Antimony Recovery from Industrial Residues—Emphasis on Leaching: A Review
Abstract
1. Introduction
2. Data Sourcing
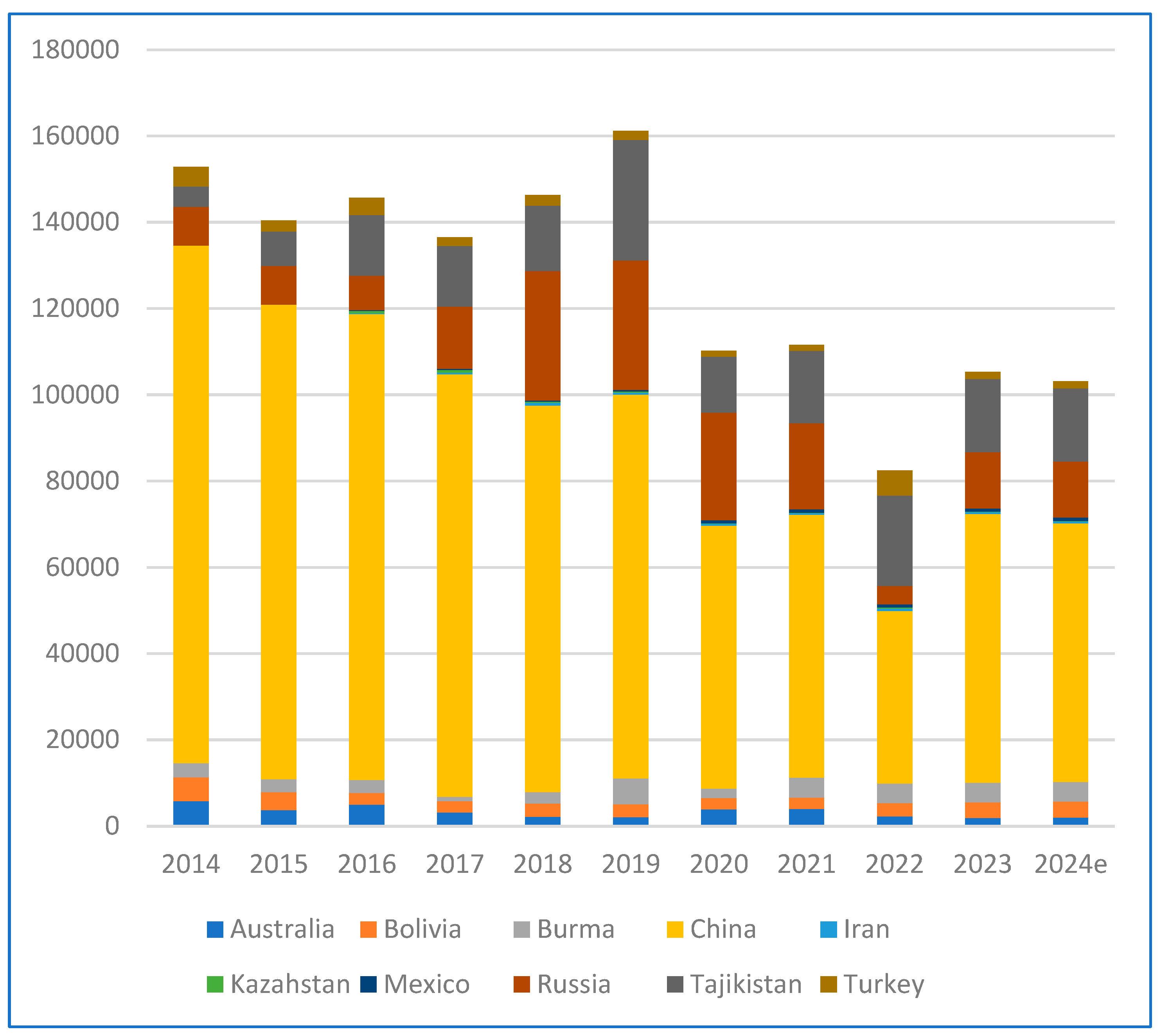
3. Antimony Availability in Industrial Residues
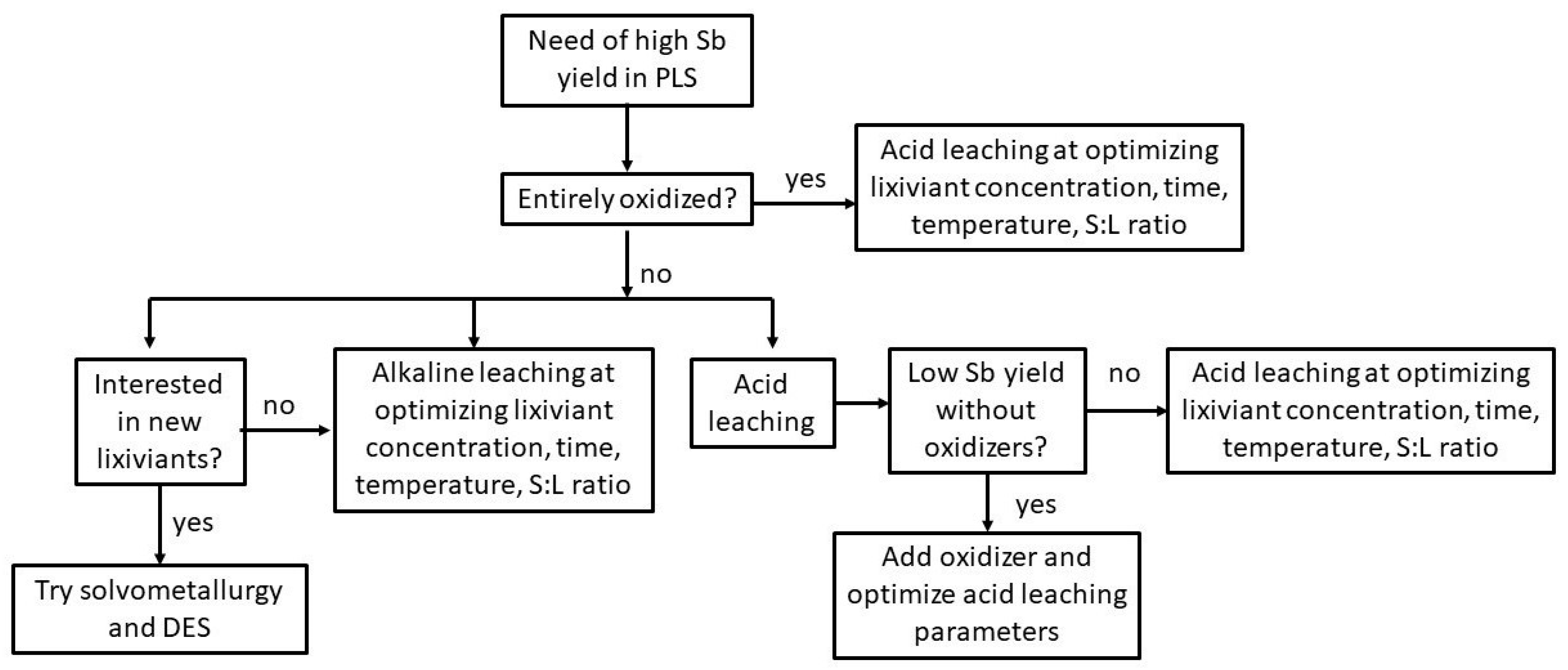
4. Antimony Leaching
4.1. Leaching in Acidic Systems
4.2. Leaching in Alkaline Sulfide Systems
4.3. Leaching in Other Solutions
4.4. Bioleaching
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Sb | Antimony |
| BASE | Bielefeld Academic Search Engine |
| CRMs | Critical raw materials |
| DESs | Deep eutectic solvents |
| DOAJ | Directory of Open Access Journals |
| EU | European Union |
| HCl | Hydrochloric acid |
| HBD | Hydrogen bond donor |
| PLS | Pregnant leach solution |
| RSCI | Russian Science Citation Index |
| NaOH | Sodium hydroxide |
| Na2S | Sodium sulfide |
| H2SO4 | Sulfuric acid |
References
- Anderson, C.G. The metallurgy of antimony. Geochemistry 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Dembele, S.; Akcil, A.; Panda, S. Technological trends, emerging applications and metallurgical strategies in antimony recovery from stibnite. Miner. Eng. 2022, 175, 107304. [Google Scholar] [CrossRef]
