Abstract
The unregulated consumption of corticosteroids causes significant adverse effects on human health. Therefore, it is important to develop methodologies that allow their analysis in pharmaceutical matrices with competitive analysis times and costs. The determination of corticosteroids by micellar electrokinetic chromatography (MEKC) using a background electrolyte (BGE) composed of phosphate buffer and a micellar pseudo-stationary phase constituted by a mixture of surfactants is proposed as an alternative quantification technique. The variables involved in the BGE: phosphate concentration, surfactant (sodium dodecyl sulfate (SDS) or sodium lauryl ether sulfate (SLES)), sodium taurocholate (STC) and the pH value were optimized using a Taguchi L9 (34) experimental design. Employing the optimal BGE, the separation of the three corticosteroids is possible in a linear range of 1.05–10.0 mg L−1, with limits of detection (LOD) of 0.28–0.35 mg L−1. The relative standard deviation (RSD) values obtained for the repeatability (n = 3) and intermediate precision (n = 9) were less than 5.0%. Pharmaceutical formulations (ointments, injectable solution and ophthalmic solution) were analyzed using the proposed methodology (MEKC) and the official methodology (high-performance liquid chromatography, HPLC), and no significant differences were found between the corticosteroid contents obtained from both methods.
1. Introduction
Corticosteroids include all steroid hormones, and are classified into two groups according to their mode of action. The first one, glucocorticoids, inhibit the production and activity of inflammatory cells, while mineralocorticoids maintain the balance of water content and sales in the human body [1]. Structurally, corticosteroids are formed by 21 carbon atoms (C), distributed in 3 rings of 6 C atoms and a ring of 5 C atoms linked together. Modifications to the base structure have a significant influence on the activity and potency of the molecule [2].
Corticosteroids are successfully applied in the treatment of various diseases, such as psoriasis, eczema, arthritis, rhinitis, asthma, hepatitis, systemic lupus erythematosus and inflammatory bowel disease [3], due to their anti-inflammatory, antipyretic and immunosuppressive properties [4]. However, administration without medical supervision causes serious adverse effects on the health of patients (diabetes, hypertension, obesity, osteoporosis, skin atrophy, Cushing’s syndrome, etc.) [5].
These drugs are dosed to patients in different forms (tablets, solutions, ointments and suspensions) and can be found in single forms (only the corticosteroid) or in combination with another active ingredient [6]. To improve quality control and reduce operation times and costs, the analysis of compounds with similar properties and structures is required; however, it represents a challenge for quality control laboratory.
Most of the methods developed for the analysis of corticosteroids in various matrices (human urine, cosmetics and pharmaceutical formulations) are chromatographic techniques [7]. Gas chromatography (GC) has been employed for the analysis of synthetic corticosteroids (prednisolone and triamcinolone); however, it requires an additional derivatization step, which complicates the instrumental analysis [8,9]. On the other hand, high-performance liquid chromatography (HPLC) has been the preferred chromatographic technique for the analysis of these compounds based on its sensitivity and precision [10,11]. Generally, samples are analyzed by the reversed-phase mode using a C18 column and a mobile phase composed of acetonitrile and water [12,13,14,15,16], employing ultraviolet-visible spectrophotometry (between 230 and 254 nm) as detector [17]. Some disadvantages of HPLC analysis are the implementation of a complex sample treatment and high consumption of organic solvents [18].
Capillary electrophoresis (CE) has been described as an alternative to chromatographic methods due to its versatility, low sample and reagent consumption, short analysis time and the possibility of modifying the composition of the background electrolyte (BGE) to separate the corticosteroids properly [19]. Although it has been used in a wide range of active compounds for pharmaceutical formulations, the separation of corticosteroids by CE can only be carried out under micellar electrokinetic chromatography (MEKC) conditions [20] due to the structural similarity and absence of charge in the pH interval of 2.0–12.0 as result of pKa values around 12 [21].
MEKC modality is based on the partitioning of analytes between an aqueous phase and a micellar pseudo-stationary phase, and the migration of the compounds is proportional to their interaction with the micelles [22]. Micelles are formed by adding surfactants to BGE [23] at a minimum concentration required for aggregate formation; the critical micellar concentration [24]. The most used ionic surfactant in the BGE is sodium dodecyl sulfate (SDS) [25,26]; this strategy has allowed the separation of dexamethasone–ciprofloxacin [27] and prednisolone–gatifloxacin mixtures [28]. However, the use of other surfactants such as sodium lauryl ether sulfate (SLES) has been less studied than SDS in electrophoretic separations, despite being environmentally friendly compared to SDS [29].
On the other hand, the separation of corticosteroids by MEKC requires the preparation of an electrolyte composed of buffer ion, surfactant and a third component that promotes the formation of mixed micelles: cyclodextrins [30,31,32,33], ionic liquids [20], organic solvents [34] and bile salts [35]. Bile salts are compounds that are poorly soluble in water due to their chemical structure, but they are successfully applied to the solubilization and separation of hydrophobic compounds [36]. Some of the bile salts evaluated in the analysis of corticosteroids are sodium taurocholate (STC) for human urine analysis [19], sodium taurodeoxycholate for contraceptive pill analysis [37] and a mixture of sodium cholate and sodium deoxycholate for pharmaceutical tablets samples [25].
