe temperature and other factors affecting signal in gas chromatography/Fourier transform infrared spectrometry. Anal. Chem. 1985, 57, 2275–2279. [Google Scholar] [CrossRef]
- Grob, R.L. Theory of gas chromatography. In Modern Practice of Gas Chromatography, 4th ed.; Grob, R.L., Barry, E.F., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2004; pp. 23–63. [Google Scholar] [CrossRef]
- Griffiths, P.R. Gas chromatography | Infrared spectroscopy. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 4, pp. 186–192. [Google Scholar] [CrossRef]
- Milman, B.L. General principles of identification by mass spectrometry. TrAC Trends Anal. Chem. 2015, 69, 24–33. [Google Scholar] [CrossRef]
- Milman, B.L.; Zhurkovich, I.K. Mass spectral libraries: A statistical review of the visible use. TrAC Trends Anal. Chem. 2016, 80, 636–640. [Google Scholar] [CrossRef]
- Sasaki, T.A.; Wilkins, C.L. Gas chromatography with Fourier transform infrared and mass spectral detection. J. Chromatogr. A 1999, 842, 341–349. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 5th ed.; Cengage Learning: Stamford, CT, USA, 2015. [Google Scholar]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66 (Suppl. C), 146–157. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Kong, D.-X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Marriott, P.J.; Shellie, R.; Cornwell, C. Gas chromatographic technologies for the analysis of essential oils. J. Chromatogr. A 2001, 936, 1–22. [Google Scholar] [CrossRef]
- Wong, Y.F.; Kulsing, C.; Marriott, P.J. Switchable enantioselective three- and four-dimensional dynamic gas chromatography–mass spectrometry: Example study of on-column molecular interconversion. Anal. Chem. 2017, 89, 5620–5628. [Google Scholar] [CrossRef]
- Wang, F.C.-Y.; Edwards, K.E. Separation of C2-naphthalenes by gas chromatography × Fourier transform infrared spectroscopy (GC × FT-IR): Two-dimensional separation approach. Anal. Chem. 2007, 79, 106–112. [Google Scholar] [CrossRef]
- Wang, F.C.-Y.; Qian, K.; Green, L.A. GC × MS of diesel: A two-dimensional separation approach. Anal. Chem. 2005, 77, 2777–2785. [Google Scholar] [CrossRef]
- Wang, F.C.-Y. GC × VUV study of diesel: A two-dimensional separation approach. Energy Fuels 2020, 34, 1432–1437. [Google Scholar] [CrossRef]
- Kulsing, C.; Nolvachai, Y.; Wong, Y.F.; Glouzman, M.I.; Marriott, P.J. Observation and explanation of two-dimensional interconversion of oximes with multiple heart-cutting using comprehensive multidimensional gas chromatography. J. Chromatogr. A 2018, 1546, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Nolvachai, Y.; Kulsing, C.; Trapp, O.; Marriott, P.J. Multidimensional gas chromatography investigation of concentration and temperature effects of oxime interconversion on ionic liquid and poly(ethylene glycol) stationary phases. Anal. Chim. Acta 2019, 1081, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kempe, J.; Bellmann, C.; Meyer, D.; Windrich, F. GC-IR based two-dimensional structural group analysis of petroleum products. Anal. Bioanal. Chem. 2005, 382, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Richardson, Z.; Perez-Guaita, D.; Kochan, K.; Wood, B.R. Determining the age of spoiled milk from dried films using attenuated reflection Fourier transform infrared (ATR FT-IR) spectroscopy. Appl. Spectrosc. 2019, 73, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Toziou, P.-M.; Barmpalexis, P.; Boukouvala, P.; Verghese, S.; Nikolakakis, I. Quantification of live Lactobacillus acidophilus in mixed populations of live and killed by application of attenuated reflection Fourier transform infrared spectroscopy combined with chemometrics. J. Pharm. Biomed. Anal. 2018, 154, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Stein, S. Mass spectral reference libraries: An ever-expanding resource for chemical identification. Anal. Chem. 2012, 84, 7274–7282. [Google Scholar] [CrossRef]
- Ausloos, P.; Clifton, C.L.; Lias, S.G.; Mikaya, A.I.; Stein, S.E.; Tchekhovskoi, D.V.; Sparkman, O.D.; Zaikin, V.; Zhu, D. The critical evaluation of a comprehensive mass spectral library. J. Am. Soc. Mass Spectrom. 1999, 10, 287–299. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).