- McNulty, B.A.; Jowitt, S.M.; Belousov, I. The importance of geology in assessing by- and co product metal supply potential; A case study of antimony, bismuth, selenium, and tellurium within the copper production stream. Econ. Geol. 2022, 117, 1367–1385. [Google Scholar] [CrossRef]
- Segura-Salazar, J.; Brito-Parada, P.R. Stibnite froth flotation: A critical review. Miner. Eng. 2021, 163, 106713. [Google Scholar] [CrossRef]
- Nishad, P.A.; Bhaskarapillai, A. Antimony, a pollutant of emerging concern: A review on industrial sources and remediation technologies. Chemosphere 2021, 277, 130252. [Google Scholar] [CrossRef]
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. How can we adapt to geological scarcity of antimony? Investigation of antimony’s substitutability and of other measures to achieve a sustainable use. Resour. Conserv. Recycl. 2016, 108, 54–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Ma, B.; Jie, X.; Xing, P. Extracting antimony from high arsenic and gold-containing stibnite ore using slurry electrolysis. Hydrometallurgy 2019, 186, 284–291. [Google Scholar] [CrossRef]
- European Commission. Study on the Critical Raw Materials for the EU 2023—Final Report; Publication Office of the European Union: Luxembourg, 2023; Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1 (accessed on 25 May 2025).