The present work proposes to develop a rapid, low-cost, robust analytical MEKC method that reduces the consumption of organic solvents and allows the determination and quantification of corticosteroids in pharmaceutical formulations.
2. Materials and Methods
2.1. The Reagents and Solutions
All solutions were prepared with deionized water (Milli-Q Merck, Millipore Darmstadt, Hesse, Germany) with a resistivity not less than 18.2 MΩ cm. All chemicals used were of analytical grade. Methylprednisolone (MEP, internal standard), dexamethasone (DEX), prednisolone (PRE), triamcinolone (TRI), sodium hydrogen taurocholate hydrate (STC), SDS, sodium hydroxide (NaOH) and dibasic sodium phosphate (Na2HPO4) were obtained from Sigma Aldrich (St Louis, MO, USA). Sodium borate (Na2B4O7·10H2O); methanol (MeOH) and hydrochloric acid (HCl) were purchased from J.T. Baker (Phillipsburg, NJ, USA). SLES was obtained from DPS Mexicana (CDMX, Mexico).
Standard solutions of three analytes (TRI, PRE and DEX) and the internal standard solution (MEP) were prepared in HPLC-grade MeOH (1000.0 mg L−1) and preserved at 4 °C. The BGE was prepared with Na2HPO4 (20.0 mM), SDS (6.0 mM) and STC (30.0 mM) adjusted to a pH value of 8.0 ± 0.02 with HCl (0.1 M).
All the pharmaceutical samples were purchased in Pachuca de Soto, Hidalgo (Mexico).
2.2. MEKC Conditions
Electrophoretic experiments were performed using a Beckman Coulter PA 800 plus (Fullerton, CA, USA) instrument with a diode array detector. The electrokinetic chromatograms obtained were analyzed using 32 Karat software (Beckman Coulter, Brea, CA, USA). An Oakton pH510 series pH/ion analyzer (Vernon Hills, IL, USA) was used to adjust the pH of the solutions.
A fused silica capillary (30.0 cm × 75.0 µm I.D.) was employed, which was activated by passing 1.0 M NaOH for 10 min, followed by 0.1 M NaOH for 10 min, deionized water for 10 min, and finally the BGE for 10 min. Between each analysis, the capillary was washed using 1.0 M NaOH for 2 min, 0.1 M NaOH for 2 min, deionized water for 2 min, and the BGE for 2 min.
The separation voltage was 14.0 kV 25.0 °C, and the sample was introduced into the capillary hydrodynamically using a pressure of 3447.37 Pa (0.5 psi) for 5.0 s.
2.3. Optimization of the BGE Composition
The influence of two ionic surfactants (SLES and SDS) on the electrophoretic separation of corticosteroids was evaluated by optimizing BGE; for the optimization, a Taguchi- design of experiments L9 (34) was applied. Four factors (pH, [phosphate] mM, [surfactant] mM and [STC] mM) were evaluated at three levels (Table 1), and the number of theoretical plates (N) was selected as the output variable. The results were analyzed using Minitab 17 statistical software.

Table 1.
Factors and levels evaluated in the optimization of the composition of the BGE.
The tests were performed by analyzing a solution containing 30.0 mg L−1 of the analytes and internal standard.
2.4. Sample Preparation
For liquid formulations (injectable and ophthalmic solutions), an aliquot equivalent to 8.0 mg of corticosteroid was transferred to a 100.0 mL volumetric flask and the volume was completed with a MeOH:H2O (1:1 v:v) [38].
For solid formulations (ointments), 1.0 g of the sample was weighed, 2.0 mL of MeOH was added, and it was subsequently mixed. Afterward, the sample was heated to 40 °C and centrifuged at 1512 units g. The supernatant phase was filtered, and the volume was completed to 50.0 mL with MeOH:H2O (1:1 v:v) [17].
2.5. Method Validation
Method validation was performed according to ICH guidelines [39]. Analytical parameters were obtained under optimal electrophoretic conditions by constructing and interpreting a calibration line. The line was constructed from ACorticosteroid/AIS ratios over a concentration interval of 2.0–10.0 mg L−1.
The limit of detection (LOD) and limit of quantification (LOQ) were calculated for a signal-to-noise ratio of 3.3 and 10, respectively. Precision was assessed by repeatability and intermediate precision by analyzing three concentration levels (5.0, 7.0 and 9.0 mg L−1) in triplicate for three days. Results were expressed as relative standard deviation (%RSD). Accuracy was evaluated by comparing the results of the analysis of five pharmaceutical formulations (Table 2) by MEKC and HPLC.

Table 2.
Chemical composition of analyzed samples.
