Abstract
The advancement of miniaturized liquid chromatography (M-LC) systems has drawn considerable attention for their ability to enhance sensitivity, expedite analysis, and minimize the environmental impact of chemical usage in various analytical processes. This review explores the fundamental principles and recent innovations in M-LC technology, including diverse pump designs, advanced column techniques, and the reduction in connection devices. Emphasizing the need for components that operate efficiently at the capillary or nanoscale with minimal dead volumes, we also discuss the development of benchtop instruments and mass spectrometry integrations. The review further highlights the growing applications of M-LC in food, environmental, and biological analyses, highlighting its potential as a powerful and emerging tool in separation science. Looking forward, addressing problems such as limited robustness, fabrication complexity, and integration with sensitive detectors will be instrumental to advancing M-LC technology. Modern innovation in microfabrication, materials science, and hyphenated methods holds great promise for allowing real-time, high-throughput, and portable analytical solutions in the near future.
1. Introduction
1.1. History of Chromatography
The conceptual roots of liquid chromatography (LC) miniaturization can be traced back to 1957–1958, when Martin and Golay introduced the concept of capillary columns in gas chromatography (GC), which laid the theoretical foundation for their later adoption in LC [1]. In 1964, J.C. Giddings proposed the use of capillary columns in LC to improve separation efficiency, marking one of the earliest theoretical advances in miniaturized LC systems [2]. Subsequently, ref. [3] applied 1 mm i.d. columns for the separation of ribonucleotides, a major practical leap forward in column size reduction. From 1973 to 1978, Ishii and colleagues developed 0.5 mm i.d. capillary columns and, importantly, engineered gradient pumps and low-volume ultra-violet detectors specifically designed for capillary formats [4].
In 1976–1884, JASCO introduced commercially available micro-LC systems (FAMILIC-100), further supporting the transition from conventional HPLC to miniaturized LC [5]. Novotny (1978) advanced the field by employing drawn glass capillary columns to conduct high-efficiency separations [6]. Then, in 1981, Jorgenson and Lukacs made a groundbreaking contribution with the introduction of capillary electrochromatography (CEC) using packed columns, setting a milestone for electrically driven miniaturized separations [7].
By 1985–1986, Ishii and Yang had proposed a unified approach to chromatography, envisioning the use of a single column format across GC, LC, and Supercritical fluid chromatography(SFC), reinforcing the convergence of separation sciences [8]. In parallel, MiLiChrome systems, a family of micro-LC instruments, emerged in Russia and gained moderate distribution until the mid-1990s, as noted by Baram et al. in a review of micro-LC innovations [9]. The 1990s and early 2000s saw an explosion of innovation: capillary monolithic columns (both silica and polymeric), chip-based columns, AI-coupled LC detection, and on-chip valves/injection technologies. Table 1 shows the milestones of LC in the past. These advances shaped the modern field of M-LC and chip-LC systems now being used in omics, toxicology, food safety, and environmental science.

Table 1.
Summary of milestones in LC miniaturization.
1.2. Liquid Chromatography
The late 1960s saw the emergence of high-performance liquid chromatography (HPLC), a significant enhancement over classical LC methods [10]. HPLC uses a liquid mobile phase in conjunction with a high-pressure infusion system to achieve separation based on the physical and chemical properties of analytes [11]. It is particularly effective for analyzing organic compounds that exhibit high boiling points, low volatility, large molecular weights, or diverse polarities—factors that limit the applicability of GC [12,13]. Known for its precision, high efficiency, rapid throughput, and broad applicability, HPLC has become the dominant technique in fields such as biochemistry, medicine, food science, and environmental monitoring [12,13]. Its development has also driven innovations in stationary phase materials, detector technology, data processing, and theoretical aspects of chromatography [11].
Moreover, ultra-high-performance liquid chromatography (UHPLC) continues to be an important technique characterized by ultra-fast analysis, high-resolution separation, and heightened sensitivity [14,15]. Building on the principles of HPLC, UHPLC incorporates innovations such as sub-2 µm particle packing, reduced system dead volume, and rapid detection methods to enhance flow rates, sensitivity, and chromatographic peak capacity [16]. Compared to HPLC, UHPLC offers improved resolution and significantly reduced analysis times. It is increasingly used in pharmaceutical research, biochemical assays, food safety testing, and environmental analysis [13,16].
1.3. Why Do We Want to Miniaturize Liquid Chromatography?
Although LC technology has advanced significantly over the past six decades, the widespread use of HPLC remains limited in certain contexts due to factors such as bulky instrumentation, high operational costs, and lengthy analysis times [17]. These limitations have spurred interest in the development of new LC-based approaches [18], with system miniaturization emerging as a prominent trend, especially in analytical chemistry [19]. M-LC offers several advantages: higher chromatographic resolution, reduced consumption of samples and organic solvents, and improved reproducibility [20].
As LC systems shrink in size, there is a corresponding miniaturization of analytical columns and continuous innovation in detector technology, which facilitates seamless coupling with mass spectrometry [21].
This review focuses on the recent progress and applications of M-LC over the past decade, particularly in the domains of food safety, environmental monitoring, and biological analysis.
1.4. Why Use M-LC for Food Safety Monitoring, Environmental Tracking, and Biological Analysis?
1.4.1. Food Safety Monitoring
The increasing prevalence of food contamination and its public health implications have made food safety and quality assurance global priorities. M-LC technologies offer effective tools for verifying food authenticity, assessing nutritional and toxicological properties, and detecting contaminants and residues via high-resolution chromatographic data [22,23]. Chip-based M-LC platforms are especially promising, allowing for rapid, on-site screening of food adulterants, toxins, and other hazards—an essential capability in settings with limited laboratory infrastructure [23].
Given the complexity of food matrices, robust analytical methods are required. Liquid chromatography-mass spectrometery (LC-MS) is a powerful solution [24], though traditional LC-MS platforms are often expensive and technically demanding. Emerging chip-based LC-MS systems aim to overcome these barriers by offering greater throughput and sensitivity [25], enabling precise detection of nutritional components, veterinary drug residues, and pesticide contaminants in a variety of food products.
1.4.2. Environmental Analysis
Environmental monitoring represents a critical application area for M-LC systems, particularly for tracking pollutants in aquatic and terrestrial environments. Water samples, in particular, are well suited for M-LC due to their relatively simple matrices, which allow direct injection with minimal risk of column clogging [26,27]. In addition to miniaturized versions of conventional HPLC (e.g., capillary and micro-LC systems), researchers are increasingly exploring the use of microchip-based platforms in place of traditional analytical columns [28,29]. These chip-based LC-MS methods significantly reduce both analysis time and solvent consumption, making them a promising advancement in environmental science [30,31].
1.4.3. Biological Analysis
Progress in bioanalytical chemistry relies heavily on innovative analytical technologies. LC-MS has established itself as a gold standard in this field, with chip-based LC-MS gaining traction as a more efficient alternative [32,33]. Omics disciplines, particularly proteomics and metabolomics, demand high-resolution and high-throughput platforms [34]. M-LC, especially when integrated with MS, offers enhanced peak resolution, enabling more comprehensive metabolite profiling and quantification [35]. Compared to conventional benchtop instruments, chip-based LC provides notable benefits, including faster analysis, higher sensitivity, reduced band broadening, and increased throughput, making it exceptionally well-suited for complex biological studies [17].
M-LC also plays an important role in advancing the goals of Green Analytical Chemistry (GAC), particularly through its significant reduction in solvent consumption and improved energy efficiency [36]. M-LC systems, such as capillary LC and nano-LC, operate with extremely low flow rates, often in the nL to mL per minute range, leading to solvent reductions of up to 1000 times compared to traditional LC systems. This not only decreases hazardous waste but also reduces costs and improves laboratory sustainability practices [36,37]. It also shows the integration of high-efficiency columns and water-rich or alternative green solvents (e.g., ethanol, glycerol, deep eutectic solvents) as key trends in greener LC system design [36].
Miniaturized systems use less thermal energy since they have smaller interior volumes and frequently operate at room temperature, which aligns with GAC’s energy efficiency principles. Coupling M-LC with MS or miniaturized detectors allows for real-time and in-process analysis, which are GAC principles centered on pollution reduction and rapid decision-making [36]. However, they acknowledge trade-offs: M-LC systems may face challenges such as reduced sample loading capacity and the need for precise control systems to manage low flow rates. Despite these limitations, M-LC stands out as a promising green technology that minimizes the ecological footprint of chemical analysis while maintaining analytical performance.
2. Development of an M-LC System
The development of M-LC systems presents a multifaceted engineering challenge, demanding innovation across multiple components [38]. Early pioneers laid the foundation for today’s advanced systems, which emphasize high precision, portability, and reduced sample consumption. One of the earliest breakthroughs came in 1986 when Otagawa et al. introduced a portable HPLC system featuring a plunger pump and an electrochemical detector [39], initiating the path toward chromatographic miniaturization. This was followed by Tulchinsky et al. in 1998, who designed an HPLC system compactly housed in an aluminum box, further enhancing its portability and practical functionality [40].
The 2010s witnessed accelerated innovation. In 2015, Ishida et al. proposed an ultra-compact HPLC system integrating a chip module that combined a separation column and an electrochemical detection cell, driven by a compact electric flow pump [41]. This marked a shift toward highly integrated M-LC platforms. In 2017, Lynch et al. developed a system using an electroosmotic flow pump and an interchangeable HPLC cartridge compatible with both UV and MS detection [42], showcasing the adaptability of modern M-LC setups. Around the same time, Chang’s group introduced a series of lightweight, field-deployable LC systems [43]. Their post-2019 models typically measured 20 × 16 × 16 cm, weighed only 1.8 kg, and featured battery-powered electrolysis micropumps (EMPs), LC microchips, and LED-based absorbance detectors—exemplifying miniaturization without sacrificing functionality.
Further developments include Foster et al.’s 2020 portable capillary LC system, which measured 20.1 × 23.1 × 32.0 cm and weighed 7.82 kg [44]. Designed for flexibility, it employed high-pressure syringe pumps (up to 10,000 psi) and supported variable flow rates and column configurations. In 2021, Chatzimichail et al. introduced a hand-portable LC system using 3D-printed components, highlighting the potential of additive manufacturing to produce customizable, low-cost chromatographic instruments [45]. Since 2021, Brett Paull and colleagues have focused on field-deployable LC/MS systems, aiming to deliver laboratory-grade analytical performance in portable formats for on-site pharmaceutical analysis [46,47]. In 2022, Campíns-Falcó’s team developed two lab-built M-LC systems emphasizing user-friendly interfaces and operational versatility [27]. The following year, Svensson et al. integrated a chip-based pressure regulator with fluorescence and MS detection in a chip-HPLC system [21], reflecting on ongoing efforts to improve precision and system compactness. In 2023, Ishida’s group further enhanced chip-based M-LC technology by introducing modular detection units designed to minimize dead volume and boost system efficiency [19].
Commercialization of M-LC began in earnest in 2005 with Yin et al.’s prototype of the HPLC-Chip/MS system from Agilent Technologies [48], setting a precedent for future market-ready designs. Since then, companies such as Sciex-Eksigent [49], Waters [50], and PharmaFluidics [51] have launched robust M-LC products with separation performance comparable to full-scale LC systems. These commercial systems emphasize user-friendly integration, offering stable fluid drives, precise chip-peripheral connectivity, and highly sensitive detectors.
Ultimately, the performance of an M-LC system hinges on the coordinated function of its key components: chips, pumps, columns, detectors, and injectors. Seamless integration among these parts is essential to achieve the high precision, sensitivity, and operational efficiency required for modern analytical applications.
2.1. Microchip
The development and application of analytical microfluidic devices play a critical role in advancing separation science across various domains [52]. M-LC systems, particularly those integrated with MS, are gaining traction due to their ability to consolidate key LC components into a compact, planar architecture [53,54]. This integration reflects a significant trend in analytical chemistry—delivering high-performance analysis while minimizing system footprint.
Xing et al. developed a hydrogel-based microchip to model complex physiological barriers, specifically the intestinal barrier and blood–brain barrier, using a combination of alginate and gelatin (Figure 1) [55]. The UV cross-linking improved stability compared to traditional ionic methods. This microchip successfully mimicked the gut–brain axis and allowed in vitro simulation of tryptophan metabolism. LC-MS confirmed realistic levels of tryptophan and kynurenine, demonstrating biological accuracy. Unlike traditional organ-on-chip (OOC) systems, this design avoided porous membranes, supported co-culture of cells and microorganisms, and allowed future integration of organoids or iPSC-derived tissues. The chip demonstrates strong potential for applications in disease modeling, drug screening, and metabolic research.
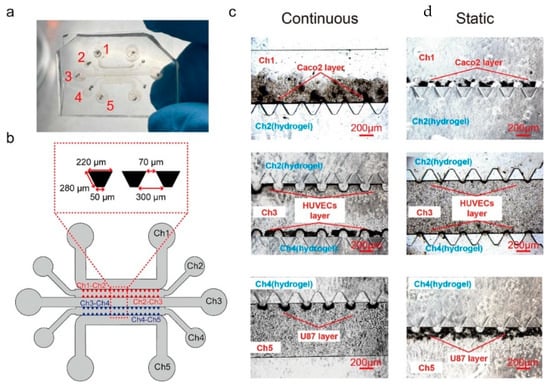
Figure 1.
Cell morphogenesis in an inoculated microchip. (a) Physical diagram of the microchip after cell inoculation. (b) Illustration of the inoculated chip. (c) Corresponding cell layer on each hydrogel surface after the continuous flow culture. (d) Corresponding cell layer on each hydrogel surface after the static culture. (Scale bar = 200 μm) [55]. Reprinted with permission from Ref. [55]. Copyright 2025, Elsevier. All rights reserved.
In summary, the continuous evolution of microchip design is largely driven by the demand for enhanced analytical performance, operational durability, and system versatility in M-LC platforms. Ongoing advances in materials science and microfabrication techniques are expected to further expand the capabilities of chip-based LC systems, opening new frontiers in analytical applications.
2.2. Pumps
Pumps are essential components of M-LC systems, generating the high pressures required to drive the mobile phase through the chromatographic column [27]. Their design directly impacts the consistency of flow rates, minimization of pulsations, and overall system stability. Various pumping mechanisms have been developed to meet the specific needs of miniaturized systems, including electroosmotic pumps (EOPs), piston pumps, and syringe pumps (Figure 2) [56].
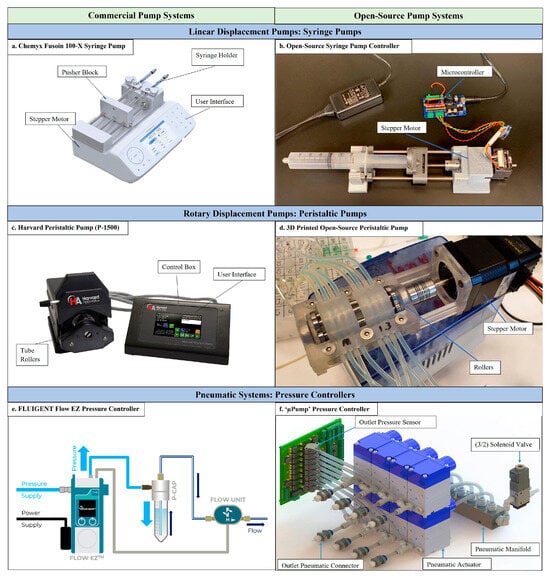
Figure 2.
Commercial and open-source pumping systems. (a) Chemyx syringe pump with embedded electronics; requires the user to input the desired flow rate that is regulated with the help of a stepper motor and pusher block. (b) An open-source syringe pump controlled with the help of a microcontroller, stepper motor, and pushing mechanism. (c) Harvard peristaltic pump with essential control box: requires the user to set the Revolutions Per Minute (RPM) of the motor, which is then translated to the rollers’ speed. (d) A 3D-printed peristaltic pump assembled with the help of a stepper motor and rollers. (e) FLUIGENT pressure controller: requires the user to set the pressure according to the desired flow rate, indicated through the flow sensor. (f) An open-source pressure controller assembled with the help of pneumatic actuators, pressure sensors, and necessary connectors [56]. Reprinted with permission from Ref. [56]. Copyright 2025, Elsevier. All rights reserved.
2.2.1. Electroosmotic Pump
EOPs have become increasingly prominent in recent decades, particularly in microanalytical and miniaturized HPLC systems [57]. EOPs generate flow by electroosmosis—moving liquid through a charged porous medium under an applied electric field [19]. This mechanism provides pulse-free flow delivery, it is well-suited for miniaturization, and it supports lab-on-a-chip (LOC) and portable LC systems [58]. Figure 3 shows a flowchart illustrating trends in system miniaturization.
Figure 3.
Flowchart illustrating trends in system miniaturization.
Microfluidic systems frequently use EOPs, but traditional high-voltage sources are large and ineffective because of adverse effects [59]. In order to overcome this, a triboelectricity-driven electroosmotic pump (TEOP) powered by a triboelectric nanogenerator (TENG) was developed (Figure 4) [60]. Through biomechanical motion, the TEOP produces powerful electric fields that allow for regulated electroosmotic flow. The TEOP achieves ≈600 nL·min−1 flow with significantly lower Joule heating (1.76 J·cm−3·nL−1) than high-voltage sources (≈50 nL·min−1, 8.12 J·cm−3·nL−1). Its efficiency, portability, safety, and low cost make it highly promising for motion-activated micro/nanofluidic transport and manipulation.
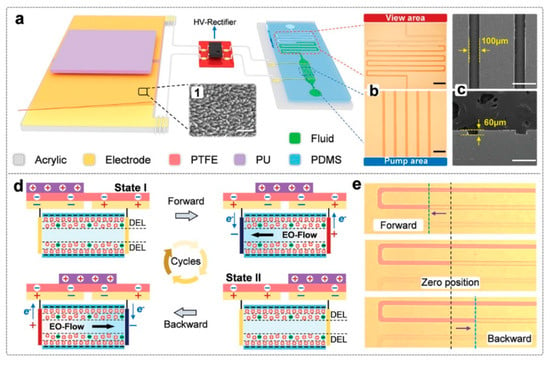
Figure 4.
Prototype of the TEOP. (a) 3D schematic diagram of the triboelectricity-driven micro-flow pump composed of a TENG component, high-voltage rectifier module, and microfluidic chip. Inset 1 (scale bar: 1 µm) shows the surface morphology of the tribo-layer. (b) Optical microscopy image of the view area (top) and pump area (bottom) in the microfluidic chip; the widths of the channels are 150 and 100 µm, respectively (scale bar: 1 mm and 500 µm). (c) Electron microscopy image of the pump area; its width and height are 100 and 60 µm, respectively (scale bar, 200 and 100 µm). (d) The step-by-step working mechanism of the EOP works under a full cycle of TENG movement. (e) Photographs of the micro-flow while operating TENG forward and backward [60]. Reprinted with permission from Ref. [60]. Copyright 2025, John Wiley and Sons. All rights reserved.
Recent research has focused on enhancing EOP performance for M-LC applications. For instance, nanoporous membranes fabricated using track-etch techniques have shown promise in boosting electroosmotic flow efficiency [61].
In conclusion, while EOPs have demonstrated significant potential, further advancements are required to ensure stable flow rates and reproducible gradient programming prerequisites for commercial adoption in chromatography. As these challenges are addressed, EOPs are expected to become central to next-generation portable analytical systems.
2.2.2. Piston and Syringe Pump
Piston and syringe pumps remain critical in M-LC systems, valued for their pressure stability and flow accuracy [62]. Piston pumps, especially single-piston and double-piston configurations (parallel or serial), have played a foundational role in LC miniaturization [63]. The single-piston design, although simple, enables precise unidirectional flow via check-valve control.
In order to improve automated ambulatory Duodopa pumps for patients with Parkinson’s disease, Pravika et al. [64] conducted a study that proposes the design of a linear electromechanical actuator for use with a positive displacement piston-type medication dispensing syringe pump [64]. A direct current (DC) servo motor with gears powers the electromechanical actuators’ lead screw mechanism, which is intended to guarantee self-locking and stop back-driving. While fault assessments addressed possible mechanical and electrical failures, in silico testing verified improved dynamic responsiveness, stability, and dependability. Frequency response plots used in stability experiments revealed that time delays or inertia mismatches could lead to instability, highlighting the necessity of the ideal DC motor sizing with an inertia ratio near 1:1 for dependable pump operation.