- Klochko, K. Antimony. In U.S. Geological Survey 2024, Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; pp. 34–35. [Google Scholar] [CrossRef]
- Multani, R.S.; Feldmann, T.; Demopoulos, G.P. Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy 2016, 164, 141–153. [Google Scholar] [CrossRef]
- Dupont, D.; Arnout, S.; Jones, P.T.; Binnemans, K. Antimony recovery from end-of-life products and industrial process residues: A critical review. J. Sustain. Metall. 2016, 2, 79–103. [Google Scholar] [CrossRef]
- Tan, C.L.; Mohseni, H. Emerging technologies for high performance infrared detectors. Nanophotonics 2018, 7, 169–197. [Google Scholar] [CrossRef]
- Nie, R.; Hu, M.; Risqi, A.M.; Li, Z.; Seok, S.I. Efficient and stable antimony selenoiodide solar cells. Adv. Sci. 2021, 8, 2003172. [Google Scholar] [CrossRef]
- Itzhaik, Y.; Bendikov, T.; Hines, D.; Kamat, P.V.; Cohen, H.; Hodes, G. Band diagram and effects of the KSCN treatment in TiO2/Sb2S3/CuSCN ETA. J. Phys. Chem. C 2016, 120, 31–41. [Google Scholar] [CrossRef]
- Parize, R.; Katerski, A.; Gromyko, I.; Rapenne, L.; Roussel, H.; Kärber, E.; Appert, E.; Krunks, M.; Consonni, V. ZnO/TiO2/Sb2S3 core-shell nanowire heterostructure for extremely thin absorber solar cells. J. Phys. Chem. C 2017, 121, 9672–9680. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, S.; Deng, H.; Ishaq, M.; Yang, X.; Hou, T.; Shah, U.A.; Song, H.; Tang, J. Controllable orientations for Sb2S3 solar cells by vertical VTD method. Prog. Photovolt. 2020, 28, 823–832. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Yan, P.; Jiang, Y.; Wang, H.; Tang, Y. Sulfur-doped reduced graphene oxide/Sb2S3 composite for superior lithium and sodium storage. Mater. Chem. Phys. 2020, 244, 122661. [Google Scholar] [CrossRef]
- Moolayadukkam, S.; Bopaiah, K.A.; Parakkandy, P.K.; Thomas, S. Antimony (Sb)-Based Anodes for Lithium–Ion Batteries: Recent Advances. Condens. Matter 2022, 7, 27. [Google Scholar] [CrossRef]
- Hwang, H.; Seong, H.; Lee, S.Y.; Moon, J.H.; Kim, S.K.; Lee, J.B.; Myung, Y.; Na, C.W.; Choi, J. Synthesis of Sb2S3 NRs@rGO Composite as High-Performance Anode Material for Sodium-Ion Batteries. Materials 2021, 14, 7521. [Google Scholar] [CrossRef]
- Wu, Y.; Shuang, W.; Wang, Y.; Chen, F.; Tang, S.; Wu, X.L.; Bai, Z.; Yang, L.; Zhang, J. Recent Progress in Sodium-Ion Batteries: Advanced Materials, Reaction Mechanisms and Energy Applications. Electrochem. Energy Rev. 2024, 7, 17. [Google Scholar] [CrossRef]
- Moosavi-Khoonsari, E.; Mostaghel, S.; Siegmund, A.; Cloutier, J.P. A Review on Pyrometallurgical Extraction of Antimony from Primary Resources: Current Practices and Evolving Processes. Processes 2022, 10, 1590. [Google Scholar] [CrossRef]
- Anderson, C.G. Antimony Production and Commodities. In SME Mineral Processing and Extractive Metallurgy Handbook; Dunne, R.C., Komar-Kawatra, S., Young, C.A., Eds.; Society for Mining, Metallurgy and Exploration, Inc.: Englewood, CO, USA, 2019; pp. 1557–1568. [Google Scholar]
- Guberman, D.E. Antimony. In U.S. Geological Survey 2016, Mineral Commodity Summaries 2016; U.S. Geological Survey: Reston, VA, USA, 2016; pp. 24–25. [Google Scholar] [CrossRef]
- Guberman, D.E. Antimony. In U.S. Geological Survey 2017, Mineral Commodity Summaries 2017; U.S. Geological Survey: Reston, VA, USA, 2017; pp. 24–25. [Google Scholar] [CrossRef]
- Klochko, K. Antimony. In U.S. Geological Survey 2018, Mineral Commodity Summaries 2018; U.S. Geological Survey: Reston, VA, USA, 2018; pp. 22–23. [Google Scholar] [CrossRef]
- Klochko, K. Antimony. In U.S. Geological Survey 2019, Mineral Commodity Summaries 2019; U.S. Geological Survey: Reston, VA, USA, 2019; pp. 22–23. [Google Scholar] [CrossRef]
- Klochko, K. Antimony. In U.S. Geological Survey 2020, Mineral Commodity Summaries 2020; U.S. Geological Survey: Reston, VA, USA, 2020; pp. 22–23. [Google Scholar] [CrossRef]
- Klochko, K. Antimony. In U.S. Geological Survey 2021, Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, VA, USA, 2021; pp. 22–23. [Google Scholar] [CrossRef]
- Klochko, K. Antimony. In U.S. Geological Survey 2022, Mineral Commodity Summaries 2022; U.S. Geological Survey: Reston, VA, USA, 2022; pp. 24–25. [Google Scholar] [CrossRef]
- Sangine, E.S. Antimony. In U.S. Geological Survey 2023, Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023; pp. 32–33. [Google Scholar] [CrossRef]
- Klochko, K. Antimony. In U.S. Geological Survey 2025, Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025; pp. 34–35. [Google Scholar] [CrossRef]
- Amoah, M.; Sovacool, B.K.; Mulvaney, D.; Bazilian, M.D.; Luarkie, R.; Cardenas, D. Critical minerals mining and Native American sovereignty: Comparing case studies of lithium, copper, antimony, nickel and graphite mining in the United States. Extr. Ind. Soc. 2024, 20, 101557. [Google Scholar] [CrossRef]
- Department of Energy. Notice of Final Determination on 2023 DOE Critical Materials List; Federal Register 88(149) (A Notice by the Energy Department on 4 August 2023); Federal Register: Washington, DC, USA, 2023. Available online: https://www.federalregister.gov/documents/2023/08/04/2023-16611/notice-of-final-determination-on-2023-doe-critical-materials-list (accessed on 25 May 2025).
- European Commission. COM(2023) 160 Final ANNEXES to the Proposal for a Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020; European Commission: Brussels, Belgium, 2023; Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:903d35cc-c4a2-11ed-a05c-01aa75ed71a1.0001.02/DOC_2&format=PDF (accessed on 25 May 2025).
- European Commission. Study on the EU’s List of Critical Raw Materials (2020); Publications Office of the European Union: Luxembourg, 2020; Available online: https://op.europa.eu/en/publication-detail/-/publication/c0d5292a-ee54-11ea-991b-01aa75ed71a1/language-en (accessed on 25 May 2025).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Materials for the EU 13.9.2017 COM(2017) 490 Final; European Commission: Brussels, Belgium, 2017; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017DC0490 (accessed on 25 May 2025).