Corticosteroids analysis was performed by HPLC using an Agilent Technologies 1260 Infinity HPLC apparatus (Agilent, DE, Waldbronn, Germany) equipped with an Agilent Zorbax Eclipse XDB-C8 Analytical 4.6 × 150.0 mm, 5.0 µm column. The mobile phase was composed of acetic acid aqueous solution 1.0% v:v (A) and ACN (B). Analytical signals were detected using a diode array detector at 250.0 nm.
3. Results and Discussion
Optimization of the Background Electrolyte Composition
For the analysis of corticosteroids by MEKC, it is necessary to incorporate linear surfactants and bile salts into the BGE to promote the formation of micelles [40]. The influence of SDS (Figure 1a), SLES (Figure 1b) and STC (Figure 1c) on the electrophoretic separation was evaluated by their individual addition to the phosphate BGE (40.0 mM, pH = 7.0). In any case, an adequate separation of corticosteroid-associated signals was not observed as result of the weak interaction between corticosteroids and simple micelles (containing only one additional component to the buffer ion). In order to improve the resolution of the signals of interest, the mixture of linear and non-linear surfactants (bile salts) contained in the BGE was evaluated, based on the formation of mixed micelles to favor the separation of corticosteroids [41]. Bile salts are surfactants with low solubility in water, and their chemical structure is formed by a steroid skeleton of lipophilic nature, which contributes to the solubilization and separation of hydrophobic compounds (corticosteroids). Likewise, the linear surfactant (SDS or SLES) interacts with the hydrophilic part of the analytes and provides charge to the micelle to perform migration and separation in aqueous medium. The stabilization of this type of micelles is attributed to the hydrogen interactions formed between the hydroxy groups of the bile salt and the ethoxy group present in the linear surfactant [42].

Figure 1.
Electrokinetic chromatograms obtained from the analysis of standard solution with 10.0 mg L−1 of corticosteroids (triamcinolone; TRI, prednisolone; PRE and dexamethasone; DEX) using a background electrolyte (BGE) (sodium tetraborate 40.0 mM, pH = 10.0) and (a) sodium dodecyl sulfate (SDS) 10.0 mM, (b) sodium lauryl ether sulfate (SLES) 3.0 mM and (c) sodium taurocholate (STC) 60.0 mM.
The evaluation of mixed micelles in the separation of corticosteroids was performed by preparing two BGE composed of (1) SDS-STC and (2) SLES-STC. The composition of separation electrolyte was optimized by applying a Taguchi design of experiments L9 (34), as it allows a robust evaluation of controlling factors with minimal experiments [43]. pH value, phosphate and surfactants concentration were selected as control variables due to these variables affecting the ionic strength, chemical form of the species and viscosity of the medium, and therefore the electrophoretic mobility. The separation is evaluated in separation systems using the number of theoretical plates (N) as the response variable [44].
Taguchi design of experiments has been used to evaluate BGE composition and its effect on the separation of racemic mixtures (levocabastine, galantamine and mitratapide), where variables such as pH, electrolyte concentration and the type and concentration of additives (cyclodextrins and organic solvents) were evaluated [45].
The design of experiments and the results obtained from each experiment are shown in Table 3. It can be observed that N is higher in the case of the BGE composed by SLES-STC; however, the separation of the analytes is possible under both conditions.

Table 3.
Orthogonal matrix and its correspondent N for each experiment.
The mean graph obtained from the analysis of the results, for both electrolytes, is presented in Figure 2. Considering that the aim is to maximize efficiency in electrophoretic separation, the conditions that provided the highest value in response variable (N) for the SDS-STC electrolyte are [Phosphate] = 20.0 mM, [SDS] = 6.0 mM and [STC] 30.0 mM adjusted to a pH value of 8.0, while for the SLES-STC electrolyte it is required to establish a working pH equal to 7.0, [Phosphate] = 30.0 mM, [SLES] = 6.0 mM and [STC] 35.0 mM.
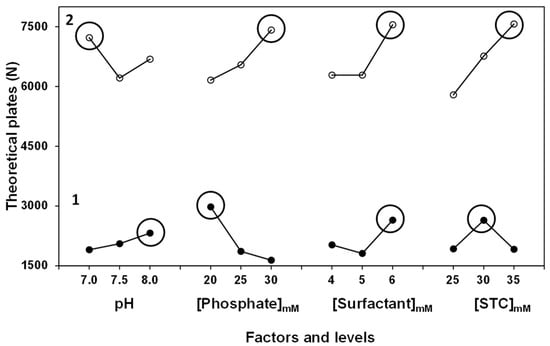
Figure 2.
Effect of the control factors over output variable (N) using the BGE SDS-STC (1) y SLES-STC (2).
Both optimized electrolytes allow for the separation of the analytes; however, a significant difference in the value of N was shown, where separation efficiency was higher using the SLES-STC electrolyte. The difference in the magnitude of N is associated to the surfactant added to each electrolyte; SLES form smaller micelle size (3 nm) compared to SDS (6 nm). The decrease in anionic micelle size increase opposition to electroosmotic flow and in consequence migration times increases, resulting in better resolution and definition for corticosteroids signals [46]. On the other hand, Figure 2 shows that all the control variables evaluated are significant in the electrophoretic separation.