Recent innovations have also improved syringe pump systems. For example, a modular LC platform using syringe pumps demonstrated performance on par with commercial systems, achieving <1.5% relative standard deviation (RSD) for peak area and <0.4% RSD for retention time [65]. Operating at pressures up to 4000 psi, the system utilized capillary columns with sub-3 μm particles and integrated miniature Z-cell detection with deep UV light-emitting diodes (LEDs) and photodiodes. Its open-architecture design, including custom-built control software, allowed flexible configuration and future expansions such as multi-wavelength detection and portable MS integration (Figure 5) [65].
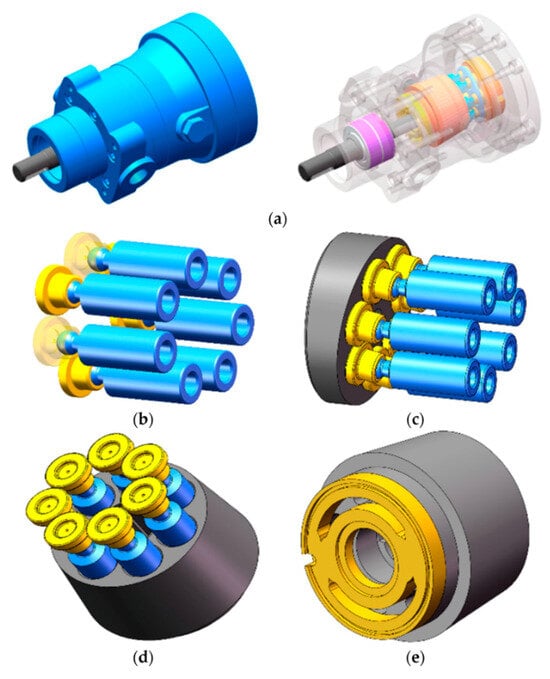
Figure 5.
Schematic diagram of the structural composition of a piston pump. (a) Three-dimensional structural model of a hydraulic piston pump. (b) Piston ball heads and slippers. (c) Slippers and swashplate. (d) Pistons and piston holes. (e) Cylinder and valve plate [62].
Despite their strengths, size and integration challenges remain. Instrument manufacturers are actively developing more compact and efficient pump designs to meet the increasing demand for M-LC systems [56,66]. These developments are crucial for enabling the next generation of high-performance, field-deployable M-LC platforms.
2.2.3. Constant-Pressure Pump
Constant-pressure pumps, also known as pneumatic pumps, utilize compressed gas to deliver the mobile phase at a steady pressure, ensuring consistent flow through the chromatographic column [67]. By maintaining stable pressure, these pumps provide reproducible flow rates, an essential requirement for reliable chromatographic separation. Their ability to suppress flow fluctuations makes them particularly advantageous in applications where even minor deviations in flow can compromise analytical quality and result in reproducibility [68]. Figure 6 shows the Angio-Jet thrombus aspiration system and constant-pressure pump [69].
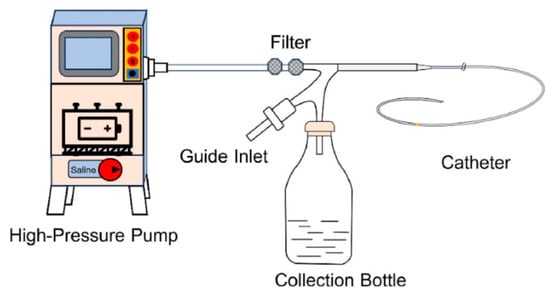
Figure 6.
Schematic of the Angio-Jet thrombus aspiration system with a constant-pressure pump [69]. Reprinted with permission from Ref. [69]. Copyright 2025, Elsevier. All rights reserved.
Recently, Bai et al. presented a novel handheld pressure pump using a disposable mini gas cylinder as its pressure source and porous polydimethylsiloxane (PDMS) films as flow regulators [70]. This design incorporated multiple parallel PDMS films with varied porosity, thickness, and cross-sectional areas, enabling precise flow modulation by selectively engaging specific branches of the flow circuit. The release of gas was finely controlled, which allowed accurate pressure regulation across the microfluidic network. This compact and lightweight pressure system demonstrated effectiveness in generating concentration gradients and producing emulsion droplets in microfluidic experiments. The authors emphasized its suitability for portable, disposable applications such as point-of-care (POC) diagnostics and analytical workflows in resource-limited settings due to its simplicity, ease of operation, and low cost.
Despite these advantages, constant-pressure pumps are generally less common in LC systems than their constant-flow counterparts. This is mainly due to challenges in maintaining precise, pulse-free flow and generating complex gradients—critical factors for many chromatographic applications [71]. Nonetheless, recent innovations have renewed interest in refining these systems, especially for cost-sensitive and portable LC configurations [68].
In summary, although constant-pressure pumps have not yet been widely adopted in mainstream LC systems, they offer specific benefits such as efficiency, simplicity, and longevity. With ongoing miniaturization and technological improvements, they present a promising direction for future M-LC developments, particularly where affordability and field deployability are prioritized [72].
2.3. Injectors
Injectors are important components in LC systems, directly influencing separation efficiency by reducing void volume, minimizing flow disruption, and ensuring precise sample delivery essential for reproducibility [73]. As M-LC technologies advance, innovations in injector design, particularly at the microfluidic scale, have significantly advanced analytical performance and system integration. Zohouri et al. systematically analyzed how microfluidic droplets can be integrated with separation methods (e.g., LC) to enable minimal sample volume workflows [74]. They showcase the benefits of droplets as discrete carriers (reducing sample diffusion and cross-contamination) but also point out that interfacing droplets with injection modules poses challenges in dead volumes, reproducibility, and pressure matching between droplet modules and separation modules. For instance, droplet-to-column coupling often requires specific valve designs or flow converters to maintain stable transfer without disrupting droplet integrity [74].
On the other hand, Chatzimichail et al. showed that their portable LC system’s 60 nL injection valve yielded retention time RSDs of 0.55 and 0.93% and peak area RSDs of 1.42 and 2.20%, almost matching a benchtop Agilent LC system’s performance, a great example of how careful injector design can deliver robust miniaturized performance in field conditions [45]. The limitations, however, remain: this portable system uses gas-driven pressure (limiting gradient flexibility), and droplet-based interfaces may struggle to maintain uniform droplet transfer under varying backpressure or complex matrices. Regarding quantitative impacts of miniaturization and performance tradeoffs, ref. [74] emphasized that droplet-based microfluidic LC hybrids can minimize sample and solvent consumption by orders of magnitude, e.g., using pico- to nL droplets instead of mL injections, while preserving or enhancing sensitivity via compartmentalization.
These miniaturized workflows also allow higher throughput, since parallel droplet generation and rapid droplet transport reduce dead times. In Chatzimichail et al.’s system, using only 5 µL injections and <150 µL/min flow, they achieved continuous operation (24 h) with a 150 mL solvent reservoir and good retention stability (e.g., 0.09–0.57% RSD in retention). Together, these cases show that miniaturization can bring >90% reductions in reagent use and 3 5× (or more) throughput improvements over classical LC runs (which often require ≥20 µL injections and 20–60 min per run) [45]. However, such gains come with tradeoffs: smaller volumes exacerbate the effects of pressure fluctuations, bubble formation, and coupling inefficiencies, especially when connecting to MS. Ensuring robustness, reproducibility, and seamless MS interfacing remains a central challenge for these systems to become routine in analytical labs.
2.3.1. Chip Injectors
Microfluidic integration has revolutionized injector technology by embedding injection mechanisms directly onto chips, thereby reducing dead volume and enhancing sample precision [75]. In M-LC systems, where stability and miniaturization are paramount, chip injectors are especially valuable for maintaining analytical sensitivity and reducing sample dispersion [45].
In 2020, Morioka et al. integrated a compact 6-port valve onto a microchip using 3D printing [76]. The manually operated design allowed seamless switching between load and injection modes, supporting sample delivery at flow rates below 100 μL/min. This system performed comparably to commercial injectors and was applied in a hydrogen peroxide assay, achieving a detection limit of 0.5 μM within 100 s post-injection. Its flexibility supports various microfluidic formats, such as microflow injection analysis (μFIA), offering high analytical accuracy and efficient sample handling. The microchip is equipped with a 6-port valve [76] (Figure 7).
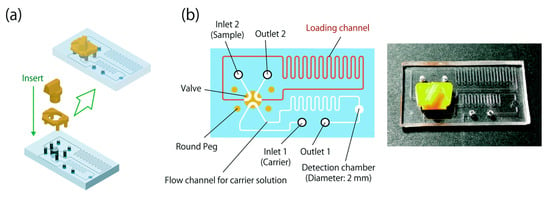
Figure 7.
(a) Schematic of integration of the valve and valve stopper on the microchip. (b) Cross-sectional drawing and photograph of the microchip equipped with the 6-port valve. The dimensions of the valve-equipped microchip are 41 mm in length, 23 mm in width, and 10 mm in height. The channels are 263 μm in width and 105 μm in depth [76].
Despite their benefits, chip injectors face several limitations. These include a susceptibility to clogging from particulate matter, challenges in maintaining steady flow rates, and compatibility issues with aggressive solvents that may degrade common polymeric materials [73]. Moreover, microfabrication costs and limited sample volume capacity restrict their broader applicability [77].
Nevertheless, advances in materials, precision flow control, and device integration continue to expand the utility of chip injectors, particularly in LOC platforms. As these systems evolve, chip injectors are poised to become essential components in next-generation diagnostic and analytical technologies [78].
2.3.2. Valve-Based Injectors
Valve-based injectors are integral to LC systems where precise sample control and consistent delivery are required [79]. Though traditional syringe-based designs remain relevant, modern valve-based systems offer improved reliability, reduced dead volume, and enhanced automation [58]. These are typically categorized into stop-flow and continuous-flow types, both designed to minimize band broadening and maintain chromatographic resolution [80,81].
A sophisticated injector was designed by Cocovi-Solberg et al. for the purpose of high-precision sample preparation and solid-phase material packing [80]. The proposed system facilitates (i) accurate loading of sub-milligram quantities of sorbent, (ii) matrix removal during sample loading, (iii) inline analyte transfer via switching valves, and (iv) automated sorbent flushing for renewable bead injection-solid phase extraction. Operating at low pressure (<25 bar), this system is compatible with commercial auto-samplers and provides high throughput (up to 51 samples/hour). Its modular sorbent selection and low solvent usage yield a 250-fold average signal enhancement. Sample volumes up to 3200 μL were processed without peak broadening, outperforming traditional large-volume injection and inline cartridge-based solid phase extraction (SPE) systems. Despite its versatility, the prototype’s pressure tolerance remains a limitation (≤120 bar), driven by the complexity of the flow path. Future improvements may include reducing wetted surfaces to support smaller sorbent particles and higher flow rates suitable for 1 mm ID columns operating at 50 mL/min. Additionally, valve switching introduces challenges in method development, as sample transfer occurs with each cycle through specific valve positions (Figure 8) in the injector prototype [80].
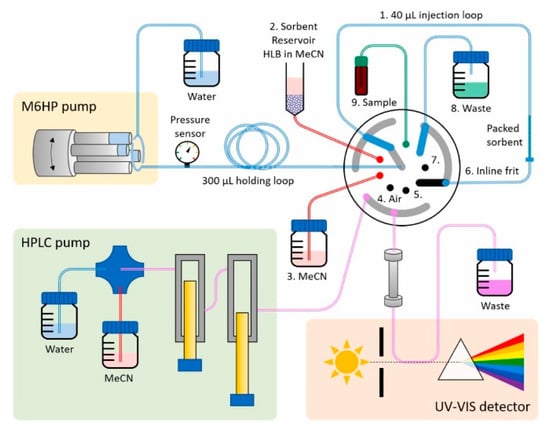
Figure 8.
Illustration of the injector prototype and its fluidic connections. The rotor mechanism links low-pressure pumps and packed columns for precise sample handling. The analytical column (Pursuit 3 PAH, 4.6 × 100 mm, 3 μm (Agilent, Santa Clara, CA, USA)) was operated at 20 °C and 1 mL min−1 using water and MeCN as the mobile phase. The pump aspirates 20 μL of MeCN from position 3, and a part of it is used to resuspend the bead material in position 2, then aspirates the desired bead suspension volume from position 2, and dispenses the train to position 1 [80].
While issues such as clogging, fabrication cost, and solvent compatibility persist, ongoing research and material innovation are steadily improving performance. These injectors are increasingly applicable in emerging areas such as personalized medicine, high-throughput screening, and environmental analysis [82,83]. As integration with microfluidic and M-LC platforms advances, valve-based injectors are positioned to become indispensable tools in future bioanalytical workflows.
2.4. Column
The analytical column is a central component in M-LC, playing a pivotal role in determining separation performance and system efficiency [84]. With increasing demand for high-resolution, low-volume analytical techniques, the development of advanced columns—especially capillary and nanoscale variants—has become essential [85]. Column design must account for multiple factors, including maintaining backpressure within acceptable limits, achieving stable flow rates, ensuring detector compatibility, and minimizing dwell volume [17]. These considerations collectively impact the chromatographic system’s reliability and analytical accuracy [86]. Miniaturized columns generally fall into two categories: open-tube capillary columns (OTCCs) and packed capillary columns [87].
A few years ago, Forster et al. presented work on portable LC systems, a miniature LED-based UV absorption detector built into a capillary column cartridge [82]. Column diameters were raised to 0.2–0.3 mm i.d. to handle the higher flow cell volume compared to the prior on-column version. Comparing kinetic plots across columns with varying lengths, particle sizes, and pressure restrictions revealed theoretical efficiency and achievable chromatographic performance, with smaller particles allowing for faster runs. The technique works best in portable applications when the volume of the mobile phase is constrained by tiny syringe pumps. Future research will optimize the trade-off between analysis time and mobile phase volume in both gradient and isocratic scenarios. Figure 9 shows a UV-absorbance detector with a column [82].
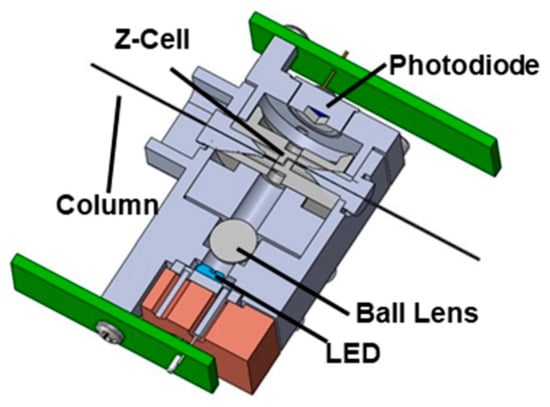
Figure 9.
Schematic of a new UV-absorbance detector module for use in a capillary column cartridge. Light from a UV-LED source is focused through a Z-shaped flow cell with a ball lens. Transmitted light is then detected by a photodiode on the opposite end of the detector flow cell optical path [82]. Reprinted with permission from Ref. [82]. Copyright 2025, Elsevier. All rights reserved.
However, miniaturized columns face specific challenges. These include reduced efficiency due to shorter lengths, lower sample capacity, and difficulties in achieving uniform particle packing [88]. The use of small particles to enhance resolution often leads to high backpressures-difficult to manage in compact systems. Additionally, precision manufacturing increases cost and fragility, while dead volume, solvent incompatibility, and inadequate thermal control further limit performance [89]. Low analyte volumes reduce detection sensitivity, and inconsistencies in fabrication processes can impair reproducibility [90]. Table 2 illustrates the different dimensions used in M-LC columns.

Table 2.
Dimensions of columns used in M-LC.
Emerging technologies such as 3D printing offer promising solutions. Three-dimensional printing enables custom column designs, reduces dead volume by integrating columns directly into microfluidic chips, and improves material compatibility and robustness [94]. It also lowers manufacturing costs, enhances reproducibility, and offers better mechanical and thermal stability-making M-LC more feasible for real-world applications, including on-site antibiotic detection in food matrices [95].
Recent advancements in micro-capillary column fabrication, such as the slurry packing of fused silica particles, have further improved column uniformity, which is vital for boosting separation efficiency and analytical reproducibility [84]. As material science and fabrication techniques continue to evolve, miniaturized columns are expected to deliver performance on par with, or superior to, their conventional counterparts—paving the way for broader adoption of M-LC across disciplines.
2.4.1. Packed Capillary Column
Packed capillary columns offer several advantages in M-LC systems. Their primary benefit lies in delivering high separation efficiency while consuming minimal amounts of sample and solvent [82]. Given that M-LC inherently operates with small sample volumes, packed capillary columns are especially suited for high-throughput applications [96]. When filled with fine particle materials, these columns can achieve exceptional resolution. Their narrow inner diameters reduce analyte diffusion, resulting in sharper peaks and more precise separations [26].
Recently, Fanali & D’Orazio et al. presented a tutorial of capillary electrochromatography for UV and MS techniques for enantiomer separation employing packed capillaries with silica–vancomycin stationary phase [97]. It describes the instrumentation, chiral stationary phase synthesis, and coupling of CEC with MS via a liquid junction interface, which provided high sensitivity. The chiral stationary phase synthesis showed strong enantio-separation and chemo-selectivity, and its practical applicability was demonstrated through analysis of plasma samples from patients undergoing venlafaxine therapy. CEC was displayed with a detector [97] (Figure 10).
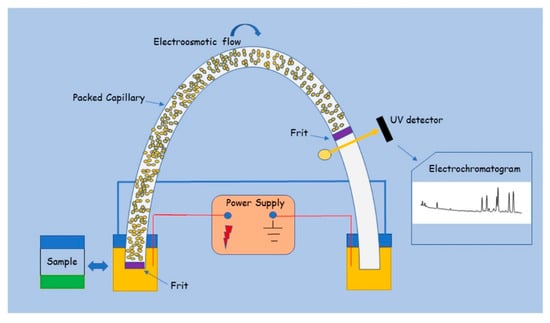
Figure 10.
Scheme of a CEC instrumentation where a UV detector is used [97]. Reprinted with permission from Ref. [97]. Copyright 2025, Elsevier. All rights reserved.
Despite these advantages, packed capillary columns present several technical challenges in M-LC contexts. One of the main limitations is the difficulty in achieving uniform packing within such narrow columns [98]. Uneven packing can result in channeling and inconsistent flow distribution, leading to reduced separation performance and reproducibility [97]. Furthermore, the use of fine particles generates high backpressures due to the small internal diameter, which imposes significant mechanical stress on system components—particularly pumps—and may reduce overall system longevity [17].
To address these issues, recent innovations have focused on improving column packing technologies and materials. Notably, the use of sub-2 µm particles and highly porous stationary phases has enabled enhanced separation efficiency while keeping backpressure at manageable levels [82].
As fabrication techniques continue to improve and as new materials and column architectures are developed, packed capillary columns are expected to play a foundational role in the evolution of high-efficiency, low-volume M-LC systems.
2.4.2. Particle-Packed Capillary Column
In particle-packed capillary columns, the size and uniformity of the packing particles are key determinants of chromatographic performance [99,100]. Recent innovations have focused on optimizing particle sizes in the range of 1.7 to 5 µm, with sub-2 µm particles gaining attention for their superior separation efficiency and improved peak resolution [34,100]. These smaller particles increase surface area and promote stronger analyte-stationary phase interactions, yielding sharper chromatographic peaks. However, this improvement comes at the cost of significantly higher backpressure, which necessitates robust and pressure-resistant system components [85].
Conversely, particle sizes in the range of 3–5 µm remain widely used for applications where lower backpressure and enhanced system robustness are prioritized over ultra-high resolution [35]. These particles offer longer operational life and are better suited for high-throughput or routine analyses.
Takuya Kubo & Toyohiro Naitoa et al. prepared a packed-bed particle model within a microfluidic LC column to quantitatively assess the impact of structural design on band broadening [84]. Si-quartz columns with integrated on-column detection were fabricated to test various structural parameters. The study found that columns with minimal variation in structural size distribution exhibited the least band broadening. Additionally, micro-columns featuring duplicated pillar size patterns demonstrated that lower duplication frequency yielded better separation performance by reducing peak dispersion at transition zones between different regions. Further, the influence of void ratio—related to packing density—was evaluated. While columns with higher void ratios exhibited more band broadening, this factor was found to have a smaller effect compared to the impact of structural size distribution. Figure 11 shows a schematic of columns integrated with Si-quartz [84]. These results provide a detailed understanding of how microstructural homogeneity and packing density affect chromatographic efficiency in particle-packed capillary systems.