- Anderson, C.G. Hydrometallurgically treating antimony-bearing industrial wastes. JOM 2001, 53, 18–20. [Google Scholar] [CrossRef]
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards mine tailings valorization: Recovery of critical materials from Chilean mine tailings. J. Clean. Prod. 2020, 263, 121555. [Google Scholar] [CrossRef]
- Khakmardan, S.; Rezai, B.; Abdollahzadeh, A.; Ghorbani, Y. From waste to wealth: Unlocking the value of copper anode slimes through systematic characterization and pretreatment. Miner. Eng. 2023, 200, 108141. [Google Scholar] [CrossRef]
- Nakhaei, F.; Corchado-Albelo, J.; Alagha, L.; Moats, M.; Munoz-Garcia, N. Progress, challenges, and perspectives of critical elements recovery from sulfide tailings. Sep. Purif. Technol. 2025, 354, 128973. [Google Scholar] [CrossRef]
- Cooper, W.C. The treatment of copper refinery anode slimes. JOM 1990, 42, 45–49. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, S.-G.; Pan, D.A.; Li, B.; Tian, J.J.; Volinsky, A.A. Antimony recovery from SbCl5 acid solution by hydrolysis and aging. Rare Met. 2015, 34, 436–439. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Xu, Z.; Tian, Q.; Feng, Q. Leaching behavior of metals from copper anode slime using an alkali fusion leaching process. Hydrometallurgy 2015, 157, 9–12. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Xu, Z.; Xu, R.; Feng, Q. Metal values separation from residue generated in alkali fusion-leaching of copper anode slime. Hydrometallurgy 2016, 165, 290–294. [Google Scholar] [CrossRef]
- Guo, X.; Yi, Y.; Shi, J.; Tian, Q. Leaching behavior of metals from high-arsenic dust by NaOH−Na2S alkaline leaching. Trans. Nonferrous Met. Soc. China 2016, 26, 575–580. [Google Scholar] [CrossRef]
- Xue, J.; Long, D.; Zhong, H.; Wang, S.; Liu, L. Comprehensive recovery of arsenic and antimony from arsenic-rich copper smelter dust. J. Hazard. Mater. 2021, 413, 125365. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Li, Y.; Xu, Z.; Wang, R.; Ma, B. Leaching behavior and kinetics of arsenic’s leaching from arsenic-antimony dust in H2SO4-H3PO4-H2O system. Sep. Purif. Technol. 2025, 360, 130934. [Google Scholar] [CrossRef]
- Wikedzi, A.; Sandström, Å.; Awe, S.A. Recovery of antimony compounds from alkaline sulphide leachates. Int. J. Miner. Process. 2016, 152, 26–35. [Google Scholar] [CrossRef]
- Wu, X. Process Flowsheet Development for Recovering Antimony from Sb-Bearing Copper Concentrates, Degree Project in Molecular Science and Engineering; KTH Royal Institute of Technology: Stockholm, Sweden, 2023; Available online: https://kth.diva-portal.org/smash/get/diva2:1811335/FULLTEXT01.pdf (accessed on 25 May 2025).
- Zha, G.; Yang, C.; Wang, Y.; Guo, X.; Jiang, W.; Yang, B. New vacuum distillation technology for separating and recovering valuable metals from a high value-added waste. Sep. Purif. Technol. 2019, 209, 863–869. [Google Scholar] [CrossRef]
- Liu, W.; Yang, T.; Zhang, D.; Chen, L.; Liu, Y. A new pyrometallurgical process for producing antimony white from by-product of lead smelting. JOM 2014, 66, 1694–1700. [Google Scholar] [CrossRef]
- Cao, H.; Chen, J.; Yuan, H.; Zheng, G. Preparation of pure SbCl3 from lead anode slime bearing high antimony and low silver. Trans. Nonferrous Met. Soc. China 2010, 20, 2397–2403. [Google Scholar] [CrossRef]
- Osmani, A.; Rizaj, M.; Terziqi, A.; Kamberaj, N. Slag valorisation of reductive smelting process by shaft furnace in the lead metallurgy of “Trepça” complex with economical and environmental effects. J. Int. Environ. Appl. Sci. 2009, 4, 198–206. [Google Scholar]
- Li, Y.; Liu, Z.; Li, Q.; Liu, F.; Liu, Z. Alkaline oxidative pressure leaching of arsenic and antimony bearing dusts. Hydrometallurgy 2016, 166, 41–47. [Google Scholar] [CrossRef]
- Li, W.; Liu, W.; Jiao, F.; Xie, L.; Qin, W. Comprehensive recovery of arsenic and valuable metals from lead smelting flue dust: Process optimization and mechanism investigation. Sep. Purif. Technol. 2025, 353, 128497. [Google Scholar] [CrossRef]
- Ling, H.; Blanpain, B.; Guo, M.; Malfliet, A. Characterization of antimony-containing metallurgical residues for antimony recovery. J. Clean. Prod. 2021, 327, 129491. [Google Scholar] [CrossRef]
- Kanarskii, A.V.; Adamov, E.V.; Krylova, L.N. Flotation concentration of the sulfide antimony-arsenic gold-bearing ore. Russ. J. Non-Ferrous Met. 2012, 53, 120–124. [Google Scholar] [CrossRef]
- Solozhenkin, P.M.; Alekseev, A.N. Innovative processing and hydrometallurgical treatment methods for complex antimony ores and concentrates. J. Min. Sci. 2010, 46, 203–209. [Google Scholar] [CrossRef]