For the electrolyte (SDS-STC), the factor with the most influence is [Phosphate], obtaining a 39.0% contribution; this is attributed to a gain in the ionic strength of the separation medium due to the increase in the concentration of phosphate ion, producing slightly growing retention times and retention factors and consequently improving the resolution for the signals associated with corticosteroids [47]. On the other hand, in SLES-STC electrolyte, [STC] is the factor with most contribution to the electrolyte (33.5%); by incorporating a surfactant that has a smaller micelle size and lower critical micellar concentration, it is necessary to add a greater amount of STC for the formation and stability of mixed micelles responsible for the partition of corticosteroids [48].
Employing the optimized composition for each BGE, a calibration curve was constructed (2.0, 4.0, 6.0, 8.0 and 10.0 mg L−1). Table 4 shows the analytical parameters obtained according to ICH criteria. The proposed methodology reached limits of detection in the interval of 0.95–1.13 mg L−1 for SDS-STC electrolyte, while the SLES-STC electrolyte obtained lower LODs (0.26–0.35 mg L−1). Both electrolytes allowed the separation and detection of corticosteroids according to the contents described for therapeutic doses (0.01–1.00% w/w).

Table 4.
Analytical parameters obtained for corticosteroids with SDS-STC and SLES-STC.
Precision was evaluated in terms of repeatability and intermediate precision; corticosteroid concentrations were determined by interpolation from a previously constructed calibration curve. Corticosteroids were analyzed at three concentration levels (5.0, 7.0 and 9.0 mg L−1); results are expressed as RSD. Repeatability was evaluated under homogeneous conditions by triplicate (n = 3), while intermediate precision was evaluated at three concentration levels in triplicate for three days, n = 9 (Table 5). Method precision improves as a result of obtaining better-resolved and more defined electrokinetic chromatograms (SLES-STC electrolyte); however, for both electrolytes, RSD values obtained were less than 5.0%. The results obtained are adequate for samples that contains analytes studied in the interval of 1.0–10.0 mg L−1 [49].

Table 5.
Corticosteroid concentration determined by the proposed methodology to determine precision using SDS-STC and SLES-STC electrolytes.
The accuracy of proposed methodology was determined by analyzing five pharmaceutical samples using HPLC and MEKC (both electrolytes). The results obtained for each sample are shown in Table 6. The determined concentration is presented as the mean of three independent determinations. For each sample, the mean concentrations of corticosteroid content obtained by MEKC (SDS or SLES) was compared to the content determined by the official method (HPLC) using a paired t-test. The values of tcalculated for the analysis with the SDS-STC electrolyte (1.1) and SLES-STC (1.0) were compared to tcritical (2.8, n = 4, α = 0.05), a null hypothesis which establishes that there are no significant differences between the results from each method was accepted. The proposed methodologies (SDS-MEKC and SLES-MEKC) are accurate and comparable to the established methodology. A chromatogram obtained from the analysis of the standard solution of analytes and internal standard (10.0 mg L−1) is showed in Figure S1.

Table 6.
Corticosteroid content (Mean and %RSD, n = 3) in pharmaceuticals formulations determined by the proposed methodology and compared with HPLC.
The robustness of the proposed methodology was evaluated by calculating the impact of minor variations introduced deliberately in the analytical process and the influence on results. The method validation consists of experiments carried out over several weeks, which allows the evaluation of variables that were identified as factors that could affect robustness: the storage time of standard solutions of corticosteroids (a week or a month) and sample composition (5 different pharmaceuticals formulations were analyzed). The composition of the sample is a relevant factor in the evaluation of the method since each evaluated formulation contains different excipients and drugs in addition to corticosteroids that can be an interference in the determination of the analytes. However, variations observed in the peak areas and migration times of the analytes were not significant (%RSD < 3.0%) and, consequently, the procedure can be considered robust.
Electrokinetic chromatograms obtained for the analysis of pharmaceutical samples using both electrolytes (SDS-STC and SLES-STC) are presented in Figure 3 and Figure 4, respectively. In both cases, additional signals to the evaluated compounds are observed, which are associated with other components present in the pharmaceutical samples; however, they do not interfere with the identification and quantification of signals associated with corticosteroids, securing a selective and reliable analysis.

Figure 3.
Electrokinetic chromatograms obtained using the SDS-STC electrolyte for the analysis of (a) standard solution containing 10.0 mg L−1 of corticosteroids (1; TRI, 2; PRE and 3; DEX) and internal standard (methylprednisolone, MEP), (b) sample #5 and (c) sample #4. Composition of BGE: [Phosphate] = 20.0 mM, [SDS] = 6.0 mM, [STC] 30.0 mM and pH = 8.0, separation voltage of 14 kV, electric current of 100.0 µA.