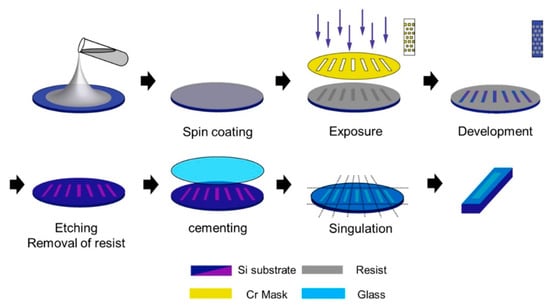
Figure 11.
Preparation of Si-Q columns [84]. Reprinted with permission from Ref. [84]. Copyright 2025, Elsevier. All rights reserved.
Despite their many advantages, particle-packed capillary columns face technical limitations. High backpressure remains a critical issue, especially in M-LC systems where pump capacities are limited [16,89]. Uniform particle packing at micro-scales is also difficult to achieve, and any inconsistencies may lead to channeling or uneven flow, degrading column performance. Moreover, the narrow internal diameter limits sample loading capacity, which can be a constraint in higher-throughput applications [101]. These columns also tend to be fragile, making them prone to damage during handling and operation.
To address these challenges, several recent innovations have emerged: 3D printing of column hardware has enabled precise fabrication of custom geometries and integrated fluidic connectors, reduced dead volume, and improved packing consistency [102,103]. Hybrid particle technologies, combining silica and polymeric materials, have expanded chemical stability and pH compatibility [89]. Nanofabrication techniques, such as electrostatic deposition, ensure uniform particle placement and reduce channeling effects [104]. Advanced surface functionalization, including zwitterionic coatings, has enhanced selectivity—particularly for polar analytes—broadening the applicability of these columns across various separation challenges [105].
2.4.3. Monolithic Packed Capillary Column
Monolithic packed capillary columns have emerged as critical components in M-LC, offering high separation efficiency and structural versatility across various analytical applications [106]. Their continuous porous architecture—formed in situ—distinguishes them from traditional particle-packed columns by eliminating interstitial voids and enabling improved mass transfer and lower backpressure [107,108]. These properties make monolithic columns particularly suitable for complex analyses in proteomics, environmental science, and biomedical research [109].
W. Li et al. recently developed a monolith using thermally induced free radical polymerization with phase separation, a porous copolymerization of 2-methyladamantan-2-yl acrylate (MADA) with ditrimethylolpropane tetraacrylate (DTTA) in fused silica. The prepared poly (MADA-co-DTTA) monolith showed adjustable permeability, developed porous structure and high thermal stability [106]. Porous structure, permeability, and surface area were optimized by modifying the reaction conditions and prepolymerization content. The monolith exhibited great thermal stability from crosslinking and increased hydrophobicity because of the adamantyl groups. It performed exceptionally well in the separation of biological materials and small molecules (PAHs, alkylbenzenes). Additionally, host-guest interactions immobilized cucurbituril (CB) onto the monolith, and capillary- LC (cLC), cLC-MS/MS analysis verified that CB could control column hydrophobicity. In order to accommodate increasingly complicated samples, this approach raises the possibility of dynamically tuning monolithic columns in the future, utilizing various host-guest systems. Schematic of monolithic column design [106] (Figure 12).
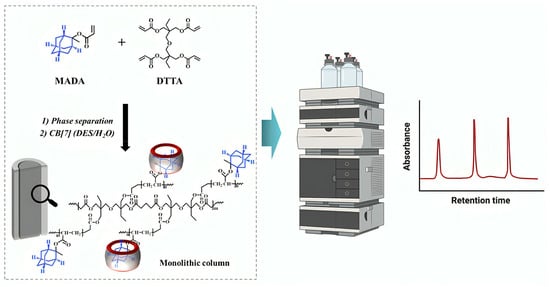
Figure 12.
Overview of a monolithic column design, highlighting its open, interconnected pore structure and high-speed separation capability [106]. Reprinted with permission from Ref. [106]. Copyright 2025, Elsevier. All rights reserved.
Typically fabricated from organic polymers or silica-based materials, monolithic columns can be tailored for specific separation targets [34]. Recent advancements in additive manufacturing, particularly 3D printing, have expanded design possibilities by enabling precise control over pore geometry and distribution [110,111]. As a result, these columns can achieve plate numbers between 50,000 and 100,000 plates per meter, depending on material selection and analyte interactions [106]. Incorporation of nanomaterials has further enhanced the functionality of monolithic columns. Metallic nanoparticles—such as gold or silver—embedded within the monolith matrix improve selectivity and introduce catalytic properties, expanding the column’s utility in biomolecular analysis, including proteomics and glycomics [107,112].
Despite their advantages, monolithic packed capillary columns face limitations that hinder broader adoption. Fabrication reproducibility remains a challenge, particularly in achieving consistent pore structures and functionalization across batches [105]. Such variability can affect analytical reliability and complicate method standardization. Furthermore, commercial availability is still limited; monolithic columns are not as widely produced or distributed as traditional packed columns, restricting their use in routine laboratory workflows [34].
Nevertheless, monolithic packed columns represent a substantial advancement in column technology. Their low backpressure, ease of integration with microfluidic platforms, and compatibility with advanced materials such as nanoparticles and 3D-printed frameworks make them ideal candidates for next-generation M-LC systems. Future research is focusing on areas such as stimuli-responsive materials for smart separation control, sustainable and scalable fabrication methods, and computational modeling for precision column design. With continued innovations in materials science and microfabrication, monolithic capillary columns are poised to play an increasingly important role in advancing the performance, reliability, and accessibility of M-LC technologies.
2.4.4. Open Tubular Capillary Column
OTCCs play a critical role in M-LC, offering advantages in efficiency, speed, and flow dynamics due to their unique structure [113]. Unlike packed or monolithic columns, OTCCs consist of a thin stationary phase coating applied to the inner wall of the capillary. This design eliminates packing material, enhancing permeability and reducing backpressure—ideal for fast, high-resolution separations in proteomics, pharmaceutical analysis, and complex sample workflows [114].
Stationary phases in OTCCs may be porous or non-porous, with film thicknesses ranging from 0.1 µm to several micrometers [115]. Non-porous coatings provide uniform analyte interaction surfaces, while porous films—incorporating materials like silica or metal–organic frameworks (MOFs)—offer greater selectivity and retention [115]. Recent advances from 2022 to 2024 introduced hybrid stationary phases (e.g., silica-polymer composites, carbon-based nanomaterials) that enhance chemical robustness and analyte-specific interactions [116].
Though OTCCs are not packed with particles, the stationary phase porosity and surface structure play crucial roles in determining peak sharpness and resolution [117]. Their inner diameters typically range from 10 to 100 µm, with column lengths spanning from 10 cm to over 1 m [118,119], offering broad application flexibility.
A study by Santos et al. evaluated key parameters of wall-coated open tubular (WCOT) columns within nano-LC- Electrospray ionization (ESI)-MS/MS systems. Parameters such as stationary phase composition, column length, and flow rate were optimized [92]. While WCOT columns demonstrated strong intrinsic separation capabilities, especially when combined with trapping columns, issues like film detachment and clogging remain challenges. The findings underscore the potential of WCOT columns as competitive tools in M-LC, with further research required to address fabrication and reliability concerns. Figure 13 shows the development of WCOT columns [92].
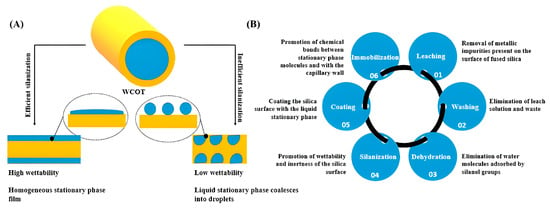
Figure 13.
(A) The difference in the silica surface’s coating with the liquid stationary phase depends on the wettability profile; (B) summary of the steps involved in developing WCOT columns and their respective functions [92].
Despite recent improvements, OTCCs face limitations in fabrication reproducibility, stationary phase uniformity, and limited sample capacity. These restrict their use in high-throughput or preparative workflows [115]. However, their unique advantages—especially in speed and selectivity—make them a compelling choice for advanced analytical applications. Continued innovation in materials science and thin-film deposition will be key to realizing their full potential.
2.4.5. Chip-Based Column
Chip-based columns represent a groundbreaking development in M-LC, integrating chromatographic functionality directly into LOC systems [120]. Fabricated using microfluidic technologies, these columns offer unmatched precision, low sample consumption, and rapid analysis times—particularly beneficial for proteomics, diagnostics, and pharmaceutical analysis [121]. Materials used for chip-based columns include silicon, glass, and various polymers [122]. Recent advancements have introduced hybrid substrates such as cyclic olefin copolymers and engineered thermoplastics to improve solvent resistance and structural durability [123]. Fabrication methods—photolithography, laser etching, and 3D printing—allow for precise control over microchannel geometry, stationary phase integration, and multi-modal system compatibility [120,124,125]. Table 3 shows a comparison of several types of M-LC systems.

Table 3.
Comparison of M-LC Systems.
In a representative study, Weise et al. reported a chip-based nano supercritical fluid chromatography MS (NanoSFC-MS) system [124]. This modular device included a packed nanobore column between two laser-etched chip modules: a pre-column microtee for sample injection and a post-column micro-cross for coupling to atmospheric pressure MS via a split-flow interface. The system enabled rapid separations of vitamins and chiral compounds in seconds, demonstrating excellent speed, efficiency, and pressure regulation. Figure 14 shows the NanoSFC setup [124].
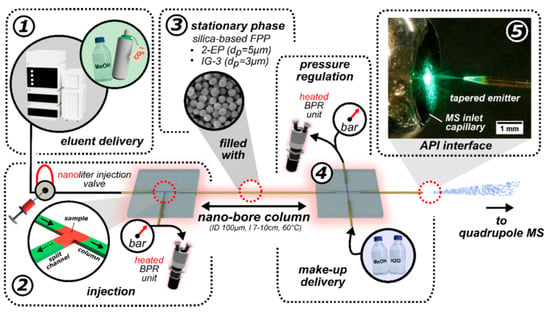
Figure 14.
Schematic illustration of the NanoSFC-MS setup, detailing pump, column, interface, and pressure sensor integration. An external nano-injection valve (C74MPKH-4674, VICI, Switzerland) with a 5 nL sample loop was installed between the solvent delivery system and the modular nano-SFC system. The proposed post-column chip module with the micro-cross structure was evaluated using a fluorescent sample (7-amino-4-methyl coumarin, c120, c = 500 μM dissolved in MeOH for the imaging, c = 100 μM for FLD measurements) [124].
Despite these advances, chip-based columns face limitations. Fabrication consistency and channel reproducibility can impact separation reliability [130]. Small sample capacities limit their use in high-throughput or preparative contexts [131], and the high cost of specialized instrumentation can deter widespread adoption [132]. Additionally, polymer-based chips may degrade under harsh chemical or thermal conditions [133].
Nonetheless, chip-based columns are transforming the M-LC landscape. Their high integration potential, customizable designs, and compatibility with advanced detection systems position them at the forefront of analytical innovation. As fabrication technologies mature and costs decrease, their adoption is expected to grow across disciplines—from environmental monitoring to clinical diagnostics.
2.5. Detector
Detectors are critical components of M-LC systems, serving to identify and quantify analytes after chromatographic separation [134]. In M-LC, detectors must be appropriately miniaturized to preserve the system’s compact form while maximizing detectability, resolution, and analytical efficiency [27]. Common detector types include absorbance detectors, also known as LEDs, laser-induced fluorescence (LIF), and electrochemical detectors (ECDs) [135]. These are often tailored for specific compound classes but can be adapted for broader analyte detection through optical, electrochemical, or signal-processing modifications [135]. Recent advancements have significantly enhanced detector sensitivity, compactness, and integration, enabling more versatile and portable LC systems [136].
A study was conducted by Jiao, Zhang, Wang, et al., where a miniaturized detector integrated with a microfluidic chip for use in portable LC platforms was developed and then reported by [54]. The system incorporates an LED light source and compact optical components within a light-shielding sleeve, allowing easy adjustment and alignment. The microfluidic chip, constructed from three layers of Poly methyl methacrylate (PMMA) features a Z-shaped detection cell in the middle layer—designed to maximize the optical path length and improve sensitivity. To reduce stray light interference, dual slits were applied to the chip surface, maintaining interference at a low 0.08%. Moreover, the Z-shaped cell design helped mitigate optical scattering caused by substrate surface roughness. This detector was successfully coupled with microchips employing different stationary phases, supporting both ion-exchange and reversed-phase LC configurations. The integrated system was used to detect metabolic markers such as glycated hemoglobin and bilirubin, demonstrating strong potential for non-invasive chronic disease screening, including diabetes and liver disorders. The LC system with a detector [54] is shown below (Figure 15).
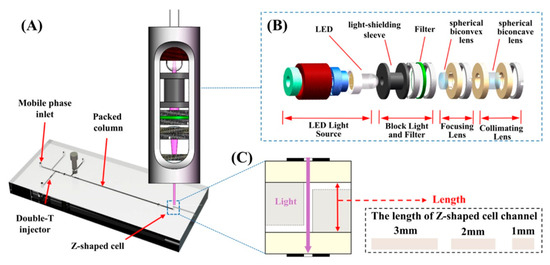
Figure 15.
(A) Schematic diagram of the LC system composed of a microfluidic chip composed of three layers of PMMA and a miniaturized detector. (B) Cross-sectional view of the miniaturized detector, including LED light source, black light-shielding sleeve, filter, spherical biconvex lens, and spherical biconcave lens. (C) A Z-shaped detection unit was processed on the middle layer chip, and the length of the Z-shaped detection unit varies with the thickness of the middle layer chip. Three lengths of 1, 2, and 3 mm were designed, making the volume of the detection cell 0.2826, 0.5652, and 0.8478 µL, respectively, which is necessary to determine the optimal path length [54]. Reprinted with permission from Ref. [54]. Copyright 2025, Elsevier. All rights reserved.
Recent advances in detector integration within M-LC systems have significantly improved analytical performance, but limitations remain. For example, Svensson et al. demonstrated that coupling chipHPLC systems with microchip pressure regulators enabled robust pressure control at the nanoflow level, which is essential for stable operation of miniaturized detectors such as UV and MS interfaces [21]. Their system achieved a stable flow rate of 400 nL/min and reproducible peak retention times with RSDs below 2%, showcasing improved reliability. However, they also noted that reproducibility was compromised at high backpressures due to the fragility of microfluidic connections, especially when interfacing with ESI-MS. Hemida et al. further emphasized challenges in coupling M-LC with mass spectrometry, particularly in chip-based LC-MS applications. While their low-footprint system enabled online MS-based reaction monitoring with rapid cycle times (<1 min), they reported that miniaturized interfaces remain prone to clogging, dead volume effects, and signal suppression due to inconsistent electrospray formation. These findings highlight the progress in precision and miniaturization while exposing reproducibility, pressure instability, and MS compatibility as ongoing technical hurdles in detector integration [47].
Miniaturization has significantly enhanced LC by reducing sample and solvent consumption, improving throughput, and lowering detection limits. According to Zheng Li et al., microfluidic chips used for nucleic acid diagnostics achieved sample volumes as low as 0.5 nL, a stark contrast to the 10–100 µL typically required in conventional LC [136].
Recent innovations in microfluidic chip-based nucleic acid detection platforms have achieved remarkable sensitivity. For instance, a CRISPR-Cas12a-assisted surface-enhanced Raman scattering (SERS) biosensor enabled detection of HIV-1 dsDNA with a limit of detection (LOD) as low as 0.3 fM (femtomolar), demonstrating strong clinical potential for ultrasensitive diagnostics [137]. These systems often integrate sample preparation, amplification, and detection within a compact format, contributing to improved specificity and automation in molecular diagnostics.
Hemida et al. reported that their compact LC-MS platform consumed less than 100 µL of solvent per run, achieved detection limits below 0.5 ng/mL, and supported automated throughput with analysis times under 2 min per sample [47]. This represents a 3–5× increase in throughput and up to a 95% reduction in solvent usage compared to traditional systems. Such quantitative improvements confirm the environmental and operational advantages of M-LC systems while also suggesting their readiness for high-frequency applications in pharmaceutical and diagnostic workflows.
To overcome these limitations, researchers are pursuing several strategies. Hybrid detection systems that integrate UV-Vis and fluorescence modalities, offering broader detection capabilities [138]. Machine learning (ML) algorithms to reduce noise and enhance signal quality in real time [134]. Advanced materials, such as self-cleaning electrodes for ECDs, are needed to improve robustness and reduce maintenance [139]. As detection technologies continue to evolve, the integration of smart detectors into M-LC systems will play a critical role in expanding their accessibility, functionality, and application scope—particularly for POC diagnostics, environmental monitoring, and resource-limited analytical contexts.
2.5.1. Absorbance Detector
Absorbance detectors have significantly advanced the analytical capabilities of M-LC, enabling accurate quantification of analytes at low concentrations [17]. These detectors are vital in pharmaceutical, environmental, and biochemical analysis, offering rapid and precise compound detection [58]. The miniaturization of absorbance detectors has extended their utility to portable systems, enabling on-site analysis, reducing sample degradation, and improving response times [140]. A major driver of this evolution is the use of LEDs, which offer compact size, low power consumption, high stability, and long operational lifespans, which are characteristics ideal for integration into portable M-LC systems [141].
In a compelling application, Prado et al. successfully displayed the application of an easy, cost-effective UV detector with the use of a 280 nm LED [142]. They made a 3D-printed structure and incorporated a custom-made quartz tube, which enabled in-flow absorbance measurements during the elution process in classical column chromatography. Combining an LED and a photodiode allowed for the establishment of a simple setup that massively reduces device costs. This detector showed that it allows real-time monitoring of elution, can facilitate the collection of UV-absorbing compounds, and also decreases the overall experimental time. Hence, by using this method and the potential use of multiple deep-UV LEDs, they predicted an efficient and affordable answer for monitoring the elution of non-colored compounds in column chromatography. Model of an LED [142] (Figure 16).
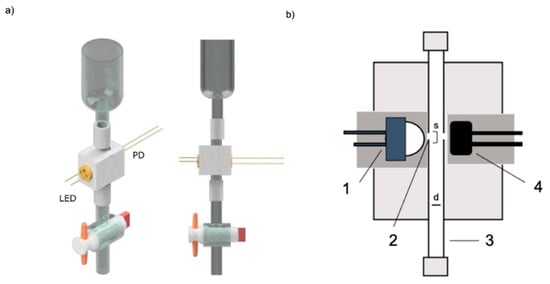
Figure 16.
(a) 3D rendered models of the proposed LED-based absorbance detector for column chromatog-raphy; (b) Cross-sectional design of the assembled detector: (1) UV-LED in positioning stage (3 mm to the slit), (2) optical slit (1.5 mm diameter), (3) quartz tube with internal diameter (d) of 3 mm, (4) signal photodiode in positioning stage (3 mm to the slit) [142]. Reprinted with permission from Ref. [142]. Copyright 2025, Elsevier. All rights reserved.
While LED and LIF detectors have substantially enhanced the analytical power of M-LC systems, several challenges remain. For UV-visible absorbance detectors, sensitivity is strongly tied to optical path length, which is constrained by miniaturization. Moreover, stray light and electronic noise can compromise signal quality and measurement accuracy [143].
To address these limitations, advanced microchip-based absorbance detectors now incorporate Z-shaped flow cells, optical slits, and collimating microlenses to extend the optical path and minimize stray light interference [144]. These advancements enhance the sensitivity [145], reproducibility [146], and overall performance while maintaining a compact footprint. Furthermore, the introduction of multi-wavelength programmable detectors has enabled simultaneous detection of multiple analytes, improving analytical efficiency and reducing instrument cost [147]. As a result, absorbance detectors have broadened the scope of M-LC applications, from pharmaceutical quality control [148] to in situ environmental monitoring [143].
Overall, absorbance detection in M-LC systems continues to evolve through material innovation, optical engineering, and miniaturized design integration, solidifying its role in portable, high-efficiency analytical platforms.
2.5.2. Electrochemical Detectors
ECDs have become integral to M-LC systems due to their high sensitivity, versatility, and adaptability to portable formats [129]. ECDs are particularly effective for detecting electrochemically active compounds that undergo redox reactions at the electrode surface [134]. This makes them highly suitable for the analysis of a wide range of substances, including organic molecules, trace metals, and bioactive compounds with electrochemical properties [149].