- Celep, O.; Alp, I.; Deveci, H. Improved gold and silver extraction from a refractory antimony ore by pretreatment with alkaline sulphide leach. Hydrometallurgy 2011, 105, 234–239. [Google Scholar] [CrossRef]
- Karimi, P.; Abdollahi, H.; Amini, A.; Noaparast, M.; Shafaei, S.Z.; Habashi, F. Cyanidation of gold ores containing copper, silver, lead, arsenic and antimony. Int. J. Miner. Process. 2010, 95, 68–77. [Google Scholar] [CrossRef]
- Yang, T.; Rao, S.; Liu, W.; Zhang, D.; Chen, L. A selective process for extracting antimony from refractory gold ore. Hydrometallurgy 2017, 169, 571–575. [Google Scholar] [CrossRef]
- Han, J.; Ou, Z.; Liu, W.; Jiao, F.; Qin, W. Recovery of antimony and bismuth from tin anode slime after soda roasting–alkaline leaching. Sep. Purif. Technol. 2020, 242, 116789. [Google Scholar] [CrossRef]
- Tan, C.; Li, H.; Li, J.; Li, L. Recovery of Sb and Fe from Sb-bearing Slags through a Reduction Roasting Process with Low Density Polyethylene. ISIJ Int. 2019, 59, 2113–2119. [Google Scholar] [CrossRef]
- Tan, C.; Li, L.; Zhong, D.; Wang, H.; Li, K. Separation of arsenic and antimony from dust with high content of arsenic by a selective sulfidation roasting process using sulfur. Trans. Nonferrous Met. Soc. China 2018, 28, 1027–1035. [Google Scholar] [CrossRef]
- Zhong, D.; Li, L. Separation of arsenic from arsenic−antimony-bearing dust through selective oxidation−sulfidation roasting with CuS. Trans. Nonferrous Met. Soc. China 2020, 30, 223–235. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, L.; Zheng, Q.; Che, X.; Cui, X.; Wei, S.; Li, H.; Shi, X. Present Situation and Research Progress of Comprehensive Utilization of Antimony Tailings and Smelting Slag. Sustainability 2023, 15, 13947. [Google Scholar] [CrossRef]
- Dembele, S.; Akcil, A.; Panda, S. Investigation of the characteristics of stibnite (Sb2S3) flotation tailings and extraction of critical metals (Sb and As): Optimization and scale-up. Miner. Eng. 2024, 216, 108883. [Google Scholar] [CrossRef]
- Chen, L.; Ren, B.; Deng, X.; Yin, W.; Xie, Q.; Cai, Z. Potential toxic heavy metals in village rainwater runoff of antimony mining area, China: Distribution, pollution sources, and risk assessment. Sci. Total Environ. 2024, 920, 170702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, P.; Ye, Z.; Wen, B.; Beckie, R.D.; Zhou, A.; Zhou, Z.; Zhou, J. Antimony mobility in soil near historical waste rock at the world’s largest Sb mine, Central China. Sci. Total Environ. 2024, 921, 17119. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ran, Y.; Wu, P.; Liu, P.; Yang, B.; Gu, X.; Zhao, P.; Liu, S.; Song, L.; Liu, Y.; et al. Antimony and arsenic migration in a heterogeneous subsurface at an abandoned antimony smelter under rainfall. J. Hazard. Mater. 2024, 470, 134156. [Google Scholar] [CrossRef]
- Kappen, P.; Ferrando-Miguel, G.; Reichmand, S.M.; Innes, L.; Welter, E.; Pigram, P.J. Antimony leaching and chemical species analyses in an industrial solid waste: Surface and bulk speciation using ToF-SIMS and XANESP. J. Hazard. Mater. 2017, 329, 131–140. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Sherman, D.M.; Ragnarsdottir, K.V.; Collins, C. An EXAFS spectroscopic study of aqueous antimony(III)-chloride complexation at temperatures from 25 to 250 °C. Chem. Geol. 1998, 151, 21–27. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, B.; Hursthouse, A.S.; Zhou, S. Antimony Ore Tailings: Heavy Metals, Chemical Speciation, and Leaching Characteristics. Pol. J. Environ. Stud. 2019, 28, 485–495. [Google Scholar] [CrossRef]
- Singh, L.N. Synthesis of potassium antimony tartrate from the antimony dross of lead smelters. Hydrometallurgy 1990, 25, 19–25. [Google Scholar] [CrossRef]
- Rahimzoda, K.S.; Anderson, C.G.; Badalov, A.B.; Kadirov, A.A.; Eshov, B.B. Hydrometallurgical Processing of Antimony Bearing Pyrometallurgical Calcines. J. Miner. Mater. Sci. 2024, 5, 1097. [Google Scholar] [CrossRef]
- Sajadi, S.A.A.; Khorablou, Z.; Naeini, M.S. Recovery of antimony from acidic and alkaline leaching solution of low-grade antimony ore by electrowinning process. Heliyon 2024, 10, e35300. [Google Scholar] [CrossRef]
- Ye, L.; Ouyang, Z.; Chen, Y.; Chen, Y. Ferric chloride leaching of antimony from stibnite. Hydrometallurgy 2019, 186, 210–217. [Google Scholar] [CrossRef]
- Padilla, R.; Caro, O.; Vega-Garcia, D.; Ruiz, M.C. Mechanism and Kinetics of Stibnite Dissolution in H2SO4-NaCl-Fe(SO4)1.5-O2. Minerals 2022, 12, 718. [Google Scholar] [CrossRef]