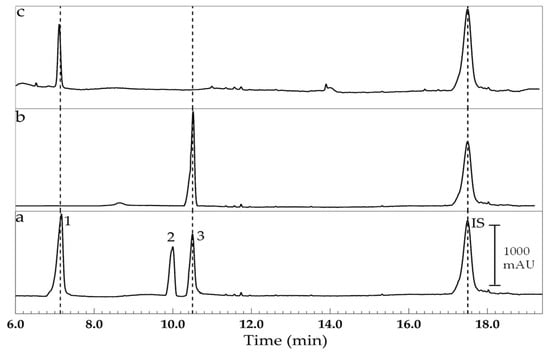
Figure 4.
Electrokinetic chromatograms obtained using the SLES-STC electrolyte for the analysis of (a) standard solution containing 10.0 mg L−1 of corticosteroids (1; TRI, 2; PRE and 3; DEX) and internal standard (methylprednisolone, MEP), (b) sample #5 and (c) sample #4. Composition of BGE: [Phosphate] = 30.0 mM, [SLES] = 6.0 mM, [STC] 35.0 mM and pH = 7.0, separation voltage of 14 kV, electric current of 100.0 µA.
On the other hand, the SLES-STC electrolyte is preferred for the analysis of corticosteroids in pharmaceutical samples because it facilitates the obtention of electrophoretic profiles with better signal resolution and lower limits of detection compared to results obtained using the SDS-STC electrolyte. Unfortunately, the retention time of the internal standard is relatively much longer than the retention times of the analytes, which prolongs the time of analysis from 11.0 min to 18.0 min.
Furthermore, according to the Danish Environmental Protection Agency, SLES is a more environmentally friendly surfactant compared to SDS [50]; this is consistent with the principle of green chemistry, which describes the use of safer solvents and auxiliaries [51].
The methodology proposed in this work emerges as viable and simple, with minimum consumption of organic solvents and a low-cost alternative for the analysis of corticosteroids in single or combined pharmaceutical samples. MEKC methodology was compared with the HPLC method using the analytical greenness metric software (AGREE, Software documentation for the Analytical Greenness Calculator v.0.5). Figure 5 shows the 12 principles of green analytical chemistry evaluated by the software; the proposed methodology (MEKC) has a more benign environmental impact than the HPLC method as result of requiring a smaller sample amount, as well as reducing the waste volumes and removing the use of organochlorine solvents.

Figure 5.
Environmental impact score according AGREE software (v. 0.5 beta) for (a) proposed methodology (MEKC) and (b) HPLC method [38].
There are few MEKC-based analytical methodologies for the determination of corticosteroids in pharmaceutical samples; however, MEKC using a BGE containing SLES-STC presents appropriate limits of detection, precision and accuracy values appropriate to the active ingredient content present in the pharmaceutical formulation samples (0.05–1.0% w/w) [52].
In general, the methods described were applied to determine a single corticosteroid (Table 7); however, this work proposes an alternative to analyze three corticosteroids simultaneously where the matrix in each sample does not interfere.

Table 7.
Comparison between methodologies used for the determination of corticosteroids in pharmaceutical formulations.
The developed methodology (MEKC) was applied in the analysis of a biological sample. The electrokinetic chromatograms obtained from the analysis of a synthetic urine sample spiked with 10.0 mg L−1 of the analytes and the internal standard are shown in Figures S2 and S3. Using both electrolytes (STC-SDS and STC-SLES), it was possible to identify the signals of interest, denoting the robustness of the method. However, a decrease in signal intensity was observed as result of matrix composition. As a perspective, the optimization of the analysis conditions in biological matrices could be carried out to improve the analytical parameters.
4. Conclusions
An MEKC-based methodology employing a separation electrolyte composed of phosphate buffer and a micellar pseudo-stationary phase constituted by a mixture of surfactants was developed for the analysis of a mixture of corticosteroids (very similar structurally) in different formulations (simple or combined), using several pharmaceutical presentations (liquid, suspension and semisolid). The proposed methodology is simple, robust and significantly reduces the use of organic solvents compared to established techniques (HPLC), and requires volumes less than 2.0 mL of BGE for the analysis of a large number of samples, which traduces into a reduction in reagents, costs and time required for the analysis of a mixture of corticosteroids. Furthermore, complex sample treatment is not necessary, and a minimal sample amount is required to perform the analysis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/separations12060154/s1: Figure S1: Chromatograms obtained employing HPLC method for the analysis of standard solution containing 10.0 mg L−1 of corticosteroids (1; TRI, 2; PRE, 3; DEX) and in-ternal standard (methylprednisolone, MEP); Figure S2: Electrokinetic chromatograms obtained using the SDS-STC electrolyte for the analysis of: (a) Synthetic urine sample containing 10.0 mg L−1 of internal standard (methylprednisolone, MEP) and (b) Synthetic urine sample containing 10.0 mg L−1 of corticosteroids (1; TRI, 2; PRE, 3; DEX) and internal standard (methylprednisolone, MEP). Composition of BGE: [Phosphate] = 20.0 mM, [SDS] = 6.0 mM, [STC] 30.0 mM and pH = 8.0, separation voltage of 14 kV, electric current of 100.0 µA; Figure S3: Electrokinetic chromatograms obtained using the SLES-STC electrolyte for the analysis of: (a) Synthetic urine sample containing 10.0 mg L−1 of internal standard (methylprednisolone, MEP) and (b) Synthetic urine sample containing 10.0 mg L−1 of corticosteroids (1; TRI, 2; PRE, 3; DEX) and internal standard (methylprednisolone, MEP). Composition of BGE: [Phosphate] = 30.0 mM, [SLES] = 6.0 mM, [STC] 35.0 mM and pH = 7.0, separation voltage of 14 kV, electric current of 100.0 µA.