An ECD typically consists of three electrodes: a working electrode (the site of redox reactions), a reference electrode (maintains a stable potential for accurate measurement), and a counter electrode (completes the electrical circuit) [150,151,152,153]. Common electrode materials include platinum, gold, glassy carbon, and carbon composites [154]. Platinum and gold are particularly favored for their superior conductivity and corrosion resistance, which ensure stable performance even in chemically harsh environments frequently encountered in M-LC [155,156].
Krishnamurthy et al. developed a new electrochemical sensor based on screen-printed electrodes, modified with carbon-supported platinum and gold, in order to detect the benzenediol isomers catechol, hydroquinone, and resorcinol in acidic environments [157]. Chronoamperometry, differential pulse voltammetry, and cyclic voltammetry were used for detection. While differential pulse voltammetry enabled the simultaneous detection of several analytes by differentiating their oxidation potentials, cyclic voltammetry allowed for the identification of individual isomers. Chronoamperometry was used in the determination of the quantification limits, which were proven to be reproducible across two potentiostats and reached 1 mM for catechol and hydroquinone and 100 nM for resorcinol. The results highlighted the potential of modified screen-printed electrodes for monitoring volatile toxic organic compounds in acidic settings by showing that they can offer a quick, easy, and affordable platform for benzenediol detection. Figure 17 shows a general description of a screen-printed electrode, where the critical elements are shown and explained [157].
Figure 17.
Scheme of sensing volatile toxic organic compounds in air and water using modified screen-printed electrodes [157]. Reprinted with permission from Ref. [157]. Copyright 2025, Elsevier. All rights reserved.
The integration of ECDs has significantly advanced analytical chemistry by enabling ultrasensitive detection of low-concentration analytes, supporting analysis in complex matrices such as biofluids or environmental samples [157], and facilitating cost-effective and portable on-site monitoring systems [158]. ECDs are also well-suited for miniaturization and battery-powered operation, aligning with the trend toward decentralized, POC diagnostics and field-deployable analytical tools [158].
In summary, the ECD continues to expand the utility of M-LC systems across biomedical, pharmaceutical, and environmental domains. With ongoing innovations in electrode design, microfabrication, and integration, ECDs are expected to play an even more pivotal role in next-generation analytical platforms.
2.5.3. Miniaturized Mass Spectrometer
Miniaturized MS have become increasingly prominent in the context of M-LC, driven by advancements in microfluidics, compact instrument design, and analytical methodologies [159,160]. These devices harness the core strengths of MS, including high sensitivity, broad molecular detection range, and precise compound identification, while adapting them to portable, low-footprint formats [104,119]. As a result, miniaturized MS systems are revolutionizing clinical diagnostics, environmental monitoring, and pharmaceutical analysis by enabling analyses to occur directly at the point of need [161]. Progress in ionization techniques has played a pivotal role in the integration of MS with M-LC. Methods such as electrospray ionization (ESI) and ambient ionization are particularly well-suited for compact systems [162]. These soft ionization approaches preserve molecular structures, making them ideal for analyzing complex biological or labile compounds without excessive fragmentation.
Svensson et al. successfully developed a chip-based pressure regulator (chipPR) integrated into a chip-HPLC system, which was further coupled with fluorescence detection and MS for various analytical applications [21]. This chipPR utilized temperature-controlled restriction for precise flow gradient generation and pinched injections, significantly reducing system complexity. The enhanced portability and integration enabled by chipPR modules make these systems more suitable for field-deployable and POC applications. The chip is composed of an injection cross, a separation channel with a packed column, and a post-column section with two outgoing channels, illustrated in Figure 18, where one could be used to introduce an additional sheath flow for MS-hyphenation [21].
Figure 18.
Overview of HPLC chips and their microscopic features. (A) A fusion-bonded borosilicate-based HPLC chip (45 × 10 × 1.1 mm) was used for fluorescence measurements; detection took place directly after the microcolumn (DFLD). (B) Modified chipHPLC interface for ESI-MS; in here a ground hydrophobized chip-based electrospray emitter is shown. (C) Insight into microscopic chip features: (1) Injection cross and column head. (2) Pressure-tight sealing of the microcolumn. (3) Sheath flow channel merging into the post-column channel prior to the emitter [21].
Despite their many advantages, miniaturized MS systems face challenges, including limited resolution and mass range compared to their full-sized counterparts [163,164]. The trade-offs between performance and portability mean that these systems may not always be suitable for highly complex or large-scale analyses [165]. Additionally, maintaining a stable vacuum in a compact form factor and ensuring consistent ionization can be technically demanding [166].
Recent breakthroughs are addressing these challenges. Innovations such as energy-efficient ion traps and hybrid mass analyzers (e.g., combining quadrupole and time-of-flight) have improved resolution, scan speed, and dynamic range in compact formats [167]. These innovations allow miniaturized MS systems to achieve higher resolution and faster scan speeds [167]. Additionally, wireless communication integration has enabled real-time data transmission and remote access, expanding the utility of miniaturized MS in collaborative research and decentralized diagnostics [168].
In summary, the evolution of miniaturized MS systems is central to the future of portable analytical science. As performance continues to improve and integration with microfluidic M-LC platforms advances, these systems are poised to become core components in flexible, POC, and on-site analytical solutions across diverse scientific fields.
3. Application
3.1. Food Analysis
3.1.1. Toxins
M-LC has emerged as a powerful approach for toxin analysis, offering advantages such as reduced matrix effects and lower analyte dilution in the mobile phase. These benefits enhance signal intensity in concentration-dependent detectors like ESI-MS [169,170].
A study conducted by Chen, Liu, et al. established a fast and automated approach for determining aflatoxins (aflatoxins B1, B2, G1, and G2) in peanut oil [171]. An SPE-based microfluidic chip and MS (SPE-Chip-MS) were presented. Aflatoxins were enhanced in the SPE channel of a microfluidic chip filled with HP C18 particles, then eluted and put into the MS for qualitative and quantitative analysis using multiple reaction monitoring mode. ESI in the positive ion mode was employed to detect aflatoxins. The SPE-Chip-MS system produced a series of good linear calibration curves with a linear range of 1.25 ng/mL to 100 ng/mL, and the detection limits (S/N = 3) of aflatoxins were 0.013–0.067 ng/mL. During this study, the sample pretreatment was entirely incorporated into a microfluidic chip for the first time, and online MS detection was performed; this technology might be extended to detect a variety of food samples.
Currently, there are many specific and well-established conventional techniques used on a regular basis for food safety assessment and regulation. These techniques are often specific, but they may have limitations in terms of speed, sensitivity, and ability to detect a wide range of toxins (Figure 19) [172].
Figure 19.
Schematic representation of various methods used for the detection of food toxins [172].
The shift from technical miniaturization in LC systems to real-world toxin detection is underpinned by advancements that directly enhance analytical performance. The incorporation of microfluidic components such as low-dead-volume injectors and integrated fluorescence sensors has enabled high sensitivity, particularly valuable in agri-food and environmental monitoring. According to Fakayode et al., fluorescent chemical sensors have achieved detection limits as low as 0.1 nM for toxins such as mycotoxins and aflatoxins in grain and dairy products, a level of precision significantly supported by M-LC platforms [169]. These sensors, when integrated into chip-based LC systems, allow real-time, label-free toxin quantification in complex matrices with minimal sample volumes. Complementing this, Ahuja et al. report that coupling M-LC to tandem MS allows marine toxin detection (e.g., domoic acid, saxitoxin) with limits below 1 µg/kg, showing clear gains in sensitivity, selectivity, and sample economy [172]. Together, these examples highlight how M-LC advances from channel geometry to optical detection coupling, which are not merely technical feats but critical enablers of high-performance toxin analysis in applied fields.
Looking ahead, the trajectory of M-LC strongly favors integration with intelligent technologies such as Artificial Intelligence (AI), sensor fusion, and mobile diagnostics. Shi et al. described how ambient ionization MS paired with portable M-LC enables field-ready analysis of food and environmental toxins without laborious sample prep. In such platforms, AI-based data analytics optimize signal extraction, peak identification, and noise suppression in real-time, drastically improving throughput and decision accuracy [162]. Alves et al. further emphasize that microfluidic platforms designed for autonomous operation can incorporate feedback mechanisms to adjust flow rates and separation conditions based on toxin concentration [173]. The addition of fluorescent or electrochemical sensors (as shown by [169]) enables rapid screening of multiple toxin classes simultaneously, even in low-resource settings [169]. These integrations support the development of fully portable, battery-operated M-LC systems with detection limits in the picomolar-to-nanomolar range, minimal sample consumption (≤1 µL) [173], and throughput improvements of up to 10× compared to conventional LC systems, positioning M-LC as a cornerstone of smart, sustainable analytical chemistry [162].
Despite its advantages, M-LC faces challenges—particularly in handling complex sample matrices and maintaining sensitivity. Miniaturized systems, while efficient, can still suffer from issues such as matrix interferences and co-elution, similar to traditional LC [129].
Recent advancements have significantly enhanced the capability of M-LC in toxin analysis. Improvements in column technology, such as reduced particle sizes, have led to higher separation efficiency [172]. Simultaneously, developments in microchip and LOC platforms have enabled the miniaturization of entire analytical workflows, reducing sample volume and cost while increasing throughput [129].
3.1.2. Drug Residues
Drug residues, especially antibiotics in food and environmental matrices, pose significant risks to public health, food safety, and ecological balance [174]. M-LC has rapidly evolved into a leading technique for trace-level detection of such residues [175]. When coupled with MS, M-LC enables highly sensitive, accurate, and selective analysis, even distinguishing between structurally similar compounds such as isomers [91]. HRMS, in particular, can detect residues in the ppt range, ensuring regulatory compliance [23].
A notable study by Xueting et al. introduced a method for simultaneously quantifying 20 pesticide and veterinary drug residues in cow and buffalo milk (Figure 20) [174]. Using a modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) extraction followed by UPLC-MS/MS, the method optimized sample treatment with 10% formic acid in acetonitrile, achieving efficient recovery. Calibration using matrix-matched curves demonstrated excellent linearity (R2 > 0.9990), and method validation showed recoveries of 73.1–111.6% with RSDs below 15%. Detection and quantification limits ranged from 0.7 to 1.7 μg/L and 2.0–5.0 μg/L, respectively, confirming the method’s sensitivity and precision.
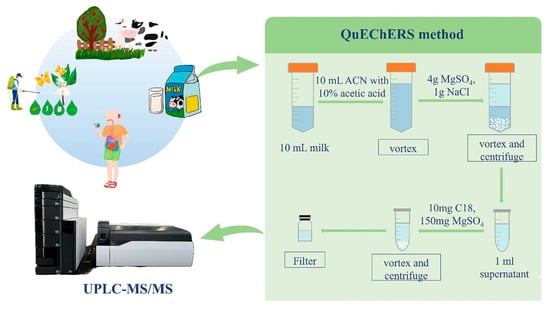
Figure 20.
Workflow for detecting 20 pesticides and veterinary drugs via QuEChERS and LC-MS/MS. Milk samples (10 mL) of 10% formic acid in acetonitrile (ACN) were added to the sample in a 50 mL polypropylene centrifuge tube, vortexed for 2 min, and then supplemented with 4 g anhydrous magnesium sulfate and 1 g sodium chloride to prevent protein coagulation. The mixture was vortexed for 1 min and centrifuged at 5000 rpm for 5 min. The supernatant (1 mL) was transferred to a 2 mL centrifuge tube containing 10 mg C18 and 150 mg anhydrous magnesium sulfate, vortexed for 1 min, and centrifuged at 10,000 rpm for 1 min. The supernatant was then filtered through a 0.22 μm membrane and analyzed by UPLC-MS/MS [174]. Reprinted with permission from Ref. [174]. Copyright 2025, Elsevier. All rights reserved.
Recent advancements in M-LC systems, particularly in sample preparation, solvent handling, and detector integration, have enabled practical improvements in the detection of veterinary drug residues in complex food matrices. For example, Zareasghari et al. demonstrated that coupling deep eutectic solvent-based pressurized liquid extraction with dispersive liquid microextraction (DLLME) significantly improved the recovery and sensitivity of organophosphorus pesticide residues in egg powder. The optimized method achieved LOD as low as 0.26 and 0.47 µg/kg with excellent repeatability (RSD < 8%) using only 10 mL of extraction solvent, a marked improvement over traditional LC workflows that typically consume higher solvent volumes and exhibit larger variation due to manual sample handling [176]. These results highlight how modern M-LC systems minimize sample and solvent consumption while maintaining high analytical performance, making them well-suited for rapid screening in food safety monitoring [177]. The enhanced pressure and flow control in miniaturized platforms also contribute to stable baselines and sharper peaks, facilitating trace detection even in complex biological matrices like egg powder, milk, or meat products [176].
Looking ahead, the integration of M-LC systems with AI-based data processing and portable MS platforms opens new avenues for on-site drug residue screening and decision-making in agri-food chains [177]. Studies conducted by Zhou et al. on deep learning-enabled miniature MS systems for therapeutic drug monitoring illustrate how real-time AI-assisted analysis can drastically enhance throughput, reducing turnaround times from hours to minutes without sacrificing accuracy [178]. Moreover, AI tools can identify retention time shifts, predict analyte behavior under varying matrix loads, and flag outliers autonomously, thereby improving reproducibility and reducing the dependency on expert operators [179]. The fusion of micro-extraction methods like DLLME with field-deployable LC-MS units is particularly promising for decentralized testing in rural or resource-limited settings [176]. As Singh et al. emphasized in their review on AI in drug design, such integrations are crucial for building predictive systems that not only detect but also anticipate contamination patterns based on environmental and historical data [179]. Together, these innovations signal a future where M-LC platforms are not only miniaturized but also intelligent, self-correcting, and mobile, radically improving how drug residues are monitored across the food supply chain.
However, M-LC also faces practical limitations. Miniaturization can impact system robustness and reproducibility, especially in complex food matrices [129]. Moreover, the cost and technical complexity of MS-based detection can hinder widespread adoption, particularly in low-resource settings [129].
To address these challenges, future work should focus on developing cost-effective, user-friendly M-LC systems that maintain analytical performance. Advances in materials science and microfabrication hold promise—for example, incorporating nanomaterials into stationary phases could enhance selectivity, while integrating microfluidics could further miniaturize and automate analytical workflows [13,28].
3.1.3. Other Organic Pollutants
M-LC has become a vital analytical tool for the precise and sensitive detection of a wide range of organic pollutants, significantly impacting food safety, environmental monitoring, and industrial quality assurance [180]. Its capacity for trace-level analysis has been notably enhanced by technological innovations [181], allowing accurate identification and quantification of complex chemical species across diverse sample matrices [182].
The growing relevance of M-LC is closely linked to heightened concerns over hazardous organic compounds such as heterocyclic aromatic amines (HAs)—formed during high-temperature cooking—and synthetic dyes like Azo dyes, which pose both health and regulatory risks [178,183]. This section explores M-LC’s role in characterizing such substances, focusing on their formation during food processing [22], the detection of toxic dyes, and recent innovations that have expanded the method’s analytical reach [180].
Advances in M-LC systems have significantly strengthened the analytical capabilities required for detecting organic pollutants in complex matrices such as environmental, food, and industrial samples. For instance, Guo et al. demonstrated how the integration of multi-walled carbon nanotube (MWCNT)-modified paper spray ionization with a miniature MS allows on-site detection of persistent organic pollutants like polychlorinated biphenyls (PCBs) at ng/mL levels within less than one minute, eliminating the need for elaborate sample prep or lab-based instrumentation [184]. These technical innovations are directly translatable to real-world monitoring, especially in field-deployable contexts. Similarly, Roy et al. designed a MoS2@ZIF-8 nanocomposite photocatalyst that achieved 98.4% degradation efficiency of methylene blue (a model organic pollutant) under visible light within 70 min, showcasing a material-based advancement that complements separation-based detection by reducing contaminant burden before analysis [185]. Together, these examples highlight how innovations in materials (e.g., catalysts, ionization aids) and device miniaturization (e.g., paper spray-MS) provide scalable, low-resource solutions to environmental pollutant detection.
Looking forward, integrating AI-powered analytics, smart sensors, and automated LC-MS platforms into M-LC workflows will define the next frontier in organic pollutant detection. Most recently, Pawar et al. showcased the potential of automated data preprocessing and algorithmic peak deconvolution in high-throughput LC analyses, which can reduce false positives/negatives and optimize method validation, especially in matrix-heavy environmental samples [186]. Sensor coupling is another transformative direction. In 2024, Chavan et al. stressed the need for real-time dye/phthalate detection in food using fluorescent sensors or electrochemical arrays that could be seamlessly interfaced with portable LC setups [180]. Additionally, Guo et al.’s miniature MS platform (already field-ready) serves as a prototype for fully deployable pollutant tracking systems, with built-in data transmission capabilities for remote monitoring [184]. Finally, Vignesh et al. advocated for coupling such systems with green extraction and low-energy workflows, aligning future developments with sustainability goals [182]. These directions collectively position M-LC not just as a lab-based tool but as a versatile, intelligent, and eco-conscious system for real-time environmental diagnostics.
3.1.4. Food Allergens
Food allergens pose a significant global public health issue, affecting millions of individuals and potentially triggering a wide spectrum of immune responses—from mild gastrointestinal discomfort and hives to severe, life-threatening anaphylaxis [187,188]. The impact of food allergies extends beyond individual health, placing a substantial burden on healthcare systems due to the need for emergency interventions, long-term management, and strict preventive measures [189].
Nelis et al. conducted a study that established a rapid LC-MS method for quantifying milk allergens by evaluating all tryptic peptides of the main milk allergens [190]. The bovine-specific αS-2 casein peptide and the allergen-epitope NAVPITPTLNR showed excellent sensitivity (LOD 1 mg·kg−1; LOQ 2 mg·kg−1) across several dairy products; it also showed good recovery in baked croissants (77% with a 10% inter-day RSD) and exhibited a linear range of 2–2000 mg·kg−1. This technique showed that it is suitable for routine detection of trace contamination with bovine milk allergens and for identifying adulteration of high-value caprine dairy products with lower-value bovine milk, thereby safeguarding consumer confidence and addressing the needs of the increasing population affected by food allergies. The specificity of the identified pan-milk and bovine-specific peptides was tested experimentally using digests from full-cream goat milk, a feta made 100% from sheep and goat milk, and a feta made from 100% bovine milk [190] (Figure 21).
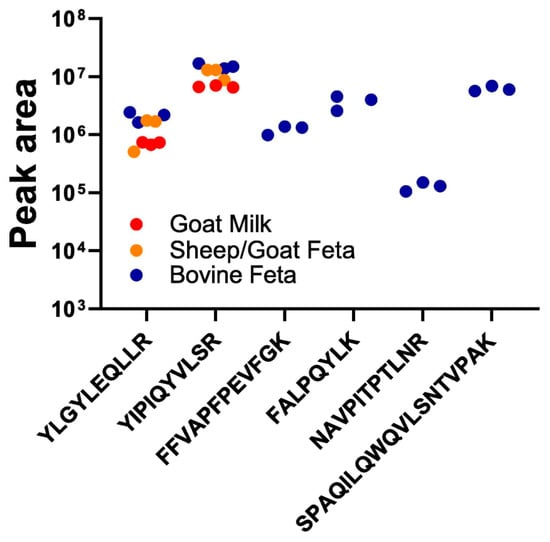
Figure 21.
Peak areas for pan-milk and bovine-specific allergen peptides detected in digests of goat milk, feta made from a mixture of sheep and goat milk, and feta made from cow milk. YIPIQYVLSR gave the best results for the pan-allergen peptides (peak area ~107 and a CV of 33% across all dairy types tested versus a peak area of ~106 and a CV of 50% for YLGYLEQLLR). Of the bovine-specific peptides, SPAQILQWQVLSNTVPAK gave the highest intensity and lowest CV (9%). NAVPITPTLNR had the lowest signal intensity and a CV of 14%. FFVAPFPEVFGK equally had a CV of 14%, while FALPQYLK had the highest CV of these peptides (22%) [190].
Recent technological advancements in M-LC have significantly impacted the detection of food allergens by enabling more efficient, sensitive, and field-deployable analytical tools. As highlighted by Jain et al., the transition from conventional LC to M-LC platforms has made it possible to integrate ultra-sensitive biosensors and compact detection modules into portable configurations [191]. This is particularly useful for detecting trace allergens like gluten, where lateral-flow immunoassays and LOC LC systems have achieved detection limits as low as 0.1 and 0.5 ppm for gliadin [191]. Moreover, Bianco et al. further reported that coupling M-LC with tandem MS (LC-MS/MS) enhances the ability to identify novel and complex protein allergens in processed foods, such as insect proteins and plant-based substitutes, with LODs reaching sub-ng/mL levels [188]. These advances provide practical improvements by reducing sample volume requirements (down to mL), minimizing analysis time (minutes vs. hours), and increasing throughput, which are essential features for screening in high-risk manufacturing environments or during food recalls. Table 4 shows applications of M-LC to food matrices.