- Li, G.; Xin, Y.-T.; Lu, X.-D.; Tian, Q.-H.; Yan, K.; Ye, L.-G. Stability constants of Sb5+ with Cl− and thermodynamics of Sb−S−Cl−H2O system involving complex behavior of Sb with Cl. Trans. Nonferrous Met. Soc. China 2020, 30, 3379–3389. [Google Scholar] [CrossRef]
- Ibrahim, A.I.I.; Aboelgamel, M.; Soylu, K.K.; Top, S.; Kursunoglu, S.; Altiner, M. Production of high-grade antimony oxide from smelter slag via leaching and hydrolysis process. Sep. Purif. Technol. 2025, 354, 129355. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Y. A hydrometallurgical process for the separation and recovery of antimony. Hydrometallurgy 2014, 143, 68–74. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, H.; Xin, Y.; Li, D.; Guo, X. Ozonation leaching of a complex sulfidic antimony ore in hydrochloric acid solution. Hydrometallurgy 2016, 159, 126–131. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Y.; Wang, H.; Tian, Q. Leaching kinetics of antimony-bearing complex sulfides ore in hydrochloric acid solution with ozone. Trans. Nonferrous Met. Soc. China 2017, 27, 2073–2081. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Y.; Wang, H.; Tian, Q. Mineralogical characterization and pretreatment for antimony extraction by ozone of antimony-bearing refractory gold concentrates. Trans. Nonferrous Met. Soc. China 2017, 27, 1888–1895. [Google Scholar] [CrossRef]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Annex 3: Codification of Statements and Pictograms; United Nations: New York, NY, USA; Geneva, Switzerland, 2021; pp. 268–385. [Google Scholar]
- Awe, S.A.; Khohkhoo, M.; Ktuger, P.; Sandtrőm, Å. Modelling and process optimisation of antimony removal from a complex copper concentrate. Trans. Nonferrous Met. Soc. China 2012, 22, 675–685. [Google Scholar] [CrossRef]
- Luo, D.; Wu, X.; Vázquez, B.; Maestre, M.; Davoise, D.; Lopez, J.; Cortina, J.L. Selective recovery of antimony from Sb-bearing copper concentrates by integration of alkaline sulphide leaching solutions and microwave-assisted heating: A new sustainable processing route. Sci. Total Environ. 2024, 951, 175576. [Google Scholar] [CrossRef]
- Rusalev, R.; Rogozhnikov, D.; Dizer, O.; Golovkin, D.; Karimov, K. Development of a Two-Stage Hydrometallurgical Process for Gold–Antimony Concentrate Treatment from the Olimpiadinskoe Deposit. Materials 2023, 16, 4767. [Google Scholar] [CrossRef]
- Dembele, S.; Akcil, A.; Panda, S. Study of alkaline hydrometallurgical process for stibnite flotation tailings reprocessing: Semi-pilot antimony leaching. Miner. Eng. 2025, 222, 109168. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Sudová, M.; Sisol, M.; Kanuchova, M.; Marcin, M.; Kurty, J. Environmentally Friendly Leaching of Antimony from Mining Residues Using Deep Eutectic Solvents: Optimization and Sustainable Extraction Strategies. Processes 2024, 12, 555. [Google Scholar] [CrossRef]
- Poll, C.G.; Nelson, G.W.; Pickup, D.M.; Chadwick, A.V.; Riley, D.J.; Payne, D.J. Electrochemical recycling of lead from hybrid organic–inorganic perovskites using deep eutectic solvents. Green Chem. 2016, 18, 2946–2955. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An emerging branch of extractive metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef]
- Palden, T.; Machiels, L.; Regadío, M.; Binnemans, K. Antimony Recovery from Lead-Rich Dross of Lead Smelter and Conversion into Antimony Oxide Chloride (Sb4O5Cl2). ACS Sustain. Chem. Eng. 2021, 9, 5074–5084. [Google Scholar] [CrossRef]
- Hafeez, I.; Nasir, S.; Zahra, S.; Aamir, M.; Mahmood, Z.; Akram, A. Metal extraction process for high grade stibnite of Kharan (Balochistan Pakistan). J. Miner. Mater. Charact. Eng. 2017, 5, 39–48. [Google Scholar] [CrossRef][Green Version]
- Muravyov, M. Two-step processing of refractory gold-containing sulfidic concentrate via biooxidation at two temperatures. Chem. Pap. 2019, 73, 173–183. [Google Scholar] [CrossRef]
- Loni, P.C.; Wu, M.; Wang, W.; Wang, H.; Ma, L.; Liu, C.; Song, Y.H.; Tuovinen, O. Mechanism of microbial dissolution and oxidation of antimony in stibnite under ambient conditions. J. Hazard. Mater. 2020, 385, 121561. [Google Scholar] [CrossRef]
- Ubaldini, S.; Veglio, F.; Toro, L.; Abbruzzese, C. Combined biohydrometallurgical process for gold recovery from refractory stibnite. Miner. Eng. 2000, 13, 1641–1646. [Google Scholar] [CrossRef]
- Čerňanský, S.; Šimonovičová, A.; Juhásová, J.; Semerád, M. Bioleaching of Arsenic and Antimony from Mining Waste. Acta Environ. Univ. Comen. 2016, 24, 5–9. [Google Scholar] [CrossRef]