Author Contributions
Conceptualization, J.A.R. and I.S.I.; methodology, K.A.E.-L. and J.L.-T.; validation, K.A.E.-L. and J.A.R.; formal analysis, K.A.E.-L. and J.A.R.; investigation, J.A.R. and I.S.I.; writing—original draft preparation, K.A.E.-L. and J.A.R.; writing—review and editing, J.A.R., J.L.-T. and I.S.I.; supervision, J.A.R.; project administration, J.A.R. and J.L.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within this article.
Acknowledgments
The authors thank the support from Secretaria de Ciencia, Humanidades, Tecnologia e Innovacion (SECIHTI) (SNI distinction as research membership and PhD scholarship No.815952).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BGE | Background electrolyte |
| CE | Capillary electrophoresis |
| DEX | Dexamethasone |
| GC | Gas chromatography |
| HPLC | High-performance liquid chromatography |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MEKC | Micellar electrokinetic chromatography |
| MEP | Methylprednisolone |
| N | Number of theoretical plates |
| PRE | Prednisolone |
| RSD | Relative standard deviation |
| SDS | Sodium dodecyl sulfate |
| SLES | Sodium lauryl ether sulfate |
| STC | Sodium taurocholate |
| TRI | Triamcinolone |
References
- Kapugi, M.; Cunningham, K. Corticosteroids. Orthop. Nurs. 2019, 38, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Gans, E.H. Topical corticosteroids, structure-activity and the glucocorticoid receptor: Discovery and development-A process of “planned serendipity”. J. Pharm. Sci. 2008, 97, 2936–2947. [Google Scholar] [CrossRef]
- Fiori, J.; Andrisano, V. LC-MS method for the simultaneous determination of six glucocorticoids in pharmaceutical formulations and counterfeit cosmetic products. J. Pharmaceut. Biomed. 2014, 91, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M. Clinical pharmacology of corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef]
- Yadav, J.P.; Lodhi, L.; Fatma, T.; Dey, K.K.; Ghosh, M. Investigation of the influence of various functional groups on the dynamics of glucocorticoids. ACS Omega 2022, 7, 43190–43209. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef]
- Görög, S. Advances in the analysis of steroid hormone drugs in pharmaceuticals and environmental samples (2004–2010). J. Pharmaceut. Biomed. 2011, 55, 728–743. [Google Scholar] [CrossRef]
- Pujos, E.; Flament-Waton, M.M.; Paisse, O.; Grenier-Loustalot, M.F. Comparison of the analysis of corticosteroids using different techniques. Anal. Bioanal. Chem. 2005, 381, 244–254. [Google Scholar] [CrossRef]
- Amendola, L.; Garribba, F.; Botrè, F. Determination of endogenous and synthetic glucocorticoids in human urine by gas chromatography-mass spectrometry following microwave-assisted derivatization. Anal. Chim. Acta 2003, 489, 233–243. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Li, J.; Wu, Z.; Wang, F.; Liu, L.; Tan, X.; Lei, F. Selective separation and determination of glucocorticoids in cosmetics using dual-template magnetic molecularly imprinted polymers and HPLC. J. Colloid Interf. Sci. 2017, 504, 124–133. [Google Scholar] [CrossRef]
- Arif, S.; Ata, S. Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics. Open Chem. 2020, 18, 962–973. [Google Scholar] [CrossRef]
- Zivanovic, L.; Zecevic, M.; Markovic, S.; Petrovic, S.; Ivanovic, I. Validation of liquid chromatographic method for analysis of lidocaine hydrochloride, dexamethasone acetate, calcium dobesilate, buthylhydroxyanisol and degradation product hydroquinone in suppositories and ointment. J. Chromatogr. A 2005, 1088, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Smits, E.A.; Smits, C.J.; Vromans, H. The development of a method to quantify encapsulated and free prednisolone phosphate in liposomal formulations. J. Pharmaceut. Biomed. 2013, 75, 47–54. [Google Scholar] [CrossRef]
- Araujo, J.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 2010, 393, 168–176. [Google Scholar] [CrossRef]
- Razzaq, S.N.; Khan, I.U.; Mariam, I.; Razzaq, S.S. Stability indicating HPLC method for the simultaneous determination of moxifloxacin and prednisolone in pharmaceutical formulations. Chem. Cent. J. 2012, 6, 1–10. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.C.; Santos, A.L.A.; Bonfilio, R.; de Araújo, M.B. A critical review of analytical methods in pharmaceutical matrices for determination of corticosteroids. Crit. Rev. Anal. Chem. 2020, 50, 111–124. [Google Scholar] [CrossRef]
- Flor, S.; Lucangioli, S.; Contin, M.; Tripodi, V. Simultaneous determination of nine endogenous steroids in human urine by polymeric-mixed micelle capillary electrophoresis. Electrophoresis 2010, 31, 3305–3313. [Google Scholar] [CrossRef]