Table 4.
Applications of M-LC to food matrices.
Looking forward, the integration of M-LC systems with AI and smart sensors offers a transformative opportunity for allergen detection. In 2024, Adedeji et al. emphasized the role of AI-powered, non-destructive optical sensors (e.g., hyperspectral imaging and near-infrared spectroscopy) in real-time allergen monitoring [203]. When linked with M-LC and cloud-based analytics, these systems can predict allergen presence, classify food types, and even automate risk assessment with over 90% accuracy in certain models. The synergy between sensor technologies and M-LC has already led to the development of prototype handheld devices for allergen detection, capable of on-site quantification of allergenic proteins using AI-enhanced signal processing [203]. Moreover, Jain et al. discussed how M-LC chips with integrated microfluidics and smart detection interfaces are enabling field-deployable testing in food processing lines, supporting compliance with labeling laws and enhancing consumer safety. Together, these innovations point to a future where food allergen testing is not only highly sensitive and accurate but also decentralized, autonomous, and responsive to the complexities of modern food systems [191].
3.2. Environmental Analysis
M-LC has emerged as a highly effective analytical tool for detecting a broad range of pollutants across diverse environmental matrices, including water, soil, and air [204]. Its outstanding sensitivity and specificity allow for the detection of contaminants at ultra-trace levels—an essential capability given the typically low concentrations of pollutants in natural environments [205,206].
For instance, M-LC has proven particularly effective in quantifying endocrine-disrupting chemicals, such as bisphenol A and phthalates, in water samples [108,204]. Table 5 shows how M-LC is applied to environmental matrices. A key advancement in M-LC is its portability, which has revolutionized environmental monitoring by enabling on-site analyses [17]. This real-time capability minimizes risks of sample degradation or contamination during transport, ensuring more reliable and representative data.

Table 5.
Applications of M-LC to environmental matrices.
One illustrative study by Serra-Mora et al. investigated the degradation kinetics and residual levels of the sulfonylurea herbicide tribenuron-methyl in different water sources [212]. Using in-tube solid-phase microextraction (IT-SPME) online-coupled with nanoLC and UV diode array detection (DAD), the researchers achieved highly sensitive detection (LOD: 0.25 ppb) without altering the original matrix. The method leveraged the enhanced extraction efficiency of IT-SPME—boosted by metal oxide nanoparticle-reinforced polymeric coatings—and the superior sensitivity of nanoLC. The study assessed tribenuron-methyl degradation under varied conditions, including UV exposure and pH fluctuations, across water types such as sea, river, ditch, and transitional waters. Results indicated substantial matrix-dependent variability in degradation rates, with tribenuron-methyl persisting from several days to weeks, depending on water composition. In Figure 22, the percentages of unchanged TBM as a function of time are depicted. As observed, significant differences in the degradation rate of TBM were found.
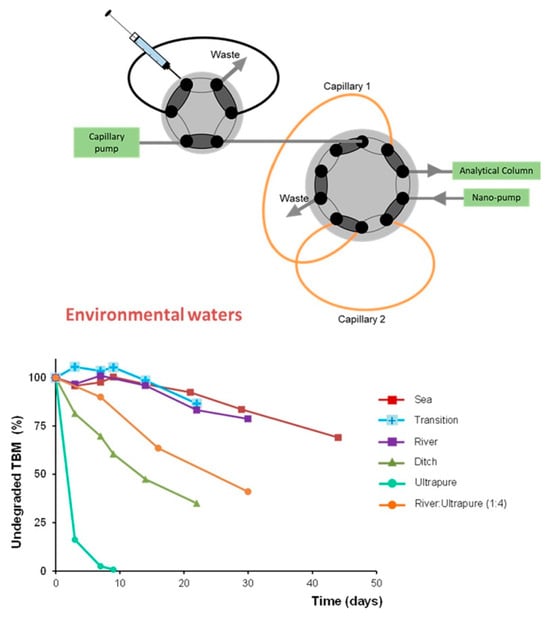
Figure 22.
Representations of the Ln of the percentage of undegraded TBM vs. time of exposure for the different water samples assayed spiked with TBM 50 ppb [212]. Reprinted with permission from Ref. [212]. Copyright 2025, Elsevier. All rights reserved.
Environmental monitoring using M-LC is challenged by issues such as limited accessibility to miniaturized instruments, insufficient training in handling low-volume flow systems, and the complexity of environmental matrices. In 2023, Vargas Medina et al. noted that although technological hurdles like low flow rates and sample handling have largely been overcome, wider adoption is hindered by practical barriers, such as cost and the need to overhaul existing HPLC infrastructure [129]. Environmental matrices, such as water, soil, and air, frequently contain interfering substances that affect reproducibility and analytical accuracy. Moreover, Lulu Shi et al. emphasized that ambient ionization MS, when integrated with M-LC, can simplify sample preparation and improve selectivity in complex field environments, making it a promising tool for environmental assessment [162]. Additionally, their work highlighted how capillary LC-MS/MS systems have been able to detect trace contaminants like perfluorooctanoic acid (PFOA) at sub-ng/L levels in river water, rivaling UHPLC while using significantly less solvent. These improvements demonstrate how miniaturized systems now offer sensitivity, portability, and sustainability, which are essential for decentralized environmental monitoring.
The integration of AI and advanced signal processing techniques presents a transformative direction for environmental M-LC applications. Most recently, Zhan et al. developed an LSTM-EEMD hybrid model for improving data reliability and pattern recognition in miniature MS systems, enabling real-time correction of baseline drifts and noise filtering in field measurements [213]. These techniques are particularly useful for environmental samples, which are prone to signal fluctuation due to varying external factors. Moreover, Internet of Things (IoT)-enabled platforms embedded with ML algorithms now allow remote monitoring and autonomous decision-making, as described in the works by Lulu Shi et al., with one example demonstrating a 20% reduction in downtime using predictive diagnostics [162]. Future M-LC systems will benefit greatly from such edge AI capabilities embedded directly into compact devices. Consequently, Ahrum Son et al. also underscored the importance of integrated microfluidics and AI-driven feedback loops for real-time diagnostics in portable LC-MS systems [127]. The convergence of AI with environmental M-LC will not only reduce the burden of data interpretation but also make these systems more autonomous, energy-efficient, and suitable for long-term deployment in remote or underserved regions.
Recent innovations have led to the development of field-deployable capillary LC-MS systems capable of on-site analysis of persistent pollutants like PFAS (Per- and polyfluoroalkyl substances) in real soil samples. One such system, described by Vargas Medina et al., combines capillary LC with a portable MS unit, weighing just under 40 kg, and is optimized with a split flow interface and ultrasound-assisted extraction for immediate field analysis. This setup achieved detection limits of 0.6 to 0.1 ng/g for PFASs with recovery rates between 70% and 110%, proving its robustness under field conditions. Moreover, it facilitates rapid data collection (under 8 min per run) and matches laboratory-grade accuracy [129]. These field-based systems are particularly relevant for monitoring pollutants in remote or contaminated zones where traditional lab infrastructure is unavailable. The synergy between sensor integration, AI-driven analytics, and miniaturized hardware is now redefining the speed and accuracy of environmental risk assessment. Going forward, the marriage of microfluidics, autonomous control algorithms, and wireless data transmission will be crucial in making miniaturized LC-MS systems a staple in routine environmental monitoring worldwide.
3.3. Biological Analysis
Biological analysis is essential for deciphering complex physiological systems, diagnosing diseases, and advancing therapeutic interventions [214]. In recent years, M-LC has emerged as a transformative technology within this domain, providing high-sensitivity and high-resolution analyses of biomolecules even when sample volumes are severely limited [99]. The compact design and advanced features of M-LC systems make them well-suited to meet the growing demand for rapid, accurate, and cost-effective analytical approaches in contemporary biomedical research [215]. From innovations in proteomics and metabolomics to POC diagnostic technologies, M-LC is reshaping how biological samples are studied and understood [127].
Particularly when integrated with HRMS, M-LC has achieved notable success in detecting biomolecules at nanomolar to picomolar concentrations—critical for studying low-abundance biomarkers in complex biological fluids [158,162]. Table 6 views the applications of M-LC towards biological matrices.

Table 6.
Applications of M-LC to biological matrices.
In order to assess glycated hemoglobin (HbA1c) quickly and accurately, Li et al. developed a small and light LC apparatus weighing 1.8 kg and measuring 20 cm by 16 cm by 16 cm [215]. The system integrates a battery-powered electrolysis micropump (EMP), a highly integrated LC microchip, and an LED-based absorbance detector. The LED detector demonstrated strong linearity (R2 = 0.9860) and a low LOD, approximately nine times lower than that of a commercial detector, thereby fulfilling the sensitivity requirements for on-chip HbA1c analysis. The accuracy (≤4.3%) and repeatability (≤2.8%) of the system were confirmed by measuring the HbA1c levels in samples of diabetes patients and contrasting the results with those from a commercial LC device. According to these results, the suggested LC system has a lot of potential for precise, portable, and POC diabetic diagnostics. Figure 23 shows that the future model for diagnostics may change from the clinical setting to community hospitals and remote regions.
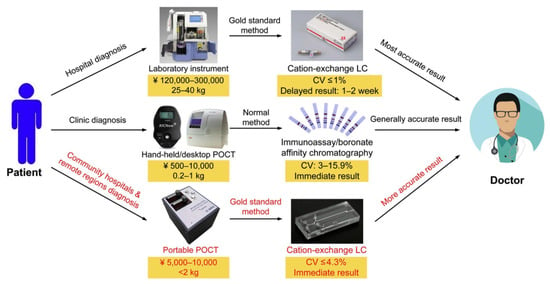
Figure 23.
Schematic comparing emerging diagnostic models for community hospitals and remote regions, and the traditional diagnostic model for hospital and clinic use. A compact and lightweight LC system was developed with an overall size of 20 cm × 16 cm × 16 cm and a weight of 1.8 kg for the fast and accurate measurement of glycated hemoglobin (HbA1c). The system mainly comprises a battery-operated EMP, a highly integrated LC microchip, and an LED-based absorbance detector. The good linearity (R2 = 0.9860) and low LOD (about nine times lower than the commercial detector) of the LED detector were measured to meet the requirement of the on-chip determination. The inaccuracy (≤4.3%) and repeatability (≤2.8%) of the LC system were successfully verified by measuring the HbA1c levels of patients with diabetes and comparing the results with those from a commercial LC instrument [215]. Reprinted with permission from Ref. [215]. Copyright 2025 Elsevier. All rights reserved.
Biological analysis using M-LC faces several technical and practical challenges, especially when transitioning from controlled laboratory settings to real-world applications. One core issue is the variability and complexity of biological matrices, such as urine, blood, and tissue extracts, which often introduce interfering substances that compromise sensitivity and reproducibility [228]. Similarly, Maciel et al. demonstrated this in their hormone analysis study using an in-house packed ODS-GO column in capillary LC, where meticulous optimization was required due to matrix effects [126]. Additionally, sample volumes are often limited in clinical or pediatric settings, which necessitates techniques capable of ultra-trace detection. That said, Zhen Long et al. compared analytical, micro-, and nano-flow LC-MS/MS platforms and found that nano-flow systems, despite offering the highest sensitivity, suffered from lower robustness and increased susceptibility to clogging, especially problematic in clinical workflows. Instrument miniaturization also introduces maintenance difficulties; for example, the tiny inner diameters of capillaries (<500 µm) demand specialized training to avoid operational errors such as overloading or leakage [129]. These limitations highlight the need for durable, user-friendly M-LC systems for consistent biological analysis.
Recent advances in M-LC platforms have begun addressing these challenges by enhancing sensitivity, portability, and system integration. Therefore, Ahrum Son et al. emphasized the power of HRMS (e.g., orbitrap, TOF) paired with M-LC in detecting low-abundance biomarkers such as dihydrotestosterone or estradiol with high specificity, hence overcoming the cross-reactivity seen in immunoassays. In 2023, Wu et al. demonstrated a successful application of miniaturized MS coupled with C18 pipette-tip solid-phase extraction for the detection of illicit drugs in urine at sub-ng/mL levels in under 10 min. This setup proved effective for rapid screening in toxicological and forensic settings, offering both portability and performance comparable to laboratory-scale systems [228]. Similarly, Maciel et al. introduced an in-house packed C18-functionalized graphene oxide column for capillary LC hormone analysis in urine, achieving LOD values as low as 0.04 µg/L with RSDs under 6.9% [126]. These innovations prove that M-LC, when engineered for compactness and sensitivity, can support clinical diagnostics and biomarker discovery at the bedside or in the field, and portability without compromising analytical performance.
Looking forward, the fusion of AI-based data analytics, sensor integration, and portable chip-based LC systems represents a transformative trajectory for biological analysis. According to Medina & Lanças et al., adoption of M-LC beyond omics will depend on simplifying operation through intelligent control and automating data workflows [129]. AI models can now predict retention times, automate optimization, and perform drift correction, therefore minimizing operator involvement. Subsequently, Henning et al. noted that AI is already being embedded into microcontroller units for real-time calibration, fault detection, and dynamic actuation [229]. LOC devices, like those developed by Rafiq et al., combine microfluidics, optical detection, and data processing into self-contained units ideal for resource-limited settings [128]. The emergence of cloud-connected, edge-AI-enabled M-LC systems is redefining biological analysis as a decentralized practice, where wearable biosensors and portable LC-MS can deliver hospital-grade diagnostics even in field hospitals or remote areas. These converging technologies not only overcome current challenges but also democratize access to high-precision bioanalytical tools.
4. Conclusions and Future Trend
Recent advancements in M-LC have substantially enhanced analytical capabilities across diverse scientific domains—including food safety, environmental monitoring, and biomedical research. By combining reduced sample and solvent consumption with enhanced sensitivity, rapid analysis, and growing potential for automation, M-LC has emerged as a cornerstone of modern analytical chemistry.
In food analysis, M-LC has demonstrated remarkable efficacy in detecting toxins, drug residues, allergens, and various organic contaminants. Its ability to accurately quantify trace compounds in complex food matrices ensures regulatory compliance and strengthens consumer health protection. Environmental applications have equally benefited from M-LC’s high sensitivity and portability. The integration of microchip platforms with advanced MS allows for real-time, on-site detection of pollutants in water, soil, and air—facilitating timely interventions and contributing to sustainable environmental management. In biological research, M-LC is transforming how we study disease biomarkers, monitor therapeutic drugs, and analyze metabolites, particularly in scenarios where sample volume is limited. These capabilities position M-LC as a vital tool in the advancement of personalized medicine and POC diagnostics.
Looking ahead, several key trends are likely to shape the future of M-LC:
- (1)
- Integration with emerging technologies: M-LC systems are expected to increasingly incorporate microfluidics, LOC designs, and ambient ionization techniques. These integrations will enhance automation, portability, and suitability for field-based and POC applications.
- (2)
- Enhanced sensitivity and selectivity: Advances in column materials, mass spectrometry interfaces, and sample preparation techniques will continue to push detection limits lower, enabling the analysis of trace-level analytes in even more complex matrices.
- (3)
- Growth in personalized medicine: M-LC will play an expanding role in therapeutic drug monitoring, pharmacokinetic studies, and biomarker discovery. Its rapid, high-sensitivity analysis of minute biological samples will support individualized treatment strategies and improve clinical outcomes.
- (4)
- Sustainability analytical practices: The environmentally friendly features of M-LC—including low solvent consumption and shorter run times—align with global priorities in green chemistry. As sustainability becomes a guiding principle in laboratory practices, M-LC adoption is expected to rise.
- (5)
- Integration with data science: As M-LC platforms generate increasingly complex datasets, advanced computational tools such as machine learning and big data analytics will become essential. These technologies will enhance data interpretation, enable predictive modeling, and unlock deeper insights into biological and environmental systems.
In conclusion, it is poised for continued innovation and broader adoption. With its versatility, precision, and adaptability, M-LC will remain a driving force in advancing analytical science and addressing the multifaceted challenges of food safety, environmental health, and biomedical research in the years to come.
Author Contributions
Investigation, H.L.; conceptualization, K.T.; data analysis H.L.; writing the original draft, K.T.; review D.P., Z.W. and Y.Z.; writing—review and editing by K.T., D.P., Z.W., Y.Z. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32572674 and 32102060; and the Key R&D Program of Ningbo, grant number 2025Z102.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Golay, M.J. Theory of chromatography in open and coated tubular columns with round and rectangular cross-sections. Gas Chromatogr. 1958, 2, 36–55. [Google Scholar][Green Version]
- Giddings, J.C. Comparison of the Theoretical Limit of Separating Ability in Gas and Liquid Chromatography. Anal. Chem. 1964, 36, 1890–1892. [Google Scholar] [CrossRef]
- Horvath, C. High-Performance Ion-Exchange Chromatography with Narrow-Bore Columns: Rapid Analysis of Nucleic Acid Constituents at the Subnanomole Level. Methods Biochem. Anal. 1973, 21, 79–154. [Google Scholar] [CrossRef]
- Tsuda, T.; Hibi, K.; Nakanishi, T.; Takeuchi, T.; Ishii, D. Studies of open-tubular micro-capillary liquid chromatography: II. Chemically bonded octadecylsilane stationary phase. J. Chromatogr. A 1978, 158, 227–232. [Google Scholar] [CrossRef]
- Henion, J. Chapter 8 Micro LC/MS Coupling. In Journal of Chromatography Library; Kucera, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 28, pp. 260–300. [Google Scholar] [CrossRef]
- Novotny, M. Contemporary capillary gas chromatography. Anal. Chem. 1978, 50, 16A–32A. [Google Scholar] [CrossRef]
- Jorgenson, J.; Luckacs, K.D. Free-zone electrophoresis in glass capillaries. Clin. Chem. 1981, 27, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Rae, T. IBM-PC,-XT,-AT 170K RAM (128K clear RAM at run time) ISBN 0 947946 56 X. TRAC Trends Anal. Chem. 2013, 7, 151. [Google Scholar]
- Baram, G.I. Portable liquid chromatograph for mobile laboratories I. Aims. J. Chromatogr. A 1996, 728, 387–399. [Google Scholar] [CrossRef]
- Akhter, M.; Alam, M.M. Chromatography Techniques. In Physical Pharmacy and Instrumental Methods of Analysis; Akhter, M., Alam, M.M., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 207–225. [Google Scholar] [CrossRef]
- Aydoğan, C.; Rigano, F.; Krcmova, L.K.; Chung, D.S.; Macka, M.; Mondello, L. Miniaturized LC in molecular omics. Anal. Chem. 2020, 92, 11485–11497. [Google Scholar] [CrossRef]
- Cruz, J.C.; de Souza, I.D.; Grecco, C.F.; Figueiredo, E.C.; Queiroz, M.E.C. Recent advances in column switching high-performance liquid chromatography for bioanalysis. Sustain. Chem. Pharm. 2021, 21, 100431. [Google Scholar] [CrossRef]
- Kanu, A.B. Recent developments in sample preparation techniques combined with high-performance liquid chromatography: A critical review. J. Chromatogr. A 2021, 1654, 462444. [Google Scholar] [CrossRef]
- Dong, M.W.; Zhang, K. Ultra-high-pressure liquid chromatography (UHPLC) in method development. TrAC Trends Anal. Chem. 2014, 63, 21–30. [Google Scholar] [CrossRef]
- Nováková, L.; Svoboda, P.; Pavlík, J. Chapter 29—Ultra-high performance liquid chromatography. In Liquid Chromatography, 2nd ed.; Fanali, S., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 719–769. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; de Toffoli, A.L.; Sobieski, E.; Domingues Nazário, C.E.; Lanças, F.M. Miniaturized liquid chromatography focusing on analytical columns and mass spectrometry: A review. Anal. Chim. Acta 2020, 1103, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Cortés Bautista, S.; Carmen Molins, L.; Pilar, C.-F.; Rosa, H.-H.; Luis Antonio, T.-G.; José González, R. Miniaturized Liquid Chromatography: From Lab to Portable Tool for Environmental, Biological, and Nanoparticles Samples; Universitat de València: Valencia, Spain, 2023; pp. 1–264. Available online: https://producciocientifica.uv.es/documentos/6876982b3383aa6556f3cc1a (accessed on 3 October 2023).