- Aghazadeh, S.; Abdollahi, H.; Gharabaghi, M.; Mirmohammadi, M. Bioleaching of zinc, copper and antimony from a tetrahedrite concentrate using acidophilic microorganisms. Hydrometallurgy 2023, 219, 106075. [Google Scholar] [CrossRef]
- Tsaplina, I.A.; Sorokin, V.V.; Zhuravleva, A.E.; Melamud, V.S.; Bogdanova, T.I.; Kondrat’eva, T.F. Oxidation of gold-antimony ores by a thermoacidophilic microbial consortium. Microbiology 2013, 82, 680–689. [Google Scholar] [CrossRef]
- de Carvalho, L.C.; da Silva, S.R.; Giardini, R.M.N.; de Souza, L.F.C.; Leão, V.A. Biooxidation of refractory gold ores containing stibnite and gudmundite. Environ. Technol. Innov. 2019, 15, 100390. [Google Scholar] [CrossRef]
- Hagarova, L.; Kupka, D.; Bartova, Z. Bioleaching of Antimony from Tetrahedrite Concentrate by Iron-Oxidizing Bacteria. In Proceedings of the 63rd Conference of Metallurgists, COM 2024, Halifax, NS, Canada, 19–22 August 2024; Metallurgy and Materials Society of CIM, Ed.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]


| Sb Content, % | Type of Raw Material | Processing Method | Basic Chemical Reactions |
|---|---|---|---|
| 5–25 | sulfide | oxide volatilization (by roasting and volatilization at 1000 °C) followed by reduction smelting or reverberatory smelting of oxides | 2Sb2S3 + 9O2 → 2Sb2O3 + 6SO2 2Sb + 1.5O2 → Sb2O3 Sb2O3 + 3CO → 2Sb + 3CO2 Sb2O3 + 3C → 2Sb + 3CO |
| 25–40 | sulfide | blast furnace smelting at 1300–1400 °C | Sb2S3 + 9O2 → 2Sb2O3 + 6SO2 2Sb2O3 + Sb2S3 → 6Sb + 3SO2 |
| sulfide | blast furnace direct reduction to metal | 2Sb2S3 + 3C → 3CS2 + 4Sb Sb2S3 + 3CO → 3COS + 2Sb | |
| 45–60 | sulfide | liquation at 550–600 °C, iron (Fe) precipitation alkaline smelting, or reduction smelting | Sb2S3(S) → Sb2S3(L) Sb2S3 + 3Fe → 2Sb + 3FeS 2Sb2S3 + 6Na2O + 3C → 4Sb + 6Na2S + 3CO2 |
| >60 | sulfide | iron precipitation | Sb2S3 + 3Fe → 2Sb + 3FeS |
| all grades | oxide | reduction smelting in reverberatory and electric or blast furnace | Sb2O3 + 3CO → 2Sb + 3CO2 CO2 + C → 2CO |
| Type of Man-Made Source | Sb, wt.% | As, wt.% | Bi, wt.% | Reference |
|---|---|---|---|---|
| Flue dust | 3.1 | 2.8 | 2.8 | [11] |
| Tin depleted anode slime | 24.6 | 2.0 | 2.0 | [42] |
| Anode slime | 5.09 | 4.1 | n.d. | [43] |
| Anode slime | 12.62 | n.d. | 1.76 | [44] |
| Flue dust | 9.55 | 6.86 | 0.12 | [45] |
| Flue dust | 17.58 | 28.72 | n.d. | [46] |
| Arsenic-antimony dust | 20.38 | 53.39 | n.d. | [47] |
| Speiss | 8.8 | 7.7 | 0.003 | [48] |
| Anode slimes from different companies worldwide | 0.5–3.4 | 0.7–4.1 | 0.1–0.77 | [39] |
| Cu mining waste—2 mines (unspecified) | 0.46–1.03 | 0.29 | n.d. | [49] |
| Flotation tailings from the copper-anode dressing-metallurgy | 15.39 | n.d. | 3.9 | [50] |
| Type of Man-Made Source | Sb, wt.% | As, wt.% | Bi, wt.% | Reference |
|---|---|---|---|---|
| Sb dust | 42.4 | 10.4 | n.d. | [51] |
| Harris dross | 8.2 | n.d. | n.d. | [11] |
| Slime | 63.6 | 4.0 | 3.3 | [52] |
| Anode slime | 12.62 | n.d. | 1.76 | [44] |
| Matte | 0.9 | 0.6 | n.d. | [53] |
| Lead anode slime | 22.7 | 23.4 | 0.86 | [54] |
| Lead smelting flue dust | 0.35 | 33.82 | n.d. | [55] |
| Lead softening slag | 17.7 | 4.4 | n.d. | [56] |
| Type of Man-Made Source | Au (g/t) | Sb, wt.% | As, wt.% | Reference |
|---|---|---|---|---|
| Refractory Sb-Au ore | 3.6 | 0.3 | 0.4 | [57] |
| Refractory Sb-Au ore | 7.4 | 16.73 | n.d. | [58] |
| Refractory Sb-Au ore | 42.2 | 28.7 | n.d. | [58] |
| Refractory Sb-Au ore | 20.0 | 1.6 | n.d. | [59] |
| Refractory Sb-Au ore | 10.5 | 0.22 | 1.67 | [60] |
| Refractory Sb-Au ore | 58.8 | 6.30 | 5.50 | [61] |
| Type of Man-Made Source | Sb, wt.% | Bi, wt.% | As, wt.% | Reference |
|---|---|---|---|---|
| Tin anode slime | 13.24 | 19.38 | 2.44 | [62] |
| Slag from As–Sb dust treating plant | 42.04 | n.d. | n.d. | [63] |
| Pyrometallurgically treated tin anode slime | 28.72 | 0.685 | 36.28 | [64,65] |
| Raw Material; Sb Content, % | Operating Conditions | Sb Recovery in PLS, % | Reference |
|---|---|---|---|
| Cu smelter dust, 17.58% | 4 M HCl, L:S = 6:1, 90 °C, and 2 h | 97.53 | [46] |
| Stibnite flotation tailings, 1.74% | 4.4 M HCl, 0.5 M NaNO3, pulp density 25%, 70 °C, and 1 h | 99.88 | [67] |
| Low-grade ore, n.a. % Fe3Si2O5(OH)4·Sb3O6(OH) | 5 M HCl, 80 °C, and 8 h | 87 | [76] |
| Slag from Sb smelting, 4.12% | 8 M HCl, 75 °C, and 3 h | 91.9 | [80] |
| Stibnite concentrate, 61.85% | 4 M HCl, L:S = 5:1, 85 °C, and 2 h (Cl2/SbCl5 solution) | 99.5 | [81] |