- Sirén, H.; Seppänen-Laakso, T.; Orešič, M. Capillary electrophoresis with UV detection and mass spectrometry in method development for profiling metabolites of steroid hormone metabolism. J. Chromatogr. B 2008, 871, 375–382. [Google Scholar] [CrossRef]
- Kravchenko, A.V.; Kolobova, E.A.; Kartsova, L.A. Usage of 3-methyl-1-β-cyclodextrinimidazole tosylate for electrophoretic separation and preconcentration of corticosteroids by capillary electrophoresis. Monatsh. Chem. 2021, 152, 1067–1074. [Google Scholar] [CrossRef]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Wishart, D.S. DrugBank 6.0: The DrugBank knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Do, D.P.; Saleh, A.M. Fundamentals of micellar electrokinetic chromatography (MEKC). Eur. J. Chem. 2011, 2, 276–281. [Google Scholar] [CrossRef]
- Xu, X.; Ni, X.; Cao, Y.; Zhuo, X.; Yang, X.; Cao, G. Amphiphilic polymeric micelle as pseudostationary phase in electrokinetic chromatography for analysis of eight corticosteroids in cosmetics. Electrophoresis 2014, 35, 827–835. [Google Scholar] [CrossRef]
- Wu, R.; Tian, M.; Shu, C.; Zhou, C.; Guan, W. Determination of the critical micelle concentration of surfactants using fluorescence strategies. Soft Matter 2022, 18, 8920–8930. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Wu, S.M. Micellar electrokinetic chromatography for simultaneous determination of six corticosteroids in commercial pharmaceuticals. J. Sep. Sci. 2005, 28, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Huang, Z.J.; Huang, X.G. Rapid determination of surfactant critical micelle concentration in aqueous solutions using fiber-optic refractive index sensing. Anal. Biochem. 2010, 401, 144–147. [Google Scholar] [CrossRef]
- Elmansi, H.; El-Awady, M.I.; Barghash, S.; El-Razeq, S.A.; Belal, F. Green versatile micellar electrokinetic chromatographic method for determination of six antimicrobial and anti-inflammatory drugs in combined dosage forms. Acta Chromatogr. 2023, 35, 233–246. [Google Scholar] [CrossRef]
- Essam, H.M.; Saad, M.N.; Elzanfaly, E.S.; Amer, S.M. Stepwise optimization and sensitivity improvement of green micellar electrokinetic chromatography method to simultaneously determine some fluoroquinolones and glucocorticoids present in various binary ophthalmic formulations. Biomed. Chromatogr. 2020, 34, e4941. [Google Scholar] [CrossRef]
- Iadarola, P.; Fumagalli, M.; Viglio, S. Micellar electrokinetic chromatography. In Analytical Separation Science, 1st ed.; Anderson, J.L., Berthod, A., Pino, V., Stalcup, A.M., Eds.; Wiley-VCH: Weinheim, Alemania, 2015; pp. 675–706. [Google Scholar]
- Bessonova, E.A.; Kartsova, L.A.; Gallyamova, V.F. Effect of 3-methyl-1-cetylimidazolium chloride ionic liquid on the electrophoretic preconcentration of steroid hormones. J. Anal. Chem. 2016, 71, 696–702. [Google Scholar] [CrossRef]
- Shakalisava, Y.; Regan, F. Determination of association constants of inclusion complexes of steroid hormones and cyclodextrins from their electrophoretic mobility. Electrophoresis 2006, 27, 3048–3056. [Google Scholar] [CrossRef]
- Marzullo, L.; Gotti, R.; Orlandini, S.; Slavíčková, P.; Jireš, J.; Zapadlo, M.; Dousa, M.; Nekvapilova, P.; Rezanka, P.; Furlanetto, S. Analytical quality by design-compliant development of a cyclodextrin-modified micellar electrokinetic chromatography method for the determination of trimecaine and its impurities. Molecules 2023, 28, 4747. [Google Scholar] [CrossRef] [PubMed]
- Kovač, I.; Jakl, M.; Šolínová, V.; Konášová, R.; Kašička, V.; Dytrtová, J.J. Micellar electrokinetic chromatography in the determination of triazoles in fruit peel. J. Chromatogr. A 2021, 1652, 462385. [Google Scholar] [CrossRef] [PubMed]
- Kartsova, L.A.; Bessonova, E.A. Determination of steroids in biological samples by micellar electrokinetic chromatography. J. Anal. Chem. 2007, 62, 68–75. [Google Scholar] [CrossRef]
- Noe, S.; Böhler, J.; Keller, E.; Frahm, A.W. Evaluation and optimisation of separation buffers for the determination of corticosteroids with micellar electrokinetic capillary chromatography (MECC). J. Pharmaceut. Biomed. 1998, 18, 911–918. [Google Scholar] [CrossRef]
- Holm, R.; Müllertz, A.; Mu, H. Bile salts and their importance for drug absorption. Int. J. Pharm. 2013, 453, 44–55. [Google Scholar] [CrossRef]
- Britz-McKibbin, P.; Ichihashi, T.; Tsubota, K.; Chen, D.D.; Terabe, S. Complementary on-line preconcentration strategies for steroids by capillary electrophoresis. J. Chromatogr A 2003, 1013, 65–76. [Google Scholar] [CrossRef]
- Farmacopea de los Estados Unidos Mexicanos; Undécima Edición; Secretaria de Salud, Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos: Ciudad de México, Mexico, 2014.