- Vissers, J.P.C.; Claessens, H.A.; Cramers, C.A. Microcolumn liquid chromatography: Instrumentation, detection and applications. J. Chromatogr. A 1997, 779, 1–28. [Google Scholar] [CrossRef]
- Ishida, A.; Nishimura, T.; Koyama, K.; Maeki, M.; Tani, H.; Tokeshi, M. A portable liquid chromatography system based on a separation/detection chip module consisting of a replaceable ultraviolet-visible absorbance or contactless conductivity detection unit. J. Chromatogr. A 2023, 1706, 464272. [Google Scholar] [CrossRef]
- Cruz, J.C.; Souza, I.D.d.; Lanças, F.M.; Queiroz, M.E.C. Current advances and applications of online sample preparation techniques for miniaturized liquid chromatography systems. J. Chromatogr. A 2022, 1668, 462925. [Google Scholar] [CrossRef]
- Svensson, K.; Weise, C.; Westphal, H.; Södergren, S.; Belder, D.; Hjort, K. Coupling microchip pressure regulators with chipHPLC as a step toward fully portable analysis system. Sens. Actuators B Chem. 2023, 385, 133732. [Google Scholar] [CrossRef]
- Mejía-Carmona, K.; Maciel, E.V.S.; Lanças, F.M. Miniaturized liquid chromatography applied to the analysis of residues and contaminants in food: A review. Electrophoresis 2020, 41, 1680–1693. [Google Scholar] [CrossRef]
- Fedorenko, D.; Bartkevics, V. Recent applications of nano-liquid chromatography in food safety and environmental monitoring: A review. Crit. Rev. Anal. Chem. 2023, 53, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Caño-Carrillo, I.; Gilbert-López, B.; Montero, L.; Martínez-Piernas, A.B.; García-Reyes, J.F.; Molina-Díaz, A. Comprehensive and heart-cutting multidimensional liquid chromatography–mass spectrometry and its applications in food analysis. Mass Spectrom. Rev. 2024, 43, 936–976. [Google Scholar] [CrossRef]
- Tang, M.; Zhao, Y.; Chen, J.; Xu, D. On-line multi-residue analysis of fluoroquinolones and amantadine based on an integrated microfluidic chip coupled to triple quadrupole mass spectrometry. Anal. Methods 2020, 12, 5322–5331. [Google Scholar] [CrossRef]
- Salido-Fortuna, S.; Bosco, C.D.; Gentili, A.; Castro-Puyana, M.; Marina, M.L.; D’Orazio, G.; Fanali, S. Enantiomeric analysis of drugs in water samples by using liquid–liquid microextraction and nano-liquid chromatography. Electrophoresis 2023, 44, 1177–1186. [Google Scholar] [CrossRef]
- Cortés-Bautista, S.; Navarro-Utiel, R.; Ballester-Caudet, A.; Campíns-Falcó, P. Towards in field miniaturized liquid chromatography: Biocides in wastewater as a proof of concept. J. Chromatogr. A 2022, 1673, 463119. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, M.; Xu, D. Integrated microfluidic chip coupled to mass spectrometry: A minireview of chip pretreatment methods and applications. J. Chromatogr. Open 2021, 1, 100021. [Google Scholar] [CrossRef]
- Yuan, X.; Oleschuk, R.D. Advances in Microchip Liquid Chromatography. Anal. Chem. 2018, 90, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Chang, H.L. Chip-based ion chromatography (chip-IC) with a sensitive five-electrode conductivity detector for the simultaneous detection of multiple ions in drinking water. Microsyst. Nanoeng. 2020, 6, 66. [Google Scholar] [CrossRef]
- Ríos, Á.; Zougagh, M. Chip-Based Separation Devices Coupled to Mass Spectrometry in Food and Environmental Chemistry. In Mass Spectrometry in Food and Environmental Chemistry; Picó, Y., Campo, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 359–379. [Google Scholar] [CrossRef]
- André, C.; Guillaume, Y.C. Development of nano Bio LC columns for the search of acetylcholinesterase molecular targets. J. Sep. Sci. 2022, 45, 2109–2117. [Google Scholar] [CrossRef]
- Santbergen, M.J.C.; van der Zande, M.; Gerssen, A.; Bouwmeester, H.; Nielen, M.W.F. Dynamic in vitro intestinal barrier model coupled to chip-based liquid chromatography mass spectrometry for oral bioavailability studies. Anal. Bioanal. Chem. 2020, 412, 1111–1122. [Google Scholar] [CrossRef]
- Khoo, H.T.; Leow, C.H. Advancements in the preparation and application of monolithic silica columns for efficient separation in liquid chromatography. Talanta 2021, 224, 121777. [Google Scholar] [CrossRef]
- Roberg-Larsen, H.; Wilson, S.R.; Lundanes, E. Recent advances in on-line upfront devices for sensitive bioanalytical nano LC methods. TrAC Trends Anal. Chem. 2021, 136, 116190. [Google Scholar] [CrossRef]
- Meher, A.K.; Zarouri, A. Green Analytical Chemistry—Recent Innovations. Analytica 2025, 6, 10. [Google Scholar] [CrossRef]
- Agrawal, A.; Keçili, R.; Ghorbani-Bidkorbeh, F.; Hussain, C.M. Green miniaturized technologies in analytical and bioanalytical chemistry. TrAC Trends Anal. Chem. 2021, 143, 116383. [Google Scholar] [CrossRef]
- Rahimi, F.; Chatzimichail, S.; Saifuddin, A.; Surman, A.J.; Taylor-Robinson, S.D.; Salehi-Reyhani, A. A Review of Portable High-Performance Liquid Chromatography: The Future of the Field? Chromatographia 2020, 83, 1165–1195. [Google Scholar] [CrossRef]
- Otagawa, T.; Stetter, J.R.; Zaromb, S. Portable liquid chromatograph for analysis of primary aromatic amines in coal-derived materials. J. Chromatogr. A 1986, 360, 252–259. [Google Scholar] [CrossRef]
- Tulchinsky, V.M.; St. Angelo, D.E. A practical portable HPLC system—MINICHROM, a new generation for field HPLC. Field Anal. Chem. Technol. 1998, 2, 281–285. [Google Scholar] [CrossRef]
- Ishida, A.; Fujii, M.; Fujimoto, T.; Sasaki, S.; Yanagisawa, I.; Tani, H.; Tokeshi, M. A Portable Liquid Chromatograph with a Battery-operated Compact Electroosmotic Pump and a Microfluidic Chip Device with a Reversed Phase Packed Column. Anal. Sci. 2015, 31, 1163–1169. [Google Scholar] [CrossRef]
- Lynch, K.B.; Chen, A.; Yang, Y.; Lu, J.J.; Liu, S. High-performance liquid chromatographic cartridge with gradient elution capability coupled with UV absorbance detector and mass spectrometer for peptide and protein analysis. J. Sep. Sci. 2017, 40, 2752–2758. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Z.; Chang, H. Chip-based nanoflow liquid chromatography for on-chip detection of glycated haemoglobin levels. Sens. Actuators B Chem. 2019, 291, 433–440. [Google Scholar] [CrossRef]
- Foster, S.W.; Xie, X.; Pham, M.; Peaden, P.A.; Patil, L.M.; Tolley, L.T.; Farnsworth, P.B.; Tolley, H.D.; Lee, M.L.; Grinias, J.P. Portable capillary liquid chromatography for pharmaceutical and illicit drug analysis. J. Sep. Sci. 2020, 43, 1623–1627. [Google Scholar] [CrossRef]
- Chatzimichail, S.; Rahimi, F.; Saifuddin, A.; Surman, A.J.; Taylor-Robinson, S.D.; Salehi-Reyhani, A. Hand-portable HPLC with broadband spectral detection enables analysis of complex polycyclic aromatic hydrocarbon mixtures. Commun. Chem. 2021, 4, 17. [Google Scholar] [CrossRef]
- Hemida, M.; Ghiasvand, A.; Gupta, V.; Coates, L.J.; Gooley, A.A.; Wirth, H.-J.; Haddad, P.R.; Paull, B. Small-Footprint, Field-Deployable LC/MS System for On-Site Analysis of Per- and Polyfluoroalkyl Substances in Soil. Anal. Chem. 2021, 93, 12032–12040. [Google Scholar] [CrossRef]
- Hemida, M.; Haddad, P.R.; Lam, S.C.; Coates, L.J.; Riley, F.; Diaz, A.; Gooley, A.A.; Wirth, H.-J.; Guinness, S.; Sekulic, S.; et al. Small footprint liquid chromatography-mass spectrometry for pharmaceutical reaction monitoring and automated process analysis. J. Chromatogr. A 2021, 1656, 462545. [Google Scholar] [CrossRef]
- Yin, H.; Killeen, K.; Brennen, R.; Sobek, D.; Werlich, M.; van de Goor, T. Microfluidic chip for peptide analysis with an integrated HPLC column, sample enrichment column, and nanoelectrospray tip. Anal. Chem. 2005, 77, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Kozlik, P.; Sanda, M.; Goldman, R. Nano reversed phase versus nano hydrophilic interaction liquid chromatography on a chip in the analysis of hemopexin glycopeptides. J. Chromatogr. A 2017, 1519, 152–155. [Google Scholar] [CrossRef]
- Kleinnijenhuis, A.J.; Ingola, M.; Toersche, J.H.; van Holthoon, F.L.; van Dongen, W.D. Quantitative Bottom Up Analysis of Infliximab in Serum Using Protein A Purification and Integrated μLC-Electrospray Chip Ionkey MS/MS Technology. Bioanalysis 2016, 8, 891–904. [Google Scholar] [CrossRef]
- Müller, J.B.; Geyer, P.E.; Colaço, A.R.; Treit, P.V.; Strauss, M.T.; Oroshi, M.; Doll, S.; Virreira Winter, S.; Bader, J.M.; Köhler, N.; et al. The proteome landscape of the kingdoms of life. Nature 2020, 582, 592–596. [Google Scholar] [CrossRef]
- Murugesan, P.; Raj, G.; Moses, J.A. Microfluidic devices for the detection of pesticide residues. Rev. Environ. Sci. Bio-Technol. 2023, 22, 625–652. [Google Scholar] [CrossRef]
- Jiao, D.; Zhang, R.; Zhang, H.; Ma, H.; Zhang, X.; Fan, X.; Chang, H. Rapid detection of glycosylated hemoglobin levels by a microchip liquid chromatography system in gradient elution mode. Anal. Chim. Acta 2024, 1288, 342186. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Zhang, R.; Wang, M.; Zhang, X.; Ma, H.; Li, M.; Chang, H. Compact photometric detector integrated with separation microchip for potential portable liquid chromatography system. J. Chromatogr. A 2024, 1731, 465175. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Li, Y.; Wu, Z.; Shang, Y.; Yao, H.; Jia, Y.; Lin, J.-M. Alginate-gelatin hydrogel scaffolds for establishing physiological barriers on a gut-brain-axis microchip. Int. J. Biol. Macromol. 2025, 312, 144084. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.A.; Huynh, V.; Doeven, E.H.; Adams, S.; Kouzani, A.; Guijt, R.M. Closed-loop control systems for pumps used in portable analytical systems. J. Chromatogr. A 2023, 1695, 463931. [Google Scholar] [CrossRef]
- Shabgard, H.; Mirbozorgi, S.A.; Niazmand, H. Numerical Simulation and Performance Analysis of Multi-Stage Electroosmotic Micropumps. Commun. Nonlinear Sci. Numer. Simul. 2024, 128, 107616. [Google Scholar] [CrossRef]
- Hemida, M.; Ghiasvand, A.; Macka, M.; Gupta, V.; Haddad, P.R.; Paull, B. Recent advances in miniaturization of portable liquid chromatography with emphasis on detection. J. Sep. Sci. 2023, 46, 2300283. [Google Scholar] [CrossRef]
- Shultz, C.; Harrison, C. Flat Panel Haptics: Embedded Electroosmotic Pumps for Scalable Shape Displays. In Proceedings of the 2023 CHI Conference on Human Factors in Computing Systems, Hamburg, Germany, 23–28 April 2023; Association for Computing Machinery: New York, NY, USA, 2023; p. 745. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Li, Z.; Tang, Q.; Chen, J.; Huang, Y.; Hu, C.; Guo, H.; Peng, Y.; Wang, Z.L. A Mobile and Self-Powered Micro-Flow Pump Based on Triboelectricity Driven Electroosmosis. Adv. Mater. 2021, 33, 2102765. [Google Scholar] [CrossRef] [PubMed]
- Kiy, A.; Dutt, S.; Notthoff, C.; Toimil-Molares, M.E.; Kirby, N.; Kluth, P. Highly Rectifying Conical Nanopores in Amorphous SiO2 Membranes for Nanofluidic Osmotic Power Generation and Electroosmotic Pumps. ACS Appl. Nano Mater. 2023, 6, 8564–8573. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Q.; Tang, S.; Khoo, B.C.; Chang, Z. Intelligent Fault Diagnosis Methods for Hydraulic Piston Pumps: A Review. J. Mar. Sci. Eng. 2023, 11, 1609. [Google Scholar] [CrossRef]
- Xing, T.; Huang, Y.; Gao, C.; Ruan, J. Characteristic Study of a Valve-Matched Flow Single-Piston Miniature Water-Hydraulic Pump. IEEE Access 2024, 12, 110504–110516. [Google Scholar] [CrossRef]
- Pravika, M.; Jacob, J.; Joseph, K.P. Design of linear electromechanical actuator for automatic ambulatory Duodopa pump. Eng. Sci. Technol. Int. J. 2022, 31, 101056. [Google Scholar] [CrossRef]
- Hemida, M.; Coates, L.J.; Lam, S.; Gupta, V.; Macka, M.; Wirth, H.-J.; Gooley, A.A.; Haddad, P.R.; Paull, B. Miniature Multiwavelength Deep UV-LED-Based Absorption Detection System for Capillary LC. Anal. Chem. 2020, 92, 13688–13693. [Google Scholar] [CrossRef]
- Lucchini, A.; Elli, S.; Burgazzi, A.; Malvestuto Grilli, L.; Pes, C.; Ferrari, K.; Fumagalli, L.; Fiorillo, C.; Giani, M.; Rezoagli, E. Simulated haemodynamic parameters and different infusion set-up affect drug delivery during syringe pump change over: A bench-top study in a laboratory setting. Intensive Crit. Care Nurs. 2025, 86, 103861. [Google Scholar] [CrossRef]
- Petrović, R.; Banaszek, A.; Andjelković, M.; Qananah, H.R.; Alnagasa, K.A. Experimental Tests of the Piston Axial Pump with Constant Pressure and Variable Flow. Designs 2024, 8, 5. [Google Scholar] [CrossRef]
- Andrade-Cedeno, R.J.; Pérez-Rodríguez, J.A.; Amaya-Jaramillo, C.D.; Rodríguez-Borges, C.G.; Llosas-Albuerne, Y.E.; Barros-Enríquez, J.D. Numerical Study of Constant Pressure Systems with Variable Speed Electric Pumps. Energies 2022, 15, 1918. [Google Scholar] [CrossRef]
- Wei, C.; Dong, J.; Yang, H.; Shi, J.; Fang, H.; Zuo, Y.; Shi, Y. Recent development of aspiration pump in the aspiration thrombectomy system. Med. Nov. Technol. Devices 2024, 22, 100292. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, X.; Han, X.; Liu, Y.; Li, G. A handheld, wide-range pressure pump for portable microfluidic applications. Sens. Actuators A Phys. 2024, 377, 115683. [Google Scholar] [CrossRef]
- Gao, R.Z.; Hébert, M.; Huissoon, J.; Ren, C.L. µPump: An open-source pressure pump for precision fluid handling in microfluidics. HardwareX 2020, 7, e00096. [Google Scholar] [CrossRef]
- Hoang, S.; Shehada, M.; Patel, Z.; Tran, M.-H.; Karydis, K.; Brisk, P.; Grover, W.H. Air-powered logic circuits for error detection in pneumatic systems. Device 2024, 2, 100507. [Google Scholar] [CrossRef]
- Işıklı, F.; Şentürk, G.; Sürmen, A. Effects of Valve, Armature, and Armature Pin Guidance on Diesel Injector Performance. Appl. Sci. 2024, 14, 5737. [Google Scholar] [CrossRef]
- Zohouri, D.; Lienard-Mayor, T.; Obeid, S.; Taverna, M.; Mai, T.D. A review on hyphenation of droplet microfluidics to separation techniques: From instrumental conception to analytical applications for limited sample volumes. Anal. Chim. Acta 2023, 1291, 342090. [Google Scholar] [CrossRef] [PubMed]
- Crucello, J.; de Oliveira, A.M.; Sampaio, N.; Hantao, L.W. Miniaturized systems for gas chromatography: Developments in sample preparation and instrumentation. J. Chromatogr. A 2022, 1685, 463603. [Google Scholar] [CrossRef]
- Morioka, K.; Sato, H.; Morita, K.; Akihide, H.; Nakajima, H.; Shoji, A.; Yanagida, A. Development of an on-chip sample injection system with a 6-port valve incorporated in a microchip. RSC Adv. 2020, 10, 35848–35855. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, J.; Zhang, X.; Jiang, J.; Zhang, M.; Li, Y.; Li, T.; Wang, J. A thread-based micro device for continuous electrochemical detection of saliva urea. Microchem. J. 2023, 190, 108634. [Google Scholar] [CrossRef]
- Gilbile, D.; Shelby, M.L.; Lyubimov, A.Y.; Wierman, J.L.; Monteiro, D.C.F.; Cohen, A.E.; Russi, S.; Coleman, M.A.; Frank, M.; Kuhl, T.L. Plug-and-play polymer microfluidic chips for hydrated, room temperature, fixed-target serial crystallography. Lab. Chip. 2021, 21, 4831–4845. [Google Scholar] [CrossRef]
- Hicks, M.B.; Mattern, K.; Fine, J.; Grosser, S.; Patel, D.; Weisel, L.; Aggarwal, P. Portable capillary LC for in-line UV monitoring and MS detection: Comparable sensitivity and much lower solvent consumption. J. Sep. Sci. 2023, 46, 2300300. [Google Scholar] [CrossRef]
- Cocovi-Solberg, D.J.; Schnidrig, S.; Miró, M.; Hann, S. Versatile injector for inline renewable solid-phase extraction: Application to cyclodextrin-based bioaccessibility assessment in environmental solids. Anal. Chim. Acta 2024, 1329, 343047. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; He, C.; Shen, H. Programmable stopped-flow injection analysis: A comparative study on the effects of adenosine and its aptamer on respiratory burst of salivary and circulatory neutrophils. Talanta 2024, 271, 125672. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.W.; Gates, E.P.; Peaden, P.A.; Calugaru, S.V.; West, W.R.; Lee, M.L.; Grinias, J.P. Column selection considerations in compact capillary liquid chromatography. J. Chromatogr. A 2023, 1701, 464067. [Google Scholar] [CrossRef]
- Sanjuan-Navarro, L.; Cortés-Bautista, S.; Moliner-Martínez, Y.; Campíns-Falcó, P. In-tube solid phase microextraction coupled to miniaturized liquid chromatography for both, noble metal nanoparticle assessment and sensitive plasmonic assay development. Anal. Chim. Acta 2021, 1171, 338665. [Google Scholar] [CrossRef]
- Kubo, T.; Ichikawa, M.; Adachi, T.; Watabe, Y.; Naito, T.; Otuka, K. Development of a particle packed bed model for homogeneity evaluation of liquid chromatography column. J. Chromatogr. A 2023, 1705, 464171. [Google Scholar] [CrossRef]
- Rozing, G. Micropillar array columns for advancing nanoflow HPLC. Microchem. J. 2021, 170, 106629. [Google Scholar] [CrossRef]
- Shih, C.H.; Ke, C.H.; Hsiao, C.C. Liquid chromatography on a centrifugal platform for separation and collection of water-soluble dyes. J. Chromatogr. A 2023, 1705, 464211. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Izumi, Y.; Hata, K.; Baron, G.V.; Bamba, T.; Desmet, G. Performance of small-domain monolithic silica columns in nano-liquid chromatography and comparison with commercial packed bed columns with 2 µm particles. J. Chromatogr. A 2020, 1616, 460804. [Google Scholar] [CrossRef]
- Debruille, K.; Mai, Y.; Hortin, P.; Bluett, S.; Murray, E.; Gupta, V.; Paull, B. Portable IC system enabled with dual LED-based absorbance detectors and 3D-printed post-column heated micro-reactor for the simultaneous determination of ammonium, nitrite and nitrate. Anal. Chim. Acta 2024, 1304, 342556. [Google Scholar] [CrossRef]
- McCalley, D.V. Understanding and managing peak shape for basic solutes in reversed-phase high performance liquid chromatography. Chem. Commun. 2023, 59, 7887–7899. [Google Scholar] [CrossRef]
- Li, W.W.; Keller, A.A. Optimization of Targeted Plant Proteomics Using Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS). ACS Agric. Sci. Technol. 2023, 3, 421–431. [Google Scholar] [CrossRef]