| Complex sulfidic Sb ore, 58.57% | 4.5 M HCl, L:S = 8:1, 65 °C, and 2 h (O3 2 L/min) | 94.3 | [82] |
| Raw Material; Sb Content, % | Operating Conditions | Sb Recovery in PLS, % | Reference |
|---|---|---|---|
| Lead silicate slag, 6.5% | 0.75 M NaOH, 100 °C, and 24 h | 83 | [48] |
| Refractory Au ore, 6.30% | 0.5 M NaOH, 1 M Na2S, L:S = 1.5:1, 50 °C, and 1.5 h | 96.64 | [61] |
| Stibnite flotation tailings, 1.74% | 2.5 M NaOH, 0.97 M Na2S, pulp density = 25%, 70 °C, and 1 h | Bench scale 99.13 Semi pilot scale 97.00 | [67] |
| Tin anode slime obtained by soda roasting, 13.21% | 0.7 M Na2S, L:S = 14:1, 85 °C, and 2 h | 98 | [62] |
| Low-grade ore, n.a. % Fe3Si2O5(OH)4·Sb3O6(OH) | 5 M NaOH, 0.5 M Na2S, 80 °C, and 8 h | 85 | [76] |
| Complex Cu concentrate, 1.69% | 0.2 M NaOH, 80 °C, and 20 h | 52 | [86] |
| Sb-bearing Cu concentrate, 1.04% | 1.5 M NaOH, 4.5 M Na2S, 140 °C, and 2 h (microwave heating) | 96 | [87] |
| Au-Sb concentrate, 19.18% | 0.4 M NaOH, 1.1 M Na2S, L:S = 4.5:1, 50 °C, and 3 h | 99 | [88] |
| Leaching System; Parameter | HCl-Based | NaOH + Na2S Based | Comments |
|---|---|---|---|
| Temperature | 65–90 | 70–100 | Keeping in mind both temperature and process duration, it could be expected that there would not be a big difference in energy consumption. |
| Time | 2–3 | 1.5–2 | |
| Reagent’s consumption | >4 M | 0.5–2.5 M NaOH 1–2 M Na2S | Keeping in mind both reagents’ consumption and price, it could be expected that there would not be a big difference in the costs related to the reagents. |
| Reagent’s price, USD/kg | 0.2–0.30 1 | NaOH 0.43–0.70 2 Na2S 0.41–0.43 3 | |
| Sb leaching efficiency | 87–99% | 85–99% | Generally, the addition of an oxidizing reagent is needed to achieve efficiency > 90% by HCl-based systems. |
| Safety issues and hazards according to the GHS 4 | H290 H314 H335 | NaOH: H290; H302; H314 Na2S: H302; H311; H314; H400 | Proper measures for protecting the workers’ health and the environment have to be considered with both leaching systems. |
| Selectivity with respect to Sb | Low | High | A decrease in the number of procedures and costs for further Sb recovery from the PLS from alkaline solutions is expected. |
| Sb separation from the PLS | Hydrolysis, conversion, and electrowinning | Precipitation, crystallization, and electrowinning | |
| Equipment corrosion | High | Low | Although the H290 code is given for both systems, it is well known from corrosion science that the acidic medium is considerably more corrosive compared to the alkaline one for general-purpose leaching equipment, especially in the pH ranges used in Sb leaching. |
| Scalability | Difficult, mainly due to corrosion problems | Relatively easy | Alkaline-based leaching predominates in real industrial applications. |
| Microorganism | Leaching Conditions | Sb Recovery in the PLS | Reference |
|---|---|---|---|
| At. ferrooxidans | pH 2, 37 °C, and 120 days | 73% | [95] |
| Sulfobacillus | pH 1.6–2.0, 50 °C and 39 °C, and 4 days | n.d. | [96] |
| Aspergillus niger | pH 8.49, 21 days | 10.8 to 13.7% | [99] |
| Mesophilic: Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, and Leptospirillum ferrooxidans Moderately thermophilic prokaryotes: Acidithiobacillus caldus, Leptospirillum ferriphilum, and Sulfobacillus | initial pH 1.8, 34 °C for mesophiles and 45 °C for thermophiles, and 17 days | 6.6% | [100] |
| Sulfobacillus, Leptospirillum, and Ferroplasma | pH 1.8–1.9, 39 °C, and 14 days | 20.6–86.2% | [101] |
| Acidithiobacillus ferrooxidans | pH 1.75, 32 °C, and 40 days | <13% | [102] |
| At. ferrooxidans, At. ferrivorans SS3, and Leptospirillum ferriphilum | pH ≅ 2, 25 °C, and 30 days | 12.7% | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panayotova, M.; Pysmennyi, S.; Panayotov, V. Antimony Recovery from Industrial Residues—Emphasis on Leaching: A Review. Separations 2025, 12, 156. https://doi.org/10.3390/separations12060156
Panayotova M, Pysmennyi S, Panayotov V. Antimony Recovery from Industrial Residues—Emphasis on Leaching: A Review. Separations. 2025; 12(6):156. https://doi.org/10.3390/separations12060156
Chicago/Turabian StylePanayotova, Marinela, Serhii Pysmennyi, and Vladko Panayotov. 2025. "Antimony Recovery from Industrial Residues—Emphasis on Leaching: A Review" Separations 12, no. 6: 156. https://doi.org/10.3390/separations12060156
APA StylePanayotova, M., Pysmennyi, S., & Panayotov, V. (2025). Antimony Recovery from Industrial Residues—Emphasis on Leaching: A Review. Separations, 12(6), 156. https://doi.org/10.3390/separations12060156