- ICH HARMONISED GUIDELINE. Validation of Analytical Procedures Q2 (R1). ICH: (2022). Geneva, Switzerland. Available online: https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf (accessed on 17 February 2025).
- Jumppanen, J.H.; Wiedmer, S.K.; Sirén, H.; Riekkola, M.L.; Haario, H. Optimized separation of seven corticosteroids by micellar electrokinetic chromatography. Electrophoresis 1994, 15, 1267–1272. [Google Scholar] [CrossRef]
- Rupp, C.; Steckel, H.; Müller, B.W. Mixed micelle formation with phosphatidylcholines: The influence of surfactants with different molecule structures. Int. J. Pharmaceut. 2010, 387, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, H.; Sarkar, M. Optical spectroscopic and TEM studies of catanionic micelles of CTAB/SDS and their interaction with a NSAID. Langmuir 2004, 20, 3551–3558. [Google Scholar] [CrossRef]
- Olvera-Ureña, E.; Lopez-Tellez, J.; Vizueto, M.M.; Hidalgo-Ledezma, J.G.; Martinez-Quiroz, B.; Rodriguez, J.A. Lipase-assisted synthesis of alkyl stearates: Optimization by Taguchi design of experiments and application as defoamers. Molecules 2023, 29, 195. [Google Scholar] [CrossRef]
- Hillaert, S.; De Beer, T.R.M.; De Beer, J.O.; Van den Bossche, W. Optimization and validation of a micellar electrokinetic chromatographic method for the analysis of several angiotensin-II-receptor antagonists. J. Chromatogr. A 2003, 984, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Jimidar, M.I.; Van Ael, W.; Van Nyen, P.; Peeters, M.; Redlich, D.; De Smet, M. A screening strategy for the development of enantiomeric separation methods in capillary electrophoresis. Electrophoresis 2004, 25, 2772–2785. [Google Scholar] [CrossRef] [PubMed]
- Terabe, S. Selectivity manipulation in micellar electrokinetic chromatography. J. Pharmaceut. Biomed. 1992, 10, 705–715. [Google Scholar] [CrossRef]
- Maher, H.M.; Alzoman, N.Z.; Alshehri, M.M.; Aljohar, H.I.; Sultan, M.A. Microemulsion electrokinetic chromatography with polarity switching stacking mode for the determination of dexamethasone and dexamethasone sodium phosphate: Application to pharmacokinetic studies in rabbit plasma. Anal. Methods 2015, 7, 3260–3267. [Google Scholar] [CrossRef]
- Ćirin, D.M.; Poša, M.M.; Krstonošić, V.S. Interactions between selected bile salts and Triton X-100 or sodium lauryl ether sulfate. Chem. Cent. J. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Escamilla-Lara, K.A.; Ibarra, I.S.; Paez-Hernandez, M.E.; Gutierrez, E.; Rodriguez, J.A. Determination of prohibited corticosteroids, dexamethasone, prednisolone and triamcinolone in cosmetic creams by combination of dispersive solid phase extraction and high-performance liquid chromatography with diode array detection (HPLC-DAD). Anal. Lett. 2025, 1–16. [Google Scholar] [CrossRef]
- Hoeman, K.W.; Culbertson, C.T. A novel, environmentally friendly sodium lauryl ether sulfate-, cocamidopropyl betaine-, cocamide monoethanolamine-containing buffer for MEKC on microfluidic devices. Electrophoresis 2008, 29, 4900–4905. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- González, A.G.; Herrador, M.Á. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. TrAC Trends Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- Gallego, J.L.; Arroyo, J.P. Determination of prednisolone acetate, sulfacetamide and phenylefrine in local pharmaceutical preparations by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 2003, 31, 873–884. [Google Scholar] [CrossRef]
- Lemus Gallego, J.M.; Pérez Arroyo, J. Micellar electrokinetic capillary chromatography as an alternative method for determination of hydrocortisone and its most important associated compounds in local pharmaceutical preparations. Chromatographia 2002, 56, 455–462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).