- Fitz, V.; El Abiead, Y.; Berger, D.; Koellensperger, G. Systematic investigation of LC miniaturization to increase sensitivity in wide-target LC-MS-based trace bioanalysis of small molecules. Front. Mol. Biosci. 2022, 9, 857505. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.G.P.; Medina, D.A.V.; Lanças, F.M. Development of Wall-Coated Open Tubular Columns and Their Application to Nano Liquid Chromatography Coupled to Tandem Mass Spectrometry. Molecules 2023, 28, 5103. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Ponce-Rodríguez, H.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Determination of caffeine in dietary supplements by miniaturized portable liquid chromatography. J. Chromatogr. A 2022, 1664, 462770. [Google Scholar] [CrossRef] [PubMed]
- Diep, T.T.; Bizley, S.; Edwards, A.D. 3D-Printed Dip Slides Miniaturize Bacterial Identification and Antibiotic Susceptibility Tests Allowing Direct Mastitis Sample Analysis. Micromachines 2022, 13, 941. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Gregucci, D.; Guardigli, M.; Michelini, E. Low-cost and sustainable smartphone-based tissue-on-chip device for bioluminescence biosensing. Biosens. Bioelectron. 2024, 261, 116454. [Google Scholar] [CrossRef]
- Plachká, K.; Pilařová, V.; Horáček, O.; Gazárková, T.; Vlčková, H.K.; Kučera, R.; Nováková, L. Columns in analytical-scale supercritical fluid chromatography: From traditional to unconventional chemistries. J. Sep. Sci. 2023, 46, 2300431. [Google Scholar] [CrossRef]
- Fanali, C.; D’Orazio, G. Capillary electrochromatography applied to the separation of enantiomers utilizing packed capillary columns with silica-vancomycin. A tutorial. J. Chromatogr. Open 2024, 6, 100171. [Google Scholar] [CrossRef]
- Rachkidi, M.; Michel, A.; Raffin, G.; Barattin, R.; Colinet, E.; Randon, J. Characterization of semi-packed columns with different cross section in high-pressure gas chromatography. J. Chromatogr. A 2024, 1722, 464869. [Google Scholar] [CrossRef]
- Sanders, K.L.; Edwards, J.L. Nano-liquid chromatography-mass spectrometry and recent applications in omics investigations. Anal. Methods 2020, 12, 4404–4417. [Google Scholar] [CrossRef] [PubMed]
- Planeta, J.; Moravcová, D.; Karásek, P.; Roth, M. Fabrication of monolithic capillary columns with inner diameter 50–530 μm employing a mixture of pentaerythritol tetraacrylate and polyhedral oligomeric silsesquioxane-methacrylate as crosslinkers. J Sep Sci. 2022, 45, 3256–3263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, K.; Shao, C.; Shi, X.; Zeng, J.; Guo, R.; Zhang, B. Performance of nanoflow liquid chromatography using core-shell particles: A comparison study. J. Chromatogr. A 2021, 1648, 462218. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Maciel, E.V.S.; de Toffoli, A.L.; Lanças, F.M. Miniaturization of liquid chromatography coupled to mass spectrometry.: 2. Achievements on modern instrumentation for miniaturized liquid chromatography coupled to mass spectrometry. TrAC Trends Anal. Chem. 2020, 128, 115910. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Maciel, E.V.S.; Lanças, F.M. Miniaturization of liquid chromatography coupled to mass spectrometry. 3. Achievements on chip-based LC–MS devices. TrAC Trends Anal. Chem. 2020, 131, 116003. [Google Scholar] [CrossRef]
- Desmet, G.; Van Geite, W.; Jimidar, I. Separation Science: The State of the Art: The Future of Column Packing Technology. LCGC Eur. 2022, 35, 430–432. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Z.J.; Desmet, G.; Cabooter, D. In-Depth Performance Analysis and Comparison of Monolithic and Particulate Zwitterionic Hydrophilic Interaction Liquid Chromatography Polymer Columns. Molecules 2023, 28, 2902. [Google Scholar] [CrossRef]
- Li, W.; Gao, C.; Wang, Y.; Zuo, H.; Bian, Y.; Li, C.; Ma, S.; Shen, Y.; Ou, J. Construction of adamantane-based monolithic column with three-dimensionally porous structure for small molecules separation and biosample analysis. Anal. Chim. Acta 2024, 1317, 342900. [Google Scholar] [CrossRef]
- Beltekin, B.; Aydoğan, C.; Alharthi, S.; El Rassi, Z. Chapter 8—Monolithic column based capillary- and nano-liquid chromatography applied to protein separation. In Biophysics at the Nanoscale; Denizli, A., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 151–166. [Google Scholar] [CrossRef]
- Castañeda, F.N.; Prince, D.L.; Peirano, S.R.; Giovannoni, S.; Echevarría, R.N.; Keunchkarian, S.; Reta, M. New sorbents for sample pretreatment: Development and applications. TrAC Trends Anal. Chem. 2024, 180, 117924. [Google Scholar] [CrossRef]
- Ashenef, A.; Tesfaye, E.; Hymete, A.; Niguss, T. Theory and Recent Applications of Nano-Liquid Chromatography. In Relevant Applications of High-Performance Liquid Chromatography in Food, Environmental, Clinical and Biological Fields; Núñez, O., Ed.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Li, M.; Zhu, C.; Yang, H.; Dong, P.; Lu, M.; Wang, W.; Cao, J.; Liu, Q.; et al. Boosting CO2 hydrogenation of Fe-based monolithic catalysts via 3D printing technology-induced heat/mass-transfer enhancements. Appl. Catal. B Environ. 2024, 340, 123211. [Google Scholar] [CrossRef]
- Zajickova, Z. Review of recent advances in development and applications of organic-silica hybrid monoliths. J. Sep. Sci. 2023, 46, 202300396. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lei, X.; Wang, S.; Lin, C.; Wu, X. Ionic liquid assisted in situ growth of nano-confined ionic liquids/metal-organic frameworks nanocomposites for monolithic capillary microextraction of microcystins in environmental waters. J. Chromatogr. A 2023, 1692, 463849. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Su, M.; Chen, P.; Yan, M.; Wang, D.; Xia, L.; Rao, L.; Xia, Z.; Fu, Q. Zirconium(IV) coordination-mediated rapid and versatile post-modification of polydopamine coating as stationary phase for open-tubular capillary electrochromatography. J. Chromatogr. A 2024, 1736, 465415. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhou, W.; Wang, C.; Liu, Z.; Chen, Z. Direct coupling in-tube solid-phase microextraction with mass spectrometry using polymer coated open-tubular column for rapid analysis of antiepileptic drugs in biofluids. Anal. Chim. Acta 2023, 1240, 340775. [Google Scholar] [CrossRef]
- Ning, W.; Xiang, Y.; Zhang, L.; Ye, N. Hydrogen-bonded organic frameworks as stationary phase for open-tubular capillary electrochromatography. Anal. Chim. Acta 2024, 1326, 343148. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Z.; Hu, C.; Li, Q.; Chen, Z. Synthesis of carbon dots-based covalent organic nanomaterial as stationary phase for open tubular capillary electrochromatography. J. Chromatogr. A 2022, 1678, 463343. [Google Scholar] [CrossRef]
- Harnmontree, W.; Somboot, W.; Kawichai, S.; Jakmunee, J.; Kanyanee, T. Cost-effective low-volume sample introduction system for open tubular capillary ion chromatography via a hybrid negative pressure approach. Talanta 2025, 284, 127243. [Google Scholar] [CrossRef]
- Ma, M.; Chen, C.; Zhu, X.; Li, X.; Du, Y.; Zhang, L.; Gan, J. A porous layer open-tubular capillary column supported with pepsin and zeolitic imidazolate framework for enantioseparation of four basic drugs in capillary electrochromatography. J. Chromatogr. A 2021, 1637, 461866. [Google Scholar] [CrossRef]
- Xiang, P.; Yang, Y.; Chen, H.; Chen, A.; Liu, S. Liquid chromatography using ≤5 μm open tubular columns. TrAC Trends Anal. Chem. 2021, 142, 116321. [Google Scholar] [CrossRef]
- Tsunoda, M. On-Chip Liquid Chromatography. Encyclopedia 2022, 2, 617–624. [Google Scholar] [CrossRef]
- Shen, Y.-F.; Zhang, X.; Mo, C.-E.; Huang, Y.-P.; Liu, Z.-S. Preparation of graphene oxide incorporated monolithic chip based on deep eutectic solvents for solid phase extraction. Anal. Chim. Acta 2020, 1096, 184–192. [Google Scholar] [CrossRef]
- Graf, A.; Hild, O.R.; Zeh, G.; Sauerwald, T.; Buecking, M. Concept for a MEMS-based GC Chip as a Part of an Easy-to-Use Handheld System. In Proceedings of the MikroSystemTechnik 2023, Dresden, Germany, 23–25 October 2023. [Google Scholar]
- Chen, P.-C.; Zhang, W.-Z.; Chen, W.-R.; Jair, Y.-C.; Wu, Y.-H.; Liu, Y.-H.; Chen, P.-Z.; Chen, L.-Y.; Chen, P.-S. Engineering an integrated system with a high pressure polymeric microfluidic chip coupled to liquid chromatography-mass spectrometry (LC-MS) for the analysis of abused drugs. Sens. Actuators B Chem. 2022, 350, 130888. [Google Scholar] [CrossRef]
- Weise, C.; Schirmer, M.; Polack, M.; Korell, A.; Westphal, H.; Schwieger, J.; Warias, R.; Zimmermann, S.; Belder, D. Modular Chip-Based nanoSFC–MS for Ultrafast Separations. Anal. Chem. 2024, 96, 13888–13896. [Google Scholar] [CrossRef]
- Chen, X.; Ye, L.; Yu, D. Terahertz sensing with a 3D meta-absorbing chip based on two-photon polymerization printing. Photon. Res. 2024, 12, 895–903. [Google Scholar] [CrossRef]
- Maciel, E.S.; Borsatto, J.V.B.; Mejia-Carmona, K.; Lanças, F.M. Application of an in-house packed octadecylsilica-functionalized graphene oxide column for capillary liquid chromatography analysis of hormones in urine samples. Anal. Chim. Acta 2023, 1239, 340718. [Google Scholar] [CrossRef] [PubMed]
- Son, A.; Kim, W.; Park, J.; Park, Y.; Lee, W.; Lee, S.; Kim, H. Mass Spectrometry Advancements and Applications for Biomarker Discovery, Diagnostic Innovations, and Personalized Medicine. Int. J. Mol. Sci. 2024, 25, 9880. [Google Scholar] [CrossRef]
- Rafiq, S.M.; Majumder, R.; Joshi, D.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Sidiqi, U.S. Lab-on-a-chip device for food quality control and safety. Food Control 2024, 164, 110596. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Lanças, F.M. What still hinders the routine application of miniaturized liquid chromatography beyond the omics sciences? J. Chromatogr. Open 2024, 6, 100149. [Google Scholar] [CrossRef]
- Li, M.; Yang, P.; Wu, J.; Ni, R.; Yuan, H.; Guo, Z.; Zou, J.; Gao, W.; Cong, H.; Jin, Q. Highly efficient and label-free sensitive detection of tumor-derived exosome with an aptasensor-based microfluidic chip. Microchem. J. 2024, 203, 110875. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; He, M.; Hu, B. Hierarchically porous covalent organic framework doped monolithic column for on-line chip-based array microextraction of nonsteroidal anti-inflammatory drugs in microlitre volume of blood. Anal. Chim. Acta 2024, 1331, 343332. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Hwang, S.; Choi, H.; Kim, J.; Lim, S.-H. UiO-66–coated hybrid micro-gas chromatography column chips: Evaluation of gas preconcentration and separation performances as a function of particle size, pore size, time, concentration, and flow rate. Chem. Eng. J. 2024, 500, 157075. [Google Scholar] [CrossRef]
- Karim, M.A.; Abdullah, M.Z.; Deifalla, A.F.; Azab, M.; Waqar, A. An assessment of the processing parameters and application of fibre-reinforced polymers (FRPs) in the petroleum and natural gas industries: A review. Results Eng. 2023, 18, 101091. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Shao, Q.; Li, Z.; Chun, Z.; Wang, Y.; Zhou, Y.; Chen, R. Screening, fingerprinting, and identification of phenolic antioxidants in Persicaria chinensis (L.) H. Gross by liquid chromatography–electrochemical detection and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2025, 1250, 124387. [Google Scholar] [CrossRef] [PubMed]
- Kuru, C.İ.; Ulucan-Karnak, F.; Akgöl, S. 4-Lab-on-a-chip sensors: Recent trends and future applications. In Fundamentals of Sensor Technology; Barhoum, A., Altintas, Z., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 65–98. [Google Scholar] [CrossRef]
- Wang, J.; Tang, S.; Zhang, K. Quantitation of polyethylene glycol by size exclusion chromatography with charged aerosol, differential refractive index, and multi-angle light scattering detectors. J. Pharm. Biomed. Anal. 2024, 238, 115854. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Wang, D.; Jiang, X. Recent advancements in nucleic acid detection with microfluidic chip for molecular diagnostics. TrAC Trends Anal. Chem. 2023, 158, 116871. [Google Scholar] [CrossRef]
- Soto-Rodríguez, L.D.; García-Rojas, N.S.; Hernández-Caricio, C.; Guillén-Alonso, H.; DeLuna, A.; Mancera, E.; Winkler, R. High-throughput detection of bottle materials of agave spirits using 3D-printed cartridges for paper-spray ionization mass spectrometry. Talanta Open 2024, 10, 100387. [Google Scholar] [CrossRef]
- Yang, L.; Lopes, I.C.; Vadgama, P. Self-cleaning sensors based on thermoresponsive polymeric film modified screen-printed platinum electrodes. Chem. Eng. J. 2023, 474, 145932. [Google Scholar] [CrossRef]
- Qiu, Y.; Shi, M.; Guo, X.; Li, J.; Wu, J.; Zhou, Y.; Sun, H.; Hang, Y.; Li, X.; Li, Y. Sensitivity improvement in the measurement of minor components by spatial confinement in fiber-optic laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2023, 209, 106800. [Google Scholar] [CrossRef]
- Lebanov, L.; Mikhail, I.E.; Paull, B. Light-emitting diode-based absorbance detectors for flow-through analysis in analytical chemistry: A tutorial. J. Chromatogr. Open 2024, 6, 100191. [Google Scholar] [CrossRef]
- Prado, D.G.; de Lima, D.M.; Gonçalves, K.T.; de Oliveira, A.; de Sousa, R.M.F.; Petruci, J.F.d.S. Adaptable 3D printed detector for absorbance monitoring of column chromatography separations using deep-UV LED at 280 nm. J. Chromatogr. A 2025, 1748, 465870. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Yang, L.; Zeng, H.; Li, Y.; Pu, Q.; Zhang, M. Three-in-One Detector by 3D Printing: Simultaneous Contactless Conductivity, Ultraviolet Absorbance, and Laser-Induced Fluorescence Measurements for Capillary Electrophoresis. Anal. Chem. 2023, 95, 2146–2151. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, X.; Zhang, R.; Zhang, H.; Jiao, D.; Chang, H. A microchip based Z-cell absorbance detector integrating micro-lenses and slits for portable liquid chromatography. J. Chromatogr. A 2024, 1730, 465099. [Google Scholar] [CrossRef]
- Mansour, F.R.; Abdallah, I.A.; Bedair, A.; Hamed, M. Analytical Methods for the Determination of Quercetin and Quercetin Glycosides in Pharmaceuticals and Biological Samples. Crit. Rev. Anal. Chem. 2023, 55, 187–212. [Google Scholar] [CrossRef]
- Cho, Y.; Park, M.; Shin, Y.J.; Kim, Y.W.; Jeong, J.-Y.; Cho, E.-S.; Kwon, S.J.; Jeon, Y. Wavelength-Tunable Textile-Based Organic Light-Emitting Diode for Multifunctional Wearable Photomedical Applications and Display with Cool White to Warm White Light. ACS Photonics 2024, 11, 5418–5429. [Google Scholar] [CrossRef]
- Mikhail, I.E.; Hemida, M.; Lebanov, L.; Astrakhantseva, S.; Gupta, V.; Hortin, P.; Parry, J.S.; Macka, M.; Paull, B. Multi-wavelength deep-ultraviolet absorbance detector based upon program-controlled pulsing light-emitting diodes. J. Chromatogr. A 2023, 1709, 464382. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Liu, F. Compact Laser-Induced Fluorescence Detector with Adjustable Laser Focal Spot for Multiple Purposes. Sensors 2024, 24, 6224. [Google Scholar] [CrossRef]
- Svitková, V.; Nemčeková, K.; Drdanová, A.P.; Imreová, Z.; Tulipánová, A.; Homola, T.; Zažímal, F.; Debnárová, S.; Stýskalík, A.; Ryba, J.; et al. Advancing wastewater treatment: The efficacy of carbon-based electrochemical platforms in removal of pharmaceuticals. Chem. Eng. J. 2024, 500, 156946. [Google Scholar] [CrossRef]
- Aymerich, J.; Ferrer-Vilanova, A.; Cisneros-Fernández, J.; Escudé-Pujol, R.; Guirado, G.; Terés, L.; Dei, M.; Muñoz-Berbel, X.; Serra-Graells, F. Ultrasensitive bacterial sensing using a disposable all-in-one amperometric platform with self-noise cancellation. Biosens. Bioelectron. 2023, 234, 115342. [Google Scholar] [CrossRef]
- Knihnicki, P.; Kusior, B.; Paluch, J.; Kościelniak, P.; Kochana, J. A novel approach of flow system with a 3D-printed Direct Injector Detector for electrochemical determination of cadmium and lead. Microchem. J. 2023, 195, 109514. [Google Scholar] [CrossRef]
- Salguero Salas, M.A.; Fuertes, V.C.; Arciniegas Jaimes, D.M.; Bajales, N.; Linarez Pérez, O.E. Electrochemical boost via thermally reduced graphene oxide for tailoring composite paste electrodes. FlatChem 2024, 48, 100766. [Google Scholar] [CrossRef]
- Shin, J.; Gwon, Y.; Hwang, S.Y.; Bae, S.; Kim, S.Y.; Rhee, C.K.; Sohn, Y. Electrochemical CO2 reduction chemistry of C1 and C2+ products on Cu/Zn electrodes via galvanic replacement. J. Alloys Compd. 2025, 1010, 177660. [Google Scholar] [CrossRef]
- Vujančević, J.; Sodnik, N.; Korent, A.; Črešnovar, Š.; Trebše, P.; Kralj, M.B.; Martelanc, M.; Samardžija, Z.; Soderžnik, K.Ž. Rapid and reliable electrochemical detection of bisphenol S in thermal paper. Sens. Bio-Sens. Res. 2024, 44, 100662. [Google Scholar] [CrossRef]
- Balkourani, G.; Brouzgou, A.; Tsiakaras, P. A review on recent advancements in electrochemical detection of dopamine using carbonaceous nanomaterials. Carbon 2023, 213, 118281. [Google Scholar] [CrossRef]
- Barhoum, A.; Hamimed, S.; Slimi, H.; Othmani, A.; Abdel-Haleem, F.M.; Bechelany, M. Modern designs of electrochemical sensor platforms for environmental analyses: Principles, nanofabrication opportunities, and challenges. Trends Environ. Anal. Chem. 2023, 38, e00199. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Samardžija, Z.; Trafela, Š.; Korent, A.; Šturm, S.; Žagar Soderžnik, K. Electrochemical detection of benzenediols using carbon-supported catalysts. Electrochim. Acta 2024, 493, 144389. [Google Scholar] [CrossRef]
- Rypar, T.; Bezdekova, J.; Pavelicova, K.; Vodova, M.; Adam, V.; Vaculovicova, M.; Macka, M. Low-tech vs. high-tech approaches in μPADs as a result of contrasting needs and capabilities of developed and developing countries focusing on diagnostics and point-of-care testing. Talanta 2024, 266, 124911. [Google Scholar] [CrossRef]
- O’Connor, E.; Micklefield, J.; Cai, Y. Searching for the optimal microbial factory: High-throughput biosensors and analytical techniques for screening small molecules. Curr. Opin. Biotechnol. 2024, 87, 103125. [Google Scholar] [CrossRef]
- Zhai, Y.; Fu, X.; Xu, W. Miniature mass spectrometers and their potential for clinical point-of-care analysis. Mass Spectrom. Rev. 2024, 43, 1172–1191. [Google Scholar] [CrossRef] [PubMed]
- Rankin-Turner, S.; Sears, P.; Heaney, L.M. Applications of ambient ionization mass spectrometry in 2022: An annual review. Anal. Sci. Adv. 2023, 4, 133–153. [Google Scholar] [CrossRef]
- Shi, L.; Habib, A.; Bi, L.; Hong, H.; Begum, R.; Wen, L. Ambient Ionization Mass Spectrometry: Application and Prospective. Crit. Rev. Anal. Chem. 2024, 54, 1584–1633. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, D.; Zhang, H.; Jiang, T.; Xu, W. Smart Miniature Mass Spectrometer Enabled by Machine Learning. Anal. Chem. 2023, 95, 5976–5984. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, H.; Hong, J.; Zhang, M.; Jiang, T.; Xu, W. Development of a High-Field “Brick” Mass Spectrometer with Extended Mass Range and Capable of Characterizing Native Proteins. Anal. Chem. 2023, 95, 13503–13508. [Google Scholar] [CrossRef]
- Smith, B.L.; Hankinson, T.; Maher, S. Portable Instrumentation for Ambient Ionization and Miniature Mass Spectrometers. Annu. Rev. Anal. Chem. 2024, 17, 69–102. [Google Scholar] [CrossRef]
- Szyszka, P.; Jendryka, J.; Sobków, J.; Zychla, M.; Białas, M.; Knapkiewicz, P.; Dziuban, J.; Grzebyk, T. MEMS quadrupole mass spectrometer. Sens. Actuators B Chem. 2024, 411, 135712. [Google Scholar] [CrossRef]
- Yang, Z.; Li, D.; Ren, Z.; Sun, J.; Zhang, H.; Cheng, L.; Wang, P.; Li, G.; Cheng, Y.; Guo, Y.; et al. Research on a miniature Mattauch-Herzog mass spectrometer designed for space exploration. Vacuum 2024, 230, 113749. [Google Scholar] [CrossRef]
- Amirian, H.; Dalvand, K.; Ghiasvand, A. Seamless integration of Internet of Things, miniaturization, and environmental chemical surveillance. Environ. Monit. Assess. 2024, 196, 582. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, S.O.; Lisse, C.; Medawala, W.; Brady, P.N.; Bwambok, D.K.; Anum, D.; Alonge, T.; Taylor, M.E.; Baker, G.A.; Mehari, T.F.; et al. Fluorescent chemical sensors: Applications in analytical, environmental, forensic, pharmaceutical, biological, and biomedical sample measurement, and clinical diagnosis. Appl. Spectrosc. Rev. 2024, 59, 1–89. [Google Scholar] [CrossRef]
- Fernandes, S.R.; Barreiros, L.; Sampaio-Maia, B.; Miró, M.; Segundo, M.A. Total analysis system for the determination of uremic toxins in human plasma based on bead injection solid phase extraction hyphenated to mass spectrometry. Anal. Chim. Acta 2023, 1277, 341668. [Google Scholar] [CrossRef]
- Chen, J.; Liu, F.; Li, Z.; Tan, L.; Zhang, M.; Xu, D. Solid phase extraction based microfluidic chip coupled with mass spectrometry for rapid determination of aflatoxins in peanut oil. Microchem. J. 2021, 167, 106298. [Google Scholar] [CrossRef]
- Ahuja, V.; Singh, A.; Paul, D.; Dasgupta, D.; Urajová, P.; Ghosh, S.; Singh, R.; Sahoo, G.; Ewe, D.; Saurav, K. Recent Advances in the Detection of Food Toxins Using Mass Spectrometry. Chem. Res. Toxicol. 2023, 36, 1834–1863. [Google Scholar] [CrossRef]
- Alves, E.; Gurupadayya, B.M.; Prabhakaran, P. Next-Generation Miniaturized Separation Platforms: Converging Detection, Automation, and Sustainable Design for Intelligent Analytical Science. Crit. Rev. Anal. Chem. 2025, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, M.; Hou, F.; He, Y.; Zhu, Y.; Dai, H.; Huang, M.; Yang, Y.; Wu, L. Multi-residue analysis of pesticides and veterinary drugs by ultra-performance liquid chromatography-tandem mass spectrometry using a modified QuEChERS method. Microchem. J. 2025, 208, 112384. [Google Scholar] [CrossRef]
- Jayasinghe, G.D.T.M.; Szpunar, J.; Lobinski, R.; Edirisinghe, E.M.R.K.B. Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp from Sri Lanka by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Fishes 2023, 8, 154. [Google Scholar] [CrossRef]
- Zareasghari, O.; Javadi, A.; Afshar Mogaddam, M.R. Deep eutectic solvent–based pressurized liquid extraction combined with dispersive liquid–liquid microextraction of organophosphorus pesticide residues in egg powder prior to high-performance liquid chromatography analysis. J. Sep. Sci. 2024, 47, 2300070. [Google Scholar] [CrossRef]
- Bo, S.Y.; Zhang, R.M.; Zhao, L.B.; Yang, S.; Lu, Z.H.; Wang, P.G. High-resolution mass spectrometry for glycoproteomics. Bioanalysis 2023, 15, 57–61. [Google Scholar] [CrossRef]
- Zhou, Y.; Ai, J.; Ye, Z.; Chen, K.; Lin, J.; Zhang, Z.; Luo, M.; Zhou, B.; Xiang, S.; Zhou, J.; et al. Fully Automated Deep Learning Enabled Miniature Mass Spectrometry System for Psychoactive Therapeutic Drug Monitoring. Adv. Sci. 2025, 12, e02721. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, N.; Gehlot, A. Application of artificial intelligence in drug design: A review. Comput. Biol. Med. 2024, 179, 108810. [Google Scholar] [CrossRef]
- Chavan, A.R.; Kaur, G. Keeping food safe: Tackling the health risks of phthalates and azo dyes. World J. Pharm. Res. 2024, 13, 1310–1325. [Google Scholar]
- Waliullah, R.M.; Rehan, A.I.; Awual, M.E.; Rasee, A.I.; Sheikh, M.C.; Salman, M.S.; Hossain, M.S.; Hasan, M.M.; Kubra, K.T.; Hasan, M.N.; et al. Optimization of toxic dye removal from contaminated water using chitosan-grafted novel nanocomposite adsorbent. J. Mol. Liq. 2023, 388, 122763. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Vasanth, K. Food contaminants: Impact of food processing, challenges and mitigation strategies for food security. Food Res. Int. 2024, 191, 114739. [Google Scholar] [CrossRef]
- Josephy, P.D.; Allen-Vercoe, E. Reductive metabolism of azo dyes and drugs: Toxicological implications. Food Chem. Toxicol. 2023, 178, 113932. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Bai, H.; Ma, Q. Rapid on-site detection of persistent organic pollutants using multiwalled carbon nanotube–modified paper spray ionization and a miniature mass spectrometer. Rapid Commun. Mass Spectrom. 2023, 37, e9509. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Darabdhara, J.; Ahmaruzzaman, M. MoS2 Nanosheets@Metal organic framework nanocomposite for enhanced visible light degradation and reduction of hazardous organic contaminants. J. Clean. Prod. 2023, 430, 139517. [Google Scholar] [CrossRef]
- Pawar, A. Recent Innovations in High-Performance Liquid Chromatography (HPLC): Method Development and Validation Strategies. J. Drug Deliv. Biother. 2024, 1, 55–61. [Google Scholar]
- Villa, C.; Costa, J.; Mafra, I. Sesame as a source of food allergens: Clinical relevance, molecular characterization, cross-reactivity, stability toward processing and detection strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 4746–4762. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Food allergen detection by mass spectrometry: From common to novel protein ingredients. Proteomics 2023, 23, 202200427. [Google Scholar] [CrossRef]
- Lin, J.F.; Chang, K.-L.; Hsieh, B.-S.; Hu, Y.-C.; Huang, E.S.; Yu, H.-S. Development of validated sandwich ELISA for detecting peanut allergen Ara h 3 in food. Food Chem. 2024, 445, 138757. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Dawson, A.L.; Bose, U.; Anderson, A.; Colgrave, M.L.; Broadbent, J.A. Safe food through better labelling; a robust method for the rapid determination of caprine and bovine milk allergens. Food Chem. 2023, 417, 135885. [Google Scholar] [CrossRef]
- Jain, S.; Lamba, B.Y.; Dubey, S.K. Recent advancements in the sensors for food analysis to detect gluten: A mini-review [2019,2020,2021,2022,2023]. Food Chem. 2024, 449, 139204. [Google Scholar] [CrossRef]
- Simone, P.; Pierri, G.; Capitani, D.; Ciogli, A.; Angelini, G.; Ursini, O.; Gentile, G.; Cavazzini, A.; Villani, C.; Gasparrini, F. Capillary methacrylate-based monoliths by grafting from/to γ-ray polymerization on a tentacle-type reactive surface for the liquid chromatographic separations of small molecules and intact proteins. J. Chromatogr. A 2017, 1498, 46–55. [Google Scholar] [CrossRef]
- Aydoğan, C. Chiral separation and determination of amino acid enantiomers in fruit juice by open-tubular nano liquid chromatography. Chirality 2018, 30, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, O.K.; Røen, B.-T.; Enger, S.; Lundanes, E.; Wilson, S.R. Multichannel Open Tubular Enzyme Reactor Online Coupled with Mass Spectrometry for Detecting Ricin. Anal. Chem. 2017, 89, 8667–8673. [Google Scholar] [CrossRef]
- Alcántara-Durán, J.; Moreno-González, D.; Gilbert-López, B.; Molina-Díaz, A.; García-Reyes, J.F. Matrix-effect free multi-residue analysis of veterinary drugs in food samples of animal origin by nanoflow liquid chromatography high resolution mass spectrometry. Food Chem. 2018, 245, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mu, T.-H. Identification and characterization of antioxidant peptides from sweet potato protein hydrolysates by Alcalase under high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2017, 43, 92–101. [Google Scholar] [CrossRef]
- Li, R.; Shao, Y.; Yu, Y.; Wang, X.; Guo, G. Pico-HPLC system integrating an equal inner diameter femtopipette into a 900 nm I.D. porous layer open tubular column. Chem. Commun. 2017, 53, 4104–4107. [Google Scholar] [CrossRef]
- Lin, S.-L.; Fuh, M.-R. Preparation and characterization of vinylimidazole-based polymer monolithic stationary phases for reversed-phase and hydrophilic interaction capillary liquid chromatography. Talanta 2018, 187, 73–82. [Google Scholar] [CrossRef]
- Fiedler, K.L.; Panda, R.; Croley, T.R. Analysis of Gluten in a Wheat-Gluten-Incurred Sorghum Beer Brewed in the Presence of Proline Endopeptidase by LC/MS/MS. Anal. Chem. 2018, 90, 2111–2118. [Google Scholar] [CrossRef]
- Bromilow, S.; Gethings, L.A.; Buckley, M.; Bromley, M.; Shewry, P.R.; Langridge, J.I.; Clare Mills, E.N. A curated gluten protein sequence database to support development of proteomics methods for determination of gluten in gluten-free foods. J. Proteom. 2017, 163, 67–75. [Google Scholar] [CrossRef]
- García-Molina, M.D.; Muccilli, V.; Saletti, R.; Foti, S.; Masci, S.; Barro, F. Comparative proteomic analysis of two transgenic low-gliadin wheat lines and non-transgenic wheat control. J. Proteom. 2017, 165, 102–112. [Google Scholar] [CrossRef]
- Arena, S.; D’Ambrosio, C.; Vitale, M.; Mazzeo, F.; Mamone, G.; Di Stasio, L.; Maccaferri, M.; Curci, P.L.; Sonnante, G.; Zambrano, N.; et al. Differential representation of albumins and globulins during grain development in durum wheat and its possible functional consequences. J. Proteom. 2017, 162, 86–98. [Google Scholar] [CrossRef]
- Adedeji, A.A.; Priyesh, P.V.; Odugbemi, A.A. The Magnitude and Impact of Food Allergens and the Potential of AI-Based Non-Destructive Testing Methods in Their Detection and Quantification. Foods 2024, 13, 994. [Google Scholar] [CrossRef]
- Yuan, Y.; Jia, H.; Xu, D.; Wang, J. Novel method in emerging environmental contaminants detection: Fiber optic sensors based on microfluidic chips. Sci. Total Environ. 2023, 857, 159563. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wu, W.; Li, M.; Li, Z.; Pan, L.; Ahamad, T.; Alshehri, S.M.; Bando, Y.; Yamauchi, Y.; Xu, X. Metal-organic framework-based nanoarchitectonics: A promising material platform for electrochemical detection of organophosphorus pesticides. Coord. Chem. Rev. 2024, 501, 215534. [Google Scholar] [CrossRef]
- Primost, M.A.; Chierichetti, M.A.; Castaños, C.; Bigatti, G.; Miglioranza, K.S.B. Persistent Organic Pollutants (POPs), Current Use Pesticides (CUPs) and Polycyclic Aromatic Hydrocarbons (PAHs) in edible marine invertebrates from a Patagonian harbor. Mar. Pollut. Bull. 2024, 207, 116940. [Google Scholar] [CrossRef]
- Murray, E.; Li, Y.; Currivan, S.A.; Moore, B.; Morrin, A.; Diamond, D.; Macka, M.; Paull, B. Miniaturized capillary ion chromatograph with UV light-emitting diode based indirect absorbance detection for anion analysis in potable and environmental waters. J. Sep. Sci. 2018, 41, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nesterenko, P.N.; Stanley, R.; Paull, B.; Macka, M. High sensitivity deep-UV LED-based z-cell photometric detector for capillary liquid chromatography. Anal. Chim. Acta 2018, 1032, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Termopoli, V.; Famiglini, G.; Palma, P.; Piergiovanni, M.; Rocio-Bautista, P.; Ottaviani, M.F.; Cappiello, A.; Saeed, M.; Perry, S. Evaluation of a liquid electron ionization liquid chromatography–mass spectrometry interface. J. Chromatogr. A 2019, 1591, 120–130. [Google Scholar] [CrossRef]
- Magrini, L.; Famiglini, G.; Palma, P.; Termopoli, V.; Cappiello, A. Boosting the Detection Potential of Liquid Chromatography-Electron Ionization Mass Spectrometry Using a Ceramic Coated Ion Source. J. Am. Soc. Mass Spectrom. 2016, 27, 153–160. [Google Scholar] [CrossRef][Green Version]
- Termopoli, V.; Famiglini, G.; Palma, P.; Piergiovanni, M.; Cappiello, A. Atmospheric Pressure Vaporization Mechanism for Coupling a Liquid Phase with Electron Ionization Mass Spectrometry. Anal. Chem. 2017, 89, 2049–2056. [Google Scholar] [CrossRef]
- Serra-Mora, P.; Herráez-Hernández, R.; Campíns-Falcó, P. Minimizing the impact of sample preparation on analytical results: In-tube solid-phase microextraction coupled on-line to nano-liquid chromatography for the monitoring of tribenuron methyl in environmental waters. Sci. Total Environ. 2020, 721, 137732. [Google Scholar] [CrossRef]
- Zhan, C.; Ju, Z.; Xie, B.; Chen, J.; Ma, Q.; Li, M. Signal processing for miniature mass spectrometer based on LSTM-EEMD feature digging. Talanta 2025, 281, 126904. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluidics 2021, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, H.; Ren, S.; Zhang, Y.; Yang, Y.; Chang, H. Portable liquid chromatography for point-of-care testing of glycated haemoglobin. Sens. Actuators B Chem. 2020, 305, 127484. [Google Scholar] [CrossRef]
- Ponce-Rodríguez, H.D.; García-Robles, A.A.; Sáenz-González, P.; Verdú-Andrés, J.; Campíns-Falcó, P. On-line in-tube solid phase microextraction coupled to capillary liquid chromatography-diode array detection for the analysis of caffeine and its metabolites in small amounts of biological samples. J. Pharm. Biomed. Anal. 2020, 178, 112914. [Google Scholar] [CrossRef]
- Li, H.; Ai, L.; Fan, S.; Wang, Y.; Sun, D. Rapid determination of 18 glucocorticoids in serum using reusable on-line SPE polymeric monolithic column coupled with LC-quadrupole/orbitrap high-resolution mass spectrometer. J. Chromatogr. B 2017, 1065–1066, 79–86. [Google Scholar] [CrossRef]
- Ohtsu, Y.; van Trigt, R.; Takama, K.; Groenendaal, D.; Takada, A.; Nakamura, T.; Noguchi, K. Quantification of ASP2151 in Human Plasma and Urine: A Pitfall Associated with Supersaturation of Analyte in Urine. Chromatographia 2017, 80, 217–227. [Google Scholar] [CrossRef]
- Wei, D.; Liu, J.; Guo, M.; Zhu, Y. Determination of betaine, l-carnitine, and choline in human urine using a self-packed column and column-switching ion chromatography with nonsuppressed conductivity detection. J. Sep. Sci. 2017, 40, 4246–4255. [Google Scholar] [CrossRef]
- Zhai, Y.; Feng, Y.; Wei, Y.; Wang, Y.; Xu, W. Development of a miniature mass spectrometer with continuous atmospheric pressure interface. Analyst 2015, 140, 3406–3414. [Google Scholar] [CrossRef]
- Wu, Q.; Tian, Y.; Li, A.; Andrews, D.; Hawkins, A.R.; Austin, D.E. A Miniaturized Linear Wire Ion Trap with Electron Ionization and Single Photon Ionization Sources. J. Am. Soc. Mass Spectrom. 2017, 28, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.-C.; Etter, R.; Schaer, M.; Siegenthaler, P.; Zenobi, R. Direct and Sensitive Detection of CWA Simulants by Active Capillary Plasma Ionization Coupled to a Handheld Ion Trap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2016, 27, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Albergamo, A.; Rigano, F.; Purcaro, G.; Mauceri, A.; Fasulo, S.; Mondello, L. Free fatty acid profiling of marine sentinels by nanoLC-EI-MS for the assessment of environmental pollution effects. Sci. Total Environ. 2016, 571, 955–962. [Google Scholar] [CrossRef]
- Aydoğan, C.; Alharthi, S. Nano-LC with New Hydrophobic Monolith Based on 9-Antracenylmethyl Methacrylate for Biomolecule Separation. Int. J. Mol. Sci. 2024, 25, 13646. [Google Scholar] [CrossRef]
- Aydoğan, C.; Günyel, Z.; Ali, A.; Göktürk, I.; Yılmaz, F.; Denizli, A. Recent advances and applications of miniaturized analytical- and on-line sample preparation- columns. J. Chromatogr. Open 2025, 8, 100247. [Google Scholar] [CrossRef]
- Aydoğan, C.; Bayındır, S.; Avcil, H.; Yurt, B. Nano-LC with new neutral monolith for biomolecule separation. Green Anal. Chem. 2025, 12, 100226. [Google Scholar] [CrossRef]
- He, B.; Di, X.; Guled, F.; Harder, A.V.E.; van den Maagdenberg, A.M.J.M.; Terwindt, G.M.; Krekels, E.H.J.; Kohler, I.; Harms, A.; Ramautar, R.; et al. Quantification of endocannabinoids in human cerebrospinal fluid using a novel micro-flow liquid chromatography-mass spectrometry method. Anal. Chim. Acta 2022, 1210, 339888. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Zhang, M.; Dong, L.; Zou, B.; Jin, J.; Liu, Y.; Yu, D.; Xu, Y.; Fan, Y.; et al. Rapid on-site detection of illicit drugs in urine using C18 pipette-tip based solid-phase extraction coupled with a miniaturized mass spectrometer. J. Chromatogr. A 2024, 1738, 465485. [Google Scholar] [CrossRef]
- Henning, R.W.; Kosheleva, I.; Šrajer, V.; Kim, I.-S.; Zoellner, E.; Ranganathan, R. BioCARS: Synchrotron facility for probing structural dynamics of biological macromolecules. Struct. Dyn. 2024, 11, 014301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).